Abstract
Guanidinylated neomycin (GNeo) can transport bioactive, high molecular weight cargo into the interior of cells in a process that depends on cell surface heparan sulfate proteoglycans. In this report, we show that GNeo-modified quantum dots bind to cell surface heparan sulfate, undergo endocytosis and eventually reach the lysosomal compartment. An N-hydroxysuccinimide activated ester of GNeo (GNeo-NHS) was prepared and conjugated to two lysosomal enzymes, β--glucuronidase (GUS) and α--iduronidase. Conjugation did not interfere with enzyme activity and enabled binding of the enzymes to heparin-Sepharose and heparan sulfate on primary human fibroblasts. Cells lacking the corresponding lysosomal enzyme took up sufficient amounts of the conjugated enzymes to restore normal turnover of glycosaminoglycans. The high capacity of proteoglycan-mediated uptake suggests that this method of delivery might be used for enzyme replacement or introduction of foreign enzymes into cells.
Introduction
Heparan sulfate proteoglycans are abundantly expressed on virtually all animal cells and bind a large number of ligands, including growth factors and morphogens, proteases and their inhibitors, as well as lipoproteins and the lipases that act on them.1,2,3 Proteoglycans undergo constitutive endocytosis, apparently through a clathrin-independent pathway,4 and eventually arrive at the lysosome.5,6 Various lysosomal proteases, sulfatases, and glycosidases subsequently degrade the proteoglycan core protein and the heparan sulfate chains.7,8 Ligands bound to the chains also undergo degradation. For example, the heparan sulfate proteoglycan syndecan-1 expressed by hepatocytes binds triglyceride-rich remnant lipoproteins, resulting in their internalization and degradation in the liver. Loss of syndecan-1 expression results in hypertriglyceridemia, demonstrating at least one physiological role for this uptake system.9
Guanidinoglycosides are derivatives of the naturally occurring aminoglycoside antibiotics in which all the ammonium groups have been converted into guanidinium groups.10,11 For example, guanidinylated neomycin (GNeo) contains six positively charged guanidinium groups in place of the naturally occurring amino groups on the four monosaccharide units that make up the antibiotic. Noncovalent attachment of GNeo to large bioactive molecules (>300 kd) by way of biotin–streptavidin interaction facilitates their transit across cell membranes.12 At low nanomolar transporter concentrations, these carriers deliver their cargo in a heparan sulfate exclusive manner based on the refractory behavior of mutant cells lacking heparan sulfate. Conjugation of GNeo to the fluorophores phycoerythrin-Cy5 and Alexa488 demonstrated uptake into subcellular organellar compartments. A GNeo conjugate containing saporin, a ribosome inactivating protein, apparently can gain access to the cytoplasm and kill the cells in a heparan sulfate–dependent manner. Whether protein ligands containing GNeo continue to progress through the vesicular system in cells to other subcellular organelles was unclear.
In this report, we demonstrate that GNeo, conjugated to quantum dots via biotin–streptavidin, enables particle entry into cells in a heparan sulfate–dependent manner and facilitates their lysosomal accumulation. We also describe an N-hydroxysuccinimide activated ester of GNeo (GNeo-NHS), which facilitates covalent conjugation under mild conditions of the transporter to lysine residues exposed on the surface of proteins. Conjugation of GNeo to β--glucuronidase (GUS) and α--iduronidase in this fashion does not interfere with enzymatic activity, endows the enzymes with the capacity to bind heparin, and enables their delivery to lysosomes. Sufficient amounts of enzymes are delivered to restore the turnover of glycosaminoglycans in cells lacking endogenous forms of these enzymes. We conclude that cell surface heparan sulfate proteoglycans have sufficient capacity to deliver therapeutic doses of enzymes, opening up the possibility of using guanidinylated glycosides as a general vehicle for enzyme delivery.
Results
GNeo-conjugated quantum dots bind to heparin and heparan sulfate, and appear in lysosomes
In previous studies, we conjugated biotinylated GNeo to Streptavidin-Alexa488 to monitor cell binding and uptake by fluorescence microscopy. Labeling studies of Chinese hamster ovary (CHO) cells showed initial uptake of the fluorophore into vesicular structures. Uptake depended on the presence of cell surface proteoglycans based on the absence of signal in mutant cells unable to make glycosaminoglycans (pgsA).12 In a similar way, we conjugated biotinylated GNeo to streptavidin-coated quantum dots (QD525), which are more photostable and resistant to degradation compared to conventional fluorophores. As a result, many consecutive focal-plane images can be reconstructed into a high-resolution three-dimensional image, and real-time tracking in cells can be achieved over extended periods of time. Each quantum dot contains from 5–10 molecules of streptavidin each containing four binding sites for biotin. Pilot experiments demonstrated that extensive binding to heparin-Sepharose occurred when the biotin acceptor sites were saturated with GNeo. GNeo-QD525 prepared in this way bound strongly to heparin-Sepharose and eluted between 0.9 and 1.8 mol/l NaCl (data not shown). Conjugation did not affect the excitation or emission spectra (data not shown).
