Abstract
Hyaluronan is a component of the extracellular matrix, which affects tissue homeostasis. In this study, we investigated the regulatory mechanisms of one of the hyaluronan-synthesizing enzymes, HAS2. Ectopic expression of Flag- and 6myc-HAS2 in COS-1 cells followed by immunoprecipitation and immunoblotting revealed homodimers; after co-transfection with Flag-HAS3, also heterodimers were seen. Furthermore, the expressed HAS2 was ubiquitinated. We identified one acceptor site for ubiquitin on lysine residue 190. Mutation of this residue led to inactivation of the enzymatic activity of HAS2. Interestingly, K190R-mutated HAS2 formed dimers with wt HAS2 and quenched the activity of wt HAS2, thus demonstrating a functional role of the dimeric configuration.
Keywords: Glycosaminoglycan, Hyaluronate, Mass Spectrometry (MS), Protein Motifs, Protein Stability, Ubiquitin
Introduction
Hyaluronan is abundantly found in tissues throughout the body and has key roles in tissue organization and homeostasis (1). Abnormal accumulation of hyaluronan in tissues and serum is associated with the progression of many diseases, such as inflammatory diseases and cancer (2–5). The biosynthesis of hyaluronan is tightly regulated by growth factors and cytokines as well as other stimuli that promote wound healing, inflammation, or transformation. External regulatory signals, such as platelet-derived growth factor (PDGF)-BB, transforming growth factor (TGF)-β, and phorbol 12-myristate 13-acetate (PMA), regulate both the size (6) and amount of the produced hyaluronan (7–14).
The hyaluronan-synthesizing enzymes (HAS)4 were cloned and characterized first in the bacterium Streptococcus pyogenes, and then in mammals, where three different HAS isoforms were characterized, HAS1, HAS2, and HAS3 (15, 16). Studies by us and other laboratories (6, 11) revealed that growth factor-mediated increase of hyaluronan synthesis is often due to induction of the HAS2 gene in fibroblasts, whereas external signals have been shown to predominantly regulate HAS1 and HAS3 transcripts in synoviocytes and keratinocytes, respectively (12, 14, 17). In addition to the regulation of HASs at the transcriptional level (6, 11, 13, 14), there is evidence that the activity of HAS isoforms is regulated by phosphorylation by protein kinase C (PKC), protein kinase A (PKA), calcium-dependent protein kinase, and extracellular signal-regulated kinase (10, 18–20). Prediction from their amino acid sequences suggests that HASs are multi-pass membrane proteins (15) that occur in the plasma membrane, but there are also evidence that much of the HAS enzymes reside at intracellular localizations including perinuclear membrane, endoplasmic reticulum (ER)-Golgi pathway, and endocytic vesicles (21–23).
Ubiquitination of proteins affects their stability, activity, interaction with other proteins as well as subcellular localization and trafficking (24, 25). Modification of a protein with K48-linked poly-Ub chains can trigger the proteasomal degradation of the protein. The functional consequences of protein ubiquitination by K63-linked poly-Ub chains include activation of proteins or alteration of their trafficking. Protein modification by monoubiquitination, at one or several positions, is associated with protein sorting at the plasma membrane and endosomes, targeting them to the interior of multivesicular bodies, which leads to their transport to lysosomes (24, 26, 27).
Because regulatory ubiquitination modulates the function of cellular proteins similar to that of phosphorylation, we investigated in the present study whether HAS2 is subjected to regulatory ubiquitination, and its consequences for the activity and stability of HAS2. Furthermore, we demonstrate that HAS2 forms homo- and/or hetero-oligomers.
MATERIALS AND METHODS
Antibodies
The following primary antibodies were used: rabbit antisera against HAS2 (CGR; (28)) and against HAS3 (Abcam); mouse monoclonal antibodies against Flag M2 (IgG1, Sigma-Aldrich), c-Myc (9E10 IgG1, Santa Cruz Biotechnology), influenza hemagglutinin A (HA) antiserum (anti-HA, Y-11; Santa Cruz Biotechnology) and to ubiquitin (P4D1 clone IgG1, Santa Cruz Biotechnology); goat anti-mouse and goat anti-rabbit horseradish peroxidase IgG (GE Healthcare).
Construction of Transcripts, Cell Culture, and Transfection
The cDNAs encoding the open reading frames of mouse HAS2 and HAS3 genes in pcNeoI plasmid (29, 30), kindly provided by Dr. A. Spicer, were excised by XhoI/XbaI digestions followed by ligation in Flag-, 6myc-, or HA-pcDNA3 vectors, fused in-frame at their N termini. Plasmids were purified (Plasmid Maxi kit, Qiagen) and sequenced to confirm that the insertions were in-frame. HA-ubiquitin wild-type (HA-Ub wt) and HA-Ub chains mutated at Lys-48 or Lys-63, HA-Ub K48R, and HA-Ub K63R, respectively, cloned in pcDNA3 vectors, were gifts from Drs I. Dikic and K. Haglund (Goethe University, Frankfurt, Germany; Ref. 31).
SV40-transformed African green monkey kidney cells (COS-1) and Chinese hamster ovarian (CHO)-KI cells were grown in Dulbecco′s modified Eagle′s medium (Sigma), 4 mm l-glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 10% fetal calf serum (Invitrogen; Complete medium). Cells were seeded at 2 × 106 cells/60-mm dish and grown overnight until 95% confluence. Transient transfection of the cells was performed using LipofectamineTM 2000 (Invitrogen), according to the manufacturer's recommendations. In some experiments, after 40 h of transfection, the cells were treated for 8 h with the proteasomal inhibitor MG132 (25 μm, Calbiochem). Co-transfection with pEGFP-C1 revealed a transfection efficiency of about 45%. For selection of stable transfected clones expressing pcDNA3/6myc or pcDNA3/6myc-HAS2, 24 h after transfection, CHO-KI cells were passaged in medium containing 600 μg of G418/ml, at 1:10 series dilutions.
