Abstract
To evaluate the contribution of bone marrow (BM) cells to treat neurological disorders, we examined the effectiveness of BM cells expressing the homeobox B4 (HoxB4) gene to cure mice with metachromatic leukodystrophy (MLD) through transplantation. Increased number of donor cells was observed in brains of the MLD mice transplanted with HoxB4-transduced BM cells (B4MLD) in contrast to those transplanted with control green fluorescent protein (GFP)-transduced BM cells (MIGMLD). Immunohistochemical staining showed that most of the GFP+ cells were Iba1+ microglia. In addition, O4+ oligodendrocytes were identified only in the B4MLD brains but not in the MIGMLD brain. Alcian blue staining showed that accumulation of sulfatide was dramatically reduced in brain tissue from B4MLD mice, and there was a corresponding improvement in the animals' ability to walk a balance beam 8 months after transplantation. Thus transplantation of BM cells overexpressing HoxB4 appears to effectively prevent the progression of MLD in this mouse model. These findings support the idea that hematopoietic stem cells (HSCs) transduced with a HoxB4 expression vector could be the useful carriers of therapeutic proteins into the brain for regeneration of oligodendrocytes to treat such demyelinating disorders as MLD, Krabbe disease, and multiple sclerosis.
Introduction
Metachromatic leukodystrophy (MLD) is an autosomal recessive inherited lysosomal storage disorder caused by a deficiency in the lysosomal enzyme arylsulfatase A (ASA), which catalyzes the degradation of galactosyl-3-sulfate ceramide (sulfatide), a major myelin sphingolipid.1 This disease is characterized pathologically by degeneration of myelin in both the central and peripheral nervous systems, and clinically by progressive motor and mental deterioration that is ultimately lethal. Currently there is no efficient therapy. The major obstacle is the blood–brain barrier, which limits the delivery of systemically administered therapeutic molecules to the brain.
Bone marrow transplantation (BMT) has been used to treat selected inborn errors of metabolism, including lysosomal storage disorder.2 The primary rationale for using BMT to treat lysosomal storage disorder is that the donor cells provide correcting enzymes both inside and outside the blood compartment, because lysosomal enzymes are secreted and taken up by the mannose-6-phoshate receptor-mediated pathway.3,4 In addition, it was reported that following BMT donor-derived cells from the myelomonocytic lineage can migrate into the central nervous system (CNS) and then differentiate to form perivascular and parenchymal (resting) microglia,5,6 therefore hematopoietic cells may be used as vehicles to carry genes into the CNS. The clinical benefit of cord blood transplantation has been recently reported in Krabbe disease, another lysosomal storage disorder, if performed in asymptomatic newborns.7 However, the efficacy of allogenic BMT in MLD patients is very limited.8,9 In the infantile form of MLD, which is the most frequent and severe type, BMT is completely ineffective against the rapid progression of cerebral demyelination and neuromotor dysfunction. Thus a new strategy for treating CNS disorders such as MLD is urgently needed.
Homeobox (Hox) transcription factors have emerged as important regulators of hematopoiesis. Hox genes encode a large family of transcription factors that contain a highly conserved DNA-binding motif, and specific expression patterns of multiple Hox genes have been detected in normal and leukemic hematopoiesis.10 One particular factor, HoxB4, has been implicated in the regulation of hematopoietic stem cell (HSC) regeneration,11 and retrovirally engineered overexpression in murine BM cells dramatically increases the stem cell pool in vitro and in vivo, resulting in faster and more complete recovery of stem cells in transplantation studies.12,13,14,15,16 Here, we describe an improved strategy for using transplantation of BM cells overexpressing HoxB4 to treat the CNS involvement of MLD.
Results
High levels of donor cells were detected in the brains of B4MLD mice
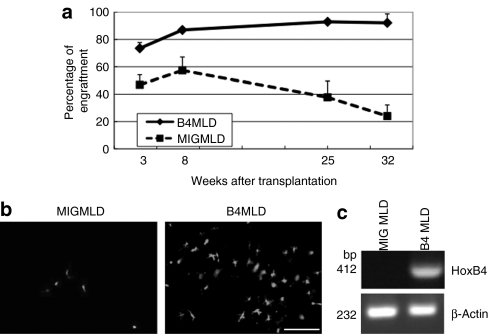
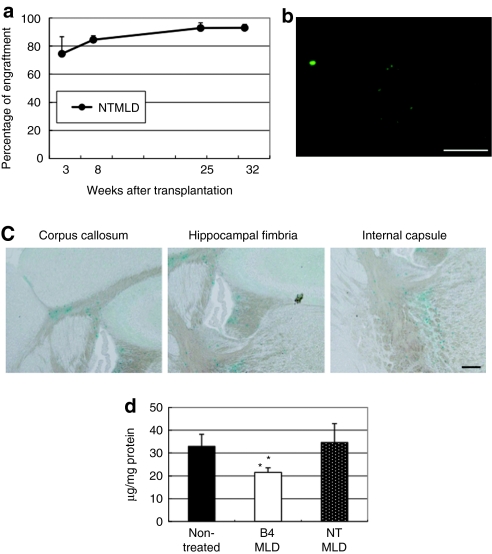
We initially examined levels of engraftment following BMT. To detect the percentage of engraftment and donor cells in the brain easily, we used C57BL/6 green fluorescent protein (GFP) mice as a donor. Peripheral blood was collected from primary recipients 3, 8, 25, and 32 weeks post-BMT, and the percentages of donor GFP+ cells present were plotted as a function of time (Figure 1a). We found that the percentage of donor cells in peripheral blood samples from MLD mice transplanted with HoxB4-transduced BM cells (B4MLD) were significantly higher than in those from MLD mice transplanted with control GFP-transduced BM cells (MIGMLD). Excluding erythrocytes, >90% of blood cells from B4MLD mice were GFP+ 25 and 32 weeks after transplantation. To determine whether donor GFP+ cells can be detected in the brain, we sacrificed transplanted mice 8 months after transplantation, and counted the GFP+ cells using fluorescence microscopy (Olympus, Tokyo, Japan) and Lumina Vision Software (Mitani, Tokyo, Japan). We found that substantially greater numbers of GFP+ donor cells were present in the brains of B4MLD mice than MIGMLD mice (2.52 ± 0.40 versus 0.15 ± 0.06%, P < 0.03) (Figures 1b and 5b). To analyze the expression of HoxB4 in brain, we extracted RNA from brain of MIGMLD or B4MLD and performed reverse transcription-PCR. Expression of HoxB4 was detected only on B4MLD but not MIGMLD (Figure 1c).
