Abstract
In platelets, STIM1 has been recognized as the key regulatory protein in store-operated Ca2+ entry (SOCE) with Orai1 as principal Ca2+ entry channel. Both proteins contribute to collagen-dependent arterial thrombosis in mice in vivo. It is unclear whether STIM2 is involved. A key platelet response relying on Ca2+ entry is the surface exposure of phosphatidylserine (PS), which accomplishes platelet procoagulant activity. We studied this response in mouse platelets deficient in STIM1, STIM2, or Orai1. Upon high shear flow of blood over collagen, Stim1−/− and Orai1−/− platelets had greatly impaired glycoprotein (GP) VI-dependent Ca2+ signals, and they were deficient in PS exposure and thrombus formation. In contrast, Stim2−/− platelets reacted normally. Upon blood flow in the presence of thrombin generation and coagulation, Ca2+ signals of Stim1−/− and Orai1−/− platelets were partly reduced, whereas the PS exposure and formation of fibrin-rich thrombi were normalized. Washed Stim1−/− and Orai1−/− platelets were deficient in GPVI-induced PS exposure and prothrombinase activity, but not when thrombin was present as co-agonist. Markedly, SKF96365, a blocker of (receptor-operated) Ca2+ entry, inhibited Ca2+ and procoagulant responses even in Stim1−/− and Orai1−/− platelets. These data show for the first time that: (i) STIM1 and Orai1 jointly contribute to GPVI-induced SOCE, procoagulant activity, and thrombus formation; (ii) a compensating Ca2+ entry pathway is effective in the additional presence of thrombin; (iii) platelets contain two mechanisms of Ca2+ entry and PS exposure, only one relying on STIM1-Orai1 interaction.
Keywords: Calcium Channels, Phosphatidylserine, Platelet, Signal Transduction, Thrombin, Coagulation, GPVI, Orai1, STIM1, Thrombus Formation
Introduction
In platelets, elevation in cytosolic [Ca2+]i is imperative to almost all functional responses. Moderate and transient rises in [Ca2+]i mediate shape change, integrin αIIbβ3 activation, thromboxane formation, and secretion of granule contents, whereas high and prolonged [Ca2+]i rises are required for the procoagulant response (1, 2). The latter is achieved by a Ca2+-activated scramblase mechanism disturbing the normal phospholipid asymmetry in the plasma membrane, with, as a result, the exposure of phosphatidylserine (PS)5 at the outer membrane surface (3, 4). Exposed PS provides high affinity binding sites for key coagulation factors and, thereby, facilitates the assembly of tenase and prothrombinase complexes, which are responsible for the formation of factor Xa and thrombin, respectively (3). Because thrombin is one of the most potent platelet agonists, the procoagulant platelet response triggers a potent positive feedback loop of platelet and coagulation activation. Recent in vivo studies have indicated that PS exposure and ensuing thrombin generation are key regulatory events in murine arterial thrombus formation (5, 6).
Whereas stored platelets may expose procoagulant PS in a Ca2+-independent way, PS exposure in activated platelets relies on a high and prolonged rise in cytosolic [Ca2+]i (7). Platelet stimulation with single G protein-coupled agonists, like thrombin and ADP, results in limited PS exposure (8, 9), but stimulation of the tyrosine kinase-linked collagen receptor glycoprotein VI (GPVI), with ligands such as collagen-related peptide (CRP) or convulxin, results in appreciable procoagulant activity (10, 11). Combined stimulation of the collagen and thrombin receptors though results in high PS exposure, likely because these agonists use different signaling pathways for mobilizing cytosolic Ca2+ (1). Although thrombin transiently activates Gqα and phospholipase Cβ2/β3 isoforms, activation of GPVI causes a more persistent activation of the phospholipase Cγ2 isoform (2, 12). For PS exposure, entry of extracellular Ca2+ is required, complementing the Ca2+-mobilizing effect of phospholipase C stimulation, to reach sufficiently high [Ca2+]i (10, 13, 14).
In platelets like other cells, Ca2+ entry can be triggered by receptor stimulation as well as by Ca2+ mobilization from stores via the processes of receptor-operated Ca2+ entry and store-operated Ca2+ entry (SOCE), respectively (15). For long, not only the responsible Ca2+ entry channels, but also the coupling mechanisms of receptor activation and Ca2+ store depletion to channel opening have remained elusive. In earlier work with platelets, roles of the TRPC1 and TRPC6 channel proteins in Ca2+ entry have been proposed (16, 17). Recent studies, however, have shown the importance of the Orai class of plasma membrane Ca2+ channels. The channel Orai1 (also called CRACM1) oligomerizes and opens, following depletion of the Ca2+ stores, by interacting with Ca2+ sensing STIM1, which is a transmembrane protein located in the endoplasmic reticulum (18–20). The homologous protein STIM2 can have a similar regulatory role in Ca2+ entry (21). Both Orai1 and STIM1 have been implicated in the physiological activation of T cells and mast cells (22, 23). Recent studies using genetically modified mice have established that STIM1 and Orai1 account for the large majority of SOCE in platelets. The importance of this SOCE pathway appeared from the finding that platelet deficiency in either Orai1 or STIM1 protects against collagen-dependent arterial thrombus formation and brain infarction in vivo (24, 25). In confirmation, others have provided evidence that a functional R93W mutation in Orai1 leads to impaired GPVI-induced platelet activation (26).
In the present paper, we investigated whether the STIM isoforms and Orai1 provide the main Ca2+ entry mechanism responsible for PS exposure and procoagulant activity in platelets stimulated by the collagen and thrombin receptors. The studies were carried out using mice with Stim1−/−, Stim2−/−, or Orai1−/− platelets.
EXPERIMENTAL PROCEDURES
Mouse Strains
Animal studies were approved by the local animal care and use committees. Mice homozygously deficient in Stim1 or Orai1 were generated from embryonic stem cell clones and germ line transmission, as described (24, 25). Because these animals suffered from early lethality and growth retardation, bone marrow chimeras were created which had normal viability. Female, 5–6-week-old C57BL/6 mice were irradiated with a single dose of 10 Gy and injected intravenously with bone marrow cells from donor Stim1−/−, Orai1−/−, or wild type mice (4 × 106 cells/animal). The recipient mice received acidified water containing 2 g/liter neomycin sulfate for 6 weeks after transplantation. Blood was taken from the chimeras after >6 weeks. Mice homozygously deficient in Stim2 had a mixed genetic background and were compared with wild types of the same background (27). Blood cell counts of all mice were in the normal range. Purified platelets from (bone marrow-transplanted) mice were subjected to Western blotting to confirm knock-out of STIM proteins. Deficiency in Orai1 transcripts was confirmed by reverse transcription-PCR analysis (25).
