Abstract
Muscle represents an attractive target tissue for adeno-associated virus (AAV) vector–mediated gene transfer for hemophilia B (HB). Experience with direct intramuscular (i.m.) administration of AAV vectors in humans showed that the approach is safe but fails to achieve therapeutic efficacy. Here, we present a careful evaluation of the safety profile (vector, transgene, and administration procedure) of peripheral transvenular administration of AAV-canine factor IX (cFIX) vectors to the muscle of HB dogs. Vector administration resulted in sustained therapeutic levels of cFIX expression. Although all animals developed a robust antibody response to the AAV capsid, no T-cell responses to the capsid antigen were detected by interferon (IFN)-γ enzyme-linked immunosorbent spot (ELISpot). Interleukin (IL)-10 ELISpot screening of lymphocytes showed reactivity to cFIX-derived peptides, and restimulation of T cells in vitro in the presence of the identified cFIX epitopes resulted in the expansion of CD4+FoxP3+IL-10+ T-cells. Vector administration was not associated with systemic inflammation, and vector spread to nontarget tissues was minimal. At the local level, limited levels of cell infiltrates were detected when the vector was administered intravascularly. In summary, this study in a large animal model of HB demonstrates that therapeutic levels of gene transfer can be safely achieved using a novel route of intravascular gene transfer to muscle.
Introduction
Adeno-associated virus (AAV) vectors can direct efficient gene transfer into a variety of target tissues.1,2,3,4,5,6 Among these, muscle is a key target for gene transfer strategies directed to the treatment of neuromuscular7 and metabolic diseases,8 and for hemophilia B (HB) when liver is compromised due to viral hepatitis.9,10 Direct intramuscular (i.m.) administration has been extensively studied in experimental animals and humans.5,8,11,12,13,14,15 In HB subjects, direct i.m. administration of an AAV-2 vector encoding human factor IX (FIX) resulted in long-term expression of the transgene, which was detectable in muscle sections >3 years after gene transfer.1 However, this delivery method failed to reach therapeutic levels of FIX in the circulation, even at a dose of ~2 × 1012 vector genomes (vg)/kg (refs. 11,12). The poor performance of AAV-2 vectors in reaching large areas of muscle when injected i.m. may also be associated with higher transgene immunogenicity. Importantly, and directly related to the delivery method,16 high levels of expression achieved locally (i.e., high antigen concentration around the injection site) may enhance the risk of immune responses to the transgene product;17,18 this holds true particularly for proinflammatory environments, such as dystrophic muscle, which constitute a danger signal per se.19 In the setting of genetic disease, lack of tolerance to the product of the donated gene may be associated with unwanted immune responses, resulting in loss of therapeutic efficacy. Although AAV gene transfer to liver is associated with induction of tolerance to the transgene product20 mediated by regulatory T-cells,2,21 there is no evidence that AAV-directed muscle gene transfer is associated with regulatory T-cells expansion.22,23,24 Another important aspect of AAV-mediated gene transfer is the T-cell response to the vector capsid; this issue emerged recently in the context of liver-directed gene transfer,25,26 in which transgene expression in human subjects with HB was detected only transiently after AAV-2 gene transfer for FIX, due to a capsid-specific CD8+ T-cell response that resulted in clearance of transduced hepatocytes. Similar findings were also described in the context of i.m. gene transfer;5,27 although the role of T-cell responses to the AAV capsid is still under study, data suggest that dose-dependent activation of capsid T-cell responses may limit the duration of the therapeutic transgene expression after both intravascular or i.m. gene transfer, with responses less marked in healthy muscle tissue where class I expression is usually low [although recently published data in a clinical trial of AAV-1 gene transfer in patients with limb-girdle muscular dystrophy type 2B (α-sarcoglycan deficiency) documented upregulation of major histocompatibility complex class I expression on muscle fibers at sites of direct injection of AAV-1; ref. 6].
We showed that regional intravascular delivery using vasoactive drugs to achieve AAV vector extravasation through the vascular endothelium results in sustained expression of the cFIX transgene in HB dogs.28 More recently, using afferent transvenular retrograde extravasation (ATVRX) in the same dog model we achieved sustained correction of the HB disease phenotype.29 Using pressure instead of pharmacological intervention to increase vascular permeability, we achieved canine FIX (cFIX) transgene expression levels up to tenfold higher than those measured with the same vector and dose, delivered by direct i.m. injection.13 For both regional intravascular and ATVRX vector delivery, transient immunosuppression (IS) was needed to prevent antibody responses to the cFIX donated gene product in hemophilic dogs.
In this study, we present the complete characterization of the safety profile of ATVRX delivery of AAV vectors encoding the cFIX transgene in HB dogs. We show that AAV vector delivery of the cFIX transgene to muscle via ATVRX under transient immunosuppression is associated with (i) limited, non-neutralizing antibody responses to the cFIX transgene characterized by almost exclusive production of IgG2 antibodies; (ii) absence of T-cell responses to the AAV capsid; (iii) secretion of interleukin (IL)-10 at high levels in response to cFIX antigen or cFIX-derived peptides in circulating peripheral blood mononuclear cells (PBMCs), and expansion of a population of CD4+IL-10+FoxP3+ T-cells in response to cFIX antigen; (iv) minimal systemic or local inflammation; and (v) minimal vector transduction of nontarget tissues. Together, these data support the safety of ATVRX vector administration for the correction of the HB disease phenotype.
Results
ATVRX administration of AAV vectors to the muscle of HB dogs under IS results in sustained expression of the cFIX transgene
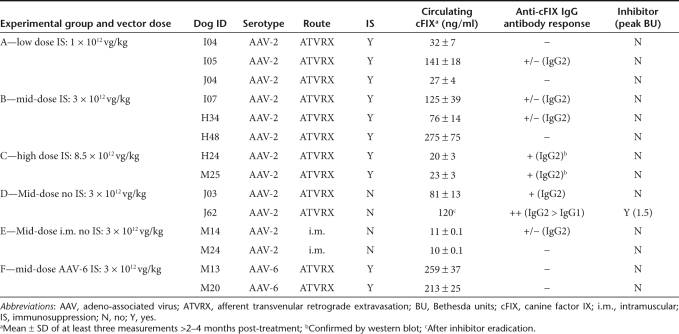
The safety of AAV-mediated muscle gene transfer via ATVRX30 was evaluated in a large cohort of HB dogs (Table 1) carrying a missense mutation in the cFIX gene (University of North Carolina at Chapel Hill colony). HB dogs received 1 × 1012 vg/kg (low dose, n = 2), 3 × 1012 vg/kg (mid-dose, n = 3), or 8.5 × 1012 vg/kg (high dose, n = 2) of an AAV-2-cFIX vector via ATVRX under transient IS with cyclophosphamide. As controls, four HB dogs received 3 × 1012 vg/kg of the same vector via ATVRX (n = 2) or i.m. (n = 2) without IS (Table 1). Two additional HB dogs received 3 × 1012 vg/kg of an AAV-6-cFIX vector via ATVRX with IS. ATVRX delivery of the AAV-2-cFIX vector in HB dogs resulted in plateau plasma levels of cFIX transgene product ranging from ~80 to ~275 ng/ml at a dose of 3 × 1012 vg/kg, compared to ~10 ng/ml of circulating cFIX obtained when the same vector at the same dose was delivered i.m. (compare group B to group E in Table 1). A further dose advantage was obtained by switching to an AAV serotype 6 vector (Table 1). Efficacy of ATVRX delivery in HB dogs is discussed elsewhere.29
Table 1. Summary of experimental design and cFIX expression data.