Figure 1.
FACS analysis of transplanted cells and detection of BM-derived cells in the brain. (a) PB samples from primary recipients were analyzed 3, 8, 25, and 32 weeks post-BMT, and the percentages of donor GFP+ cells are plotted against time. The percentage of GFP+ donor cells was significantly higher in HoxB4-transduced cells. (b) BM-derived cells were detected in the brain 8 months after transplantation. Greater numbers of GFP+ donor cells were detected in the brains of mice transplanted with HoxB4-transduced cells. (Bar = 100 µm) (c) expression of HoxB4 in the brain of B4MLD mice. RNAs were extracted from fixed brain of transplanted mice (MIGMLD or B4MLD) and analyzed by RT-PCR using primers specific for the human HoxB4 or β-actin. BM, bone marrow; B4MLD, HoxB4-transduced BM cells transplanted MLD mice; BMT, bone marrow transplantation; FACS, fluorescence-activated cell sorting; GFP, green fluorescent protein; MIGMLD, GFP-transduced BM cells transplanted MLD mice.
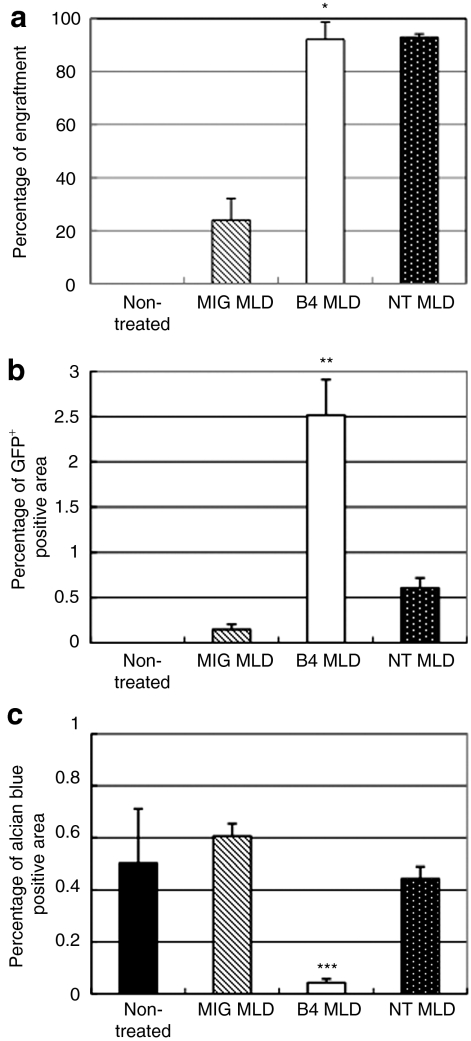
Figure 5.
Overexpression of HoxB4 is important for increasing the numbers of cells in the brain. Eight months after transplantation, mice were sacrificed and analyzed. (a) Percentage of engraftment detected by flow cytometry. *P < 0.05 compared to MIGMLD, (b) percentage of GFP+ in the brain. GFP+ area in the brain was calculated using Lumina Vision Software (Mitani). **P < 0.003 compared to MIGMLD and NTMLD, and (c) percentage of alcian blue+ in the brain. Alcian blue+ area in the brain was calculated by Lumina Vision Software. ***P < 0.01 compared to MIGMLD and NTMLD. Overexpression of HoxB4 is important for increasing the numbers of donor cells in the brain and for reduced the amount of stored sulfatides. B4MLD, HoxB4-transduced BM cells transplanted MLD mice; GFP, green fluorescent protein; MIGMLD, GFP-transduced BM cells transplanted MLD mice; NTMLD, nontransduced BM-transplanted MLD.