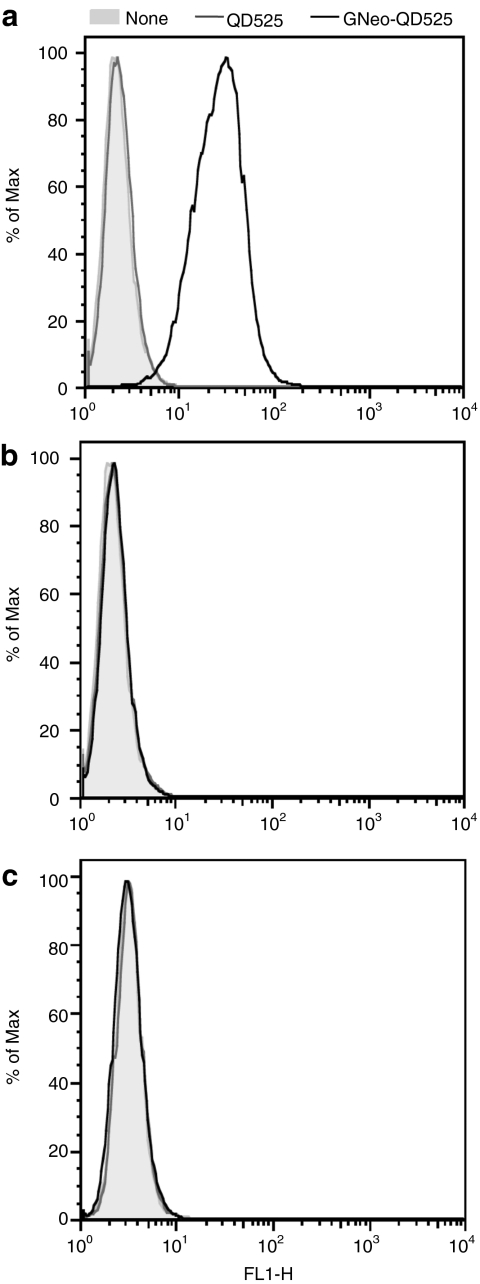
Incubation of wild-type CHO cells with 5 nmol/l GNeo-QD525 led to fluorescent labeling of the cells, which was easily quantified by flow cytometry (Figure 1a, black line). Significant fluorescence was observed compared to controls in which the quantum dots were not added to the cells (shaded area in Figure 1a) or in cells incubated with quantum dots not derivatized with GNeo (QD525, gray line). Under these conditions, the majority of the fluorescence signal was due to uptake of GNeo-QD525 because the cells were treated with trypsin prior to flow cytometry. Control experiments in which cells were incubated with GNeo-QD525 at 4 °C showed that nearly all of the cell-associated material was released by trypsin treatment at low temperature (Supplementary Figure S1). In contrast, at 37 °C about 20% of GNeo-QD525 was resistant to trypsin treatment, a value that increased with time. Uptake was completely dependent on glycosaminoglycans because pgsA-745 cells lacking both heparan sulfate and chondroitin sulfate did not show a significant signal by flow cytometry (Figure 1b). Similar results were obtained in pgsD-677 cells, which lack heparan sulfate and make about two- to threefold more chondroitin/dermatan sulfate (Figure 1c). Thus, GNeo-conjugated quantum dots behaved much like GNeo-streptavidin-Alexa488 and GNeo-streptavidin-phycoerythrin-Cy5 conjugates described previously in terms of their high selectivity for heparan sulfate.12
Figure 1.
GNeo-QD525 uptake depends on heparan sulfate. (a) Wild-type Chinese hamster ovary cells, (b) mutant pgsA-745 deficient in heparan sulfate and chondroitin sulfate, and (c) mutant pgsD-677 deficient in heparan sulfate were incubated with 5 nmol/l GNeo-QD525 (black), QD525 (dark gray), or untreated (none, light gray area) for 2 hours under normal growth conditions. The cells were rinsed twice and lifted with trypsin/EDTA before being analyzed by flow cytometry.
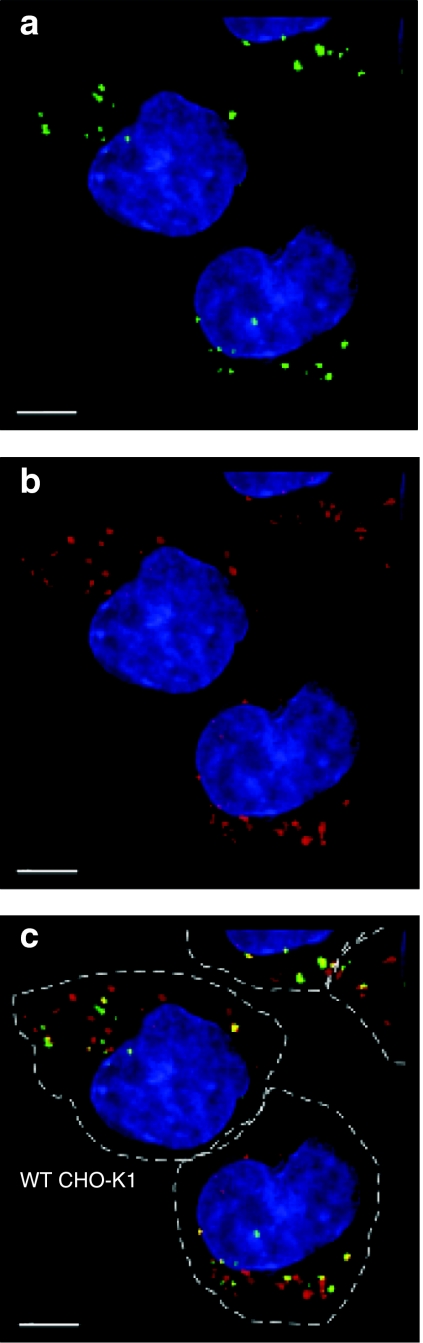
Imaging of the cells by deconvolution fluorescence microscopy showed that GNeo-QD525 was present in punctate structures (Figure 2a, green). Uptake was a relatively slow process because GNeo-QD525 did not appear inside the cells for ~45 minutes (data not shown). Many of the punctate structures containing GNeo-QD525 at 37 °C also co-stained with LysoTracker (red) (Figure 2b,c). A three-dimensional volumetric rendering and fly-through is shown in the Supplementary Video S1, which demonstrated that the majority (~90%) of internalized GNeo-conjugated quantum dots colocalized with lysosomes after 3 hours. These findings indicated that GNeo could deliver very high molecular weight cargo (estimated size of the streptavidinylated quantum dots >107 Da) to lysosomes by way of cell surface heparan sulfate proteoglycans.
Figure 2.
GNeo-QD525 colocalize in lysosomes. Wild-type Chinese hamster ovary cells were incubated with 5 nmol/l GNeo-QD525 in growth medium for 30 minutes. After rinsing the cells three times, fresh medium was added, and 2.5 hours later, they were rinsed with Hank's balanced salt solution and labeled with Hoechst dye and LysoTracker Red. Images were captured with a DeltaVision Restoration microscope system and were deconvolved to show the localization of (a) GNeo-QD525, (b) lysosomes in a single Z-stack plane. The merged images from a and b are shown in c with the outline of cells (hatched line) drawn based on a phase contrast micrograph. Bar = 5 µm. A three-dimensional reconstruction is shown in Supplementary Video S1 to better appreciate the extensive colocalization of quantum dots with the lysosomes.
