Abstract
Helper-dependent adenoviral vectors (HDAd) are effective tools for liver-directed gene therapy because they can mediate long-term transgene expression in the absence of chronic toxicity. However, high vector doses required for efficient hepatocyte transduction by intravascular delivery result in systemic vector dissemination and dose-dependent activation of the innate immunity. Therefore, strategies to achieve high-efficiency hepatocyte transduction using low vector doses and/or to reduce the acute elevations of proinflammatory cytokines and chemokines may have significant clinical potential. Vasoactive intestinal peptide (VIP) is an endogenous neuropeptide involved in the regulation of hepatic blood flow and plays an important role as modulator of immune functions. Here, we show that VIP pretreatment in mice is able to increase hepatocyte transduction by HDAd, decrease vector uptake by the spleen, reduce elevation of proinflammatory serum cytokines interleukin (IL)-6 and IL-12, and reduce serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) following intravenous HDAd injection. VIP pretreatment also resulted in a reduction in the expression of the chemokines macrophage-inflammatory protein 2 (MIP-2), monocyte chemotactic protein 1 (MCP-1), and regulated on activation normal T-cell expressed and secreted (RANTES) in the livers of mice injected with HDAd. These results suggest that VIP can improve the therapeutic index of HDAd by increasing hepatocyte transduction efficiency while reducing cytokine and chemokine expression following intravascular delivery of HDAd.
Introduction
Helper-dependent adenoviral vectors (HDAd), which are devoid of all viral coding sequences, have demonstrated a tremendous potential for liver-directed gene therapy because they can transduce hepatocytes to direct long-term transgene expression in the absence of chronic toxicity.1,2 However, the high doses required for efficient hepatocyte transduction result in widespread vector dissemination as well as dose-dependent activation of the innate immune response resulting in acute toxicity with potentially severe and lethal consequences.3,4,5 This acute activation of the innate immune response is characterized by high levels of serum inflammatory cytokines and chemokines within a few hours postinjection.3 In order to overcome this major obstacle toward clinical implementation of HDAd gene therapy, new strategies are needed to achieve high-efficient hepatocyte transduction using low vector doses.
It has been shown that the size of liver sinusoidal endothelial fenestrae (SEF) (≤100 nm) is a critical determinant of hepatocyte transduction by adenoviral vectors with a virion diameter of ≥100 nm.6 The size of the SEF can be enlarged by means of drugs such as Na-decanoate6 or N-acetylcysteine combined with transient liver ischemia7 or by increasing the intrahepatic pressure by hydrodynamic injection.8 However, further studies are necessary to determine the real clinical potential of these drugs, and hydrodynamic injection as performed in rodents is not suitable for human application due to the rapid injection of a large volume.
Vasoactive intestinal peptide (VIP) is a 28-amino acid peptide originally described in the lung and small intestine that exerts a broad spectrum of actions including the control of liver, spleen, and intestine microcirculation and modulation of the innate and adaptive immunity.9,10,11 Previous studies have demonstrated that systemic VIP administration in rodents results in the enlargement of SEF mediated by an increase in liver blood flow and a concomitant reduction in the splenic microcirculation.12,13,14,15,16 Regarding its role in the innate immunity, VIP is released from immune cells during inflammatory reactions and inhibits the production and release of several proinflammatory cytokines and chemokines including tumor necrosis factor–α, interleukin (IL)-6, IL-12, regulated on activation normal T-cell expressed and secreted (RANTES), monocyte chemotactic protein 1 (MCP-1), and macrophage-inflammatory protein (MIP-2) from activated macrophages as well as negatively regulating the expression of Toll-like receptors.9,17,18,19,20,21 VIP exerts therapeutic effects by acting as a potent anti-inflammatory factor in many different disease models, including septic shock, rheumatoid arthritis, and inflammatory bowel disease.21,22,23,24 The mechanisms by which VIP exerts these immunological actions are mediated by the interaction with three different G-coupled receptors: VPAC1, VPAC2, and PAC1. Using a cyclic adenosine monophosphate-dependent pathway, VIP acts as a potent vasodilator whereas through cyclic adenosine monophosphate-dependent or independent pathways VIP negatively regulates downstream players involved in the innate and adaptive immune responses, including nuclear factor-κB, Jak1-2/STAT1/IRFs, ERK1/2, and p38/MAP kinases.9,17,18,24,25 It is well known that the ERK1/2, p38/mitogen-activated protein kinase pathways and nuclear factor-κB activation play a pivotal role in the adenovirus induction of the innate immune response.26,27,28 For these reasons we choose to investigate VIP pretreatment as a pharmacological approach to improve the therapeutic index of HDAd by increasing hepatocyte transduction efficiency and reducing the innate immune response.
Results
VIP increases hepatic transduction and reduces splenic vector uptake
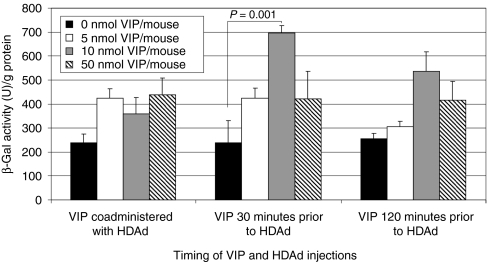
It has been reported that supraphysiologic doses of systemically injected VIP in rodents is able to enlarge SEF and increase the liver blood flow.14,15 Therefore, given the critical role of SEF in the efficiency of adenovirus-mediated liver transduction,6,7,8 we hypothesized that VIP might improve the efficiency of hepatocyte transduction by HDAd as a result of SEF enlargement. To test this hypothesis, various doses of VIP (0, 5, 10, 50 nmol/mouse) were administered to wild-type C57BL/6 mice by intraperitoneal injection. HDΔ28E4LacZ, a HDAd bearing a murine cytomegalovirus-LacZ expression cassette,3 at a dose of 1 × 1012 vector particles (vp)/kg, was administered by retro-orbital injection at the same time as VIP administration, or at 30 or 120 minutes after VIP administration. To determine hepatic transduction, livers were harvested 48 hours postvector, total proteins were extracted and the amount of β-galactosidase activity was determined. The results indicated that VIP increases the efficiency of hepatic transduction with the highest level of β-galactosidase activity achieved at the dose of 10 nmol/mouse given 30 minutes before HDΔ28E4LacZ administration; a 2.9-fold increase over no VIP (P = 0.01) (Figure 1). Therefore, for all subsequent experiments, 10 nmol VIP was given to each mouse by intraperitoneal injection 30 minutes before HDAd administration.