Materials
H-Phe-Pro-Arg chloromethyl ketone (PPACK) was obtained from Calbiochem. Annexin A5 labeled with fluorescein isothiocyanate (FITC), Fura-2 and Fluo-4 acetoxymethyl esters, and pluronic F-127 were from Invitrogen. Thrombin substrate, Z-Gly-Gly-Arg aminomethyl coumarin (Z-GGR-AMC), was from Bachem. Fibrillar type I collagen (Horm) was from Nycomed. Recombinant human tissue factor was from Dade Behring. Apyrase (grade V), bovine serum albumin (BSA), heparin, SKF96365, and thrombin were from Sigma. The GPVI agonist CRP was synthesized and cross-linked by Tana Laboratories. The agonist convulxin was purified as described (11). FITC-labeled anti-mouse P-selectin monoclonal antibody was from Emfret Analytics. Bovine coagulation factors and other materials were from sources indicated before (28).
Blood Collection and Platelet Preparation
Mouse blood was obtained via orbital puncture under anesthesia. For perfusion studies in the absence of coagulation, blood was collected into a mixture of 40 μm PPACK, 5 units/ml heparin, and 40 units/ml fragmin. For later perfusions with coagulation, blood was collected into 12.9 mm trisodium citrate. Citrate-anticoagulated blood was also used to prepare platelet-rich plasma (PRP), normalized with autologous platelet-poor plasma to a platelet count of 1 × 108/ml. Washed platelets were prepared from PRP by supplementation with ACD solution (85 mm sodium citrate, 78 mm citric acid, and 11 mm d-glucose) (1:25). After centrifugation, platelets were suspended in Hepes buffer, pH 7.45 (136 mm NaCl, 5 mm Hepes, 2.7 mm KCl, 2 mm MgCl2, 0.42 mm Na2HPO4, 5 mm glucose, and 0.1% BSA). Cells were counted with a Coulter counter.
Activation of Suspended Platelets
Washed mouse platelets were loaded with the ratiometric Ca2+ probe Fura-2, as described (24). Platelets suspended in the presence or absence of 1 mm CaCl2 were preincubated with 100 μm SKF96365 and activated with collagen and/or thrombin receptor agonists, as indicated. Changes in fluorescence were measured with a PerkinElmer Life Sciences 55 fluorometer. Excitation was alternated between 340 and 380 nm, and emission was measured at 509 nm. Calibration parameters of nanomolar [Ca2+]i were obtained by lysis with 1% Triton X-100 and addition of a surplus of EGTA. For flow cytometry, unloaded washed platelets (1 × 108/ml) were activated in the presence of CaCl2 with the indicated agonists for 10 min; stirring was absent to prevent platelet aggregation (29). Surface expression of PS was detected in the presence of CaCl2 (2 mm) with FITC-annexin A5 (0.5 μg/ml).
Thrombus Formation on Collagen
Glass coverslips were coated with fibrillar type I collagen and blocked with BSA-containing Hepes buffer, pH 7.45. The coverslips were mounted in a transparent, 50 μm-deep parallel-plate poly(methyl) methacrylate flow chamber (30). Mouse blood was perfused through the flow device at a defined shear rate under physiological, millimolar concentrations of divalent cations. For experiments in the absence of coagulation, PPACK/heparin-treated blood was flowed over collagen at 1000 s−1 for 4 min. For experiments in the presence of coagulation, citrate-anticoagulated blood was recalcified directly before entering the flow chamber, using a two-pump system (29). Briefly, 1-ml syringes were filled with citrate-anticoagulated blood or isotonic CaCl2/MgCl2 solution (110 mm NaCl, 13.3 mm CaCl2, and 6.7 mm MgCl2). The syringes were connected to the flow chamber via a v-shaped inlet, designed to give optimal fluid mixing. By co-infusing both fluids into the chamber at an equal flow rate (final shear rate, 1000 s−1), coagulation was started by collagen- and factor XII-dependent activation (31). The thrombi on coverslips were postlabeled by perfusion with FITC-annexin A5 (0.5 μg/ml) in Hepes buffer, pH 7.45, containing 2 mm CaCl2 and 1 unit/ml heparin. Bright-field and fluorescence images were recorded from at least 10 randomly chosen microscopic fields (32). Images were analyzed with ImagePro software (Media Cybernetics). The procoagulant index of thrombi was determined as the ratio of surface coverage of PS-exposing platelets (FITC-annexin A5) to the coverage of total platelets (33).
Single-platelet Ca2+ Fluxes under Flow
Mouse platelets were loaded with Fluo-4 (34) and added to PPACK/fragmin- or citrate-anticoagulated blood from the same genotype (10% labeled platelets). During blood flow over collagen, 16-bit digital fluorescence images were recorded at high speed (5 Hz) using an EM-CCD camera (31). Regions of interest representing single adhered platelets were analyzed off-line for changes in fluorescence (F) (34). Pseudo-ratio F/Fo values were converted into nanomolar concentrations of [Ca2+]i using predefined calibration parameters (35). For quantitative purposes, traces from individual cells were superimposed so that [Ca2+]i rises started at the same frame number.
Prothrombinase Activity
Prothrombinase-stimulating activity was determined at linear assay conditions, as previously assessed for human platelets (36). Washed mouse platelets were diluted in Hepes buffer, pH 7.45, containing 3 mm CaCl2 to a count of 4 × 105/ml and incubated with 0.5 μm prothrombin, 2 nm factor Va, and 1 nm factor Xa (37 °C). Samples were taken after exactly 3 min and transferred to vials containing 0.5 mm thrombin substrate S2238 for chromogenic measurement of the thrombin formed.
Thrombin Generation
Thrombin generation was measured in citrate-anticoagulated PRP (28). The PRP, pooled from three animals with the same (chimeric) genotype, was diluted with autologous platelet-poor plasma to a count of 1.5 × 108 platelets/ml. Samples were activated with convulxin (100 ng/ml), ionomycin (20 μm), or vehicle for 15 min. Aliquots (4 volumes) were then transferred to a polystyrene 96-wells plate (Immulon 2HB, Dynex Technologies), already containing 1 volume of buffer A (20 mm Hepes, 140 mm NaCl, 0.5% BSA, and 6 pm tissue factor). Coagulation was started by adding 1 volume of buffer B (2.5 mm Z-GGR-AMC, 20 mm Hepes, 140 mm NaCl, 100 mm CaCl2, and 6% BSA). First-derivative curves were converted into nanomolar thrombin concentrations using a calibrator for human α-thrombin (28). All analyses were in duplicate.
