Abstract
The transition from latent to lytic phases of the Epstein-Barr virus life cycle is triggered by expression of a viral transactivator, BZLF1, that then induces expression of the viral immediate-early and early genes. The BZLF1 protein is post-translationally modified by a small ubiquitin-related modifier-1 (SUMO-1). Here we found that BZLF1 is conjugated at lysine 12 not only by SUMO-1 but also by SUMO-2 and 3. The K12R mutant of BZLF1, which no longer becomes sumoylated, exhibits stronger transactivation than the wild-type BZLF1 in a reporter assay system as well as in the context of virus genome with nucleosomal structures. Furthermore, exogenous supply of a SUMO-specific protease, SENP, caused de-sumoylation of BZLF1 and enhanced BZLF1-mediated transactivation. Immunoprecipitation experiments proved that histone deacetylase 3 preferentially associated with the sumoylated form of BZLF1. Levels of the sumoylated BZLF1 increased as lytic replication progressed. Based on these observations, we conclude that sumoylation of BZLF1 regulates its transcriptional activity through histone modification during Epstein-Barr virus productive replication.
Keywords: Herpesvirus, Histone Acetylase, Histone Deacetylase, Oncogenic Viruses, Sumoylation, BZLF1, EBV
Introduction
The Epstein-Barr virus (EBV)2 is a human γ-herpesvirus that predominantly establishes latent infection in B lymphocytes and epithelial cells. Only a small percentage of infected cells switch from the latent stage into the lytic cycle to produce progeny viruses. This reactivation has been associated with the emergence of human cancers (1, 2), suggesting that the EBV switching mechanism is a key determinant of EBV pathogenesis. Although the details of EBV reactivation in vivo are not fully understood, it is known that viral lytic replication can be achieved by treatment of latently infected B cells with some chemical or biological reagents such as 12-O-tetradecanoylphorbol-13-acetate, calcium ionophore, sodium butyrate, or anti-immunoglobulin. Through different pathways these reagents lead to expression of two transcriptional regulators, BZLF1 (also known as Zta or ZEBRA) and BRLF1 (Rta), which are the products of the two immediate-early genes, BZLF1 and BRLF1. BZLF1 is a transcriptional activator that shares structural similarities to the basic leucine zipper (b-Zip) family transcriptional factors and is involved in the activation of replication origin, oriLyt, used in the lytic cycle. BZLF1 expression alone can trigger the entire reactivation cascade, suggesting that this is a primary event (3–5).
In latently infected cells, EBV DNA is present as multicopy episomes assembled into nucleosomal structures that are similar to cellular chromatin (6). Changes in histone acetylation in the BZLF1 promoter region results in activation of BZLF1 gene expression leading to reactivation from latency, as treatment with histone deacetylase (HDAC) inhibitors can activate viral lytic gene expression (7–9). It is reported that, in latently infected cells, the silent state of the virus genome is maintained, at least in part, by MEF2-mediated recruitment of HDAC proteins to the BZLF1 promoter region (10). Once BZLF1 protein is expressed, the viral transcriptional activator recruits cAMP-response element-binding protein (CREB)-binding protein (CBP), a histone acetyltransferase, to BZLF1-responsive sequences to activate viral early promoters (11–15) followed by a coordinated cascade of viral lytic steps such as viral DNA replication, late gene expression, and progeny virus production. Thus, interplay between histone deacetylation and acetylation is associated with repression or activation of transcription, regulating latency and the replicative cycle of EBV.
Reversible posttranslational modifications are widely used to dynamically regulate protein activity. Proteins can be modified by small chemical groups, sugars, lipids, and even by covalent attachment of other polypeptides. Conjugation of target proteins by the small ubiquitin-related modifier (SUMO) (16) is a polypeptide post-translational modification that takes place at the lysine residue(s) of the target protein. SUMO is an 11-kDa protein that is structurally related to ubiquitin, and its covalent modification of proteins regulates various important cellular functions, such as nuclear transport, activation/suppression of signal transduction, cell cycle progression, and protein degradation (17–19). Three SUMO homologs have been described in mammals. SUMO-1 and SUMO-2/3 appear to partly share their substrates. Thus, some substrates may be simultaneously modified by SUMO-1 and SUMO-2/3, whereas RanGAP1, for example, is predominantly modified by SUMO-1, and topoisomerase II is also predominantly modified by SUMO-2/3 (20, 21).
Adamson and Kenney first reported in 2001 (22) that BZLF1 protein is modified by SUMO-1, most likely at lysine 12, and that BZLF1 is responsible for disruption of promyelocytic leukemia bodies upon induction of EBV lytic replication. However, sumoylation of BZLF1 does not appear to be involved in promyelocytic leukemia body disruption (22). Subsequently, with a sumoylation-defective mutant of BZLF1 (Zm12/13), Adamson (23) demonstrated that the SUMO-1 modification decreases the transcriptional activity of BZLF1 without its degradation. The physiological significance of the SUMO modification of BZLF1, however, remains elusive.
In this study we show that BZLF1 is conjugated not only by SUMO-1 but also by SUMO-2 and 3 and that sumoylated BZLF1 is deconjugated by SENP reversibly. SUMO modification of BZLF1 negatively modulated its transcriptional activity. We further found that transcriptional repression by SUMO modification is correlated with association of repressor complexes, which at least include HDAC3 in the context of infection. These results indicate that the virus utilizes SUMO modification to provide favorable conditions for its optimal replication in the host cell.
EXPERIMENTAL PROCEDURES
Cell Culture and Reagents
GTC-4, AGS-CR2/GFP-EBV, HEK293T, EBV-Bac/Zp-luc, and BZLF1KO cells were maintained in Dulbecco's modified Eagle's medium (Sigma) supplemented with 10% fetal bovine serum. GTC-4 is a cell line established from an EBV-positive gastric cancer (24). To prepare AGS-CR2/GFP-EBV cells, an EBV-negative cell line from gastric cancer, AGS, was stably transfected with CR2 (CD21, the receptor for EBV) expression vector (25) and infected with GFP-EBV (26) followed by G418 selection. EBV-Bac/Zp-luc cells were prepared by transfection of Bac/Zp-luc DNA into HEK293 cells (27). Anti-HA antibodies were purchased from Roche Applied Science, and anti-tubulin antibodies were from Cell Signaling. Anti-GFP, -CBP, and -HDAC3 antibodies were from MBL, Santa Cruz, and Abcam, respectively. Rabbit anti-BZLF1, -BMRF1, -BALF2, and -BALF5 antibodies were as reported previously (27). Horseradish peroxidase (HRP)-linked goat antibodies to mouse/rabbit IgG and TrueBlot ULTRA HRP anti-rabbit/mouse IgG were from Amersham Biosciences and eBioscience, respectively. Tricostatin A (TSA) was purchased from Sigma.
