Abstract
Vacuolar proton-translocating ATPases (V-ATPases) are responsible for organelle acidification in all eukaryotic cells. The yeast V-ATPase, known to be regulated by reversible disassembly in response to glucose deprivation, was recently reported to be regulated by extracellular pH as well (Padilla-López, S., and Pearce, D. A. (2006) J. Biol. Chem. 281, 10273–10280). Consistent with those results, we find 57% higher V-ATPase activity in vacuoles isolated after cell growth at extracellular pH of 7 than after growth at pH 5 in minimal medium. Remarkably, under these conditions, the V-ATPase also becomes largely insensitive to reversible disassembly, maintaining a low vacuolar pH and high levels of V1 subunit assembly, ATPase activity, and proton pumping during glucose deprivation. Cytosolic pH is constant under these conditions, indicating that the lack of reversible disassembly is not a response to altered cytosolic pH. We propose that when alternative mechanisms of vacuolar acidification are not available, maintaining V-ATPase activity becomes a priority, and the pump is not down-regulated in response to energy limitation. These results also suggest that integrated pH and metabolic inputs determine the final assembly state and activity of the V-ATPase.
Keywords: Metabolic Regulation, Organellar pH Homeostasis, Proton Pumps, Subcellular Organelles, Vacuolar Acidification, Vacuolar ATPase
Introduction
Two primary proton pumps, Pma1 at the plasma membrane and the vacuolar proton-translocating ATPase (V-ATPase)2 on multiple organelle membranes, are central players in pH homeostasis in fungi and plants (1). Pma1 is a single-subunit P-type proton pump that exports H+ from the cytosol. It is largely responsible for energization of the plasma membrane and thus is required for activity of multiple secondary transporters, and it is also regarded as the major determinant of cytosolic pH. Consistent with the central role of Pma1 in pH homeostasis, yeast mutants lacking Pma1 activity are inviable (2). V-ATPases are multisubunit enzymes that bear the sites for ATP hydrolysis in a complex of peripheral membrane subunits (V1) that is attached to a complex of integral membrane subunits (Vo) containing the proton pore. V-ATPases are present in the lysosome/vacuole, endosomes, and Golgi apparatus of all eukaryotic cells and are responsible for acidification of these organelles (3). Yeast mutants lacking V-ATPase activity are viable but have wide ranging growth defects; specifically, they are able to grow at extracellular pH 5 but are unable to grow above pH 7; are sensitive to elevated levels of Ca2+, heavy metals, and multiple drugs; and are also intolerant of certain metals (3). In yeast, the V-ATPase contributes to cytosolic pH homeostasis, both directly through removal of cytosolic protons and indirectly through effects on Pma1 localization (4, 5). These pumps, acting in concert with an array of exchangers and cellular buffers, allow fungi to survive over a wide range of extracellular pH.
Pma1 is regulated post-translationally in response to changes in extracellular glucose or pH (6). Glucose addition to glucose-starved cells activates the ATPase, resulting in a decreased Km, increased Vmax, and shift toward higher optimum pH (7). These kinetic changes are attributed to phosphorylation of the C-terminal tail of Pma1p, which relieves obstructive interactions of the C terminus with the catalytic site (8, 9). Recently, mass spectrometry experiments have implicated serine 911 and threonine 912 as the critical targets for glucose activation of Pma1 (9), although previous work has suggested other possible sites as well (8). Several kinases directly required for Pma1 phosphorylation are known; Npr family kinase Ptk2 has been shown to phosphorylate a C-terminal tail peptide, and the related Hrk1 has also been associated with glucose activation (10, 11). However, multiple other glucose-activated pathways have also been associated with glucose activation of Pma1, so the full route to activation is not known (6). Pma1 is also activated in response to reduced cytosolic pH, both as a result of metabolic activity during growth and as an acute response to the addition of weak acids (12, 13). The kinetic changes associated with activation in response to pH, primarily lowered Km, are different from those resulting from glucose activation, suggesting a distinct mechanism (6, 12). It was recently shown that changes in cytosolic pH can indirectly activate Pma1 by modulating pH-dependent regulatory interactions with Trk1, a K+ transporter that imports K+ and thus relieves the plasma membrane potential generated by electrogenic proton transport through Pma1 (14). Because Pma1 activity can be limited by plasma membrane potential, pH-dependent activation of Trk1 also activates Pma1.
V-ATPases are activated by glucose as well (15). The addition of glucose to briefly glucose-deprived yeast cells results in a drop in vacuolar pH that is entirely dependent on V-ATPase function (5). During even a brief (2–5-min) glucose deprivation, V1 subunits are dissociated from membrane-bound Vo sectors, inhibiting V-ATPase activity, and V-ATPase activation in response to glucose probably arises from reassembly of the V1 and Vo sectors (15). The pathway of glucose activation of V-ATPase reassembly remains unclear; protein kinase A activity (16–18) and phosphatidylinositol 3-kinase (19) have been implicated as well as interactions with glycolytic enzymes, such as aldolase (20, 21), and the assembly factor RAVE (22, 23). Transient phosphorylation of the V1 C subunit during reassembly has been observed in Manduca sexta, and this may well be a critical target in the signaling pathways (17).
