Abstract
Although the importance of natural killer (NK) cells in innate immune responses against tumors or viral infections are well documented, their ability to directly recognize pathogens is less well defined. We have recently reported FimH, a bacterial fimbrial protein, as a novel Toll-like receptor (TLR)4 ligand that potently induces antiviral responses. Here, we investigated whether FimH either directly or indirectly can activate human and murine NK cells. We demonstrate that FimH potently activates both human and murine NK cells in vitro to induce cytokines [interferon (IFN)-γ and tumor necrosis factor (TNF)-α] and cytotoxicity. Importantly, NK cells directly recognize FimH-expressing pathogens as FimH+, but not FimH−, bacteria were able to activate human NK cells. FimH activation of NK cells required TLR4 and MyD88 signaling, as NK cells from both TLR4−/− and MyD88−/− mice as well as human NK-92 cells, which lack TLR4, were all unresponsive to FimH. In addition, TLR4 neutralization significantly abrogated the response of human NK cells to FimH. Activation of purified NK cells by FimH was independent of lipopolysaccharide (LPS) or other bacterial contaminations. These data demonstrate for the first time that highly purified NK cells directly recognize and respond to FimH via TLR4–MyD88 pathways to aid innate protection against cancer or microbial infections.
Introduction
Natural killer (NK) cells constitute a unique subset of lymphocytes that are an important part of host innate antimicrobial and antitumoral defense.1,2 Upon microbial infection, NK cells are activated by cytokines produced by surrounding innate cells, particularly antigen-presenting cells (APCs), and produce cytokines including interferon (IFN)-γ and tumor necrosis factor (TNF)-α that tailor innate and adaptive immune responses by modulating the growth and differentiation of monocytes, dendritic cells, and granulocytes.1,3,4 NK cells are well-known primary producers of IFN-γ that helps to clear some intracellular pathogens.5 NK cell–derived inflammatory cytokines are also known to play a role in tumor clearance via the restriction of tumor-driven angiogenesis and the generation of tumor-specific immunity.6 Given the fact that NK cells can target virally infected cells and tumor cells, a number of strategies for the therapeutic application of NK cells have been proposed based on mediators that can directly or indirectly activate NK cells endogenously.7,8,9 Much is known about the effector function of NK cells, however, the activation of these cells through Toll-like receptor (TLR) detection of microbial components is less well studied. Recently, we and others have demonstrated that human NK cells express most TLRs and can be activated by several microbial components, known as pathogen-associated molecular patterns (PAMPs), including flagellin, lipopolysaccharide (LPS), peptidoglycan, and double-stranded RNA.3,10,11,12,13,14
Recognition of microbial pathogens by TLRs is a prerequisite for the activation of innate and adaptive immune responses. Several PAMPs of bacterial or viral origin have been well appreciated to bind directly to TLRs to initiate host innate defense against infections and cancers.15,16,17,18,19 Of the bacterial PAMPs, peptidoglycan binds to TLR2, LPS binds to TLR4, flagellin binds to TLR5, and bacterial DNA is recognized by TLR9. Upon PAMP ligation all TLRs, except TLR3, signal through the common adaptor molecule MyD88. TLR4 can also alternatively utilize Toll/interleukin-1 receptor (TIR) domain containing adaptor inducing-IFN-b (TRIF) to activate distinctive downstream signaling cascades leading to type 1 IFN and proinflammatory cytokine release.18,20,21 Bacterial DNA containing unmethylated CpG dinucleotides has become an important immunomodulator in the context of protection against pathogens and cancer immunotherapy.22,23 The outer membrane protein of Klebsiella pneumoniae (KpOmpA) and flagellin have been shown to activate NK-cell proliferation and function directly as well as through priming dendritic cells.10,24 Recent advancement with fusion proteins of known antigens and TLR ligands have been effectively utilized as adjuvant.25,26 TLR characterizations have unveiled the mode of action of the adjuvant and their potential benefits.18,27,28 We have recently discovered that uropathogenic Escherichia coli (UPEC) fimbrial type 1 pilli, FimH adhesin, acts as a novel ligand for TLR4 (ref. 29) enhancing antiviral responses in a TLR4–MyD88-dependent manner.30 In this study, we investigated the potential role of FimH in human and murine NK-cell activation, inhibition of UPEC colonization in bladder and kidney and antitumor activity. We demonstrate that FimH directly activates both human and murine NK cells in vitro to induce cytokines, activation phenotypes, and cytotoxicity. This FimH-induced NK-cell activation was independent of LPS or other bacterial contaminations and required TLR4 and MyD88 signaling. Further, NK cells were able to directly recognize and discriminate pathogenic FimH+ E. coli from nonpathogenic (FimH-deficient) E. coli. More importantly, FimH potently prevented transurethral UPEC infections as well as melanoma tumor challenge in an NK cell-dependent fashion in mouse model.
Results
FimH activates human and murine NK cells directly
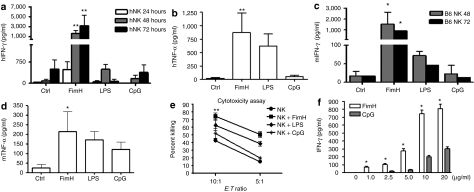
Several TLR ligands have been shown to activate NK cell functions.3,10,13 We have recently discovered that FimH can act as a novel ligand for TLR4 (ref. 29), and induces a strong antiviral response against herpes simplex virus-2 infections.30 However, the effects of FimH on NK-cell function have not been well characterized. Here, we examined the ability of FimH to activate human CD56+ NK cells to produce innate cytokines and acquire cytolytic ability in vitro. NK cells were purified from human peripheral blood mononuclear cell (PBMCs), stimulated with FimH for up to 72 hours and cell-free supernatants were assayed for IFN-γ and TNF-α production. As illustrated in Figure 1a,b, both IFN-γ and TNF-α production were substantially elevated after FimH stimulation. Interestingly, NK cell responses to FimH were greater than those observed with either LPS or CpG. We then examined the potential of FimH to induce cytokine production in murine C57BL/6 CD49b (DX5)+ NK-cell populations purified from splenocytes. Similarly, comparable levels of cytokines were detected from FimH-activated murine NK cells (Figure 1c,d), although FimH induced greater amounts of IFN-γ and TNF-α compared to CpG and LPS. We then examined the ability of FimH to stimulate CD56+ NK-cell-mediated cytotoxicity utilizing a standard 4-hour chromium release assay with human K562 tumor cells as targets. The treatment of NK cells with FimH markedly increased the cytotoxic response against K562 cancer cells (Figure 1e), and this response including IFN-γ production (Figure 1f) was higher than that induced with either LPS and/or CpG stimulation.
Figure 1.
FimH directly activates human and murine NK cell cytokine release and cytotoxicity. Human peripheral blood NK cells were cultured in the absence or presence of FimH (10 µg/ml), LPS (100 ng/ml), or CpG (10 µg/ml) for up to 72 hours and then cell-free supernatants harvested and analyzed for (a) IFN-γ and (b) TNF-α by specific enzyme-linked immunosorbent assay (ELISA). (Data are mean ± SEM of five separate experiments using NK cells from five donors cultured in triplicate wells.) Splenic NK cells from C57BL/6 mice were unstimulated or stimulated with FimH (10 µg/ml), LPS (100 ng/ml), or CpG (10 µg/ml) for 72 hours, cell-free supernatants harvested, and (c) IFN-γ or (d) TNF-α concentrations measured by specific ELISA. (Data are mean ± SEM of three individual experiments using NK cells from each of three mice cultured in triplicate wells.) (e) Human peripheral blood NK cells were cultured with or without FimH (10 µg/ml), LPS (100 ng/ml), or CpG (10 µg/ml) stimulations for 48 hours, co-cultured with chromium-labeled target K562 cells in triplicate wells at two different effector to target (E:T) ratios, 10:1 and 5:1, for 4 hours and were analyzed for cytotoxicity. (Data are mean ± SEM, n = 3.) (f) Human NK cells were untreated or treated with varying concentrations of FimH or CpG as shown in the figure for 48 hours and IFN-γ levels were measured in cell-free supernatants by ELISA. Data are mean ± SD from at least two separate experiments using two different volunteers. *P < 0.05, **P < 0.01, ***P < 0.001, comparisons were shown between FimH treatments and untreated controls and/or FimH treatments and LPS or CpG treatments. h, human; IFN, interferon; LPS, lipopolysaccharide; m, murine; NK, natural killer; TNF, tumor necrosis factor.