Plasmid Construction
The expression vector for BZLF1 was constructed as detailed previously (28), and the K12R point mutation was introduced by PCR using appropriate primers. Vectors for HA-SUMO-1, -2, and -3 and SENPs were also described previously (29–32) as was construction of the pZp-luc and pBALF2-luc reporter plasmids (28, 33). pCMV-Rluc was from Promega. The pcDNA3-based expression vector for SUMO-2/BZLF1 fusion protein was made by PCR using appropriate primers.
Immunoprecipitation and Immunoblotting
To detect sumoylated BZLF1, we carried out immunoprecipitation under stringent conditions (34). In brief, cells were solubilized and sonicated in 100 μl of SDS(+) lysis buffer (10 mm Tris-HCl, pH 7.8, 150 mm NaCl, 1 mm EDTA, 0.2% Nonidet P-40, 1% SDS, and a protease inhibitor mixture). Lysates were boiled for 5 min to completely denature proteins and disrupt non-covalent interactions. Cell lysates were then diluted with 900 μl of SDS(−) lysis buffer (10 mm Tris-HCl, pH 7.8, 150 mm NaCl, 1 mm EDTA, 0.2% Nonidet P-40, and protease inhibitor mixture) and precleared with protein G-Sepharose (Amersham Biosciences). Supernatants were then incubated with anti-BZLF1antibodies, and immunocomplexes were recovered by interaction with protein-G-Sepharose for 1 h, after which the resin was washed five times with SDS(−) lysis buffer. Samples were subjected to SDS-PAGE followed by immunoblotting with indicated antibodies. Immunoblotting was carried out as described previously (28). For measurement of protein levels, imaging, and densitometry software (Lumi Vision Analyzer version 2.1, AISIN SEIKI, Co.) were used. To detect associations with sumoylated BZLF1, we used the lysis buffer described previously (28) with the slight modification that 10 mm N-ethylmaleimide was added to preserve the sumoylation of cellular proteins (35).
Transfection and Luciferase Assays
Plasmid DNA was transfected into HEK293T cells by lipofection or electroporation using Lipofectamine 2000 reagent (Invitrogen) or Microporator (Digital Bio). The total amounts of plasmid DNAs were standardized by the addition of an empty vector. Proteins were extracted from cells with the lysis buffer supplied in a Dual-Luciferase Reporter Assay System (Promega) kit, and luciferase activity was measured using the kit. The counts for firefly luciferase were normalized to those for renilla luciferase.
Genetic Manipulation of EBV-Bac DNA
EBV-Bac DNA was provided by Hammerschmidt and co-workers (36). Homologous recombination was carried out in Escherichia coli as described previously (27).
To prepare Bac/Zp-luc, transfer DNA fragment for the first recombination was prepared by PCR using PpsL-neo (Gene Bridges) as the template with the primers 5′-AGGAGGCTGGTGCCTTGGCTTTAAAGGGGAGATGTTAGACAGGTAACTCACTAAACATTGGGCCTGGTGATGATGGCGGGATC-3′ and 5′-ATTTATTAATATTCCATTAGTAAACGAGGCGTGAAGCAGGCGTGGTTTCAATAACGGGAGTCAGAAGAACTCGTCAAGAAGG-3′. After the recombination, kanamycin-resistant colonies were selected and checked to make the intermediate, dBZLF1/NeoSt. The NeoSt+ cassette in the intermediate DNA was then replaced using the next transfer vector DNA which contained the firefly luciferase gene with Zp. The transfer vector was made by PCR using pZp-luc (28) as the template with the primers 5′-GCCATGCATATTTCAACTGG-3′ and 5′-AGAGAGCCGACAGGAAGATATTTATTAATATTCCATTAGTAAACGAGGCGTGAAGCAGGCGTGGTTTCAATAACGGGAGTTACACGGCGATCTTGCCGC-3′. Streptomycin-resistant colonies were cloned and checked to make Bac/Zp-luc. To construct the BZLF1KO virus, the following primers were used for PCR: 5′-GCTCCTGAGAATGCTTATCAAGCTTATGCAGCACCTCAGCTGTTCCCAGTCTCCGACATAGGCCTGGTGATGATGGCGGGATC-3′ and 5′-CCGGCATTTTCTGGAAGCCACCCGATTCTTGTATCGCTTTATTTCTAGTTCAGAATCGCATCAGAAGAACTCGTCAAGAAGG-3′. Electroporation for E. coli was performed using Gene Pulser III (Bio-Rad), and purification of EBV-Bac DNA was achieved with NucleoBond Bac100 (Macherey-Nagel). Recombinant EBV-Bac viruses were confirmed in supplemental Fig. 1.
Chromatin Immunoprecipitation Assay
This assay was performed as described previously (28). The recovered DNA was amplified by PCR using the following primers: for BZLF1 promoter, 5′-TAGCCTCGAGGCCATGCATATTTCAACTGG-3′ and 5′-GCCAAGCTTCAAGGTGCAATGTTTAGTGAG-3′; for BALF2 promoter, 5′-ACCAAGCTTGATGCCCAAGGTATCGCCCCG-3′ and 5′-CTGGCCCTCGCTAGCAGACTCTGGTTTGCG-3′; for Ori-Lyt, 5′-CCGGCTCGCCTTCTTTTATCCTC-3′ and 5′-CCTGGTTCAACCCTATGGAGGGGAC-3′; for the EBNA-1 open reading frame, 5′-GTCATCATCATCCGGGTCTC-3′ and 5′-TTCGGGTTGGAACCTCCTTG-3′.
RESULTS
BZLF1 Protein Is a Substrate for SUMO-1, -2, and -3 Modifications
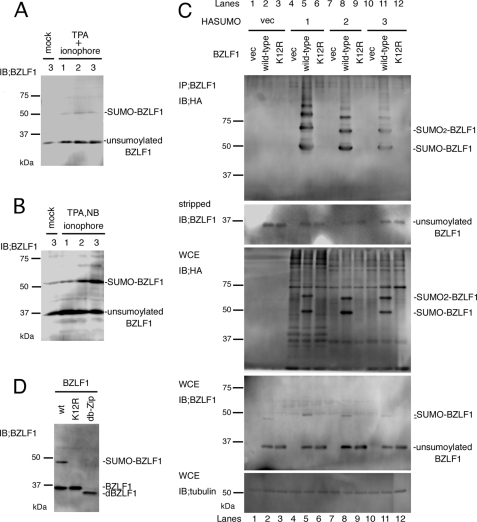
It was previously reported that the BZLF1 protein of EBV is modified by SUMO-1, most likely at lysine 12 (22). We first examined the conjugation in the lytic replication-induced cells (Fig. 1, A and B). Densitometry analysis demonstrated in GTC-4 (Fig. 1A) cells that unsumoylated BZLF1 levels were 31% without induction (mock) and reached 91, 100, and 95% 1, 2, and 3 days after induction, respectively, when sumoylated BZLF1 levels were 1.8, 7.0, 13, and 9.8% in mock, 1-, 2-, and 3-day samples (value of the highest density (unsumoylated BZLF1 band, day 2 in Fig. 1A) was set 100%). The sumoylation was more conspicuous in AGS-CR2/GFP-EBV cells (Fig. 1B). A significant amount of sumoylated and unsumoylated BZLF1 was spontaneously produced in the cells even without lytic induction (22 and 54%, respectively), as AGS cells support significantly high levels of persistent lytic infection of the virus (37). Unsumoylated BZLF1 levels reached a maximum on day 1 (100%) and gradually declined on days 2 and 3 (75 and 64%, respectively), whereas levels of SUMO-conjugated BZLF1 rose 29, 36, and 41% on days 1, 2, and 3. In EBV-positive lymphocytes, however, the SUMO modification of BZLF1 was very low (data not shown), suggesting the physiological significance of the modification, especially in epithelial cells.