Figure 1.
Effect of timing and dose of VIP on liver transduction efficiency by HDΔ28E4LacZ. Hepatic β-galactosidase activity was determined 48 hours postinjection of 1 × 1012 vp/kg HDΔ28E4LacZ into C57BL/6 mice treated with VIP at various doses and times by intraperitoneal injection. Means ± SD shown (n = 5). HDAd, helper-dependent adenoviral vectors; VIP, vasoactive intestinal peptide; vp, vector particles.
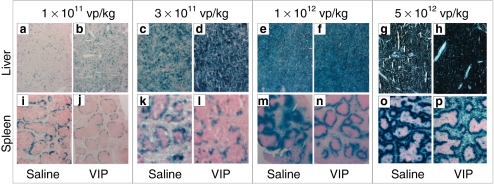
We next determined the effect of vector dose on hepatic transduction in mice pretreated with VIP. To accomplish this, saline or 10 nmol of VIP was administered to the mice and then, 30 minutes later, various doses of HDΔ28E4LacZ (1 × 1011, 3 × 1011, 1 × 1012, and 5 × 1012 vp/kg) were administered by retro-orbital injection and the livers were harvested 48 hours later for analyses. At 1 × 1011 and 3 × 1011 vp/kg, saline pretreatment (Figure 2a,c) yielded qualitatively less β-galactosidase positive cells than VIP pretreatment (Figure 2b,d) as determined by X-gal histochemistry. At the higher doses of 1 × 1012 and 5 × 1012 vp/kg, qualitative differences in the number of β-galactosidase positive cells were not distinguishable between saline (Figure 2e,g) or VIP (Figure 2f,h) pretreatment because all cells appeared β-galactosidase positive as determined by X-gal histochemistry, likely because these high doses were saturating. In contrast to the liver, VIP pretreatment at all doses appeared to yield qualitatively less β-galactosidase positive cells than saline pretreatment in the spleen as determined by X-gal histochemistry (Figure 2i–p).
Figure 2.
Liver and spleen transduction efficiency. X-gal histochemistry of the (a–h) liver or (i–p) spleen 48 hours following injection of various doses of HDΔ28E4LacZ into mice pretreated 30 minutes prior with either saline or VIP (10 nmol/mouse) by intraperitoneal injection. The results from one representative mouse from each treatment group is shown (n ≥ 5 per treatment). The liver and spleen shown for each treatment condition are from the same mouse. VIP, vasoactive intestinal peptide; vp, vector particles.
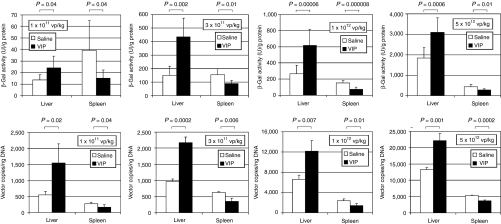
To quantitate the amount of transgene expression, total protein was extracted from the livers and the amount of β-galactosidase activity was determined by enzymatic assay. The results revealed that VIP pretreatment yielded higher activities than saline pretreatment in the liver at all doses (Figure 3); 1.8-fold higher at 1 × 1011 vp/kg (P = 0.04), 2.9-fold higher at 3 × 1011 vp/kg (P = 0.002), 2.3-fold higher at 1 × 1012 vp/kg (P = 0.00006), and 1.7-fold higher at 5 × 1012 vp/kg (P = 0.006). In contrast to the liver, saline pretreatment yielded higher activities than VIP pretreatment in the spleen at all doses (Figure 3); 2.6-fold higher at 1 × 1011 vp/kg (P = 0.04), 1.7-fold higher at 3 × 1011 vp/kg (P = 0.01), twofold higher at 1 × 1012 vp/kg (P = 0.000008), and 1.6-fold higher at 5 × 1012 vp/kg (P = 0.01). To determine the amount of vector genomes taken up by the liver, total DNA was extracted and subjected to quantitative real-time PCR to determine HDAd genome copies. The results revealed that VIP pretreatment yielded greater number of vector genomes per ng of liver DNA than saline pretreatment at all doses (Figure 3); 2.8-fold at 1 × 1011 vp/kg (P = 0.02), 2.2-fold at 3 × 1011 vp/kg (P = 0.0002), 1.8-fold at 1 × 1012 vp/kg (P = 0.007), and 1.7-fold at 5 × 1012 vp/kg (P = 0.001). In contrast to the liver, saline pretreatment yielded greater number of vector genomes per ng of spleen DNA than VIP pretreatment at all doses (Figure 3); 1.6-fold at 1 × 1011 vp/kg (P = 0.04), 1.8-fold at 3 × 1011 vp/kg (P = 0.006), 1.7-fold at 1 × 1012 vp/kg (P = 0.01), and 1.4-fold at 5 × 1012 vp/kg (P = 0.0002).
Figure 3.