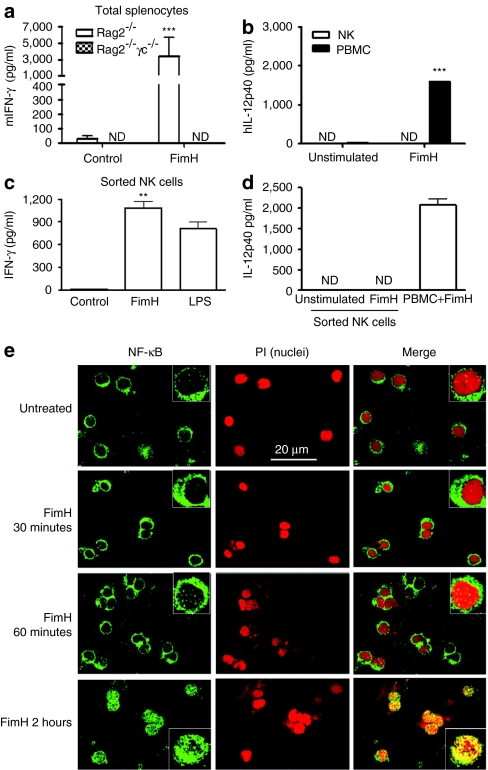
NK cells can be activated directly following TLR ligand stimulation10,13 or indirectly via cytokines produced by surrounding APCs.14 To demonstrate that NK cells, but not T cells or macrophages, were the source of IFN-γ following FimH activation, we treated splenocytes from RAG2−/− (lacking B and T cells) and RAG-2−/−γc−/− (lacking B, T, NK, and NKT cells) with FimH and measured IFN-γ levels. Interestingly, in contrast to the substantial secretion of IFN-γ by RAG2−/− splenocytes, RAG-2−/−/γc−/− splenocytes completely failed to produce any detectable IFN-γ upon FimH stimulation (Figure 2a), which indicates the possible secretion of IFN-γ from NK cells upon stimulation by FimH. We then evidenced that FimH directly activates purified human NK cells by analyzing the presence of interleukin (IL)-12 in supernatants from purified NK cells and PBMCs after FimH treatment. As illustrated in Figure 2b, CD56+ NK cells did not release detectable IL-12p40, whereas PBMC populations produced significant quantities of IL-12p40. Similar results were observed with purified murine NK cells (data not shown). Finally, using flow cytometry sorted highly purified (98.8%) human NK cells, we confirmed that FimH directly activates NK cells to release IFN-γ (Figure 2c,d). To further confirm that NK cell activation by FimH is a direct effect, we analyzed a key signaling molecule, NF-κB (nuclear factor κ-light-chain-enhancer of activated B cells) activation upon FimH stimulations. Purified human peripheral NK cells were stimulated with FimH (10 µg/ml) for various time points and NF-κB nuclear translocation was evaluated by confocal immunofluorescence imaging. Importantly, short-term stimulations, even up to 60 minutes, by FimH could not activate NF-κB, whereas stimulations for 2 hours strongly activated NF-κB with complete nuclear translocation (Figure 2e).
Figure 2.
FimH activation of NK cells is direct. (a) Total splenocytes from RAG2−/− (n = 5) and RAG2−/−γc−/− (n = 3) mice were cultured with or without FimH (10 µg/ml) stimulations for 48 hours, and cell-free supernatants were assessed for IFN-γ production by enzyme-linked immunosorbent assay (ELISA). (b) Human peripheral blood mononuclear cells (PBMCs) and purified NK cells were cultured in absence or presence of FimH (10 µg/ml) for 24 hours and cell-free supernatants assessed for IL-12p40 secretions by ELISA (n = 3, mean ± SD). (c) Human peripheral blood NK cells initially purified by EasySep negative selection NK enrichment kit were further sorted by flow cytometry (98.8% pure NK cells) and cultured in triplicate wells with or without FimH (10 µg/ml) or LPS (100 ng/ml) for 48 hours and cell-free supernatants were assayed for IFN-γ by ELISA. (d) EasySep negative selection purified followed by flow cytometry sorted NK cells or PBMCs (as positive control) were induced with FimH (10 µg/ml) for 24 hours and supernatants were measured by ELISA for IL-12p40 production. Data for (c) and (d) are mean ± SD from two separate experiments with two donors). (e) Purified human peripheral blood NK cells were untreated or stimulated with FimH (10 µg/ml) for the indicated time periods, cells were then transferred onto slides by Shandon cytospin 4, acetone fixed and stained for NF-κB (green) using Alxafluor-488-labeled secondary antibody or counterstained (nuclear staining) with propidium iodide (red). Images were gained using LSM 510 confocal microscope using ×63 objectives (insets are higher magnification images). Bar = 20 µm. h, human; IFN, interferon; IL, interleukin; LPS, lipopolysaccharide; NK, natural killer; m, murine.
FimH+, but not FimH−, UPEC induces NK-cell activation
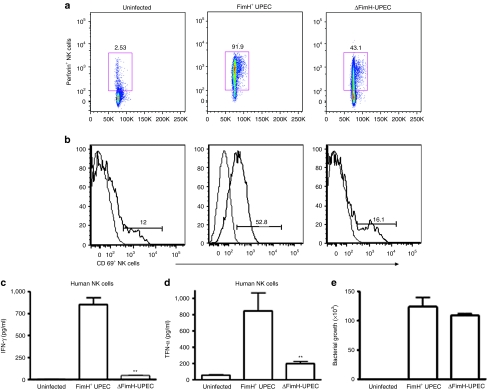
We further evaluated whether human NK cells could directly recognize FimH+ UPEC bacteria. To this end, we co-cultured highly purified human CD56+ NK cells along with FimH+ (strain Nu14) or FimH− (strain Nu14-1) UPEC bacteria and analyzed the expression of CD69 and perforin, the key NK cell activation markers, by flow cytometry. Interestingly, purified NK cells co-cultured with FimH+ UPEC displayed highly elevated expression of CD69 and perforin compared to those co-cultured with FimH− UPEC (Figure 3a,b). We further examined the cell-free supernatants from the above NK cells and UPEC co-cultures for IFN-γ and TNF-α production. Similarly, the FimH-deficient strain of UPEC bacteria had a diminished capacity to activate IFN-γ and TNF-α release by NK cells, as illustrated in Figure 3c,d. On the contrary, FimH+ bacteria induced significant levels of IFN-γ and TNF-α production. We then evaluated whether NK cells have any impact on the growth of FimH+ or FimH− UPEC. As shown in Figure 3e, NK cells apparently had no influence on the growth of either FimH+ or FimH− UPEC bacterial strains.
Figure 3.