No postphlebitic syndrome or postprocedure angiopathy has been noted in any of the animals. Transient IS, given around the time of ATVRX administration of the vector in HB dogs, efficiently prevented inhibitory antibody responses to the cFIX transgene product at vector doses up to 3 × 1012 vg/kg. However, at a vector dose of 8.5 × 1012 vg/kg, declining cFIX transgene expression levels were observed even under IS, and the formation of non-neutralizing IgG2 antibodies to cFIX was documented by enzyme-linked immunosorbent assay and western blot shortly after IS discontinuation. ATVRX administration of 3 × 1012 vg/kg of the AAV-2-cFIX vector with no concomitant administration of cyclophosphamide resulted in the development of a neutralizing antibody to the cFIX in one animal (J62), peaking at 1.5 Bethesda units and slowly disappearing over time; in this dog, antibody subclass analysis showed the production of both IgG1 and IgG2, with the titer for IgG2 > IgG1. None of the other HB injected dogs developed neutralizing anti-cFIX antibodies; however, several dogs developed low-titer (<1,000 ng/ml) non-neutralizing IgG2 antibodies >3 months after vector administration (Table 1). These antibodies did not seem to affect the levels of circulating cFIX transgene product (Table 1).
We next undertook a complete evaluation of local and systemic immune responses in these experimental groups.
Intravascular or i.m. AAV vector administration results in IgG2 anti-capsid antibody formation but no capsid-triggered IFN-γ secretion detected by ELISpot
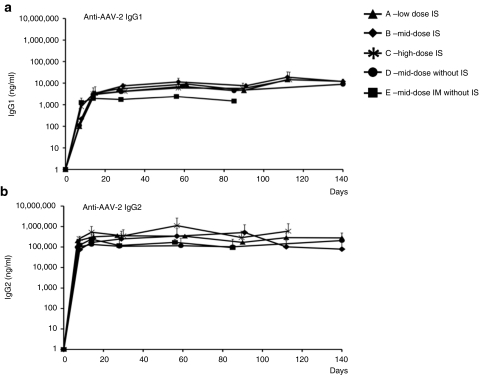
All naive animals had no detectable anti-AAV antibodies before vector administration. Following gene transfer, all animals developed high-titer antibodies to the AAV capsid (Figure 1). No significant difference in the titer was observed among the experimental groups, except for a ~twofold higher titer measured in the mid-dose ATVRX dogs compared to dogs injected i.m. with the same vector and dose (3 × 1012 vg/kg), probably reflecting the more extensive systemic exposure to the vector. Anti-AAV antibody subclass analysis showed the prevalence of a Th2 response, characterized by the secretion of IgG2 (Figure 1b), with limited secretion of IgG1 (Figure 1a). Similar differences in relative titers of IgG subclasses were noted in dogs receiving AAV-1 or AAV-6 i.m. or via ATVRX, respectively (data not shown).
Figure 1.
Humoral responses to capsid proteins after ATVRX or intramuscular (i.m.) vector delivery. Anti-AAV-2 capsid (a) IgG1 and (b) IgG2 subclass antibody titer measured by enzyme-linked immunosorbent assay; values are represented as average (n = 2 per time point) ± SD; animals received AAV-2 under immunosuppression (IS) by ATVRX at a dose of 1 × 1012 vg/kg (group A), 3 × 1012 vg/kg (group B), 8 × 1012 vg/kg (group C) or without IS at a dose of 3 × 1012 vg/kg either by ATVRX (group D) or i.m. (group E); x axis, time in days. ATVRX, afferent transvenular retrograde extravasation.
A capsid-specific interferon (IFN)-γ enzyme-linked immunosorbent spot (ELISpot) assay was used to follow T-cell responses to the AAV capsid. For antigen stimulation, a peptide library of 15-mers overlapping in sequence by 10 amino acids and spanning the sequence of the VP-1 capsid protein was used for the screening; alternatively, purified AAV empty capsids were also used. No T-cell responses to the AAV capsid were detected at baseline or at any time point tested in the dogs in the study (Supplementary Table S1). These results were also confirmed in HB dogs re-administered with an AAV-2 or an AAV-6 vector expressing the cFIX transgene via ATVRX (data not shown).
To test whether alternate AAV serotypes with higher tropism for muscle tissue than AAV-2 were more immunogenic, we also injected a normal dog (J53) i.m. with an AAV-1-null vector (J53) and monitored T-cell responses to the capsid by IFN-γ ELISpot; similar to the other animals, no response was detectable at any time point in J53 (Supplementary Table S1).
T-cell responses to the cFIX transgene product are characterized by secretion of IL-10 detected in an ELISpot assay
T-cell responses directed to the cFIX transgene were analyzed by IFN-γ and IL-10 ELISpots to detect antigen-specific Th1 and Th2 responses, respectively. No IFN-γ responses to peptides derived from cFIX were detected at any time (Supplementary Table S1), consistent with the results of anti-cFIX antibody subclass analysis showing prevalent secretion of non-neutralizing anti-cFIX IgG2 antibodies (Table 1). Absence of IFN-γ response to the cFIX transgene product was also documented in dog J62 although the inhibitor to cFIX persisted (Table 1).
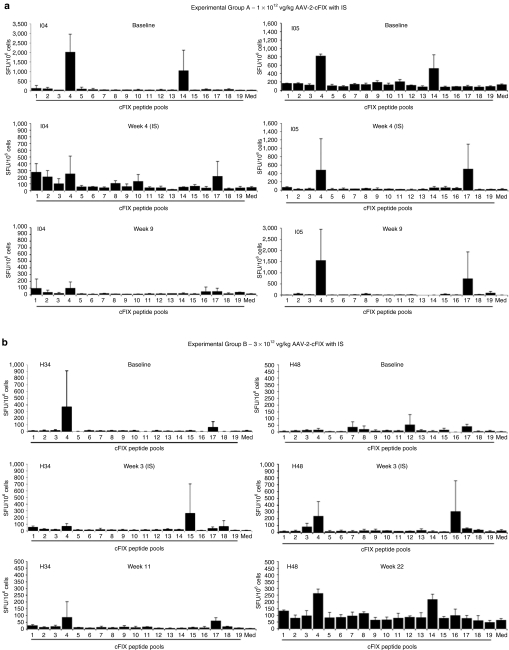
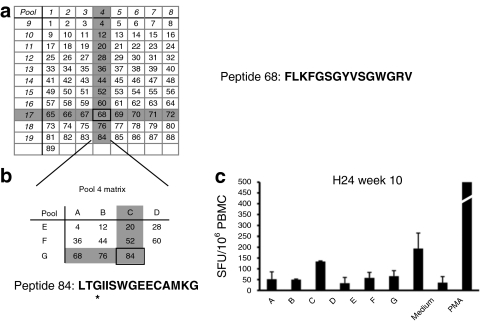
An IL-10 ELISpot assay was used to monitor Th2 responses triggered by the cFIX transgene product. A library of peptides (15-mers overlapping by 10 amino acids in sequence) was designed based on the wild-type cFIX sequence and organized in 19 pools to form a matrix as previously described.26 We analyzed PBMCs collected from the animals in experimental groups A–D (Table 1) at baseline, during IS administration, and after IS discontinuation. All dogs showed some level of reactivity to cFIX peptide pools (Figure 2); at baseline, 3/8 animals tested showed a marked IL-10 ELISpot response to cFIX [>400 spot-forming units/106 PBMCs] while the remaining animals had either a lower or no response at baseline. In some of the animal tested, IS administration was associated with a marked decrease in the IL-10 response to cFIX (Figure 2, dogs I04, I05, and H34), which then returned to detectable levels after IS discontinuation. Analysis of reactive pools 4 and 17, the two pools which scored consistently positive in most of the IL-10 ELISpot assays, led to the identification of a peptide epitope candidate, peptide 68 (Figure 3a). We further investigated the peptides contained in pool 4 by rearranging them into smaller pools (Figure 3b) and testing these pools in the IL-10 ELISpot assay. With this approach, we showed reactivity for another peptide within cFIX, peptide 84 (Figure 3c), in one of the two dogs receiving the highest dose of vector (8.5 × 1012 vg/kg) via ATVRX (H24). Of note, the peptide (84 amino acids) sequence spans the cFIX Chapel Hill mutation responsible for HB in this dog colony.31
Figure 2.