FIGURE 1.
BZLF1 is modified by SUMO. A and B, sumoylation of BZLF1 in infected cells is shown. A, GTC-4 cells were treated with or without 12-O-tetradecanoylphorbol-13-acetate (TPA, 20 ng/ml) and calcium ionophore A23187 (0.5 mm) for 1, 2, or 3 days. Cell lysates were subjected to immunoblotting (IB) with anti-BZLF1 antibodies. B, likewise, AGS-CR2/GFP-EBV cells were treated with or without 12-O-tetradecanoylphorbol-13-acetate (20 ng/ml), sodium butyrate (NB.5 mm), and calcium ionophore A23187 (0.5 mm) for 1, 2, or 3 days. Cell lysates were subjected to immunoblotting with anti-BZLF1 antibodies. NB, sodium butyrate. C, BZLF1 protein is a substrate for SUMO-1, 2, and -3 modification. HEK293T cells were transfected with pcDNABZLF1(wild type) or pcDNABZLF1(K12R) and pcHASUMO-1, -2, or 3. Immunoprecipitation (IP) was carried out using anti-BZLF1 antibodies. After immunoblotting (IB) with anti-HA antibodies (top panel), the membrane was stripped and reprobed with anti-BZLF1 antibodies (second panel). As controls, WCE from the same samples were also stained with HA (third panel), BZLF1 (fourth panel), and tubulin (bottom panel) antibodies. D, the b-Zip domain of BZLF1 is crucial for its sumoylation. HEK293T cells were transfected with pcDNABZLF1(wild type), pcDNABZLF1(K12R), or pcDNABZLF1(db-Zip). Cell proteins were harvested for immunoblotting using anti-BZLF1 antibodies.
We then performed a detailed biochemical analysis of the modification. In humans, three paralogs of SUMO proteins, SUMO-1, -2, and -3, have been identified. Despite the similarities between the proteins, recent studies suggest that SUMO-1 and SUMO-2/3 conjugate to distinct substrates in some cases. Thus, we then tested if BZLF1 is also modified by SUMO-2 and SUMO-3. In Fig. 1C, HEK293T cells were transfected with wild-type or K12R mutant of BZLF1 expression vector together with each of HA-tagged SUMO-1, -2, and -3 expression vectors. An aliquot of whole cell extract was reserved for immunoblotting, whereas the remainder of the sample was subjected to immunoprecipitation using anti-BZLF1 antibody followed by immunoblotting. Unsumoylated BZLF1 proteins (wild type and K12R) with a molecular mass of 35 kDa were detectable in the whole cell extracts (WCE; Fig. 1C, fourth panel) and in the immunoprecipitated samples (IP;BZLF1) (second panel), proving comparable expression of the proteins and successful immunoprecipitation. When co-expressed with either of the SUMO proteins, wild-type BZLF1 gave evidence of multiple sumoylation (top panel, lanes 5, 8, and 11), whereas the K12R mutant did not at all (lanes 6, 9, and 12). In addition, sumoylation of BZLF1 was so efficient that at least mono- and di-sumoylated forms of BZLF1 became visible even in the HA staining of WCE (third panel, lanes 5, 8, and 11). Together, BZLF1 is modified not only by SUMO-1 but also by SUMO-2 and -3 at lysine 12 residue. We also found that when the b-Zip domain of BZLF1 was removed, sumoylation of the protein diminished (Fig. 1D), just as reported in Kaposi's sarcoma-associated herpesvirus (KSHV) (38).
K12R Mutant BZLF1 Activates BZLF1-responsive Promoters More Efficiently Than Wild-type BZLF1
To assess the effects of BZLF1 sumoylation on its transcriptional activity, we analyzed whether a sumoylation-defective mutant of BZLF1 enhances BZLF1-responsive promoter activity (Fig. 2). We used the BALF2 promoter, which is responsive to BZLF1 (33). Although the expression of wild-type BZLF1 exhibited 106-fold transactivation, expression of the K12R mutant exhibited 228-fold transactivation. As shown in Fig. 2C, expression levels of wild-type and K12R BZLF1 were comparable, and the K12R mutation disrupted SUMO conjugation. Thus, the mutation enhanced transcription from the BALF2 promoter by 2.2-fold. Likewise, BZLF1-mediated transcriptional activity of the BZLF1 promoter was also up-regulated by K12R mutation by 1.6-fold (Fig. 2B). These results clearly support the previous report that sumoylation of BZLF1 results in attenuated transactivation using a reporter assay system with the EBV BMRF1 promoter (23).
FIGURE 2.
K12R mutant BZLF1 activates promoters with BZLF1-responsive elements more efficiently than wild-type BZLF1. A, HEK293T cells were transfected with 10 ng of BALF2-luc, 1 ng of pCMV-RL, and 10 ng of pcDNABZLF1(wild type) or pcDNABZLF1(K12R). Counts are shown as -fold activation of that without the BZLF1 expression vector (vec). B, HEK293T cells were transfected with 10 ng of pZp-luc, 1 ng of pCMV-RL, and 10 ng of pcDNABZLF1(wild type) or pcDNABZLF1(K12R). Counts were shown as -fold activation of that without the BZLF1 expression vector. C, the expression levels of BZLF1 and tubulin proteins in the assay were measured by immunoblotting (IB).
