Abstract
Members of the protein kinase C (PKC) family of serine-threonine kinases are important regulators of immune cell survival. Ingenol 3-angelate (PEP005) activates a broad range of PKC isoforms and induces apoptosis in acute myeloid leukemia cells by activating the PKC isoform PKCδ. We show here that, in contrast to its effect on leukemic cells, PEP005 provides a strong survival signal to resting and activated human T cells. The antiapoptotic effect depends upon the activation of PKCθ. This PKC isoform is expressed in T cells but is absent in myeloid cells. Further studies of the mechanism involved in this process showed that PEP005 inhibited activated CD8+ T cell apoptosis through the activation of NFκB downstream of PKCθ, leading to increased expression of the antiapoptotic proteins Mcl-1 and Bcl-xL. Transfection of CD8+ T cells with dominant-negative PKCθ diminished the prosurvival effect of PEP005 significantly. Ectopic expression of PKCθ in the acute myeloid leukemia cell line NB4 turned their response to PEP005 from an increased to decreased rate of apoptosis. Therefore, in contrast to myeloid leukemia cells, PEP005 provides a strong survival signal to T cells, and the expression of functional PKCθ influences whether PKC activation leads to an anti- or proapoptotic outcome in the cell types tested.
Keywords: Apoptosis, Cell/Apoptosis, Diseases/Cancer/Leukemia, Immunology, Immunology/Cellular Response, Phosphorylation/Kinases/Serine-Threonine
Introduction
Many cellular processes, including proliferation, differentiation, and apoptosis, are regulated by serine-threonine protein kinases belonging to the PKC2 isoenzyme family. Due to their structural variation and the resulting activation requirements of PKC family members, these have been categorized into three subclasses: The classical PKCs (α, βI, βII, and γ) are calcium-dependent and diacylglycerol and phorbol ester-responsive; novel PKCs (δ, ϵ, η, and θ) lack the C2 domain and thus are calcium-insensitive but retain responsiveness to diacylglycerol and phorbol ester; atypical PKCs (ζ, ι, and λ) also lack the C2 domain and do not bind diacylglycerol or phorbol ester/diacylglycerol (reviewed in Ref. 1). Depending on their activation requirements, subcellular localization, and substrate targets, the function of the PKC isoforms can be extremely diverse (2).
The ability of PKC activators to induce apoptosis in a wide range of leukemia-derived cell lines has made them attractive targets for the development of antileukemic drugs (3). In contrast to leukemic cells, activation of a broad range of PKC isoforms in T lymphocytes inhibits apoptosis and increases proliferation (4). Currently, this differential response to broad range PKC activators is not fully understood.
The novel PKC-activating agent ingenol-3-angelate (PEP005) has been identified as the active component of the sap of the plant Euphorbia peplus. This sap has been used in traditional medicine to treat skin conditions such as actinic keratosis (5) and basal cell carcinoma (6). PEP005 has been shown to activate a broad range of PKC isoforms and binds to the C1 domain of classical and novel PKC isoforms (7). More recently, it has been shown to induce apoptosis in a range of myeloid leukemia cell lines and in primary human acute myeloid leukemia cells (8) at concentrations in the low nanomolar range. The outcome of PKC activation depends on several factors, including the isoforms expressed and activated in a particular cell, their direction of translocation upon activation (dictating their substrate pool) and their down-regulation by proteasomal degradation (1). The proapoptotic effect of PEP005 on leukemia cells depends on the activation of PKCδ (8). In contrast, treatment of T cells with broad range activators results in their proliferation and survival. Expression of PKCδ is widespread, but T cells also express PKCθ, an isoform that is present at high levels only in T cells and myocytes (9). PKCθ is highly homologous to PKCδ, but the biological functions of these two isoforms differ considerably. While activation of PKCδ is commonly associated with proapoptotic effects (10), the outcome of PKCθ activation is generally antiapoptotic (11). Studies using PKCθ-deficient mice have demonstrated that PKCθ is an essential factor for the survival of activated primary T cells (11). PKCθ is a link in the chain of signals leading from T cell receptor (TCR) ligation to the activation of NFκB, an important regulator of T cell survival. PKCθ translocates to the site of contact with the antigen presenting cell, the immunological synapse (12, 13), and interacts with signalosome constituents, including CARMA1 (caspase recruitment domain membrane associated guanylate kinase 1), Bcl-10 (B cell lymphoma 10) and MALT 1 (mucosa-associated lymphoid tissue 1), as well as the IκB kinase complex. Within this complex, PKCθ phosphorylates CARMA1 (14) and is required to initiate NFκB activation (15, 16).
T cell survival is largely regulated by the availability of antiapoptotic factors. These survival factors exert their function through the regulation of expression and subcellular localization of pro and antiapoptotic members of the Bcl-2 family. Among these, particularly in T cells, Bim exerts its proapoptotic effect while antiapoptotic Bcl-2, Mcl-1, and Bcl-xL act as its counterparts (17, 18).
In the present study, we demonstrate the antiapoptotic effect of the broad range PKC activator PEP005 on activated and resting T cells. This activity contrasts with the proapoptotic response induced in myeloid leukemic cells. The data show that PEP005 induced survival of T cells and that this was dependent on activation of PKCθ and correlated with up-regulation of Mcl-1 and Bcl-xL. Furthermore, our work emphasizes the role of PKCθ in the divergent response of primary CD8+ T cells and myeloid leukemic cells to PKC activation.
EXPERIMENTAL PROCEDURES
Antibodies and Pharmacological Inhibitors and Activators
Antibodies used in isolation of CD8+ T cells by negative selection were mouse IgG-specific for CD4 (clone RFT4; Royal Free Hospital, London, UK), CD11b (OKM1), CD11c (BU15), CD19 (BU12; University of Birmingham), CD14 (UCHM1), CD16 (3G8), TCRγδ (IMMU510) (Beckman Coulter, High Wycombe, UK), and glycophorin A (GA-R2; BD Biosciences, Oxford, UK). Antibodies used in multiparameter cell sorting included CD8-PE (MEM-31, ImmunoTool), CD45RA-PECy5 (MEM56, Serotec), and CCR7-FITC (R&D Systems, Abingdon, UK). Mouse anti-CD95 antibody (CH11, Upstate) was used to induce apoptosis in Jurkat T cells. Goat polyclonal α-human IL-2 was used to neutralize IL-2 (Abcam, Cambridge, UK). PEP005 was supplied by Peplin, Inc., (Brisbane, Australia). Bisindolylmaleimide I (BisI), Gö6976, 3-(1-(3-imidazol-1-yl-propyl)-1H-indol-3-yl)-4-anilino-1H-pyrrole-2,5-dione, and myristoylated pseudosubstrate peptide inhibitor of PKCθ were used to inhibit PKC, classical PKC isoforms, PKCβ, and PKCθ, respectively (all from Calbiochem, Nottingham, UK). BAY11-7085 was used to inhibit NFκB (Alexis Biochemicals, Nottingham, UK).