Time course of IL-10 ELISpot responses to canine FIX (cFIX) in dogs receiving AAV-2-cFIX vectors by ATVRX. Secretion of IL-10 from PBMCs after stimulation with peptides spanning the cFIX sequence organized in 19 pools. Samples were collected and analyzed at baseline, during IS and after IS discontinuation. Responses were considered positive when the number of spot-forming units (SFU) per million PBMCs were >50 and at least threefold higher than the media control (Med). All test conditions were assayed in triplicate. (a) Group A (1 × 1012 vg/kg with IS); (b) Group B (3 × 1012 vg/kg with IS); (c) Group C (8.5 × 1012 vg/kg with IS); (d) Group D (3 × 1012 vg/kg without IS). Baseline sample for J62 was not available; a sample obtained 1 week after vector delivery was analyzed instead. ATVRX, afferent transvenular retrograde extravasation; IL, interleukin; IS, immunosuppression; PBMC, peripheral blood mononuclear cell.
Figure 3.
Analysis of canine FIX (cFIX) peptide pools which showed reactivity in the IL-10 ELISpots. (a) A peptide library of 89 peptides spanning the sequence of cFIX was organized into a matrix in which each peptide was represented in two orthogonal pools. Highlighted in grey are the pools that scored positive in most of the dogs analyzed. Positivity in two orthogonal pools identified a single stimulatory peptide, peptide 68. Peptide 68 amino acid sequence is indicated. (b) Peptides contained in pool 4 were rearranged into a smaller orthogonal matrix, allowing the identification of another reactive peptide that spanned the Chapel Hill mutation (peptide 84). *Chapel Hill mutation (glycin→glutamic acid) within peptide 84. (c) IL-10 secretion detected by ELISpot in PBMCs obtained form H24 (group C, ATVRX, 8.5 × 1012 vg/kg with IS) stimulated with the matrix represented in b. ELISpot, enzyme-linked immunosorbent assay; IL, interleukin; PBMC, peripheral blood mononuclear cell.
IL-10 responses to cFIX are associated with expansion of a population of CD4+IL-10+FoxP3+ T-cells
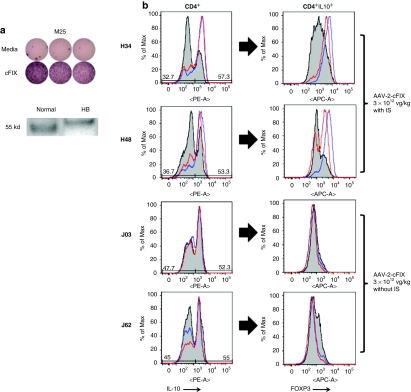
IL-10 responses to the cFIX antigen were detectable by ELISpot in naive HB dogs tested with both the cFIX pepide library (Figure 2) and cFIX whole protein (Figure 4a). The detection of cFIX in the liver lysate of these dogs (Figure 4a) may explain why HB dogs do have responses to the protein even in the absence of circulating cFIX. We further investigated the role of IL-10 secretion in response to cFIX pool 4, the pool that scored positive for most of the animals tested. PBMCs collected between week 10 and 12 (after IS discontinuation) from dogs H34 and H48 (AAV-2-cFIX, 3 × 1012 vg/kg via ATVRX with IS), and dogs J03 and J62 (AAV-2-cFIX, 3 × 1012 vg/kg via ATVRX without IS) were cultured in vitro with an irrelevant cFIX peptide pool, serving as negative control, peptide 68, or purified cFIX protein. After a 7-day stimulation in the presence of canine IL-2 and antigen, a population of CD4+ T-cells expressing IL-10 was detectable to some extent in all animals for all restimulation conditions (Figure 4b, left panels). FoxP3 staining of CD4+IL-10+ T-cells showed a marked difference between the animals which received the vector under IS (H34 and H48) and those which did not (J03 and J62), with the detection of a population of CD4+IL-10+FoxP3+ T-cells only in dogs H34 and H48 and only in response to restimulation with cFIX protein or peptide 68, but not with the negative control (Figure 4b, right panels). When assayed for suppressor activity, cFIX or peptide 68 expanded cells induced a slight reduction (compared to mock-stimulated PBMCs) in secretion of IFN-γ from autologous, unexpanded CD4+ T-cells activated with an anti-CD3 antibody (data not shown).
Figure 4.
Expansion of CD4+IL-10+FoxP3+ T-cells in response to canine FIX (cFIX) antigens in dogs receiving an AAV-2-cFIX vector via ATVRX. (a) Top: ELISpot assay for IL-10 secretion after stimulation with purified canine FIX protein. Bottom: western blot analysis of cFIX protein in liver lysates from normal and HB dogs. (b) Staining for CD4+IL-10+FoxP3+ T-cells after in vitro restimulation with either cFIX protein (red line), peptide 68 from cFIX (blue line), or cFIX irrelevant pool (gray shaded area). Left panels, histogram plots showing IL-10 intracellular staining on CD4+ T-cells; right panels, FoxP3 staining on cells gated on CD4+IL-10+ T-cells. Cells were gated on lymphocytes, CD4+CD8−. ATVRX, afferent transvenular retrograde extravasation; AAV, adeno-associated virus; ELISpot, enzyme-linked immunosorbent spot; HB, hemophilia B; IL, interleukin.
Bioinformatics analysis of peptide 68 using a major histocompatibility complex class II binding model developed for human alleles32 showed that the N-terminal 9 amino acids of this peptide (FLKFGSGYV) have high binding affinity for several human leukocyte antigen alleles. This binding promiscuity potentially explains why the same peptide scored positive in several outbred animals.
AAV-mediated gene transfer to muscle via ATVRX is associated with minimal systemic inflammation
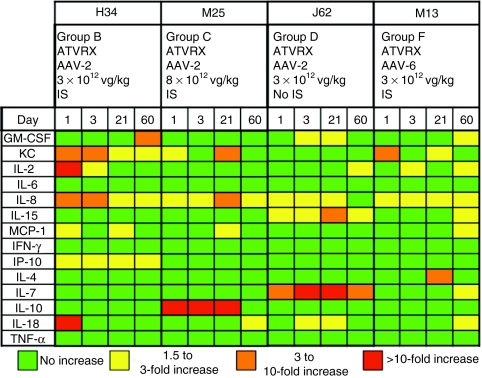
Serum samples collected at baseline and at day 1, 3, 21, and 60 after ATVRX gene transfer from animals in experimental groups B (H34, AAV-2-cFIX, 3 × 1012 vg/kg with IS), C (M25, AAV-2-cFIX, 8.5 × 1012 vg/kg with IS), D (J62, AAV-2-cFIX, 3 × 1012 vg/kg without IS), and F (M13, AAV-6-cFIX, 3 × 1012 vg/kg with IS) were assayed for cytokine and chemokine secretion in a luminex-based assay (Figure 5). Fourteen analytes, including markers of innate and adaptative immune responses, were included in the screening. None of the dogs showed secretion of early inflammation markers like IL-6 or TNF-α at any point after gene transfer. An elevation of IL-8 and keratinocyte-derived chemokine was measured in most of the dogs tested; these two markers are typically associated with vascular damage and remodeling,33,34 and their detection early after ATVRX delivery of vector may reflect vascular damage/stress from the procedure itself rather than from exposure to AAV. J62, the HB dog that developed an inhibitor after vector administration without IS, had high levels of circulating IL-7, perhaps reflecting the ongoing, Th1-driven immune response to cFIX.
Figure 5.