Stimulation of BZLF1-responsive Promoter Activity by K12R Mutation in the Context of the Viral Genome
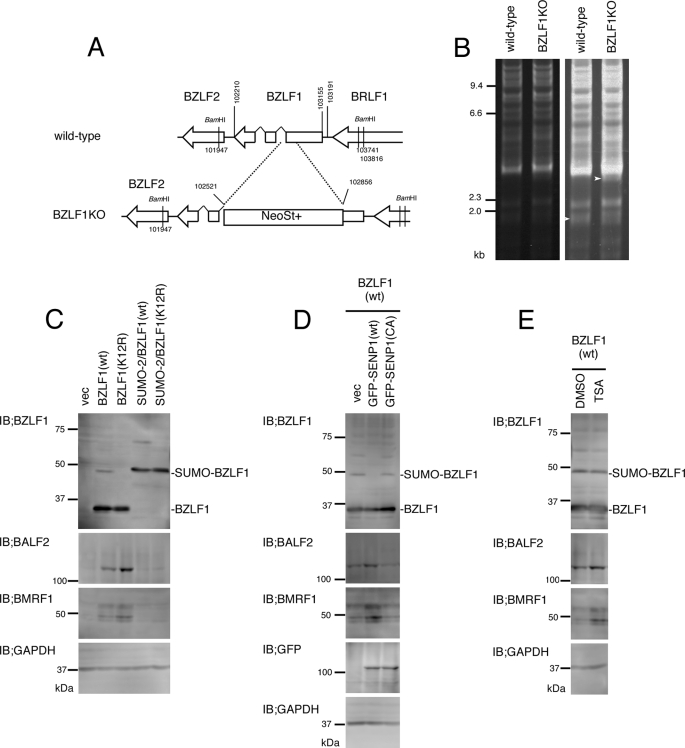
To extend the above reporter gene experiment, we further examined whether SUMO conjugation of BZLF1 could influence its transcriptional activity on the BZLF1 promoter of the viral genome with nucleosomal structures. To this end we generated EBV-Bac DNA in which the entire open reading frame of the BZLF1 gene was replaced with the firefly luciferase gene (Fig. 3A, Bac/Zp-luc). As this Bac DNA contains the luciferase gene under the control of native BZLF1 gene promoter, reporter activity reflects transcription from the promoter in the EBV genome. The Bac DNA construction was confirmed by BamHI digestion followed by electrophoresis (Fig. 3B). Bac DNA was introduced into HEK293 cells followed by hygromycin selection to establish cell lines in which multiple copies of Bac/Zp-luc DNA were maintained as an episome. We confirmed that viral lytic gene expression of elements such as BMRF1 and BALF2 could be induced by the BZLF1 expression vector into cells (data not shown). As shown in Fig. 3C, expression of wild-type BZLF1 induced luciferase reporter activity 111-fold, whereas K12R mutant BZLF1 induced 206-fold activity. Expression levels of wild-type and K12R BZLF1 proteins were comparable, and the mutation disrupted SUMO conjugation (Fig. 4D). Thus, the sumoylation of BZLF1 at Lys-12 repressed BZLF1-dependent transcription by 47% in the context of the viral genome.
FIGURE 3.
The K12R mutant activates the BZLF1 promoter more efficiently than wild-type BZLF1 in the context of the viral genome. A, shown is a schematic arrangement of the recombination of the EBV genome using tandemly arranged neomycin resistance and streptomycin sensitivity genes (NeoSt+). The region between nucleotide 102,210 and 103,191 of the B95-8 genome (V01555) was replaced with the NeoSt+ cassette to make the intermediate, dBZLF1/NeoSt. The NeoSt+ cassette was then replaced with a vector sequence with firefly luciferase (F-luc) flanked with the 5′- and 3′-UTRs of the BZLF1 gene to construct Bac/Zp-luc. The Bac DNA was introduced into HEK293 cells followed by hygromycin selection. Resultant cell clones were tested for lytic induction, and one of the typical clones was used as EBV-Bac/Zp-luc cells in the next panels. B, electrophoresis of the recombinant viruses is shown. EBV-Bac DNAs were digested with BamHI and separated in an agarose gel. Brightness was enhanced in the right panel to clearly show BamHI-Z fragment of the virus. White arrowheads indicate the size of BamHI-Z fragment (wild type) or the sizes of the fragments plus inserts. C, EBV-Bac/Zp-luc cells were transfected with 1 ng of pCMV-RL and 30 ng of pcDNABZLF1(wild type) or pcDNABZLF1(K12R). Counts are shown as -fold activation of that without the BZLF1 expression vector. D, the expression levels of BZLF1 and tubulin proteins in the assay were measured by immunoblotting (IB).
FIGURE 4.
SENP proteins deconjugate SUMO from BZLF1. A and B, HEK293T cells were transfected with 10 ng of pcDNABZLF1(wild type) and 50 ng of GFP-tagged, wild type (wt), or inactive (CA) SENP expression vectors (vec) together with 10 ng of pZp-luc and 1 ng of pCMV-RL. Cell proteins were subjected to luciferase assay (A) and immunoblotting (IB) using anti-BZLF1 (upper panel) and GFP (lower panel) antibodies (B). C and D, EBV-Bac/Zp-luc cells were transfected with 1 ng of pCMV-RL and 30 ng of pcDNABZLF1(wild type) or pcDNABZLF1(K12R). Counts are shown as -fold activation of that without the BZLF1 expression vector. Cell proteins were subjected to luciferase assay (C) and immunoblotting using anti-BZLF1 (upper panel) and GFP (lower panel) antibodies (D).
SENP Proteins Deconjugate SUMO from BZLF1 and Enhance BZLF1-mediated Transcription
We then focused on the desumoylation of BZLF1. As cleavage of isopeptide bonds between SUMO and its targets is mediated by the SUMO-specific protease, SENP, we examined whether the protease could affect BZLF1 transcription through sumoylation (Fig. 4B). Two SENP proteins, 1 and 2, and their CA mutants, in which the conserved cysteine residue within the catalytic domain is changed to alanine, were used. SUMO-conjugated BZLF1 was detected in cells without SENP proteins (lane 1), and expression of SENP1 or SENP2 completely abrogated the modification (lanes 2 and 4). In contrast, catalytically inactive mutants of SENP proteins failed to deconjugate the modification (lanes 3 and 5). These results clearly confirmed the BZLF1 protein to be sumoylated as the SUMO-specific proteases cleaved the conjugation.
Because the desumoylation by the proteases was confirmed, we examined whether an exogenous supply of SENPs might affect the BZLF1-mediated transcription of the BALF2 promoter in a reporter gene assay (Fig. 4A). Without SENPs, the addition of the BZLF1 expression vector activated the BALF2 promoter by 81-fold. In the presence of wild-type SENP1 or -2, it reached 146- or 185-fold activation, respectively. In contrast, when catalytically inactive mutants of SENP1 or -2 were transfected, the transactivation became 74- or 78-fold, respectively, which was essentially the same as the level of transactivation obtained with BZLF1 transfection itself.
We then tested if SUMO deconjugation of BZLF1 by SENP could influence its transcriptional activity on the BZLF1 promoter of the viral genome using Bac/Zp-luc (Fig. 4, C and D). As expected, desumoylation of BZLF1 by either SENP1 or SENP2 resulted in increased transcription from the viral genome. These results support our data that sumoylation of BZLF1 results in repression of its transcriptional activity and also provide evidence that BZLF1 sumoylation is a reversible process.
Involvement of Histone Deacetylation in Transcriptional Repression by BZLF1 Sumoylation
It has been reported that BZLF1 activates viral gene expression by interacting with the CBP possessing histone transacetylase activity (11–15). Immunoprecipitation assays indicated that BZLF1 did associate with CBP, but the interaction between sumoylated BZLF1 and CBP was somehow attenuated (data not shown), suggesting that conjugation of SUMO molecule to BZLF1 protein might reduce its association with the CBP transcriptional co-activator.
Next, we also examined whether BZLF1 interacts with histone deacetylase (Fig. 5). Surprisingly, sumoylated BZLF1 protein, but not the unmodified form, was pulled down with HDAC3 (Fig. 5, lane 2). A similar result was obtained when anti-HDAC7 antibodies were used (not shown).