Determination of Synthesized Hyaluronan and HAS2 Expression by the Transfectants
Hyaluronan synthesized de novo by untransfected COS-1 cells or COS-1 cells transiently transfected with pcDNA3 vector alone (mock) as well as with tagged HAS2 or HAS3 constructs, was determined after different time periods by using a microtiter-based assay, as described previously (28). Similarly, the hyaluronan-synthesizing capacity of CHO-KI cells stably transfected with 6myc-HAS2, was also determined. This assay is based on the specific binding of hyaluronan to the G1 globular domain of aggrecan. Briefly, conditioned media obtained at different time points after the transfections were added to a 96-well microtiter plate (MaxiSorp Nunc-Immuno plates, Nalge Nunc International) precoated with G1 protein. As a standard, highly purified hyaluronan (0–100 ng/ml, Q-Med, Uppsala, Sweden) were used. After 1 h of incubation at 37 °C, samples were incubated for another hour with biotinylated G1 protein; its binding to hyaluronan was then determined by incubation for 1 h with peroxidase-conjugated streptavidin (1:1600 diluted; RPN 1051, GE Healthcare), followed by washings and incubation for 15 min with 3,3′,5,5′-tetramethyl-benzidine liquid substrate for ELISA (Supersensitive; T4444, Sigma). The reaction was stopped with H2SO4, and the absorbance was measured at 450 nm using an ELISA reader. The intervariation of this assay is less than 10%.
Cell monolayers were washed with cold phosphate-buffered saline and scraped into sucrose buffer: 10 mm Hepes, pH 7.1, 0.5 mm dithiothreitol (DTT), and 0.25 m sucrose, supplemented with protein inhibitors (10 μg/ml leupeptin, 3 μg/ml pepstatin A, 5 μg/ml aprotinin, 0.5 μg/ml pefabloc, 1 mm Na3VO4, 50 mm NaF, 1 mm DTT). Then samples were sonicated and subjected to centrifugation in a Beckman Ultra-Centrifuge TL-100 with a TLS-55 rotor at 43,000 rpm (150,000 × g) for 60 min at 4 °C, using Ultra-Clear centrifuge tubes (11 × 34 mm, Beckman). The high speed pellets (membrane fractions) were resuspended in 600 μl of TBS/Ca2+ buffer (25 mm Tris, 150 mm NaCl, 1 mm CaCl2, pH 7.8), supplemented with 0.1% SDS and 0.5% Nonidet P-40 as well as the protease inhibitors described above, and incubated for 1 h at 4 °C. In some of the experiments, the TBS/Ca2+ buffer was in addition to protease inhibitors supplemented with 1% SDS or 1% digitonin. After centrifugation at 14,000 rpm for 10 min at 4 °C, the protein concentration in the supernatant was measured using the BCA Protein Assay Reagent kit (Pierce). Then, samples were divided to be processed for immunoprecipitation (250 μg of protein each) and immunoblotting (30 μg of protein each).
For immunoblotting, aliquots from membrane fractions were subjected to SDS-PAGE in 10% polyacrylamide gels and the proteins were transferred to nitrocellulose membranes (Hybond-C extra). All samples were reduced with DTT and alkylated with iodoacetamide. Nonspecific binding sites on membranes were blocked for 3 h at room temperature with 3% nonfat dried milk in TBS-T (Tris-buffered saline containing 0.1% Tween 20), followed by overnight incubation with anti-Flag, anti-Myc, or anti-HA monoclonal antibody at dilutions of 1:1000, 1:800, or 1:200 in1% bovine serum albumin/TBS-T, respectively. HASs were also detected with anti-HAS2 IgG (5 μg/ml; Ref. 28) and anti-HAS3 IgG (1 μg/ml). For ubiquitination studies, the anti-ubiquitin P4D1 antibodies were used at a dilution of 1:200. After four washes, membranes were incubated for 1 h with horseradish-peroxidase-conjugated goat anti-mouse IgG at a 1:10,000 dilution in blocking solution. The immunocomplexes were visualized using enhanced chemiluminescence according to the manufacturer's instructions. For re-blotting, the membranes were stripped with stripping buffer (0.2 m NaOH, 0.5 m NaCl) for 10 min and reblotted with antibodies, as specified above. Silver staining was performed, as described before (32).
For co-immunoprecipitation, sample aliquots from membrane fractions solubilized in TBS/Ca2+ buffer containing 0.1% SDS and 0.5% Nonidet P-40 or 1% digitonin were precleared by protein G-Sepharose (50% slurry in TBS/Ca2+ buffer; GE Healthcare) before 2 μg of anti-Flag, 1 μg of anti-Myc, or 1 μg of anti-HA monoclonal antibodies were added followed by 2 h incubation at 4 °C. Samples solubilized with TBS/Ca2+ buffer containing 1% SDS, were diluted 2-fold before pre-clearing. Then, 30 μl of protein G-Sepharose was added, and the mixtures were incubated for an additional 1 h, at 4 °C, followed by washing (3500 rpm for 3 min). After resuspension in loading buffer, samples were subjected to immunoblotting.
Analysis of Proteins by MALDI-TOF Mass Spectrometry
Bands observed at molecular masses of 53–58 kDa (for the Flag-HAS2 transfectants), 65–74 kDa (for the 6myc-HAS2 transfectants) and molecular mass species of about 120 kDa were excised from silver-stained gels and treated for in-gel digestion, as described (33). In brief, the bands were destained and dried, then trypsin (sequence grade porcine, modified, Promega) was added, and the samples were digested overnight at 30 °C. The peptide mixtures were concentrated and desalted on a μZipTip C18 (Millipore) and applied directly to the sample target. The peptide mass fingerprints were obtained on an Ultraflex TOF/TOF MALDI mass spectrometer (Bruker Daltonics) using α-cyano-4-hydroxy-cinnamic acid as matrix. The identities of the protein bands were obtained by scanning the sequence data base NCBInr using the search engine ProFound with the peptide mass lists.
The potential presence of Ub in samples of slower migrating species with about 5–9 kDa higher molecular mass was analyzed using GPMAW (Lighthouse Data, Odense, Denmark) by allowing the addition of tryptic peptides from the C-terminal part of Ub to a lysine residue, i.e. GG (114.05 Da) or LRGG (383.24 Da), to the mass of the predicted tryptic peptides of HAS2.