NK cells directly recognize FimH+ UPEC pathogens and become activated. Human peripheral blood CD56+ NK cells were either uninfected or infected ex vivo with FimH+ UPEC (Nu14) or δFimH-UPEC (Nu14-1) bacteria at an multiplicity of infection of 20 as described in Materials and Methods for 16 hours, fluorescence-activated cell sorting stained for intracellular perforin or surface expression of CD69, known NK-cell activation markers and analyzed by flow cytometry. CD56+CD3− lymphocyte populations (NK cells) expressing (a) perforin or (b) CD69 were gated to show NK cell activation phenotypes. Data represent three independent and reproducible experiments. Human peripheral blood NK cells were uninfected or infected with FimH+ or δFimH-UPEC bacteria similarly as mentioned earlier for 24 hours and cell-free supernatants were assessed for (c) IFN-γ or (d) TNF-α release by enzyme-linked immunosorbent assay. (e) Human peripheral NK cells were co-cultured with FimH+ or FimH− UPEC at NK:UPEC ratio of 1:20 for 2 hours and cells washed, lysed, and cells lysates were plated onto Luria–Bertani agar for overnight and bacterial colony-forming units (CFU) were counted. Data represent mean ± SEM of at least two independent experiments involving two different donors. **P < 0.01. IFN, interferon; NK, natural killer; TNF, tumor necrosis factor; UPEC, uropathogenic E. coli.
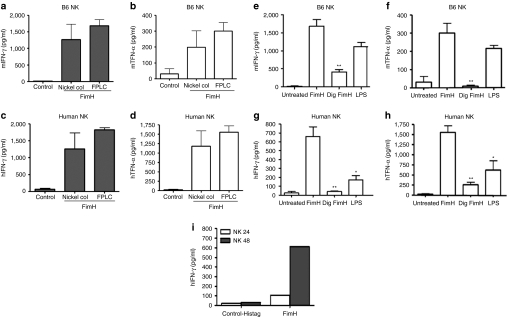
FimH activation of NK cells require TLR4–MyD88 signaling pathway
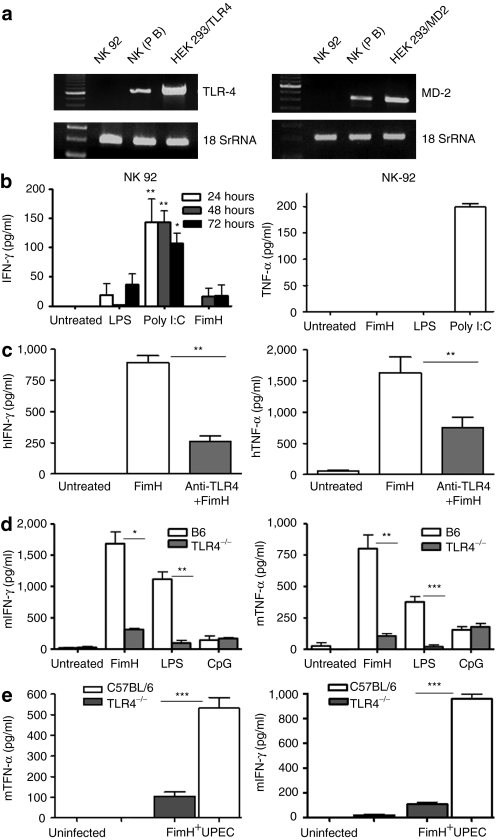
We have recently established that FimH is a potent ligand for TLR4 in the absence of MD-2 or CD14, factors essential for LPS signaling via TLR4 (ref. 29). Here, we examined whether or not FimH requires TLR4 to stimulate human or murine NK cells. Therefore, we first examined the expression of TLR4 and MD-2 on purified primary human NK cells or a human-transformed NK cell line (NK-92). Although freshly isolated human NK cells expressed TLR4 and MD-2 mRNA, NK-92 cells were negative for both TLR4, and MD-2 mRNA expressions (Figure 4a). To confirm whether the FimH driven NK cell activation is TLR4-dependent, human NK-92 cells (deficient in TLR4), as well as purified DX5+ NK cells from TLR4−/− mice were incubated for 48 hours with FimH, LPS, or CpG and the supernatants were then assayed for IFN-γ and TNF-α production. Figure 4b demonstrates that FimH failed to activate TLR4-deficient human NK cells. In addition, preincubation of purified human NK cells with anti-TLR4 antibody significantly abrogated the FimH-induced IFN-γ and TNF-α production (Figure 4c), which strongly suggests a requirement of TLR4 for NK-cell activation. As well, FimH stimulation of the TLR4−/− mouse NK cells ex vivo induced significantly lower amounts of IFN-γ and TNF-α as compared to C57BL/6 NK cells (Figure 4d). Furthermore, co-culture of purified NK cells from TLR4−/− mice with FimH+ UPEC produced significantly less TNF-α or no IFN-γ compared to NK cells from B6 mice (Figure 4e) confirming the requirement of TLR4 signaling.
Figure 4.
FimH activation of NK cells requires TLR4 signaling. (a) Total RNA was isolated from human peripheral blood purified NK cells, NK-92 cells and 293t expressing TLR4/MD-2 cells and assessed mRNA expressions for TLR4 and MD-2 by reverse transcription-PCR. (b) Human NK-92 cells were untreated or stimulated with FimH, LPS, and poly I:C for up to 72 hours and cell-free supernatants were measured for IFN-γ and TNF-α productions by specific enzyme-linked immunosorbent assay (ELISA). Data represent mean ± SEM of three separate experiments done in triplicate wells. (c) Human peripheral blood NK cells were untreated, preincubated with anti-TLR4 mAb for 1 hour and/or treated with FimH (10 µg/ml), cell-free supernatants were harvested after 24 and 48 hours and analyzed for TNF-α and IFN-γ concentrations, respectively (n = 3, mean ± SEM). (d) Purified splenic NK cells from B6 or TLR4−/− mice were either untreated or treated with FimH (10 µg/ml), LPS (100 ng/ml), or CpG (10 µg/ml) and cell-free supernatants assessed for IFN-γ and TNF-α productions by ELISA (n = 5, mean ± SEM). (e) Purified splenic NK cells from B6 or TLR4−/− mice were co-cultured with FimH+ UPEC bacteria similarly as mentioned earlier for 24 hours and cell free supernatants were assessed for TNF-α or IFN-γ production by ELISA (data are mean ± SD). Experiments were repeated two times. *P < 0.05, **P < 0.01, ***P < 0.001. IFN, interferon; LPS, lipopolysaccharide; NK, natural killer; TLR4, Toll-like receptor 4; TNF, tumor necrosis factor; UPEC, uropathogenic E. coli.
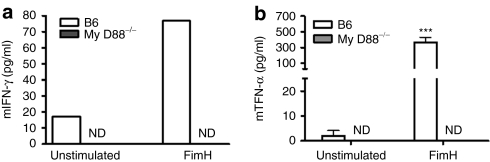
Upon ligand binding, TLR4 can signal either through the common adaptor molecule MyD88 or via TRIF21 to induce transcriptional activation of proinflammatory cytokines and type 1 IFNs. In this study, we sought to explore whether FimH activation of NK cell functions through TLR4 signaling requires the adaptor protein MyD88. To achieve this, we compared IFN-γ and TNF-α production from MyD88−/− splenocytes with those from C57BL/6 following FimH stimulation. As demonstrated in Figure 5a,b, splenocytes from MyD88−/− mice were unable to induce detectable IFN-γ and TNF-α, in response to FimH stimulations compared to substantial productions by C57BL/6 splenocytes. Not surprisingly, NK cells from MyD88−/− mice were unresponsive to FimH (data not shown).
Figure 5.
NK cells activation by FimH depends on MyD88 signaling. Total splenocytes from C57BL/6 (n = 3) or MyD88−/− (n = 5) mice were isolated and cells were either left untreated or stimulated with FimH (10 µg/ml) for 24 hours for TNF-α and 48 hours for IFN-γ production. Cell-free supernatants were then harvested and analyzed by enzyme-linked immunosorbent assay for (a) IFN-γ and (b) TNF-α measurements (data are mean ± SEM of three repeated experiments). ***P < 0.001. ND, not detected; NK, natural killer; IFN, interferon; TNF, tumor necrosis factor.
