Abstract
T-cell-based adoptive immunotherapy is widely used to treat graft rejection and relapse after stem cell transplantation (SCT). However, this approach is hampered by a high risk of life-threatening graft-versus-host-disease (GvHD). Clinical trials have demonstrated the value of suicide genes to modify T cells for the effective control of GvHD. Herewith, we show that the combination of a codon-optimized B-cell antigen (CD20op) with a selection marker based on a cytoplasmic truncated version of the human stem cell antigen CD34 (tCD34) allows the generation of highly enriched gene-modified T cells. We demonstrate coordinate co-expression of both transgenes and high expression of CD20op resulting in an increased susceptibility to Rituximab (RTX)-induced cell death. In addition, T cells partially retained their alloreactive potential and their CD4/CD8 ratio after transduction and expansion. Long-lasting transgene expression was sustained in vivo after adoptive transfer into Rag-1−/− mice. Moreover, gene-modified T cells were quickly and efficiently depleted from peripheral blood (PB) and secondary lymphoid organs of transplanted animals after RTX treatment. These results warrant further steps toward a clinical application of CD20op as a suicide gene for adoptive immunotherapy.
Introduction
Haploidentical stem cell transplantation is an attractive treatment option for patients suffering from malignant diseases including high-risk acute lymphoblastic leukemia.1 Transplantation of T-cell-depleted grafts effectively prevents graft-versus-host-disease (GvHD) but increases the risk of leukemia relapse and opportunistic infections leading to high mortality rates.2 To support early immune recovery and to potentially protect against disease re-occurrence, adoptive immunotherapy employing donor lymphocyte infusions represents a potent treatment strategy.3,4 However, the widespread exploitation of donor lymphocyte infusion has been greatly hampered by the occurrence of life-threatening GvHD.4 Due to the tight clinical association between graft-versus-leukemia and GvHD,5 one challenge in donor lymphocyte infusion post-SCT is to exploit the graft-versus-leukemia effect while controlling GvHD. A promising concept developed >10 years ago involves the genetic modification of donor T cells with suicide genes.6,7 In combination with the herpes simplex virus thymidine kinase gene, this strategy has been shown to be effective and safe in the context of allogeneic SCT in clinical trials with adult patients6,8,9 and has recently been proven to be also feasible in haploidentical SCT settings.10 Despite the remarkable clinical efficacy of the thymidine kinase system, several disadvantages have become apparent with its use. A potential limitation is represented by its reported immunogenicity in immunocompetent patients11,12 leading to the undesired elimination of gene-modified T cells. Furthermore, reported rates of T-cell elimination in vivo are rather slow upon ganciclovir treatment.13 To overcome these limitations, several alternative suicide systems have been developed and tested in preclinical studies in recent years. These include for example FAS (CD95) fused to FK506-binding protein variants in combination with chemical inducers of dimerization,14 as well as the human thymidylate kinase system, where specific cell death is induced by the conversion of azidothymidine to its toxic AZT-triphosphate.15 In addition, the B-cell surface antigen CD20 has been used to genetically modify T cells, which are readily eliminated upon exposure to the anti-CD20 monoclonal antibody Rituximab (RTX).16,17,18 However, our initial attempts to use CD20 for both purification and elimination of transduced T cells resulted in low recovery rates after purification and poor killing efficiencies.
Here, we describe the use of a bicistronic retroviral vector encoding an optimized CD20 sequence (CD20op) linked to tCD34 using a 2A ribosomal skip element sequence for gene marking of T cells.19 We demonstrate efficient purification and elimination of a CD20op/tCD34-transduced T-cell line and primary human T cells by different effector mechanisms in vitro and show that adoptively transferred CD20op-expressing primary T cells can be rapidly and effectively depleted in vivo after RTX treatment.
Results
Codon optimization improves CD20 expression and substantially increases susceptibility to complement-dependent lysis
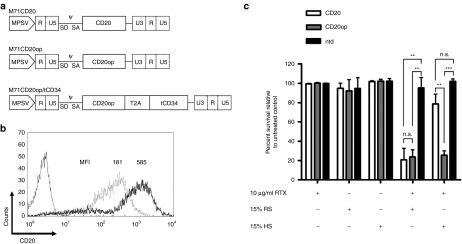
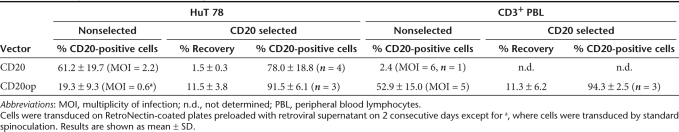
A myeloproliferative sarcoma virus–based retroviral vector containing the coding sequence for CD20 (M71CD20) was constructed for adoptive T-cell immunotherapy (Figure 1a). Initial attempts to transduce T-cell lines and primary T cells failed due to low titers (<1 × 105 transducing units/ml; n = 4). Using a RetroNectin-based protocol a mean transduction efficiency of 61% was obtained in the human T-cell line HuT 78, whereas primary human T cells could not be transduced (Table 1). The level of CD20 expression in HuT 78 cells was low (Figure 1b) and immunoselection based on CD20 using clinical applicable reagents suffered from both low recovery and purity (Table 1). Furthermore, CD20 HuT 78 cells were weakly susceptible to RTX-mediated complement-dependent cytotoxicity (CDC) when human serum was used as a source of complement (Figure 1c). Due to these unsatisfying results, a codon-optimized CD20 sequence (CD20op) was synthesized generating the vector M71CD20op (Figure 1a). Codon optimization increased retroviral titers up to 35-fold allowing transduction of HuT 78 cells by standard spinoculation-based protocols.17 Primary human T cells were efficiently transduced using vector preloading (Table 1). Moreover, CD20 expression was enhanced threefold based on median fluorescence intensities (Figure 1b and data not shown). CD20op-expressing cells showed an increased susceptibility to human serum in RTX-mediated CDC, which was comparable to rabbit serum (Figure 1c). Immunoselection of CD20op-transduced cells resulted in an overall increase in purity rates, but at low recovery rates (Table 1).
Figure 1.
Codon optimization improves CD20 expression and complement-dependent depletion of gene-modified T cells. (a) Schematic diagram of the γ-retroviral vectors used in this study. The long terminal repeats were derived from myeloproliferative sarcoma virus (MPSV). CD20 denotes the open reading frame (ORF) of wild-type CD20, CD20op the ORF of the codon-optimized CD20, CD20opT2AtCD34 indicates the fusion construct of the optimized CD20 sequence linked to the truncated version of CD34 (tCD34) by a T2A element (2A sequence of Thosea asigna virus). Further elements indicated: splice donor (SD) and splice acceptor (SA) sites, packaging signal (ψ). (b) The human T-cell line HuT 78 was transduced either with M71CD20 (light gray line) or M71CD20op (black line) and immunoselected using anti-CD20 beads. CD20 expression was analyzed by flow cytometry. Numbers indicate the median fluorescence intensity (MFI). An isotype control antibody was used as a negative control (dark gray line). (c) Rituximab-mediated complement-dependent depletion (CDC) of gene-modified T cells. For CDC, HuT 78 cells expressing either CD20 (white bars) or CD20op (gray bars) were immunoselected and incubated with or without 10 µg/ml Rituximab and 15% rabbit (RS) or human (HS) serum for 4 hours at 37 °C. Cells were analyzed by flow cytometry using 7-aminoactinomycin D to discriminate between viable and dead cells. Nontransduced HuT 78 cells (ntd, black bars) served as a negative control. Results are expressed as cell survival relative to the untreated control and shown as the averages ± SD from three independent experiments. **P < 0.01, ***P < 0.001, n.s., not significant.