Site-directed Mutagenesis
Point mutants of Lys-190 in pcDNA3-Flag-HAS2 and pcDNA3–6myc-HAS2 were generated by site-directed mutagenesis. The following mutagenic primer and its complementary were designed to change Lys to Arg (R) at position 190 (K190R; primer is shown in the sense orientation with the altered codon in boldface, 5′-ATGCAAAAATGGGGTGGAAGGAGAGAAGTCATGTACACAGCC-3′).
The site-directed mutagenesis was performed using two QuikChange (Stratagene), according to the manufacturer's instructions. The pcDNA3 plasmid containing either the Flag-HAS2 or the 6myc-HAS2 transcripts were used as a template for the primer extension reactions with the pair of mutagenic primers. Mutant strand synthesis reaction was performed using the polymerase chain reaction (PCR) and pfu DNA polymerase. PCR was performed for 18 cycles: 95 °C for 15 s, 55 °C for 15 s, and 78 °C for 3 min. The amplification products were treated with DpnI to digest the parental methylated and hemimethylated DNA at 37 °C for 1 h. The digested reaction was transformed into DH5α competent cells, purified using the Nucleospin Plasmid (Macherey-Nagel), and colonies were screened for the desired mutations by sequencing isolated DNA using ABI Prism 310 genetic Analyzer (Applied Biosystems).
RESULTS
HAS2 and HAS3 Proteins Form Homo- and Hetero-oligomers
The expression and activity of Flag-HAS2 and 6myc-HAS2 proteins were studied in transiently transfected COS-1 cells. Both constructs gave rise to active HAS molecules, as revealed by increased hyaluronan synthesis (Fig. 1A and subsequent figures).
FIGURE 1.
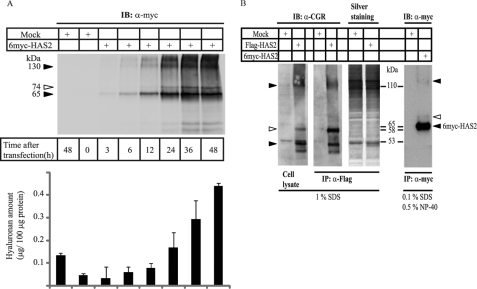
Structure and activity of HAS2 expressed in COS-1 cells. A, membrane fractions from COS-1 cells transiently transfected with 6myc-HAS2 or with empty vector (mock), were solubilized in TBS/Ca2+ buffer supplemented with 1% SDS. Total cell lysates were separated by SDS-PAGE followed by immunoblotting (IB) with Myc antibodies to detect the expression of 6myc-HAS2. The hyaluronan content in conditioned media at different time points was determined as described under “Materials and Methods.” The error bars indicate ranges of duplicate samples. B, membrane fractions from COS-1 cells transiently transfected with 6myc-HAS2, Flag-HAS2, or transfected with empty vectors were solubilized in TBS/Ca2+ buffer supplemented with 1% SDS or 0.1% SDS and 0.5% Nonidet P-40. Total cell lysates or immunoprecipitated (IP) material were separated by SDS-PAGE, and the gels were subjected to silver-staining or IB, as described under “Materials and Methods.” Immunoreactive bands were visualized with antibodies against HAS2 (α-CGR) and myc. Filled arrowheads indicate the position of oligomeric- and polymeric-tagged HAS2 species. Open arrowheads indicate a slower migrating species of tagged HAS2. Data in panels A and B represent one of two and one of three experiments with similar results, respectively.
Total cell lysate as well as anti-Flag- or anti-Myc-immunoprecipitated material were subjected to SDS-PAGE followed by immunoblot analysis (Fig. 1, A and B). Initially, as a way to ensure efficient solubilization of Flag- and 6myc-HAS2 proteins from COS-1 membrane fraction, the ionic detergent SDS at a concentration of 1% was used. In addition to the 65-kDa 6myc-HAS2 species, bands of 74 kDa and about 130–170 kDa (Fig. 1A) were detected. Notably, the 74-kDa component (marked with an open arrowhead) was readily detected 24–48 h after transfection, coincident with increase in the amount of synthesized hyaluronan by 6myc-HAS2 transfectants. Similarly, in addition to the Flag-HAS2 band of a molecular mass of about 53 kDa other immunoreactive bands of 58 kDa and about 110 kDa were seen using anti-CGR antibodies against the Flag-HAS2 (Fig. 1B and Ref. 28). Similar results were obtained by extraction with TBS/Ca2+ buffer supplemented with 1% digitonin (supplemental Fig. S1) or supplemented with 0.1% SDS and 0.5% Nonidet P-40, which was used for all subsequent experiments (Fig. 1B). No differences in the pattern of immunoreactive bands were observed when the SDS-PAGE was performed under non-reducing compared with reducing conditions (data not shown).
To identify the silver-stained proteins (Fig. 1B), analysis by mass spectrometry and peptide mass fingerprinting was performed. This analysis identified the 53-kDa band to be the mouse HAS2 isoform (Table 1, sequence coverage was 32%). In addition, material excised from the 110-kDa species of silver-stained gels indicated the presence of HAS2 (the sequence coverage was 18%), suggesting that HAS2 may form dimers (data not shown).
TABLE 1.