β-Galactosidase activity and vector genome copy number in the livers and spleens of mice 48 hours following injection of various doses of HDΔ28E4LacZ into mice pretreated 30 minutes prior with either saline or VIP (10 nmol/mouse) by intraperitoneal injection. Means ± SD shown (n ≥ 5). Background β-galactosidase activity (no vector control) was 12.5 ± 2.5 U/g protein for the liver and 9.3 ± 0.5 U/g protein for the spleen. Vector genome was undetectable in no vector control animals. VIP, vasoactive intestinal peptide; vp, vector particles.
Taken together, these data show that VIP pretreatment improves liver transduction efficiency by HDAd while reducing splenic uptake of the vector.
VIP reduces HDAd-mediated acute cytokine activation and liver toxicity
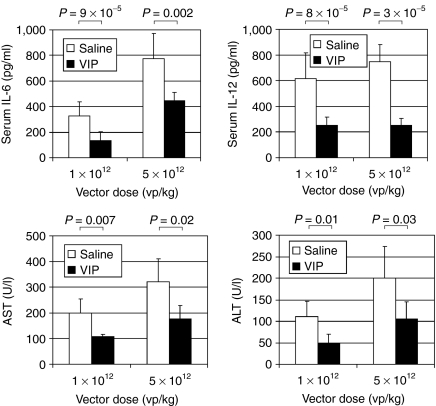
Systemic intravascular HDAd injection results in activation of the innate inflammatory response, marked by elevations of proinflammatory cytokines, the magnitude of which is dose dependent.3,26,29,30 Therefore, we next compared the serum levels of proinflammatory cytokines IL-6 and IL-12 in the mice injected with either 1 × 1012 or 5 × 1012 vp/kg presented above at 6 hours postinjection. For IL-6, the results revealed that saline pretreatment yielded higher levels than VIP pretreatment at both vector doses; 2.5-fold at 1 × 1012 vp/kg (P = 9 × 10−5) and 1.7-fold at 5 × 1012 vp/kg (P = 0.002) (Figure 4). Likewise, for IL-12, saline pretreatment yielded higher levels than VIP pretreatment at both vector doses; 2.4-fold at 1 × 1012 vp/kg (P = 8 × 10−6) and 2.9-fold at 5 × 1012 vp/kg (P = 3 × 10−5) (Figure 4). The two lower HDAd doses, 1 × 1011 and 3 × 1011 vp/kg, did not result in elevation of serum IL-6 and IL-12 over baseline values regardless of saline or VIP pretreatment (not shown).
Figure 4.
Serum levels of IL-6, IL-12, AST, and ALT from mice injected with either 1 × 1012 or 5 × 1012 vp/kg of HDΔ28E4LacZ pretreated 30 minutes prior with either saline or VIP (10 nmol/mouse) by intraperitoneal injection. IL-6 and IL-12 levels were measured 6 hours postvector. AST and ALT levels were measured 24 hours postvector. Means ± SD shown (n ≥ 5). Background levels were 11.2 ± 8.5 pg/ml for IL-6, 24.8 ± 2.8 pg/ml for IL-12, 43 ± 14.3 U/l for AST, and 29 ± 4.2 U/l for ALT. ALT, alanine aminotransferase; AST, aspartate aminotransferase; IL, interleukin; VIP, vasoactive intestinal peptide.
Systemic intravascular injection of HDAd also results in acute but transient hepatoxicity, the severity of which is also dose dependent. Therefore, we also compared the levels of serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in mice injected with 1 × 1012 or 5 × 1012 vp/kg presented above at 24 hours postinjection. For AST, the results revealed that saline pretreatment yielded higher levels than VIP pretreatment at both vector doses despite lower efficiencies of hepatic transduction; 1.8-fold at both 1 × 1012 vp/kg (P = 0.007) and 5 × 1012 vp/kg (P = 0.02) (Figure 4). For ALT, the results also showed that saline pretreatment yielded higher levels than VIP pretreatment at both vector doses despite lower efficiencies of hepatic transduction; 2.3-fold (P = 0.01) and 1.9-fold (P = 0.03) at 1 × 1012 and 5 × 1012 vp/kg, respectively (Figure 4). Taken together, these results indicate that VIP pretreatment reduces HDAd-mediated activation of the innate immune response and hepatotoxicity.
The livers and spleens were obtained from mice 24 hours after injection of HDAd at 5 × 1012 vp/kg with and without VIP pretreatment for hematoxylin and eosin histology. In the livers of four mice injected with HDAd without VIP pretreatment, multiple small clusters of acute parenchymal necrosis with no special zonal distribution and small foci with microsteatosis were observed in two animals (Supplementary Figure S1a), whereas in the other two animals a few foci of sinusoidal inflammation with virtually no necrosis or steatosis were observed (data not shown). Variation was observed in the livers of four mice injected with HDAd with VIP pretreatment. One animal had extensive ductular proliferation and small foci of hepatocyte or duct cell necrosis at the interface, as well as focal lobular necrosis, and hepatic vein endothelialitis (Supplementary Figure S1b). Another animal had similar abnormalities which were less extensive (data not shown). Two other animals had only focal cell necrosis without ductular proliferation (Supplementary Figure S1c). Animals injected with VIP alone had lobular inflammation and very rare single cell necrosis with very little focal steatosis (Supplementary Figure S1d). The spleens of all animals injected with HDAd with or without VIP pretreatment presented with similar findings; depletion of lymphocytes from interfollicular regions and depletion of megakaryocytes, as well as an increased number of neutrophils (Supplementary Figure S1e). The white pulp was unaffected. Spleens from animals injected with VIP alone appeared normal (Supplementary Figure S1f). These data show that histological abnormalities in the liver and spleen are associated with HDAd, with or without VIP pretreatment.