Cell Isolation and Culture
Peripheral blood mononuclear cells were isolated by gradient centrifugation using Ficoll-Paque (Amersham Biosciences, Buckinghamshire, UK). CD8+ T cells were isolated by negative selection using antibodies specific for CD4, CD11b, CD11c, CD19, CD14, CD16, TCRγδ, and glycophorin A. Goat anti-mouse IgG antibody-coated MACS® microbeads and MACS® LS columns were used to remove labeled cells (Miltenyi Biotech). The remaining CD8+ T cells routinely were >96% pure. CD8+ T cells were cultured in complete medium (RPMI 1648; Sigma-Aldrich) with 10% v/v heat-inactivated fetal calf serum (Biosera), 100 μg/ml streptomycin, 60 μg/ml pencillin, 2 mm l-glutamine, and 10 mm HEPES (all from Sigma-Aldrich)) containing 50 units/ml IL-2 (Proleukin), 5 × 104/ml gamma-irradiated Epstein-Barr virus-transformed lymphoblastoid cells, and 9 μg/ml phytohemagglutinin (HA15-PHA, Biostat). Cells were then incubated at 37 °C with an atmosphere of 5% CO2 and maintained in complete medium containing 50 units/ml IL-2. Cells were restimulated with the same protocol weekly and were used for experiments on day 7 after stimulation. The leukemic cell line NB4 (DSMZ ACC 207) was purchased from the DSMZ (Braunschweig, Germany). Cells were maintained in complete medium, RPMI 1640 (Sigma-Aldrich) supplemented with 10% (v/v) heat-inactivated fetal calf serum, 100 μg/ml streptomycin, 100 units/ml penicillin, 2 mm l-glutamine, and 10 mm HEPES (all from Sigma-Aldrich), at 37 °C and in a humidified 5% CO2 atmosphere.
Survival and Apoptosis Assays
For assessment of survival, CD8+ T cells were cultured at 1 × 106 cells/ml in serum-free medium with RPMI 1640, 2 mm glutamine, 100 units/ml penicillin G, 10 μg/ml streptomycin, 10 mm HEPES, 1 mm sodium pyruvate, 1% (v/v) nonessential amino acids, 52.5 μg/ml leucine, ITS+3 medium supplement (10 μg/ml insulin, 5.5 μg/ml transferrin, 5 ng/ml selenium, 0.5 mg/ml bovine serum albumin, 4.7 μg/ml linoleic acid, and 4.7 μg/ml oleic acid) (all from Sigma-Aldrich), in the presence or absence of PEP005. Jurkat T cells and NB4 cells were cultured at 1 × 106 cells/ml in serum-free medium, in the presence of absence of PEP005. Apoptosis was assessed by flow cytometric detection of activated caspase-3. Briefly, cells were fixed and permeabilized using Caltag Fix and Perm kit (Caltag Laboratories, Inc.), and activated caspase-3 was detected using a rabbit antibody specific for the active caspase-3 (BD Biosciences) and revealed with an a goat anti-rabbit heavy and light chain fluorescein isothiocyanate-conjugated secondary antibody (Southern Biotechnology Associates, Inc.).
Simultaneous Detection of Proliferation and Apoptosis
CD8+ T cells were cultured as described above for survival assays with or without PEP005 at 20 nm. At 24 h from the start of each experiment, cells were pulsed with 10 μm of bromodeoxyuridine (BrdUrd, BD Biosciences) for 2 h in dark at 37 °C. Cells were then washed twice in culture medium and resuspended in 200 μl of serum-free medium with and without PEP005 corresponding to their original culture conditions. At 48 h, cells were resuspended and washed once in PBS and stained for BrdUrd and active caspase-3 using the BD BrdUrd flow kit (BD Biosciences). Briefly, cells were resuspended in 50 μl Cytofix/Cytoperm buffer and incubated at room temperature for 20 min, followed by one washing step using 200 μl of 1× Perm/Wash buffer. Cells were then resuspended in 50 μl of Cytoperm plus buffer and incubated on ice for 10 min before incubation with 50 μl of Cytofix/Cytoperm buffer at room temperature for 20 min, followed by one washing step. Each sample of cells was resuspended in 30 μl of 30 μg/μl DNase I plus 20 μl PBS and incubated at 37 °C for 1 h. Cell were washed once in 200 μl of 1× Perm/Wash buffer and then resuspended in 50 μl of 1× Perm/Wash buffer plus 10 μl of diluted mouse anti-BrdUrd-APC (allophycocyanin) and rabbit antiactive caspase-3 monoclonal antibody in 1× Perm/Wash buffer. Cells were incubated in the dark at room temperature for 20 min followed by one washing step. Cells were then resuspended in 50 μl of goat anti-rabbit heavy and light chain fluorescein isothiocyanate secondary antibody in 2% bovine serum albumin/PBS and incubated in the dark at room temperature for 30 min before washing and analysis on a FACSCaliburTM flow cytometer (BD Biosciences).
Isolation of Human CD8+ T Cell Subsets by Multiparameter Cell Sorting and Survival Assays
CD8+ T cell subpopulations were purified by multiparameter cell sorting on a Coulter MoFloTM cell sorter. Cells expressing a high level of CD8 were chosen for further gating for isolation of different CD8+ T cell subsets. Four subsets of CD8+ T cells, namely, naïve, central memory, effector memory and CD45RA-expressing effector memory cells were isolated by their corresponding phenotypes identified by expression of CD45RA and CCR7. Purity of the sorted cells routinely was >95%. Harvested subpopulations were cultured in serum-free medium at 1 × 106 cells/ml and in the presence/absence of 20 nm PEP005. On day 7, the percentage of apoptosis was analyzed by flow cytometry for active caspase-3.
Western Blotting
2 × 105 T cells were lysed in SDS-PAGE sample buffer, and total cellular proteins were separated by SDS-PAGE and transferred to polyvinylidene difluoride membrane (Geneflow, Fradley, UK). Blots were blocked in 5% (w/v) nonfat milk powder and 0.1% (v/v) Tween 20 in Tris-buffered saline (10 mm Tris, pH 7.5, 150 mm NaCl). Primary antibodies used were mouse anti-Bcl-xL (2H12, BD Pharmingen, Oxford, UK), rat anti-Bim (14A8, Calbiochem, Nottingham, UK), rabbit anti-Mcl-1 (S-19, Santa Cruz Biotechnology, Inc., Heidelberg, Germany), mouse anti-PKCθ (R&D Systems), and β-actin (AC74, Sigma). Horseradish peroxidase-linked sheep anti-mouse IgG, donkey anti-rabbit IgG, and goat anti-rat IgG were used as secondary antibodies.