Transdifferentiation into oligodendrocytes was detected in B4MLD mice
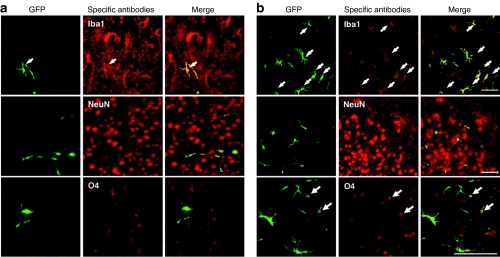
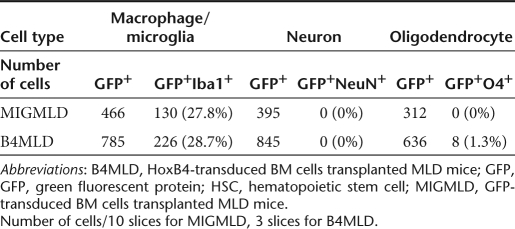
To determine which cell types were transdifferentiated in the brain, we immunostained brain sections using anti-Iba1 for detection of microglia, anti-NeuN for neurons, and anti-O4 for oligodendrocytes. As shown in Figure 2, the majority of GFP+ cells were Iba1+ glial cells, and there was no difference in the percentages of Iba1+ cells between the MIGMLD and B4MLD mice (27.8 versus 28.7%) (Table 1). In addition, although the efficiency of the transdifferentiation of O4+ cells was not high (1.3%), we were able to detect O4+ cells in the B4MLD mice, but not MIGMLD mice (Figure 2 and Table 1).
Figure 2.
BM cells overexpressing HoxB4 transdifferentiate into oligodendrocytes. Immunostaining brain sections from (a) MIGMLD and (b) B4MLD mice with anti-Iba1, anti-NeuN, and anti-O4 revealed that the majority of GFP+ cells were Iba1+ glial cells. O4+ oligodendrocytes were also detected in B4MLD mice but not in MIGMLD mice. (Bar = 100 µm). BM, bone marrow; B4MLD, HoxB4-transduced BM cells transplanted MLD mice; GFP, green fluorescent protein; MIGMLD, GFP-transduced BM cells transplanted MLD mice.
Table 1. Differentiation of HoxB4-expressing HSCs in the brain.
Therapeutic effects of BMT on MLD
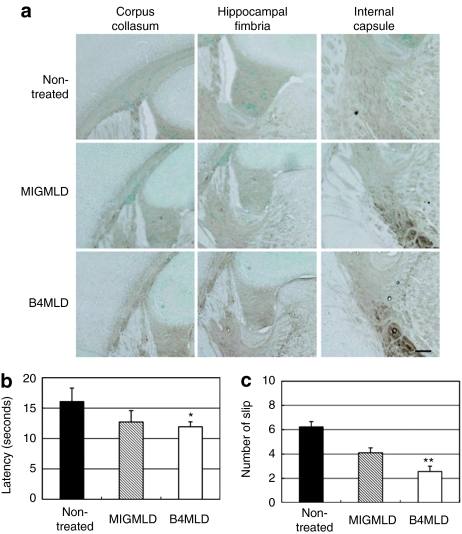
To analyze the therapeutic effects of BMT, we stained tissue samples with alcian blue to detect the presence of sulfatides, which specifically accumulates in various tissues in MLD. We found that sulfatide is detectable especially in corpus callosum, hippocampal fimbria, and internal capsule from 10-month-old nontreated and MIGMLD mice. By contrast, no significant sulfatide accumulation was detected in the B4MLD mice (Figure 3a). Furthermore, in the behavior test, B4MLD mice showed a corresponding improvement in their ability to traverse narrow balance beams, as compared to nontreated mice (latency: 11.7 ± 0.8 versus 16.2 ± 2.2 seconds, P < 0.05; slips: 2.6 ± 0.4 versus 6.2 ± 0.4 times, P < 0.001) (Figure 3b,c).
Figure 3.
Correction of sulfatide storage in the CNS and balance beam test of B4MLD mice. (a) Sections of corpus callosum, hippocampal fimbria, and internal capsule were stained with alcian blue. Cells accumulating sulfatides were detected in nontreated and MIGMLD mice. By contrast, no significant sulfatide accumulation was detected in B4MLD mice. (Bar = 200 µm) (b,c) balance and motor coordination on the balance beam. Nontreated (n = 15), MIGMLD (n = 9), and B4MLD (n = 13) mice were trained to walk across round beams having a cross-sectional diameter of 20 mm or less to reach an enclosed safety platform. (b) The time needed (latency) to cross and (c) the number of slips were recorded in each trial. All animals were given three trials on the beams. *P < 0.05, **P < 0.001 compared to nontreated. B4MLD, HoxB4-transduced BM cells transplanted MLD mice; CNS, central nervous system; MIGMLD, GFP-transduced BM cells transplanted MLD mice.
