Abstract
Nucleotide-binding oligomerization domain protein 1 (Nod1) is an intracellular protein involved in recognition of the bacterial component peptidoglycan. This recognition event induces a host defense response to eliminate invading pathogens. The genetic variation of Nod1 has been linked to several inflammatory diseases and allergies, which are strongly affected by environmental factors. We have found that many of the bacteria that contain DAP-type peptidoglycan release Nod1 ligands into the environment. However, the structures of natural Nod1 ligands in the environment are not well understood. Herein, we report the isolation and structural elucidation of natural human Nod1 (hNod1) ligands from the Escherichia coli K-12 culture supernatant. The supernatant was fractionated with reversed-phase high performance liquid chromatography (RP-HPLC), resulting in the isolation of several hNod1 stimulatory fractions. Structural characterization studies demonstrated that the molecular structure of the most active fraction was the native hNod1 ligand GlcNAc-(β1–4)-(anhydro)MurNAc-l-Ala-γ-d-Glu-meso-DAP. We also found other peptidoglycan fragments using the 7-(diethylamino)coumarin-3-carbonyl labeling method to enhance sensitivity in mass spectroscopy studies. These results suggested that DAP-containing bacteria release certain hNod1 ligands to the environment, and these ligands would accumulate in the environment and regulate the immune system through Nod1.
Keywords: Bacteria, Glycoconjugate, Innate Immunity, Mass Spectrometry (MS), Peptides
Introduction
The innate immune system represents the first line of defense against invading pathogens. Various pattern-recognition receptors are involved in recognizing microbial components known as pathogen-associated molecular patterns (1–3). Several pattern-recognition receptors have been identified, including Toll-like receptors (4–6), nucleotide-binding oligomerization domain (NOD)-like3 receptors (7–9), and retinoic acid-inducible gene I-like receptors (10, 11). NOD-like receptors Nod1 and Nod2 are intracellular receptors that recognize peptidoglycan (PGN), a bacterial cell wall component (12–15), have been previously reported by our group (12, 14) and also by a French group (13, 15) independently. The receptor proteins are composed of three main domains: the leucine-rich repeat domain, which is involved in ligand recognition located at the carboxyl-terminal site; the NOD domain, which facilitates self-oligomerization and has ATPase activity located at the central site; and the caspase-recruitment domain at the amino-terminal site. The target molecule, PGN, is known as a potent immunopotentiator and an adjuvant for antibody production. The structural features of PGN include polysaccharide chains having alternating (β1–4)-linked N-acetylglucosamine (GlcNAc) and N-acetylmuramic acid (MurNAc) residues. The polysaccharide chains are connected to a peptide network to form a three-dimensional rigid structure. The branched position of the peptide usually has diamino acids such as l-lysine (in many Gram-positive bacteria) or meso-diaminopimelic acid (meso-DAP) (in most Gram-negative and some Gram-positive bacteria). Nod1 recognizes the site of DAP-containing fragments such as γ-d-Glu-meso-diaminopimelic acid (iE-DAP), whereas Nod2 recognizes muramyl dipeptide. Several studies have investigated and shown that genetic variations of Nod1 and Nod2 are associated with susceptibility to diseases including asthma and Crohn disease, respectively, and also to other allergic diseases (16–23). An important function of Nod1 and Nod2 also were revealed recently; that is, Nod1 and Nod2 are critical for the autophagic response to invasive bacteria, with the recruitment of the autophagy protein ATG16L1 to the plasma membrane at the bacterial entry site (24). Autophagy induction also was observed with iE-DAP-containing PGN fragments in insect systems (25).
In contrast, we found that DAP-containing bacteria release Nod1 ligands (relatively more than Nod2), based on the result that the human Nod1 (hNod1) stimulatory activities of various bacteria, tested with their bacterial bodies and also with the culture supernatants (26). The supernatants of some bacteria such as Escherichia coli and Bacillus species showed more potent hNod1 stimulation than the bacterial bodies. It also was exhibited that Nod1 ligands were found to be more stable than the Nod2 or Toll-like receptor 4 ligands in higher temperatures or under acidic or basic conditions. These results suggest a mechanism in which bacteria present in the environment stimulate the host immune system through Nod1, and this event may be related, in some circumstances, to the development of allergic diseases.