FIGURE 5.
SUMO modification of BZLF1 mediates HDAC3. Proteins from HEK293T cells transfected with wild-type pcDNABZLF1 were subjected to immunoprecipitation (IP) using normal IgG or anti-HDAC3 followed by immunoblotting (IB) with anti-BZLF1 (upper panel) and HDAC3 (lower panel) antibodies. WCE (1/50) from the same lysate was also blotted.
From these observations we hypothesized that the transcriptional inhibition by BZLF1 sumoylation is mediated mainly by HDAC recruitment. To test this possibility, we analyzed the effect of TSA, a potent inhibitor of HDACs, on BZLF1-mediated transcription from the BALF2 and BZLF1 promoters in reporter gene assays. HEK293T cells were transfected, incubated in the presence or absence of TSA, harvested, and subjected to luciferase assays (Fig. 6, A and B) and immunoblotting (Fig. 6C). TSA treatment reversed the inhibitory action of BZLF1 sumoylation. We further examined the effect of TSA in the context of the viral genome (Fig. 6, D and E). As expected, TSA treatment abrogated the inhibitory action of BZLF1 sumoylation. Collectively, our results strongly suggested that BZLF1 represses BZLF1-responsive promoters by recruiting histone deacetylase complexes through sumoylation.
FIGURE 6.
An HDAC inhibitor reverses the effect of BZLF1 sumoylation. A and B, HEK293T cells were transfected with 10 ng of BALF2-luc (A) or pZp-luc (B), 1 ng of pCMV-RL, and 10 ng of pcDNABZLF1(wild type) or pcDNABZLF1(K12R). After 4 h, cells were then exposed to TSA (100 μm) for 20 h. Counts are shown as -fold activation of that without the BZLF1 expression vector (vec). C, the expression levels of BZLF1 and tubulin proteins in the assay were measured by immunoblotting (IB). D, EBV-Bac/Zp-luc cells were transfected with 1 ng of pCMV-RL and 30 ng of pcDNABZLF1(wild type) or pcDNABZLF1(K12R). After 4 h cells were then exposed to TSA (100 μm) for 20 h. Counts are shown as -fold activation of that without the BZLF1 expression vector. E, the expression levels of BZLF1 and tubulin proteins in the assay were measured by immunoblotting.
HDAC Is Recruited to BZLF1-responsive Promoters through Association with BZLF1
Next, we determined whether HDAC is actually recruited to viral promoters bound by sumoylated BZLF1. However, the experiment was hampered by low levels of SUMO conjugation. Thus, an expression vector for SUMO-2 and BZLF1 fusion protein was constructed to mimic sumoylated BZLF1, as it has been reported that SUMO fusion to target proteins produces similar effects (39–41). We speculated that HDAC could be recruited onto BZLF1-responsive promoters to repress transcription in the presence of the SUMO-2/BZLF1 fusion protein. We first examined the expression of the fusion protein (Fig. 7A).
FIGURE 7.
HDAC is recruited to BZLF1-responsive promoters through association with SUMO-BZLF1. A, pcDNASUMO-2/BZLF1, the expression vector for SUMO-2/BZLF1 fusion protein, was constructed as described under “Experimental Procedures,” and its expression in HEK293T cells was confirmed by immunoblotting (IB). B, EBV-Bac/Zp-luc cells were transfected with 1 ng of pCMV-RL and 30 ng of pcDNABZLF1(wild type) or pcDNASUMO-2/BZLF1. Counts are shown as -fold activation of that without the BZLF1 expression vector (vec). C, SUMO fusion with BZLF1 mediates HDAC3. Proteins from HEK293T cells transfected with pcDNASUMO-2/BZLF1 were subjected to immunoprecipitation (IP) using normal IgG or anti-HDAC3 followed by immunoblotting with anti-BZLF1 (upper panel) and HDAC3 (lower panel) antibodies. WCE (1/50) from the same lysate was also blotted. D, proteins from HEK293T cells transfected with pcDNABZLF1(K12R) or pcDNASUMO-2/BZLF1(K12R) were subjected to immunoprecipitation using normal IgG or anti-BZLF1 followed by immunoblotting with anti-HDAC3 (top panel), CBP (middle panel), and BZLF1 (lower panel) antibodies. WCE (1/50) from the same lysate was also blotted. E, chromatin immunoprecipitation experiments were carried out using cross-linked DNA-protein complexes from EBV-Bac/Zp-luc cells transfected with pcDNABZLF1 or pcDNASUMO-2/BZLF1. DNA-protein complexes were precipitated using normal IgG, anti-BZLF1, anti-CBP, or anti-HDAC3 followed by DNA extraction and PCR reactions to detect the BZLF1 and BALF2 promoters or Ori-Lyt, which contains binding sites for BZLF1. A fragment for the EBNA-1 open reading frame was also detected as a negative control.
We then tested the effect of the fusion protein on the BZLF1 promoter of the viral genome using Bac/Zp-luc (Fig. 7B). As expected, SUMO-2/BZLF1 fusion protein failed to transactivate the BZLF1 promoter, whereas wild-type BZLF1 protein clearly did transactivate the promoter by 66-fold.
Results of immunoprecipitation assay using anti-HDAC3 antibody confirmed the SUMO-2/BZLF1 fusion protein to be associated with HDAC3, just like sumoylated BZLF1 (Fig. 7C). Because the SUMO-2/BZLF1 fusion protein acted as expected, we further confirmed the interactions. When anti-BZLF1 antibody was used for precipitation (Fig. 7D), HDAC3 co-precipitated more efficiently with SUMO-2/BZLF1 fusion protein than BZLF1 protein. On the other hand, CBP preferentially associated with BZLF1, but the association was weak with SUMO-2/BLZF1. We here used BZLF1 K12R and SUMO-2/BZLF1 K12R mutants to eliminate the SUMO conjugation at the residue, which might provoke confusion.
To examine if HDAC is actually recruited to BZLF1-responsive elements in viral promoters, we performed chromatin immunoprecipitation assays in the presence of the SUMO-2/BZLF1 fusion protein (Fig. 7E). With either wild-type or SUMO-2-fused BZLF1, BZLF1 and BALF2 promoter sequences and oriLyt, all of which contain BZLF1-responsive elements, co-precipitated with anti-BZLF1 antibodies (Fig. 7E, lanes 3 and 7), demonstrating that BZLF1 binds to BZLF1-responsive elements even when N-terminal-fused with SUMO-2. Immunoprecipitation with anti-HDAC3 antibodies (Fig. 7E, lanes 4 and 8) revealed the SUMO-2/BZLF1 fusion protein to exhibit more efficient recruitment of the deacetylase to the BZLF1 and BALF2 promoters and oriLyt than wild-type BZLF1 protein. This finding is consistent with our speculation that BZLF1 represses BZLF1-responsive promoters by recruiting HDAC complexes through sumoylation.