VIP downregulates chemokine expression in the liver
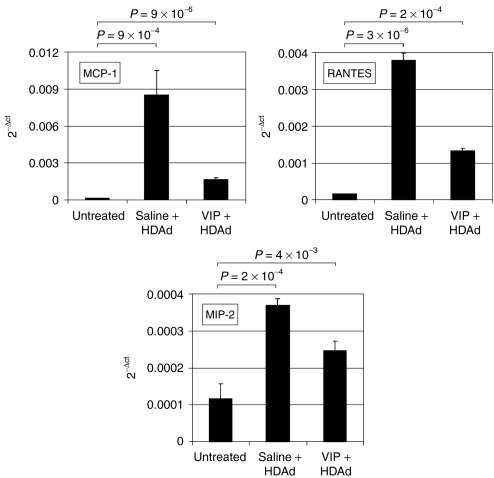
The liver is the major site of adenovirus vector deposition following intravascular administration causing an increased expression of chemokines in that organ.26 Therefore, we next analyzed the in vivo effect of VIP on hepatic chemokine expression following intravenous HDAd administration. To accomplish this, 1 × 1012 vp/kg of HDΔ28E4LacZ was administered into saline or VIP pretreated mice by retro-orbital injection. Three hours later, the livers were harvested and the expression levels of MCP-1, MIP-2, and RANTES were determined by quantitative real-time PCR. As shown in Figure 5, injection of HDΔ28E4LacZ into saline pretreated mice resulted in a 53-fold increase in hepatic MCP-1 expression compared to untreated mice (P = 9 × 10−4). In contrast, injection of HDΔ28E4LacZ into VIP pretreated mice resulted in only a tenfold increase in MCP-1 expression in the liver compared to untreated mice (P = 9 × 10−6) (Figure 5). Likewise, injection of HDΔ28E4LacZ into saline pretreated mice resulted in a 24-fold increase in hepatic RANTES expression compared to untreated mice (P = 3 × 10−6). In contrast, injection of HDΔ28E4LacZ into VIP pretreated mice resulted in only an 8.6-fold increase in RANTES expression compared to untreated mice. In the case of MIP-2, injection of HDΔ28E4LacZ resulted in a threefold increase in expression (P = 0.0002) compared to untreated mice. In contrast, injection of HDΔ28E4LacZ into VIP pretreated mice resulted in only a twofold increase in MIP-2 expression compared to untreated mice (P = 0.004). These data indicated that VIP pretreatment down regulates cytokine and chemokine expression in the liver following administration of HDAd in vivo.
Figure 5.
Expression levels of MCP-1, MIP-2, and RANTES in the livers of mice 3 hours after injection of 1 × 1012 vp/kg of HDΔ28E4LacZ into mice pretreated 30 minutes prior with either saline or VIP (10 nmol/mouse) by intraperitoneal injection. Shown as mean ± SD of three to four independent experiments, each performed in duplicate. HDAd, helper-dependent adenoviral vectors; MCP-1; monocyte chemotactic protein 1; MIP-2, macrophage-inflammatory protein 2; VIP, vasoactive intestinal peptide.
VIP pretreatment does not affect long-term transgene expression from HDAd
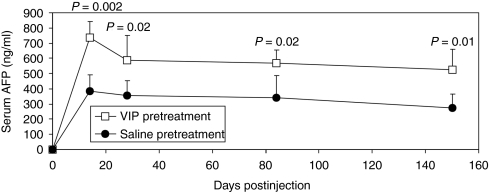
Long-term transgene expression is desirable feature of HDAds. Therefore, we next tested whether VIP pretreatment affects the duration of transgene expression from HDAd. To accomplish this, mice were first pretreated with VIP or saline and then 1 × 1011 vp/kg of HDΔ21.7-PEPCK-bAFP-WL was administered by retro-orbital injection. This vector expresses the α-fetoprotein (AFP) from a liver-specific promoter.31 As expected, VIP pretreatment yielded 1.7–1.9-fold higher levels of serum AFP than saline pretreated mice (P value 0.002–0.02) (Figure 6). Importantly, VIP pretreatment did not affect the duration of transgene expression because, like saline pretreatment, high levels of AFP were observed for the duration of the experiment of at least 150 days (Figure 6).
Figure 6.
The mean ± SD levels of serum AFP from C57BL/6 mice (n = 5) following injection of 1 × 1011 vp/kg of HDΔ21.7-PEPCK-bAFP-WL in VIP (10 nmol/mouse) or saline pretreated mice. AFP, α-fetoprotein; VIP, vasoactive intestinal peptide.