Expression of HoxB4 but not high level of engraftment is essential for increased number of donor cells in the brain
To determine whether the increased number of donor cells in the brain is dependent on the level of engraftment, we examined nontransduced (without in vitro culture) BM-transplanted MLD (NTMLD) mice to assess the importance of a high level of engraftment. Although >90% engraftment was obtained in NTMLD mice, which is about the same as in B4MLD mice (Figure 4a), there was no significant increase in the number of GFP+ cells (Figure 4b), as compared to nontreated or MIGMLD mice. Thus improved engraftment efficiency is not sufficient for increased number of BM-derived cells in brain. Moreover, alcian blue staining showed that there was no therapeutic effect of engraftment in NTMLD mice (Figure 4c). Figure 4d showed quantitative analysis of sulfatide contents by biochemical assay. Decrease of the amount of stored sulfatide was detected in B4MLD but not in NTMLD (21.6 ± 2.0 versus 34.1 ± 8.3 µg/mg protein, P < 0.05). Summarized in Figure 5 are the percentages of engraftment, GFP+, and alcian blue+ cells in the brain. Note that HoxB4-mediated expansion of repopulating cells appears to be essential for their increased number of GFP+ cells in the brain and therapeutic effect against MLD, and that these donor cells could serve as effective carriers of therapeutic proteins for secretion in the brain.
Figure 4.
The percentage of engraftment did not correlate with increased number of GFP+ cells in the brain. (a) Percentages of engraftment, (b) GFP+ cells in the brain (Bar = 100 µm), and (c) alcian blue staining in NTMLD mice (Bar = 200 µm). (d) Quantitative analysis of sulfatide contents by biochemical assay. *P < 0.05 compared to NTMLD. B4MLD, HoxB4-transduced BM cells transplanted MLD mice; GFP, green fluorescent protein; MIGMLD, GFP-transduced BM cells transplanted MLD mice; NTMLD, nontransduced BM-transplanted MLD.
Increased number of GFP+ cells is a result of increased local proliferation rather than enhanced migration
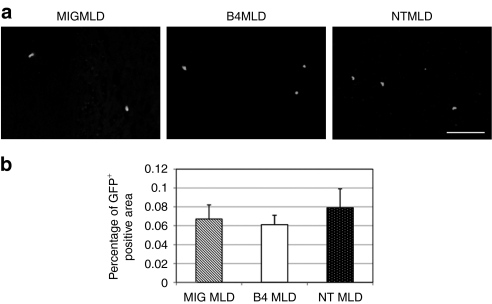
To analyze the mechanism of the increased number of GFP+ cells in the brain, we examined GFP+ cells in the brain at early stage after transplantation. We did not find any GFP+ cells in the brain in B4MLD, MIGMLD, or NTMLD mice three days after transplantation (data not shown). GFP+ donor cells were detected in the brain 7 days after transplantation (Figure 6a). There was no significant difference in the number of GFP+ cells in B4MLD, as compared to MIGMLD or NTMLD mice (Figure 6b). Almost same results were obtained when we examined GFP+ cells in the brain 10 days after transplantation (data not shown). From these results, we speculate that increased number of GFP+ cells 8 months after transplantation is a result of increased local proliferation or skewed differentiation rather than enhanced migration.
Figure 6.
BM-derived donor GFP+ cells detected in the brain 7 days after transplantation. (a) Seven days after transplantation, mice were sacrificed and analyzed GFP+ cells in the brain. GFP+ donor cells were detected in the brains of B4MLD (n = 4), MIGMLD (n = 4), or NTMLD (n = 5) mice. (Bar = 100 µm) (b) percentage of GFP+ in the brain. GFP+ area in the brain was calculated using Lumina Vision Software (Mitani). BM, bone marrow; B4MLD, HoxB4-transduced BM cells transplanted MLD mice; GFP, green fluorescent protein; MIGMLD, GFP-transduced BM cells transplanted MLD mice; NTMLD, nontransduced BM-transplanted MLD.
Discussion
Matzner and colleagues previously showed that gene therapy with BM cells harboring a retroviral vector encoding human ASA produces no significant improvement of biochemical or pathological findings in MLD mice.17,18 The limited success of this approach suggests that unexpectedly high levels of ASA are required to correct of the metabolic defect underlying MLD. This requirement could be met only through high-frequency HSC transduction and robust therapeutic gene expression in their progeny. One strategy for achieving high levels of ASA in the CNS is to transduce BM cells using a lentiviral vector encoding ASA. With this approach, the development of CNS disease manifestations has been prevented, although sulfatide levels in brain were not measured.19,20 Another strategy to get high levels of ASA into the CNS is to increase the number of cells in the brain. We found that HoxB4-transduced cells were detected in the brain much more efficiently than untransduced cells and could simultaneously reduce the amount of stored sulfatides and improve performance in a behavior test measuring clinical motor skills. This suggests that transplantation of HoxB4-expressing BM cells could be a potentially useful treatment for some neurological disorders.
Our immunohistochemical analysis of transdifferentiated cells in the brain revealed that the majority of GFP+ cells were Iba1+ glial cells. This finding is consistent with an earlier report showing that transplanted BM cells transdifferentiated into glial cells, but not into neurons or oligodendrocytes.5 To our surprise, however, we detected some O4+ cells in B4MLD mice, which is consistent with the findings of Nicolay et al., who reported that expression of HoxB4 may be involved in the transdifferentiation of oligodendrocytes.21 Notably, regeneration of oligodendrocytes is thought to be potentially useful for the treatment of demyelinating disorders such as MLD, Krabbe disease, and multiple sclerosis. Because oligodendrocytes are a major target in MLD, our finding that overexpression of HoxB4 leads to production of oligodendrocytes could represent an important advantage for the treatment of MLD.