Activation of Viral Early Genes in BZLF1KO Virus-infected Cells
In Fig. 3 we assayed the effect of BZLF1 sumoylation on the BZLF1 promoter. Because viral early genes such as BALF2 and BMRF1 are also responsive to BZLF1, we made the BZLF1KO virus (Fig. 8, A and B) and examined how the sumoylation affected on its early gene expressions (Fig. 8, C–E). As shown in Fig. 8C, K12R mutant of BZLF1 induced BALF2 and BMRF1 proteins more efficiently than wild-type BZLF1. On the other hand, with or without K12R mutation, SUMO-2/BZLF1 fusion protein did not enhance the early genes (Fig. 8C). Co-expression of wild-type SENP1 correlated with the disappearance of sumoylated BZLF1 and increased early gene productions (Fig. 8D). Finally, a HDAC inhibitor TSA augmented amounts of early gene products without affecting the BZLF1 sumoylation levels (Fig. 8E). These results support the idea that sumoylated BZLF1 represses transcription from BZLF1-responsive promoters and that histone acetylation levels play a significant role in that process.
FIGURE 8.
Viral early gene expression was decreased by BZLF1 sumoylation. A, shown is a schematic arrangement of the recombination of the EBV genome using the neomycin resistance and streptomycin sensitivity genes (NeoSt+). The region between nucleotide 102,521 and 102,856 of the B95-8 genome (V01555) was replaced with the NeoSt+ cassette to make the BZLF1KO virus. The Bac DNA was introduced into HEK293 cells followed by hygromycin selection. Resultant cell clones were tested for lytic induction, and one of the typical clones was used as BZLF1KO cells in the next panels. B, electrophoresis of the recombinant viruses is shown. EBV-Bac DNAs were digested with BamHI and separated in an agarose gel. Brightness was enhanced in the right panel to clearly show BamHI-Z fragment of the virus. White arrowheads indicate the size of BamHI-Z fragment (wild type) or the size of the fragment plus the cassette. C, effect of BZLF1 mutants on early gene expressions of BZLF1KO virus. HEK293 cells with the BZLF1KO virus were transfected with indicated BZLF1 mutants. After 15 h, cell lysates were subjected to immunoblotting (IB) using anti-BZLF1, BALF2, BMRF1, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies. D, the effect of SENP on early gene expressions of BZLF1KO virus is shown. HEK293 cells with the BZLF1KO virus were transfected with BZLF1(wt) together with GFP-SENP expression vectors. After 15 h, cell lysates were subjected to immunoblotting using anti-BZLF1, BALF2, BMRF1, and glyceraldehyde-3-phosphate dehydrogenase antibodies. E, shown is the effect of an HDAC inhibitor on early gene expressions of BZLF1KO virus. HEK293 cells with the BZLF1KO virus were transfected with BZLF1(wt) expression vectors. After 4 h, TSA was added to the medium followed by incubation for 11 h. Cell lysates were subjected to immunoblotting using anti-BZLF1, BALF2, BMRF1, and glyceraldehyde-3-phosphate dehydrogenase antibodies.
DISCUSSION
In this report we document evidence that SUMO conjugation of BZLF1 negatively affects its transcriptional activity. In contrast to ubiquitination, which most frequently is regarded as an earmark for proteasome-dependent degradation, sumoylation has a wide range of substrate-specific functions. With transcription factors, SUMO modification is mostly related to repression. We also suggest that negative regulation is brought by epigenetic alterations of virus gene promoters. It has been previously demonstrated that the BZLF1 protein interacts physically with CBP through its N-terminal activation domain to enhance viral early gene transcription (11–14). As BZLF1 sumoylation takes place at the N terminus, it is tempting to speculate that structural alteration caused by the conjugation weakens the association, thereby inhibiting the transcriptional activity.
By means of simple immunoblotting experiments, we could show that the level of the sumoylated form of BZLF1 is much less than with its un-sumoylated counterpart. Nevertheless, the K12R mutant of BZLF1, which cannot be sumoylated, enhanced more efficiently than the wild-type BZLF1. Therefore, we speculate that there must be much more active and powerful repression mechanism rather than just inhibition of the interaction with CBP. Recently, it has been reported that sumoylation of cellular transcription factors promotes interaction with repressor complexes including HDAC (42–48). We, therefore, tested the interaction between SUMO-modified BZLF1 and HDAC and showed that at least HDAC3 associates with the sumoylated form of BZLF1 but not clearly with the un-sumoylated form, suggesting that SUMO-mediated acetylation levels of chromatin affect the BZLF1-dependent transcription.
Sumoylation is often regulated by phosphorylation at a neighboring residue of the target protein. It is very well established that a phosphorylation-dependent sumoylation motif (ΦKX(D/E)XX(S/T)P, where Φ is a hydrophobic amino acid residue, X represents any residue, and D or E is an acidic residue) is present in some transcription factors, including heat-shock factor 1, MEF2, and estrogen-related receptors. Phosphorylation at the phosphorylation-dependent sumoylation motif, which contains a SUMO consensus motif (ΦKX(D/E)) and an adjacent proline-directed phosphoacceptor, up-regulates SUMO conjugation of those transcription factors (49–51). Although phosphorylation-dependent sumoylation motif is not a feature of BZLF1, we found a remarkable similarity except in the Thr-14 residue in BZLF1 when the surrounding sequence of BZLF1 Lys-12 (11VKFTP15) was compared with the consensus motif for SUMO modification (ΦKX(D/E)). It could be envisaged that the non-functional motif, ΦKX(S/T), might be converted to a functional substrate for sumoylation after phosphorylation at Ser or Thr residues (17). In fact, BZLF1 Thr-14 is strongly phosphorylated (52). We, thus, examined if a single point mutation at Thr-14 affected the BZLF1 sumoylation. However, the levels of sumoylation were neither increased nor decreased by the T14D or T14A mutation of BZLF1 (data not shown), indicating that phosphorylation at Thr-14 has little or no effect on the conjugation.
The information about KSHV K-bZIP sumoylation provided by Izumiya et al. (38) may be of great value in determining the nature of sumoylation of the EBV homologue, BZLF1. K-bZIP is thought to be a structural homolog of EBV BZLF1, as they share the b-Zip domain located near the C terminus and a presumptive regulatory region in the N terminus, although the homology of the sequences is not very high (about 37%). Interestingly, although the EBV BZLF1 gene product functions as a transcriptional activator, K-bZIP has strong repression activity. Izumiya et al. (38) have indicated that transcriptional repression by K-bZIP is caused by SUMO. As they showed a physical interaction between K-bZIP and the SUMO E2 ligase, Ubc9, we also examined the possibility of such interaction with EBV BZLF1. Co-immunoprecipitation assays in fact did reveal a weak association (data not shown) suggesting that the affinity with the ligase may at least in part define the opposite actions of the viral b-Zip protein.