FimH-induced NK cell activation is not due to LPS or other bacterial contaminants
As we have produced our recombinant FimH in E. coli, the His-tag purified protein had low levels of LPS contaminations (7–10 pg of LPS per 1 µg of FimH). We have performed several experiments to confirm that the effect of FimH on NK cells was not due to LPS or other possible bacterial contaminants. We also further purified FimH protein by fast protein liquid chromatography (FPLC) and then compared the abilities of nickel–column- and FPLC-purified FimH preparations in the context of IFN-γ and TNF-α production by human and murine NK cells. Although FPLC-purified FimH had undetectable levels of LPS (data not shown) compared to our previously nickel-column-purified FimH, both FimH preparations induced similar levels of cytokines by NK cells (Figure 6a,d). We then subjected FimH to digestion by proteinase-K or trypsin. The digested FimH failed to activate both murine (Figure 6e,f) and human (Figure 6g,h) NK cells as displayed by diminished or no production of IFN-γ or TNF-α. Notably, although the initial nickel-column-purified FimH had only traces of LPS, we were unable to detect any IFN-γ or TNF-α even when human and murine NK cells were treated with 100 pg/ml of LPS (data not shown). Finally, stimulation of purified human NK cells by control His-tag recombinant protein did not induce any IFN-γ secretion, whereas recombinant FimH preparation did (Figure 6i), which further excludes any possibility of LPS contamination.
Figure 6.
FimH-stimulated NK cell activation is free of LPS contamination. (a,b) Purified splenic NK cells from C57BL/6 mice (n = 3), and (c,d) peripheral human NK cells (n = 3) were untreated or treated with column- and/or FPLC-purified recombinant FimH (10 µg/ml) for 24 and 48 hours, and cell-free supernatants were assessed by specific enzyme-linked immunosorbent assay (ELISA) for murine or human IFN-γ and TNF-α productions, respectively (data are mean ± SEM of three independent experiments). (e,f) Purified NK cells from C57BL/6 mice (n = 3) or from (g,h) human peripheral blood (n = 3) were cultured alone or in presence of FimH (10 µg/ml), proteinase-K/trypsin degraded FimH (10 µg/ml), and 100 ng/ml of LPS, cell-free supernatants harvested after 24 and 48 hours were then assessed by ELISA for TNF-α and IFN-γ, respectively (data are mean ± SEM of three separate experiments). (i) purified human peripheral NK cells were stimulated with recombinant vector His-tagged or recombinant (His-tagged) FimH protein for 48 hours and cell-free supernatants obtained at 24 and 48 hours were assayed for IFN-γ production by ELISA. Representative data from one donor is shown. *P < 0.05, **P < 0.01. FPLC, fast protein liquid chromatography; IFN, interferon; LPS, lipopolysaccharide; NK, natural killer; TNF, tumor necrosis factor.
FimH induces innate protection against melanoma tumors and UPEC urinary tract infection in vivo
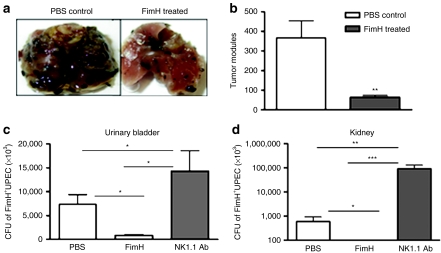
In order to evaluate whether FimH-induced activation of NK cell functions could have therapeutic implications, we performed two sets of in vivo mouse model experiments. It is well established that protection against B16F10 melanoma cells is mediated via activated NK cells. Here, we injected phosphate-buffered saline (PBS) or FimH into the foot pad of B6 mice and 2 days later mice were challenged with B16F10 melanoma cells intravenously. Two weeks later, lungs were removed and examined for tumor nodules. We found significantly higher number of tumor nodules in the lungs harvested from PBS-injected mice whereas FimH-injected mice displayed significantly less tumor nodules (Figure 7a,b). We then examined whether FimH has potential to protect natural urinary tract infection. We therefore chose intraurethral natural route of infection with UPEC in mouse model. To this end, B6 mice were injected intravenously with FimH or PBS or anti-NK1.1 antibody followed by intraurethral infection with FimH+ UPEC bacteria. Urinary bladder and kidney cultures from NK-depleted (NK1.1 Ab) mice displayed robust colonization of bacteria and modest in PBS-treated mice (Figure 7c,d). However, to our surprise, FimH-treated mice had diminished bacterial loads both in urinary bladder and kidney (Figure 7c,d).
Figure 7.
FimH-induced protection against tumor and UPEC urinary tract infection are mediated by NK cells. (a) C57BL/6 mice were injected through foot pad in the hindlimb with PBS or FimH (50 µg). Forty-eight hours later, mice were challenged with 5 × 105 B16F10 melanoma cells intravenously. (b) Lungs were harvested after 2 weeks and tumor nodules in the lungs were photographed and enumerated (n = 5–6/group, data are mean ± SEM). (c,d) B6 mice were injected with PBS or 50 µg of FimH on day 1 preinfection or with anti-NK1.1 antibody (200 µg) on days 2 and 1 preinfection. Twenty-four hours later, 108 CFU of FimH+UPEC (strain Nu14) bacteria were delivered into the urinary bladder through a soft catheter. Twenty-four hours later (c) urinary bladder and (d) kidney were removed, homogenized, plated onto LB agar and bacterial CFU enumerated. (n = 5–6, data are mean ± SEM). *P < 0.05, **P < 0.01, ***P < 0.001. LB, Luria–Bertani; CFU, colony-forming units; NK, natural killer; PBS, phosphate-buffered saline; TNF, tumor necrosis factor; UPEC, uropathogenic E. coli.
Discussion
There is increasing evidence that some bacterial components such as LPS, flagellin, peptidoglycan, or unmethylated bacterial DNA (CpG) are potent inducers of innate defense against microbial infections or cancers.15,16,17,18,19 In searching for such microbial PAMPs, we have recently discovered that FimH, a UPEC type 1 fimbrial adhesin, is a novel TLR4 ligand.29 Here, we demonstrate that FimH directly activates human and murine NK cells to release innate cytokines (IFN-γ and TNF-α), induces the expression of NK cell activation markers including CD69 and perforin, and triggers NK cell cytotoxicity. The FimH-induced NK cell activities are clearly dependent on TLR4-mediated MyD88 signaling pathways. Of particular interest, we have shown that purified human NK cells are able to differentially recognize FimH+ pathogenic E. coli from FimH-deficient E. coli.