Follow-up of cytokine profile in dogs receiving AAV vectors via ATVRX. Markers of innate and adaptive immune responses were analyzed simultaneously with a multiplex luminex-based assay in samples corresponding to baseline and days 1, 3, 21, and 60 postvector infusion by ATVRX. For each cytokine, the magnitude of the increase over baseline levels is color-coded. AAV, adeno-associated virus; ATVRX, afferent transvenular retrograde extravasation.
Biodistribution of AAV vector genome following ATVRX delivery shows vector is found predominantly in the muscle of the target limb
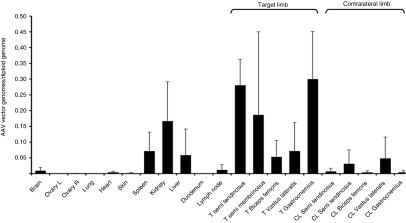
Two hemostatically normal dogs were used to perform vector biodistribution studies. The animals received 8.5 × 1012 vg/kg of an AAV-2-cFIX vector via ATVRX and were killed 20 weeks after gene transfer. Genomic DNA was extracted from several tissues, including muscle collected at different sites in the target and contralateral limbs. For the real-time quantitative PCR used in the assay, a probe placed across a splice junction in cFIX was used to ensure that the reaction would not amplify genomic DNA but only the donated gene. Results of biodistribution studies are shown in Figure 6. Widespread muscle transduction was detected in the target limb, whereas minimal muscle transduction was evident in the contralateral leg. Little or no PCR signal was evident in other organs, with the exception of liver, spleen, and kidneys. This result reflects the systemic exposure to the AAV vector and is in agreement with previous studies of AAV-2 gene transfer to liver in nonhuman primates,2 in which the same vector dose was given through the hepatic artery, showing prevalent vector distribution to spleen and liver, with no altered tropism related to IS administration. Although it is not clear which splenocyte cell subpopulation(s) was mainly positive for AAV vector genomes, no enhanced immune responses to either the vector capsid or the transgene product, or both, were evident compared to other routes of vector administration targeting the muscle or the liver.1,2,13,35 This may reflect the poor immunogenic profile of AAV vectors, at least in experimental animal models.36,37
Figure 6.
Biodistribution of vector genomes following ATVRX delivery of an AAV-2-cFIX vector. Quantitative PCR analysis of vector gene copy numbers/diploid genome in DNA samples extracted from different tissues 6 months postvector delivery by ATVRX. Results are shown as mean ± SD, n = 2. AAV, adeno-associated virus; ATVRX, afferent transvenular retrograde extravasation; cFIX, canine FIX.
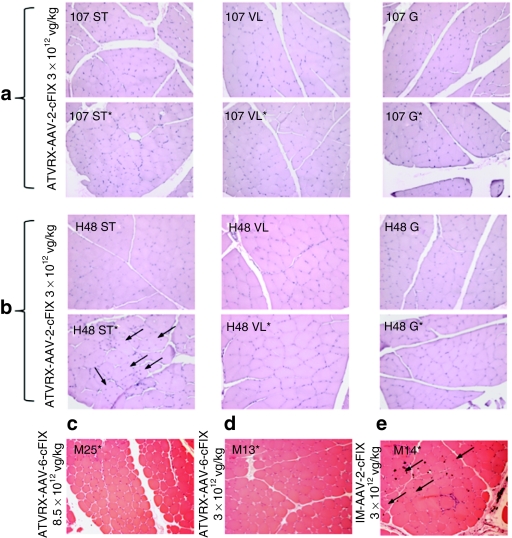
ATVRX delivery of AAV vectors is associated with limited muscle alterations
We have shown that ATVRX delivery of an AAV-2-cFIX vector to muscle of HB dogs results in detection of cFIX locally in transduced muscle tissue.29 Here, we evaluated the histology of muscle biopsies from ATVRX-injected dogs from experimental groups B—mid-dose AAV-2 (3 × 1012 vg/kg with IS, H48, I07), C—high dose AAV-2 (8.5 × 1012 vg/kg with IS, M25), and F—mid-dose AAV-6 (3 × 1012 vg/kg with IS, M13) collected ~6 months after gene transfer. Muscle biopsies from dog M14, which received an AAV-2-cFIX vector i.m. at 3 × 1012 vg/kg, were also analyzed. Hematoxylin and eosin staining of muscle sections were free of muscle alterations in dogs I07 (Figure 7a), M25 (Figure 7c), and M13 (Figure 7d), a result consistent with all other end points analyzed in the study (IFN-γ ELISpot for AAV capsid and transgene product, luminex assay for cytokines, and cFIX transgene expression). In contrast to these results, muscle sections collected at the same time from one of the dogs receiving 3 × 1012 vg/kg of the AAV-2-cFIX vector, H48, showed evidence of muscle inflammation and regeneration in the semi-tendinosus muscle (Figure 7b). Similarly, the HB dog injected i.m. with the same vector, M14, had signs of local inflammation at the vector injection sites (Figure 7e). Of note, cellular infiltrates in dog H48 were detectable only in one of the muscles analyzed (Figure 7b) and did not interfere with cFIX transgene expression. No signs of muscle inflammation or regeneration were evident in animal M13, which received 3 × 1012 vg/kg of an AAV-6-cFIX vector (Figure 7d). Immunostaining of muscle sections from dogs H48 and M14, the two dogs with signs of muscle inflammation, showed minimal presence of CD4+ and CD8+ T-cells in muscle; some positive staining for leukocytes (CD45) was present in dog H48 (Supplementary Figure S1).
Figure 7.
Histology of muscle biopsies in dogs receiving AAV vectors by ATVRX or i.m. Hematoxylin and eosin staining of muscle biopsies obtained from the ATVRX dogs 6 months after gene transfer: (a) I07 and (b) H48, AAV-2, 3 × 1012 vg/kg with IS; (c) M25, AAV-2, 8.5 × 1012 vg/kg with IS; or (d) M13, AAV-6, 3 × 1012 vg/kg with IS. (e) Hematoxylin and eosin staining on muscle from dog M14, AAV-2, 3 × 1012 vg/kg, i.m. without IS. Asterisk denotes target limb. Black arrows indicate central nuclei, evidence of muscle regeneration. Images were all taken at a ×200 magnification. AAV, adeno-assocaited virus; ATVRX, afferent transvenular retrograde extravasation; G, gastrocnemius muscle; i.m., intramuscular; IS, immunosuppression; ST, semi-tendinosus muscle; VL, vastus lateralis muscle.
Discussion
Muscle is an important target tissue for gene transfer–based therapies for neuromuscular, metabolic, and hemorrhagic diseases. For HB, achieving safe, effective gene transfer in skeletal muscle would afford a therapeutic alternative to those patients with advanced liver disease due to hepatitis C contracted from plasma-derived coagulation concentrates. In this work, we present the first complete characterization of the safety profile of a novel route of vector administration to muscle in a large cohort of immunocompetent, outbred dogs with HB.
Regardless of the route of administration and the serotype used, we documented a robust humoral response to the AAV capsid after gene transfer, a common finding in both i.m. or systemic vector administration studies in experimental models and humans. To the limit of sensitivity of the ELISpot assay used in this study,38 we did not detect any capsid-specific T-cell response. These results also agree with several other published studies in which the lack of cellular immunity to the AAV capsid was documented in the context of preclinical studies where an AAV vector was delivered intravascularly to the muscle,16,39,40 with only a few exceptions.41,42 In particular, our results agree with a recently published study39 in which a careful evaluation of T-cell responses to the AAV capsid was performed using an IFN-γ ELISpot assay and no T-cell response to the AAV capsid was found. Whether these results reflect the real absence of T-cell reactive to the AAV capsid, or their very low frequency in peripheral blood compared to other lymphoid organs25 is not clear at this point.