The interplay between V-ATPase activity and extracellular pH is suggested by the pH-dependent growth phenotypes of mutants lacking V-ATPase subunits (vma mutants) (24). Optimal growth of vma mutants at an extracellular pH of 5 was initially attributed to endocytic transport of acidic medium to the vacuole (24, 25). This was subsequently disputed, but partial acidification of vacuoles in vma mutants through passive proton transport was demonstrated to occur (26). Specifically, the authors found that ammonium ion, which is added to yeast medium as a nitrogen source, acted as a vacuolar proton shuttle. They proposed that ammonium ion transporters might facilitate vacuolar acidification at low pH but acknowledged that the distinct complement of transporters in the plasma membrane and vacuole could allow other weak electrolytes to contribute to vacuolar acidification as well (26). These data suggest that although the V-ATPase is the primary player in organelle acidification, alternative acidification mechanisms may operate in tandem with the proton pump, particularly at low extracellular pH, where concentrations of permeant acids are higher. It has also been suggested that the V-ATPase itself might be regulated by extracellular pH. Padilla-López and Pearce (27) found much higher V-ATPase activity in vacuoles isolated from cells grown at an extracellular pH of 7.5 than in vacuoles from cells grown at pH 4. They attributed this difference primarily to higher levels of V1 assembly in the vacuoles from cells maintained at high pH.
Here we examine the activity of the yeast V-ATPase under different extracellular pH conditions, in both the presence and absence of glucose. We find increased activity in vacuoles isolated from cells grown at high extracellular pH although only in minimal medium. Remarkably, under these conditions, disassembly of the V-ATPase in response to glucose deprivation is largely suppressed. These results suggest that activity of V- ATPases on intracellular organelles can respond to extracellular pH conditions and that retention of V-ATPase activity may become a cellular priority at high pHext, even under conditions of energy limitation.
EXPERIMENTAL PROCEDURES
Strains and Media
Wild type yeast strain SF838-5A (MATα leu3-3,112 ura3-52 ade6 his4-519) was used for all experiments except the cytosolic and vacuolar pH measurements in Figs. 5 and 6, which generally use BY4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) background. All mutant strains used in Fig. 6, except the snf1Δ strain, are kanMX deletions of the indicated gene in the BY4741 background that were purchased from Open Biolabs. The snf1Δ10 strain is in the W303-1A background (MATa trp1 leu2 his3 ura3 ade2 can1) and was a gift from Marian Carlson. Cells were grown overnight to log phase (A600 = 0.6–0.8) in synthetic complete medium (28) buffered to pH 5 or pH 7 with 50 mm MES or yeast extract, peptone, 2% dextrose (YEPD) medium buffered to pH 5 or 7 with 50 mm succinate, 50 mm phosphate buffer or with 50 mm MES, as indicated.
FIGURE 5.
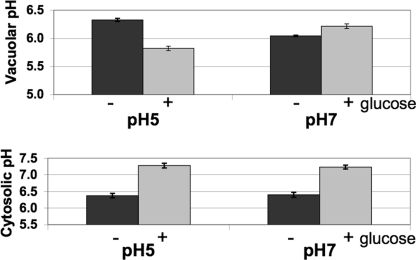
Cytosolic pH differences do not correlate with vacuolar pH differences in cells grown at pH 5 or 7. A, vacuolar pH responses of wild type yeast strain BY4741 were measured as described in the legend to Fig. 2A. B, cytosolic pH was measured by transforming BY4741 cells with yeast pHluorin as described under “Experimental Procedures.” Cells were deprived of glucose for 20–30 min to obtain the glucose-deprived (−) measurement, and then glucose was added, and a second measurement was taken after 5 min (+). Extracellular pH was maintained at pH 5 or 7 during measurement of vacuolar and cytosolic pH. Error bars, S.E.
FIGURE 6.
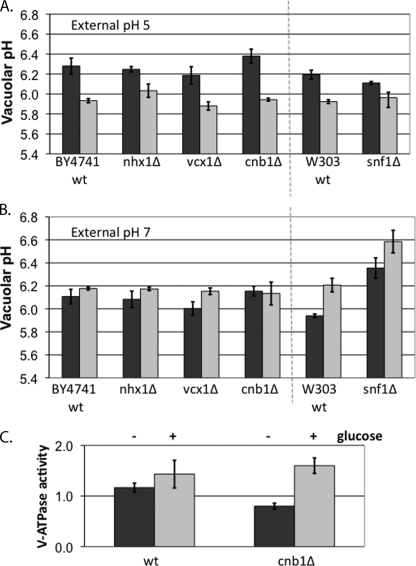
Vacuolar pH responses in mutants lacking proton antiporters and alkaline pH signaling components. A and B, vacuolar pH responses were monitored using BCECF-AM as described in the legend to Fig. 2A. The indicated strains were grown to log phase in SC buffered to pH 5 (A) or pH 7 (B), and vacuolar pH was measured after a 20–30-min glucose deprivation (dark bars) then 5 min after glucose addition (light bars). Values shown are the means of three independent determinations ± S.E. The light dotted line separates mutant strains in the BY4741 background from the snf1Δ mutant and its W303 wild type background control. C, V-ATPase activity in vacuolar vesicles from wild type (BY4741) (wt) or the congenic cnb1Δ mutant strain. Cells were grown to log phase in SC buffered to pH 7 with MES, converted to spheroplasts, and then incubated in the presence of glucose (+) or the absence of glucose (−) for 30 min before lysis, as described in the legend to Fig. 2. V-ATPase activity is expressed as μmol of Pi produced/min/mg of total vacuolar protein, and the mean of three independent vacuole preparations ± S.E. (error bars) is shown.
Reagents
2′,7′-Bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein, acetoxymethyl ester (BCECF-AM) and Alexa Fluor 488 goat anti-mouse antibody used for immunofluorescence were purchased from Invitrogen. Concanamycin A and protease inhibitors were purchased from Sigma; Zymolyase-100T was purchased from U.S. Biological. MES was purchased from GenScript. Sodium succinate and potassium phosphate were purchased from Sigma. Peptone, yeast extract, and glucose were purchased from Fisher. Yeast nitrogen base was purchased from Difco. Amino acids and Ficoll PM400 were from Sigma.