Identification of mouse HAS2 by peptide mass fingerprint analysis
The 53 kDa band from silver-stained SDS-PAGE was subjected to tryptic in-gel digestion and analysis of peptides by MALDI-TOF. An unbiased search (all species, sizes up to 3000 kDa, one-allowed missed cleavage) resulted in identification of HAS2 from mouse with high probability (9.7 × 10−7). This table shows the 17 tryptic peptides identified making up 32% of the mouse HAS2 sequence.
| Measured mass | Calculated mass | Error (Da) | Residues Start To | Missed cut | Sequence | |
|---|---|---|---|---|---|---|
| 806.446 | 806.447 | −0.001 | 6 | 11 | 0 | FLCVLR |
| 926.430 | 926.428 | 0.002 | 421 | 428 | 0 | SSFASCLR |
| 944.493 | 944.482 | 0.010 | 347 | 353 | 0 | WLNQQTR |
| 1015.495 | 1015.479 | 0.015 | 192 | 199 | 0 | EVMYTAFR |
| 1171.600 | 1171.580 | 0.019 | 191 | 199 | 1 | REVMYTAFR |
| 1228.595 | 1228.569 | 0.026 | 260 | 268 | 0 | YWMAFNIER |
| 1393.712 | 1393.690 | 0.022 | 336 | 346 | 0 | CLTETPIEYLR |
| 1458.736 | 1458.714 | 0.022 | 248 | 259 | 0 | YDSWISFLSSVR |
| 1523.721 | 1523.701 | 0.020 | 361 | 371 | 0 | EWLYNAMWFHK |
| 1568.815 | 1568.815 | −0.000 | 166 | 179 | 0 | ESSQHVTQLVLSNK |
| 1996.949 | 1996.956 | −0.007 | 84 | 100 | 0 | TVALCIAAYQEDPDYLR |
| 2068.035 | 2068.050 | −0.015 | 228 | 247 | 0 | VLEEDPMVGGVGGDVQILNK |
| 2125.046 | 2125.050 | −0.004 | 84 | 101 | 1 | TVALCIAAYQEDPDYLRK |
| 2353.017 | 2353.010 | 0.007 | 269 | 288 | 0 | ACQSYFGCVQCISGPLGMYR |
| 2369.038 | 2369.005 | 0.033 | 269 | 288 | 0 | ACQSYFGCVQCISGPLGMoxYR |
| 2620.314 | 2620.271 | 0.043 | 156 | 179 | 1 | GPGETEESHKESSQHVTQLVLSNK |
| 2674.247 | 2674.180 | 0.067 | 204 | 227 | 0 | SVDYVQVCDSDTMLDPASSVEMVK |
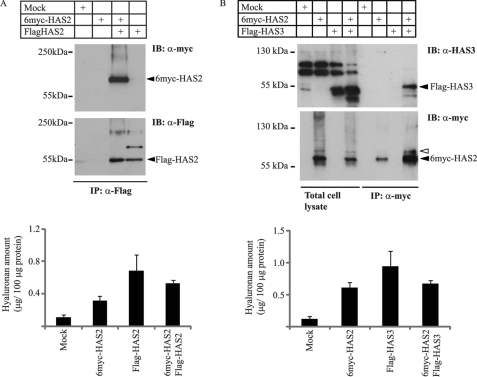
To verify that HAS2 forms homodimers, a co-immunoprecipitation experiment was performed. Immunoprecipitation by Flag antibodies of lysate from COS-1 cells transfected with Flag-HAS2 and 6myc-HAS2, followed by immunoblotting with Myc antibodies revealed a band at the expected size (Fig. 2A), suggesting the formation of dimers or oligomers. To investigate whether the HAS2 protein in addition to homo-oligomers, can form hetero-oligomers with another HAS isoform, COS-1 cells were co-transfected with 6myc-HAS2 and Flag-HAS3. After immunoprecipitation with Myc anti-serum, immunoblotting with anti-HAS3 antibodies revealed a band of a molecular mass of about 55 kDa, i.e. a similar size as that of the HAS3 band seen in total cell lysates (Fig. 2B). As a control, the filter was then stripped and blotted with a Myc antiserum, which revealed the monomeric 6myc-HAS2 band and the about 8 kDa higher immunoreactive band (marked with an open arrowhead). These data demonstrate that HAS2 and HAS3 isoforms interact and form heteromeric complexes. The functionality of the transfected 6myc-HAS2 and Flag-HAS3, as well as combinations thereof, was confirmed by the production of hyaluronan in the conditioned media (Fig. 2, A and B).
FIGURE 2.
6myc-HAS2 forms homo-oligomers and hetero-oligomers with Flag-HAS3. 6myc-HAS2, Flag-HAS2, and/or Flag-HAS3 were transiently transfected into COS-1 cells. Lysates were subjected to immunoprecipitation with antibodies against Flag- or 6myc-HAS2, samples were resolved by SDS-PAGE and possible homo-oligomerization (A) as well as hetero-oligomerization (B) were analyzed with immunoblotting with anti-Myc or anti-Flag antibodies, as well as with an antiserum against HAS3. The hyaluronan content in the 48 h conditioned media was measured as described under “Materials and Methods.” Data in panels A and B are the mean of duplicate ± variation from a representative experiment out of two with similar results.
HAS2 Is Both Mono- and Polyubiquitinated
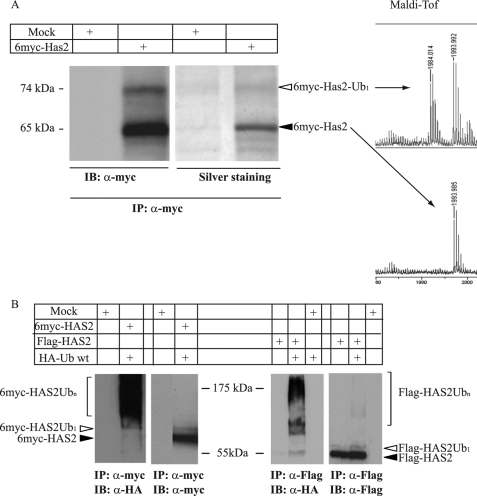
Because monoubiquitination of proteins is characterized by about an 8-kDa mobility shift on SDS-PAGE, we hypothesized that the slower migrating components of apparent molecular masses of 5–8 kDa higher than the monomeric HAS2 isoforms (denoted with open arrowheads, Figs. 1 and 2) could reflect monoubiquitination of Flag-HAS2 (58 kDa) and 6myc-HAS2 (74 kDa). As shown in Fig. 3A, MALDI-TOF analyses of the 65 and 74 kDa bands of the silver-stained species indicated that they were both derived from HAS2 (minimum sequence coverage of 30%). Furthermore, the peptide mass lists were scanned for the addition of GG or LRGG (the C-terminal tryptic peptides of ubiquitin) onto lysine residues in HAS2. A peptide of mass 1984.014 Da was found in the 74 kDa form, but not in the 65 kDa form, suggesting that the peptide 187-WGGK*REVMYTAFR-199, was ubiquitinated at Lys-190 (K190; indicated by an asterisk); the presence of the bulky Ub on Lys-190 may explain why trypsin did not cleave after Lys-190 or Arg-191 during the digestion of 6myc-HAS2. Monoubiquitination on Lys-190 could also be determined in another cell type, i.e. CHO cells stably transfected with 6myc-HAS2, that exhibits a hyaluronan-synthesizing capacity of about 0.3 μg of hyaluronan per 100 μg of protein (supplemental Fig. S2).