However, in one animal, H48, receiving the mid-dose of the AAV-2-cFIX vector via ATVRX, which resulted in the highest levels of cFIX expression of all AAV-2-injected dogs, we documented some level of muscle infiltrates and regeneration; this was associated with the lack of detectable T-cell responses to either the capsid or the transgene. Muscle infiltrates in dog H48 appeared to be localized in defined areas, in particular in the semi-tendinosus muscle, which showed high levels of vector transduction, suggesting that high levels of antigen were expressed locally. The fact that histological changes were also documented in M14, a dog injected i.m. with an AAV-2-cFIX vector at a dose of 3 × 1012 vg/kg, and that these changes were not accompanied by detection of T-cell responses to transgene or capsid, or loss of cFIX transgene expression, may indicate that there is a relationship between high levels of antigen expressed locally and the presence of muscle inflammation. Some levels of inflammation have been reported in the literature in experimental animals24,43 and humans6 in the presence of transgene expression. One possible explanation of the phenomenon is that reactive T cells infiltrating the muscle fail to clear transduced tissues.44
Another intriguing finding in our study is that we did not observe the same pattern of muscle infiltration in M13, the AAV-6-cFIX ATVRX dog, which received the same vector dose as H48 and had similar levels of cFIX expression. The fact that AAV-6 is more efficient in diffusing through the muscle fibers, achieving more widespread transduction,45 would suggest that a more uniform expression of the transgene product may help preventing local muscle inflammation.46
In humans, T-cell responses directed to the capsid antigen were documented in lipoprotein lipase–deficient subjects undergoing i.m. gene transfer with an AAV-1 vector expressing lipoprotein lipase,27 in α1-antitrypsin (AAT)-deficient subjects receiving i.m. injection of an AAV-1-AAT vector,5 and in a study on AAV-1-α-sarcoglycan in limb-girdle muscular dystrophy subjects.6
Although it appears clear that in humans T-cell responses to AAV vectors become readily detectable after gene transfer in a dose-dependent fashion, data presented here and previous findings indicate that several factors play a role in shaping these responses, including immunomodulatory properties of the transgene product,47 danger signals,19 levels of antigen being expressed locally,17,18 or possibly the silencing of reactive T-cells at the local level.44 Most importantly, the systemic exposure of vector, documented by the biodistribution profile of vector genomes after ATVRX delivery, and the detection of cytokines associated with vascular damage and remodeling (danger signal), are elements to be taken into consideration when evaluating safety of intravascular delivery of AAV vectors to muscle. Given these considerations, AAV muscle- and liver-directed gene transfer may have very similar safety profiles and both may require transient immunomodulation.
Immune tolerance to antigens expressed in the liver has been well documented20,48 and relies on the induction of antigen-specific regulatory T-cells.2,21 In contrast, muscle is typically chosen as the target tissue for vaccines because it is more immunogenic than other tissues. In AAV-mediated gene transfer for HB, and in protein replacement therapy,49 the risk of developing immune responses to the FIX-donated gene product varies according to the genetic background of the host.50,51 Clearance of anti-FIX antibodies or absence of responses to the FIX transgene product has been documented in some instances in AAV-FIX muscle gene transfer in mice and dogs;22,52 in one study, immune tolerance to FIX was described in mice after AAV-1 gene transfer with no involvement of regulatory T cells.23 Our results represent the first evidence, developed in a large animal model of HB, that AAV-FIX gene transfer to muscle is associated with the expansion of a population of CD4+FoxP3+ T-cells secreting large amounts of IL-10 in response to cFIX-derived epitopes. Although these cells seem to be detectable also at baseline in some dogs (perhaps reflecting previous cFIX infusions or the fact that cFIX is detectable in liver), muscle gene transfer seems to further induce their expansion. This finding may explain why the humoral responses observed in the context of this study were predominantly Th2-driven and non-neutralizing. Importantly, the ability to expand CD4+IL-10+FoxP3+ T-cells in vitro seemed to correlate with the outcome of gene transfer in terms of development of inhibitory antibodies to the cFIX transgene product, with the two non-IS dogs J03 and J62 showing no expansion of these cells. Whether IS contributes to the induction of this CD4+IL-10+FoxP3+ T-cells population is not clear; however, it should be noted that a similar phenomenon has been described in the past for other IS regimens.53 The identification of a population of CD4+IL-10+FoxP3+ T-cells has implications for the design of future studies in humans in which vectors are administered with concurrent immunosuppression, as interference with tolerance induction may lead to development of harmful immune responses to the transgene product.2 The detection of vector genomes in the liver suggests that a portion of cFIX transgene product measured in the bloodstream after ATVRX may have been produced in the liver. It is likely, however, that cytomegalovirus promoter-driven liver expression of the cFIX transgene, if present, was only transient,54 a scenario more consistent with the inhibitor development observed in dog J62, which would argue against liver-induced tolerance to the transgene product.20
In summary, our work shows that intravascular administration of an AAV vector encoding cFIX to HB dogs is safe and results in the induction of a population of CD4+IL-10+FoxP3+ T-cells that have suppressor activity and may contribute to modulation of immune responses to the transgene product in AAV-mediated gene transfer. This study lays the groundwork for future studies aimed at translating ATVRX-mediated AAV gene transfer of FIX to muscle in HB subjects.
Materials and Methods
Animal procedures. Both hemostatically normal and HB dogs from the colony at the University of North Carolina at Chapel Hill were used in this study. This HB canine model is characterized by the presence of a missense mutation within the region of the gene encoding the catalytic domain of FIX, resulting in complete absence of FIX antigen or activity in plasma, mimicking severe human HB.31 At the time of gene transfer, all animals were naive for antibodies to AAV capsid, with the exception of dogs used in re-administration experiments. Sedated dogs were administered AAV vectors by either i.m. injection or ATVRX, as previously described.30 At the time of vector administration, all HB dogs were supplemented with pooled normal canine plasma at doses calculated to achieve at least 10–20% of normal cFIX plasma activity. Dogs received 6 weekly intravenous infusions of cyclophosphamide (200 mg/m2 of body surface area) starting 1 week before vector delivery as previously described.28 Animals were bled periodically to obtain plasma and PBMCs. All procedures were approved by the Institutional Animal Care and Use Committee at the Children's Hospital of Philadelphia and the University of North Carolina at Chapel Hill.
Vector production. AAV vectors were produced by triple transfection of HEK293 cells followed by cesium chloride gradient–based purification as previously described.13 All vector preps used were devoid of empty capsids. Vectors were titered by dot-blot hybridization or real-time PCR; titers were expressed as vg/ml. An AAV expression cassette with the cFIX complementary DNA under transcriptional control of the cytomegalovirus immediate-early enhancer–promoter, a chimeric β-globin/cytomegalovirus intron, and the human growth hormone polyadenylation signal13 was used in most of the experiments in HB and normal dogs. An AAV “null” vector carrying an antisense copy of the β-galactosidase complementary DNA under the control of a cytomegalovirus promoter was used in a normal dog. Vectors were pseudotyped with either AAV-1, AAV-2, or AAV-6 capsids.
cFIX antigen detection. cFIX antigen levels were measured by immunoassay using a polyclonal matched-pair antibody set for cFIX enzyme-linked immunosorbent assay (Affinity Biologicals, Ancaster, Ontario, Canada) following manufacturer's instructions. Serial dilutions of normal pooled canine plasma were used as standards. With this methodology, cFIX was undetectable in untreated HB animals.