Vacuolar and Cytosolic pH Measurements
Vacuolar pH measurements were performed using the pH-sensitive ratiometric dye BCECF-AM, as previously described (5, 29). After overnight growth to log phase, cells were collected by centrifugation, and 200 mg of cell mass was resuspended in 200 μl of the original medium. Cells were then incubated with 50 μm BCECF-AM for 30 min at 30 °C with shaking. After this, cells were washed three times with medium lacking glucose in order to remove excess dye, resuspended at the same density in medium with no glucose, and kept on ice before measurement. (Cells were deprived of glucose for a total of 20–30 min before measurement.) 20 μl of cell suspension was added to 2 ml of 1 mm MES/TEA, pH 5 or 7, and the mixture was stirred in the cuvette at 30 °C. Fluorescence intensity at excitation wavelengths of 450 and 490 nm was measured in triplicate for each sample at constant emission wavelength of 535 nm with an SPEX Fluorolog-3-21 fluorometer. Vacuolar pH in the presence of glucose was measured 5 min after the addition of glucose to 50 mm final concentration. Calibration of fluorescence with pH was carried out for each sample as described (5, 29). Briefly, 20 μl of cell suspension was added to 2 ml of calibration buffer and adjusted to a desired pH with HCl and TEA or NaOH. 75 μm monensin and 10 μm nigericin were added to allow for equilibration of the intracellular pH with the external pH of the buffer. After a 30–45-min incubation at 30 °C, the ratio of fluorescence at the two excitation wavelengths was measured. Calibration curves were constructed for a pH range from 5.3 to 6.7 for each sample and used to calculate the vacuolar pH values from the observed fluorescence ratios.
Cytosolic pH measurements were performed on wild type strain BY4741, as described previously (5, 30), but used a different plasmid, which expresses the pH-sensitive, ratiometric green fluorescent protein, yeast pHluorin, under control of the phosphoglycerate kinase promoter (a generous gift from Dr. Rajini Rao, The Johns Hopkins University). A plasmid containing the phosphoglycerate kinase promoter-driven yeast pHluorin was transformed into yeast cells as described, and transformants were selected on supplemented minimal medium lacking uracil (SC−uracil), followed by microscopic confirmation of green fluorescent protein expression. Transformants were grown to log phase and collected by centrifugation, and 200 mg of cell mass was washed three times in SC−uracil lacking glucose and before resuspension at the same density. (Cells were deprived of glucose for a total of 20–30 min before the measurements were initiated.) 20 μl of cell suspension was added to 2 ml of 50 mm MES, pH 5 or 7, and the mixture was stirred in the cuvette at 30 °C. Fluorescence at excitation wavelengths of 405 and 485 nm was measured at a constant emission wavelength of 508 nm as described above. Cytosolic pH response to glucose was measured 5 min after the addition of glucose to 50 mm final concentration. Calibration curves were constructed for each sample as described above but for a pH range from 6.3 to 7.6.
Vacuolar Vesicle Preparation and Characterization
Vacuolar vesicles were isolated as described previously (31, 32). After treatment with zymolyase, spheroplasts were washed and resuspended in growth medium at the desired pH with or without glucose and recovered for 30 min at 30 °C. The spheroplasts were then pelleted by centrifugation. Cell lysis and isolation of vacuolar vesicles by flotation on Ficoll gradients were performed identically for all samples.
The rates of ATP hydrolysis were determined on freshly prepared vacuolar vesicles by a coupled enzyme assay as described (33). Concanamycin inhibition was measured by adding concanamycin A directly to the assay mixture to a final concentration of 100 nm. Specific V-ATPase activity represents the rate of concanamycin A-sensitive ATPase hydrolysis, expressed as μmol of ATP consumed/min/mg of vacuolar protein. Greater than 85% of the total ATPase activity was typically inhibited by concanamycin in the purified vacuolar vesicles.
Proton pumping was measured by the 9-amino-6-chloro-2-methoxyacridine quenching assay as described previously (33). 10–17 μg of vacuolar vesicles were added each assay. Pumping was initiated by the addition of ATP and MgSO4 to final concentrations of 0.5 and 1.0 mm, respectively. Concanamycin inhibition was measured by adding concanamycin A directly to the assay mixture to a final concentration of 100 nm. The rate of proton pumping is represented by the initial rate (first 15 s following the addition of MgATP) of 9-amino-6-chloro-2-methoxyacridine fluorescence quenching in the presence or absence of concanamycin A and is normalized to the amount of vacuolar protein (determined by a Lowry assay) added.
For detection of vacuolar protein levels by immunoblotting, vacuolar vesicles were solubilized at 65 °C with cracking buffer (8 m urea, 5% SDS, 1 mm EDTA, 50 mm Tris-HCl, pH 6.8, 5% β-mercaptoethanol), separated by SDS-PAGE, and transferred to nitrocellulose. Mouse monoclonal antibodies 8B1, 13D11, 7A2, and 10D7 were used to detect the yeast V-ATPase subunits A, B, C, and a, respectively, as described previously (33). The loads of vacuolar vesicles required to achieve subsaturating signals on immunoblots for each antibody were determined in parallel experiments (34). Signals from individual subunits were quantitated using NIH Image J software (version 1.37). In order to compare ATPase activities and subunit levels across multiple experiments, samples for vacuolar vesicles prepared in parallel were quantitated from a single immunoblot and then normalized to subunit levels from the sample grown at pH 5 and maintained in glucose through the recovery (pH 5 + glucose). ATPase activities were normalized similarly to obtain the comparisons plotted in Fig. 4. Linear regression analysis was performed using Microsoft Excel on the quantitated data from all four conditions.
FIGURE 4.