FIGURE 3.
Mono- and polyubiquitination of HAS2 expressed by COS-1 cells. COS-1 cells were transfected with 6myc-HAS2, Flag-HAS2, or a mock construct in the absence or presence of HA-Ub. A, lysates were subjected to immunoprecipitation by Myc antibody followed by SDS-PAGE and immunoblot analysis with antibodies against Myc or silver staining. The immunoreactive bands of 65 and 74 kDa were cut out from the silver-stained gel and analyzed with MALDI-TOF. B, immunoprecipitation was also performed with antibodies against myc-HAS2 (myc) or Flag-HAS2 (Flag), followed by immunoblot analysis with Myc or Flag as well as with antibodies against HA-Ub (HA). Data in A and B are representative experiments out of two and three experiments with similar results, respectively.
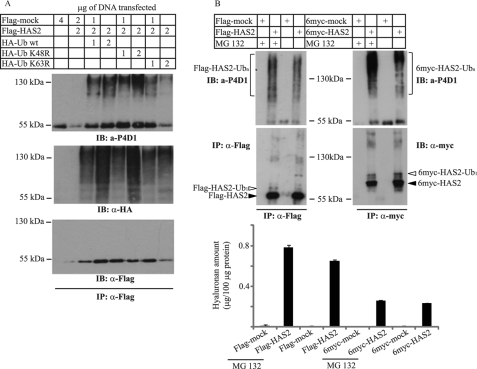
To investigate further the ubiquitination of HAS2, COS-1 cells were co-transfected with 6myc- or Flag- HAS2 and HA-tagged ubiquitin (HA-Ub wt) (Fig. 3B). Smeary bands were detected by immunoblotting with HA antibodies from 6myc-HAS2 or Flag-HAS2 immunoprecipitates, indicating that HAS2 is post-translationally modified by polyubiquitin molecules, in addition to monoubiquitination (denoted with open arrowheads). We then investigated whether the polyubiquitin chains on the HAS2 protein were linked at Lys-48 and/or Lys-63 of ubiquitin, by comparing the incorporation into HAS2 of wt ubiquitin (HA-Ub wt), and K48R (HA-Ub K48R) or K63R (HA-Ub K63R) point mutants of Ub. Immunoprecipitation of transfected HAS2 followed by immunoblotting by the antibody P4D1 which recognizes both poly- and monoubiquitinated protein and an anti-HA antibody revealed strong smeary bands after transfection of wt HA-Ub, as well as K48R and K63R mutant ubiquitin (Fig. 4A). Altogether, these results suggest that HAS2 molecules are modified with polyubiquitin chains linked to both Lys-48 and Lys-63 residues.
FIGURE 4.
Effects of MG132 on polyubiquitination and activity of tagged HAS2. A, Flag-pcDNA3 vector and Flag-HAS2 alone or together with HA-Ub (either wt or the K48R and K63R Ub mutants) were expressed in COS-1 cells at different amounts. Cell extracts were subjected to immunoprecipitation with antibodies against Flag-HAS2 (Flag) and sequential immunoblot analysis with antibodies against HA-Ub (P4D1), Flag-HAS2 (Flag), and anti-HA. B, mock-transfected and Flag- or 6myc-HAS2 transfected cells were treated with the proteasomal inhibitor MG132 (25 μm) for 8 h. Cell lysates were subjected to immunoprecipitation with antibodies against Flag-HAS2 (Flag) and 6myc-HAS2 (myc), followed by immunoblot analysis with antibodies against tagged HAS2, then the membranes were stripped and reprobed with anti-HA-Ub (P4D1). Conditioned media, after 48 h of incubation, were analyzed for hyaluronan content as described under “Materials and Methods.” Each value depicts mean of triplicates ± S.D. Data in A and B represent one of three experiments performed with similar results.
Because Lys-48-linked polyubiquitination is linked to protein turnover by targeting for degradation in proteasomes, we evaluated the physiological importance of polyubiquitination for the proteasomal degradation of HAS2. As shown in Fig. 4B, ectopic overexpression of Flag- and 6myc-HAS2 in the presence of the proteasomal inhibitor MG132 increased slightly the pool of polyubiquitinated HAS2, as determined by the anti-ubiquitin antibody P4D1. Notably, blocking of proteasome-mediated degradation did not significantly affect the expression levels of Flag- and 6myc-HAS2 proteins, nor either their hyaluronan-synthesizing capacity (Fig. 4B), suggesting that only a minor pool of HAS2 were polyubiquitinated, possibly reflecting not properly folded molecules.