Conjugation of GNeo to enzymes confers heparin binding
The discovery that GNeo-conjugated quantum dots appeared in lysosomes suggested the use of GNeo to deliver lysosomal enzymes to cells. Initial discussions with W.S.S. (St Louis University, St Louis, MO) and Elizabeth Neufeld (University of California–Los Angeles, Los Angeles, CA) suggested that GUS was an ideal enzyme to initiate these studies due to the availability of the purified bovine and recombinant human enzymes, its relative stability compared to other lysosomal enzymes, and the availability of human fibroblasts from mucopolysaccharidosis (MPS) VII patients (Sly's syndrome) lacking endogenous GUS.
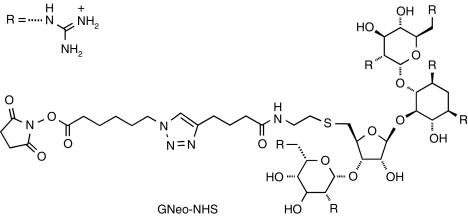
To attach GNeo to the enzyme, a linker containing a terminal NHS-activated ester was conjugated to GNeo (GNeo-NHS, Figure 3). A key step in fabricating this relatively reactive derivative is a 2+3 cycloaddition reaction (commonly referred to as a Click reaction) between an NHS-activated azido carboxylic acid and an alkyne-linked guanidinoglycoside derivative (see Supplementary Scheme S1 and Supplementary Materials and Methods for synthetic procedures and analytical data). The necessary building blocks, including the singly modified neomycin core, were prepared according to previously published procedures.13,14 Enzyme conjugates were prepared by reacting bovine GUS (bGUS) with GNeo-NHS at various ratios (e.g., ten-, 50-, and 100-fold molar excess of the NHS derivative). A high molar excess (>50-fold) was necessary to achieve conjugation levels that enabled binding to heparin-Sepharose. Under these conditions, about two-thirds of the treated enzyme did not bind to the resin, like native bGUS. The remainder required 0.3–0.9 mol/l NaCl to elute from the resin (Supplementary Figure S2a). The addition of GNeo had at most only a minor effect on enzyme-specific activity (~30% in the unfractionated preparation, but no effect on the modified enzyme that eluted at 0.3 and 0.6 mol/l NaCl; Supplementary Figure S2b).
Figure 3.
Generation of GNeo-N-hydroxysuccinimide (GNeo-NHS) by “Click” chemistry.
Uptake of GNeo-GUS by cells depends on heparan sulfate
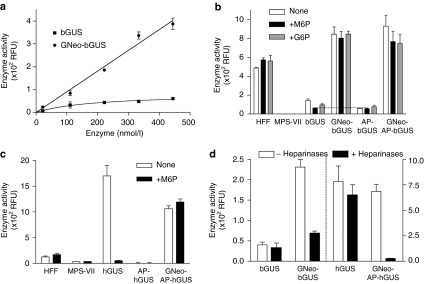
To test whether GNeo-bGUS was taken up by GUS-deficient cells, enzyme activity was measured in MPS VII fibroblasts after 2-hour incubation with various concentrations of unmodified bGUS and GNeo-bGUS (Figure 4a). Poor uptake of bGUS occurred at all concentrations tested, whereas uptake of GNeo-bGUS was proportional to concentration up to 450 nmol/l, the highest concentration tested. When cells were incubated with 20 nmol/l of GNeo-bGUS for 2 hours, the cells took up 0.5% of the enzyme, whereas in the absence of GNeo conjugation, cells took up only 0.06% of the added enzyme. Neither bGUS nor GNeo-bGUS was degraded in cell-free extracts based on enzymatic activity assays, suggesting that incorporated enzyme was stable.
Figure 4.
GNeo delivery of GUS is M6P-independent. (a) MPS VII fibroblasts were treated with the indicated concentration of bGUS or GNeo-bGUS for 2 hours. The cells were washed, trypsin treated, sedimented by centrifugation, washed three times and subsequently assayed for β-glucuronidase activity. (b) MPS VII cells were treated with 5 nmol/l of the indicated bovine enzyme preparations in growth medium in the absence (open bars) or in the presence of 5 mmol/l mannose-6-phosphate (filled bars) or glucose-6-phosphate (gray bars), and assayed for β-glucuronidase activity. Assays of normal human foreskin fibroblasts (HFF) and MPS VII cells are shown for comparison. The difference between bGUS uptake in the presence of M6P was significant, whereas the inhibition by G6P was not. Analysis of variance showed that these differences were significant (P = 0.0104). The differences within the other data sets were not significant (P = 0.2915 for HFF cells, P = 0.8595 for GNeo-bGUS, P = 0.4577 for AP-bGUS, and P = 0.4513 for GNeo-AP-bGUS). (c) MPS VII cells were treated with 1 nmol/l of the indicated human enzymes in the presence (filled bars) and absence (open bars) of 5 mmol/l mannose-6-phosphate and assayed for β-glucuronidase activity. Assays of normal human fibroblasts (HFF) and untreated MPS VII cells are shown for comparison. (d) MPS VII cells were treated with medium in the absence (open bars) or with a mixture of heparin lyases (filled bars) 15 minutes prior to addition of 5 nmol/l bGUS or GNeo-bGUS or 1 nmol/l of hGUS or GNeo-AP-hGUS. Error bars are the SEM of triplicate uptake experiments. The difference between GNeo-bGUS uptake after heparinase digestion and bGUS was significant (P = 0.009) and probably reflected incomplete digestion by heparinases. The difference in hGUS uptake after heparinase was not significant (P = 0.2). The data were compared by Student's t-test. G6P, glucose 6-phosphate; M6P, mannose-6-phosphate; MPS, mucopolysaccharidosis; RFU, relative fluorescence units.