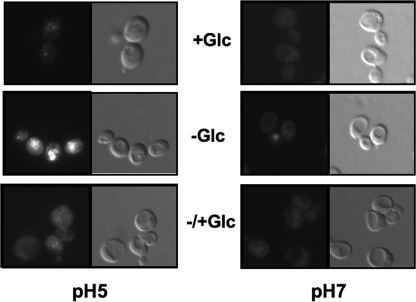
Immunofluorescence microscopy reveals limited disassembly of V-ATPase complexes at pH 7. Yeast cells were grown to log phase in SC at pH 5 or 7 as described above. Cells were then pelleted, washed, and suspended in the same medium containing glucose (+glc) or in the same medium lacking glucose (−glc). The −glc samples were incubated in the absence of glucose for 20 min at 30 °C. Fixative (4% formaldehyde) was then added, and the incubation continued for 60 min. Glucose was added to the −/+ Glc cells after the 20-min glucose deprivation, and the incubation was continued for 15 min before the addition of fixative. Fixative was added to the glucose-containing samples after 20 min of incubation in fresh glucose-containing medium. Fixed cells were prepared for immunofluorescence microscopy as described (31) and probed with mouse monoclonal antibody 10D7, which recognizes the V0 a subunit only when V1 is not bound, followed by goat anti-mouse antibody conjugated to Alexa Fluor 488. For each set of cells, the same field viewed under fluorescein fluorescence (left) and Nomarski optics (right) is shown. All fluorescence images were obtained under identical exposure times.
Indirect Immunofluorescence Microscopy
Indirect immunofluorescence of subunit a of the V-ATPase was performed as previously described (31, 32). Cells were grown overnight to log phase and then were either 1) maintained in glucose until fixation, 2) transferred to medium lacking glucose for 20 min before fixation, or 3) deprived of glucose for 20 min and then provided glucose for 15 min before fixation. V-ATPase subunit a was detected by incubation with undiluted cultured supernatant containing mouse monoclonal antibody 10D7, followed by Alexa Fluor 488 goat anti-mouse secondary antibody (1:300 dilution). Immunofluorescence imaging was done using a Zeiss Imager.Z1 fluorescent microscope, under Nomarski optics and fluorescein fluorescence optics with equal exposure time for all fluorescence samples.
RESULTS
pH-dependent Activity Changes in the V-ATPase Isolated from Cells Grown in Minimal Medium at pH 7
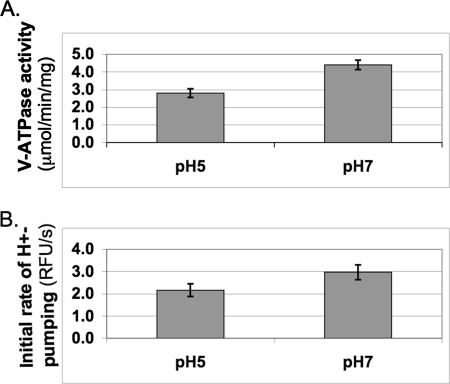
We grew cells to log phase in synthetic complete medium (SC) buffered to pH 5 or pH 7 with MES. Padilla-López and Pearce (27) had reported that V-ATPase activity depended on extracellular pH in cells grown in this medium. After overnight growth of the cells in the 50 mm MES, pH 5 and 7, medium, the medium pH had dropped to 4.2 and 6.2, respectively. Cells were converted to spheroplasts, recovered for 20–30 min in glucose-containing medium buffered to pH 5 or 7, and then lysed, and vacuolar vesicles were isolated without further pH adjustment. Interestingly, the specific V-ATPase activity in vacuolar vesicles isolated from cells grown in the pH 7 medium was significantly higher than the activity in vacuoles from cells grown in pH 5 medium (Fig. 1A). Proton pumping was also somewhat higher in the vacuoles from cells maintained at the more alkaline pHext (Fig. 1B), but there was not as much difference as in the ATPase activity. These results suggest that there is activation of V-ATPase activity when cells are grown at elevated extracellular pH.
FIGURE 1.
V-ATPase activity is higher in vacuolar vesicles isolated from cells grown in SC buffered to neutral pH. Wild type yeast cells (SF838–5Aα) were grown to log phase in SC buffered to pH 5 with 50 mm MES. Vacuolar vesicles were isolated as described under “Experimental Procedures.” Concanamycin-sensitive ATPase specific activity (V-ATPase activity; A) and the initial rate of proton pumping upon MgATP addition (B) expressed as relative fluorescence units (RFU) per second were measured in the vesicles from cells grown at each pH. The mean ± S.E. (error bars) for n = 4 independent vacuolar vesicle preparations is shown.
Activation of the plasma membrane proton pump, Pma1p, involves both decreased Km for ATP and increased Vmax (6, 7). We measured the kinetics of concanamycin-dependent ATPase hydrolysis in the vesicles from pH 5- versus pH 7-grown cells and found that there was no significant difference in overall Michaelis-Menten kinetic behavior or Km for ATP in vesicles derived from cells under the two conditions. Km for ATP was 184 ± 15 μm in vesicles from pH5-grown cells and 161 ± 25 μm in vesicles from pH 7-grown cells (both expressed as mean ± range of two independent measurements). These values are comparable with previously reported values (35) and clearly cannot account for differences in ATPase activity measured at 2 mm ATP. This result indicates that Vmax differences account for variations in ATPase activity. V-ATPase activity is electrogenic, and in other systems, the enzyme can establish a sufficient membrane potential to inhibit its activity. We added low concentrations of detergent (1% C12E9) to vacuolar membranes and retested ATPase activity. The difference in activity between vesicles from pH 5- and 7-grown cells was maintained in the presence of detergent, suggesting that differences in membrane potential could not account for the activity difference.
Surprisingly, we found that the V-ATPase exhibits no response to pHext in rich medium (YEPD) buffered with phosphate/succinate buffer. In addition, we measured vacuolar pH in vivo using the ratiometric pH-sensitive dye BCECF-AM. The initial vacuolar pH, after 20–30 min of glucose deprivation, was not significantly different for cells grown at pH 5 or 7 before glucose deprivation. The vacuolar pH had decreased by about 0.3 pH units for both samples 5 min after the addition of glucose. Weak acids and bases in growth media have been implicated in adjusting vacuolar pH independent of V-ATPase activity (26), and YEPD contains multiple components, as well as the phosphate and succinate in our buffer system, that could act as weak acids. Use of an impermeant buffer (MES) in YEPD did not alter V-ATPase activity or V-ATPase-dependent pH responses in vivo.