However, the identification of the hNod1 ligands in the environment has not been elucidated, although synthetic studies have revealed that the recognition core of hNod1 stimulatory molecules is an iE-DAP-containing molecule. We were thus interested in the structure of natural hNod1 ligands in the culture supernatant of bacteria. The E. coli K-12 strain was used because hNod1 stimulatory activity was observed with its supernatant (26) and because the required cultivation in a minimum salt medium simplifies structural analysis. We expected that E. coli K-12 would excrete various PGN fragments containing meso-DAP in the culture supernatant. We adopted a strategy in which the synthetic library of PGN fragments was used as an authentic sample for structural elucidation, as the amount of each fragment isolated from the complex bacterial supernatants likely was to be insufficient to elucidate both the structure and the biological activity. Pure preparations of various PGN fragments containing meso-DAP were accessed by chemical synthesis with the development of newer synthetic methods, and the hNod1 stimulatory activities of these fragment molecules have been reported recently (27).
In previous investigations on molecular events of the bacterial cell wall during cell growth, ∼60% of the parental cell wall may be recycled, entailing a degradative processing of the older cell wall, entry into the cell, and reuse of the components for de novo biosynthesis (28–31). Lytic transglycosylases are important bacterial enzymes in the degradation of the bacterial cell wall, and this degradation process yields N-acetyl-1,6-anhydro-muramyl [(anh)MurNAc] moieties as the product (32, 33). The presence of N-acetyl-1,6-anhydro-muramyl [(anh)MurNAc] moiety in the cell wall was first reported in 1975 by Taylor et al. (34). GlcNAc-(β1–4)-(anh)MurNAc-l-Ala-γ-d-Glu-meso-DAP-d-Ala (tracheal cytotoxin; TCT), originally found in Bordetela pertussis (35), is thought to be a fragment released to the environment during recycling of the cell wall (36).
TCT activates murine Nod1 (37) but not hNod1 (27). In addition, sltY-deficient E. coli mutant MHD63 (amiA−, amiB−, amiC−, and sltY−) culture supernatant, which is thought to produce scarcely N-acetyl-1,6-anhydro-muramyl moieties, was found to induce hNod1 stimulation (26). In contrast, when germ-free mice were reconstituted with E. coli mutants or wild-type, E. coli mutant MHD79 (sltY−, mltA−, mltB−, mltC−, mltD−, and emtA−) (38), which release low amounts of PGNs, containing mainly GlcNAc-(β1–4)-MurNAc-l-Ala-γ-d-Glu-meso-DAP-d-Ala and TCT, the induction of isolated lymphoid follicles in germ-free mice was abrogated as compared with wild-type E. coli MC1061 (39). It also was shown that the isolated lymphoid follicles were induced only with Nod1 ligand and not with Nod2 ligand.
In this study, we found and analyzed the natural hNod1 ligand structures in E. coli K-12 culture media. A major fragment, GlcNAc-(β1–4)-(anh)MurNAc-l-Ala-γ-d-Glu-meso-DAP, was isolated and characterized by using MS and NMR. Other fragments were also detected by MS analysis. The biological activities of the isolated fragments were characterized by using synthetic samples. The present study provides key information to understand the molecular basis for stimulation of our immune system through Nod1 by accumulated stimulants from the environment, even in the absence of physical contact with bacteria.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Growth Conditions
The E. coli strain used in this study was a K-12 strain (wild-type) obtained from National BioResource Project (National Institute of Genetics, Japan). The bacteria were grown in 1.5 liters (×2) of M9 minimal medium (Na2HPO4, 6 g/liter; KH2PO4, 3 g/liter; NaCl, 0.5 g/liter; NH4Cl, 1 g/liter; CaCl2, 3 mg/liter; MgSO4, 1 mm, pH 7.0) containing 20% glucose for 24 h at 37 °C with vigorous aeration, and it was used as a seed medium. The entire volume of the seed culture was used to inoculate 40 liters of M9 minimal medium in a 60-liter fermentor, and the bacterial cells were grown for 40 h at 30 °C with agitation (26). The fermentation broth was then centrifuged and passed through a 0.22-μm filter (Steritop-GV; Millipore, MA) to collect the supernatant.
Isolation of hNod1 Active Fractions
The E. coli supernatant (22 liters) was then passed through an ODS (Cosmosil 75C18-OPN, Nakalai Tesque, Kyoto) open column to remove the medium salts eluting with mixtures of 0.1% trifluoroacetic acid (TFA) in water (mobile phase A) and 0.1% TFA in MeCN (mobile phase B); A/B (volume for 5 liters of supernatant): 100/0 (3 liters), 90/10 (2 liters), 70/30 (2.5 liters), and then 30/70. All eluted fractions were lyophilized, and the hNod1 stimulatory activity of each fraction was assessed (26). Nod1 stimulatory activity was observed at the fraction eluted with the 90/10 mixture. The crude powder (53.7 mg) of the fraction was dissolved in water, and the solution was purified further (three times) by RP-HPLC (column, Cosmosil 5C18 AR300 (Nacalai Tesque, Inc.) 10 × 250 mm; mobile phase A, 0.1% TFA in water; B, 0.1% TFA in MeCN; and UV detection, 220 nm). In the first cycle, the retention time of the most active fraction was 27–32 min (isocratic 0% B solution for 10 min, and then linear gradient was applied up to 30% B solution for 50 min at 4 ml/min). In the second cycle, the retention time of the most active fraction was 6–9 min (isocratic 9% B solution at 3.5 ml/min). In the third cycle, the retention time of the most active fraction was 7.34–7.69 min (isocratic 9% mobile phase B solution at 3.5 ml/min).