Discussion
HDAds have shown to be a very promising vector for liver-directed gene therapy. Several studies indicate that a single injection of HDAd can result in therapeutic levels of transgene expression that can be maintained for several years.1,31,32,33,34,35 However, the major limitation of these vectors is the acute toxicity occurring rapidly after systemic injection of high vector doses. This dose-dependent toxic response is due to the activation of the innate immunity, which may have lethal consequences.3 In this study, we found that VIP, a potent anti-inflammatory factor and vasodilator, increases liver transduction efficiency by HDAd, and attenuates vector-mediated acute toxicity. The improvement in liver transduction is presumably a consequence of the effect of VIP on liver blood flow and the increase in the SEF diameter.11,12,14,15 Oda et al. showed that the enlargement of the diameter of SEF can occur either as a secondary effect mediated by an increase in the portal blood flow or by a direct action on the liver sinusoidal endothelial cells.15 There are several potential additional mechanisms that could explain the enlargement of SEF, including the activation of cyclic adenosine monophosphate and the Ca2+-calmodulin–actomyosin system which is modulated by VIP.14,15 Interestingly, we found that splenic uptake of HDAd was also reduced by VIP. This effect may be due to the increased liver transduction resulting in less available vector for splenic uptake or a result of a specific action on the splenic microcirculation. As suggested from a previous study,12 it is possible that VIP reduces HDAd splenic uptake by decreasing the splenic blood flow through an active mechanism. According to previous studies, the spleen is a major organ involved in the release of some proinflammatory cytokines and chemokines after intravascular administration of adenovirus.36,37 Therefore, we speculate that reducing splenic uptake of HDAd may reduce the systemic release of cytokines. Although we did not determine the source(s) of cytokines in the serum, it is possible that the change in the vector biodistribution might account, at least in part, for the reduction in the cytokine and chemokines observed. Moreover, it is well known that in pathologic stimulation such as presence of lipopolysaccharide, VIP is released in vivo by activated T cells and interacts with VPAC1 or VPAC2, two G-coupled receptors present on the macrophage plasma membrane.19,38 The signaling cascade culminates in the repression of the mitogen-activated protein kinase/p38/ERK pathway and ultimately in the inhibition of nuclear factor-κB nuclear translocation, leading to reduction in promoter activation of several cytokines and chemokines.19,25,38,39 Because the mitogen-activated protein kinase/p38/ERK and nuclear factor-κB are key pathways involved in the innate immune response to adenovirus, it is possible that VIP pretreatment might repress these molecular mediators and attenuate the early inflammatory response initiated by HDAds. Previous studies have shown that adenovirus-induced cytokine/chemokine recruitment of immune effector cells into the liver results in hepatotoxicity.26,40 It is remarkable that although the liver transduction was increased by VIP, elevations in AST and ALT were concomitantly reduced. This finding appears inconsistent with previous studies.41,42 However, it is possible that the VIP can counteract the proinflammatory effect of HDAd by preventing the expression and/or release of chemokines and cytokines by Kupffer cells and/or other parenchymal cells of the liver. In our in vivo experiments, we were not able to exactly identify the cell types involved in the anti-inflammatory effects of VIP.
The ability of VIP to attenuate some aspects of the HDAd immune response suggests a new and unexpected role for this neuropeptide in the context of adenovirus-host interaction. It has been proposed that VIP, through its ability to induce the synthesis of interferons and to modulate the function of important immune cells such as dendritic cells and natural killer cells, plays a protective role against viral infection.43,44 VIP and other related neuroimmune mechanisms may therefore be involved in the modulation of immune responses to natural adenovirus infection by acting through these additional mechanisms. VIP is well tolerated in clinical studies in human volunteers45 and currently, there are several studies being conducted involving VIP as an immunosuppressant/vasodilator in chronic obstructive pulmonary disease, sepsis, asthma among others with several ongoing clinical trials in different phases with active recruitment (ClinicalTrials.gov identifiers: NCT00272896, NCT00464932, NCT00004494, NCT00255320).
In conclusion, our study shows that VIP pretreatment (i) increases liver transduction by HDAd, (ii) reduces splenic uptake of HDAd, and (iii) attenuates the HDAd-mediated innate immune response as evident by reduction in cytokine and chemokine expression, and attenuates HDAd-mediated hepatotoxicity as evident by reductions in AST and ALT. Thus, VIP pretreatment could represent a potentially useful strategy to improve the therapeutic index of HDAds.
Materials and Methods
Vectors. HDΔ28E4LacZ bears a murine cytomegalovirus-LacZ expression cassette46 and HDΔ21.7E4PEPCK-bAFP-WL contains a liver-restricted baboon AFP (bAFP) expression cassette and has been described previously.31 HDAd was produced in 116 cells47 with the helper virus AdNG163 as described elsewhere.48 Helper virus contamination levels were determined as described elsewhere47 and were found to be <0.05%. DNA analyses of HDAd genomic structure was confirmed as described elsewhere.47 All vector preparations were tested using Multi-test Limulus Amebocyte Lysate (Pyrogent; Biowhittaker, Walkersville, MD) for the presence of endotoxin and were found to be below the limit of detection (endotoxin <0.5 endotoxin units/ml).
Mice and injections. Nine- to twelve-week-old male C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME) were used for all the experiments. Intravenous HDAd vector administrations were performed in saline solution by retro-orbital injection with a volume of 200 µl. VIP (Calbiochem; EMD Bioscience, La Jolla, CA), resuspended in saline, was administered by intraperitoneal injection in a volume of 200 µl. For mice injected with HDΔ28E4LacZ, blood was collected retro-orbitally for analyses at 6 hours postinjection and at the time of sacrifice at 3 or 48 hours postinjection. Upon sacrifice, the liver and spleen were harvested and kept on dry ice or at −80 °C until analyses. For mice injected with HDΔ21.7E4PEPCK-bAFP-WL, blood was collected retro-orbitally weekly for analyses. Serum was frozen immediately and stored at −80°C until analyses.
Analyses of tissue and blood. X-gal histochemistry was performed on liver and spleen and from mice as described previously.8 Total protein was extracted from the liver and spleen and the β-galactosidase activity was determined using the β-Galactosidase Enzyme Assay System with Reporter Lysis Buffer (Promega, Madison, WI) following quantification using the Micro BCA Protein Assay Kit (Pierce, Rockford, IL). Total DNA was extracted from liver and spleen using the QIAamp DNA extraction kit (Qiagen, Valencia, CA) and quantitated by absorbance at 260 nm. Quantitative real-time PCR was performed using the LightCycler FastStart DNA Master SYBR Green I (Roche, Indianapolis, IN) in a total volume of 20 µl with 2 µl of template DNA, 4 mmol/l MgCl2, and 5 mol/l each HDAd-specific primer (5′-TCT GAA TAA TTT TGT GTT ACT CAT AGC GCG-3′ and 5′-CCC ATA AGC TCC TTT TAA CTT TTA AAG TC-3′). Cycling conditions consisted of 95 °C for 10 minutes followed by 45 cycles at 95 °C for 10 seconds, 60 °C for 7 seconds, and 72 °C for 20 seconds. Serial dilutions of a plasmid bearing the PCR target sequence were used as a control to determine the amounts of HDAd and results were analyzed with LightCycler software version 3.5 (Roche). Serum levels of IL-6 and IL-12 were determined by enzyme-linked immunosorbent assay (Biosource; Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Serum bAFP was measured by immunoassay, using antihuman AFP (ADVIA Centaur; Bayer Diagnostics, Tarrytown, NY). Total RNA from livers was isolated using RNeasy mini kit (Qiagen) according to manufacturer' instructions. Statistical analyses were performed with the t-test.