V-ATPase Disassembly in Response to Glucose Deprivation Is Suppressed at High pHext
The addition of glucose to briefly glucose-deprived cells lowers vacuolar pH in cells grown in YEPD. We performed the same measurement for cells grown in SC buffered with MES, and the results are shown in Fig. 2A. Although the final vacuolar pH after the glucose addition is comparable, the yeast cells that had been grown at pH 7 maintained a lower vacuolar pH during glucose deprivation. The yeast V-ATPase shows a characteristic disassembly of the V1 and Vo sectors in response to glucose deprivation (3), and we attributed the higher vacuolar pH in glucose-deprived cells to V-ATPase inhibition arising from disassembly (5).
FIGURE 2.
Disassembly of the V-ATPase in response to glucose deprivation is suppressed at high extracellular pH. A, vacuolar pH was measured using the ratiometric fluorescent dye BCECF-AM loaded into the vacuoles of log phase cells grown in SC buffered to pH 5 or 7 with 50 mm MES and maintained at the same pH during the measurement. Cells were deprived of glucose for 20–30 min before the initial pH measurement (−), and then glucose was added to a final concentration of 50 mm, and the vacuolar pH was measured again 5 min after the glucose addition (+), as described under “Experimental Procedures.” Fluorescence measurements were performed at the same extracellular pH as the growth medium. Vacuolar pH is plotted as mean ± S.E. (error bars) for n = 4 independent measurements. B and C, cells were grown as described in A, converted to spheroplasts, and then incubated for 30 min in SC containing 1.2 m sorbitol and buffered to pH 5 or 7, either in the presence of 50 mm glucose (+) or in the absence of added glucose (−). After this incubation, spheroplasts were lysed, and vacuolar vesicles were isolated as described under “Experimental Procedures.” V-ATPase activity (B) and proton pumping (C) were assayed as described in the legend to Fig. 1 and under “Experimental Procedures.” RFU, relative fluorescence units. D, immunoblot of vacuolar vesicles prepared as described in B and C. Vesicles were solubilized, separated by SDS-PAGE, and transferred to nitrocellulose as described under “Experimental Procedures.” For detection of the V1 A and B subunits, 1 μg of vacuolar vesicle protein was loaded on the gel, whereas for detection of V0 a and V1 C, 4 μg was loaded from each preparation. The blots were probed with subunit-specific mouse monoclonal antibodies (see “Experimental Procedures”), followed by alkaline phosphatase-conjugated goat anti-mouse antibody and colorimetric development.
We therefore tested whether disassembly of the V-ATPase in response to glucose deprivation was affected at pH 7 in MES-buffered minimal medium. Cells grown overnight in SC buffered with MES to pH 5 and 7 were converted to spheroplasts as described above, and then incubated in the same pH 5- or 7-buffered medium (with osmotic support) for 20 min with or without glucose before lysis and vacuole isolation. As shown in Fig. 2, B and C, vacuolar vesicles from pH 5-grown cells responded as expected to glucose deprivation. ATPase specific activity was decreased by 79% and proton pumping by 67% after glucose deprivation. In contrast, only a 33% decrease in ATPase activity and 8% decrease in proton pumping activity is seen in the cells grown at pH 7, suggesting that there was little down-regulation of ATPase activity in response to glucose deprivation. We confirmed reduced disassembly at pH 7 with immunoblots of vacuolar vesicles. Although similar levels of Vo a subunit are present in vacuolar vesicles from the pH 5-grown cells with or without glucose deprivation, the levels of V1 subunits A, B, and C are drastically reduced by glucose deprivation (Fig. 2D). The cellular levels of these subunits are unchanged by pH or a 20-min glucose deprivation (data not shown), indicating that the subunits have been released from the vacuolar membrane by glucose deprivation. In contrast, there is relatively little loss of the V1 A, B, and C subunits in vacuolar vesicles isolated after glucose deprivation of pH 7-grown cells. This is consistent with retention of activity and indicates that disassembly of the V-ATPase in response to glucose deprivation is suppressed in these cells.
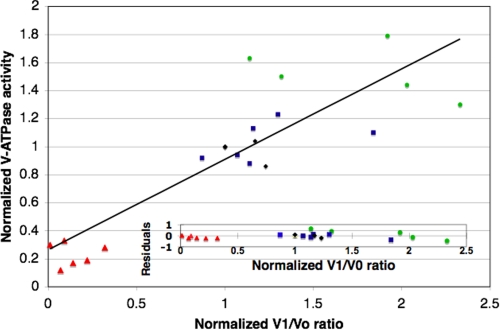
In order to more quantitatively determine the extent to which differences in V1 subunit assembly with Vo sectors account for differences in V-ATPase activity across the varied conditions, we measured the levels of V1 subunit A or C and Vo subunit a in immunoblots from several independent experiments where pH and/or glucose were varied in buffered minimal medium. For each blot, we calculated the ratio of V1/Vo and normalized to the level of a sample grown at pH 5 and maintained in glucose on the same blot as an internal control. We also normalized V-ATPase activities to the same samples and then plotted the normalized V-ATPase activities against the normalized V1/Vo ratio for all conditions, as shown in Fig. 3. This plot suggests a linear relationship between activity and V1/Vo ratio across all of the conditions. We calculated an R2 value of 0.75 for the data shown in Fig. 3. A plot of the residuals (inset) indicates that neither pH nor glucose deprivation was responsible for a consistent deviation from the linear relationship. From these data, we conclude that V1-Vo assembly is probably the major, although probably not the only, factor determining the level of activity under varied pH conditions and glucose levels.