Table 1. Efficiency of immunoselection using CD20.
T cells co-expressing human CD20op and tCD34 are efficiently immunoselected and prone to RTX-mediated lysis in vitro
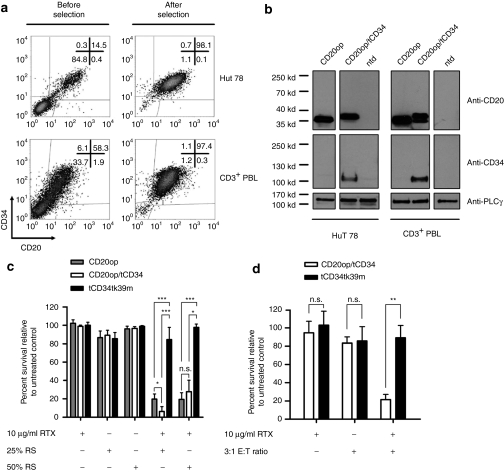
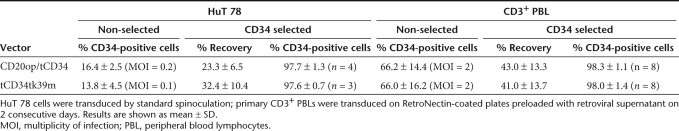
To improve immunoselection, we constructed a retroviral vector containing the optimized CD20 linked the truncated version of CD34 (tCD34)14,20 by a T2A-based ribosomal skip mechanism (M71CD20op/tCD34) (Figure 1a). Average titers of 2.3 ± 0.9 × 106 transducing units/ml (n = 8) were achieved enabling efficient transduction of HuT 78 cells by standard spinoculation whereas primary T cells were transduced with an efficiency of 66 ± 14% using vector preloading. Cell surface expression of both proteins was shown by flow cytometric analysis in transduced HuT 78 and primary CD3+ T cells (Figure 2a, left panel). Immunoselection based on CD34 led to improved recovery and purity rates that were comparable to that achieved in cells transduced with the monocistronic tCD34tk39m control vector14 (Table 2 and Figure 2a, right panel). Protein expression and efficient cleavage at the 2A site was verified by western blot analysis (Figure 2b). CD20op/tCD34-expressing HuT 78 cells could be effectively lysed by RTX-mediated CDC (Figure 2c). Primary T cells co-expressing CD20op and tCD34 were eliminated with a mean cell killing of 79 ± 6% by antibody-dependent cellular cytotoxicity using autologous natural killer cells whereas control vector–transduced cells were not affected (Figure 2d).
Figure 2.
Transduced T cells co-expressing CD20op and tCD34 can be depleted in vitro by complement- and cell-mediated lysis. (a) The human T-cell line HuT 78 (upper panel) and CD3+ peripheral blood lymphocytes (PBLs) (lower panel) were transduced with the CD20op/tCD34 vector and immunoselected using anti-CD34 beads. CD20op and tCD34 expression was analyzed before (left panel) and after (right panel) purification by flow cytometry with CD20- and CD34-specific antibodies. (b) Protein lysates from immunoselected HuT 78 (left panel) and CD3+ PBLs (right panel) transduced with the CD20op or CD20op/tCD34 vector were separated by sodium dodecyl sulfate–polyacrylamide gel, transferred onto polyvinylidene fluoride membrane and probed with anti-CD20 and anti-CD34 antibodies. Nontransduced (ntd) HuT 78 cells and CD3+ PBLs served as a negative control, respectively. As loading control, blots were reprobed with an anti-PLCγ antibody. CD20op has a T2A peptide attached to its C terminus in the context of the fusion construct and thus migrates at a slightly higher position in sodium dodecyl sulfate–polyacrylamide gels. (c) Rituximab-mediated complement-dependent depletion (CDC). For CDC, CD20op (gray bars) and CD20op/tCD34 (white bars) vector-transduced and immunoselected HuT 78 cells were incubated with or without 10 µg/ml Rituximab and 25% rabbit (RS) or 50% human (HS) serum for 4 hours at 37 °C. Cells were analyzed by flow cytometry using 7-aminoactinomycin D to discriminate between viable and dead cells. Immunoselected cells transduced with a tCD34tk39m vector (black bars) served as a negative control. Results are expressed as cell survival relative to the untreated control and shown as the averages ± SD from four independent experiments. (d) Rituximab-mediated cell-dependent depletion (ADCC) of CD20op/tCD34 vector-transduced CD3+ peripheral blood lymphocytes (PBLs). For ADCC, purified autologous NK cells were used as effector cells. CD3+ PBLs were transduced with the CD20op/tCD34 vector (white bars) and incubated with or without 10 µg/ml Rituximab and effector cells at a 3:1 effector:target ratio (E:T) overnight at 37 °C. Target cell depletion was measured by a quantitative four-color flow cytometric analysis50 to discriminate between target and effector cells. tCD34tk39m vector or nontransduced CD3+ PBL cells (black bars) served as a negative control. Results are expressed as cell survival relative to untreated control cells and averages ± SD from three independent donors are shown. *P < 0.05, **P < 0.01, ***P < 0.001, n.s., not significant.
Table 2. Efficiency of immunoselection using CD34.
Gene-modified T cells maintain central memory phenotype and proliferate in response to alloantigens
Phenotypic analysis of gene-modified and nontransduced primary human T cells revealed that the majority of cells had acquired a central memory phenotype (CD45RA−CD62L+) 7 days after stimulation, which could be maintained up to at least day 11 (Supplementary Figure S1a). After transduction, a CD4/CD8 ratio comparable to nontransduced cells was observed at day 7 and day 11 (Supplementary Figure S1b). The alloreactive potential of gene-modified, carboxyfluorescein succinimidyl ester–labeled T cells was evaluated by co-culture with irradiated allogeneic PB mononuclear cells. Freshly isolated T cells were used as a positive control. A considerable proportion of gene-modified T cells remained responsive to the allogeneic challenge and proliferated, thereby reducing the carboxyfluorescein succinimidyl ester signal intensity (Supplementary Figure S1c). However, a slightly reduced proliferation capacity of both CD4 and CD8 subsets was detected for gene-modified T cells in comparison to freshly isolated T cells (Supplementary Figure S1d).