Monoubiquitination of HAS2 Is Important for Its Hyaluronan-synthesizing Capacity
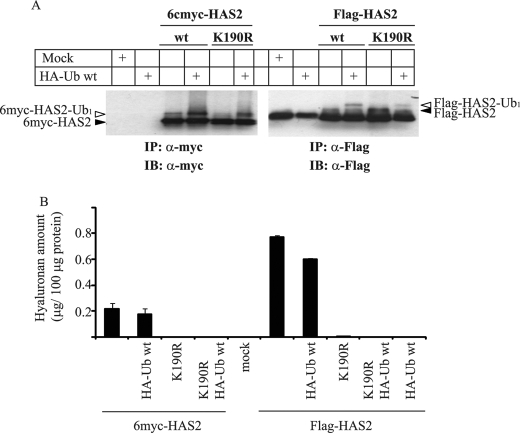
To verify that HAS2 is monoubiquitinated on Lys-190, we created tagged HAS2 K190R point mutants. COS-1 cells were co-transfected with HA-Ub along with Flag- and 6myc-HAS2, as well as Flag- and 6myc-HAS2 K190R mutants. The cells were then lysed, followed by immunoprecipitation by Flag or Myc antibodies; samples were subjected to SDS-PAGE and immunoblot analysis with antibodies to Myc or Flag. A slower migrating species of tagged wt HAS2 were seen in the presence of HA-Ub wt (open arrowheads, Fig. 5A); however, such bands were less evident upon ectopic expression of the Flag- or 6myc-HAS2 K190R mutants. These findings verified that Lys-190 is a major, although not the only, ubiquitinated site. The size of the modified HAS2 molecule suggests that Lys-190 is monoubiquitinated, but we cannot exclude the possibility that it is also poly- and/or multiubiquitinated.
FIGURE 5.
Lys-190 in Flag- and 6myc-HAS2 is monoubiquitinated and crucial for HAS2 enzymatic activity. A, COS-1 cells were transfected with 2 μg of either 6myc- or Flag-HAS2 constructs alone, as well as with 2 μg of their respective K190R mutants. Some cell cultures were also transfected with wt HA-Ub (1 μg). Samples were subjected to immunoprecipitation with antibodies against 6myc-HAS2 (myc) or Flag-HAS2 (Flag), followed by immunoblot analysis with antibodies against Myc and Flag, respectively. Filled arrowheads indicate the position of ubiquitinated-tagged HAS2 species. Open arrowheads point out the slower migrating monoubiquitinated forms (Ub1). B, conditioned media, after 48 h of transfection, were analyzed for hyaluronan content, as described under “Materials and Methods.” Each value depicts means of duplicates ± variation. Data in A and B represent one of three experiments performed with similar results.
Monoubiquitination has been demonstrated to regulate localization and activity of certain proteins by proteasome-independent cellular processes (34, 35). To explore the physiological role of Lys-190 monoubiquitination of the HAS2 protein and its possible importance for HAS2 activity, we investigated the hyaluronan-synthesizing capacity of HAS2 K190R mutants in comparison to wt HAS2. Whereas overexpression of 6myc-HAS2 or Flag-HAS2 induced higher levels of hyaluronan production compared with mock transfectants, overexpression of the corresponding K190R HAS2 mutants did not induce any hyaluronan production (Fig. 5B), suggesting that monoubiquitination of HAS2 at Lys-190 is important for the enzymatic activity of HAS2.
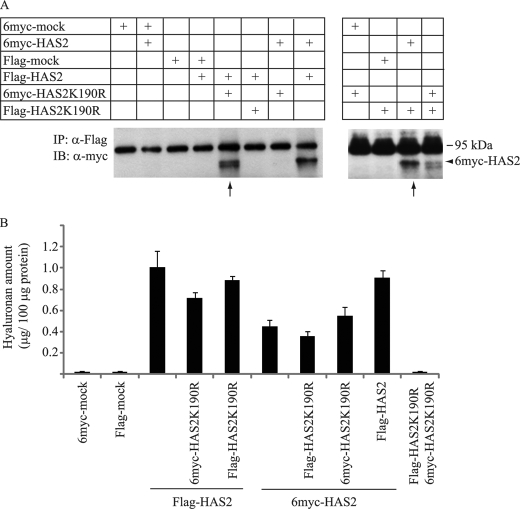
We then examined the role of Lys-190 monoubiquitination for the formation of oligomeric HAS2 structures. Immunoprecipitation with antibodies against Flag, followed by immunoblotting with anti-Myc antibodies, revealed heterodimers or oligomers between wt HAS2 and HAS2 K190R mutant molecules (Fig. 6A; thin arrows), as well as homomeric complexes of wt or K190R mutant HAS2 molecules (Fig. 6A). Thus, monoubiquitination is not necessary for dimerization of HAS2.
FIGURE 6.
HAS2 K190R mutants can form oligomers with wt HAS2. A, COS-1 cells were transfected with 2 μg of either wt 6myc- or Flag-HAS2 constructs with or without 2 μg of their respective K190R mutants. Samples were then subjected to immunoprecipitation with antibodies against Flag-HAS2 (Flag), followed by immunoblot analysis with antibodies against Myc. Thin arrows indicate co-immunoprecipitation of wt 6myc- and Flag-HAS2 with K190R mutants. B, bar graph shows the hyaluronan content in 48 h conditioned media of cells transfected with the indicated vectors. Each value depicts the means of triplicates ± S.D. Data in A and B, are representative experiments out of four experiments performed with similar results.
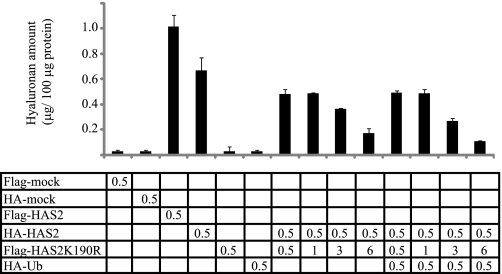
Importantly, analysis of the hyaluronan content in the conditioned media of COS-1 cells co-transfected with equal amounts of HAS2 wt and K190R mutant constructs, revealed slightly lower levels of hyaluronan compared with conditioned medium of COS-1 cells transfected with wt HAS2 (Fig. 6B). To evaluate further the functional significance of monoubiquitination of HAS2 at the Lys-190 residue, COS-1 cells were co-transfected with wt HAS2 and increasing concentrations of the HAS2 K190R mutant (Fig. 7). COS-1 cells transfected with wt Flag or HA-tagged HAS2 synthesized hyaluronan whereas Flag-HAS2K190R mutants did not exhibit any detectable hyaluronan-synthesizing capacity. Expression of increasing amounts of the K190R mutant HAS2 molecule gradually decreased the synthesis of hyaluronan (Fig. 7). Because wt and K190R mutant HAS2 form dimers, these results suggest that the functional form of HAS2 is a dimer and that monoubiquitination of Lys-190 is crucial for the activity of HAS2 in the dimer. In addition, overexpression of wt HA-Ub did not significantly decrease the HAS2-induced hyaluronan production, suggesting that HAS2 ubiquitination at other sites than Lys-190 does not regulate HAS2 hyaluronan synthesizing activity and does not interfere with the structural organization of the oligomeric forms.