Antibody assays. The presence of cFIX inhibitory antibodies was determined as previously reported by Bethesda assay.22 One Bethesda unit represents an amount of antibody sufficient to neutralize 50% of FIX activity. Antibody subclasses against cFIX were evaluated by enzyme-linked immunosorbent assay capture immunoassay. Briefly, microtiter plates were coated with 1 µg/ml of purified plasma-derived cFIX (Enzyme Research, South Bend, IN). Plates were washed and blocked for 2–4 hours with 3% bovine serum albumin 0.05% Tween 20 in phosphate-buffered saline. Samples were diluted 1/20 or 1/40 in LowCross-Buffer (Candor Bioscience, Weissensberg, Germany) and incubated for 2 hours at 37 °C. Antigen-antibody complexes were detected by 2-hour incubation at 37 °C with horseradish peroxidase–conjugated goat anti-dog IgG1 or sheep anti-dog IgG2 (Bethyl Laboratories, Montgomery, TX). Similarly, to detect antibody responses against viral proteins, plates were coated with serotype-specific AAV empty capsids at 1 µg/ml. Samples were diluted 1/200 to 1/800 in 3% bovine serum albumin 0.05% Tween 20 in phosphate-buffered saline and incubated overnight at 4 °C. In both cases, a standard curve generated by coating serially diluted dog reference serum with known quantities of IgGs (Bethyl Laboratories) was used for quantification of results.
ELISpot assays. One-color ELISpot assays were used to measure IFN-γ or IL-10 responses to cFIX or AAV capsid proteins in PBMCs isolated from AAV-treated dogs. Peptide libraries of 15-mers overlapping in sequence by 10 amino acids and spanning the sequence of cFIX and of the AAV capsid serotype-specific VP-1 protein were synthesized (Mimotopes, Clayton, Australia). Peptides were arranged into a matrix as previously described,26 or alternatively assembled into pools of 10–24 peptides each. ELISpot assays were performed as previously described.55 Briefly, a 1/60 dilution of anti-canine IFN-γ or anti-canine IL-10 antibody (R&D Systems, Minneapolis, MN) was used to coat ELISpot plates (Millipore, Bedford, MA) overnight at 4 °C. After blocking of free binding sites for 2 hours, 100 µl of culture media (2-mixed leukocyte culture 4% heat-inactivated fetal bovine serum20) containing the antigens were added to each well. Media containing a mixture of 0.05 µg/ml of phorbol 12-myristate 13-acetate (Sigma, St Louis, MO) and 1 µg/ml of ionomycin (Sigma) served as positive control, while plain media served as negative control. In all experiments, the positive control gave a robust response (>1,000 spot-forming units/106 PBMCs), confirming good cell viability. Cryopreserved PBMCs were thawed, washed, and adjusted to a concentration of 2–2.5 × 106 cells/ml in culture media, and 100 µl of the suspension was carefully added to each well. Each condition was assayed in triplicate. Cultures were incubated for 24 hours (IFN-γ) or 48 hours (IL-10) at 37 °C, 10% CO2. Plates were subsequently washed, and a biotinylated anti-canine IFN-γ antibody or anti-canine IL-10 antibody diluted 1/60 in phosphate-buffered saline 1% bovine serum albumin was added. Finally, plates were washed and developed after addition of streptavidin–alkaline phosphatase (Mabtech, Cincinnati, OH) and a chromogenic substrate (BCIP/MBT; KPL, Gaithersburg, MA). The number of spots was determined using an ELISpot reader (CTL, Cleveland, OH) and analyzed with Immunospot version 3.2 software (CTL). A response was considered to be positive if the average number of spot-forming units per million PBMCs was at least 50 and also thre times greater than the media control.
T-cell expansion experiments and flow cytometry. A volume of 1 × 106 PBMCs were resuspended in ~500 µl of 4% fetal bovine serum 2-mixed leukocyte culture media,20 along with an equal volume and concentration of irradiated feeder cells, and cultured at 37 °C and 5% CO2. Irradiated feeder cells were from the same initial PBMC population. Peptides were added at a concentration of 10–50 µg/ml on day 0 to stimulate PBMCs for 1 week. To sustain culture growth, canine IL-2 (R&D Systems) was added every 2 days at a 10 ng/ml concentration. On the final day, all cells were re-stimulated with peptide 68, and monensin was added (Golgi Stop, BD Biosciences, San Jose, CA) according to manufacturer's directions. After a further 4–5 hours of incubation, cells were harvested into fluorescence-activated cell sorting tubes, washed twice with FACS buffer (2% fetal bovine serum in phosphate-buffered saline, 0.05% sodium azide), and stained with anti-canine CD4-FITC (AbD Serotec, Kidlington, UK) for 1 hour at 4 °C. Cells were washed twice with FACS buffer, then incubated in Fixation/Permeabilization Buffer (eBioscience, San Diego, CA) for 1 hour at 4 °C. Cells were washed twice with Permeabilization Wash Buffer (eBioscience), and incubated in biotin-binding NeutrAvidin (Invitrogen, Carlsbad, CA) for 30 minutes at room temperature. After two more washes in Permeabilization Wash Buffer, cells were intracellularly stained with previously conjugated anti-canine IL10-biotin (R&D Systems) to streptavidin–phycoerythrin (Invitrogen), along with anti-Foxp3-APC (eBioscience) for 1 hour at 4 °C. Finally, cells were washed twice with Permeabilization Wash Buffer, and resuspended in 4% paraformaldehyde. For the suppression assay, PBMCs were expanded in the presence of cFIX or Peptide 68 for 5 days (suppressor cells), and plated at a 5:1 T suppressor:T effector ratio with unexpanded autologous effector cells labeled with carboxyfluorescein succinimidyl ester (Molecular Probe, Invitrogen, Carlsbad, CA) to distinguish them from the suppressor cell population. This was done in anti-CD3 coated plates to stimulate target T-cell IFN-γ production (0.5 µg/well for 96 wells), with 4 × 105 of expanded suppressor cells in 100 µl and 1 × 105 effector cells in 100 µl. Cells were incubated for 1 hour, after which monensin was added. After an additional 4-hour incubation at 37 °C, 5% CO2, cells were harvested and stained with anti-CD4 PacBlue, anti-CD8 PE, and anti-IFN-γ Alexa 647 antibodies (AbD Serotec, Kidlington, UK). IFN-γ secretion was measured on CFSE+CD4+ and CFSE+CD8+ T-cells. All samples were read on a FACS CantoII flow cytometer (BD Biosciences); data were analyzed using the FACS Diva Software (BD Biosciences) and Flowjo (Treestar, Ashland, OR).
Cytokine profile. Serum samples were analyzed at baseline and at days 1, 3, 21, and 60 after vector delivery by multiplex assay on a Luminex 100 instrument at the Radioimmunoassay and Biomarker core of the University of Pennsylvania with the Milliplex Canine Cytokine/Chemokine Panel (Millipore, Billerica, MA) for the simultaneous detection and quantification of 14 canine cytokines. The sensitivity of this assay was 14.4 pg/ml for granulocyte–macrophage colony-stimulating factor, 1.6 pg/ml for keratinocyte-derived chemokine, 6.4 pg/ml for IL-2, 12.1 pg/ml for IL-6, 20.3 pg/ml for IL-8, 14.8 pg/ml for IL-15, 8.6 pg/ml for monocyte chemotactic protein -1, 4.4 pg/ml for IFN-γ, 2.4 pg/ml for interferon-γ inducible protein -10, 28.8 pg/ml for IL-4, 4.6 pg/ml for IL-7, 1.6 pg/ml for IL-10, 4.6 pg/ml for IL-18, and 0.4 pg/ml for TNF-α.
Biodistribution. Total DNA was isolated with either QIAamp DNA Mini Kit (Qiagen, Valencia, CA) or MasterPureDNA Purification Kit (Epicentre Biotechnologies, Madison, WI). Vector genome copy number in 100–200 ng of genomic DNA was determined by quantitative real-time PCR with a set of primers and probe placed across a splicing junction in the cFIX complementary DNA. Forward primer: 5′-AAC GTC ACC CAA CCG CTT AA-3′ reverse primer: 5′-ATG ATG GAA CCT CCG CAG AA-3′ probe: 5′-FAM-CCA GGT CAA TTC CCT TGG CAG GTC C-Quencher-3′ (Applied Biosystems, Foster City, CA). Serial dilutions of a linearized plasmid bearing the cFIX expression cassette used in the study supplemented with 200 ng of commercially available dog genomic DNA (Zyagen, San Diego, CA) were used to build a reference standard.