Reverse transcription and real-time quantitative PCR. Complementary DNA was synthesized from 1 µg of total RNA by reverse transcription using Superscript II according to the manufacturer's instruction (Invitrogen). Samples were then diluted 1:10 in nuclease free water and used as template for real-time quantitative PCR. Real-time quantitative PCR was performed using the Light Cycler Faststart DNA Master SYBR Green I (Roche) in a total volume of 20 µl with 2 µl of template DNA, 4 mmol/l MgCl2, and 5 µmol/l of each specific primer. Sequences of the mouse primer used in real-time PCR were as follows: MCP-1: 5′-CGG AAC CAA TGA GAT CAG AAC CTA C-3′ and 5′-AAT TAA GGC ATC ACA GTC CGA GTC AC-3'. MIP-2: 5′-GCC CAG ACA GAA GTC ATA GCC A-3′ and 5′-GAC AGC GAG GCA CAT CAG GTA CGA-3′. RANTES: 5′-AGG ATA GAG GGT TTC TTG ATT CTG-3′ and 5′-CAT TTT CCC AGG ACC GAG T-3′. GAPDH: 5′-CGA CCC CTT CAT TGA CC TCA ACT-3′ and 5′-GGC CTC ACC CCA TTT GAT GTT AG-3′. Results were obtained using the 2−ΔCT method (normalized for GAPDH as housekeeping gene). Cycling conditions consisted of 95 °C for 10 seconds, 50–65 °C for 7 seconds, and 72 °C for 20 seconds. The results were analyzed with LightCycler software version 3.5 (Roche).
SUPPLEMENTARY MATERIAL Figure S1. Hematoxylin and eosin histology of liver and spleen from mice 24 hours postinjection.
Acknowledgments
This work was supported by the National Institutes of Health R01 DK067324 (P.N.) and NIH R00 DK077447 (N.B.-P.). We thank the Morphology Core Laboratory of the Gulf Coast Digestive Disease Center and Dorene M. Rudman and Pamela Parson for enzyme histology.
Supplementary Material
Hematoxylin and eosin histology of liver and spleen from mice 24 hours postinjection.
REFERENCES
- Brunetti-Pierri N, Stapleton GE, Law M, Breinholt J, Palmer DJ, Zuo Y, et al. Efficient, long-term hepatic gene transfer using clinically relevant HDAd doses by balloon occlusion catheter delivery in nonhuman primates. Mol Ther. 2009;17:327–333. doi: 10.1038/mt.2008.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti-Pierri N., and, Ng P. Progress towards liver and lung-directed gene therapy with helper-dependent adenoviral vectors. Curr Gene Ther. 2009;9:329–340. doi: 10.2174/156652309789753310. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Palmer DJ, Beaudet AL, Carey KD, Finegold M., and, Ng P. Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Hum Gene Ther. 2004;15:35–46. doi: 10.1089/10430340460732445. [DOI] [PubMed] [Google Scholar]
- Morral N, O'Neal WK, Rice K, Leland MM, Piedra PA, Aguilar-Córdova E, et al. Lethal toxicity, severe endothelial injury, and a threshold effect with high doses of an adenoviral vector in baboons. Hum Gene Ther. 2002;13:143–154. doi: 10.1089/10430340152712692. [DOI] [PubMed] [Google Scholar]
- Nunes FA, Furth EE, Wilson JM., and, Raper SE. Gene transfer into the liver of nonhuman primates with E1-deleted recombinant adenoviral vectors: safety of readministration. Hum Gene Ther. 1999;10:2515–2526. doi: 10.1089/10430349950016852. [DOI] [PubMed] [Google Scholar]
- Lievens J, Snoeys J, Vekemans K, Van Linthout S, de Zanger R, Collen D, et al. The size of sinusoidal fenestrae is a critical determinant of hepatocyte transduction after adenoviral gene transfer. Gene Ther. 2004;11:1523–1531. doi: 10.1038/sj.gt.3302326. [DOI] [PubMed] [Google Scholar]
- Snoeys J, Lievens J, Wisse E, Jacobs F, Duimel H, Collen D, et al. Species differences in transgene DNA uptake in hepatocytes after adenoviral transfer correlate with the size of endothelial fenestrae. Gene Ther. 2007;14:604–612. doi: 10.1038/sj.gt.3302899. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Palmer DJ, Mane V, Finegold M, Beaudet AL., and, Ng P. Increased hepatic transduction with reduced systemic dissemination and proinflammatory cytokines following hydrodynamic injection of helper-dependent adenoviral vectors. Mol Ther. 2005;12:99–106. doi: 10.1016/j.ymthe.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Arranz A, Juarranz Y, Leceta J, Gomariz RP., and, Martínez C. VIP balances innate and adaptive immune responses induced by specific stimulation of TLR2 and TLR4. Peptides. 2008;29:948–956. doi: 10.1016/j.peptides.2008.01.019. [DOI] [PubMed] [Google Scholar]
- Chorny A, Gonzalez-Rey E, Ganea D., and, Delgado M. Vasoactive intestinal peptide generates CD4+CD25+ regulatory T cells in vivo: therapeutic applications in autoimmunity and transplantation. Ann N Y Acad Sci. 2006;1070:190–195. doi: 10.1196/annals.1317.011. [DOI] [PubMed] [Google Scholar]
- Richardson PD., and, Withrington PG. Liver blood flow. II. Effects of drugs and hormones on liver blood flow. Gastroenterology. 1981;81:356–375. [PubMed] [Google Scholar]
- Finke U., and, Seifert J. Differential effects of gastrointestinal hormones on the blood flow of the alimentary tract of the dog. Res Exp Med (Berl) 1986;186:151–165. doi: 10.1007/BF01851996. [DOI] [PubMed] [Google Scholar]