Statistics
Differences between groups were tested for significance with the nonparametric Mann-Whitney U test. Paired data were compared with Student's t test. The statistical package for social sciences (SPSS 15.0) was used.
RESULTS
Deficiency in Platelet STIM1 or Orai1 Impedes GPVI-mediated PS Exposure and Thrombus Formation
For the experiments, chimeric mice were generated by transplantation of irradiated wild type animals with Stim1−/−, Orai1−/−, or wild type bone marrow cells. Platelets from these mice, isolated at least 4 weeks after transplantation, were checked for the absence of STIM1 protein or Orai1 transcript (supplemental Fig. 1, A and B). The platelets from chimeric Stim1−/− and Orai1−/− mice were greatly impaired in Ca2+ rises evoked by the GPVI agonist, CRP (a triple-helical collagen peptide), or by thrombin, most markedly when extracellular CaCl2 was present (supplemental Fig. 1C), which is in full agreement with earlier data (24, 25). In addition, Stim1−/− platelets showed reduced Ca2+ rises in the absence of CaCl2 and Ca2+ entry, supporting the concept that STIM1 controls the filling state of Ca2+ stores (24).
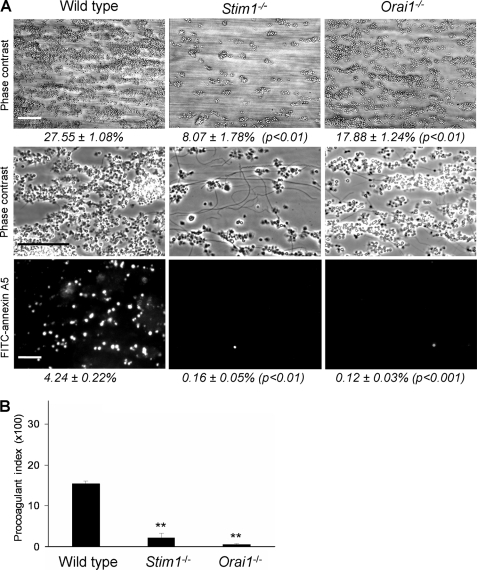
Blood from these chimeric mice was anticoagulated with PPACK/heparin and perfused at high shear flow rate over a collagen surface. By using these thrombin inhibitors, blood coagulation was ablated, whereas high, physiological concentrations of free Ca2+ and Mg2+ were maintained. In this test of collagen-induced thrombus formation, platelets in contact with the collagen fibers become activated via GPVI and respond by Ca2+ elevation, PS exposure, and secretion of autocrine mediators, as a result platelet aggregate formation (5, 33, 37). Strikingly, with blood from chimeric Stim1−/− and Orai1−/− mice, no or only small aggregates were formed, whereas PS exposure was almost completely absent, as apparent from the lack of staining with FITC-labeled annexin A5 (Fig. 1A). This contrasted to the large aggregates and many PS-exposing platelets observed with blood from corresponding wild type mice. Analysis of microscopic images pointed to a markedly reduced deposition of Stim1−/− (−70%) and Orai1−/− (−46%) platelets compared with wild type. The procoagulant index, i.e. the relative formation of PS-exposing platelets compared with all adhered platelets (33), was also greatly reduced in either knock-out (Fig. 1B).
FIGURE 1.
Deficiency in STIM1 or Orai1 impedes GPVI-dependent thrombus formation and PS exposure under flow. PPACK/heparin-anticoagulated blood of C57BL/6 mice transplanted with bone marrow from wild type, Stim1−/− or Orai1−/− animals was flowed over collagen at a shear rate of 1000 s−1. A, representative contrast images after 4 min, captured at low (top panels) or high (middle panels) magnification. Bottom panels, fluorescence images after staining with FITC-annexin A5. Percentages in italic indicate area covered with platelets (scale bars, 50 μm). B, procoagulant index representing relative number of PS-exposing platelets. Data are percentage fractions of adhered platelets exposing PS. Means ± S.E. (error bars) are shown. n = 6–8; **, p < 0.01 versus wild type.
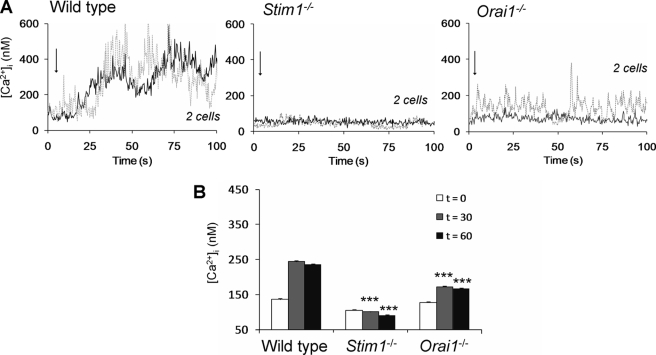
Mouse platelets were loaded with Fluo-4 and back-added to blood of the same genotype to measure Ca2+ rises in the cells during blood flow over collagen. Wild type platelets showed high Ca2+ responses shortly after adhesion (Fig. 2A). However, adhered Stim1−/− platelets gave only minute Ca2+ spikes, in a manner resembling the low responses of Fcer1g−/− platelets, known to lack GPVI signaling activity (34). Many of the adhered Orai1−/− platelets gave similar, minute Ca2+ spikes (∼50%), but the remaining Orai1−/− cells showed a short series of medium amplitude spikes (Fig. 2A). Quantitative analysis demonstrated a nearly complete ablation of the average Ca2+ signal in Stim1−/− platelets and a greatly reduced signal in Orai1−/− platelets (Fig. 2B). Together, these results point to important roles of both STIM1 and Orai1 in collagen-dependent Ca2+ signaling, PS exposure, and thrombus formation under noncoagulant conditions.
FIGURE 2.
Deficiency in STIM1 or Orai1 impedes GPVI-dependent Ca2+ responses of collagen-adhered platelets under flow. PPACK/heparin-anticoagulated blood of wild type or chimeric Stim1−/− or Orai1−/− mice was supplemented with 10% Fluo-4-loaded platelets of the same genotype. Blood samples were flowed over collagen at 1000 s−1, and fluorescence images from the collagen surface were recorded at 5 Hz. A, single-cell rises in Ca2+ of two representative platelets per genotype. Arrows indicate time point of adhesion. B, quantitative analysis of Ca2+ responses at 30 and 60 s after initial Ca2+ rises. Means ± S.E. are shown. n = 35–45 cells; ***, p < 0.001 compared with wild type.