FIGURE 7.
Increased expression of Flag-HAS2 K190R suppresses the HA-HAS2-induced hyaluronan production. COS-1 cells were transfected with 0.5 μg of HA-wt HAS2, Flag-HAS2 K190R, HA-Ub, or mock alone. Some of the HA-HAS2 transfected cultures were co-transfected with increasing amounts of Flag-HAS2 K190R constructs (0.5–6 μg) without or together with 0.5 μg of wt HA-Ub. Conditioned media, after 48 h of transfection, were analyzed for hyaluronan content as described under “Materials and Methods.” Each value depicts means of triplicates ± S.D. The data shown are representative of one out of four experiments with similar results.
DISCUSSION
We show in the present report that HAS2 forms dimers or oligomers, and that HAS2 activity is regulated by ubiquitination. HAS2 dimerization or oligomerization was demonstrated by co-immunoprecipitation of differentially tagged HAS2 molecules. Interestingly, HAS2 oligomers could be detected in SDS-PAGE gels after extraction in TBS/Ca2+ buffer, supplemented with 0.1% SDS and 0.4% Nonidet P-40, or with the mild non-anionic detergent digitonin (supplemental Fig. S1), but also after extraction in TBS/Ca2+ buffer supplemented with 1% SDS (Fig. 1). Evidence for SDS-resistant HAS2 dimers in CHO cells stably transfected by HAS2 was also obtained (data not shown). Thus, the dimers are kept together by strong interactions. In a recent study it was demonstrated that HAS1 forms multimers through intermolecular disulfide bonds (36). However, the HAS2 dimers we observed were seen both in the presence and absence of reducing agents, making it unlikely that disulfide bonds are involved. The nature of the bonds that keep the SDS-resistant HAS2 dimers together remains to be elucidated. The observation of a dimeric structure of HAS2 is consistent with our earlier findings that the HAS activity may reside in a large protein complex (37).
An indication that HAS2 dimerization may be of functional importance came from the other finding of our study, i.e. our demonstration that HAS2 is ubiquitinated. We identified one acceptor site for ubiquitination, i.e. Lys-190 (Table 1), in transiently transfected COS cells (Fig. 3A) and in stably transfected CHO cells (supplemental Fig. S2). Interestingly, mutation of this residue led to inactivation of the enzymatic activity of HAS2. Moreover, the K190R mutant HAS2 was shown to dimerize with wt HAS2, and suppressed the activity of wt HAS2 in such dimers. Our data suggest that monoubiquitination of Lys-190 of both HAS2 subunits are necessary for activity.
Another conclusion from the observation that K190R mutant HAS2 can inhibit the activity of HAS2 is that a dimeric, or possibly oligomeric, structure of HAS2 is necessary for its function. The reason for the need for dimerization or oligomerization of HAS2 for its activity remains to be elucidated. It should be noted, however, that hyaluronan is secreted through the plasma membrane in conjunction with its synthesis and that HAS isoforms are rather small transmembrane proteins; possibly more than one HAS molecule is needed to form a pore through which hyaluronan is extruded. Notably, there are indications that phospholipids, such as cardiolipin, are required for Streptococcus pyogenes HAS activity (38, 39). It is possible that the activity of mammalian HAS can also be affected by the lipids in the plasma membrane. Furthermore, it has been reported that an ABC transporter system is involved in the transport of hyaluronan to the extracellular space (40, 41). Thus, it is possible that the activities of mammalian enzymes and the amounts of hyaluronan synthesized, are affected by the oligomeric state of HAS molecules, monoubiquitination, ABC transport systems, and phospholipid microenvironment.
Another interesting question for future studies is to elucidate the exact role of ubiquitination for HAS2 function. It is possible that ubiquitination of Lys-190 is needed for the docking of the substrates required for hyaluronan synthesis, or for the actual catalysis. Noteworthy, Lys190 is located in the HAS2 glycosyltransferase-2 conserved domain. Significantly, amino acid sequence alignment between vertebrate HASs within the glycosyltransferase region, shows that Lys-190 is conserved among all other HAS isoforms, suggesting that it may have a key role for the function of HAS (42). Interestingly, some ubiquitination remained in the K190R mutant, suggesting that there are also other sites of ubiquitination in HAS2, the function of which remains to be determined.
Supplementary Material
This work was supported in part by grants from the Swedish Cancer Society, Wenner-Gren Foundation, and Mizoutani Foundation for Glycoscience.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- HAS
- hyaluronan synthase
- wt
- wild type
- Ub
- ubiquitin
- MALDI-TOF
- matrix-assisted laser desorption/ionization-time of flight
- HA
- hemagglutinin.