In this study, we provide experimental evidence that both human and murine NK cells recognize FimH and are activated to release innate cytokines (IFN-γ and TNF-α) to a comparable and/or higher level than that produced by LPS, CpG, or polyionosinic–polycytidylic acid stimulations. NK cells, but not APCs or neutrophils, were the source of IFN-γ because IFN-γ was not detectable when splenocytes from RAG-2−/−/γc−/− mice (deficient in NK and NKT cells) were stimulated with FimH. In contrast, RAG2−/− (deficient in B and T cells, but have NK cells) splenocytes produced copious amounts of IFN-γ. NK cells can be activated by many cytokines, mainly IL-12, produced from surrounding activated APCs. In this study, the absence of IL-12 in supernatants from FimH-stimulated magnetic bead purified CD56+ NK cells as well as in flow cytometry sorted highly purified NK cells eliminated any possible APC contaminations of NK cells. Finally, utilizing the flow cytometry sorting of NK cells after NK enrichment purification (yielded 98.8% pure CD56+CD3− NK cell populations), we confirmed that FimH directly activates human NK cells to release significant amounts of IFN-γ. Recently, Leishmania lipophosphoglycan11 and LeIF,31 and K. pneumoniae KpOmpA3 were shown to activate NK cells directly. We then show novel experimental evidence that human NK cells can directly recognize FimH containing UPEC and become subsequently activated to eliminate the infections. This was evident by the fact that exposure of CD56+ NK cells to FimH-expressing, but not FimH-deleted, UPEC bacteria induced elevated expression of IFN-γ and TNF-α. Similarly, Mycobacterium bovis BCG infections stimulate NK cell activation directly and indirectly through triggering APC-derived cytokine production.32,33,34,35 Mechanistically, NK cell-derived IFN-γ regulates tumor immunity36,37 by affecting proliferation and metabolic activity as well by death-receptor-mediated apoptosis of tumor cells.38 Thus, FimH could have the potential to be a target protein ligand to study NK cell IFN-γ-mediated tumor immunity. Notably, NK cell alone had no effects on UPEC growth irrespective of the presence or absence of FimH, the underlying reasons yet remain obscure. It is more likely that NK cells cannot clear UPEC directly; however, NK-cell IFN-γ may trigger APCs or macrophages to act on UPEC. How NK cells along with accessory cells interact with UPEC remains an interesting topic to explore. In addition to cytokine release, NK cells mainly achieve killing of infected or cancer cells through perforin and granzyme B–mediated granular exocytosis39,40 besides a number of activating or inhibitory receptors to maintain innate host defense.41 Resting human peripheral blood NK or murine splenic NK cells harbor poor cytotoxic potential because of reduced expression of perforin and granzyme B, which are induced upon stimulations or infections.42,43 But most NK cell priming mechanisms remain to be unveiled. Here, we show FimH as a potent priming agonist for NK cell cytolytic activity that FimH-treated NK cells displayed greater response to kill K562 cells than LPS or CpG. More importantly, human NK cells can sense and respond to FimH+ UPEC in the absence of APCs and elicited pronounced expression of perforin, which we speculate could have been involved in mediating the upregulation of NK cell killing capacity.
We then examined whether FimH requires TLR4 signaling for the direct activation of NK cell functions. To address this, we analyzed TLR4-deficient human NK-92 and TLR4−/− mouse NK cells for TNF-α and IFN-γ production after FimH treatments. Importantly, FimH stimulations of TLR4-deficient NK cells had significantly diminished productions of cytokines compared to TLR4-expressing control NK cells indicating the requirement of TLR4 for FimH stimulations of NK cells. In addition, blocking of FimH-induced human NK-cell TNF-α and IFN-γ by neutralizing anti-TLR4 antibody strongly supports this assertion. More interestingly, purified NK cells from TLR4−/− mice when co-cultured with FimH+ UPEC or directly stimulated by FimH, produced significantly less TNF-α and IFN-γ compared to robust secretions by C57B/6 NK cells. These data strongly suggest that direct activation of NK cells by FimH requires the presence of TLR4 signal. In line with this, we have previously demonstrated that TLR4−/− mice had significantly higher bacterial colonization in the urinary bladder compared to B6 mice although challenged by FimH+ UPEC in vivo.30 Also FimH-induced antiviral response against herpes simplex virus-2 was found to be TLR4 signal dependent.30 Although we have focused on NK cells in these studies, a recent study suggested that UPEC could activate distinct TLR4-dependent and TLR4-independent inflammatory pathways in renal duct epithelial cells.44 TLR4 ligation signals through the common adaptor molecule MyD88 (ref. 21). Utilizing MyD88 null mice, we provide further experimental evidence that the adaptor protein MyD88 is required for TLR4-mediated FimH activation of NK cells as total lymphocyte populations (including NK/NKT cells) from MyD88−/− mice failed to induce detectable TNF-α or IFN-γ upon FimH stimulations. In contrast to our results, UPEC infection has recently been shown to block MyD88-dependent and activate MyD88-independent pathways,45 this controversy is likely due to experimental differences between the two studies. However, using in vivo mouse model, we previously reported that FimH-induced type 1 IFN and antiviral responses require MyD88 signaling.30
Recombinant proteins expressed in Gram-negative bacteria are often subject to LPS or endotoxin contamination, which may obscure the activities of the target recombinant proteins.46,47 To exclude the possibilities of any unwanted bacterial contamination, including LPS, in FimH preparations, we employed Limulus Amebocyte Lysate assay that showed only traces (7–r10 pg LPS in 1 µg of FimH) of LPS, significantly less than the minimal dose required to induce any NK cell activation. Nevertheless, we conducted a series of experiments to prove that the FimH activities are independent of LPS. To this end, FimH preparations were digested by proteinase-K and trypsin, which rendered loss of FimH, but not LPS, activity on human and murine NK cells. In addition, we treated NK cells with 100 pg/ml of LPS (the amount higher than that detected in FimH preparations) and measured cytokines. Interestingly, these low LPS-treated NK cells failed to induce any detectable TNF-α or IFN-γ (data not shown). We then further purified our FimH preparations running through FPLC that induces even greater response to NK cell functions. Finally, we have been able to exclude any possibility of LPS contamination by demonstrating a diminished or no IFN-γ secretion by purified human NK cells upon stimulations with control His-tag recombinant protein, whereas recombinant FimH preparation induced significant amount of IFN-γ. Moreover, we have reported that His-atgged PapG proteins failed to protect mice against herpes simplex virus-2 viral challenge but His-tagged FimH elicited complete protection.30 We therefore conclude that FimH-induced NK cell activations are independent of LPS or any other bacterial contaminants.
The most salient findings from this study are the robust protection of urinary tract infections from FimH+ UPEC and lung tumor nodule formation by B16F10 melanoma cells following FimH treatments in in vivo mouse models. Interestingly, these protections were mediated by NK cells as NK cell depleted mice had significantly higher bacterial loads in urinary bladder and kidneys compared to wild-type mice. Off the note, mouse melanoma tumor model utilizing B16F10 cells is exclusively an NK cell dependent model. It was our goal to further investigate the role of FimH in activation of NK cells in the context of innate immune response to tumors. In this study, we have not only linked human and murine NK cell activation with the TLR4 ligand, but have described possible functional outcomes in vivo against murine melanoma tumor challenge.
In conclusion, our data demonstrate that human and murine NK cells can directly recognize and respond to FimH stimulations or FimH+ pathogens leading to subsequent TNF-α and IFN-γ release. Cytotoxic potentials of NK cells also significantly accelerated, possibly through upregulation of perforin levels. FimH-induced NK-cell effector functions require TLR4-mediated MyD88 signaling and are not synergized by LPS or other bacterial contaminants. These findings may have important implications to utilize FimH as a promising candidate for NK cell based therapeutic application against urinary tract infections and cancer.