Deficiency in STIM1 or Orai1 Does Not Abolish PS Exposure and Thrombus Formation under Coagulant Conditions
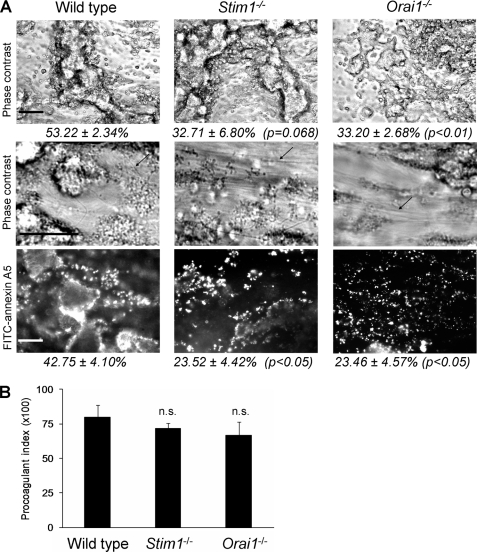
GPVI-induced platelet activation has been shown to control collagen-dependent thrombus formation also under conditions favoring coagulation (29). To determine the role of STIM1 and Orai1 in this setting, citrate-anticoagulated blood from chimeric animals was co-infused with CaCl2/MgCl2 to achieve again millimolar free Ca2+ and Mg2+ concentrations. Perfusion of the recalcified blood over collagen leads to onset of coagulation via collagen-dependent activation of factor XII (31). With wild type blood, massive and dense thrombi (clots) were formed within 4 min of perfusion, which were covered with PS-exposing platelets and connected by fibrin fibers (Fig. 3A). Surprisingly, dense fibrin-containing thrombi were also formed with Stim1−/− or Orai1−/− blood. These thrombi were also surrounded by PS-exposing platelets, but to a lesser degree compared with wild type blood. However, the procoagulant index was similarly high for images recorded from wild type, Stim1−/−, and Orai1−/− thrombi (Fig. 3B). These results thus indicate that neither STIM1 nor Orai1 is essential for collagen-dependent thrombus formation and PS exposure under coagulant conditions.
FIGURE 3.
Deficiency in STIM1 or Orai1 permits GPVI-dependent thrombus formation and PS exposure in the presence of coagulation. Citrate-anticoagulated blood of the indicated mice was recalcified with CaCl2/MgCl2 and flowed over collagen for 4 min. Thrombi with platelets and fibrin (arrows) were poststained with FITC-annexin A5. A, representative phase contrast and fluorescence images after 4 min (scale bars, 50 μm). Percentages in italic indicate area covered with (fluorescent) platelets. B, procoagulant index of relative number of PS-exposing platelets. Means ± S.E. (error bars) are shown. n = 5–7; n.s., difference between groups not significant.
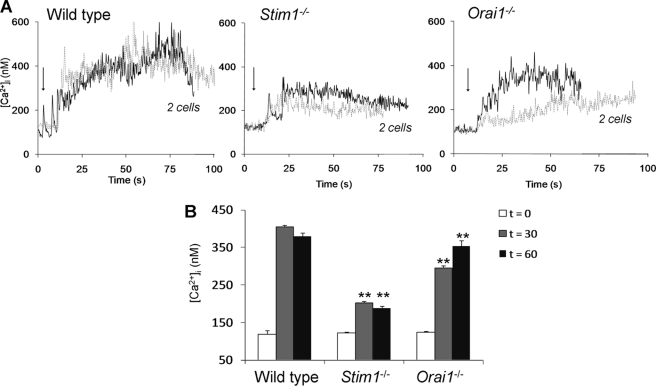
Fluo-4-loaded platelets were then used to determine rises in Ca2+ under the same conditions of flow and coagulation. Wild type platelets, adhered to collagen, showed prolonged, high Ca2+ responses (Fig. 4A), which were higher in level than those measured in anticoagulated blood (Fig. 2B). With the thrombin inhibitor hirudin present, the mean Ca2+ rises reduced from about 400 to 250 nm, which confirmed the contribution of thrombin to the Ca2+ signal. Interestingly, in recalcified blood, collagen-adhered Stim1−/− and Orai1−/− platelets also showed prolonged Ca2+ responses, but these remained lower in magnitude than those of wild type platelets (Fig. 4B). The effect of STIM1 knock-out was again more pronounced than that of Orai1 deficiency. Hence, under these conditions of in situ formation of thrombin, platelet Ca2+ responses seem to be sufficiently high for PS exposure even in the absence of the STIM1-Orai1-SOCE pathway.
FIGURE 4.
Deficiency in STIM1 or Orai1 partly reduces Ca2+ signaling in the presence of coagulation. Citrate-anticoagulated blood of the indicated mice was supplemented with 10% Fluo-4-loaded platelets of the same genotype and recalcified. During blood flow over collagen, fluorescence images from the collagen surface were recorded at 5 Hz. A, single-cell rises in [Ca2+]i of two representative platelets per genotype. Arrows indicate time point of adhesion. B, quantitative analysis of Ca2+ responses at 30 and 60 s after initial [Ca2+]i rises. Means ± S.E. (error bars) are shown. n = 15–29 cells; **, p < 0.01 compared with wild type.
Deficiency in STIM1 or Orai1 Does Not Affect GPVI-Induced PS Exposure and Prothrombinase Activity with Thrombin Present
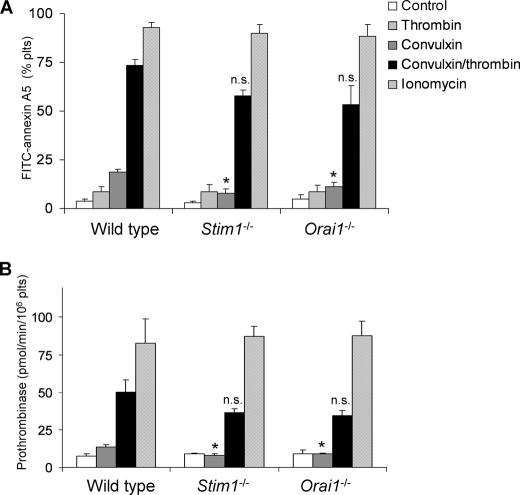
To investigate this further, washed platelets were stimulated with collagen (GPVI)- and/or thrombin-receptor agonist, and staining of the cells was observed with the PS probe, FITC-annexin A5. Extracellular CaCl2 was present to allow Ca2+ entry. The GPVI agonist convulxin was used for these studies (12), which is similarly effective as CRP in wild type and Orai1-deficient platelets (25). Flow cytometric analysis showed that convulxin stimulation of wild type platelets resulted in about 20% PS-exposing platelets (Fig. 5A). This fraction increased to 73% with thrombin as co-agonist, whereas thrombin alone had no more than little effect. Stimulation of platelets from chimeric Stim1−/− and Orai1−/− mice with convulxin resulted in impaired PS exposure, comparable with stimulation with thrombin alone. However, combined stimulation with convulxin and thrombin resulted in 53–58% PS-positive platelets, which was similar to that of wild type platelets. Control experiments indicated that all platelets responded nearly completely when stimulated with the Ca2+ ionophore, ionomycin. As an alternative approach, we studied the capacity of platelets to support prothrombinase activity, i.e. the PS-dependent cleavage of prothrombin by factors Xa and Va (13). In wild type platelets, but not Stim1−/− or Orai1−/− platelets, convulxin caused a moderate increase in prothrombinase activity (Fig. 5B). The combination of convulxin and thrombin induced a 5-fold increased prothrombinase activity with wild type platelets and a slightly lower increase with knock-out platelets, but this was not significantly different from wild type. Stimulation with ionomycin/CaCl2 caused high, maximal prothrombinase activity in all groups.