Lysosomal enzymes are normally sorted to lysosomes by way of receptors that recognize mannose-6-phosphate (M6P)-terminated, asparagine-linked glycans.15,16,17 A portion of the cation-independent M6P receptors (CI-MPR) appears at the cell surface and mediates the uptake of “high-uptake” forms of enzymes that contain the M6P-terminated glycans. High concentrations of M6P (5 mmol/l) block this uptake route, whereas glucose 6-phosphate (G6P) does not. As shown in Figure 4b, endogenous GUS activity in human foreskin fibroblasts (HFF) was not affected by M6P or G6P. Uptake of bGUS was reduced by 50%, however, by 5 mmol/l M6P but not by G6P. Analysis of variance showed that these differences were significant (P = 0.0104). Treatment of bGUS with alkaline phosphatase (AP), which removes the terminal 6-phosphate group necessary for recognition, reduced uptake by ~50% as well, and the residual uptake was insensitive to M6P, consistent with the presence of a M6P-independent receptor on fibroblasts.18 In comparison, uptake of human recombinant GUS (hGUS) was robust (Figure 4c), and the addition of M6P or treatment with AP-hGUS greatly inhibited uptake.
To test whether GNeo could confer high-uptake properties to GUS isoforms, conjugates of bGUS, AP-bGUS, or AP-hGUS were generated and added to human fibroblasts. The addition of GNeo dramatically increased enzyme uptake compared to the unmodified enzymes, exceeding the endogenous activity observed in untreated cells (Figure 4b,c). Importantly, free M6P had little, if any, effect on uptake, suggesting that the conjugated enzymes were not internalized via the CI-MPR pathway. Instead, uptake of the GNeo-conjugated enzymes depended on heparan sulfate, based on loss of uptake by prior treatment of the cells with heparin lyases, which depolymerizes the heparan sulfate chains on the surface of the cell. The incomplete inhibition of uptake of GNeo-bGUS by heparinase to the level observed with unmodified bGUS probably reflects incomplete digestion of heparan sulfate in this experiment and the presence of M6P-modified enzyme. The greater sensitivity of GNeo-AP-hGUS to heparinase probably reflects the lack of any M6P targeting signals in this preparation and the lower concentration of enzyme compared to bGUS (1 nmol/l versus 5 nmol/l, respectively). Uptake of unconjugated bGUS and hGUS was insensitive to treatment with heparin lyases.
Internalized enzymes restore normal GAG turnover
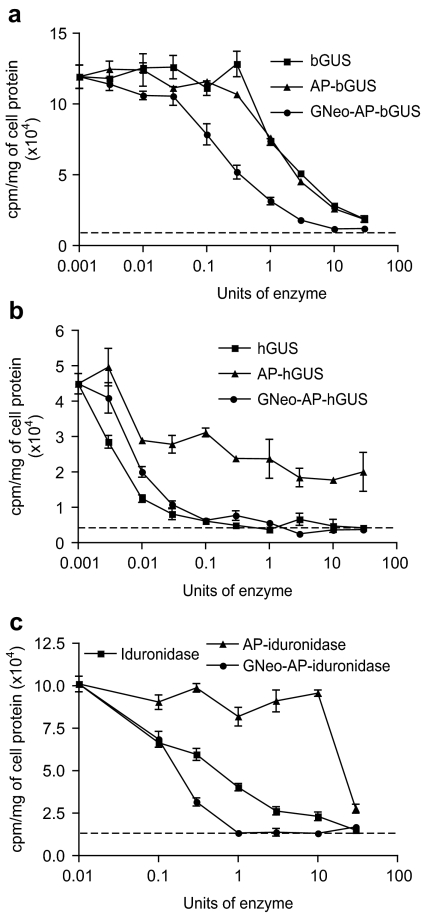
To test whether internalized GUS was functionally localized in lysosomes, we utilized a label-chase format in which cells were incubated with 35S-labeled sulfate for 24 hours to radiolabel the sulfated glycosaminoglycans. The medium was changed, and after 24 hours, the amount of [35S]glycosaminoglycans that remained associated with the cells was quantitated. Under these conditions, MPS VII fibroblasts retained about tenfold more [35S]glycosaminoglycans than normal HFF (Figure 5a).
Figure 5.
GNeo delivery of lysosomal enzymes enhances turnover of glycosaminoglycans in mucopolysaccharidosis (MPS) fibroblasts. (a,b) Normal and MPS VII or (c) MPS I fibroblasts were radiolabeled with 35SO4 and chased for 24 hours with the indicated concentration of bovine β-glucuronidases (a), human β-glucuronidases (b), and human α--iduronidase (c). The amount of [35S]glycosaminoglycan remaining was measured (Materials and Methods). (a) GUS (squares), AP-GUS (triangles), or GNeo-AP-GUS (filled circles). (b) hGUS (squares), AP-hGUS (triangles), or GNeo-AP-GUS (filled circles). (c) Iduronidase (squares), AP-iduronidase (triangles), or GNeo-AP-iduronidase (filled circles). The dotted line represents the amount of [35S]glycosaminoglycan remaining in normal fibroblasts (HFF) without enzyme supplementation. The experiment was performed twice, in triplicate.
Incubation of the cells with GNeo-AP-bGUS induced turnover, with an ED50 value of 150 mU of enzyme activity (Figure 5a). bGUS and AP-bGUS also enhanced the turnover of the [35S]glycosaminoglycans, but the ED50 values were tenfold higher, ~1,500 mU (Figure 5a). The uptake mechanism of AP-bGUS has not been well characterized, and may involve other receptors or fluid-phase pinocytosis.18 Recombinant hGUS, which is extensively modified with M6P, stimulated [35S]glycosaminoglycans turnover with a low ED50 (~3 mU) and treatment with AP reduced its potency (ED50 ~300 mU) (Figure 5b). The addition of GNeo to AP-hGUS restored its efficacy to a level comparable to hGUS (ED50 ~10 mU) and with a similar dose–response curve.
To demonstrate the general utility of GNeo as a transporter, we applied the same coupling method to α--iduronidase, a lysosomal enzyme missing in MPS I patients (Hurler, Hurler–Scheie, and Scheie syndromes). Like MPS VII cells, MPS I fibroblasts also stored [35S]glycosaminoglycans compared to wild-type HFF (Figure 5c). As expected, recombinant therapeutic α--iduronidase (Aldurazyme) restored turnover, whereas AP-α iduronidase was comparatively ineffective (ED50 = 1 U versus 30 U, respectively). Conjugating GNeo to AP-α--iduronidase enhanced its uptake, shifting the ED50 to 0.2 units, making it as effective or better than native Aldurazyme in restoring [35S]glycosaminoglycan turnover.