Reverse Phase Liquid Chromatography Electrospray Ionization-Quadrupole Time-of-Flight MS Analysis
Mass spectra were recorded on an electrospray ionization-quadrupole time-of-flight (ESI-QTOF) mass spectrometer (Q-TOF Micro; Micromass, Manchester, UK) equipped with a CapLC system (Waters, Milford, MA) using an AtlantisTM dC18 Trap column (Waters, C18, 5-μm particles, 0.18 × 23.5 mm) and an Atlantis dC18 column (Waters, C18, 3 μm, 75 μm × 150 mm). The analytical column was coupled to a PicoTip needle (New Objective, Inc., Woburn, MA). [Glu1]fibrinopeptide B (Sigma) was used for continuous calibration. LC and MS analyses were performed by using Mass-Lynx software. For LC/MS analysis, samples (20 μl) were applied onto the precolumn, which was rinsed with 0.1% formic acid for 4 min (15 μl/min). Bound compounds were eluted using a gradient consisting mobile phase A (5% acetonitrile, 0.1% formic acid) and mobile phase B (95% acetonitrile, 0.1% formic acid). The elution gradient was as follows: from 0 to 4 min at 5% B; from 4 to 45 min to 40% B; from 45 to 53 min to 70% B; from 53 to 58 min to 95% B; from 58 to 60 min at 95%; and from 60 to 70 min to 5% B. Q-TOF instrument parameters were as follows: source temperature, 100 °C; electrospray voltage, 1.8 kV; and cone voltage, 30 V. When the Nanospray ionization system was used, the instrument parameters were as follows: source temperature, 80 °C; electrospray voltage, 3.1 kV; and cone voltage, 45 V. For MS/MS, the scan time was set to 2.4 s, and the collision energy set to around 30 eV according to the m/z of the precursor and the charge state.
HEK293T Bioassay for Nod1 Stimulation
Ligand-dependent NF-κB activation was determined by using 0.5 × 105 HEK 293T cells transfected with expression plasmids of Nod1 (0.17 ng of pCMV-SPORT6-Nod1), in the presence of the reporter plasmids NF-κB-dependent pBxIV-luciferase and control pEF1BOS-β-galactosidase as described previously (26). Briefly, HEK293T cells were transfected with expression plasmids by using the calcium phosphate method, and 8 h after transfection, the cells were treated with a medium containing various ligands. 24 h after transfection, ligand-dependent NF-κB activation was determined by the luciferase reporter assay.
ESI-QTOF MS Studies of N-terminal Labeling of PGN Fragments with the Diethylamino Coumarin (DEAC) Group
Labeling of amino groups of PGN fragments in the active fraction were performed as follows: the active fraction from the second ODS separation cycle (750 μg) was dissolved in 0.1 m NaHCO3 aqueous solution (6 μl). A solution of 7-diethylamino coumarin-3-carboxylic acid succinimidyl ester (Invitrogen) (25 μg, 70 nmol) in dimethyl formamide (2.5 μl) was prepared immediately before the reaction and added to the sample solution. The reaction was mixed at 23 °C for 17 h. The crude reaction mixture was diluted with CH3CN (1:100), and the reaction products were analyzed by LC/ESI-QTOF MS.
NMR Spectroscopy
NMR spectra were recorded at 599.48 MHz for proton by using a Varian INOVA 600 spectrometer at 30 °C with a 5-mm Varian 1H(13C/15N) XYZ PFG triple resonance probe, using Varian standard homonuclear pulse programs such as one-dimensional 1H and two-dimensional 1H-1H gradient enhanced correlation spectroscopy (gCOSY). All samples of the isolated active fraction solution were prepared with D2O using 5-mm microcell NMR tubes (Shigemi Co., Tokyo, Japan). The chemical shifts in D2O are given in δ values, with the HDO signal (δ = 4.718 ppm at 30 °C) as a reference. The presaturation method was also used for suppression of the solvent signal.