FIGURE 3.
Comparison of V1/Vo ratios and V-ATPase activity. Vacuolar vesicles were isolated from cells grown in SC buffered to pH 5 and 7 and incubated with or without glucose before lysis as described in Fig. 2. Equal amounts of total vacuolar protein, loaded at levels previously determined to allow a linear response with the anti-V-ATPase monoclonal antibodies on immunoblots (34), were separated by SDS-PAGE and transferred to nitrocellulose. The bands for Vo subunit a and V1 subunits A and C were quantitated as described under “Experimental Procedures” and normalized to a sample prepared and separated in parallel that was grown at pH 5 and maintained in glucose. (There proved to be no significant difference in the final analysis if the A or C subunit was used to calculate the V1/Vo ratio.) Concanamycin A-sensitive ATPase activity (V-ATPase) was normalized in the same way for each set of samples, and the normalized V-ATPase activity was plotted against the normalized V1/Vo ratio for all samples. Linear regression analysis provided the trend line shown, and the residuals (calculated as the difference between the actual value for the ATPase activity and the value predicted from the regression analysis) are shown in the inset. The symbols designate the growth conditions before lysis and vacuole preparation: red triangles, pH 5 − glucose (cells grown at pH 5 and deprived of glucose before lysis); black diamonds, pH 5 + glucose (cells grown at pH 5 and maintained in glucose); blue squares, pH 7 − glucose; green circles, pH 7 + glucose.
We also sought to confirm reduced V-ATPase disassembly with glucose deprivation at pH 7 by an independent technique. The 10D7 monoclonal antibody recognizes a cryptic epitope on the Vo subunit a only when V1 is not bound (36), making it a useful probe for “free” Vo sectors. Disassembly of the V-ATPase in response to glucose deprivation unmasks this epitope, and reassembly of the complex upon glucose readdition sequesters it (32). We looked for binding of the 10D7 antibody by immunofluorescence microscopy in cells grown and fixed in MES-buffered minimal medium at pH 5 and 7. As shown in Fig. 4, there is relatively little staining in cells maintained in glucose at either pH, although we consistently see more staining overall at pH 5. However, a 20-min glucose deprivation extensively unmasks the 10D7 epitope in pH 5-grown cells but results in little staining of pH 7-grown cells. The readdition of glucose to deprived cells reduces staining to that of the cells maintained in glucose. These results are completely consistent with those from vacuole isolation (Fig. 2) and indicate that the V-ATPase largely fails to disassemble in cells buffered to pH 7.
Cytosolic pH Is Not Changed under Conditions That Suppress Reversible Disassembly
The difference in disassembly at high pHext could arise from variations in cytosolic pH under these conditions. Because of technical difficulties in measuring cytosolic pH in the wild type strain used for vacuole preparations, we measured both vacuolar and cytosolic pH in an alternate wild type strain, BY4741. As shown in Fig. 5A, this strain also maintained a lower vacuolar pH in cells deprived of glucose at pH 7 than in those deprived of glucose at pH 5, similar to results shown in Fig. 3A and consistent with a lack of V-ATPase disassembly at pH 7. In this strain, however, the addition of glucose to cells deprived of glucose at pH 5 gave the typical drop in vacuolar pH, whereas the addition of glucose to cells at pH 7 resulted in a slight increase in vacuolar pH. We measured cytosolic pH with a cytoplasmically localized, pH-sensitive green fluorescent protein (5, 30). As shown in Fig. 5B, there was no difference in cytosolic pH in cells grown and maintained at pH 5 from those maintained at pH 7. Comparing the cytosolic and vacuolar pH values before and after glucose addition, it appears that in cells grown at low pHext, the pH gradient across the vacuolar membrane is lost upon glucose deprivation, whereas in pH 7-grown cells, a pH gradient is maintained. These results do not support cytosolic pH as a major factor dictating V1-Vo assembly, although transient pH changes would not necessarily have been detected in this experiment.
Do Mutations in Vacuolar/Endosomal Proton Exchangers and Alkaline pH Response Pathways Alter pH Responsiveness of the V-ATPase?
As an initial approach to determining the mechanistic basis for pH control of V-ATPase activity and assembly, we surveyed vacuolar pH responses in vivo for several mutants lacking vacuolar H+ exchangers or signaling components implicated in alkaline pH responses. As shown in Fig. 6, deletion of either of two exchangers, the endosomal Na+/H+ exchanger Nhx1, which has previously been implicated in vacuolar pH control (29), and the vacuolar Ca2+/H+ exchanger Vcx1, has no significant effect on vacuolar pH or response to glucose at external pH 5 or 7. Similarly, there is no significant change in vacuolar pH responses in a cnb1Δ mutant, which lacks calcineurin activity. In contrast, the snf1Δ mutant, which lacks the yeast AMP-dependent kinase, resembled its congenic wild type strain (W303) at pH 5 but had a significantly higher vacuolar pH at extracellular pH 7, both before and after the glucose addition. Consistent with the alkaline pH growth defect reported previously (37), the snf1Δ mutant grew very slowly in medium buffered to pH 7 with MES, and this precluded isolation of vacuolar vesicles from this strain. We did isolate vacuolar vesicles from the cnb1Δ mutant grown at pH 5 and 7, however. When the cnb1Δ mutant was grown at pH 5, glucose deprivation led to a loss of 75% of the V-ATPase activity in isolated vacuolar vesicles, similar to the results shown for wild type in Fig. 2B. When grown at external pH 7 and maintained in glucose, the V-ATPase activity of wild type and cnb1Δ mutant vacuolar vesicles were both higher than for pH5-grown cells and were not significantly different. Glucose deprivation caused a somewhat larger reduction in V-ATPase activity in the cnb1Δ mutant (50% decrease) than in wild type cells (14% decrease). However, even with this increased disassembly, the activity of the cnb1Δ mutant vacuoles from cells maintained in glucose at pH 5 (0.9 μmol/min/mg) is comparable with the activity in vacuoles from pH 7-grown cells deprived of glucose (0.8 μmol/min/mg), consistent with the wild type data in Fig. 3. This may account for the lack of a significant change in vacuolar pH and suggests that calcineurin may help to maintain V-ATPase assembly during glucose deprivation at pH 7 but is unlikely to be the only factor involved.