Histology. Biopsies of muscles were obtained after vector administration. Muscle tissue was either fixed in 10% formalin or frozen in cooled isopentane followed by liquid nitrogen. Cross-sections were stained with hematoxylin and eosin. For cellular infiltrate staining, muscle serial cryosections were incubated with an anti-CD4 monoclonal antibody (1:200 dilution; AbD Serotec, Oxford, UK), an anti-CD8 monoclonal antibody (1:50 dilution; AbD Serotec, Oxford, UK), or an anti-CD45 monoclonal antibody (1:200 dilution; AbD Serotec, Oxford, UK) followed by horseradish peroxidase staining. Images were obtained with an Eclipse E800 microscope (Nikon, Tokyo, Japan).
SUPPLEMENTARY MATERIAL Table S1. Summary of IFN-γ responses monitored by antigen-specific ELISpot. Figure S1. Characterization of muscle infiltrates in dogs H48 and M14.
Acknowledgments
This work was supported by a grant from the National Institute of Health (HL64190 to K.A.H), the Howard Hughes Medical Institute, and the Center for Cellular and Molecular Therapeutics at the Children's Hospital of Philadelphia. We would like to acknowledge Toby Cohen and Annie de Groot for their collaboration with bioinformatics studies and Alex Tai and Yi Zhao for their assistance with AAV vectors preparation. F.M., V.R.A, J.F.W., and K.A.H. are consultants for companies that are developing AAV-based therapies not in the field of hemophilia or hold patents related to AAV gene therapy. All other authors declare no competing financial interests.
Supplementary Material
Summary of IFN-γ responses monitored by antigen-specific ELISpot.
Characterization of muscle infiltrates in dogs H48 and M14.
REFERENCES
- Jiang H, Couto LB, Patarroyo-White S, Liu T, Nagy D, Vargas JA, et al. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006;108:3321–3328. doi: 10.1182/blood-2006-04-017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F, Hasbrouck NC, Basner-Tschakarjan E, Edmonson SA, Hui DJ, Sabatino DE, et al. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood. 2007;110:2334–2341. doi: 10.1182/blood-2007-03-080093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F, et al. Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374:1597–1605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- Brantly ML, Chulay JD, Wang L, Mueller C, Humphries M, Spencer LT, et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc Natl Acad Sci USA. 2009;106:16363–16368. doi: 10.1073/pnas.0904514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell JR, Rodino-Klapac LR, Rosales-Quintero X, Kota J, Coley BD, Galloway G, et al. Limb-girdle muscular dystrophy type 2D gene therapy restores α-sarcoglycan and associated proteins. Ann Neurol. 2009;66:290–297. doi: 10.1002/ana.21732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir LA., and, Chamberlain JS. Emerging strategies for cell and gene therapy of the muscular dystrophies. Expert Rev Mol Med. 2009;11:e18. doi: 10.1017/S1462399409001100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroes ES, Nierman MC, Meulenberg JJ, Franssen R, Twisk J, Henny CP, et al. Intramuscular administration of AAV1-lipoprotein lipase S447X lowers triglycerides in lipoprotein lipase-deficient patients. Arterioscler Thromb Vasc Biol. 2008;28:2303–2304. doi: 10.1161/ATVBAHA.108.175620. [DOI] [PubMed] [Google Scholar]
- Patel H., and, Heathcote EJ. When to treat and the benefits of treating hepatitis C in patients with haemophilia. Haemophilia. 2009;15:20–32. doi: 10.1111/j.1365-2516.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- Larson PJ., and, High KA. Gene therapy for hemophilia B: AAV-mediated transfer of the gene for coagulation factor IX to human muscle. Adv Exp Med Biol. 2001;489:45–57. doi: 10.1007/978-1-4615-1277-6_4. [DOI] [PubMed] [Google Scholar]
- Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR, et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A, et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000;24:257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- Herzog RW, Yang EY, Couto LB, Hagstrom JN, Elwell D, Fields PA, et al. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med. 1999;5:56–63. doi: 10.1038/4743. [DOI] [PubMed] [Google Scholar]
- Lai Y, Yue Y, Liu M, Ghosh A, Engelhardt JF, Chamberlain JS, et al. Efficient in vivo gene expression by trans-splicing adeno-associated viral vectors. Nat Biotechnol. 2005;23:1435–1439. doi: 10.1038/nbt1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankinship MJ, Gregorevic P., and, Chamberlain JS. Gene therapy strategies for Duchenne muscular dystrophy utilizing recombinant adeno-associated virus vectors. Mol Ther. 2006;13:241–249. doi: 10.1016/j.ymthe.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Toromanoff A, Adjali O, Larcher T, Hill M, Guigand L, Chenuaud P, et al. Lack of immunotoxicity after regional intravenous (RI) delivery of rAAV to nonhuman primate skeletal muscle. Mol Ther. 2010;18:151–160. doi: 10.1038/mt.2009.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Cao O, Swalm B, Dobrzynski E, Mingozzi F., and, Herzog RW. Major role of local immune responses in antibody formation to factor IX in AAV gene transfer. Gene Ther. 2005;12:1453–1464. doi: 10.1038/sj.gt.3302539. [DOI] [PubMed] [Google Scholar]
- Herzog RW, Fields PA, Arruda VR, Brubaker JO, Armstrong E, McClintock D, et al. Influence of vector dose on factor IX-specific T and B cell responses in muscle-directed gene therapy. Hum Gene Ther. 2002;13:1281–1291. doi: 10.1089/104303402760128513. [DOI] [PubMed] [Google Scholar]
- Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Liu YL, Dobrzynski E, Kaufhold A, Liu JH, Wang Y, et al. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest. 2003;111:1347–1356. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao O, Dobrzynski E, Wang L, Nayak S, Mingle B, Terhorst C, et al. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood. 2007;110:1132–1140. doi: 10.1182/blood-2007-02-073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda VR, Schuettrumpf J, Herzog RW, Nichols TC, Robinson N, Lotfi Y, et al. Safety and efficacy of factor IX gene transfer to skeletal muscle in murine and canine hemophilia B models by adeno-associated viral vector serotype 1. Blood. 2004;103:85–92. doi: 10.1182/blood-2003-05-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly ME, Zhuo J, Bharadwaj AS., and, Chao H. Induction of immune tolerance to FIX following muscular AAV gene transfer is AAV-dose/FIX-level dependent. Mol Ther. 2009;17:857–863. doi: 10.1038/mt.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Dobrzynski E, Schlachterman A, Cao O., and, Herzog RW. Systemic protein delivery by muscle-gene transfer is limited by a local immune response. Blood. 2005;105:4226–4234. doi: 10.1182/blood-2004-03-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JE, et al. CD8+ T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Meulenberg JJ, Hui DJ, Basner-Tschakarjan E, Hasbrouck NC, Edmonson SA, et al. 2009AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells Bloodepub ahead of print). [DOI] [PMC free article] [PubMed]
- Arruda VR, Stedman HH, Nichols TC, Haskins ME, Nicholson M, Herzog RW, et al. Regional intravascular delivery of AAV-2-F.IX to skeletal muscle achieves long-term correction of hemophilia B in a large animal model. Blood. 2005;105:3458–3464. doi: 10.1182/blood-2004-07-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda VR, Stedman HH, Haurigot V, Buchlis G, Baila S, Favaro P, et al. 2010Peripheral transvenular delivery of adeno-associated viral vectors to skeletal muscle as a novel therapy for hemophilia B Bloodepub ahead of print). [DOI] [PMC free article] [PubMed]