FIGURE 5.
Deficiency in STIM1 or Orai1 permits GPVI-dependent procoagulant activity in the presence of thrombin. Platelets in buffer containing 2 mm CaCl2 (1 × 108/ml) were stimulated for 10 min with convulxin (100 ng/ml), thrombin (4 nm), or ionomycin (20 μm), as indicated. A, flow cytometric analysis of binding of FITC-annexin A5 to platelets (n = 5). B, determination of prothrombinase activity of platelet suspensions. Activity is expressed as pmol of thrombin/min per 106 platelets. n = 7–8. Means ± S.E. (error bars) are shown. *, p < 0.05; n.s., not significant compared with wild type.
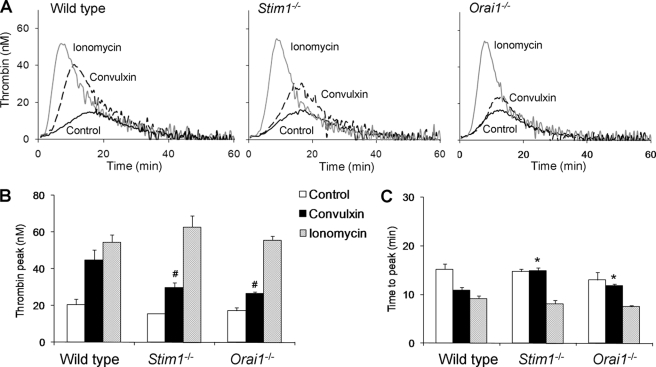
The procoagulant response of platelets was also studied in natural plasma environment, by activating PRP with tissue factor/CaCl2 and measuring generation of thrombin (5). Similar thrombin generation curves were obtained with PRP from wild type, Stim1−/−, and Orai1−/− mice (Fig. 6A). In all groups, prestimulation with ionomycin caused a 3–4-fold increase in thrombin generation, indicating that the knock-out platelets were normally capable to support thrombin generation (Fig. 6B). Prestimulation with convulxin resulted in a significant increase in thrombin peak height in all groups, but in this case STIM1- or Orai1-deficient PRP was less effective than wild type PRP compared with prestimulation with ionomycin. The time-to-peak was less shortened in convulxin-stimulated knock-out PRP compared with wild type (Fig. 6C). Taken together, these results show that, in the presence of thrombin (either externally added or formed in situ), GPVI-dependent PS exposure and procoagulant activity are only partly affected by deficiency in platelet STIM1 or Orai1.
FIGURE 6.
Deficiency in STIM1 or Orai1 reduces GPVI-dependent thrombin generation. Citrate-anticoagulated PRP (1 × 108 platelets/ml) of the indicated mice was preincubated with vehicle solvent (control), convulxin (100 ng/ml), or ionomycin (20 μm). Thrombin generation was triggered with tissue factor/CaCl2. A, representative thrombin generation curves per genotype. B, quantification of thrombin peak height. C, quantification of time to peak. Means ± S.E. (error bars) are shown. n = 4–6. #, p < 0.05 compared with wild type (ionomycin); *, p < 0.05 compared with control.
Roles of Ca2+ Entry Channels Other than Orai1
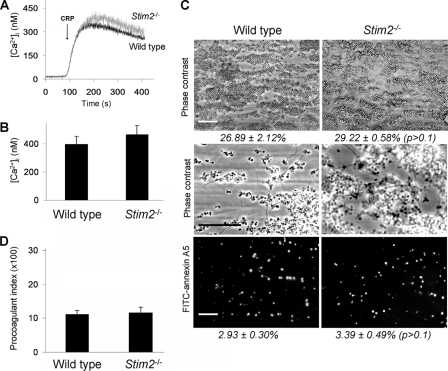
The modest roles of STIM1 and Orai1 in GPVI-dependent platelet procoagulant activity in the presence of thrombin suggest the presence of a compensating Ca2+ entry mechanism, that, for instance, could be triggered in a receptor-operated fashion. In other cell types, it has been shown also that STIM2 regulates Ca2+ entry by controlling cytoplasmic Ca2+ levels (38). Because mouse platelets highly express this isoform (supplemental Fig. 1B), we studied the possibility that STIM2-mediated Ca2+ regulation controls the extent of PS exposure. Mice homozygously deficient in STIM2 are viable and have normal platelet counts (27). Stimulation of Fura-2-loaded Stim2−/− platelets showed unaltered Ca2+ responses upon stimulation of GPVI (Fig. 7, A and B) or thrombin-receptors (not shown). Flow perfusion experiments over collagen, performed with blood from the Stim2−/− mice, indicated the formation of large thrombi and the presence of many PS-exposing platelets (Fig. 7C). Furthermore, detailed image analysis pointed to an unchanged procoagulant index of Stim2−/− thrombi compared with Stim2+/+ thrombi (Fig. 7D).
FIGURE 7.
Unchanged GPVI-dependent Ca2+ responses and thrombus formation of STIM2-deficient platelets. A, representative Ca2+ rises of Fura-2-loaded platelets from wild type and Stim2−/− mice, induced by CRP (10 μg/ml) plus CaCl2 (1 mm). B, quantification of maximal Ca2+ rises. C and D, thrombus formation after flow of PPACK/heparin-anticoagulated blood over collagen at high shear rate (4 min). C, representative contrast images captured at low (top panels) or high (middle panels) magnification. Bottom panels, fluorescence images after staining with FITC-annexin A5 (scale bars, 50 μm). D, procoagulant index of relative number of PS-exposing platelets. Means ± S.E. (error bars) are shown. n = 4–6. The difference between groups was not significant.