Discussion
Tremendous progress has been made in recent years in the application of arginine-rich protein transduction domains (also named cell-penetrating peptides) in either chimerically expressed recombinant proteins or as tags for cellular delivery.19,20,21 The intricacies of entry, localization, and release of these peptide-based transporters remain somewhat controversial.22 Multiple uptake pathways are likely to operate simultaneously, and their relative significance might depend on the specific sequences and cell types used.23 Compared to arginine-rich transduction peptides, guanidinoglycoside-based transporters, a family of synthetic derivatives where all the ammonium groups of aminoglycosides have been converted into guanidinium groups, display a unique entry pathway. At low carrier concentrations (nanomolar range), a high selectivity for cell surface heparan sulfate proteoglycans was observed. This finding suggested that guanidinoglycosides were likely to enter the cell complexed to glycosaminoglycan chains and immediately sparked the hypothesis that such carriers could effectively deliver high molecular weight cargo to organelles where heparan sulfate was stored and metabolized.12
To experimentally investigate this hypothesis and to provide a proof of concept, we showed that GNeo-conjugation to quantum dots enabled the uptake of this very high molecular weight cargo (>107 Da) in a heparan sulfate–dependent manner (Figure 1). Although the specific vesicular route of uptake remains to be determined, uptake occurred relatively slowly with a t1/2 of >30 minutes, consistent with other studies suggesting that proteoglycans are internalized in a clathrin-independent manner, possibly dependent on lipid rafts and/or macropinocytosis.4 Further studies are underway to identify the relevant proteoglycan(s) and their mechanism of internalization.
Regardless of the mechanism of uptake, internalization led to the colocalization of the quantum dots with LysoTracker Red, suggesting delivery to lysosomes. To prove that the GNeo carrier was capable of delivering bioactive cargo, we developed an effective conjugation method for proteins using an activated ester derivative (GNeo-NHS). We selected GUS as the cargo due to its availability and stability, and because cell lines lacking the enzyme have been derived from deficient patients. The enzyme exists as a 300 kd tetramer and contains 27 lysine residues per monomer.24 An X-ray crystal structure shows that many of the lysines are accessible on the protein surface (PDB structure: 1BHG). It is unclear how many of these residues underwent modification by GNeo-NHS, but in other data not reported here, we have shown that GNeo by itself binds avidly to heparin (L. Fischer, A. Dix, S. Sarrazin, J. Esko, and Y. Tor, unpublished results). Thus, purification of the GUS-GNeo conjugates was facilitated by “affinity” chromatography on heparin-Sepharose (Supplementary Figure S2). Rewardingly, the GNeo-conjugated enzyme was found to maintain its hydrolytic activity as determined by in vitro fluorescence-based assays. In addition, the enzyme conjugates were found to retain their activity upon exposure to cells and cell extracts. Future studies will address the question of how binding and uptake are affected by the extent of modification by GNeo, which might be best approached using synthetic scaffolds where the number of GNeo moieties can be controlled.
Normal routing of newly made lysosomal enzymes to lysosomes occurs in sorting vesicles in the interior of the cell via recognition of M6P-terminated glycans on the enzyme by M6P receptors.16 A portion of cation-independent M6P-receptors present on the cell surface can internalize exogenous enzymes bearing the M6P recognition determinant, which forms the basis of current enzyme replacement therapies for some lysosomal storage disorders.25 Exposing GUS-deficient MPS VII fibroblasts to recombinant hGUS, and to a lesser extent bGUS, restored enzymatic activity in this manner and treatment of these enzymes with AP inhibited uptake. Uptake was fully restored by conjugation of the phosphatase-treated preparations to GNeo, as measured by enzyme activity and turnover of [35S]glycosaminoglycans. As expected, removal of cell surface heparan sulfate had no impact on the M6P-mediated uptake of GUS, but considerably diminished the uptake of GNeo-GUS further supporting the heparan sulfate–dependent delivery of the conjugates into cells. Importantly, uptake of GNeo-AP-hGUS and GNeo-bGUS was as effective as uptake of the “high-uptake” form of hGUS containing M6P. In fact, conjugation of GNeo to AP-α--iduronidase enhanced its uptake compared to Aldurazyme, the high-uptake form currently in clinical use for treatment of MPS I patients.
Previous studies have shown that conjugation of GUS to the 11-amino-acid HIV Tat protein transduction domain or to an enhanced modified form of the peptide allowed cells to take up GUS, albeit less efficiently than M6P-receptor-mediated uptake.26 Uptake of Tat-modified enzyme was thought to be mediated, in part, through binding of the positively charged peptide to cell surface proteoglycans based on the ability of heparin, heparan sulfate, and polylysine to block enzyme uptake, consistent with other studies showing that polyarginine containing protein transduction domains can mediate transport through heparan sulfate–dependent pathways.27,28 The use of GNeo as the carrier differs significantly from Tat and other transduction mechanisms in that binding, and uptake occurs at very low concentration of the carrier and depends entirely on cell surface heparan sulfate.12 Uptake under these conditions was as efficient as M6P-mediated pathway for delivery of GUS and more efficient for delivery of α-iduronidase (Figures 4 and 5). Other mechanisms for delivery of GUS have been advanced,29,30,31 but the relative efficacy of these methods compared to GNeo-conjugated enzyme has not yet been determined.
Further studies are underway to determine the plasma clearance properties of GNeo-conjugated enzymes in mice and whether the carrier confers tissue specificity for enzyme delivery. The ease of conjugation of the NHS-derivative of GNeo to virtually any protein containing accessible lysine residues suggests that this carrier may prove useful for delivery of various proteins to cells. Because all animal cells express heparan sulfate, guanidinylated glycosides may represent a generally useful class of transporters for delivery of reagents. The lack of specific tissue targeting could prove advantageous for treating lysosomal storage disorders, which afflicts all tissues. We do not know whether GNeo conjugates target specific subdomains of heparan sulfate or whether the conjugates will exhibit selectively for heparan sulfates expressed in different tissues or cell types. We have already demonstrated uptake of GNeo-conjugated agents in CHO cells, human fibroblasts, as well as a variety of tumor cell lines.11,12 Furthermore, we have demonstrated delivery of active enzymes to the cytoplasm as well as the lysosome,12 suggesting that other cell compartments might be amenable to targeting. Additional studies are underway to determine how the stoichiometry of substitution affects binding affinity and uptake, and whether guanidinylated glycosides bind to specifically sulfated sequences in heparan sulfate, which might affect delivery in a cell type–specific manner.