It remains unclear why cells with enforced expression of HoxB4 were detected in the CNS more efficiently than control cells. Despite a high percentage of donor cells in the peripheral blood of NTMLD mice, efficient GFP+ cells in the brain was not detected (Figure 4b), suggesting that expression of HoxB4 is essential for increased number of BM-derived cells in the brain. HoxB4 overexpression increases the self-renewal and regeneration of HSC. Therefore, one explanation is that the number of primitive HSCs in B4MLD mice is higher than in NTMLD mice, and although similar numbers of cells migrate into the brain, only primitive HSCs are able to efficiently regenerate and differentiate after the migration (increased local proliferation), thereby explaining the therapeutic effect. Our results (Figure 6) support this explanation that increased number of GFP+ cells is a result of increased local proliferation or skewed differentiation rather than enhanced migration. Alternatively, even though similar levels of chimerism were seen in B4MLD and NTMLD, there could be significant differences in distributions of cell types (myeloid or lymphoid). As far as we examined, we did not find any significant difference of cell types in the BM between B4MLD and NTMLD (myeloid: 35.2 ± 1.8 versus 35.6 ± 2.7%, lymphoid: 8.2 ± 2.5 versus 6.5 ± 0.5%). To elucidate the molecular mechanism increased number of HoxB4 expressing cells in the brain, we need more detailed experiments such as monitoring the content of Colony-Forming Unit-Granulocyte, Macrophage, short-term repopulating cells of transplant recipients or additional transplantation experiment using BM cells without in vitro expansion after transduction rather than after a further 5 days in culture.
Recently, Zhang et al. demonstrated that two of two dogs and one of two macaque monkeys developed myeloid leukemia ~2 years after transplantation with cells overexpressing HoxB4 transduced using a gammaretroviral vector.22 In our experiment, we detected no side effects, including leukemogenesis, of retroviral transduction in B4MLD mice (data not shown). Most likely a number of factors contribute to the development of leukemia in transplanted animals—e.g., expression level and/or constitutive expression of HoxB4, type of expression vector used, and the species of the recipient animals. The importance of all of these potential factors should be analyzed carefully and vectors can be developed where HoxB4 expression is regulated to avoid leukemogenesis.
In summary, we developed the BMT strategy using HoxB4-expressing BM cells to treat MLD mice. HoxB4-expressing BM cells transplanted into MLD mice were detected efficiently in the brain, the amount of stored sulfatide was dramatically reduced, and motor skills were significantly improved. Moreover, we found that HoxB4-expressing BM cells transdifferentiated into oligodendrocytes. These results suggest that HoxB4-expressing BM cells can be used as the carriers of therapeutic proteins in the brain and regeneration of oligodendrocytes in the treatment of demyelinating disorders such as MLD, Krabbe disease, and multiple sclerosis.
Materials and Methods
Mice. ASA knockout mice (MLD mice) were obtained from the laboratory of Dr Volkmar Gieselmann.23 These mice have been extensively characterized and are widely used as a mouse model for MLD.4,24,25,26,27 GFP mice originated from C57/BL6.28 All animal experiments were performed in accordance with the regulations of the Ethics Committee of Nippon Medical School (Tokyo, Japan).
Retroviral vectors. MSCV-HOXB4-IRES-GFP (MIGB4) retroviral vector encoding both HoxB4 and GFP and MSCV-IRES-GFP (MIG) retroviral control vector encoding only GFP were kindly provided by Dr Keith Humphries (Terry Fox Laboratory, Vancouver, British Columbia, Canada).16,29
Transduction and transplantation of murine BM cells. Transduction and transplantation of murine BM cells were performed as described previously.29 In brief, BM cells were extracted from the femurs and tibiae of wild-type C57BL/6 GFP mice treated with 5-flurouracil (150 mg/kg; Dainihonnseiyaku, Tokyo, Japan) 4 days before harvest and cultured to a density of 0.5 × 106 cells/ml for 48 hours in X-Vivo medium (X-Vivo15; BioWhittaker, Walkersville, MD) supplemented with 1% bovine serum albumin (Stem Cell Technologies, Vancouver, British Columbia, Canada), 0.1 mmol/l β-mercaptoethanol (Sigma-Aldrich, St Louis, MO), 2 mmol/l -glutamine (Sigma-Aldrich), 100 U/ml penicillin, 100 mg/ml streptomycin (Sigma-Aldrich), 50 ng/ml murine stem cell factor, 10 ng/ml murine interleukin-3 and 50 ng/ml human interleukin-6 (R&D Systems, Minneapolis, MN). For transduction, the cells were transferred to retronectin (Takara Shuzo, Otsu, Japan)-coated 24-well plates that had been preloaded with 1 ml of supernatant containing MIG or MIGB4 virus and incubated for 1 hour at 37 °C. After retroviral transduction, the BM cells were cultured for 5 days. On day 5, initial 1.5 × 105 unsorted BM cells were transplanted into lethally irradiated (950 cGy) MLD mice. To test the effects of high-level engraftment in the absence of retroviral transduction, nontransduced (without in vitro culture) BM cells from 5-flurouracil-treated C57BL/6 GFP mice (2 × 106) were also transplanted into lethally irradiated MLD mice.