Synthesized PGN Fragments
Authentic samples of PGN fragments were prepared by using our previously reported chemical synthesis methods (12, 27, 40). The following fragments were synthesized: iE-DAP (12), A-iE-DAP (40), MS-3P(DAP), MS-4P(DAP), MS-5P(DAP), DS-3P(DAP), DS-4P(DAP), DS(anh)-3P, and DS(anh)-4P (27). The conditions of RP-HPLC analysis were as follows: column, Cosmosil 5C18 AR300 (Nacalai Tesque, Inc.) 10 × 250 mm; mobile phase A, 0.1% TFA in water; B, 0.1% TFA in CH3CN; isocratic 9% mobile phase B solution at 3.5 ml/min; and UV detection, 220 nm.
RESULTS AND DISCUSSION
To isolate natural Nod1 ligands from E. coli culture media, wild-type E. coli K-12 cells were fermented in M9 minimum medium in a 50-liter fermentor, and the culture supernatant was obtained. The incubation conditions (30 °C for 40 h in M9 medium) were the same as those previously reported in a study in which the E. coli K-12 culture supernatant but not the cell body showed much Nod1 stimulatory activity (26). Use of the M9 minimum medium also aids in simplification of the structural analysis. Using the filtered culture supernatant, we separated and purified the Nod1 stimulatory fractions as outlined in Fig. 1.
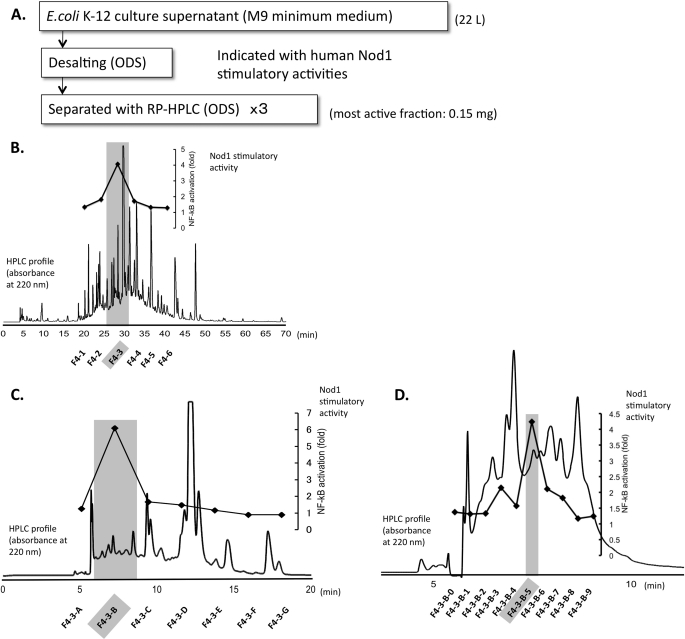
FIGURE 1.
A, isolation of fractions with Nod1 stimulatory activity from E. coli K-12 culture supernatant (M9 minimum medium). B, the HPLC profile and Nod1 stimulatory activities of fractions from the first cycle of RP-HPLC separation. Nod1 stimulatory activities were measured by using a 10 μg/ml sample prepared from lyophilized residues (F4–3: 8 mg in total). C, the second cycle of RP-HPLC separation. The dark-colored fraction from the first RP-HPLC separation was further separated with a second RP-HPLC purification. Nod1 stimulatory activities were measured by using a 5 μg/ml sample prepared from lyophilized residues (F4–3-B, 3.6 mg in total). D, the third cycle of RP-HPLC separation. The dark-colored fraction from the second RP-HPLC separation was further separated with the third RP-HPLC condition. Nod1 stimulatory activities were measured by using a 0.1 μg/ml sample prepared from the lyophilized residues (F4–3-B-5, 0.15 mg in total). The dark-colored fraction was corrected and analyzed with ESI-QTOF MS, MS/MS, and NMR. Details on the HPLC conditions are as described under “Experimental Procedures.”