Materials and Methods
GNeo derivatives. The synthesis and characterization of GNeo-NHS are described in Supplementary Materials and Methods. Stock solutions were stored at 20 mmol/l in DMSO. Biotinylated GNeo was described previously.12
Cell culture. Wild-type CHO cells (CHO-K1) were obtained from the American Type Culture Collection, Manassas, VA (CCL61). Culture conditions and CHO mutants pgsA-745 (deficient in all sulfated glycosaminoglycans) and pgsD-677 (deficient in heparan sulfate) were described previously.32,33 HFF, and fibroblasts from MPS I and MPS VII patients were obtained from Coriell (GM06214 and GM00121) and grown in Eagle's minimal essential medium supplemented with 15% of fetal bovine serum and penicillin/streptomycin (Invitrogen, Carlsbad, CA).
Enzyme sources. bGUS was obtained from Sigma (St Louis, MO), hGUS and AP-hGUS were prepared as described,34 and human recombinant α--iduronidase (Aldurazyme) was manufactured by Genzyme (Cambridge, MA).
Uptake of GNeo-QD525 in CHO cells. GNeo-biotin (12 µmol/l) was added to 300 nmol/l Streptavidin-QD525 (Invitrogen, 5–10 streptavidin/quantum dot) in Hank's balanced salt solution for 30 minutes at room temperature. Under these conditions, the conjugate contains up to 40 GNeo moieties. The GNeo-QD525 preparation was then diluted in normal growth medium to 5 nmol/l of quantum dots. Flow cytometry experiments were performed after incubation of cells for 1 hour with GNeo-QD525 under otherwise normal growth conditions. After uptake, cells were lifted using 10× trypsin/EDTA (0.5%/4.8 mmol/l, Invitrogen) for 15 minutes and rinsed twice in phosphate-buffered saline (PBS) before being analyzed by FACS. Unconjugated streptavidin-QD525 was used as a negative control. For fluorescent microscopy imaging, cells were incubated with GNeo-QD525 for 30 minutes. After three washes in medium, fresh medium was added to the cells. After 2.5 hours, cells were rinsed in Hank's balanced salt solution, labeled with Hoechst and LysoTracker Red following instructions provided by the manufacturer (Invitrogen). Images were captured with a DeltaVision Restoration system (Applied Precision, Issaquah, WA) seated on an Olympus IX70 microscope (Olympus, Center Valley, PA). Optical sections were acquired in 0.2 µm steps in the z axis using a ×100 Nikon (NA 1.3) oil immersion objective (Nikon, Melville, NY). Images were deconvolved on a softWoRx workstation (Applied Precision) and reconstituted in three dimensions using Volocity software (PerkinElmer, Waltham, MA).
Linkage of GNeo-NHS to enzymes. bGUS was dissolved in 50 mmol/l sodium acetate buffer (pH 5.2), and protein concentration was estimated by BCA assay (Pierce, Rockford, IL). The optimal concentration of GNeo-NHS was empirically determined based on the binding of the conjugated enzyme to heparin-Sepharose. Thus, the conjugation of the GNeo-NHS to GUS and α--iduronidase was performed at 4 °C using 50 and 100 molar excess ratio, respectively, of GNeo-NHS (5.56 µmol/l GUS with 278 µmol/l GNeo-NHS, 5 µmol/l α--iduronidase with 500 µmol/l GNeo-NHS) in PBS (pH 7.4). After 2 hours, excess GNeo-NHS was removed by desalting on a Zeba Desalting Column (Pierce). Conjugated enzyme was purified by heparin affinity chromatography (Hi-Trap Heparin-HP 1 ml; GE Biosciences, Piscataway, NJ). The column was equilibrated in PBS, and samples were loaded onto the column and incubated for 10 minutes at room temperature. Nonbound material was removed with 3 ml of PBS (FT1). A second washing step was performed (FT2) before elution using 3 ml each of 0.3, 0.6, 0.9, 1.2, and 2 mol/l NaCl. Each fraction was desalted on Zeba column, and the protein concentration was measured. The majority of conjugated enzyme was eluted with 0.6 mol/l NaCl.
Enzyme activity assays. GUS activity was measured by assaying the conversion of 4-methylumbelliferyl β--glucuronide (MUG; Sigma) into the fluorochrome 4-methylumbelliferone. The assay was performed in 96-well plates, using 100 µl of reaction buffer containing 50 mmol/l sodium acetate (pH 5.2), 10 mmol/l EDTA, 0.01% bovine serum albumin, 0.1% Triton X-100, 14.3 µg of MUG, and enzyme. After 1 hour at 37 °C, fluorescent product was measured by fluorimetry (excitation at 360 nm, emission at 460 nm) and quantified using a standard curve of 4-methylumbelliferone. One unit (U) of activity is defined as the liberation of 1 µg of 4-methylumbelliferone per hour at pH 5.2, 37 °C. α--iduronidase activity was measured in a similar way using 4-methylumbelliferyl α--iduronide (Glycosynth, Warrington, UK) as substrate. The assay was performed in 10 µl of 50 mmol/l sodium formate (pH 3) buffer containing 10 mmol/l NaCl, 0.1 mg/ml bovine serum albumin, 50 µmol/l substrate, and 2 µl of enzyme.
The 6-O-phosphate group on the M6P recognition site of GUS or α--iduronidase was removed with 250 U of AP (Sigma) per mg of protein (5 hours, 37 °C in a 25 mmol/l Tris/HCl buffer (pH 8) containing 140 mmol/l NaCl).