Gene-modified murine T cells repopulate Rag-1-deficient mice and co-express CD20op and tCD34 at a sustained level
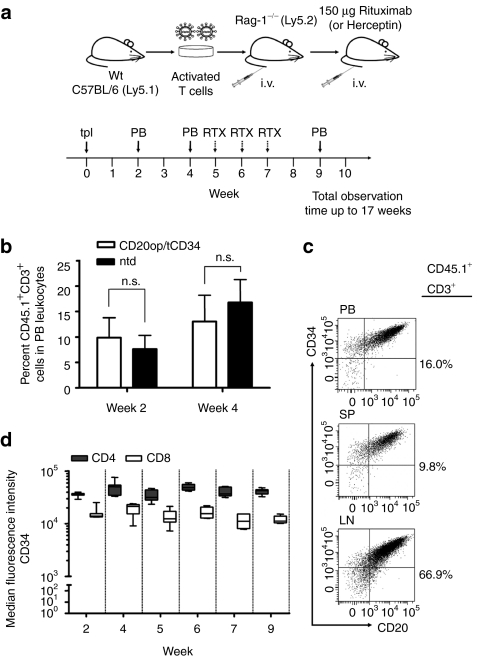
We used Rag-1−/− mice to evaluate long-term expression of CD20op and tCD34 and to assess the susceptibility of vector-transduced T cells to RTX in vivo (Figure 3a). Mature T cells (CD45.1) were transduced and immunoselected to a purity of ~98% using anti-CD34 beads (n = 2) and injected into the tail vein of Rag-1−/− recipients (CD45.2). As shown in Figure 3b, circulating CD45.1+CD3+ donor T cells could be detected in the PB of recipient animals 2 and 4 weeks after adoptive transfer. Importantly, no difference in the repopulation was observed after transplanting the animals with either transduced, immunoselected (n = 20), or nontransduced (n = 9) donor T cells (P > 0.05) (Figure 3b). Donor cells detected were primarily CD4+ T cells (78 ± 7%) with >90% expressing CD20op and tCD34 (data not shown). Long-term expression of both CD20op and tCD34 was demonstrated in PB and donor cells isolated from spleen and lymph nodes of a representative animal at week 17 (Figure 3c). As shown in Figure 3d, tCD34 expression levels remained constant in both CD4 and CD8 subsets for up to 9 weeks in these animals (n = 7) and sustained expression was detectable until the end of the observation time (17 weeks, data not shown).
Figure 3.
Experimental design and repopulation of Rag-1-deficient mice with CD20op/tCD34-transduced T cells. (a) Mature T cells were isolated from C57BL/6.Ly5.1 mice, stimulated with anti-CD3/CD28 beads, and 100 U/ml interleukin 2, and transduced 72 and 96 hours after stimulation. At day 7, vector-transduced T cells were immunoselected using anti-CD34 beads and transplanted into Rag-1-deficient recipient mice (indicated by solid arrow at week 0). Blood was collected from recipient mice 2 and 4 weeks after transplantation [peripheral blood (PB), indicated by solid arrows] and analyzed by flow cytometry for the presence of engrafted CD45.1+CD3+ donor T cells as well as for CD20op and tCD34 surface expression in transduced cells. At week 5, 6, and 7 mice received 150 µg Rituximab intravenously for cell depletion (indicated by dashed arrows). Herceptin was used as a negative control. Two days after each injection, blood was analyzed by flow cytometry. Animals were observed for up to 17 weeks. (b) Repopulation of Rag-1-deficient mice with vector-transduced, immunoselected (white bars; n = 20) and nontransduced (black bars; n = 9) donor T cells. Blood was collected at weeks 2 and 4 after adoptive transfer and analyzed for CD45.1+CD3+ donor T cells. Analysis gate was set on PB leukocytes and shown are the mean percentages of CD45.1+CD3+ donor cells ± SD from two independent experiments (n.s., not significant, P > 0.05). (c) Co-expression of CD20op and tCD34 in murine T cells. CD3+ cells obtained at week 17 from peripheral blood, spleen (SP), and lymph nodes (LNs) of control animals were analyzed for CD20op and tCD34 surface expression. Analysis gate was set on CD45.1+CD3+ leukocytes and scatter plots show the expression of CD20op and tCD34 in PB donor cells and T cells isolated from SP and LNs from one representative animal. Numbers next to each scatter plot indicate the percentage of CD45.1+CD3+ cells. (d) Stability of tCD34 expression levels in adoptively transferred T cells in Rag-1-deficient mice. Peripheral blood was collected at the indicated time points and stained for tCD34 expression in CD4 donor T cells. Analysis gate was set on CD45.1+CD3+ donor cells in peripheral blood leukocytes. The box–whisker plot indicates the median fluorescence intensity of CD34 staining in both CD4 (gray boxes) and CD8 (white boxes) subsets from 7 animals.
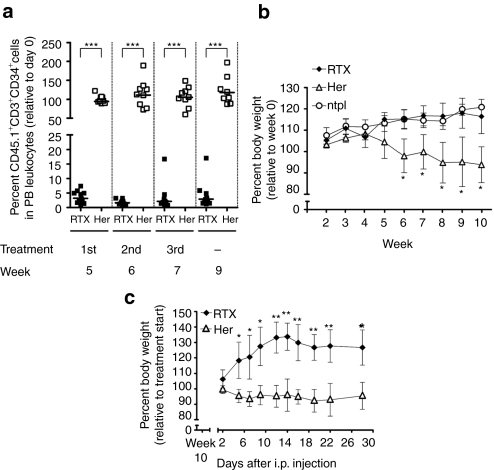
Administration of RTX depletes adoptively transferred gene-modified T cells and controls severe T cell–induced colitis in recipient mice
Five weeks after T-cell transfer, the efficiency of RTX-mediated elimination of gene-modified T cell was assessed in vivo. The treatment was repeated at week 6 and 7 (see schema in Figure 3a). RTX treatment resulted in a depletion of ~96% circulating gene-modified T cells 2 days after the first injection, whereas no reduction was observed in control animals (Figure 4a). Animals were further monitored at frequent intervals and only low levels of gene-modified donor cells were detected until the end of the observation time (Supplementary Figure S2a and data not shown). Residual circulating gene-modified cells were found to have a 2.5-fold lower surface expression of CD20op in comparison to Herceptin-treated animals as determined by median fluorescence intensities (data not shown). In line with these results, quantitative PCR analysis on genomic DNA isolated from PB of RTX-treated animals showed an at least tenfold reduction of gene-modified cells at the end point of the study in comparison to pretreatment values (Supplementary Figure S3). Flow cytometric and quantitative PCR analysis were performed on spleen and lymph node material isolated at week 17 demonstrating an effective and sustained depletion of gene-modified T cells not only from PB, but also from lymphoid tissue (Supplementary Figure S2a,b). The elimination of CD20op/tCD34 cells was specific, as RTX treatment of animals transplanted with a mixture of nontransduced, eGFP- and CD20op/tCD34-expressing donor cells led to the targeted depletion of CD20op/tCD34-expressing cells, whereas eGFP-positive and nontransduced T cells were not eliminated from the circulation (Supplementary Figure S4).
Figure 4.
Rituximab efficiently depletes adoptively transferred gene-modified T cells and controls T cell–induced colitis in Rag-1-deficient recipients. Vector-transduced, immunoselected donor T cells were infused into Rag-1-deficient recipient mice. For depletion, mice were treated with a three-dose regimen of 150 µg Rituximab (RTX) intravenously (i.v.) each 5, 6, and 7 weeks after transplantation. Herceptin (Her) was used as a negative control. (a) Blood was collected from antibody treated animals 2 days after each injection i.v. and 2 weeks after the last treatment. Samples were analyzed by flow cytometry for the presence of CD45.1+CD3+CD34+ donor cells. Analysis gate was set on peripheral blood (PB) leukocytes and quadrants were set according to isotype controls and control staining of blood samples from animals transplanted with nontransduced donor T cells. Scatter dot plot diagram shows the median percentage of CD45.1+CD3+CD34+ donor cells in PB leukocytes relative to pretreatment values (day 0) for animals receiving either Rituximab (closed squares; n = 11) or Herceptin (open squares, n = 9) in two independent experiments. ***P < 0.001 (Mann–Whitney test). (b) As a reflection of donor T cell–induced colitis, weight loss was monitored once a week starting at the day of transplantation (week 0). Mean percent body weight ± SD for animals receiving Rituximab (closed diamonds, n = 7), Herceptin (open triangles, n = 7) and nontransplanted Rag-1-deficient mice (open circles, n = 3) relative to week 0 is shown. *P < 0.05 versus Rituximab-treated animals. (c) At week 10, Herceptin-treated animals (n = 4) were injected with 150 µg Rituximab intraperitoneally (i.p.) each and received a second and third injection during the following 2 weeks. Remaining animals (n = 3) served as controls and were treated with 150 µg Herceptin. Body weight of mice was monitored twice a week and the graph shows the mean percent body weight ± SD relative to the pre-RTX treatment value. Rituximab-treated animals (closed diamonds), Herceptin-treated animals (open triangles), *P < 0.05, **P < 0.01 versus Herceptin-treated group.