Immunoprecipitation of Activated PKCθ
15 × 106 activated CD8+ T cells were were first treated either with or without 10 μm myristoylated pseudosubstrate peptide inhibitor of PKCθ at 37 °C for 30 min and then either with or without 20 nm PEP005 at 37 °C for 15 min. Cells were washed twice in ice-cold PBS and resuspended in 1 ml of lysis buffer (5 mm sodium orthovanadate, 5 mm sodium pyrophosphate, 5 mm sodium fluoride, 5 mm EDTA, 50 mm sodium chloride, 2% (v/v) Igepal, 50 mm HEPES, pH 7.4, protease inhibitor mixture, which contains 1.04 mm 4-(2-aminoethyl)benzenesulphonyl fluoride chloride (AEBSF), 800 nm aprotinin, 20 μm leupeptin, 40 μm bestatin, 15 μm pepstatin A, and 14 μm E-64) and incubated on ice for 30 min. Cell debris was removed by centrifugation for 10 min at 10,000 × g at 4 °C. Cell lysates were then incubated with 5 μl of rabbit polyclonal pThr-219 PKCθ antibody raised against phosphothreonine-containing peptide sequence, NH2-INSREpThr-219MFHKE-COOH, coupled to keyhole limpet hemocyanin (KLH) (kind gift from Dr. Gottfried Baier, Innsbruck Medical University), overnight at 4 °C. Protein G microbead suspension (Miltenyi Biotec, Surrey, UK) was used to label the immune complex at 4 °C for 1 h. pThr-219PKCθ-specific immunocomplexes were isolated by separation columns attached to a μMACS separator (both from Miltenyi Biotec, Surrey, UK) with four washes of lysis buffer and one wash of 20 mm TrisHCl, pH 7.5 and eluted with hot (95 °C) SDS loading buffer. Unbound cell lysate was mixed with 0.2 volumes of 5-fold concentrated SDS loading buffer and kept for analysis of β-actin as a control for equal cell input.
Transfection of CD8+ T Cells with Kinase-inactive PKCθ
CD8+ T cells were isolated by negative selection using a CD8+ T cell isolation kit from Miltenyi Biotech. The isolated cells were routinely >95% CD8+ T cells and were transfected using the AMAXA T cell transfection kit. Briefly, 2 × 106 cells were resuspended in 100 μl of nucleofector solution V and mixed with 1 μg of pmaxGFP and 5 μg of pEFPKCθK/R (19). Cells were electroporated with a Nucleofector II device (Lonza, Germany) using program U-014. 500 μl of prewarmed transfection culture medium (RPMI 1640 supplemented with 2 mm l-glutamine, 10 mm HEPES, 10% (v/v) fetal calf serum and adjusted to 4.5g/liters glucose) was added to cells and transferred to 1.5 ml prewarmed culture medium. Transfected cells were incubated at 37 °C, 5% CO2 for 6 h and then incubated in complete medium for 24 h in the presence or absence of 20 nm PEP005. Levels of apoptosis were assessed by staining for active caspase-3 as described above. A phycoerythrin-labeled secondary goat anti-rabbit antibody was used for detection. For analysis of transfected cells, GFP-expressing cells were selected.
Transfection of NB4 Cells with PKCθ
An empty plasmid (pEFneo) and a plasmid encoding wild-type PKCθ (pEFwtPKCθneo) were kind gifts from Dr. Gottfried Baier (University of Innsbruck). pmaxGFP (0.5 μg/μl) is provided in the Cell Line Nucleofector Kit V to monitor transfection efficiency and cell sorting of transfected cells. Transfection was performed using the Cell Line Nucleofector Kit V (Lonza). Briefly, 2 × 106 cells were resuspended in 100 μl Nucleofector solution V and mixed with 1 μg pmaxGFP and either 1.5 μg pEFneo or pEFwtPKCθneo. Cells were electroporated with a Nucleofector II device (Lonza) using the program X-001. 500 μl of prewarmed transfection culture medium (RPMI 1640 supplemented with 2 mm l-glutamine, 10 mm HEPES, and 10% (v/v) fetal calf serum and adjusted to 4.5 g/liters glucose) was added to cells and transferred to 1.5 ml of prewarmed culture medium. Transfected cells were incubated at 37 °C, 5% CO2 for 6 h. GFP-positive transfected cells were isolated using a MoFloTM cell sorter (Beckman Coulter) with a purity of 97% and then incubated in complete medium for 10 h in the presence or absence of 20 nm PEP005. Levels of apoptosis were assessed by staining for active caspase-3.
Detection of NFκB (p65) Activation
CD8+ T cells were treated in the conditions stated, and nuclear extracts were obtained with a commercial kit (Active Motif). Briefly, cells were washed twice in PBS-based phosphatase inhibitor buffer (PBS/PIB; 6.25 mm sodium fluoride, 12.5 mm β-glycerophosphate, 12.5 mm para-nitrophenyl-phosphate, and 1.25 mm sodium vanadate and then resuspended in hypotonic buffer (20 mm HEPES, pH 7.5, 5 mm sodium fluoride, 10 μm sodium molybdate, and 0.1 mm EDTA) at 56.8 μl/106 cells and incubated on ice for 15 min. Nonidet P-40 was added to a final concentration of 0.5% (v/v), and samples were mixed. Nuclear fractions were harvested by centrifugation at 14,000 × g at 4 °C for 30 s. Nuclear pellets were resuspended in lysis buffer (5 mm dithiothreitol, protease inhibitor mixture, and lysis buffer AM2, supplied in kit) at 5.68 μl/106cells and agitated on ice at 150 rpm for 30 min. Nuclear extracts were harvested after centrifugation at 14,000 × g at 4 °C for 10 min. Protein concentration of nuclear extracts was determined by BCATM protein quantification kit (Perbio Science, Cramlington, UK). Activated p65 was quantified using the TransAM p65 activation kit (Active Motif). Briefly, 10 μg of nuclear extract was plated on microwells coated with DNA oligonucleotides containing activated p65 consensus binding elements. After binding and washing, an antibody specific for DNA-bound p65 was added. An anti-rabbit IgG horseradish peroxidase antibody was used for signal detection and quantification.