DISCUSSION
Fungi have a robust capacity to adapt to extracellular pH ranging from 2.5 to 8.5. Activation of the plasma membrane pump (Pma1p) at acidic extracellular pH, particularly in the presence of permeant acids, is well established and is critical for maintenance of cytosolic pH (6, 12, 13). The central question posed in this work is whether the activity of V-ATPases, which are primarily responsible for controlling pH of intracellular compartments, responds to the pH of the extracellular environment. Activation of V-ATPases as part of adaptation to the weak acid 2,4-dichlorophenoxyacetic acid was reported previously (38). In contrast, Padilla-López and Pearce (27) reported that V-ATPase activity was higher in cells grown at elevated pHext. Our results indicate that the V-ATPase is responsive to extracellular pH but that the relationship between pHext and V-ATPase activity is not a simple one.
The most striking results presented here are the high V-ATPase activity in vacuolar vesicles isolated from cells grown in minimal medium buffered to pH 7 and the relative insensitivity of these V-ATPases to disassembly in response to glucose deprivation. The analysis in Fig. 3 indicates that varied levels of V1 assembly are largely responsible for both the pH- and glucose-dependent differences in ATPase activity. These results are partially consistent with work of Padilla-López and Pearce (27). Their studies indicated that higher V-ATPase activity at pHext of 7.5 arose from higher levels of V1-Vo assembly, but they reported V-ATPase disassembly upon glucose deprivation at both pH 4 and pH 7.5. However, those glucose deprivation experiments were done in unbuffered medium, where pHext changes may have occurred during growth.
Our results suggest that yeast V-ATPase complexes are stabilized at elevated pHext, rendering them more active and less susceptible to dissociation, but we do not yet know the structural basis of this effect. Significantly, our results do not support direct control of V-ATPase stability and activity by cytosolic pH, because we measured nearly identical values for cytosolic pH at extracellular pH 5 and 7 (Fig. 5). In addition, direct incubation of isolated vacuolar vesicles with buffers at pH from 5.5 to 7.5 did not result in higher V-ATPase activities at high pH (39). Our results suggest interplay between glucose and pH regulation of the V-ATPase. Even the structural basis of glucose regulation, which has been known for some time, is incompletely understood. Much current interest centers on the V1 C subunit as a potential target for regulation by protein kinase A during acute shifts in glucose levels (16–18). There are also data supporting an important role for direct interactions between the V-ATPase and glycolytic enzymes and/or the RAVE complex in glucose regulation (20, 34), but it is not known if any of these interactions are responsive to extracellular pH. Only the interaction of endosomal V-ATPase subunit a2 and ARNO in mammalian cells has been shown to be directly pH-sensitive, and this interaction is sensitive to endosomal lumen, not cytosolic or extracellular, pH (40).
One major question is whether glucose and extracellular pH modulate V-ATPase activity and assembly through a common mechanism or provide separate inputs that combine to determine the enzyme's assembly. Yeast cells grow optimally at acidic pH and mount a coordinated response to alkaline pH stress that involves several signaling pathways (37). Two of these pathways involve calcineurin activation and activity of the AMP-dependent protein kinase Snf1. We measured vacuolar pH responses in mutants lacking these pathways. The snf1Δ mutant grew very poorly in minimal medium buffered to pH 7. Under these conditions, vacuolar pH was elevated both in the presence and absence of glucose, suggesting a general defect in vacuolar acidification at elevated extracellular pH. The in vivo vacuolar pH responses of the cnb1Δ mutant are not significantly different from wild type, but there is somewhat less retention of V-ATPase activity in isolated mutant vacuoles upon glucose deprivation at pH 7. Further investigation of V-ATPase assembly in strains with alkaline pH growth defects (41, 42) could be pursued in the future. It is also possible that a signaling pathway responsible for triggering disassembly of the V-ATPase in response to glucose deprivation is suppressed at elevated pH. However, such a mechanism does not easily account for the substantial increase in V-ATPase activity at pHext 7 in the presence of glucose (Figs. 2 and 3), which might require a “positive” assembly signal. In addition, potential signaling components are poorly compatible with a common pathway for glucose and pH effects. For example, Snf1 is activated by both glucose deprivation and alkaline pH (43), but these two growth conditions have opposite effects on V-ATPase assembly. Glucose metabolism is required for maintaining V-ATPase assembly (44), but the glucose uptake rate is slower at pH 7 than at pH 5 (45). Based on these observations, we believe that more than one pH- and glucose-sensitive input probably determines the final level of V-ATPase assembly.
Two other notable features of the pH regulation of the V-ATPase are its persistence through vacuole purification and its dependence on medium composition. Somewhat surprisingly, vacuoles isolated under identical conditions retain differences in activity that reflect growth conditions before cell lysis. Vacuole isolation involves multiple washes and incubations, so transient effects of medium components on vacuolar content should be removed. For example, contributions from ammonium ion in the medium can certainly affect in vivo measurement of vacuolar pH but were also shown to be rapidly reversible (26) and thus cannot account for the activity differences observed in isolated vacuoles. (There is no ammonium in solutions used for vacuole preparation.) In contrast, the results presented here indicate that growth of cells at elevated extracellular pH elicits a relatively long term change in V-ATPase activity and stability. We failed to see significant pHext-dependent differences in V-ATPase activity in YEPD, regardless of whether we added a cell-permeant buffer system (phosphate/succinate) or the relatively impermeant MES buffer. YEPD has a relatively high concentration of buffering agents; consistent with this, growth of yeast cells to saturation in unbuffered YEPD brings the medium to a final pH of ∼5, but growth in SC without added buffer results in a final pH of 2.7–2.9, even after a smaller number of cell divisions. The vulnerability of yeast cultures to acid production during extended growth in minimal medium has been highlighted in chronological aging experiments, where production and handling of acetic acid have recently been invoked as major factors limiting chronological life span (46). There are also very significant metabolic differences between growth of yeast cells in minimal and rich medium that could impact V-ATPase stability and regulation as well; dissection of these effects will require further investigation.