Rag-1−/− mice injected with nontransduced or gene-modified T cells develop massive colitis characterized by weight loss and a dense lymphocytic infiltrate, crypt abscesses, and intraepithelial apoptosis in the gut (Supplementary Figure S5 and ref. 21). Immunostaining for CD20 in Herceptin-treated animals clearly showed the presence of gene-modified T cells in the intestine (Supplementary Figure S5c). In contrast to Herceptin-treated mice, RTX-infused animals did not show any signs of disease or weight loss, reflecting the efficient elimination of the majority of gene-modified T cells (Figure 4b). Furthermore, RTX application to Herceptin-treated animals at week 10, a time point where the animals were already severely ill, rescued these mice from colitis progression resulting in a substantial and rapid gain of body weight up to 34 ± 9% relative to that in the beginning of treatment (Figure 4c) along with a depletion of 90% gene-modified T cells (data not shown). The Herceptin-treated control group (n = 3) further lost or kept its body weight constant.
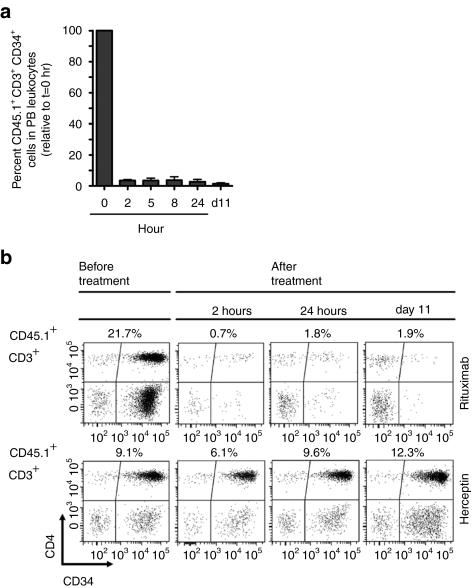
In addition, we determined the kinetics of donor T-cell depletion in vivo. T-cell infused Rag-1-deficient animals received a single dose of 150 µg RTX (n = 5) or Herceptin (n = 2) intravenously at week 5. Remarkably, 96.5 ± 0.6% of transduced donor T cells could be eliminated from PB at 2 hours after RTX treatment, which further increased to 98.6% by day 11 (Figure 5a). Fluorescence-activated cell sorting scatter plots acquired at different time points are shown for a representative animal from each treatment group in Figure 5b.
Figure 5.
Kinetics of Rituximab-induced depletion of gene-modified T cells. Rag-1-deficient recipient mice transplanted with transduced, immunoselected donor T cells were treated with a single dose of 150 µg Rituximab intravenously at week 5 after cell infusion. Blood was collected 2, 5, 8, and 24 hours after injection and analyzed by flow cytometry. Herceptin was used as a negative control. Animals were killed at day 11 after treatment for final analysis. (a) Blood samples collected at the indicated time points were analyzed by flow cytometry for CD45.1+CD3+CD34+ donor cells. Analysis gate was set on peripheral blood (PB) leukocytes. Mean percentages ± SD of CD45.1+CD3+CD34+ donor cells in PB leukocytes of Rituximab-treated animals (n = 4) are shown relative to the pretreatment values. (b) Selected fluorescence-activated cell sorting scatter plots from the kinetic study are shown for a representative animal from each treatment group (upper panel: Rituximab, lower panel: Herceptin). PB leukocytes were gated on CD45.1+CD3+ donor cells and scatter plots show CD4+CD34+ and CD8+CD34+ subsets before treatment, 2 and 24 hours as well as at day 11 after treatment. Numbers above each scatter plot indicate the percentage of CD45.1+CD3+ donor cells in PB leukocytes.
Discussion
CD20 has been proposed as a powerful single genetic marker for both the purification and killing of gene-modified T cells.16,17,18 However, our initial attempts to use CD20 for these purposes failed due to cytotoxic effects during CD20-based immunoselection (I. Vogler, unpublished results) in conjunction with poor recovery rates. Moreover, CDC of CD20-transduced T cells using human serum failed presumably due to the low expression of the CD20.22 To circumvent these problems, a codon-optimized CD20 sequence was synthesized. Besides an increase in viral titers, an enhanced expression in CD20op levels was achieved most likely due to an increased mRNA stability and/or transport as previously shown for other optimized sequences.23 Despite these advances, recovery rates of immunoselected cells remained low excluding CD20 as a marker for selection of gene-modified T cells. To this end, we combined the CD20 strategy with the truncated version of CD34 (tCD34), which allows a clinically established purification protocol.20 The T2A peptide was used to link CD20op with tCD34 as this approach has recently been shown to yield high expression levels of the T-cell receptor α and β chains.24 By using this strategy, we could show coordinate expression of CD20op and tCD34 in vitro and were further able to demonstrate stable and prolonged expression in adoptively transferred murine T cells in vivo. Importantly, the suitability of CD34-based immunoselection of gene-modified T cells was confirmed due to acceptable purity and recovery rates.
Although the residual 2A peptide present at the C terminus of CD20op might induce unwanted immune responses similar to those observed in the thymidine kinase clinical trials,11,12 prediction analysis of epitope binding did not reveal peptides binding to common HLA alleles suggesting a low immunogenic potential.25,26 Also, the ectopic expression of CD20op and tCD34 in primary T cells may represent a safety concern due to unphysiologic signaling. Although the function of CD20 is not yet fully elucidated, it has been suggested that CD20 serves as a regulator of B-cell proliferation and differentiation27 and there is evidence of CD20 involvement in store-operated calcium entry.28 As for tCD34, truncation of the C-terminal part of CD20op may prevent potential signaling cascades. However, a short membrane-proximal cytoplasmic sequence (residues 219–225) is required for the localization to lipid rafts28 important for the activation of the complement system29 and downstream apoptotic signaling.30 In addition, several C-terminal deletions impaired or completely abolished CD20 surface expression.31 Previous in vitro studies have not observed phenotypic and functional changes in lymphocytes expressing CD20 or tCD34 of human origin16,17,20 and a clinical phase I trial employing human tCD34 as an immunoselection marker was recently approved in the UK (Wassem Qasim, University College London, personal communication). Despite this, an effect of tCD34 on T-cell properties cannot be completely excluded, as a recent study has shown effects of full-length murine CD34 on the homing capacities of differentiated blood cells32. In our in vivo model, both CD20op/tCD34-transduced and nontransduced T cells repopulated Rag-1−/− mice to a similar extent and no significant differences in the percentages of donor cells were detected in spleen and lymph nodes of control animals 17 weeks after infusion (data not shown). To date, we have not observed an influence of human CD20op or tCD34 on T-cell properties in vitro or in vivo.