Flow cytometry. Peripheral blood samples were collected from the retro-orbital sinus of mice, after which the cells were pelleted and washed twice with phosphate-buffered saline containing 2% fetal calf serum, and the red blood cells were lysed with ammonium chloride (NH4Cl; Stem Cell Technologies). The dead cells were removed after straining with 7-aminoactinomycin D (Sigma-Aldrich). The remaining cells were subjected to flow cytometry using a FACS Calibur (Becton Dickinson, San Jose, CA), and results were analyzed using Cell Quest software (Becton Dickinson).
Immunohistochemical analysis. Mice were anesthetized and then perfused with phosphate-buffered saline followed by 4% paraformaldehyde. The brains were then dissected, fixed again overnight at 4 °C, and immersed in phosphate-buffered saline containing 20% sucrose. Thereafter, they were placed in OCT compound (Tissue-Tek, Tokyo, Japan), and tissue sections were cut to a thickness of 20 µm and mounted on glass slides. Migrated GFP+ cells were detected by fluorescence microscopy (Olympus). In addition, the sections were immunostained for neurons, microglia, and oligodendrocytes by incubation for 16 hours at 4 °C with mouse anti-NeuN antibody (1:500; Millipore, Billerica, MA), rabbit anti-Iba1 polyclonal antibody (1:500; Wako, Osaka, Japan), and mouse anti-oligodendrocyte marker O4 monoclonal antibody (1:50; Millipore), respectively. After washing the sections three times with phosphate-buffered saline, they were stained with Texas-red-conjugated donkey anti-rabbit or anti-mouse immunoglobulin G (1:200; Dako, Glostrup, Denmark). Finally, the sections were examined using a confocal laser scanning microscope (Leica, Wetzlar, Germany), and the collected signals were digitally color enhanced before superimposition.
Quantification of sulfatide levels. Sulfatides was extracted from the organs and purified essentially as described.30 Quantification of sulfatide levels was analyzed as described previously.31 Amounts of sulfatide were quantitatively determined by densitometric scanning with a DT-20 MCP (Atto, Tokyo, Japan). Standard Sulfatide was obtained from Wako.
Alcian blue staining. To detect the accumulation of sulfatides, brain sections were stained with alcian blue (Sigma-Aldrich) as described previously.32
Quantitative analysis. Quantitative analysis of GFP+ and alcian blue+ area was analyzed with a computerized image analyzer consisting of a personal computer running Lumina Vision software (Mitani) interfaced with a Olympus digital CCD camera (DP70) mounted on an BX60 microscope (Olympus). The percentages of GFP+ or alcian blue+ area were calculated by the following formula: GFP or alcian blue+ area (%) = [GFP or alcian blue+ area (µm2)]/[area of subfield (114,855 µm2)] × 100.33
Reverse transcription-PCR for detection of HoxB4 expression. RNA were extracted from fixed brain of transplanted mice (MIGMLD or B4MLD) 8 months after transplantation using RecoverALL kit (Ambion, Foster City, CA). Reverse transcription-PCR was performed as described previously34 using human HoxB4 specific primers (sense: 5′-GTCTGTCCCCTC GGGCTCCTG-3′ and antisense: 5′-TTGGGCAACTTGTGGTCTTTT-3′). β-Actin was also amplified as a control.35
Behavior test. Motor coordination and balance were assessed in the mice by measuring their ability to traverse a graded series of narrow beams to reach an enclosed safety platform.36 The beams consisted of long (1 m) stainless steel rods, starting at a cross-sectional diameter of 20 mm (O'Hara, Tokyo, Japan). The beam was placed horizontally, 50 cm above the bench surface, with one end mounted on a narrow support and the other end attached to an enclosed box (20 cm2) into which a mouse could escape. The mice were trained to traverse the beam to the enclosed box. The time required to traverse each beam (“latency”) and the number of times the hind feet slipped off a beam (“slips”) were recorded for each trial. Analysis of each measure was based on the mean scores of three trials.
Statistical analysis. Results are expressed as means ± SE of duplicate or more data obtained from three or more independent experiments. Data were analyzed using two-tailed Student's t-tests. Values of P < 0.05 were considered significant.
Acknowledgments
We thank Dr Keith Humphries for kindly providing a retroviral vector encoding HoxB4, and Dr Volkmar Gieselmann for kindly providing MLD model mice. We also thank Dr Tsutomu Igarashi, Motoko Yamamoto, Manami Okabe, and Nagisa Asakawa for technical assistance. This work was supported in part by grants from the Ministry of Health and Welfare of Japan and the Ministry of Education, Science, and Culture of Japan.