REFERENCES
- 1.Laurent T. C., Fraser J. R. E. (1992) FASEB J. 6, 2397–2404 [PubMed] [Google Scholar]
- 2.Stuhlmeier K. M. (2006) Wien. Med. Wochenschr. 156, 563–568 [DOI] [PubMed] [Google Scholar]
- 3.Toole B. P. (2004) Nat. Rev. Cancer 4, 528–539 [DOI] [PubMed] [Google Scholar]
- 4.Toole B. P., Slomiany M. G. (2008) Semin. Cancer Biol. 18, 244–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Itano N., Kimata K. (2008) Semin. Cancer Biol. 18, 268–274 [DOI] [PubMed] [Google Scholar]
- 6.Jacobson A., Brinck J., Briskin M. J., Spicer A. P., Heldin P. (2000) Biochem. J. 348, 29–35 [PMC free article] [PubMed] [Google Scholar]
- 7.Heldin P., Laurent T. C., Heldin C. H. (1989) Biochem. J. 258, 919–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heldin P., Asplund T., Ytterberg D., Thelin S., Laurent T. C. (1992) Biochem. J. 283, 165–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heldin P., Pertoft H. (1993) Exp. Cell Res. 208, 422–429 [DOI] [PubMed] [Google Scholar]
- 10.Suzuki M., Asplund T., Yamashita H., Heldin C. H., Heldin P. (1995) Biochem. J. 307, 817–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Recklies A. D., White C., Melching L., Roughley P. J. (2001) Biochem. J. 354, 17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karvinen S., Pasonen-Seppänen S., Hyttinen J. M., Pienimäki J. P., Törrönen K., Jokela T. A., Tammi M. I., Tammi R. (2003) J. Biol. Chem. 278, 49495–49504 [DOI] [PubMed] [Google Scholar]
- 13.Berdiaki A., Zafiropoulos A., Fthenou E., Katonis P., Tsatsakis A., Karamanos N. K., Tzanakakis G. N. (2008) Biochim. Biophys. Acta 1780, 194–202 [DOI] [PubMed] [Google Scholar]
- 14.Stuhlmeier K. M., Pollaschek C. (2004) J. Biol. Chem. 279, 8753–8760 [DOI] [PubMed] [Google Scholar]
- 15.Weigel P. H., Hascall V. C., Tammi M. (1997) J. Biol. Chem. 272, 13997–14000 [DOI] [PubMed] [Google Scholar]
- 16.Spicer A. P., Kaback L. A., Smith T. J., Seldin M. F. (1998) J. Biol. Chem. 273, 25117–25124 [DOI] [PubMed] [Google Scholar]
- 17.Sayo T., Sugiyama Y., Takahashi Y., Ozawa N., Sakai S., Ishikawa O., Tamura M., Inoue S. (2002) J. Invest. Dermatol. 118, 43–48 [DOI] [PubMed] [Google Scholar]
- 18.Klewes L., Prehm P. (1994) J. Cell. Physiol. 160, 539–544 [DOI] [PubMed] [Google Scholar]
- 19.Goentzel B. J., Weigel P. H., Steinberg R. A. (2006) Biochem. J. 396, 347–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bourguignon L. Y., Gilad E., Peyrollier K. (2007) J. Biol. Chem. 282, 19426–19441 [DOI] [PubMed] [Google Scholar]
- 21.Müllegger J., Rustom A., Kreil G., Gerdes H. H., Lepperdinger G. (2003) Biol. Chem. 384, 175–182 [DOI] [PubMed] [Google Scholar]
- 22.Hascall V. C., Majors A. K., De La Motte C. A., Evanko S. P., Wang A., Drazba J. A., Strong S. A., Wight T. N. (2004) Biochim. Biophys. Acta 1673, 3–12 [DOI] [PubMed] [Google Scholar]
- 23.Rilla K., Siiskonen H., Spicer A. P., Hyttinen J. M., Tammi M. I., Tammi R. H. (2005) J. Biol. Chem. 280, 31890–31897 [DOI] [PubMed] [Google Scholar]
- 24.Salmena L., Pandolfi P. P. (2007) Nat. Rev. Cancer 7, 409–413 [DOI] [PubMed] [Google Scholar]
- 25.Acconcia F., Sigismund S., Polo S. (2008) Exp. Cell Res. 315, 1610–1618 [DOI] [PubMed] [Google Scholar]
- 26.Pickart C. M., Fushman D. (2004) Curr. Opin. Chem. Biol. 8, 610–616 [DOI] [PubMed] [Google Scholar]
- 27.Mukhopadhyay D., Riezman H. (2007) Science 315, 201–205 [DOI] [PubMed] [Google Scholar]
- 28.Li L., Asteriou T., Bernert B., Heldin C. H., Heldin P. (2007) Biochem. J. 404, 327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spicer A. P., Augustine M. L., McDonald J. A. (1996) J. Biol. Chem. 271, 23400–23406 [DOI] [PubMed] [Google Scholar]
- 30.Spicer A. P., Olson J. S., McDonald J. A. (1997) J. Biol. Chem. 272, 8957–8961 [DOI] [PubMed] [Google Scholar]
- 31.Haglund K., Di Fiore P. P., Dikic I. (2003) Trends Biochem. Sci. 28, 598–603 [DOI] [PubMed] [Google Scholar]
- 32.Schevchenko V. A., Akayeva E. A., Yeliseyeva I. M., Yelisova T. V., Yofa E. L., Nilova I. N., Syomov A. B., Burkart W. (1996) Mutat. Res. 361, 29–34 [DOI] [PubMed] [Google Scholar]
- 33.Hellman U. (2000) EXS. 88, 43–54 [DOI] [PubMed] [Google Scholar]
- 34.Hicke L., Dunn R. (2003) Annu. Rev. Cell Dev. Biol. 19, 141–172 [DOI] [PubMed] [Google Scholar]
- 35.Leithe E., Rivedal E. (2004) J. Biol. Chem. 279, 50089–50096 [DOI] [PubMed] [Google Scholar]
- 36.Ghosh A., Kuppusamy H., Pilarski L. M. (2009) J. Biol. Chem. 284, 18840–18850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asplund T., Brinck J., Suzuki M., Briskin M. J., Heldin P. (1998) Biochim. Biophys. Acta 1380, 377–388 [DOI] [PubMed] [Google Scholar]
- 38.Tlapak-Simmons V. L., Baggenstoss B. A., Clyne T., Weigel P. H. (1999) J. Biol. Chem. 274, 4239–4245 [DOI] [PubMed] [Google Scholar]
- 39.Weigel P. H., DeAngelis P. L. (2007) J. Biol. Chem. 282, 36777–36781 [DOI] [PubMed] [Google Scholar]
- 40.Prehm P., Schumacher U. (2004) Biochem. Pharmacol. 68, 1401–1410 [DOI] [PubMed] [Google Scholar]
- 41.Schulz T., Schumacher U., Prehm P. (2007) J. Biol. Chem. 282, 20999–21004 [DOI] [PubMed] [Google Scholar]
- 42.Spicer A. P., McDonald J. A. (1998) J. Biol. Chem. 273, 1923–1932 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.