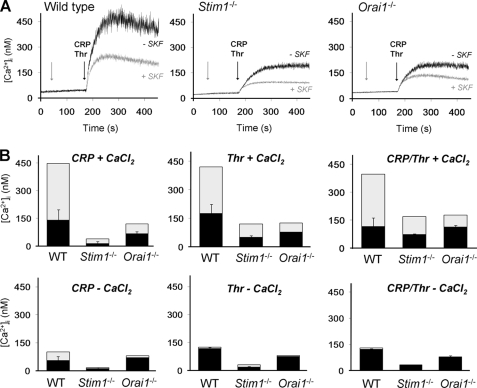
The putative role of other Ca2+ entry channels was investigated by using the established Ca2+ entry blocker SKF96365 (39). To compare with earlier established Ca2+ traces (supplemental Fig. 1), Fura-2-loaded platelets were activated with GPVI agonist CRP, thrombin, or a combination of both. In wild type platelets, pretreatment with SKF96365 inhibited the Ca2+ responses to all agonists with 60–70%, only if CaCl2 was present in the incubation medium (Fig. 8A). On the other hand, when no CaCl2 was added and Ca2+ entry was prevented, SKF96365 did not have a significant effect (Fig. 8B). Pretreatment with SKF96365 had a similar, 70% inhibitory effect on the Ca2+ response evoked by convulxin, again only in the presence of CaCl2. Strikingly, in Stim1−/− and Orai1−/− platelets, SKF96365 still suppressed both the CRP- and thrombin-induced Ca2+ responses by 40–60%, again if extracellular CaCl2 was present (Fig. 8B). For all genotypes, the inhibition by SKF96365 was significant in the presence of CaCl2 (p < 0.001), but not without CaCl2 (p = 0.05–0.38). The Stim1−/− and Orai1−/− platelets responded similarly, but the former had a lower residual Ca2+ signal in the presence of SKF96365, which is explained by the reduced Ca2+ store content in the these cells. Comparison of time traces of knock-out platelets shows that the SKF96365-inhibitable Ca2+ signal is a relatively slow component, operating after ∼10–60 s (Fig. 8A).
FIGURE 8.
Residual receptor-induced Ca2+ entry in STIM1- and Orai1-deficient platelets. Calcium responses were measured of Fura-2-loaded platelets from wild type (WT), chimeric Stim1−/−, or Orai1−/− mice, induced by CRP (10 μg/ml) and/or thrombin (Thr, 0.9 nm) and CaCl2 (1 mm), as indicated. A, representative traces of SKF96365 (SKF, 100 μm) effect on Ca2+ rises. Arrows indicate addition of SKF96365 and agonist(s), respectively. B, quantitative effect of SKF96365 on Ca2+ rises. Total bars give rises without SKF96365; black bars indicate rises with SKF96365; and gray bars represent inhibition by SKF96365 (means ± S.E., n = 3–4).
Further experiments demonstrated that the SKF96365-inhibitable Ca2+ entry pathway was important for procoagulant activity because it suppressed the GPVI/thrombin-mediated PS exposure in both wild type (-73 ± 8%) and Orai1−/− platelets (−34 ± 6%, p < 0.05). Together, these data point to the involvement of a second Ca2+ entry pathway in platelet PS exposure that is different from the Orai1 channels.
DISCUSSION
This study examines the contribution of recently discovered Ca2+ entry pathways to the regulation of platelet procoagulant activity and thrombus formation. A first key finding is that deficiency in the Ca2+ sensor STIM1 or the Ca2+-selective channel Orai1 greatly reduces GPVI-dependent PS exposure and thrombus formation under conditions of flow and absence of coagulation. This corresponds to a major reduction in the Ca2+ responses of collagen-adhered Stim1−/− and Orai1−/− platelets. Thus, it can be concluded that the reduced GPVI-induced Ca2+ signaling leads to impaired PS exposure and platelet aggregation. Interestingly, platelets from Stim2−/− mice are normally active in thrombus formation and PS exposure under the same flow conditions, indicating that the STIM2 homolog is not implicated in these platelet responses.
Detailed single-cell analysis yet points to discernible differences between the genotypes, in that a subpopulation of the collagen-adhered Orai1−/− platelets, but not of the Stim1−/− platelets, displays residual spiking in Ca2+. This residual Ca2+ signal will be a consequence of the appreciable GPVI-induced Ca2+ mobilization from internal stores of Orai1-deficient platelet (25), in contrast to the markedly reduced Ca2+ store mobilization of STIM1-deficient platelets (24). This difference between the genotypes was confirmed in CRP- and thrombin-induced activation studies with Fura-2-loaded platelets. The present findings agree with the recent evidence that a dysfunctional R93W mutation of the Orai1 channel leads to partial impairment of GPVI-induced PS exposure (26). Collectively, our data underline the importance of both SOCE-regulating proteins in shear-dependent platelet activation by collagen under conditions where the contribution of thrombin is absent or limited.
The second key finding is that, under conditions of flow and coagulation, deficiency in platelet STIM1 or Orai1 does not abolish the capability to PS exposure and formation of a fibrin-containing thrombus. Under these conditions, collagen-adhered Stim1−/− and Orai1−/− platelets still show prolonged rises in Ca2+, although these are lower in magnitude compared with wild type platelets. Although the number of PS-exposing Stim1−/− and Orai1−/− platelets is still reduced, the procoagulant index is similar for all genotypes. This suggests that the GPVI-induced SOCE pathway mediated by STIM1 and Orai1 becomes redundant in the regulation of thrombus formation in cases where thrombin acts as a co-agonist. This conclusion is supported by the data showing that stimulation of isolated Stim1−/− or Orai1−/− platelets with a combination of GPVI and thrombin receptor agonists results in near unchanged PS exposure and prothrombinase activity. Furthermore, these platelets are partly inhibited in tissue factor-triggered thrombin generation when stimulated with GPVI ligand. It has been shown that polyphosphates released from platelets significantly contribute to thrombin generation in PRP by activating factor XII (40). The above findings suggest that the polyphosphate contribution is still effective in the absence of STIM1 or Orai1.
The standard protocol for assessment of SOCE is measurement of entry of extracellular Ca2+ after Ca2+ store depletion, e.g. by the reticular Ca2+-ATPase inhibitor thapsigargin. This Ca2+ entry is almost totally blocked in Stim1−/− and Orai1−/− platelets, implying that the STIM1-Orai1 interaction forms the principal SOCE mechanism (24, 25). A similar conclusion has been drawn for other secretory cells, e.g. T cells and mast cells (41). However, it now appears that both Stim1−/− and Orai1−/− platelets show significant Ca2+ responses and PS exposure, when co-stimulated via GPVI and thrombin receptors. This is suggestive for the presence of another, compensatory mechanism of Ca2+ entry, acting in a (thrombin) receptor-operated fashion.