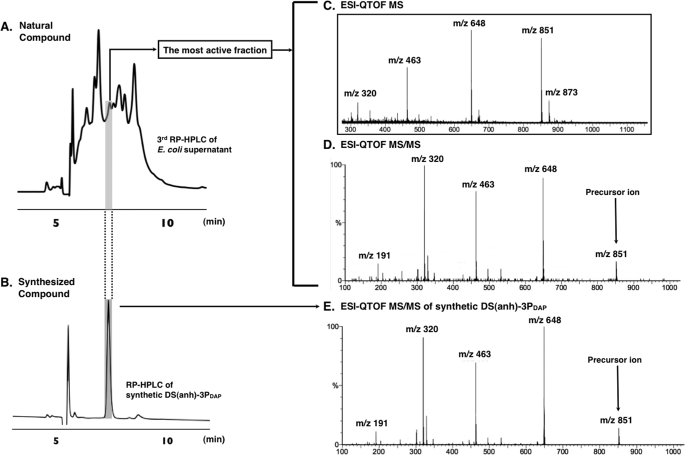
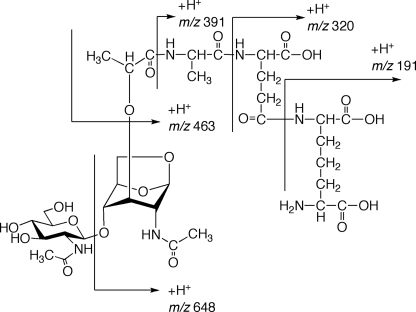
The culture supernatant was first desalted by filtration through an ODS open short column. The active fraction was lyophilized and then further purified by RP-HPLC three times as shown in Fig. 1, B–D. The hNod1 stimulatory activities of all fractions were observed in these sequential HPLC separations. In the third separation (Fig. 1D), we obtained a strong hNod1 stimulatory fraction. This fraction was analyzed by ESI-QTOF MS (Fig. 2, A and C) and MS/MS (Fig. 2D). The observed HPLC retention time and MS were identical with the retention time (Fig. 2B) and ESI-QTOF MS/MS (Fig. 2E) of the synthesized PGN fragment DS(anh)-3P(DAP) (GlcNAc-(β1–4)-(anhydro) MurNAc-l-Ala-γ-d-Glu-meso-DAP) (27). The analysis of the fragmentation pattern in the MS/MS is shown in Fig. 3. The NMR spectra (1H-1D NMR and H-H correlation spectroscopy (COSY)) of the active fraction also were obtained, and we observed consistent results between the natural and the synthetic compounds (supplemental Fig. S1). The amino acid analysis of F4-B indicated that this fraction contained DAP (supplemental material), a fragment structure important for Nod1 recognition. The fractions obtained from the final RP-HPLC separation were evaluated for hNod1 (Fig. 1D) and murine Nod1 (supplemental Fig. S2) stimulatory activities, and the most active fraction for hNod1 (F4–3-B-5) did not activate murine Nod1. These results are consistent with previous reports that DS(anh)-3P(DAP), the fragment identified in the active fraction, did not activate murine Nod1 (37).
FIGURE 2.
The HPLC profiles of the natural fractions (the third cycle of RP-HPLC) from the E. coli K-12 culture supernatant (A) and synthesized DS(anh)-3P(DAP) (B) (26). ESI-QTOF MS (C) and ESI-QTOF MS/MS (D) of the most active fraction (F4–3-B-5) from the E. coli K-12 culture supernatant. ESI-QTOF MS/MS (E) of synthesized DS(anh)-3P(DAP).
FIGURE 3.
MS/MS fragment ion analysis of the most active fraction for human Nod1 stimulatory activity (Fig. 2D).
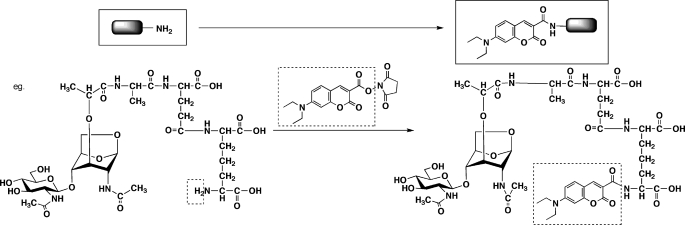
During isolation of the most active hNod1 ligand from the bacterial culture, several PGN fragments were observed in mass spectral analysis. However, these results were not always comprehensive because of the presence of many other compounds in the culture. We thus introduced a fluorescent tag, DEAC, to enhance the sensitivity of MS (41) to the PGN fragments (F4–3-B). The DEAC tags were coupled to amino groups in the PGN fragments through N-hydroxysuccinimidyl ester intermediates to generate amides (Fig. 4).
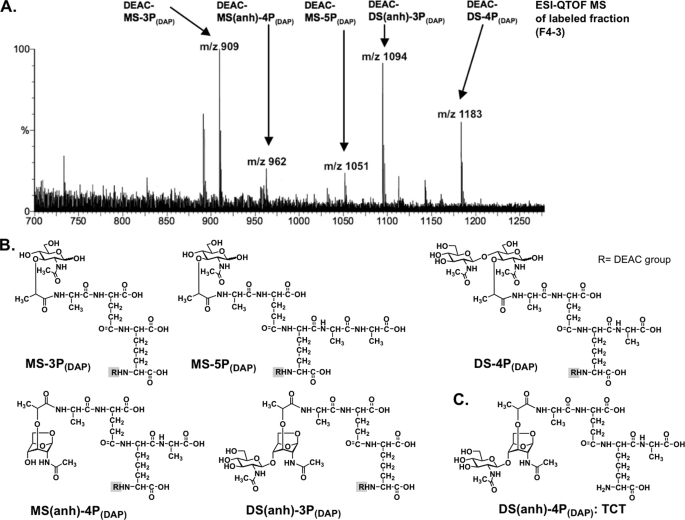
FIGURE 4.