Materials and Methods
Isolation of human and murine NK cells and culture conditions. PBMCs from 10 healthy individuals (age and sex matched) were purified by Ficoll-Paque Plus (Amersham-Pharmacia Biotech, Baie d'Urfé, Quebec, Canada) density-gradient centrifugation. NK cells were purified using EasySep Human CD56 Positive Selection Kit (StemCell Technologies, Vancouver, British Columbia, Canada) according to the manufacturer's protocol. Spleens from healthy mice were harvested, red blood cells lysed and single cell suspensions were then processed as for NK cell purification using EasySep Mouse panNK Positive Selection Kit (StemCell Technologies). CD56+ CD3− lymphocytes (human NK cells) and CD49b+DX5+ splenic leukocytes (murine NK cells) were then analyzed by flow cytometry and used for in vitro FimH activation assays. The purity of human CD56+ CD3− NK cells and murine NK1.1+CD3−NK cells were 90–92% and >95%, respectively. In addition, human NK cells purified from PBMCs by negative selection NK enrichment EasySep kit (Stem cell technologies) were further sorted by flow cytometry (BD Biosciences, San Diego, CA) and purity of sorted NK cells was 98.8% as analyzed using antihuman CD56-PE and antihuman CD3-APC (BD Biosciences). Human and murine mononuclear cells or NK cells were grown in RPMI medium 1640 (supplemented with 10% fetal bovine serum, 2 mmol/l -glutamine, 1% penicillin–streptomycin–neomycin, 2-ME), at a concentration of 2.0 × 106 cells/ml recombinant human IL-2 (50 U/ml; Genzyme Diagnostic, Cambridge, MA) was supplemented to the NK cell culture medium. TLR ligands were added to the culture at the following concentrations: polyionosinic–polycytidylic acid (Sigma-Aldrich, Oakville, Ontario, Canada), 10 µg/ml; synthetic CpG phosphorothioate oligodeoxynucleotides (ODN) (2006) 5′-TCGTCGTTTTGTCGTTTTGTCGTT-3′ murine CpG 1826 (MOBIX Lab, McMaster University), 10 µg/ml; LPS (Sigma-Aldrich), 100 ng/ml and FimH, 10 µg/ml. The plates were then incubated at 37 °C for 24, 48, and 72 hours and cell-free supernatants were obtained and analyzed for cytokine measurements.
Cytokine measurements. Frozen cell-free supernatants collected from stimulated and nonstimulated splenocytes, PBMCs, or NK cells were thawed on ice, then analyzed using DuoSet ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions to assess the production of IFN-γ, TNF-α, and IL-12p70. TNF-α and IL-12p70 were tested after 24 hours, whereas IFN-γ was tested after all three time points. The enzyme-linked immunosorbent assay plates were read using the Sapphire ELISA plate reader at 450 nm wavelength.
Chromium (51Cr) release assay for NK cytotoxicity. A 4-hour standard chromium release assay was employed to analyze the FimH-activated human and murine NK cell cytotoxic killing ability against human K562 or murine YAC-1 target cancer cells following the method as described earlier.10,48
Mice. Inbred 6- to 8-week-old female C57BL/6 mice were purchased from Charles River Laboratories (Quebec, Canada), Rag2−/− and Rag2−/−γc−/− mice (Balbc background) were purchased from Taconic Farms (Germantown, NY), and TLR4−/− mice (B6 background) were purchased from Jackson Laboratories (Bar Harbor, ME). The MyD88−/− mice (B6 background) were kindly provided by Dr Akira (via Dr Golenbock). All animals were housed in the McMaster University Central Animal Facility under the guidelines of the Canadian Council on Animal Care.
FimH purification and degradation. FimH was prepared according to the method as previously described.29 Briefly, the fimH gene from E. coli strain EC99 (O78) was cloned into pQE-30 and expressed in BL-21 competent E. coli. FimH expression and purification were performed as previously described.49 The initial nickel-column-purified recombinant FimH was further purified using gel filtration FPLC. The level of contaminating LPS in the preparations was determined to be no more than 40 pg/µg of FimH protein using the Limulus Amebocyte Lysate assay (Associates of Cape Cod, East Falmouth, MA). In order to analyze LPS contents, if any, in our FimH preparations, protein samples were degraded by 100 µg/ml of proteinase-K (Sigma-Aldrich) or trypsin (Roche, Mississauga, Ontario, Canada) at 37 °C for 2 hours and the reaction was stopped by heat inactivation at 95 °C for 15 minutes.
Flow cytometry. Mouse antihuman and anti-mouse-conjugated monoclonal antibodies (mAb) from BD Pharmingen (San Diego, CA) used were: CD56 (NCAM 16.2)-phycoerythrin (PE), antihuman CD3-fluorescein isothiocyanate (FITC), antihuman-CD69-PE-Cy7, antihuman-perforin-FITC; and for murine NK cells purity analysis, anti-mouse-NK1.1-PE, anti-mouse-CD3-PerCPCy5 and respective isotype matching IgG antibodies for human antibodies, and mouse Fc blocker (anti-mouse CD 16/32) for murine antibodies. Direct surface staining using 20 µl of all antibodies, except antihuman-perforin, to samples at 4 °C for 30 minutes was followed by three washes in PBS supplemented with 0.2% bovine serum albumin. For intracellular perforin staining, cells were permeabilized on ice for 20 minutes with Cytoperm buffer (BD Biosciences) before staining with FITC-conjugated antihuman-perforin mAb (6 µl/106 cells in 100 µl for 30 minutes. Flow cytometry was performed using BD Biosciences FACS LSRII using BD Diva and data was analyzed using Tree Star FlowJo version 8.2 software (Tree Star, Ashland OR).
Immunofluorescence staining for confocal microscopy. Highly purified (NK enrichment negative selection kit) human NK cells were stimulated with FimH (10 µg/ml) for the indicated time periods. Cells were then transferred onto clean glass slides by Shandon Cytospin 4 (Thermo Electron, Waltham, MA), air-dried and fixed with cold acetone. After blocking with 5% normal goat serum containing buffer with 0.1% Triton X100, cells were incubated overnight at 4 °C with NF-κB antibody (c-20; Santa Cruz Biotechnology, Santa Cruz, CA) followed incubation with Alexafluor-488-labeled secondary antibody and counterstained for nuclei with propidium iodide and covered with cover slip in Vectasheild hard-set mounting medium (Vector, Burlington, Ontario, Canada) and images were captured by LSM 510 (inverted) confocal microscope (Zeiss, Oberkochen, Germany) using ×63 objective. Images were analyzed using LSM 510 software (Zeiss, version 3.2) and adobe photoshop.
TLR4 neutralization. In order to confirm that FimH-induced responses are TLR4-dependent, neutralizing human anti-TLR4, CD284 (Clone HTA125), and mouse anti-TLR4, CD284/MD-2 (clone MTS510) monoclonal antibodies were purchased from Cedarlane Laboratories (Hornby, Ontario, Canada). Purified human or murine NK cells were preincubated with the mAbs for 1 hour before treatment with TLR ligands, and cell-free supernatants were assayed for TNF-α and IFN-γ by ELISA.
Cell lines. The human NK cell line NK-92 (CRL-2407; ATCC, Manassas, VA) was maintained in α-MEM supplemented with 2 mmol/l -glutamine, 1 mmol/l nonessential amino acids, 100–200 U/ml recombinant human IL-2 (Genzyme, Minneapolis, MN), 0.05 mmol/l 2-mercaptoethanol, and 20% fetal bovine serum (Invitrogen, Burlington, Ontario, Canada). The HEK293T cells transfected with hTLR4 or MD-2 (CRL-11268; ATCC, Manassas, VA) were maintained in α-MEM supplemented with 10% fetal bovine serum 1% -glutamine, 1% penicillin–streptomycin, and 1% HEPES (Invitrogen). Cell lines were maintained under normal growth conditions at 37 °C in 5% CO2.