REFERENCES
- von Figura K, Gieselmann V., and, Jaeken J.2001. Metachromatic Leukodystrophy. In: Scriver, CR, Beaudet, AL, Valle, D, Sly, WS, Childs, B, Kinzler, KW et al. (eds). The Metabolic and Molecular Bases of Inherited Disease, 8th edn. McGraw-Hill: New York, pp.3695–3724. [Google Scholar]
- Krivit W. Allogeneic stem cell transplantation for the treatment of lysosomal and peroxisomal metabolic diseases. Springer Semin Immunopathol. 2004;26:119–132. doi: 10.1007/s00281-004-0166-2. [DOI] [PubMed] [Google Scholar]
- Hille-Rehfeld A. Mannose 6-phosphate receptors in sorting and transport of lysosomal enzymes. Biochim Biophys Acta. 1995;1241:177–194. doi: 10.1016/0304-4157(95)00004-b. [DOI] [PubMed] [Google Scholar]
- Matzner U, Herbst E, Hedayati KK, Lüllmann-Rauch R, Wessig C, Schröder S, et al. Enzyme replacement improves nervous system pathology and function in a mouse model for metachromatic leukodystrophy. Hum Mol Genet. 2005;14:1139–1152. doi: 10.1093/hmg/ddi126. [DOI] [PubMed] [Google Scholar]
- Asheuer M, Pflumio F, Benhamida S, Dubart-Kupperschmitt A, Fouquet F, Imai Y, et al. Human CD34+ cells differentiate into microglia and express recombinant therapeutic protein. Proc Natl Acad Sci USA. 2004;101:3557–3562. doi: 10.1073/pnas.0306431101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priller J, Flügel A, Wehner T, Boentert M, Haas CA, Prinz M, et al. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med. 2001;7:1356–1361. doi: 10.1038/nm1201-1356. [DOI] [PubMed] [Google Scholar]
- Escolar ML, Poe MD, Provenzale JM, Richards KC, Allison J, Wood S, et al. Transplantation of umbilical-cord blood in babies with infantile Krabbe's disease. N Engl J Med. 2005;352:2069–2081. doi: 10.1056/NEJMoa042604. [DOI] [PubMed] [Google Scholar]
- Malatack JJ, Consolini DM., and, Bayever E. The status of hematopoietic stem cell transplantation in lysosomal storage disease. Pediatr Neurol. 2003;29:391–403. doi: 10.1016/j.pediatrneurol.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Krivit W, Aubourg P, Shapiro E., and, Peters C. Bone marrow transplantation for globoid cell leukodystrophy, adrenoleukodystrophy, metachromatic leukodystrophy, and Hurler syndrome. Curr Opin Hematol. 1999;6:377–382. doi: 10.1097/00062752-199911000-00004. [DOI] [PubMed] [Google Scholar]
- Buske C., and, Humphries RK. Homeobox genes in leukemogenesis. Int J Hematol. 2000;71:301–308. [PubMed] [Google Scholar]
- Sauvageau G, Lansdorp PM, Eaves CJ, Hogge DE, Dragowska WH, Reid DS, et al. Differential expression of homeobox genes in functionally distinct CD34+ subpopulations of human bone marrow cells. Proc Natl Acad Sci USA. 1994;91:12223–12227. doi: 10.1073/pnas.91.25.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsteinsdottir U, Sauvageau G., and, Humphries RK. Enhanced in vivo regenerative potential of HOXB4-transduced hematopoietic stem cells with regulation of their pool size. Blood. 1999;94:2605–2612. [PubMed] [Google Scholar]
- Sauvageau G, Thorsteinsdottir U, Eaves CJ, Lawrence HJ, Largman C, Lansdorp PM, et al. Overexpression of HOXB4 in hematopoietic cells causes the selective expansion of more primitive populations in vitro and in vivo. Genes Dev. 1995;9:1753–1765. doi: 10.1101/gad.9.14.1753. [DOI] [PubMed] [Google Scholar]
- Klump H, Schiedlmeier B, Vogt B, Ryan M, Ostertag W., and, Baum C. Retroviral vector-mediated expression of HoxB4 in hematopoietic cells using a novel coexpression strategy. Gene Ther. 2001;8:811–817. doi: 10.1038/sj.gt.3301447. [DOI] [PubMed] [Google Scholar]
- Antonchuk J, Sauvageau G., and, Humphries RK. HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell. 2002;109:39–45. doi: 10.1016/s0092-8674(02)00697-9. [DOI] [PubMed] [Google Scholar]
- Antonchuk J, Sauvageau G., and, Humphries RK. HOXB4 overexpression mediates very rapid stem cell regeneration and competitive hematopoietic repopulation. Exp Hematol. 2001;29:1125–1134. doi: 10.1016/s0301-472x(01)00681-6. [DOI] [PubMed] [Google Scholar]
- Matzner U, Schestag F, Hartmann D, Lüllmann-Rauch R, D'Hooge R, De Deyn PP, et al. Bone marrow stem cell gene therapy of arylsulfatase A-deficient mice, using an arylsulfatase A mutant that is hypersecreted from retrovirally transduced donor-type cells. Hum Gene Ther. 2001;12:1021–1033. doi: 10.1089/104303401750214258. [DOI] [PubMed] [Google Scholar]
- Matzner U, Harzer K, Learish RD, Barranger JA., and, Gieselmann V. Long-term expression and transfer of arylsulfatase A into brain of arylsulfatase A-deficient mice transplanted with bone marrow expressing the arylsulfatase A cDNA from a retroviral vector. Gene Ther. 2000;7:1250–1257. doi: 10.1038/sj.gt.3301232. [DOI] [PubMed] [Google Scholar]