Uptake of enzymes by fibroblasts. Normal HFF, MPS VII, and MPS I fibroblasts were cultured in Earl's Minimal Essential Medium supplemented with 15% of fetal bovine serum and penicillin/streptomycin (Invitrogen). Approximately 8 × 104 cells in 0.4 ml of medium were seeded in each well of a 24-well plate. After 3 days, cells were incubated in fresh medium with and without 5 mmol/l of M6P or glucose 6-phosphate (G6P) (Sigma). After 10 minutes, enzymes were added to the well at the concentrations indicated in the figure legends. The cells were incubated for 2 hours at 37 °C, washed twice with PBS, treated with trypsin/EDTA, and then combined with complete medium to inhibit the trypsin. Cells were sedimented by centrifugation, and then resuspended in 30 µl of lysis buffer [activity assay buffer containing 0.5% Triton X-100 and a mixture of protease inhibitors (Sigma)]. In some experiments, cells were treated 3 hours with recombinant heparin lyases I, II, and III (5 mU/ml) in serum-free medium, and uptake was measured as described above in medium containing heparinases. Enzyme activity in the cell extracts was measured in triplicate using 5 µg of total cell protein. All assays were performed in triplicate, and each experiment was repeated at least twice on difference occasions.
Turnover of [ 35S]glycosaminoglycans. Normal and MPS fibroblasts were incubated in DMEM/F12 medium supplemented with 10% dialyzed fetal bovine serum in order to increase the radiospecific activity of added 35SO4. Cells were seeded in a 12-well plate, and at confluence, 50 µCi of Na[35S]O4 (PerkinElmer) was added in 1 ml of fresh medium. After 24 hours, the cells were rinsed twice with PBS, exposed to GUS or α--iduronidase for 2 hours, rinsed twice, and then chased for 24 hours in complete growth medium. The cells were harvested with trypsin, centrifuged (500g, 5 minutes), and washed once. The sedimented cells were then resuspended in 100 µl of RIPA buffer (Sigma) and counted by liquid scintillation spectrometry using Scintillator Ultima Gold XR (PerkinElmer).
SUPPLEMENTARY MATERIAL Figure S1. Trypsin removes GNeo-QD525 binding. Figure S2. Purification of GNeo-GUS by heparin affinity chromatography. Scheme S1. Synthesis of an NHS-GNeo (compound 7) molecular transporter. Video S1. Fly-through rendering that demonstrates colocalization of GNeo-QD525 with lysotracker. Materials and Methods.
Acknowledgments
This work was supported by grants GM077471 (Y.T. and J.D.E.) and GM33063 (J.D.E.) from the National Institutes of Health and a grant from Fondation pour la Recherche Medicale (S.S.).
Supplementary Material
Trypsin removes GNeo-QD525 binding.
Purification of GNeo-GUS by heparin affinity chromatography.
Synthesis of an NHS-GNeo (compound 7) molecular transporter.
Fly-through rendering that demonstrates colocalization of GNeo-QD525 with lysotracker.
REFERENCES
- Bernfield M, Götte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, et al. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- Bishop JR, Schuksz M., and, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446:1030–1037. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- Bishop JR, Stanford KI., and, Esko JD. Heparan sulfate proteoglycans and triglyceride-rich lipoprotein metabolism. Curr Opin Lipidol. 2008;19:307–313. doi: 10.1097/MOL.0b013e3282feec2d. [DOI] [PubMed] [Google Scholar]
- Wilsie LC, Gonzales AM., and, Orlando RA. Syndecan-1 mediates internalization of apoE-VLDL through a low density lipoprotein receptor-related protein (LRP)-independent, non-clathrin-mediated pathway. Lipids Health Dis. 2006;5:23. doi: 10.1186/1476-511X-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuki IV, Meyer ME., and, Williams KJ.2000Transmembrane and cytoplasmic domains of syndecan mediate a multi-step endocytic pathway involving detergent-insoluble membrane rafts Biochem J 351Pt 3): 607–612. [PMC free article] [PubMed] [Google Scholar]
- Burbach BJ, Friedl A, Mundhenke C., and, Rapraeger AC. Syndecan-1 accumulates in lysosomes of poorly differentiated breast carcinoma cells. Matrix Biol. 2003;22:163–177. doi: 10.1016/s0945-053x(03)00009-x. [DOI] [PubMed] [Google Scholar]
- Winchester BG. Lysosomal metabolism of glycoconjugates. Subcell Biochem. 1996;27:191–238. doi: 10.1007/978-1-4615-5833-0_7. [DOI] [PubMed] [Google Scholar]
- Bame KJ. Heparanases: endoglycosidases that degrade heparan sulfate proteoglycans. Glycobiology. 2001;11:91R–98R. doi: 10.1093/glycob/11.6.91r. [DOI] [PubMed] [Google Scholar]
- Stanford KI, Bishop JR, Niesman IR, Witztum JL., and, Esko JD. Syndecan-1 is the primary heparan sulfate proteoglycan mediating hepatic clearance of triglyceride-rich lipoproteins in vivo. J Clin Invest. 2009;119:3236–3245. doi: 10.1172/JCI38251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedtke NW, Baker TJ, Goodman M., and, Tor Y. Guanidinoglycosides: a novel family of RNA ligands. J Am Chem Soc. 2000;122:12035–12036. [Google Scholar]
- Luedtke NW, Carmichael P., and, Tor Y. Cellular uptake of aminoglycosides, guanidinoglycosides, and poly-arginine. J Am Chem Soc. 2003;125:12374–12375. doi: 10.1021/ja0360135. [DOI] [PubMed] [Google Scholar]
- Elson-Schwab L, Garner OB, Schuksz M, Crawford BE, Esko JD., and, Tor Y. Guanidinylated neomycin delivers large, bioactive cargo into cells through a heparan sulfate-dependent pathway. J Biol Chem. 2007;282:13585–13591. doi: 10.1074/jbc.M700463200. [DOI] [PubMed] [Google Scholar]
- Baker TJ, Luedtke NW, Tor Y., and, Goodman M. Synthesis and anti-HIV activity of guanidinoglycosides. J Org Chem. 2000;65:9054–9058. doi: 10.1021/jo001142e. [DOI] [PubMed] [Google Scholar]