This hypothesis is supported by our finding that the imidazole antagonist SKF96365, which is a well studied inhibitor of Ca2+ entry in platelets (39), is still capable of suppressing the Ca2+ and procoagulant responses of platelets deficient in STIM1 or Orai1. Unfortunately, this compound cannot be used in measurements of thrombus formation because it rapidly inactivates in the presence of blood plasma (data not shown). In Fura-2-loaded Stim1−/− and Orai1−/− platelets, SKF96365 still inhibits 40–60% of the Ca2+ signal independently of the agonist (GPVI ligand and/or thrombin). The slow, non-Orai1 Ca2+ entry most likely does not involve the TRPC1 channel (16) because Trpc1−/− platelets have a fully intact Ca2+ signaling machinery (42). Other candidate target proteins of SKF96365 are the non-SOCE channels, TRPC6 and Orai3, both of which act in a receptor-operated way and are expressed in platelets (17, 19). Further studies are required to identify this channel.
A pending question is whether the SOCE mechanism per se is implicated in the transmembrane scrambling of phospholipids, which is the underlying event of PS exposure. Such a role of SOCE has been proposed by others (43). However, the current findings do not support this because Stim1−/− and Orai1−/− platelets (both devoid of SOCE) almost normally expose PS upon combined collagen- and thrombin-receptor stimulation. This is in agreement with the finding that platelets from Scott syndrome patients, which have a defect in PS exposure, show unchanged Ca2+ signals in response to collagen/thrombin (44). Together, this strongly argues against a role of SOCE or other Ca2+ channels as phospholipid scramblase proteins.
The present findings support the idea that the Orai1 channel is an attractive target for pharmacological treatment of thrombosis. Previous results have demonstrated that the deficiency of Orai1 in mouse platelets protects from arterial thrombus formation, particularly in thrombosis models that are collagen-dependent (24, 25). Because collagen-independent thrombosis models mostly rely on tissue factor exposure and thrombin generation (6), it is plausible that specific targeting of Orai1 (or STIM1) restricts platelet procoagulant activity and thrombus formation only under conditions, where the role of tissue factor is limited. Hence, targeting of Orai1 may be more effective to prevent thrombosis in arteries, where (tissue factor-dependent) thrombin accumulation is confined by the high shear flow conditions. In contrast, blockage of Orai1 may not impair bleeding from wounds where tissue factor is abundantly exposed. Indeed, mice deficient in Orai1 have only a mild prolongation of the tail bleeding time (25). Whether inhibition of the non-Orai1 (non-SOCE) channels targeted by SKF96365 also provides antithrombotic protection still needs to be investigated.
Supplementary Material
Acknowledgment
We thank Dr. E. M. Bevers for critical discussions and for correcting the manuscript.
This work was supported in part by Nederlandse Organisatie voor Wetenschappelijk Onderzoek Grant 11.400.0076, The Netherlands Heart Foundation Grant 2005-B079, and Deutsche Forschungsgemeinschaft Grants Sonderforschungsbereich 688 and 487.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- PS
- phosphatidylserine
- BSA
- bovine serum albumin
- CRP
- collagen-related peptide
- FITC
- fluorescein isothiocyanate
- GPVI
- glycoprotein VI
- PPACK
- H-Phe-Pro-Arg chloromethyl ketone
- PRP
- platelet-rich plasma
- SOCE
- store-operated Ca2+ entry
- STIM
- stromal-interacting molecule
- Z-GGR-AMC
- Z-Gly-Gly-Arg aminomethyl coumarin.
REFERENCES
- 1.Heemskerk J. W., Bevers E. M., Lindhout T. (2002) Thromb. Haemost. 88, 186–193 [PubMed] [Google Scholar]
- 2.Heemskerk J. W., Feijge M. A., Henneman L., Rosing J., Hemker H. C. (1997) Eur. J. Biochem. 249, 547–555 [DOI] [PubMed] [Google Scholar]
- 3.Zwaal R. F., Schroit A. J. (1997) Blood 89, 1121–1132 [PubMed] [Google Scholar]
- 4.Heemskerk J. W., Kuijpers M. J., Munnix I. C., Siljander P. R. (2005) Trends Cardiovasc. Med. 15, 86–92 [DOI] [PubMed] [Google Scholar]
- 5.Munnix I. C., Strehl A., Kuijpers M. J., Auger J. M., van der Meijden P. E., van Zandvoort M. A., oude Egbrink M., Nieswandt B., Heemskerk J. W. (2005) Arterioscler. Thromb. Vasc. Biol. 25, 2673–2678 [DOI] [PubMed] [Google Scholar]
- 6.Kuijpers M. J., Munnix I. C., Cosemans J. M., van Vlijmen B. J., Reutelingsperger C. P., oude Egbrink M. G., Heemskerk J. W. (2008) Microcirculation 15, 269–282 [DOI] [PubMed] [Google Scholar]
- 7.Schoenwaelder S. M., Yuan Y., Josefsson E. C., White M. J., Yao Y., Mason K. D., O'Reilly L. A., Henley K. J., Ono A., Hsiao S., Willcox A., Roberts A. W., Huang D. C., Salem H. H., Kile B. T., Jackson S. P. (2009) Blood 114, 663–666 [DOI] [PubMed] [Google Scholar]
- 8.Léon C., Ravanat C., Freund M., Cazenave J. P., Gachet C. (2003) Arterioscler. Thromb. Vasc. Biol. 23, 1941–1947 [DOI] [PubMed] [Google Scholar]
- 9.van der Meijden P. E., Feijge M. A., Giesen P. L., Huijberts M., van Raak E. P., Heemskerk J. W. (2005) Thromb. Haemost. 93, 1128–1136 [DOI] [PubMed] [Google Scholar]
- 10.Thiagarajan P., Tait J. F. (1991) J. Biol. Chem. 266, 24302–24307 [PubMed] [Google Scholar]
- 11.Siljander P., Farndale R. W., Feijge M. A., Comfurius P., Kos S., Bevers E. M., Heemskerk J. W. (2001) Arterioscler. Thromb. Vasc. Biol. 21, 618–627 [DOI] [PubMed] [Google Scholar]
- 12.Nieswandt B., Watson S. P. (2003) Blood 102, 449–461 [DOI] [PubMed] [Google Scholar]