Bacterial strains, co-culture with NK cells, and intraurethral mice injection. UPEC strain Nu14, well-characterized type 1 pilus-positive pathogenic strains and FimH-deleted mutant E. coli strain Nu14-1 were kindly gifted from Dr Coombs (McMaster University, Canada). The bacterial strains were grown overnight in Luria–Bertani (LB) medium with 50 µg/ml of streptomycin. For NK cells co-culture experiments, overnight cultures of E. coli strains were diluted 1:50 in fresh LB medium and grown to a early exponential phage (OD600 = 0.5–1.0) at 37 °C in a shaking incubator. The concentration of viable bacteria was calculated using standard growth curves. Bacteria were then centrifuged at 4500g for 10 minutes at room temperature, washed with PBS, and resuspended in 10 ml of RPMI 1640. A volume of 100 µl of this suspension (2 × 107 colony-forming units) was used to infect purified human or murine NK cells or PBMCs with a multiplicity of infections = 20. For transurethral mouse model infection, bacteria were grown overnight in LB broth, washed in 0.85% saline, and resuspended in saline to a concentration of ~109 colony-forming units/ml. C57BL/6 mice were injected, intravenously, with FimH (50 µg/mouse) on day-1 postinfection. To deplete NK cells, anti-NK1.1 antibody (200 µg/mouse) was delivered via i.p. on days-2 and -1 postinfection. Mice were then infected with 108 colony-forming units (in 100 µl) of FimH+ UPEC bacterial suspension. A soft catheter (0.7 mm) was placed in the urethra of anesthetized mice and the bacteria were delivered into the bladder. At 24 hours after infection, the bladders and kidneys were removed, homogenized, cultured onto LB agar plates, and the bacterial loads were enumerated. To further evaluate the growth of FimH+ or FimH− UPEC in presence of NK cells, purified human NK cells were co-cultured with both of the bacterial strains at NK:UPEC ratio of 1:20 for 2 hours at 37 °C, cell were then lysed and lysates were cultured onto LB agar plates overnight and bacterial colonies were enumerated.
Melanoma tumor challenge experiment. C57BL/6 mice were injected PBS or FimH (50 µg/mouse) through foot pad in the hind limb. After 48 hours, B16F10 melanoma cells (5 × 105) were injected intravenously into the mice and monitored regularly by palpation for lung tumor nodule development. Two weeks later, mice were killed and lung tumor nodules were photographed and counted.
RNA extraction and reverse transcription-PCR. Total RNA extracted from human peripheral blood NK cells from healthy donors, and human cell lines (NK-92, 293T, 293/CD14/MD-2/TLR4) were obtained using TRIzol reagents (Invitrogen) following the manufacturers protocol. Reverse transcription-PCR was performed as described29 using the following primer pairs: hTLR4, forward, 5′-CTGGACCTCTCTCAGTGTC-3′, reverse, 5′-GGCAGAGCTGAAATGGAGG-3′ hMD-2, forward, 5′-GAAGCTCAGAAGCAGTATTGGGTC-3′, reverse, GGTTGGTGTAGGATGACAAACTCC-3′ and h18SrRNA, forward, 5′-GCATTCGTATTGCGCCGCTA-3′, reverse, 5′-AGCTGCCCGGCGGGTC-3′.
Statistical analysis. Statistical analysis was performed using GraphPad Prism 4 (GraphPad Prism Software, La Jolla, CA) and Student's t-test wherever appropriate. A P value <0.05 was considered significant.
Acknowledgments
This work was supported by grants from Canadian Institute of Health Research (CIHR) and Canadian Breast Cancer Foundation (CBCF, Ontario chapter) to A.A.A. We thank Dr Akira through Dr Golenbock for breeding pair of MyD88−/− mice. We also thank Dr Hultgren (Washington University, St Louis, MO) for providing FimH-expressing and FimH-deficient UPEC. A.A.A. is a recipient of career award in Health Sciences from Rx&D/CIHR.
REFERENCES
- Biron CA, Nguyen KB, Pien GC, Cousens LP., and, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- Wu J., and, Lanier LL. Natural killer cells and cancer. Adv Cancer Res. 2003;90:127–156. doi: 10.1016/s0065-230x(03)90004-2. [DOI] [PubMed] [Google Scholar]
- Chalifour A, Jeannin P, Gauchat JF, Blaecke A, Malissard M, N'Guyen T, et al. Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers α-defensin production. Blood. 2004;104:1778–1783. doi: 10.1182/blood-2003-08-2820. [DOI] [PubMed] [Google Scholar]
- Raulet DH. Interplay of natural killer cells and their receptors with the adaptive immune response. Nat Immunol. 2004;5:996–1002. doi: 10.1038/ni1114. [DOI] [PubMed] [Google Scholar]
- Biron CA., and, Brossay L. NK cells and NKT cells in innate defense against viral infections. Curr Opin Immunol. 2001;13:458–464. doi: 10.1016/s0952-7915(00)00241-7. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Wallace ME, Nutt SL, Yagita H, Godfrey DI., and, Hayakawa Y. Sequential activation of NKT cells and NK cells provides effective innate immunotherapy of cancer. J Exp Med. 2005;201:1973–1985. doi: 10.1084/jem.20042280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth MJ, Cretney E, Kershaw MH., and, Hayakawa Y. Cytokines in cancer immunity and immunotherapy. Immunol Rev. 2004;202:275–293. doi: 10.1111/j.0105-2896.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- Farag SS., and, Caligiuri MA. Cytokine modulation of the innate immune system in the treatment of leukemia and lymphoma. Adv Pharmacol. 2004;51:295–318. doi: 10.1016/S1054-3589(04)51013-X. [DOI] [PubMed] [Google Scholar]
- Brandau S, Riemensberger J, Jacobsen M, Kemp D, Zhao W, Zhao X, et al. NK cells are essential for effective BCG immunotherapy. Int J Cancer. 2001;92:697–702. doi: 10.1002/1097-0215(20010601)92:5<697::aid-ijc1245>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Lauzon NM, Mian F, MacKenzie R., and, Ashkar AA. The direct effects of Toll-like receptor ligands on human NK cell cytokine production and cytotoxicity. Cell Immunol. 2006;241:102–112. doi: 10.1016/j.cellimm.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Becker I, Salaiza N, Aguirre M, Delgado J, Carrillo-Carrasco N, Kobeh LG, et al. Leishmania lipophosphoglycan (LPG) activates NK cells through toll-like receptor-2. Mol Biochem Parasitol. 2003;130:65–74. doi: 10.1016/s0166-6851(03)00160-9. [DOI] [PubMed] [Google Scholar]
- Iho S, Yamamoto T, Takahashi T., and, Yamamoto S. Oligodeoxynucleotides containing palindrome sequences with internal 5′-CpG-3′ act directly on human NK and activated T cells to induce IFN-γ production in vitro. J Immunol. 1999;163:3642–3652. [PubMed] [Google Scholar]
- Schmidt KN, Leung B, Kwong M, Zarember KA, Satyal S, Navas TA, et al. APC-independent activation of NK cells by the Toll-like receptor 3 agonist double-stranded RNA. J Immunol. 2004;172:138–143. doi: 10.4049/jimmunol.172.1.138. [DOI] [PubMed] [Google Scholar]
- Sivori S, Falco M, Della Chiesa M, Carlomagno S, Vitale M, Moretta L, et al. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc Natl Acad Sci USA. 2004;101:10116–10121. doi: 10.1073/pnas.0403744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulou L, Holt AC, Medzhitov R., and, Flavell RA. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Medzhitov R., and, Janeway C., Jr Innate immunity. N Engl J Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- Janeway CA., Jr, and, Medzhitov R. Introduction: the role of innate immunity in the adaptive immune response. Semin Immunol. 1998;10:349–350. doi: 10.1006/smim.1998.0142. [DOI] [PubMed] [Google Scholar]