Transduction of CD3/CD28-activated T cells yielded high transduction efficiencies, targeting both CD4 and CD8 subsets to a similar extent. As previously shown, anti-CD3/CD28 bead activation in combination with low doses of interleukin (IL)-2 generated a central memory T-cell phenotype expressing CD62L, which persist after adoptive transfer and display GvHD reactivity in a xenograft model 33. Because phenotypic analysis is only a surrogate marker for functionality, we studied the alloreactive potential of transduced central memory T cells. A similar proportion of gene-modified T cells was found to respond to the allogeneic challenge, albeit a lower proliferation capacity was observed in comparison to freshly isolated T cells. Recently, enhanced alloreactivity of anti-CD3/CD28-activated T cells was achieved by culturing in the presence of the homeostatic cytokines IL-7 and IL-15.34 In addition, lentiviral vectors pseudotyped with measles virus glycoproteins rendered quiescent T cells permissive to transduction.35 The combination of these new developments with our bicistronic CD20op/tCD34 cassette may result in an improved application of this technology for adoptive immunotherapy.
The Rag-1-deficient mouse model used in our studies was chosen to gain insight into the persistence of CD20op/tCD34 gene-modified T cells in vivo and to examine the long-term expression of CD20op and tCD34 in mice. In addition, we wanted to study the efficiency of RTX-mediated depletion of CD20op-expressing cells. Rag-1−/− are devoid of T and B cells36 and thus allow a quick and efficient engraftment of adoptively transferred cells. In contrast to a classical or haploidentical model, where the animals succumb very quickly due to severe GvHD symptoms, the Rag-1−/− model is suitable for long-term monitoring of the cell surface expression of CD20op and tCD34 by flow cytometry. Moreover, Rag-1−/− mice develop a T cell–induced colitis with symptoms such as weight loss, diarrhea, and impaired movement highly resembling a mild GvHD reaction. The observed symptoms are comparable to a syngeneic GvHD developing after lethal irradiation and bone marrow transplantation from a syngeneic donor.37 In our study, T cell–induced colitis could be effectively controlled upon infusion of RTX resulting in a delay of weight loss for up to 5 weeks. Due to the efficient depletion of CD20op/tCD34 donor T cells, the lymphopenic situation inherent in the Rag-1−/− mouse model was reestablished providing a proliferative stimulus to residual donor T cells. Hence, nontransduced donor T cells infused with the immunoselected T-cell graft (<2.0%) proliferated extensively and had repopulated the recipient mice to a considerable extent by week 9. This observation reinforces the relevance of the purity of CD20op/tCD34-transduced T-cell population for clinical application. Clinical scale immunoselection of hematopoietic stem cells based on CD34 expression normally results in highly pure populations (>98%),38 which can be further enriched by a second selection round. Thus, the technology to purify tCD34-expressing T cells to almost homogeneity is available and will contribute to the safe application of gene-modified T cells.
RTX is an attractive antibody for the rapid and efficient depletion of CD20-expressing T cells as RTX is usually well tolerated,39 and the inevitable depletion of B cells that may delay full B-cell reconstitution in a transplantation setting, can be overcome by the infusion of hyperimmunoglobulins. Also RTX is widely used for the treatment of Epstein–Barr virus viremia, but according to our experience, the incidence of Epstein–Barr virus viremia after SCT is rare and thus the unwanted depletion of gene-modified T cells in cases where RTX has to be used for the treatment of Epstein–Barr virus represents only a minor problem. Indeed, targeting both the T- and B-cell responses in the treatment of GvHD may be clinically useful as various studies have reported significant improvements in patients suffering from GvHD after RTX treatment (reviewed by Kapur et al.40) and recently, a prophylactic effect of RTX against acute GvHD was suggested.41 The depletion of gene-modified T cells with RTX might thus have a yet underappreciated additional therapeutic effect by depleting B cells.
Although the actual mechanism of RTX action in vivo is still unclear, various reports from in vitro studies support a role for CDC and antibody-dependent cellular cytotoxicity42 and several apoptotic pathways have been implicated.30 In our experiments, the infusion of RTX led to a fast and efficient depletion of adoptively transferred T cells comparable to the elimination of B cells in patients suffering from non-Hodgkin lymphoma.43 Importantly, already a single dose of 150 µg RTX per mouse was effective and in line with previously published clinical data, clearance of CD20op-expressing T cells from the PB occurred within 2 hours.44 Additionally, we observed efficient depletion from spleen and lymph node tissue. Even though a high percentage of gene-modified T cells could be depleted in vivo, we could not demonstrate a complete clearance. One potential explanation may relate to the use of murine T cells, as binding of RTX to its epitope has been shown to be highly dependent on the conformation of the extracellular loop structure. Due to the possible absence of accessory proteins normally associated with human CD20, the stable binding of RTX might not be favored by murine cells.45 Given the inferior interaction of the human IgG1 constant region of RTX with murine effector cells,46 our system might thus be more potent in the human setting. Also resistances have been described for RTX-based therapies for non-Hodgkin lymphoma,47 but more potent, second generation anti-CD20 antibodies are currently under development that have been shown to outperform RTX by means of CDC and a superior clearance of B cells from secondary lymphoid tissues.46,48 Thus, these promising developments might additionally favor the concept of CD20 gene-modified T-cell donor lymphocyte infusion.
Taken together, our results using a codon-optimized sequence of CD20 in combination with tCD34 reinforce the suitability of a RTX-dependent strategy for the effective control of life-threatening GvHD in the context of immunotherapy after haploidentical SCT. In combination with current advances optimizing the functionality of gene-modified T cells, our RTX “safety switch” system is appropriate for clinical evaluation for an effective and safe application of adoptive immunotherapy.
Materials and Methods
Cell lines. Human embryonal kidney cells (293T) and murine embryonal fibroblasts (SC-1) were cultured in Dulbecco's modified Eagle's medium (Gibco, Eggenstein, Germany) supplemented with 10% fetal calf serum (Pan Biotech, Aidenbach, Germany), 2% -glutamine and 1% penicillin/streptomycin (both PAA, Pasching, Austria). The human T-cell line HuT 78 was cultured in Roswell Park Memorial Institute (Gibco) with supplements as described for Dulbecco's modified Eagle's medium.
Animals. C57BL/6.Ly5.1 [B6.SJL-PtprcaPepb/BoyJ (CD45.1)] and Rag-1-deficient mice [B6.129S7-Rag-1tm1Mom/J (CD45.2)] were obtained from Charles River Laboratories (Sulzfeld, Germany) and Jackson Laboratory (Bar Harbor, ME). Mice were bred in the animal facility of the Georg-Speyer-Haus and housed in individually ventilated cages under pathogen-free-like conditions. The experiments were performed in compliance with the local animal experimentation guidelines. Animal experiments were approved by the regional council (VI63-19c20/15-F123/21; Regierungspräsidium, Darmstadt, Germany).
Retroviral vectors. The M71tCD34tk39m(w) vector has been described previously14 and was used in this study without the woodchuck hepatitis post-transcriptional regulatory element. The CD20 complementary DNA was a kind gift of W. Wels (Georg-Speyer-Haus, Frankfurt, Germany)49 and cloned into the MP71 retroviral backbone.20 The codon-optimized CD20 complementary DNA (CD20op) was synthesized by GeneArt (Regensburg, Germany; accession number FN555175). Rarely used and thus rate-limiting codons were avoided to improve translational efficiency. Besides codon optimization, the GC content was increased from 43% (wild-type CD20 sequence) to 60% and a putative poly A site was removed as well as several RNA instability motifs. CD20op was inserted into the retroviral backbone M71 via NotI and SalI restriction sites resulting in the vector M71CD20op. The eGFP-encoding vector M71eGFPw was a kind gift of A. Schambach (Hannover Medical School, Germany). The construction of the M71CD20op/tCD34 vector was done by standard cloning techniques. Retroviral vector supernatants were produced using transient co-transfection of 293T cells according to standard protocols and titrated on HuT 78 or SC-1 cells.