- 13.Bevers E. M., Comfurius P., van Rijn J. L., Hemker H. C., Zwaal R. F. (1982) Eur. J. Biochem. 122, 429–436 [DOI] [PubMed] [Google Scholar]
- 14.Dachary-Prigent J., Pasquet J. M., Freyssinet J. M., Nurden A. T. (1995) Biochemistry 34, 11625–11634 [DOI] [PubMed] [Google Scholar]
- 15.Sage S. O. (1997) Exp. Physiol. 82, 807–823 [DOI] [PubMed] [Google Scholar]
- 16.Rosado J. A., Brownlow S. L., Sage S. O. (2002) J. Biol. Chem. 277, 42157–42163 [DOI] [PubMed] [Google Scholar]
- 17.Hassock S. R., Zhu M. X., Trost C., Flockerzi V., Authi K. S. (2002) Blood 100, 2801–2811 [DOI] [PubMed] [Google Scholar]
- 18.Soboloff J., Spassova M. A., Tang X. D., Hewavitharana T., Xu W., Gill D. L. (2006) J. Biol. Chem. 281, 20661–20665 [DOI] [PubMed] [Google Scholar]
- 19.Zhang S. L., Kozak J. A., Jiang W., Yeromin A. V., Chen J., Yu Y., Penna A., Shen W., Chi V., Cahalan M. D. (2008) J. Biol. Chem. 283, 17662–17671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luik R. M., Wang B., Prakriya M., Wu M. M., Lewis R. S. (2008) Nature 454, 538–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parvez S., Beck A., Peinelt C., Soboloff J., Lis A., Monteilh-Zoller M., Gill D. L., Fleig A., Penner R. (2008) FASEB J. 22, 752–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baba Y., Nishida K., Fujii Y., Hirano T., Hikida M., Kurosaki T. (2008) Nat. Immunol. 9, 81–88 [DOI] [PubMed] [Google Scholar]
- 23.Oh-Hora M., Yamashita M., Hogan P. G., Sharma S., Lamperti E., Chung W., Prakriya M., Feske S., Rao A. (2008) Nat. Immunol. 9, 432–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varga-Szabo D., Braun A., Kleinschnitz C., Bender M., Pleines I., Pham M., Renné T., Stoll G., Nieswandt B. (2008) J. Exp. Med. 205, 1583–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braun A., Varga-Szabo D., Kleinschnitz C., Pleines I., Bernder M., Austinat M., Bösl M., Stoll G., Nieswandt B. (2009) Blood 113, 2056–2063 [DOI] [PubMed] [Google Scholar]
- 26.Bergmeier W., Oh-Hora M., McCarl C. A., Roden R. C., Bray P. F., Feske S. (2009) Blood 113, 675–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berna-Erro A., Braun A., Kraft R., Kleinschnitz C., Schuhmann M. K., Stegner D., Wultsch T., Eilers J., Meuth S. G., Stoll G., Nieswandt B. (2009) Sci. Signal. 2, ra67. [DOI] [PubMed] [Google Scholar]
- 28.Vanschoonbeek K., Wouters K., van der Meijden P. E., van Gorp P. J., Feijge M. A., Herfs M., Schurgers L. J., Hofker M. H., de Maat M. P., Heemskerk J. W. (2008) Arterioscler. Thromb. Vasc. Biol. 28, 2023–2029 [DOI] [PubMed] [Google Scholar]
- 29.Munnix I. C., Kuijpers M. J., Auger J. M., Thomassen C. M., Panizzi P., van Zandvoort M. A., Rosing J., Bock P. E., Watson S. P., Heemskerk J. W. (2007) Arterioscler. Thromb. Vasc. Biol. 27, 2484–2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuijpers M. J., Schulte V., Bergmeier W., Lindhout T., Brakebusch C., Offermanns S., Fässler R., Heemskerk J. W., Nieswandt B. (2003) FASEB J. 17, 685–687 [DOI] [PubMed] [Google Scholar]
- 31.van der Meijden P. E., Munnix I. C., Auger J. M., Govers-Riemslag J. W., Cosemans J. M., Kuijpers M. J., Spronk H. M., Watson S. P., Renné T., Heemskerk J. W. (2009) Blood 114, 881–890 [DOI] [PubMed] [Google Scholar]
- 32.Nieswandt B., Brakebusch C., Bergmeier W., Schulte V., Bouvard D., Mokhtari-Nejad R., Lindhout T., Heemskerk J. W., Zirngibl H., Fässler R. (2001) EMBO J. 20, 2120–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siljander P. R., Munnix I. C., Smethurst P. A., Deckmyn H., Lindhout T., Ouwehand W. H., Farndale R. W., Heemskerk J. W. (2004) Blood 103, 1333–1341 [DOI] [PubMed] [Google Scholar]
- 34.Auger J. M., Kuijpers M. J., Senis Y. A., Watson S. P., Heemskerk J. W. (2005) FASEB J. 19, 825–827 [DOI] [PubMed] [Google Scholar]
- 35.Heemskerk J. W., Willems G. M., Rook M. B., Sage S. O. (2001) J. Physiol. 535, 625–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bevers E. M., Comfurius P., Reutelingsperger C. P., Zwaal R. F. (1996) in Platelets: A Practical Approach (Watson S. P., Authi K. S. eds) pp. 319–340, IRL Press, Oxford, UK [Google Scholar]
- 37.Lecut C., Schoolmeester A., Kuijpers M. J., Broers J. L., van Zandvoort M. A., Vanhoorelbeke K., Deckmyn H., Jandrot-Perrus M., Heemskerk J. W. (2004) Arterioscler. Thromb. Vasc. Biol. 24, 1727–1733 [DOI] [PubMed] [Google Scholar]
- 38.Brandman O., Liou J., Park W. S., Meyer T. (2007) Cell 131, 1327–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merritt J. E., Armstrong W. P., Benham C. D., Hallam T. J., Jacob R., Jaxa-Chamiec A., Leigh B. K., McCarthy S. A., Moores K. E., Rink T. J. (1990) Biochem. J. 271, 515–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Müller F., Mutch N. J., Schenk W. A., Smith S. A., Esterl L., Spronk H. M., Schmidbauer S., Gahl W. A., Morrissey J. H., Renné T. (2009) Cell 139, 1143–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hewavitharana T., Deng X., Wang Y., Ritchie M. F., Girish G. V., Soboloff J., Gill D. L. (2008) J. Biol. Chem. 283, 26252–26262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Varga-Szabo D., Authi K. S., Braun A., Bender M., Ambily A., Hassock S. R., Gudermann T., Dietrich A., Nieswandt B. (2008) Pflugers Arch. 457, 377–387 [DOI] [PubMed] [Google Scholar]
- 43.Martin S., Laude-Lemaire I., Kerbiriou-Nabias D., Freyssinet J. M., Martínez M. C. (2000) Biochem. Biophys. Res. Commun. 279, 639–645 [DOI] [PubMed] [Google Scholar]
- 44.Munnix I. C., Harmsma M., Giddings J. C., Collins P. W., Feijge M. A., Comfurius P., Heemskerk J. W., Bevers E. M. (2003) Thromb. Haemost. 89, 687–695 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.