- Medzhitov R., and, Janeway CA., Jr Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L, Thomas V, Schnare M, Lobet Y, Anguita J, Schoen RT, et al. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat Med. 2002;8:878–884. doi: 10.1038/nm732. [DOI] [PubMed] [Google Scholar]
- Bowie AG., and, Haga IR. The role of Toll-like receptors in the host response to viruses. Mol Immunol. 2005;42:859–867. doi: 10.1016/j.molimm.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- Link BK, Ballas ZK, Weisdorf D, Wooldridge JE, Bossler AD, Shannon M, et al. Oligodeoxynucleotide CpG 7909 delivered as intravenous infusion demonstrates immunologic modulation in patients with previously treated non-Hodgkin lymphoma. J Immunother. 2006;29:558–568. doi: 10.1097/01.cji.0000211304.60126.8f. [DOI] [PubMed] [Google Scholar]
- Fujii H, Trudeau JD, Teachey DT, Fish JD, Grupp SA, Schultz KR, et al. In vivo control of acute lymphoblastic leukemia by immunostimulatory CpG oligonucleotides. Blood. 2007;109:2008–2013. doi: 10.1182/blood-2006-02-002055. [DOI] [PubMed] [Google Scholar]
- Tsujimoto H, Uchida T, Efron PA, Scumpia PO, Verma A, Matsumoto T, et al. Flagellin enhances NK cell proliferation and activation directly and through dendritic cell-NK cell interactions. J Leukoc Biol. 2005;78:888–897. doi: 10.1189/jlb.0105051. [DOI] [PubMed] [Google Scholar]
- Cuadros C, Lopez-Hernandez FJ, Dominguez AL, McClelland M., and, Lustgarten J. Flagellin fusion proteins as adjuvants or vaccines induce specific immune responses. Infect Immun. 2004;72:2810–2816. doi: 10.1128/IAI.72.5.2810-2816.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wille-Reece U, Flynn BJ, Loré K, Koup RA, Kedl RM, Mattapallil JJ, et al. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc Natl Acad Sci USA. 2005;102:15190–15194. doi: 10.1073/pnas.0507484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R., and, Janeway CA., Jr Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- Beutler B, Hoebe K, Du X., and, Ulevitch RJ. How we detect microbes and respond to them: the Toll-like receptors and their transducers. J Leukoc Biol. 2003;74:479–485. doi: 10.1189/jlb.0203082. [DOI] [PubMed] [Google Scholar]
- Mossman KL, Mian MF, Lauzon NM, Gyles CL, Lichty B, Mackenzie R, et al. Cutting edge: FimH adhesin of type 1 fimbriae is a novel TLR4 ligand. J Immunol. 2008;181:6702–6706. doi: 10.4049/jimmunol.181.10.6702. [DOI] [PubMed] [Google Scholar]
- Ashkar AA, Mossman KL, Coombes BK, Gyles CL., and, Mackenzie R. FimH adhesin of type 1 fimbriae is a potent inducer of innate antimicrobial responses which requires TLR4 and type 1 interferon signalling. PLoS Pathog. 2008;4:e1000233. doi: 10.1371/journal.ppat.1000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges MM, Campos-Neto A, Sleath P, Grabstein KH, Morrissey PJ, Skeiky YA, et al. Potent stimulation of the innate immune system by a Leishmania brasiliensis recombinant protein. Infect Immun. 2001;69:5270–5277. doi: 10.1128/IAI.69.9.5270-5277.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcenaro E, Ferranti B, Falco M, Moretta L., and, Moretta A. Human NK cells directly recognize Mycobacterium bovis via TLR2 and acquire the ability to kill monocyte-derived DC. Int Immunol. 2008;20:1155–1167. doi: 10.1093/intimm/dxn073. [DOI] [PubMed] [Google Scholar]
- Ferlazzo G, Morandi B, D'Agostino A, Meazza R, Melioli G, Moretta A, et al. The interaction between NK cells and dendritic cells in bacterial infections results in rapid induction of NK cell activation and in the lysis of uninfected dendritic cells. Eur J Immunol. 2003;33:306–313. doi: 10.1002/immu.200310004. [DOI] [PubMed] [Google Scholar]
- Suttmann H, Jacobsen M, Reiss K, Jocham D, Böhle A., and, Brandau S. Mechanisms of bacillus Calmette-Guerin mediated natural killer cell activation. J Urol. 2004;172:1490–1495. doi: 10.1097/01.ju.0000131944.52354.63. [DOI] [PubMed] [Google Scholar]
- Newman KC., and, Riley EM. Whatever turns you on: accessory-cell-dependent activation of NK cells by pathogens. Nat Rev Immunol. 2007;7:279–291. doi: 10.1038/nri2057. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Old LJ., and, Schreiber RD. The roles of IFNγ in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13:95–109. doi: 10.1016/s1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- Street SE, Cretney E., and, Smyth MJ. Perforin and interferon-γ activities independently control tumor initiation, growth, and metastasis. Blood. 2001;97:192–197. doi: 10.1182/blood.v97.1.192. [DOI] [PubMed] [Google Scholar]
- Wallace ME., and, Smyth MJ. The role of natural killer cells in tumor control–effectors and regulators of adaptive immunity. Springer Semin Immunopathol. 2005;27:49–64. doi: 10.1007/s00281-004-0195-x. [DOI] [PubMed] [Google Scholar]
- Trapani JA., and, Smyth MJ. Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol. 2002;2:735–747. doi: 10.1038/nri911. [DOI] [PubMed] [Google Scholar]
- van Dommelen SL, Sumaria N, Schreiber RD, Scalzo AA, Smyth MJ., and, Degli-Esposti MA. Perforin and granzymes have distinct roles in defensive immunity and immunopathology. Immunity. 2006;25:835–848. doi: 10.1016/j.immuni.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Lodoen MB., and, Lanier LL. Natural killer cells as an initial defense against pathogens. Curr Opin Immunol. 2006;18:391–398. doi: 10.1016/j.coi.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryceson YT, March ME, Ljunggren HG., and, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107:159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehniger TA, Cai SF, Cao X, Bredemeyer AJ, Presti RM, French AR, et al. Acquisition of murine NK cell cytotoxicity requires the translation of a pre-existing pool of granzyme B and perforin mRNAs. Immunity. 2007;26:798–811. doi: 10.1016/j.immuni.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Chassin C, Goujon JM, Darche S, du Merle L, Bens M, Cluzeaud F, et al. Renal collecting duct epithelial cells react to pyelonephritis-associated Escherichia coli by activating distinct TLR4-dependent and -independent inflammatory pathways. J Immunol. 2006;177:4773–4784. doi: 10.4049/jimmunol.177.7.4773. [DOI] [PubMed] [Google Scholar]
- Bhushan S, Tchatalbachev S, Klug J, Fijak M, Pineau C, Chakraborty T, et al. Uropathogenic Escherichia coli block MyD88-dependent and activate MyD88-independent signaling pathways in rat testicular cells. J Immunol. 2008;180:5537–5547. doi: 10.4049/jimmunol.180.8.5537. [DOI] [PubMed] [Google Scholar]
- Jeannin P, Renno T, Goetsch L, Miconnet I, Aubry JP, Delneste Y, et al. OmpA targets dendritic cells, induces their maturation and delivers antigen into the MHC class I presentation pathway. Nat Immunol. 2000;1:502–509. doi: 10.1038/82751. [DOI] [PubMed] [Google Scholar]
- Soulas C, Baussant T, Aubry JP, Delneste Y, Barillat N, Caron G, et al. Outer membrane protein A (OmpA) binds to and activates human macrophages. J Immunol. 2000;165:2335–2340. doi: 10.4049/jimmunol.165.5.2335. [DOI] [PubMed] [Google Scholar]
- Mian MF, Lauzon NM, Stämpfli MR, Mossman KL., and, Ashkar AA. Impairment of human NK cell cytotoxic activity and cytokine release by cigarette smoke. J Leukoc Biol. 2008;83:774–784. doi: 10.1189/jlb.0707481. [DOI] [PubMed] [Google Scholar]
- Kariyawasam S, Wilkie BN., and, Gyles CL. Resistance of broiler chickens to Escherichia coli respiratory tract infection induced by passively transferred egg-yolk antibodies. Vet Microbiol. 2004;98:273–284. doi: 10.1016/j.vetmic.2003.10.022. [DOI] [PubMed] [Google Scholar]