Immunofluorescence and Quantification of p65 in CD8+ T Cells
2 × 106/ml CD8+ T cells in serum-free medium were first treated with or without 10 μm myristoylated pseudosubstrate peptide inhibitor of PKCθ at 37 °C for 30 min and then with or without 20 nm PEP005 for 15 min. Cells were transferred to glass slides in a cytocentrifuge (Shandon). Cells were fixed in 4% formaldehyde in PBS (v/v) at room temperature and permeabilized in absolute methanol at −20 °C. Blocking was performed with 10% horse serum (v/v) in PBS for 1 h. Monoclonal rabbit anti-p65 (clone C22B4, Cell Signaling) diluted in 1% bovine serum albumin/PBS (w/v) was applied for 1 h followed by three washes with PBS. TRITC-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories) was applied for 1 h followed by three washes in PBS. The nuclei were counterstained with Hoechst 33258 at 20 μg/ml. Images were acquired using a confocal immunofluorescence microscope (Zeiss LSM 510 based on a Zeiss Axiophot M100). Contrast and brightness were enhanced using Zeiss LSM software. Mean pixels per μm2 of total p65 staining and p65 staining co-localized with Hoechst in unprocessed images of 10 cells for each slide were quantified using the LSM 510 software (Zeiss).
Statistical Analysis
Data presented here represent a minimum of three experiments and, where appropriate, data are expressed as mean ± S.D. Statistical significance was assessed by paired two-tail Student's t test, and p < 0.05 was taken as a significantly different value. Multiple comparisons were analyzed for statistical significance by one and two-way analysis of variance, as appropriate. An asterisk denotes p < 0.05, whereas ns denotes p > 0.05.
RESULTS
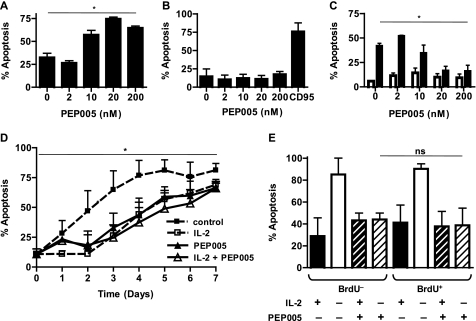
The plant-derived PKC activator PEP005 initiates apoptosis in a range of cell lines derived from myeloid leukemia, including NB4, as well as primary leukemic cells from patients with acute myeloid leukemia (8). As shown in Fig. 1a, we confirm that apoptosis is triggered in NB4 cells at nanomolar concentrations of PEP005. In contrast, PEP005 did not affect the survival of Jurkat cells (Fig. 1b), a human T cell leukemia cell line (20). Activated human T cells enter apoptosis spontaneously if deprived of survival factors such as IL-15 or IL-2. This spontaneous entry into the apoptotic pathway can be inhibited by PEP005 (Fig. 1c) in normal activated human T cells. In the experiment shown in Fig. 1c, activated CD8+ T cells were exposed to PEP005 in the absence and presence of 25 units/ml IL-2. The same concentrations of PEP005 that induced apoptosis in NB4 cells significantly inhibited apoptosis of IL-2 deprived CD8+ T cells. Survival of activated CD4+ T cells was affected to a similar extent, in the presence of fetal calf serum apoptosis was delayed, but the antiapoptotic effect of PEP005 was not affected (data not shown). Time course experiments shown in Fig. 1d revealed that this is not a permanent block of apoptosis but rather a delay of apoptosis to the same extent as addition of optimal concentrations of IL-2. Detection of an epitope specific for activated caspase 3 was used as a readout for apoptosis. Mitochondrial depolarization was assessed by detection of JC-1 incorporation in parallel experiments, yielding consistent results (data not shown).
FIGURE 1.
PEP005 induces apoptosis in NB4 cells and does not affect survival of Jurkat cells but inhibits apoptosis of activated CD8+ T cells. NB4 (A) and Jurkat T (B) cells were treated with indicated concentrations of PEP005, and levels of apoptosis were analyzed by intracellular staining of active caspase-3 on day 2. C, activated CD8+ T cells were treated in the presence (empty bars) and absence of 25 units/ml IL-2 (filled bars) and/or indicated concentrations of PEP005. Levels of apoptosis were analyzed by intracellular staining of active caspase-3 on day 2. D, survival assays were set up similarly to C with an extended time course to day 7. dashed line and filled squares, untreated cells; dashed line and empty squares, cells treated with 25 units/ml IL-2, solid line and empty triangles, cells treated with 20 nm PEP005 with 25 units/ml IL-2; and solid line and filled triangles, cells treated with 20 nm PEP005 without 25 units/ml IL-2. E, simultaneous detection of proliferation and apoptosis by BrdUrd/caspase-3 double staining shows no difference between the effect of PEP005 on proliferating cells compared with nonproliferating cells. CD8+ T cells were cultured as described above for survival assays with and without 25 units/ml IL-2 and/or 20 nm PEP005. At 24 h from the start of each experiment, cells were pulsed with BrdUrd for 2 h. At 48 h, cells were labeled with antibodies to BrdUrd and activated caspase-3. BrdUrd-positive and -negative cells were gated, and the percentage of cells with activated caspase-3 is shown in E. Data are mean ± S.D. from four separate experiments. An asterisk denotes p < 0.05, whereas ns denotes p > 0.05.
The effects of many modulators of apoptosis are restricted to cells in the active phases of the cell cycle (21). Therefore, we tested whether the effect of PEP005 was restricted to proliferating T cells. For this purpose, an assay was developed to distinguish between cells that had been actively proliferating during a 2-h time window in which the cells were exposed to BrdUrd. At the end of a 48-h incubation period, the cells were stained for active caspase-3 and for incorporation of BrdUrd. As shown in Fig. 1e, PEP005 inhibited apoptosis both in T cells that had been actively proliferating during the BrdUrd exposure and cells that had not been in S-phase. We conclude from these experiments that PEP005 does not preferentially affect proliferating T cells.
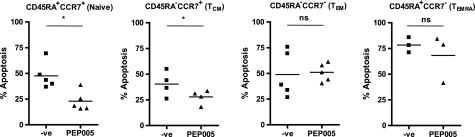
Although the initial experiments focused on the effects of PEP005 on in vitro-activated CD8+ T cells, the next series of experiments was directed at the effects of PEP005 on freshly isolated resting human CD8+ T cells. Depending on the parameters used, these cells can be subdivided into a number of functionally distinct subpopulations (22). Co-labeling with antibodies detecting CCR7 and CD45RA allows distinction of four subsets: naïve CD8+ T cells coexpressing CCR7 and CD45RA, central memory T cells expressing CCR7 but not CD45RA, effector memory T cells negative for both CCR7 and CD45RA, and a smaller subset of T cells expressing CD45RA but not CCR7 (Fig. 2). Sorting and culturing these cells in the presence and absence of PEP005 showed that although naïve and central memory T cells had a significant increase in their survival, there was no significant change in the apoptosis of effector memory or CCR7−CD45RA+CD8+ T cells.
FIGURE 2.