DEAC-labeling reaction of amino groups in the Nod1 stimulatory fraction.
After introduction of the DEAC group to the compounds in fraction F4–3-B, the ESI-QTOF MS was obtained (Fig. 5). In the mass spectrum, the following ion peaks were observed: m/z 1183 (DEAC-DS-4P(DAP)), m/z 1094 (DEAC-DS(anh)-3P(DAP)), m/z 1081 (DEAC-MS-5P(DAP)), m/z 962 (DEAC-MS(anh)-4P(DAP)), and m/z 909 (DEAC-MS-3P(DAP)). Without labeling, only the ion peaks m/z 851 (DS(anh)-3P(DAP)) and m/z 1011 (DS-4P(DAP)) were observed, indicating that labeling with the DEAC group enhanced sensitivities. DS(anh)-4P(DAP) (TCT) also was observed in the F4–3-C fraction without DEAC labeling.
FIGURE 5.
A, ESI-QTOF MS spectrum of DEAC-labeled fraction (F4–3-B in Fig. 1C) from the E. coli K-12 culture supernatant. B, the observed structures with DEAC labeling. C, DS(anh)-4P(DAP) (TCT) also was observed in the F4–3-C in Fig. 1C.
The results showed that the active fraction has fragments containing both 1,6-anhydro- and non-anhydro-GlcNAc moieties. Tripeptide-containing fragments such as MS-3P(DAP) and DS(anh)-3P(DAP) are expected to have potent hNod1 stimulatory activities, based on the results of chemically synthesized hNod1 ligands (27). The fact that MurNAc-containing (non-anhydro-type) PGN fragments were released might explain the results of hNod1 activation with the sltY-deficient E. coli culture supernatant.
In conclusion, we revealed that hNod1 ligands in the E. coli K-12 culture supernatant for the first time, based on our previously reported results that showed many kinds of bacteria, which contain DAP-type PGN, release the Nod1 ligands to their environment (26). The most Nod1 stimulatory structure was determined to be DS(anh)-3P(DAP), but other PGN fragments also were observed. These results were consistent with the hNod1 stimulatory activities, which were demonstrated by using chemically synthesized PGN fragments (27). In our analysis of PGN fragments from the E. coli culture supernatant, we also demonstrated that the DEAC-labeling method enhances the sensitivity of mass spectral analysis. Revealing the molecular structures of stable hNod1 ligands in the environment might be a key to understanding what types of molecules accumulate and affect the human immune system in the environment.
Supplementary Material
Acknowledgments
We thank Professor Seiki Kuramitsu and Hiromasa Oyama (Osaka University) for kind assistance in fermentation, Seiji Adachi (Osaka University) for dedicated efforts on NMR measurements, and Makoto Nakata (Peptide Institute, Inc.) for amino acid analysis and helpful discussion.
This work was supported in part by grants-in-aid for scientific research (17510178 and 19310144) from the Japan Society for the Promotion of Science, the Institute for Fermentation, Osaka (IFO), the Osaka University Global Center of Excellence program (Frontier Biomedical Science Underlying Organelle Network Biology), and the Naito Foundation.

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Experimental Procedures” and Figs. S1 and S2.
- NOD
- nucleotide-oligomerization domain
- DAP
- diaminopimelic acid
- iE-DAP
- γ-D-glutamyl-meso-diaminopimelic acid
- hNod1
- human Nod1
- LC
- liquid chromatography
- GlcNAc
- N-acetylglucosamyl
- ESI-QTOF
- electrospray ionization-quadrupole time-of-flight
- MurNac
- N-acetylmuramyl
- PGN
- peptidoglycan
- MS
- mass spectrometry
- MS/MS
- tandem MS
- LPS
- lipopolysaccharide
- HEK293
- human embryonic kidney 293
- DEAC
- diethylamino coumarin
- TCT
- tracheal cytotoxin
- TFA
- trifluoroacetic acid
- RP-HPLC
- reversed-phase high performance liquid chromatography
- ESI-QTOF
- electrospray ionization-quadrupole time-of-flight.