EBV BZLF1 sumoylation takes place at the N terminus, and the residue for K-bZIP is in the C terminus region. Despite the difference in residues for the SUMO conjugation, we found another similarity. Just like truncation of the b-Zip domain from KSHV K-bZIP abrogates SUMO modification of the protein (38), SUMO conjugation to EBV BZLF1 did not take place when the EBV BZLF1 b-Zip motif was removed (Fig. 2). This result suggests that DNA binding of the proteins may play significant roles in SUMO conjugation. Alternatively, the SUMO E2 ligase, Ubc9, may not be able to associate with the b-Zip null mutant of BZLF1, just as is the case with K-bZIP (38) and some other b-Zip proteins (53, 54). The SUMO-specific protease SENP might be involved in this process, as it deconjugates SUMO molecules from BZLF1 (Fig. 5).
KSHV K-bZIP sumoylation is known to be decreased by phosphorylation at Thr-111 of K-bZIP by the virus PK (55). Because the BGLF4 PK of EBV, the structural and functional homolog of KSHV vPK, interacts with and phosphorylates BZLF1 (56, 57), its effects on BZLF1 sumoylation were examined (data not shown). EBV BZLF1 sumoylation was also decreased by the virus BGLF4 PK, but unlike K-bZIP, phosphorylation of EBV BZLF1 at Ser-209 by BGLF4 (57) did not influence the SUMO modification (data not shown). Those results were clearly repeated by Hagemeier et al. in their very recent article (58). We assume that BGLF4 PK may suppress BZLF1 sumoylation through phosphorylation of another factor. One possibility is that PK might structurally inhibit SUMO conjugation of BZLF1 through their interaction because K102I mutation of BGLF4 PK disables its association with BZLF1 (57).
When viral lytic replication was induced in GTC-4 or AGS cells latently infected with EBV, BZLF1 sumoylation levels were relatively low at 24 h and then increased at 48 and 72 h (Fig. 1, A and B). This indicates that transcriptional activity of BZLF1 is relatively intact at 24 h or earlier and then suppressed by SUMO conjugation thereafter. The virus may be taking advantage of the cellular SUMO system to improve its replication efficiency because transcriptional activity of BZLF1 is necessary for enhancement of viral early gene expressions at earlier times after lytic induction and is not needed any longer after viral gene production is complete. We also tested if EBV-positive lymphocytes, including Akata, B95-8, and Raji, provide BZLF1 sumoylation. However, sumoylation levels of endogenous BZLF1 in those cell lines were very low, suggesting a significant role of the modification in epithelial cells.
To summarize, EBV reactivation from latency is dependent on the availability and association with other transcriptional co-factors and post-translational modification of BZLF1. The overall regulation appears very complicated, but with sumoylation, EBV may exploit the modification to fine-tune the levels of viral lytic replication. Further elucidation is needed for comprehensive understanding of the molecular mechanism that governs EBV reactivation from latency.
Supplementary Material
Acknowledgments
We are grateful to Drs. W. Hammerschmidt, H. J. Delecluse, S. Maruo, and M. Tajima for providing the EBV-Bac DNA, HEK293, AGS-CR2/GFP-EBV, and GTC-4 cells, respectively. We thank Dr. T. Nishida for providing SENP expression vectors. We also express our appreciation to Y. Yasui and C. Sakuragi for technical assistance.
This work was supported by Grants-in-aid for Scientific Research 20390137 and 21022055 (to T. T.) and 20790362 and 22790448 (to T. M.) from the Ministry of Education, Science, Sports, Culture, and Technology of Japan and in part by the Uehara Memorial Research Fund (to T. T.) and the Japan Leukaemia Research Fund (to T. M.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- EBV
- Epstein-Barr virus
- SUMO
- small ubiquitin-related modifier
- CBP
- cAMP-response element-binding protein (CREB)-binding protein
- HDAC
- histone deacetylase
- b-Zip
- basic leucine zipper
- TSA
- tricostatin A
- WCE
- whole cell extracts
- KSHV
- Kaposi's sarcoma-associated herpesvirus
- GFP
- green fluorescent protein
- HA
- hemagglutinin
- K-bZIP
- KSHV b-Zip.
REFERENCES
- 1.Joab I., Nicolas J. C., Schwaab G., de-Thé G., Clausse B., Perricaudet M., Zeng Y. (1991) Int. J. Cancer 48, 647–649 [DOI] [PubMed] [Google Scholar]
- 2.Feng W. H., Cohen J. I., Fischer S., Li L., Sneller M., Goldbach-Mansky R., Raab-Traub N., Delecluse H. J., Kenney S. C. (2004) J. Natl. Cancer Inst. 96, 1691–1702 [DOI] [PubMed] [Google Scholar]
- 3.Speck S. H., Chatila T., Flemington E. (1997) Trends Microbiol. 5, 399–405 [DOI] [PubMed] [Google Scholar]
- 4.Amon W., Farrell P. J. (2005) Rev. Med. Virol. 15, 149–156 [DOI] [PubMed] [Google Scholar]
- 5.Tsurumi T., Fujita M., Kudoh A. (2005) Rev. Med. Virol. 15, 3–15 [DOI] [PubMed] [Google Scholar]
- 6.Tempera I., Lieberman P. M. (2010) Biochim. Biophys. Acta 1799, 236–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jenkins P. J., Binné U. K., Farrell P. J. (2000) J. Virol. 74, 710–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Countryman J. K., Gradoville L., Miller G. (2008) J. Virol. 82, 4706–4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller G., El-Guindy A., Countryman J., Ye J., Gradoville L. (2007) Adv. Cancer Res. 97, 81–109 [DOI] [PubMed] [Google Scholar]
- 10.Gruffat H., Manet E., Sergeant A. (2002) EMBO Rep. 3, 141–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adamson A. L., Kenney S. (1999) J. Virol. 73, 6551–6558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zerby D., Chen C. J., Poon E., Lee D., Shiekhattar R., Lieberman P. M. (1999) Mol. Cell. Biol. 19, 1617–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen C. J., Deng Z., Kim A. Y., Blobel G. A., Lieberman P. M. (2001) Mol. Cell. Biol. 21, 476–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng Z., Chen C. J., Zerby D., Delecluse H. J., Lieberman P. M. (2001) J. Virol. 75, 10334–10347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng Z., Chen C. J., Chamberlin M., Lu F., Blobel G. A., Speicher D., Cirillo L. A., Zaret K. S., Lieberman P. M. (2003) Mol. Cell. Biol. 23, 2633–2644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumoto J., Ohshima T., Isono O., Shimotohno K. (2005) Oncogene 24, 1001–1010 [DOI] [PubMed] [Google Scholar]