PEP005 inhibits apoptosis in resting peripheral blood naive and effector memory CD8+ T cells ex vivo. Resting human peripheral blood CD8+ T cells were sorted according to their expression of CD45RA and CCR7. Ex vivo sorted CD8+ T cells were cultured in serum-free medium in the presence/absence (-ve) of PEP005, and levels of apoptosis were analyzed by intracellular staining of active caspase-3 on day 7. Data show mean of a minimum of three separate experiments. TCM, central memory; TEM, effector memory; TEMRA, CD45RA-expressing effector memory. An asterisk denotes p < 0.05, whereas ns denotes p > 0.05.
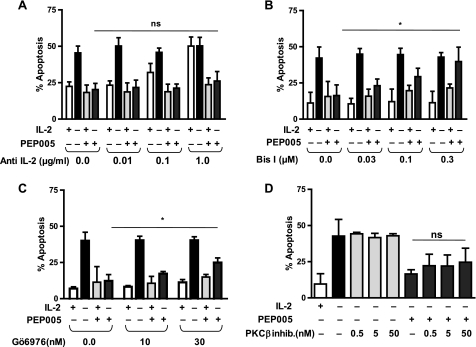
T cells stimulated to produce IL-2 can extend their own survival and proliferation by an autocrine loop (23). To test whether such an autocrine loop was mediating the antiapoptotic effect of PEP005 on T cells, a blocking antibody to IL-2 was added to the cultures. As shown in Fig. 3a, whereas the blocking antibody could eliminate the effect of externally added IL-2, it did not inhibit PEP005-mediated survival of T cells. These observations suggest that the inhibition of T cell death cannot be explained by PEP005-induced IL-2 production.
FIGURE 3.
Antiapoptotic effect of PEP005 requires PKC activation and is not dependent on autocrine IL-2 production. Activated CD8+ T cells were treated in the presence/absence of an anti-IL-2 antibody (A), indicated concentrations of pan-PKC inhibitor BisI (B), cPKC inhibitor (inhib.) Gö6976 (C), PKCβ-specific inhibitor (D) and 25 units/ml IL-2 and/or 20 nm PEP005 for 2 days. Levels of apoptosis were analyzed by intracellular staining of active caspase-3. Although the survival effect depends on PKC activation, inhibition of classical PKC by Gö6976 only had a partial effect, and inhibition of PKCβ did not reduce significantly the antiapoptotic effect of PEP005. Data are mean ± S.D. from three separate experiments. An asterisk denotes p < 0.05, whereas ns denotes p > 0.05.
PEP005 can activate a broad range of PKC isoforms; many of these can up- or down-regulate the rate of apoptosis. To test whether the antiapoptotic effect of PEP005 was dependent on the activation of PKC, BisI, a PKC inhibitor with broad specificity for classical and novel PKC isoforms was added to the cultures. As shown in Fig. 3b, BisI can completely abrogate the survival effect of PEP005. The inhibitor of classical PKC isoforms Gö6976, on the other hand, only had a partial effect on the PEP005-mediated inhibition of T cell apoptosis (Fig. 3c). An inhibitor specific for PKCβ did not have a significant effect on the inhibition of T cell apoptosis mediated by PEP005 (Fig. 3d). This observation suggested an involvement of novel PKC isoforms in the antiapoptotic effect of PEP005.
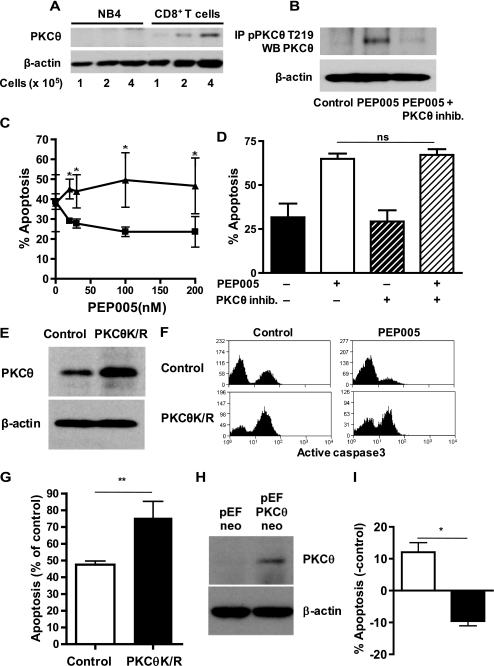
Among the novel PKC isoforms, PKCθ is an important regulator of T cell survival. As described previously, PKCθ is not expressed in myeloid cells (9). Titration of varying cell numbers of NB4 and activated CD8+ T cells into Western blots confirmed the reports that PKCθ is expressed by T cells but not by NB4 cells (Fig. 4a). Positive evidence for activation of PKCθ by PEP005 was yielded by experiments involving immunoprecipitation of PKCθ phosphorylated on Thr-219, a site associated with enzymatic activity (Fig. 4b). Although the phosphorylation of PKCθ at Thr-219 was induced by PEP005, it could be inhibited by the PKCθ-specific inhibitor. We therefore hypothesized that PKCθ expression by T cells may explain the observation that broad range PKC activation leads to an antiapoptotic signal in T cells while it induces apoptosis in myeloid leukemia cell lines. In the next series of experiments, we used a specific peptide inhibitor of PKCθ. As shown in Fig. 4c, inhibition of PKCθ could completely abrogate the survival effect of PEP005 on T cells. In contrast, the antileukemic effect of PEP005 on leukemia cells was not affected by the PKCθ inhibitor (Fig. 4d). This result suggests that the inhibition of PKCθ does not affect the proapoptotic effect mediated by PKCδ, which is highly homologous to PKCθ (24) and allows separation of the proapoptotic effect of PEP005 on leukemic cells from the antiapoptotic effect on T cells. Transfection of peripheral blood CD8+ T cells with a dominant-negative, kinase-inactive mutant of PKCθ significantly reduced the antiapoptotic effect of PEP005 (Fig. 4, e–g). As expected for a kinase involved in the inhibition of apoptosis, transfection with a dominant-negative form of PKCθ also increased the amount of constitutive apoptosis (Fig. 4f). We further confirmed the role of PKCθ in the inhibition of apoptosis by transfection of NB4 cells with a plasmid coding for full-length PKCθ (Fig. 4h). Whereas PEP005 treatment increased apoptosis in NB4 cells transfected with empty control vector, the PKCθ-transfected cells responded to PEP005 with inhibition of apoptosis (Fig. 4i). Due to the technical difficulty of transfecting promyelocytic cells without inducing apoptosis, the differences observed were not very dramatic; however, the observed effect was highly reproducible. These data support the contention that expression of PKCθ determines whether a hematopoietic cell receives a pro- or antiapoptotic stimulus when exposed to broad range PKC-activating agents.