REFERENCES
- 1.Medzhitov R. (2007) Nature 449, 819–826 [DOI] [PubMed] [Google Scholar]
- 2.Janeway C. A., Jr., Medzhitov R. (2002) Annu. Rev. Immunol. 20, 197–216 [DOI] [PubMed] [Google Scholar]
- 3.Sansonetti P. J. (2006) Nat. Immunol. 7, 1237–1242 [DOI] [PubMed] [Google Scholar]
- 4.Beutler B. A. (2009) Blood 113, 1399–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar H., Kawai T., Akira S. (2009) Biochem. Biophys. Res. Commun. 388, 621–625 [DOI] [PubMed] [Google Scholar]
- 6.Akira S., Uematsu S., Takeuchi O. (2006) Cell 124, 783–801 [DOI] [PubMed] [Google Scholar]
- 7.Franchi L., Warner N., Viani K., Nuñez G. (2009) Immunol. Rev. 227, 106–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meylan E., Tschopp J., Karin M. (2006) Nature 442, 39–44 [DOI] [PubMed] [Google Scholar]
- 9.Inohara, Chamaillard, McDonald C., Nuñez G. (2005) Annu. Rev. Biochem. 74, 355–383 [DOI] [PubMed] [Google Scholar]
- 10.Yoneyama M., Fujita T. (2009) Immunol. Rev. 227, 54–65 [DOI] [PubMed] [Google Scholar]
- 11.Yoneyama M., Onomoto K., Fujita T. (2008) Adv. Drug Deliv. Rev. 60, 841–846 [DOI] [PubMed] [Google Scholar]
- 12.Chamaillard M., Hashimoto M., Horie Y., Masumoto J., Qiu S., Saab L., Ogura Y., Kawasaki A., Fukase K., Kusumoto S., Valvano M. A., Foster S. J., Mak T. W., Nuñez G., Inohara N. (2003) Nat. Immunol. 4, 702–707 [DOI] [PubMed] [Google Scholar]
- 13.Girardin S. E., Boneca I. G., Carneiro L. A., Antignac A., Jéhanno M., Viala J., Tedin K., Taha M. K., Labigne A., Zähringer U., Coyle A. J., DiStefano P. S., Bertin J., Sansonetti P. J., Philpott D. J. (2003) Science 300, 1584–1587 [DOI] [PubMed] [Google Scholar]
- 14.Inohara N., Ogura Y., Fontalba A., Gutierrez O., Pons F., Crespo J., Fukase K., Inamura S., Kusumoto S., Hashimoto M., Foster S. J., Moran A. P., Fernandez-Luna J. L., Nuñez G. (2003) J. Biol. Chem. 278, 5509–5512 [DOI] [PubMed] [Google Scholar]
- 15.Girardin S. E., Boneca I. G., Viala J., Chamaillard M., Labigne A., Thomas G., Philpott D. J., Sansonetti P. J. (2003) J. Biol. Chem. 278, 8869–8872 [DOI] [PubMed] [Google Scholar]
- 16.Hysi P., Kabesch M., Moffatt M. F., Schedel M., Carr D., Zhang Y., Boardman B., von Mutius E., Weiland S. K., Leupold W., Fritzsch C., Klopp N., Musk A. W., James A., Nunez G., Inohara N., Cookson W. O. (2005) Hum. Mol. Genet. 14, 935–941 [DOI] [PubMed] [Google Scholar]
- 17.Hugot J. P., Chamaillard M., Zouali H., Lesage S., Cézard J. P., Belaiche J., Almer S., Tysk C., O'Morain C. A., Gassull M., Binder V., Finkel Y., Cortot A., Modigliani R., Laurent-Puig P., Gower-Rousseau C., Macry J., Colombel J. F., Sahbatou M., Thomas G. (2001) Nature 411, 599–603 [DOI] [PubMed] [Google Scholar]
- 18.Ogura Y., Bonen D. K., Inohara N., Nicolae D. L., Chen F. F., Ramos R., Britton H., Moran T., Karaliuskas R., Duerr R. H., Achkar J. P., Brant S. R., Bayless T. M., Kirschner B. S., Hanauer S. B., Nuñez G., Cho J. H. (2001) Nature 411, 603–606 [DOI] [PubMed] [Google Scholar]
- 19.McGovern D. P., Hysi P., Ahmad T., van Heel D. A., Moffatt M. F., Carey A., Cookson W. O., Jewell D. P. (2005) Hum. Mol. Genet. 14, 1245–1250 [DOI] [PubMed] [Google Scholar]
- 20.Kabesch M., Peters W., Carr D., Leupold W., Weiland S. K., von Mutius E. (2003) J. Allergy Clin. Immunol. 111, 813–817 [DOI] [PubMed] [Google Scholar]
- 21.Weidinger S., Klopp N., Rümmler L., Wagenpfeil S., Baurecht H. J., Gauger A., Darsow U., Jakob T., Novak N., Schäfer T., Heinrich J., Behrendt H., Wichmann H. E., Ring J., Illig T. (2005) Clin. Exp. Allergy 35, 866–872 [DOI] [PubMed] [Google Scholar]