- Leister I, Mbachu EM, Post S, Samel ST, Stojanovic T, Gutt CN, et al. Vasoactive intestinal polypeptide and gastrin-releasing peptide attenuate hepatic microvasculatory disturbances following intestinal ischemia and reperfusion. Digestion. 2002;66:186–192. doi: 10.1159/000066761. [DOI] [PubMed] [Google Scholar]
- Oda M, Han JY., and, Yokomori H. Local regulators of hepatic sinusoidal microcirculation: recent advances. Clin Hemorheol Microcirc. 2000;23:85–94. [PubMed] [Google Scholar]
- Oda M, Azuma T, Watanabe N, Nishizaki Y, Nishida J, Ishii K, et al. Regulatory mechanisms of the hepatic microcirculation. Involvement of the contraction and dilatation of sinusoids and sinusoidal endothelial fenestrae. Prog Appl Microcirc. 1990;17:103–128. [Google Scholar]
- Braet F., and, Wisse E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp Hepatol. 2002;1:1. doi: 10.1186/1476-5926-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M., and, Ganea D. Inhibition of IFN-gamma-induced janus kinase-1-STAT1 activation in macrophages by vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. J Immunol. 2000;165:3051–3057. doi: 10.4049/jimmunol.165.6.3051. [DOI] [PubMed] [Google Scholar]
- Delgado M., and, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit nuclear factor-κB-dependent gene activation at multiple levels in the human monocytic cell line THP-1. J Biol Chem. 2001;276:369–380. doi: 10.1074/jbc.M006923200. [DOI] [PubMed] [Google Scholar]
- Delgado M, Gomariz RP, Martinez C, Abad C., and, Leceta J. Anti-inflammatory properties of the type 1 and type 2 vasoactive intestinal peptide receptors: role in lethal endotoxic shock. Eur J Immunol. 2000;30:3236–3246. doi: 10.1002/1521-4141(200011)30:11<3236::AID-IMMU3236>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Yin H, Cheng H, Yu M, Zhang F, Lin J, Gao Y, et al. Vasoactive intestinal peptide ameliorates synovial cell functions of collagen-induced arthritis rats by down-regulating NF-kappaB activity. Immunol Invest. 2005;34:153–169. [PubMed] [Google Scholar]
- Gomariz RP, Arranz A, Abad C, Torroba M, Martinez C, Rosignoli F, et al. Time-course expression of Toll-like receptors 2 and 4 in inflammatory bowel disease and homeostatic effect of VIP. J Leukoc Biol. 2005;78:491–502. doi: 10.1189/jlb.1004564. [DOI] [PubMed] [Google Scholar]
- Abad C, Martinez C, Juarranz MG, Arranz A, Leceta J, Delgado M, et al. Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of Crohn's disease. Gastroenterology. 2003;124:961–971. doi: 10.1053/gast.2003.50141. [DOI] [PubMed] [Google Scholar]
- Juarranz Y, Abad C, Martinez C, Arranz A, Gutierrez-Cañas I, Rosignoli F, et al. Protective effect of vasoactive intestinal peptide on bone destruction in the collagen-induced arthritis model of rheumatoid arthritis. Arthritis Res Ther. 2005;7:R1034–R1045. doi: 10.1186/ar1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez C, Abad C, Delgado M, Arranz A, Juarranz MG, Rodriguez-Henche N, et al. Anti-inflammatory role in septic shock of pituitary adenylate cyclase-activating polypeptide receptor. Proc Natl Acad Sci USA. 2002;99:1053–1058. doi: 10.1073/pnas.012367999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M., and, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase activating polypeptide inhibit the MEKK1/MEK4/JNK signaling pathway in LPS-stimulated macrophages. J Neuroimmunol. 2000;110:97–105. doi: 10.1016/s0165-5728(00)00359-3. [DOI] [PubMed] [Google Scholar]
- Muruve DA, Barnes MJ, Stillman IE., and, Libermann TA. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum Gene Ther. 1999;10:965–976. doi: 10.1089/10430349950018364. [DOI] [PubMed] [Google Scholar]
- McCaffrey AP, Fawcett P, Nakai H, McCaffrey RL, Ehrhardt A, Pham TT, et al. The host response to adenovirus, helper-dependent adenovirus, and adeno-associated virus in mouse liver. Mol Ther. 2008;16:931–941. doi: 10.1038/mt.2008.37. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Bowen GP, Wong NC, Libermann TA., and, Muruve DA. Adenovirus vector-induced expression of the C-X-C chemokine IP-10 is mediated through capsid-dependent activation of NF-kappaB. J Virol. 2000;74:3941–3947. doi: 10.1128/jvi.74.9.3941-3947.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell MA, Zhang Y, Tazelaar J, Gao GP, Yu QC, Qian R, et al. 2001Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors Mol Ther 35 Pt 1): 708–722. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chirmule N, Gao GP, Qian R, Croyle M, Joshi B, et al. 2001Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages Mol Ther 35 Pt 1): 697–707. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Ng T, Iannitti DA, Palmer DJ, Beaudet AL, Finegold MJ, et al. Improved hepatic transduction, reduced systemic vector dissemination, and long-term transgene expression by delivering helper-dependent adenoviral vectors into the surgically isolated liver of nonhuman primates. Hum Gene Ther. 2006;17:391–404. doi: 10.1089/hum.2006.17.391. [DOI] [PubMed] [Google Scholar]