FIGURE 4.
Role of PKCθ in the response of NB4 cells and T cells to PEP005. A, expression of PKCθ in NB4 cells and CD8+ T cells was determined by Western blotting of cell lysate obtained from 1, 2, and 4 × 105 cells with an anti-PKCθ antibody. B, activation of PKCθ upon PEP005 exposure was detected by immunoprecipitation of activated PKCθ phosphorylated at threonine 219 and detection of PKCθ by Western blotting. Phosphorylation of PKCθ was blocked by preincubation of the cells with PKCθ inhibitor (PKCθ inhib.) Detection of β-actin in the unbound lysate was used to control for equivalent cell input. C, activated CD8+ T cells were preincubated with (triangles) and without (squares) 10 μm PKCθ inhibitor and then with indicated concentrations of PEP005 in the absence of IL-2. Levels of apoptosis were analyzed by intracellular staining of active caspase-3 on day 2. D, NB4 cells were cultured in the presence and absence of 10 μm PKCθ inhibitor in the presence/absence of 20 nm PEP005. Levels of apoptosis were analyzed on day 2 by detection of activated caspase 3. E, peripheral blood CD8+ T cells were transfected with an expression vector coding for kinase-inactive PKCθ (PKCθK/R). Western blots of cell lysates of control transfected or kinase-inactive PKCθ-transfected cells were probed with an anti-PKCθ antibody. F, after 6 h of transfection, control and PKCθK/R transfected CD8+ T cells were incubated in the presence or absence of PEP005 for 24 h. The percentage of apoptotic cells was determined by staining for activated caspase-3 and gating on the GFP expressing cell population. G, the percentage of apoptotic cells in PEP005-treated cells from three independent experiments on CD8+ T cells transfected with GFP and kinase-inactive PKCθ is shown. H, PKCθ expression was detected in GFP+-sorted NB4 cells co-transfected with empty vector or PKCθ-coding vector. I, GFP+-sorted NB4 cells co-transfected with PKCθ-coding (black bar) and control vector (empty bar) were treated with 20 nm PEP005 for 10 h, and apoptosis was detected by intracellular staining of active caspase-3. Data shown are for PEP005-treated cells and are expressed as mean ± S.D. for the percent increase or decrease of apoptosis over untreated cells. Data shown are mean ± S.D. from a minimum of three independent experiments. An asterisk denotes p < 0.05, double asterisks denote p < 0.01, whereas ns denotes p > 0.05.
In a further series of experiments, the molecular mechanism causing the antiapoptotic signal induced by PEP005 was investigated. The relative levels of expression of Bcl-2 family members govern the decision between survival and apoptosis in many aspects of T cell biology (18). The expression of the proapoptotic protein Bim is thought to initiate mitochondrial depolarisation in T cells, which can be counterbalanced by expression of Bcl-2, Bcl-xL, or Mcl-1. The levels of expression of these proteins were investigated in T cells treated either for 15 min or for 24 h with PEP005. As shown in Fig. 5, PEP005 did not lead to any obvious changes in Bcl-2 levels. However, within 24 h, an increase of Mcl-1 and Bcl-xL levels was observed. The level of expression of BimEL, the highest molecular weight isoform of Bim, increased at 24 h. The increase in BIMEL was dramatically reduced in the presence of PEP005. In cells treated with PEP005, we also observed appearance of a higher molecular weight band. This band may correspond to the phosphorylated form of BimEL. The addition of either BisI, or the PKCθ inhibitor, prevented the increase in expression of Mcl-1 and Bcl-xL. The reduced levels of BimEL expression in cells cultured with PEP005 were not affected by inhibition of PKC or PKCθ. The lower levels of BimEL may either be caused by an inhibition of the increase in BimEL levels or by accelerated PKC-independent degradation. We conclude from our investigation of the effect of PEP005 on relative levels of Bcl-2 family members, that PKCθ activation by PEP005 increases cell survival by alteration of the balance between pro- and antiapoptotic members of the Bcl-2 family.
FIGURE 5.
Induction of Mcl-1 and Bcl-xL and reduction of BimEL in activated CD8+ T cells by PEP005. A, 1 × 106/ml activated CD8+ T cells in serum-free medium were treated in the presence/absence of 300 nm BisI or 10 μm PKCθ inhibitor (inhib.) for 30 min and then in the presence/absence of 20 nm PEP005 for the duration indicated. Cell lysates were analyzed by immunoblotting with antibodies specific for Bcl-2, Mcl-1, Bcl-xL, and Bim; β-actin was measured as a loading control. B, abundance of Mcl-1, Bcl-xL, and BimEL in cell lysates were quantified by densitometry of immunobands. Density of immunobands were first normalized to that of β-actin and then normalized to values of untreated cells of 15 min in culture (Mcl-1 and Bcl-xL) or untreated cells of 24 h in culture (BimEL). Data shown are mean ± S.D. from five separate experiments. An asterisk denotes p < 0.05, whereas ns denotes p > 0.05.
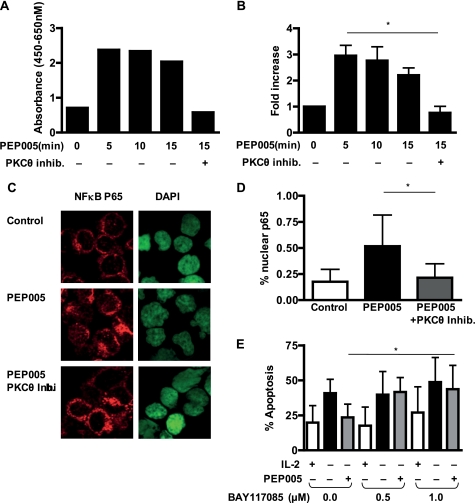
Expression of Mcl-1 as well as Bcl-xL is regulated by the canonical NFκB pathway; an important regulator of cell survival that is a known target for PKC. The effect of PEP005 on the activity of NFκB p65 was assayed by a plate-bound assay of nuclear translocation of p65 (Fig. 6, a and b). PEP005 treatment led to rapid nuclear translocation of p65. This activation was blocked entirely by the PKCθ inhibitor. We further confirmed these observations by staining cytospin preparations of activated CD8+ T cells with anti-NFκB p65 antibody. Cells were treated either with or without PEP005 after pretreatment with or without PKC θ inhibitor (Fig. 6c). Nuclear translocation was quantified and showed a significant increase in the nuclear p65 level that was inhibited in the cells pretreated with the PKCθ inhibitor (Fig. 6d).
FIGURE 6.