- Kirk SR, Luedtke NW., and, Tor Y. Neomycin–acridine conjugate: a potent inhibitor of Rev-RRE binding. J Am Chem Soc. 2000;122:980–981. [Google Scholar]
- Kaplan A, Achord DT., and, Sly WS. Phosphohexosyl components of a lysosomal enzyme are recognized by pinocytosis receptors on human fibroblasts. Proc Natl Acad Sci USA. 1977;74:2026–2030. doi: 10.1073/pnas.74.5.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S. Trafficking of lysosomal enzymes. FASEB J. 1987;1:462–468. doi: 10.1096/fasebj.1.6.3315809. [DOI] [PubMed] [Google Scholar]
- Ghosh P, Dahms NM., and, Kornfeld S. Mannose 6-phosphate receptors: new twists in the tale. Nat Rev Mol Cell Biol. 2003;4:202–212. doi: 10.1038/nrm1050. [DOI] [PubMed] [Google Scholar]
- González-Noriega A., and, Michalak C. Mannose 6-phosphate-independent endocytosis of beta-glucuronidase. II. Purification of a cation-dependent receptor from bovine liver. Biochim Biophys Acta. 2001;1538:152–161. doi: 10.1016/s0167-4889(00)00139-7. [DOI] [PubMed] [Google Scholar]
- Tilstra J, Rehman KK, Hennon T, Plevy SE, Clemens P., and, Robbins PD. Protein transduction: identification, characterization and optimization. Biochem Soc Trans. 2007;35 Pt 4:811–815. doi: 10.1042/BST0350811. [DOI] [PubMed] [Google Scholar]
- Vives E. Cellular uptake [correction of utake] of the Tat peptide: an endocytosis mechanism following ionic interactions. J Mol Recognit. 2003;16:265–271. doi: 10.1002/jmr.636. [DOI] [PubMed] [Google Scholar]
- Futaki S. Oligoarginine vectors for intracellular delivery: design and cellular-uptake mechanisms. Biopolymers. 2006;84:241–249. doi: 10.1002/bip.20421. [DOI] [PubMed] [Google Scholar]
- Foerg C., and, Merkle HP. On the biomedical promise of cell penetrating peptides: limits versus prospects. J Pharm Sci. 2008;97:144–162. doi: 10.1002/jps.21117. [DOI] [PubMed] [Google Scholar]
- Nakase I, Takeuchi T, Tanaka G., and, Futaki S. Methodological and cellular aspects that govern the internalization mechanisms of arginine-rich cell-penetrating peptides. Adv Drug Deliv Rev. 2008;60:598–607. doi: 10.1016/j.addr.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Oshima A, Kyle JW, Miller RD, Hoffmann JW, Powell PP, Grubb JH, et al. Cloning, sequencing, and expression of cDNA for human beta-glucuronidase. Proc Natl Acad Sci USA. 1987;84:685–689. doi: 10.1073/pnas.84.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrow TA, Hopkin RJ, Leslie ND, Tinkle BT., and, Grabowski GA. Enzyme reconstitution/replacement therapy for lysosomal storage diseases. Curr Opin Pediatr. 2007;19:628–635. doi: 10.1097/MOP.0b013e3282f161f2. [DOI] [PubMed] [Google Scholar]
- Orii KO, Grubb JH, Vogler C, Levy B, Tan Y, Markova K, et al. Defining the pathway for Tat-mediated delivery of beta-glucuronidase in cultured cells and MPS VII mice. Mol Ther. 2005;12:345–352. doi: 10.1016/j.ymthe.2005.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs SM., and, Raines RT. Pathway for polyarginine entry into mammalian cells. Biochemistry. 2004;43:2438–2444. doi: 10.1021/bi035933x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakase I, Tadokoro A, Kawabata N, Takeuchi T, Katoh H, Hiramoto K, et al. Interaction of arginine-rich peptides with membrane-associated proteoglycans is crucial for induction of actin organization and macropinocytosis. Biochemistry. 2007;46:492–501. doi: 10.1021/bi0612824. [DOI] [PubMed] [Google Scholar]
- Grubb JH, Vogler C, Tan Y, Shah GN, MacRae AF., and, Sly WS. Infused Fc-tagged beta-glucuronidase crosses the placenta and produces clearance of storage in utero in mucopolysaccharidosis VII mice. Proc Natl Acad Sci USA. 2008;105:8375–8380. doi: 10.1073/pnas.0803715105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb JH, Vogler C, Levy B, Galvin N, Tan Y., and, Sly WS. Chemically modified beta-glucuronidase crosses blood-brain barrier and clears neuronal storage in murine mucopolysaccharidosis VII. Proc Natl Acad Sci USA. 2008;105:2616–2621. doi: 10.1073/pnas.0712147105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBowitz JH, Grubb JH, Maga JA, Schmiel DH, Vogler C., and, Sly WS. Glycosylation-independent targeting enhances enzyme delivery to lysosomes and decreases storage in mucopolysaccharidosis type VII mice. Proc Natl Acad Sci USA. 2004;101:3083–3088. doi: 10.1073/pnas.0308728100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esko JD, Stewart TE., and, Taylor WH. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc Natl Acad Sci USA. 1985;82:3197–3201. doi: 10.1073/pnas.82.10.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidholt K, Weinke JL, Kiser CS, Lugemwa FN, Bame KJ, Cheifetz S, et al. A single mutation affects both N-acetylglucosaminyltransferase and glucuronosyltransferase activities in a Chinese hamster ovary cell mutant defective in heparan sulfate biosynthesis. Proc Natl Acad Sci USA. 1992;89:2267–2271. doi: 10.1073/pnas.89.6.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands MS, Vogler CA, Ohlemiller KK, Roberts MS, Grubb JH, Levy B, et al. Biodistribution, kinetics, and efficacy of highly phosphorylated and non-phosphorylated beta-glucuronidase in the murine model of mucopolysaccharidosis VII. J Biol Chem. 2001;276:43160–43165. doi: 10.1074/jbc.M107778200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trypsin removes GNeo-QD525 binding.
Purification of GNeo-GUS by heparin affinity chromatography.
Synthesis of an NHS-GNeo (compound 7) molecular transporter.
Fly-through rendering that demonstrates colocalization of GNeo-QD525 with lysotracker.