Isolation and transduction of primary T cells. Primary human PB mononuclear cells were isolated from PB of healthy donors collected in EDTA Monovettes (Sarstedt, Nürnbrecht, Germany) after informed consent or from Buffy Coats (German Red Cross blood donor service, Frankfurt, Germany) by Pancoll density centrifugation (Pan Biotech). For the enrichment of T cells, PB was incubated with a RosetteSep human T-cell enrichment cocktail before gradient centrifugation according to the manufacturer's instruction (Stem Cell Technologies, Cologne, Germany). This method has the advantage of leaving the CD3/T-cell receptor complex unlabeled. Human PB mononuclear cells were cultured in X-Vivo 10 (Lonza, Verviers, Belgium) supplemented with 5% human AB serum, 2% -alanyl-glutamine (both from Sigma, Deisenhofen, Germany) and 1% penicillin/streptomycin in the presence of 100 U/ml IL-2 (Proleukin S; Chiron, Munich, Germany). T cells were activated using anti-CD3/anti-CD28 antibodies conjugated to magnetic beads at a 3:1 bead:T-cell ratio (Dynabeads CD3/CD28; Invitrogen, Karlsruhe, Germany) and cultured for 3 days before transduction. Primary murine mononuclear cells were isolated from C57BL/6.Ly5.1 donor mice. Cells were stimulated and cultured as described elsewhere21 and transduced 3 days after isolation. Transduction was performed on 2 consecutive days using RetroNectin (Takara Bio, Otsu, Japan) coated non-tissue-culture plates (BD Biosciences, Franklin Lakes, NJ) preloaded with viral supernatant before the addition of cells. After transduction, T cells were propagated at 1 × 106 cells/ml and 50 U/ml IL-2.
Immunoselection. At 70 hours after the last transduction, gene-modified cells were enriched by immunoselection. Before enrichment, magnetic beads used for activation of primary T cells were removed by a magnetic device. For positive selection, CD20 or CD34 MicroBead Kits (as indicated in Results) were used according to the manufacturer's instructions (Miltenyi Biotech, Bergisch-Gladbach, Germany). Purity of selected cells was analyzed by flow cytometry.
CDC assay. For complement-dependent depletion, 2 × 105 transduced and immunoselected HuT 78 cells were incubated with or without 10 µg/ml RTX (Roche, Basel, Switzerland) and varying concentrations of rabbit (Calbiochem, Darmstadt, Germany) or human serum (collected from PB of healthy donors) as a source of complement. After incubation for 4 hours at 37 °C, cells were stained with 7-aminoactinomycin D (BD Biosciences) to discriminate between viable and dead cells and analyzed by flow cytometry. Results are expressed as survival relative to untreated control.
Antibody-dependent cellular cytotoxicity assay. Autologous effector cells were isolated from PB mononuclear cells after CD3 depletion using CD56 positive selection (both MicroBead Kits from Miltenyi Biotech). Cells were cultured in X-Vivo 10 media supplemented with 2% human AB serum, 2% -alanyl-glutamine, 1% penicillin/streptomycin, and 1,000 U/ml IL-2 for 7–12 days before usage. Stimulated effector cells were added to 2 × 105 gene-modified T cells at 3:1 effector:target ratio in the presence or absence of 10 µg/ml RTX. After incubation for 24 hours, cells were harvested and stained for target and effector cells with anti-CD34, anti-CD56, and anti-CD3 antibodies. 7-Aminoactinomycin D was used to exclude dead cells. Cell counting beads (BD Biosciences) were added and acquisition was stopped after 2,500 gated bead events. The absolute number of CD3+CD34+ and 7-aminoactinomycin D cells was determined and results are expressed as the survival relative to untreated control.
Transplantation of gene-modified T cells in Rag-1-deficient mice and RTX treatment. Six- to 8-week-old Rag-1-deficient animals were infused with 0.5–1.0 × 107 gene-modified donor T cells (CD45.1) directly after immunoselection by injection into the tail vein. PB was collected from the tail at frequent intervals starting at week 2 and analyzed by flow cytometry for the presence of gene-modified T cells. Starting at week 5, animals received a three-dose treatment regimen consisting of 150 µg RTX each (in 200 µl phosphate-buffered saline) for 3 consecutive weeks. Herceptin (Roche) was used as a negative control antibody. Two days after each injection, PB was analyzed for the presence of transduced donor T cells. After an observation time of 17 weeks, animals were killed and cells were isolated from spleen and lymph nodes for further analysis.
Statistical analysis. Statistical analysis was performed with a two-tailed Student t-test for unpaired samples or a Mann–Whitney test (as indicated in the figure legends) using Prism Software (Graph Pad, San Diego, CA).
SUPPLEMENTARY MATERIAL Figure S1. Vector-transduced T cells maintain central memory phenotype and the capacity to respond to alloantigens. Figure S2. Depletion of gene-modified T cells from lymphoid tissues. Figure S3. Three-dose Rituximab regimen efficiently depletes gene-modified T cells from peripheral blood. Figure S4. Specific elimination of adoptively transferred donor T cells expressing CD20op and tCD34. Figure S5. Rag-1−/− mice develop a T cell-induced colitis. Supplementary Material and Methods.
Acknowledgments
We thank Hana Kunkel, Margarita Diaz, Janine Kimpel, Christian Wichmann, and Sybille Wehner for technical assistance and advice and Stefan Stein for helpful discussions. The presented publication is part of the PhD thesis of I.V. This study was supported by the Deutsche Forschungsgemeinschaft (Bonn, Germany) within the graduate study program GRK 1172 and the Verein “Hilfe für Krebskranke Kinder Frankfurt” e.V.
Supplementary Material
Vector-transduced T cells maintain central memory phenotype and the capacity to respond to alloantigens.
Depletion of gene-modified T cells from lymphoid tissues.
Three-dose Rituximab regimen efficiently depletes gene-modified T cells from peripheral blood.
Specific elimination of adoptively transferred donor T cells expressing CD20op and tCD34.
Rag-1−/− mice develop a T cell-induced colitis.