Activation of NFκB by PEP005 inhibits apoptosis of activated CD8+ T cells. A, activated CD8+ T cells were treated in the presence/absence of 10 μm PKCθ inhibitor (inhib.) and then in the presence/absence of 20 nm PEP005 for 5, 10, and 15 min. Nuclear extracts were analyzed for activated p65 with the TransAM NFκB p65 activation kit. A, data shown are mean ± S.D. of the corrected absorbance (A450 nm–A650 nm). B, fold increase values were obtained by normalization of absorbance values from untreated cells. C, immunofluorescence staining for p65 on activated CD8+ T cells treated for 15 min with 20 nm PEP005 with and without pretreatment with PKCθ inhibitor. D, the percentage of p65 in the nuclei of activated CD8+ T cells treated as detailed above. Data shown are mean ± S.D. of the nuclear p65 expression detected as a percentage of the total cellular level of p65. E, activated CD8+ T cells were treated in the presence/absence of indicated concentration of BAY11-7085 prior to addition of 25 units/ml IL-2 or 20 nm PEP005. Levels of apoptosis were analyzed by intracellular staining of active caspase-3 on day 2. Data are mean ± S.D. from five independent experiments. An asterisk denotes p < 0.05, whereas ns denotes p > 0.05.
Inhibition of NFκB activity led to a titratable increase of the rate of spontaneous apoptosis. The antiapoptotic effect of PEP005 was significantly reduced by addition of BAY11-7085 (Fig. 6e). Taken together, these data suggest that PEP005 treatment-induced PKCθ activation leads to nuclear translocation of NFκB p65 and that the antiapoptotic effect depends on NFκB activation.
DISCUSSION
The observations made in this study provide an explanation for the difference in the response of myeloid leukemia cell lines and T cells to PKC activation. Whereas many cell lines enter apoptosis upon treatment with PEP005, T cells receive a strong antiapoptotic signal. The data shown here indicate that this antiapoptotic signal is mediated by activation of PKCθ in T cells. Downstream of PKCθ activation, we observed activation of the canonical NFκB pathway and a change in the ratio between expression of proapoptotic Bcl-2 family member BimEL and antiapoptotic family members Bcl-xL and Mcl-1. Most cytotoxic drugs used in chemotherapy target rapidly proliferating cells and therefore suppress the function of the immune system. PKC activators, however, induce apoptosis in many myeloid leukemia cell types in vivo and in vitro and at the same time act as potential immune stimulants. They were shown to cause T cell activation, proliferation, and secretion of cytokine such as IL-2 (4). Previous work from our group has shown that nanomolar concentrations of PEP005 triggered apoptosis of cells lines derived from patients with acute myeloid leukemia (8), and, in the other independent study, PEP005 enhanced cytotoxic functions of neutrophils in vitro and prevented relapse of grafted skin tumors in vivo (25). The only previous study of effects of PEP005 on T cells was investigating the cytokine production by T cells of acute myeloid leukemia patients in vitro. If added in conjunction with stimulating anti-CD3 antibody, PEP005 increased cytokine release (including IL-2 and interferon-γ) from T cells (26). The previous finding that PEP005 can increase IL-2 production raised the possibility that the underlying survival mechanism in T cells was an autocrine loop of increased IL-2 production. Inhibition with blocking anti-IL-2 antibodies, however, did not prevent the antiapoptotic effect of PEP005 on T cells. Looking for an alternative mechanism, we investigated the effect of PEP005 on PKCθ. The essential role of PKCθ signaling for activation of NFκB in T cell activation and survival is well established (15, 16). Our own studies on survival of activated CD8+ T cells have indeed shown that PEP005 activates PKCθ. Phosphorylation of Thr-219, an autophosphorylation site critical for NFκB activation, was clearly demonstrated (27). The NFκB pathway is an important regulator of the balance between pro- and antiapoptotic Bcl-2 family proteins. These regulate CD8+ T cell apoptosis at different development and differentiation stages; it has been shown that cytokine-deprivation mediated apoptosis of activated CD8+ T cells and viral antigen-specific CD8+ T cells from patients with chronic viral infection were caused by loss of Mcl-1 and increased expression of BimEL (28, 29). On the other hand, Bcl-xL up-regulation mediated by T cell activation and PKCθ-NFκB signaling supports survival of activated T cells (11, 30, 31). The ability to express these antiapoptotic Bcl-2 members autonomously can enhance the lifespan of effector T cells. That regulation Bcl-2 itself was not observed in our system may be due the larger nonregulated nuclear pool of Bcl-2, which can mask regulation of the mitochondrial level of Bcl-2 (32). Our finding recapitulates the role of antiapoptotic Bcl-2 members in CD8+ T cell apoptosis, as the loss of Mcl-1 and elevated BimEL expression followed by cytokine deprivation was reversed by PEP005, together with the up-regulation of Bcl-xL. When PKCθ was inhibited, PEP005-induced activation of NFκB was completely abrogated, and this, together with the failure of Mcl-1 and Bcl-xL up-regulation, subsequently led to activation of caspase-3 and apoptosis.
Homeostatic control of naïve CD8+ T cell numbers is highly regulated by levels of common γ-chain cytokines IL-7 and IL-15 and stimulation of MHC-I molecules (33). Following antigen clearance, declined availability of survival factors renders activated CD8+ T cells highly susceptible to apoptosis. PEP005 inhibited this cytokine-deprivation induced apoptosis by delaying the process of apoptosis of freshly isolated naïve and also central memory CD8+ T cells in vitro. It is interesting, in this context, that central memory CD8+ T cells, in particular, were found to be the most effective CD8+ T cell subset in generating anti-tumor responses in vivo (34).
We conclude from these studies, that broad range PKC activation can be a survival factor for activated and resting T cells, whereas it exerts a powerful proapoptotic signal to leukemia cells. This divergent response can be explained by the difference in expression of PKCθ between T cells and leukemic cells derived from acute myeloid leukemia patients.
Acknowledgments
We thank Hema Chahal and Dr. Deborah Hardie for expert technical assistance and Gottfried Baier (Innsbruck, Austria) for the kind gift of pThr-219 PKCθ antibody, pEFwtPKCθneo, and pEFwtPKCθK/Rneo vector.
This work was funded by an Arthritis Research UK Career Development Fellowship (to D. S.-T.), an Arthritis Research UK programme grant (to M. S. and J. M. L.), the European Commission (LSHB-CT-2004-503467), a project grant from Peplin, Inc., and a Birmingham University PhD studentship (to W.-y. L.).
- PKC
- protein kinase C
- BisI
- bisindolylmaleimide I
- BrdUrd
- bromodeoxyuridine
- wt
- wild-type
- IL
- interleukin
- PBS
- phosphate-buffered saline
- TCR
- T cell receptor
- GFP
- green fluorescent protein
- TRITC
- tetramethylrhodamine isothiocyanate
- BimEL
- extra long form of Bim (Bcl-2 interacting mediator of cell death).