- Biffi A, De Palma M, Quattrini A, Del Carro U, Amadio S, Visigalli I, et al. Correction of metachromatic leukodystrophy in the mouse model by transplantation of genetically modified hematopoietic stem cells. J Clin Invest. 2004;113:1118–1129. doi: 10.1172/JCI19205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffi A, Capotondo A, Fasano S, del Carro U, Marchesini S, Azuma H, et al. Gene therapy of metachromatic leukodystrophy reverses neurological damage and deficits in mice. J Clin Invest. 2006;116:3070–3082. doi: 10.1172/JCI28873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolay DJ, Doucette JR., and, Nazarali AJ. Hoxb4 in oligodendrogenesis. Cell Mol Neurobiol. 2004;24:357–366. doi: 10.1023/B:CEMN.0000022768.34389.4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XB, Beard BC, Trobridge GD, Wood BL, Sale GE, Sud R, et al. High incidence of leukemia in large animals after stem cell gene therapy with a HOXB4-expressing retroviral vector. J Clin Invest. 2008;118:1502–1510. doi: 10.1172/JCI34371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess B, Saftig P, Hartmann D, Coenen R, Lüllmann-Rauch R, Goebel HH, et al. Phenotype of arylsulfatase A-deficient mice: relationship to human metachromatic leukodystrophy. Proc Natl Acad Sci USA. 1996;93:14821–14826. doi: 10.1073/pnas.93.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittke D, Hartmann D, Gieselmann V., and, Lüllmann-Rauch R. Lysosomal sulfatide storage in the brain of arylsulfatase A-deficient mice: cellular alterations and topographic distribution. Acta Neuropathol. 2004;108:261–271. doi: 10.1007/s00401-004-0883-6. [DOI] [PubMed] [Google Scholar]
- Sevin C, Benraiss A, Van Dam D, Bonnin D, Nagels G, Verot L, et al. Intracerebral adeno-associated virus-mediated gene transfer in rapidly progressive forms of metachromatic leukodystrophy. Hum Mol Genet. 2006;15:53–64. doi: 10.1093/hmg/ddi425. [DOI] [PubMed] [Google Scholar]
- D'Hooge R, Coenen R, Gieselmann V, Lüllmann-Rauch R., and, De Deyn PP. Decline in brainstem auditory-evoked potentials coincides with loss of spiral ganglion cells in arylsulfatase A-deficient mice. Brain Res. 1999;847:352–356. doi: 10.1016/s0006-8993(99)02085-5. [DOI] [PubMed] [Google Scholar]
- Consiglio A, Quattrini A, Martino S, Bensadoun JC, Dolcetta D, Trojani A, et al. In vivo gene therapy of metachromatic leukodystrophy by lentiviral vectors: correction of neuropathology and protection against learning impairments in affected mice. Nat Med. 2001;7:310–316. doi: 10.1038/85454. [DOI] [PubMed] [Google Scholar]
- Okabe M, Ikawa M, Kominami K, Nakanishi T., and, Nishimune Y. ‘Green mice' as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- Miyake N, Brun AC, Magnusson M, Miyake K, Scadden DT., and, Karlsson S. HOXB4-induced self-renewal of hematopoietic stem cells is significantly enhanced by p21 deficiency. Stem Cells. 2006;24:653–661. doi: 10.1634/stemcells.2005-0328. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M., and, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Iwamoto N, Watanabe A, Yamamoto M, Miyake N, Kurai T, Teramoto A, et al. Global diffuse distribution in the brain and efficient gene delivery to the dorsal root ganglia by intrathecal injection of adeno-associated viral vector serotype 1. J Gene Med. 2009;11:498–505. doi: 10.1002/jgm.1325. [DOI] [PubMed] [Google Scholar]
- Scott JE., and, Dorling J. Differential staining of acid glycosaminoglycans (mucopolysaccharides) by alcian blue in salt solutions. Histochemie. 1965;5:221–233. doi: 10.1007/BF00306130. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Okazaki M, Takata K, Kitamura Y, Ohta S, Sekino Y, et al. Endogenous adenosine protects CA1 neurons from kainic acid-induced neuronal cell loss in the rat hippocampus. Eur J Neurosci. 1999;11:3617–3625. doi: 10.1046/j.1460-9568.1999.00781.x. [DOI] [PubMed] [Google Scholar]
- Miyake K, Inokuchi K, Dan K., and, Nomura T. Alterations in the deleted in colorectal carcinoma gene in human primary leukemia. Blood. 1993;82:927–930. [PubMed] [Google Scholar]
- Tahara I, Miyake K, Hanawa H, Kurai T, Hirai Y, Ishizaki M, et al. Systemic cancer gene therapy using adeno-associated virus type 1 vector expressing MDA-7/IL24. Mol Ther. 2007;15:1805–1811. doi: 10.1038/sj.mt.6300225. [DOI] [PubMed] [Google Scholar]
- Carter RJ, Lione LA, Humby T, Mangiarini L, Mahal A, Bates GP, et al. Characterization of progressive motor deficits in mice transgenic for the human Huntington's disease mutation. J Neurosci. 1999;19:3248–3257. doi: 10.1523/JNEUROSCI.19-08-03248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]