- 22.Weidinger S., Klopp N., Rummler L., Wagenpfeil S., Novak N., Baurecht H. J., Groer W., Darsow U., Heinrich J., Gauger A., Schafer T., Jakob T., Behrendt H., Wichmann H. E., Ring J., Illig T. (2005) J. Allergy Clin. Immunol. 116, 177–184 [DOI] [PubMed] [Google Scholar]
- 23.Eder W., Klimecki W., Yu L., von Mutius E., Riedler J., Braun-Fahrländer C., Nowak D., Holst O., Martinez F. D.ALEX-Team (2006) Allergy 61, 1117–1124 [DOI] [PubMed] [Google Scholar]
- 24.Travassos L. H., Carneiro L. A., Ramjeet M., Hussey S., Kim Y. G., Magalhães J. G., Yuan L., Soares F., Chea E., Le Bourhis L., Boneca I. G., Allaoui A., Jones N. L., Nuñez G., Girardin S. E., Philpott D. J. (2010) Nat. Immunol. 11, 55–62 [DOI] [PubMed] [Google Scholar]
- 25.Yano T., Mita S., Ohmori H., Oshima Y., Fujimoto Y., Ueda R., Takada H., Goldman W. E., Fukase K., Silverman N., Yoshimori T., Kurata S. (2008) Nat. Immunol. 9, 908–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasegawa M., Yang K., Hashimoto M., Park J. H., Kim Y. G., Fujimoto Y., Nuñez G., Fukase K., Inohara N. (2006) J. Biol. Chem. 281, 29054–29063 [DOI] [PubMed] [Google Scholar]
- 27.Kawasaki A., Karasudani Y., Otsuka Y., Hasegawa M., Inohara N., Fujimoto Y., Fukase K. (2008) Chem. Eur. J. 14, 10318–10330 [DOI] [PubMed] [Google Scholar]
- 28.Jacobs C., Huang L. J., Bartowsky E., Normark S., Park J. T. (1994) EMBO J. 13, 4684–4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Pedro M. A., Donachie W. D., Höltje J. V., Schwarz H. (2001) J. Bacteriol. 183, 4115–4126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suvorov M., Lee M., Hesek D., Boggess B., Mobashery S. (2008) J. Am. Chem. Soc. 130, 11878–11879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park J. T., Uehara T. (2008) Microbiol. Mol. Biol. Rev. 72, 211–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheurwater E. M., Clarke A. J. (2008) J. Biol. Chem. 283, 8363–8373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hesek D., Lee M., Zhang W., Noll B. C., Mobashery S. (2009) J. Am. Chem. Soc. 131, 5187–5193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor A., Das B. C., van Heijenoort J. (1975) Eur. J. Biochem. 53, 47–54 [Google Scholar]
- 35.Goldman W. E., Klapper D. G., Baseman J. B. (1982) Infect. Immun. 36, 782–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cloud-Hansen K. A., Peterson S. B., Stabb E. V., Goldman W. E., McFall-Ngai M. J., Handelsman J. (2006) Nat. Rev. Microbiol. 4, 710–716 [DOI] [PubMed] [Google Scholar]
- 37.Magalhaes J. G., Philpott D. J., Nahori M. A., Jéhanno M., Fritz J., Le Bourhis L., Viala J., Hugot J. P., Giovannini M., Bertin J., Lepoivre M., Mengin-Lecreulx D., Sansonetti P. J., Girardin S. E. (2005) EMBO Rep. 6, 1201–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heidrich C., Ursinus A., Berger J., Schwarz H., Höltje J. V. (2002) J. Bacteriol. 184, 6093–6099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bouskra D., Brézillon C., Bérard M., Werts C., Varona R., Boneca I. G., Eberl G. (2008) Nature 456, 507–510 [DOI] [PubMed] [Google Scholar]
- 40.Hasegawa M., Kawasaki A., Yang K., Fujimoto Y., Masumoto J., Breukink E., Nuñez G., Fukase K., Inohara N. (2007) J. Biol. Chem. 282, 11757–11764 [DOI] [PubMed] [Google Scholar]
- 41.Meesters R. J., Duisken M., Jähnigen H., Hollender J. (2008) J. Chromatogr. B 875, 444–450 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.