REFERENCES
- Aversa F, Tabilio A, Velardi A, Cunningham I, Terenzi A, Falzetti F, et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med. 1998;339:1186–1193. doi: 10.1056/NEJM199810223391702. [DOI] [PubMed] [Google Scholar]
- Ciceri F, Labopin M, Aversa F, Rowe JM, Bunjes D, Lewalle P, et al. Acute Leukemia Working Party (ALWP) of European Blood and Marrow Transplant (EBMT) Group. (2008A survey of fully haploidentical hematopoietic stem cell transplantation in adults with high-risk acute leukemia: a risk factor analysis of outcomes for patients in remission at transplantation Blood 1123574–3581. [DOI] [PubMed] [Google Scholar]
- Bader P, Kreyenberg H, Hoelle W, Dueckers G, Handgretinger R, Lang P, et al. Increasing mixed chimerism is an important prognostic factor for unfavorable outcome in children with acute lymphoblastic leukemia after allogeneic stem-cell transplantation: possible role for pre-emptive immunotherapy. J Clin Oncol. 2004;22:1696–1705. doi: 10.1200/JCO.2004.05.198. [DOI] [PubMed] [Google Scholar]
- Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, et al. European Group for Blood and Marrow Transplantation Working Party Chronic Leukemia. (1995Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients Blood 862041–2050. [PubMed] [Google Scholar]
- Dazzi F., and, Goldman JM. Adoptive immunotherapy following allogeneic bone marrow transplantation. Annu Rev Med. 1998;49:329–340. doi: 10.1146/annurev.med.49.1.329. [DOI] [PubMed] [Google Scholar]
- Bonini C, Ferrari G, Verzeletti S, Servida P, Zappone E, Ruggieri L, et al. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997;276:1719–1724. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- Cohen JL, Boyer O, Thomas-Vaslin V., and, Klatzmann D. Suicide gene-mediated modulation of graft-versus-host disease. Leuk Lymphoma. 1999;34:473–480. doi: 10.3109/10428199909058474. [DOI] [PubMed] [Google Scholar]
- Tiberghien P, Ferrand C, Lioure B, Milpied N, Angonin R, Deconinck E, et al. Administration of herpes simplex-thymidine kinase-expressing donor T cells with a T-cell-depleted allogeneic marrow graft. Blood. 2001;97:63–72. doi: 10.1182/blood.v97.1.63. [DOI] [PubMed] [Google Scholar]
- Fehse B, Ayuk FA, Kröger N, Fang L, Kühlcke K, Heinzelmann M, et al. Evidence for increased risk of secondary graft failure after in vivo depletion of suicide gene-modified T lymphocytes transplanted in conjunction with CD34+-enriched blood stem cells. Blood. 2004;104:3408–3409. doi: 10.1182/blood-2004-07-2813. [DOI] [PubMed] [Google Scholar]
- Ciceri F, Bonini C, Stanghellini MT, Bondanza A, Traversari C, Salomoni M, et al. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I–II study. Lancet Oncol. 2009;10:489–500. doi: 10.1016/S1470-2045(09)70074-9. [DOI] [PubMed] [Google Scholar]
- Traversari C, Marktel S, Magnani Z, Mangia P, Russo V, Ciceri F, et al. The potential immunogenicity of the TK suicide gene does not prevent full clinical benefit associated with the use of TK-transduced donor lymphocytes in HSCT for hematologic malignancies. Blood. 2007;109:4708–4715. doi: 10.1182/blood-2006-04-015230. [DOI] [PubMed] [Google Scholar]
- Berger C, Flowers ME, Warren EH., and, Riddell SR. Analysis of transgene-specific immune responses that limit the in vivo persistence of adoptively transferred HSV-TK-modified donor T cells after allogeneic hematopoietic cell transplantation. Blood. 2006;107:2294–2302. doi: 10.1182/blood-2005-08-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiberghien P. Use of suicide gene-expressing donor T-cells to control alloreactivity after haematopoietic stem cell transplantation. J Intern Med. 2001;249:369–377. doi: 10.1046/j.1365-2796.2001.00809.x. [DOI] [PubMed] [Google Scholar]
- Junker K, Koehl U, Zimmerman S, Stein S, Schwabe D, Klingebiel T, et al. Kinetics of cell death in T lymphocytes genetically modified with two novel suicide fusion genes. Gene Ther. 2003;10:1189–1197. doi: 10.1038/sj.gt.3301977. [DOI] [PubMed] [Google Scholar]
- Sato T, Neschadim A, Konrad M, Fowler DH, Lavie A., and, Medin JA. Engineered human tmpk/AZT as a novel enzyme/prodrug axis for suicide gene therapy. Mol Ther. 2007;15:962–970. doi: 10.1038/mt.sj.6300122. [DOI] [PubMed] [Google Scholar]
- Introna M, Barbui AM, Bambacioni F, Casati C, Gaipa G, Borleri G, et al. Genetic modification of human T cells with CD20: a strategy to purify and lyse transduced cells with anti-CD20 antibodies. Hum Gene Ther. 2000;11:611–620. doi: 10.1089/10430340050015798. [DOI] [PubMed] [Google Scholar]
- Serafini M, Manganini M, Borleri G, Bonamino M, Imberti L, Biondi A, et al. Characterization of CD20-transduced T lymphocytes as an alternative suicide gene therapy approach for the treatment of graft-versus-host disease. Hum Gene Ther. 2004;15:63–76. doi: 10.1089/10430340460732463. [DOI] [PubMed] [Google Scholar]
- van Meerten T, Claessen MJ, Hagenbeek A., and, Ebeling SB. The CD20/αCD20 ‘suicide' system: novel vectors with improved safety and expression profiles and efficient elimination of CD20-transgenic T cells. Gene Ther. 2006;13:789–797. doi: 10.1038/sj.gt.3302705. [DOI] [PubMed] [Google Scholar]
- Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, Vanin EF, et al. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving' 2A peptide-based retroviral vector. Nat Biotechnol. 2004;22:589–594. doi: 10.1038/nbt957. [DOI] [PubMed] [Google Scholar]
- Fehse B, Kustikova OS, Li Z, Wahlers A, Bohn W, Beyer WR, et al. A novel ‘sort-suicide' fusion gene vector for T cell manipulation. Gene Ther. 2002;9:1633–1638. doi: 10.1038/sj.gt.3301828. [DOI] [PubMed] [Google Scholar]
- Newrzela S, Cornils K, Li Z, Baum C, Brugman MH, Hartmann M, et al. Resistance of mature T cells to oncogene transformation. Blood. 2008;112:2278–2286. doi: 10.1182/blood-2007-12-128751. [DOI] [PubMed] [Google Scholar]
- van Meerten T, van Rijn RS, Hol S, Hagenbeek A., and, Ebeling SB. Complement-induced cell death by rituximab depends on CD20 expression level and acts complementary to antibody-dependent cellular cytotoxicity. Clin Cancer Res. 2006;12:4027–4035. doi: 10.1158/1078-0432.CCR-06-0066. [DOI] [PubMed] [Google Scholar]
- Moreno-Carranza B, Gentsch M, Stein S, Schambach A, Santilli G, Rudolf E, et al. Transgene optimization significantly improves SIN vector titers, gp91phox expression and reconstitution of superoxide production in X-CGD cells. Gene Ther. 2009;16:111–118. doi: 10.1038/gt.2008.143. [DOI] [PubMed] [Google Scholar]
- Yang S, Cohen CJ, Peng PD, Zhao Y, Cassard L, Yu Z, et al. Development of optimal bicistronic lentiviral vectors facilitates high-level TCR gene expression and robust tumor cell recognition. Gene Ther. 2008;15:1411–1423. doi: 10.1038/gt.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffioen M, van Egmond HM, Barnby-Porritt H, van der Hoorn MA, Hagedoorn RS, Kester MG, et al. Genetic engineering of virus-specific T cells with T-cell receptors recognizing minor histocompatibility antigens for clinical application. Haematologica. 2008;93:1535–1543. doi: 10.3324/haematol.13067. [DOI] [PubMed] [Google Scholar]