REFERENCES
- 1.Newton A. C. (2001) Chem. Rev. 101, 2353–2364 [DOI] [PubMed] [Google Scholar]
- 2.Deacon E. M., Pongracz J., Griffiths G., Lord J. M. (1997) Mol. Pathol. 50, 124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mackay H. J., Twelves C. J. (2007) Nat. Rev. Cancer 7, 554–562 [DOI] [PubMed] [Google Scholar]
- 4.Mohr H., Pettit G. R., Plessing-Menze A. (1987) Immunobiol. 175, 420–430 [DOI] [PubMed] [Google Scholar]
- 5.Green A. C., Beardmore G. L. (1988) Australas. J. Dermatol. 29, 127–130 [DOI] [PubMed] [Google Scholar]
- 6.Weedon D., Chick J. (1976) Med. J. Aust. 1, 928. [PubMed] [Google Scholar]
- 7.Kedei N., Lundberg D. J., Toth A., Welburn P., Garfield S. H., Blumberg P. M. (2004) Cancer Res. 64, 3243–3255 [DOI] [PubMed] [Google Scholar]
- 8.Hampson P., Chahal H., Khanim F., Hayden R., Mulder A., Assi L. K., Bunce C. M., Lord J. M. (2005) Blood 106, 1362–1368 [DOI] [PubMed] [Google Scholar]
- 9.Baier G., Telford D., Giampa L., Coggeshall K. M., Baier-Bitterlich G., Isakov N., Altman A. (1993) J. Biol. Chem. 268, 4997–5004 [PubMed] [Google Scholar]
- 10.Cross T., Griffiths G., Deacon E., Sallis R., Gough M., Watters D., Lord J. M. (2000) Oncogene 19, 2331–2337 [DOI] [PubMed] [Google Scholar]
- 11.Barouch-Bentov R., Lemmens E. E., Hu J., Janssen E. M., Droin N. M., Song J., Schoenberger S. P., Altman A. (2005) J. Immunol. 175, 5126–5134 [DOI] [PubMed] [Google Scholar]
- 12.Monks C. R., Freiberg B. A., Kupfer H., Sciaky N., Kupfer A. (1998) Nature 395, 82–86 [DOI] [PubMed] [Google Scholar]
- 13.Monks C. R., Kupfer H., Tamir I., Barlow A., Kupfer A. (1997) Nature 385, 83–86 [DOI] [PubMed] [Google Scholar]
- 14.Wang D., Matsumoto R., You Y., Che T., Lin X. Y., Gaffen S. L., Lin X. (2004) Mol. Cell. Biol. 24, 164–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coudronniere N., Villalba M., Englund N., Altman A. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 3394–3399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Z., Arendt C. W., Ellmeier W., Schaeffer E. M., Sunshine M. J., Gandhi L., Annes J., Petrzilka D., Kupfer A., Schwartzberg P. L., Littman D. R. (2000) Nature 404, 402–407 [DOI] [PubMed] [Google Scholar]
- 17.Hildeman D. A., Zhu Y., Mitchell T. C., Kappler J., Marrack P. (2002) Curr. Opin. Immunol. 14, 354–359 [DOI] [PubMed] [Google Scholar]
- 18.Zhu Y., Swanson B. J., Wang M., Hildeman D. A., Schaefer B. C., Liu X., Suzuki H., Mihara K., Kappler J., Marrack P. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 7681–7686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baier-Bitterlich G., Uberall F., Bauer B., Fresser F., Wachter H., Grunicke H., Utermann G., Altman A., Baier G. (1996) Mol. Cell. Biol. 16, 1842–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillis S., Watson J. (1980) J. Exp. Med. 152, 1709–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evan G. I., Vousden K. H. (2001) Nature 411, 342–348 [DOI] [PubMed] [Google Scholar]
- 22.Sallusto F., Geginat J., Lanzavecchia A. (2004) Annu. Rev. Immunol. 22, 745–763 [DOI] [PubMed] [Google Scholar]
- 23.Duke R. C., Cohen J. J. (1986) Lymphokine Res. 5, 289–299 [PubMed] [Google Scholar]
- 24.Osada S., Mizuno K., Saido T. C., Suzuki K., Kuroki T., Ohno S. (1992) Mol. Cell. Biol. 12, 3930–3938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Challacombe J. M., Suhrbier A., Parsons P. G., Jones B., Hampson P., Kavanagh D., Rainger G. E., Morris M., Lord J. M., Le T. T., Hoang-Le D., Ogbourne S. M. (2006) J. Immunol. 177, 8123–8132 [DOI] [PubMed] [Google Scholar]
- 26.Ersvaer E., Hampson P., Hatfield K., Ulvestad E., Wendelbo Ø., Lord J. M., Gjertsen B. T., Bruserud Ø. (2007) Cancer Immunol. Immunother. 56, 913–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thuille N., Heit I., Fresser F., Krumböck N., Bauer B., Leuthaeusser S., Dammeier S., Graham C., Copeland T. D., Shaw S., Baier G. (2005) EMBO J. 24, 3869–3880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bosque A., Marzo I., Naval J., Anel A. (2007) Mol. Immunol. 44, 1446–1453 [DOI] [PubMed] [Google Scholar]
- 29.Lopes A. R., Kellam P., Das A., Dunn C., Kwan A., Turner J., Peppa D., Gilson R. J., Gehring A., Bertoletti A., Maini M. K. (2008) J. Clin. Invest. 118, 1835–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manicassamy S., Gupta S., Huang Z., Sun Z. (2006) J. Immunol. 176, 6709–6716 [DOI] [PubMed] [Google Scholar]
- 31.Saibil S. D., Jones R. G., Deenick E. K., Liadis N., Elford A. R., Vainberg M. G., Baerg H., Woodgett J. R., Gerondakis S., Ohashi P. S. (2007) J. Immunol. 178, 2932–2939 [DOI] [PubMed] [Google Scholar]
- 32.Scheel-Toellner D., Raza K., Assi L., Pilling D., Ross E. J., Lee W. Y., Curnow S. J., Buckley C. D., Akbar A. N., Lord J. M., Salmon M. (2008) Apoptosis 13, 109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gett A. V., Sallusto F., Lanzavecchia A., Geginat J. (2003) Nat. Immunol. 4, 355–360 [DOI] [PubMed] [Google Scholar]
- 34.Klebanoff C. A., Gattinoni L., Torabi-Parizi P., Kerstann K., Cardones A. R., Finkelstein S. E., Palmer D. C., Antony P. A., Hwang S. T., Rosenberg S. A., Waldmann T. A., Restifo N. P. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 9571–9576 [DOI] [PMC free article] [PubMed] [Google Scholar]