- 17.Melchior F., Schergaut M., Pichler A. (2003) Trends Biochem. Sci. 28, 612–618 [DOI] [PubMed] [Google Scholar]
- 18.Seeler J. S., Dejean A. (2001) Oncogene 20, 7243–7249 [DOI] [PubMed] [Google Scholar]
- 19.Geiss-Friedlander R., Melchior F. (2007) Nat. Rev. Mol. Cell Biol. 8, 947–956 [DOI] [PubMed] [Google Scholar]
- 20.Saitoh H., Hinchey J. (2000) J. Biol. Chem. 275, 6252–6258 [DOI] [PubMed] [Google Scholar]
- 21.Azuma Y., Arnaoutov A., Dasso M. (2003) J. Cell Biol. 163, 477–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adamson A. L., Kenney S. (2001) J. Virol. 75, 2388–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adamson A. L. (2005) Biochem. Biophys. Res. Commun. 336, 22–28 [DOI] [PubMed] [Google Scholar]
- 24.Kanamori M., Tajima M., Satoh Y., Hoshikawa Y., Miyazawa Y., Okinaga K., Kurata T., Sairenji T. (2000) Virus Genes 20, 117–125 [DOI] [PubMed] [Google Scholar]
- 25.Katsumura K. R., Maruo S., Wu Y., Kanda T., Takada K. (2009) J. Gen. Virol. 90, 2331–2341 [DOI] [PubMed] [Google Scholar]
- 26.Maruo S., Yang L., Takada K. (2001) J. Gen. Virol. 82, 2373–2383 [DOI] [PubMed] [Google Scholar]
- 27.Murata T., Isomura H., Yamashita Y., Toyama S., Sato Y., Nakayama S., Kudoh A., Iwahori S., Kanda T., Tsurumi T. (2009) Virology 389, 75–81 [DOI] [PubMed] [Google Scholar]
- 28.Murata T., Sato Y., Nakayama S., Kudoh A., Iwahori S., Isomura H., Tajima M., Hishiki T., Ohshima T., Hijikata M., Shimotohno K., Tsurumi T. (2009) J. Biol. Chem. 284, 8033–8041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohshima T., Shimotohno K. (2003) J. Biol. Chem. 278, 50833–50842 [DOI] [PubMed] [Google Scholar]
- 30.Nishida T., Terashima M., Fukami K. (2006) Biochem. Biophys. Res. Commun. 345, 1536–1546 [DOI] [PubMed] [Google Scholar]
- 31.Nishida T., Kaneko F., Kitagawa M., Yasuda H. (2001) J. Biol. Chem. 276, 39060–39066 [DOI] [PubMed] [Google Scholar]
- 32.Muraoka A., Maeda A., Nakahara N., Yokota M., Nishida T., Maruyama T., Ohshima T. (2008) Biochem. Biophys. Res. Commun. 377, 1031–1035 [DOI] [PubMed] [Google Scholar]
- 33.Nakayama S., Murata T., Murayama K., Yasui Y., Sato Y., Kudoh A., Iwahori S., Isomura H., Kanda T., Tsurumi T. (2009) J. Biol. Chem. 284, 21557–21568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murata T., Shimotohno K. (2006) J. Biol. Chem. 281, 20788–20800 [DOI] [PubMed] [Google Scholar]
- 35.Kuo H. Y., Chang C. C., Jeng J. C., Hu H. M., Lin D. Y., Maul G. G., Kwok R. P., Shih H. M. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 16973–16978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delecluse H. J., Hilsendegen T., Pich D., Zeidler R., Hammerschmidt W. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 8245–8250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng W. H., Kraus R. J., Dickerson S. J., Lim H. J., Jones R. J., Yu X., Mertz J. E., Kenney S. C. (2007) J. Virol. 81, 10113–10122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Izumiya Y., Ellison T. J., Yeh E. T., Jung J. U., Luciw P. A., Kung H. J. (2005) J. Virol. 79, 9912–9925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ross S., Best J. L., Zon L. I., Gill G. (2002) Mol. Cell 10, 831–842 [DOI] [PubMed] [Google Scholar]
- 40.Shalizi A., Gaudillière B., Yuan Z., Stegmüller J., Shirogane T., Ge Q., Tan Y., Schulman B., Harper J. W., Bonni A. (2006) Science 311, 1012–1017 [DOI] [PubMed] [Google Scholar]
- 41.Bossis G., Malnou C. E., Farras R., Andermarcher E., Hipskind R., Rodriguez M., Schmidt D., Muller S., Jariel-Encontre I., Piechaczyk M. (2005) Mol. Cell. Biol. 25, 6964–6979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang S. H., Sharrocks A. D. (2004) Mol. Cell 13, 611–617 [DOI] [PubMed] [Google Scholar]
- 43.Yang S. H., Sharrocks A. D. (2006) Ernst Schering Res. Found. Workshop 57, 193–209 [DOI] [PubMed] [Google Scholar]
- 44.Kim J. H., Choi H. J., Kim B., Kim M. H., Lee J. M., Kim I. S., Lee M. H., Choi S. J., Kim K. I., Kim S. I., Chung C. H., Baek S. H. (2006) Nat. Cell Biol. 8, 631–639 [DOI] [PubMed] [Google Scholar]
- 45.Jacobs A. M., Nicol S. M., Hislop R. G., Jaffray E. G., Hay R. T., Fuller-Pace F. V. (2007) Oncogene 26, 5866–5876 [DOI] [PubMed] [Google Scholar]
- 46.Wang W. L., Lee Y. C., Yang W. M., Chang W. C., Wang J. M. (2008) Nucleic Acids Res. 36, 6066–6079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oshima M., Mimura J., Sekine H., Okawa H., Fujii-Kuriyama Y. (2009) J. Biol. Chem. 284, 11017–11026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ouyang J., Shi Y., Valin A., Xuan Y., Gill G. (2009) Mol. Cell 34, 145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu B., Shuai K. (2008) Curr. Opin. Cell Biol. 20, 288–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hietakangas V., Anckar J., Blomster H. A., Fujimoto M., Palvimo J. J., Nakai A., Sistonen L. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohideen F., Capili A. D., Bilimoria P. M., Yamada T., Bonni A., Lima C. D. (2009) Nat. Struct. Mol. Biol. 16, 945–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.El-Guindy A. S., Paek S. Y., Countryman J., Miller G. (2006) J. Biol. Chem. 281, 3085–3095 [DOI] [PubMed] [Google Scholar]
- 53.Eloranta J. J., Hurst H. C. (2002) J. Biol. Chem. 277, 30798–30804 [DOI] [PubMed] [Google Scholar]
- 54.Antoine K., Prosperi M. T., Ferbus D., Boule C., Goubin G. (2005) Mol. Cell. Biochem. 271, 215–223 [DOI] [PubMed] [Google Scholar]
- 55.Izumiya Y., Izumiya C., Van Geelen A., Wang D. H., Lam K. S., Luciw P. A., Kung H. J. (2007) J. Virol. 81, 1072–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Asai R., Kato A., Kato K., Kanamori-Koyama M., Sugimoto K., Sairenji T., Nishiyama Y., Kawaguchi Y. (2006) J. Virol. 80, 5125–5134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Asai R., Kato A., Kawaguchi Y. (2009) J. Gen. Virol. 90, 1575–1581 [DOI] [PubMed] [Google Scholar]
- 58.Hagemeier S. R., Dickerson S. J., Meng Q., Yu X., Mertz J. E., Kenney S. C. (2010) J. Virol. 84, 4383–4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.