- Leisegang M, Engels B, Meyerhuber P, Kieback E, Sommermeyer D, Xue SA, et al. Enhanced functionality of T cell receptor-redirected T cells is defined by the transgene cassette. J Mol Med. 2008;86:573–583. doi: 10.1007/s00109-008-0317-3. [DOI] [PubMed] [Google Scholar]
- Tedder TF., and, Engel P. CD20: a regulator of cell-cycle progression of B lymphocytes. Immunol Today. 1994;15:450–454. doi: 10.1016/0167-5699(94)90276-3. [DOI] [PubMed] [Google Scholar]
- Li H, Ayer LM, Lytton J., and, Deans JP. Store-operated cation entry mediated by CD20 in membrane rafts. J Biol Chem. 2003;278:42427–42434. doi: 10.1074/jbc.M308802200. [DOI] [PubMed] [Google Scholar]
- Cragg MS, Morgan SM, Chan HT, Morgan BP, Filatov AV, Johnson PW, et al. Complement-mediated lysis by anti-CD20 mAb correlates with segregation into lipid rafts. Blood. 2003;101:1045–1052. doi: 10.1182/blood-2002-06-1761. [DOI] [PubMed] [Google Scholar]
- Deans JP, Li H., and, Polyak MJ. CD20-mediated apoptosis: signalling through lipid rafts. Immunology. 2002;107:176–182. doi: 10.1046/j.1365-2567.2002.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans JP, Kalt L, Ledbetter JA, Schieven GL, Bolen JB., and, Johnson P. Association of 75/80-kDa phosphoproteins and the tyrosine kinases Lyn, Fyn, and Lck with the B cell molecule CD20. Evidence against involvement of the cytoplasmic regions of CD20. J Biol Chem. 1995;270:22632–22638. doi: 10.1074/jbc.270.38.22632. [DOI] [PubMed] [Google Scholar]
- Lange C, Li Z, Fang L, Baum C., and, Fehse B. CD34 modulates the trafficking behavior of hematopoietic cells in vivo. Stem Cells Dev. 2007;16:297–304. doi: 10.1089/scd.2006.0056. [DOI] [PubMed] [Google Scholar]
- Bondanza A, Valtolina V, Magnani Z, Ponzoni M, Fleischhauer K, Bonyhadi M, et al. Suicide gene therapy of graft-versus-host disease induced by central memory human T lymphocytes. Blood. 2006;107:1828–1836. doi: 10.1182/blood-2005-09-3716. [DOI] [PubMed] [Google Scholar]
- Kaneko S, Mastaglio S, Bondanza A, Ponzoni M, Sanvito F, Aldrighetti L, et al. IL-7 and IL-15 allow the generation of suicide gene-modified alloreactive self-renewing central memory human T lymphocytes. Blood. 2009;113:1006–1015. doi: 10.1182/blood-2008-05-156059. [DOI] [PubMed] [Google Scholar]
- Frecha C, Costa C, Nègre D, Gauthier E, Russell SJ, Cosset FL, et al. Stable transduction of quiescent T cells without induction of cycle progression by a novel lentiviral vector pseudotyped with measles virus glycoproteins. Blood. 2008;112:4843–4852. doi: 10.1182/blood-2008-05-155945. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S., and, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Bryson JS, Zhang L, Goes SW, Jennings CD, Caywood BE, Carlson SL, et al. CD4+ T cells mediate murine syngeneic graft-versus-host disease-associated colitis. J Immunol. 2004;172:679–687. doi: 10.4049/jimmunol.172.1.679. [DOI] [PubMed] [Google Scholar]
- Koehl U, Bochennek K, Esser R, Brinkmann A, Quaritsch R, Becker M, et al. ISHAGE-based single-platform flowcytometric analysis for measurement of absolute viable T cells in fresh or cryopreserved products: CD34/CD133 selected or CD3/CD19 depleted stem cells, DLI and purified CD56+CD3− NK cells. Int J Hematol. 2008;87:98–105. doi: 10.1007/s12185-007-0018-7. [DOI] [PubMed] [Google Scholar]
- Kimby E. Tolerability and safety of rituximab (MabThera) Cancer Treat Rev. 2005;31:456–473. doi: 10.1016/j.ctrv.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Kapur R, Ebeling S., and, Hagenbeek A. B-cell involvement in chronic graft-versus-host disease. Haematologica. 2008;93:1702–1711. doi: 10.3324/haematol.13311. [DOI] [PubMed] [Google Scholar]
- Christopeit M, Schütte V, Theurich S, Weber T, Grothe W., and, Behre G. Rituximab reduces the incidence of acute graft-versus-host disease. Blood. 2009;113:3130–3131. doi: 10.1182/blood-2009-01-200527. [DOI] [PubMed] [Google Scholar]
- Glennie MJ, French RR, Cragg MS., and, Taylor RP. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol Immunol. 2007;44:3823–3837. doi: 10.1016/j.molimm.2007.06.151. [DOI] [PubMed] [Google Scholar]
- Maloney DG, Grillo-López AJ, White CA, Bodkin D, Schilder RJ, Neidhart JA, et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood. 1997;90:2188–2195. [PubMed] [Google Scholar]
- Harjunpää A, Wiklund T, Collan J, Janes R, Rosenberg J, Lee D, et al. Complement activation in circulation and central nervous system after rituximab (anti-CD20) treatment of B-cell lymphoma. Leuk Lymphoma. 2001;42:731–738. doi: 10.3109/10428190109099335. [DOI] [PubMed] [Google Scholar]
- Teeling JL, Mackus WJ, Wiegman LJ, van den Brakel JH, Beers SA, French RR, et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol. 2006;177:362–371. doi: 10.4049/jimmunol.177.1.362. [DOI] [PubMed] [Google Scholar]
- Beers SA, Chan CH, James S, French RR, Attfield KE, Brennan CM, et al. Type II (tositumomab) anti-CD20 monoclonal antibody out performs type I (rituximab-like) reagents in B-cell depletion regardless of complement activation. Blood. 2008;112:4170–4177. doi: 10.1182/blood-2008-08-172999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz C., and, Schuler M. Molecular mechanisms of resistance to Rituximab and pharmacologic strategies for its circumvention. Leuk Lymphoma. 2009;50:873–885. doi: 10.1080/10428190902878471. [DOI] [PubMed] [Google Scholar]
- Milani C., and, Castillo J. Veltuzumab, an anti-CD20 mAb for the treatment of non-Hodgkin's lymphoma, chronic lymphocytic leukemia and immune thrombocytopenic purpura. Curr Opin Mol Ther. 2009;11:200–207. [PubMed] [Google Scholar]
- Müller T, Uherek C, Maki G, Chow KU, Schimpf A, Klingemann HG, et al. Expression of a CD20-specific chimeric antigen receptor enhances cytotoxic activity of NK cells and overcomes NK-resistance of lymphoma and leukemia cells. Cancer Immunol Immunother. 2008;57:411–423. doi: 10.1007/s00262-007-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann SY, Esser R, Rohrbach E, Klingebiel T., and, Koehl U. A novel four-colour flow cytometric assay to determine natural killer cell or T-cell-mediated cellular cytotoxicity against leukaemic cells in peripheral or bone marrow specimens containing greater than 20% of normal cells. J Immunol Methods. 2005;296:63–76. doi: 10.1016/j.jim.2004.10.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Vector-transduced T cells maintain central memory phenotype and the capacity to respond to alloantigens.
Depletion of gene-modified T cells from lymphoid tissues.
Three-dose Rituximab regimen efficiently depletes gene-modified T cells from peripheral blood.
Specific elimination of adoptively transferred donor T cells expressing CD20op and tCD34.
Rag-1−/− mice develop a T cell-induced colitis.