- Kim IH, Józkowicz A, Piedra PA, Oka K., and, Chan L. Lifetime correction of genetic deficiency in mice with a single injection of helper-dependent adenoviral vector. Proc Natl Acad Sci USA. 2001;98:13282–13287. doi: 10.1073/pnas.241506298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toietta G, Mane VP, Norona WS, Finegold MJ, Ng P, McDonagh AF, et al. Lifelong elimination of hyperbilirubinemia in the Gunn rat with a single injection of helper-dependent adenoviral vector. Proc Natl Acad Sci USA. 2005;102:3930–3935. doi: 10.1073/pnas.0500930102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Nichols TC, McCorquodale S, Merricks E, Palmer DJ, Beaudet AL, et al. Sustained phenotypic correction of canine hemophilia B after systemic administration of helper-dependent adenoviral vector. Hum Gene Ther. 2005;16:811–820. doi: 10.1089/hum.2005.16.811. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Stapleton GE, Palmer DJ, Zuo Y, Mane VP, Finegold MJ, et al. Pseudo-hydrodynamic delivery of helper-dependent adenoviral vectors into non-human primates for liver-directed gene therapy. Mol Ther. 2007;15:732–740. doi: 10.1038/sj.mt.6300102. [DOI] [PubMed] [Google Scholar]
- Koizumi N, Yamaguchi T, Kawabata K, Sakurai F, Sasaki T, Watanabe Y, et al. Fiber-modified adenovirus vectors decrease liver toxicity through reduced IL-6 production. J Immunol. 2007;178:1767–1773. doi: 10.4049/jimmunol.178.3.1767. [DOI] [PubMed] [Google Scholar]
- Sakurai H, Tashiro K, Kawabata K, Yamaguchi T, Sakurai F, Nakagawa S, et al. Adenoviral expression of suppressor of cytokine signaling-1 reduces adenovirus vector-induced innate immune responses. J Immunol. 2008;180:4931–4938. doi: 10.4049/jimmunol.180.7.4931. [DOI] [PubMed] [Google Scholar]
- Delgado M., and, Ganea D. Inhibition of endotoxin-induced macrophage chemokine production by VIP and PACAP in vitro and in vivo. Arch Physiol Biochem. 2001;109:377–382. doi: 10.1076/apab.109.4.377.4237. [DOI] [PubMed] [Google Scholar]
- Delgado M., and, Ganea D. Vasoactive intestinal peptide inhibits IL-8 production in human monocytes by downregulating nuclear factor κB-dependent transcriptional activity. Biochem Biophys Res Commun. 2003;302:275–283. doi: 10.1016/s0006-291x(03)00149-9. [DOI] [PubMed] [Google Scholar]
- Muruve DA, Cotter MJ, Zaiss AK, White LR, Liu Q, Chan T, et al. Helper-dependent adenovirus vectors elicit intact innate but attenuated adaptive host immune responses in vivo. J Virol. 2004;78:5966–5972. doi: 10.1128/JVI.78.11.5966-5972.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayakhmetov DM, Li ZY, Ni S., and, Lieber A. Analysis of adenovirus sequestration in the liver, transduction of hepatic cells, and innate toxicity after injection of fiber-modified vectors. J Virol. 2004;78:5368–5381. doi: 10.1128/JVI.78.10.5368-5381.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi N, Kawabata K, Sakurai F, Watanabe Y, Hayakawa T., and, Mizuguchi H. Modified adenoviral vectors ablated for coxsackievirus-adenovirus receptor, αv integrin, and heparan sulfate binding reduce in vivo tissue transduction and toxicity. Hum Gene Ther. 2006;17:264–279. doi: 10.1089/hum.2006.17.264. [DOI] [PubMed] [Google Scholar]
- Azzari C, Rossi ME, Resti M, Caldini AL, Carbonella R, Ciappi S, et al. VIP restores natural killer cell activity depressed by hepatitis B surface antigen. Viral Immunol. 1992;5:195–200. doi: 10.1089/vim.1992.5.195. [DOI] [PubMed] [Google Scholar]
- Fabricius D, O'Dorisio MS, Blackwell S., and, Jahrsdörfer B. Human plasmacytoid dendritic cell function: inhibition of IFN-α secretion and modulation of immune phenotype by vasoactive intestinal peptide. J Immunol. 2006;177:5920–5927. doi: 10.4049/jimmunol.177.9.5920. [DOI] [PubMed] [Google Scholar]
- Hansen JM, Sitarz J, Birk S, Rahmann AM, Oturai PS, Fahrenkrug J, et al. Vasoactive intestinal polypeptide evokes only a minimal headache in healthy volunteers. Cephalalgia. 2006;26:992–1003. doi: 10.1111/j.1468-2982.2006.01149.x. [DOI] [PubMed] [Google Scholar]
- Palmer D., and, Ng P. Methods for the Production and Characterization of Helper-Dependent Adenoviral Vectors. Cold Spring Harbor Press: Cold Spring Harbor, NY; 2007. [DOI] [PubMed] [Google Scholar]
- Palmer D., and, Ng P. Improved system for helper-dependent adenoviral vector production. Mol Ther. 2003;8:846–852. doi: 10.1016/j.ymthe.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Palmer DJ., and, Ng P. Physical and infectious titers of helper-dependent adenoviral vectors: a method of direct comparison to the adenovirus reference material. Mol Ther. 2004;10:792–798. doi: 10.1016/j.ymthe.2004.06.1013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hematoxylin and eosin histology of liver and spleen from mice 24 hours postinjection.