- Su LT, Gopal K, Wang Z, Yin X, Nelson A, Kozyak BW, et al. Uniform scale-independent gene transfer to striated muscle after transvenular extravasation of vector. Circulation. 2005;112:1780–1788. doi: 10.1161/CIRCULATIONAHA.105.534008. [DOI] [PubMed] [Google Scholar]
- Evans JP, Brinkhous KM, Brayer GD, Reisner HM., and, High KA. Canine hemophilia B resulting from a point mutation with unusual consequences. Proc Natl Acad Sci USA. 1989;86:10095–10099. doi: 10.1073/pnas.86.24.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot AS, Moise L, McMurry JA, Wambre E, Van Overtvelt L, Moingeon P, et al. Activation of natural regulatory T cells by IgG Fc-derived peptide “Tregitopes”. Blood. 2008;112:3303–3311. doi: 10.1182/blood-2008-02-138073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober A. Chemokines in vascular dysfunction and remodeling. Arterioscler Thromb Vasc Biol. 2008;28:1950–1959. doi: 10.1161/ATVBAHA.107.161224. [DOI] [PubMed] [Google Scholar]
- Okada M, Matsumori A, Ono K, Furukawa Y, Shioi T, Iwasaki A, et al. Cyclic stretch upregulates production of interleukin-8 and monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 in human endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18:894–901. doi: 10.1161/01.atv.18.6.894. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Gray JT, McIntosh J, Ng CY, Zhou J, Spence Y, et al. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007;109:1414–1421. doi: 10.1182/blood-2006-03-010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SW, Hensley SE, Tatsis N, Lasaro MO., and, Ertl HC. Recombinant adeno-associated virus vectors induce functionally impaired transgene product-specific CD8+ T cells in mice. J Clin Invest. 2007;117:3958–3970. doi: 10.1172/JCI33138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veron P, Allo V, Rivière C, Bernard J, Douar AM., and, Masurier C. Major subsets of human dendritic cells are efficiently transduced by self-complementary adeno-associated virus vectors 1 and 2. J Virol. 2007;81:5385–5394. doi: 10.1128/JVI.02516-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janetzki S, Panageas KS, Ben-Porat L, Boyer J, Britten CM, Clay TM, et al. ELISpot Proficiency Panel of the CVC Immune Assay Working Group. (2008Results and harmonization guidelines from two large-scale international Elispot proficiency panels conducted by the Cancer Vaccine Consortium (CVC/SVI) Cancer Immunol Immunother 57303–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodino-Klapac LR, Montgomery CL, Bremer WG, Shontz KM, Malik V, Davis N, et al. Persistent expression of FLAG-tagged micro dystrophin in nonhuman primates following intramuscular and vascular delivery. Mol Ther. 2010;18:109–117. doi: 10.1038/mt.2009.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodino-Klapac LR, Janssen PM, Montgomery CL, Coley BD, Chicoine LG, Clark KR, et al. A translational approach for limb vascular delivery of the micro-dystrophin gene without high volume or high pressure for treatment of Duchenne muscular dystrophy. J Transl Med. 2007;5:45. doi: 10.1186/1479-5876-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Storb R, Lee D, Kushmerick MJ, Chu B, Berger C, et al. Immune responses to AAV in canine muscle monitored by cellular assays and noninvasive imaging. Mol Ther. 2009;18:617–624. doi: 10.1038/mt.2009.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amado D, Mingozzi F, Hui D, Bennicelli JL, Wei Z, Chen Y, et al. Safety and efficacy of subretinal re-administration of a viral vector in large animals to treat congenital blindness. Sci Transl Med. 2010;2:21ra16. doi: 10.1126/scitranslmed.3000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Allen JM, Riddell SR, Gregorevic P, Storb R, Tapscott SJ, et al. Immunity to adeno-associated virus-mediated gene transfer in a random-bred canine model of Duchenne muscular dystrophy. Hum Gene Ther. 2007;18:18–26. doi: 10.1089/hum.2006.093. [DOI] [PubMed] [Google Scholar]
- Velazquez VM, Bowen DG., and, Walker CM. Silencing of T lymphocytes by antigen-driven programmed death in recombinant adeno-associated virus vector-mediated gene therapy. Blood. 2009;113:538–545. doi: 10.1182/blood-2008-01-131375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankinship MJ, Gregorevic P, Allen JM, Harper SQ, Harper H, Halbert CL, et al. Efficient transduction of skeletal muscle using vectors based on adeno-associated virus serotype 6. Mol Ther. 2004;10:671–678. doi: 10.1016/j.ymthe.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Toromanoff A, Chérel Y, Guilbaud M, Penaud-Budloo M, Snyder RO, Haskins ME, et al. Safety and efficacy of regional intravenous (r.i.) versus intramuscular (i.m.) delivery of rAAV1 and rAAV8 to nonhuman primate skeletal muscle. Mol Ther. 2008;16:1291–1299. doi: 10.1038/mt.2008.87. [DOI] [PubMed] [Google Scholar]
- Hudig D, Haverty T, Fulcher C, Redelman D., and, Mendelsohn J. Inhibition of human natural cytotoxicity by macromolecular antiproteases. J Immunol. 1981;126:1569–1574. [PubMed] [Google Scholar]
- Follenzi A, Battaglia M, Lombardo A, Annoni A, Roncarolo MG., and, Naldini L. Targeting lentiviral vector expression to hepatocytes limits transgene-specific immune response and establishes long-term expression of human antihemophilic factor IX in mice. Blood. 2004;103:3700–3709. doi: 10.1182/blood-2003-09-3217. [DOI] [PubMed] [Google Scholar]
- Oldenburg J, Schröder J, Brackmann HH, Müller-Reible C, Schwaab R., and, Tuddenham E. Environmental and genetic factors influencing inhibitor development. Semin Hematol. 2004;41 1 Suppl 1:82–88. doi: 10.1053/j.seminhematol.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Herzog RW, Mount JD, Arruda VR, High KA., and, Lothrop CD., Jr Muscle-directed gene transfer and transient immune suppression result in sustained partial correction of canine hemophilia B caused by a null mutation. Mol Ther. 2001;4:192–200. doi: 10.1006/mthe.2001.0442. [DOI] [PubMed] [Google Scholar]
- Cao O, Hoffman BE, Moghimi B, Nayak S, Cooper M, Zhou S, et al. Impact of the underlying mutation and the route of vector administration on immune responses to factor IX in gene therapy for hemophilia B. Mol Ther. 2009;17:1733–1742. doi: 10.1038/mt.2009.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn EF, Zhuo J, Kelly ME., and, Chao HJ. Efficient induction of immune tolerance to coagulation factor IX following direct intramuscular gene transfer. J Thromb Haemost. 2007;5:1227–1236. doi: 10.1111/j.1538-7836.2007.02522.x. [DOI] [PubMed] [Google Scholar]
- Chung DT, Korn T, Richard J, Ruzek M, Kohm AP, Miller S, et al. Anti-thymocyte globulin (ATG) prevents autoimmune encephalomyelitis by expanding myelin antigen-specific Foxp3+ regulatory T cells. Int Immunol. 2007;19:1003–1010. doi: 10.1093/intimm/dxm078. [DOI] [PubMed] [Google Scholar]
- Nakai H, Herzog RW, Hagstrom JN, Walter J, Kung SH, Yang EY, et al. Adeno-associated viral vector-mediated gene transfer of human blood coagulation factor IX into mouse liver. Blood. 1998;91:4600–4607. [PubMed] [Google Scholar]
- Bennicelli J, Wright JF, Komaromy A, Jacobs JB, Hauck B, Zelenaia O, et al. Reversal of blindness in animal models of leber congenital amaurosis using optimized AAV2-mediated gene transfer. Mol Ther. 2008;16:458–465. doi: 10.1038/sj.mt.6300389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of IFN-γ responses monitored by antigen-specific ELISpot.
Characterization of muscle infiltrates in dogs H48 and M14.