In the vma mutants, loss of V-ATPase activity abolishes growth at alkaline pHext, suggesting a central role for V- ATPases in pH homeostasis under these conditions. The results presented here are completely consistent with this conclusion. Increasing V-ATPase activity at higher pHext and preservation of V-ATPase activity during periods of glucose deprivation may protect acidification of intracellular compartments, such as the vacuole, from the competing export of cytosolic protons across the plasma membrane by Pma1. Consistent with this, vacuolar pH rises in response to glucose addition in the absence of V-ATPase activity, whether V-ATPase activity is inhibited acutely by concanamycin A or chronically absent as a result of a vma mutation (5). Under these conditions, glucose activation of Pma1 probably results in export of both metabolically generated protons and vacuolar protons “expended” by antiporters utilizing the pH gradient for vacuolar import of nutrients. It may be that stabilization of the V-ATPase at high pHext is necessary to balance the contributions of Pma1 and the V-ATPase to cytosolic and organellar pH. Future efforts will focus on further probing coordinated function and regulation of V-ATPases and Pma1p.
Acknowledgments
We thank Dr. Rajini Rao (The Johns Hopkins University) for the gift of the plasmid containing the yeast pHluorin under control of the phosphoglycerate kinase promoter and the Duncan/Cross laboratory for use of the fluorometer.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 GM50322 (to P. M. K.).
- V-ATPase
- vacuolar proton-translocating ATPase
- BCECF-AM
- 2′,7′-bis-(2-carboxyethyl)-5-(and 6)-carboxyfluorescein, acetoxymethyl ester
- MES
- 2-(N-morpholino)ethanesulfonic acid
- YEPD
- yeast extract/peptone/dextrose medium
- SC
- synthetic complete medium
- pHext
- extracellular pH
- TEA
- triethanolamine.
REFERENCES
- 1.Serrano R. (1991) in The Molecular and Cellular Biology of the Yeast Saccharomyces, Vol. 1 (Broach J. R., Pringle J. R., Jones E. W. eds) pp. 523–585, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 2.Serrano R., Kielland-Brandt M. C., Fink G. R. (1986) Nature 319, 689–693 [DOI] [PubMed] [Google Scholar]
- 3.Kane P. M. (2006) Microbiol. Mol. Biol. Rev. 70, 177–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perzov N., Nelson H., Nelson N. (2000) J. Biol. Chem. 275, 40088–40095 [DOI] [PubMed] [Google Scholar]
- 5.Martínez-Muñoz G. A., Kane P. M. (2008) J. Biol. Chem. 283, 20309–20319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Portillo F. (2000) Biochim. Biophys. Acta 1469, 31–42 [DOI] [PubMed] [Google Scholar]
- 7.Serrano R. (1983) FEBS Lett. 156, 11–14 [DOI] [PubMed] [Google Scholar]
- 8.Eraso P., Portillo F. (1994) J. Biol. Chem. 269, 10393–10399 [PubMed] [Google Scholar]
- 9.Lecchi S., Nelson C. J., Allen K. E., Swaney D. L., Thompson K. L., Coon J. J., Sussman M. R., Slayman C. W. (2007) J. Biol. Chem. 282, 35471–35481 [DOI] [PubMed] [Google Scholar]
- 10.Eraso P., Mazón M. J., Portillo F. (2006) Biochim. Biophys. Acta 1758, 164–170 [DOI] [PubMed] [Google Scholar]
- 11.Goossens A., de La Fuente N., Forment J., Serrano R., Portillo F. (2000) Mol. Cell. Biol. 20, 7654–7661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eraso P., Gancedo C. (1987) FEBS Lett. 224, 187–192 [DOI] [PubMed] [Google Scholar]
- 13.Carmelo V., Santos H., Sá-Correia I. (1997) Biochim. Biophys. Acta 1325, 63–70 [DOI] [PubMed] [Google Scholar]
- 14.Yenush L., Merchan S., Holmes J., Serrano R. (2005) Mol. Cell. Biol. 25, 8683–8692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kane P. M. (2000) FEBS Lett. 469, 137–141 [DOI] [PubMed] [Google Scholar]
- 16.Rein J., Voss M., Blenau W., Walz B., Baumann O. (2008) Am. J. Physiol. Cell Physiol. 294, C56–C65 [DOI] [PubMed] [Google Scholar]
- 17.Voss M., Vitavska O., Walz B., Wieczorek H., Baumann O. (2007) J. Biol. Chem. 282, 33735–33742 [DOI] [PubMed] [Google Scholar]
- 18.Bond S., Forgac M. (2008) J. Biol. Chem. 283, 36513–36521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sautin Y. Y., Lu M., Gaugler A., Zhang L., Gluck S. L. (2005) Mol. Cell. Biol. 25, 575–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu M., Ammar D., Ives H., Albrecht F., Gluck S. L. (2007) J. Biol. Chem. 282, 24495–24503 [DOI] [PubMed] [Google Scholar]
- 21.Lu M., Sautin Y. Y., Holliday L. S., Gluck S. L. (2004) J. Biol. Chem. 279, 8732–8739 [DOI] [PubMed] [Google Scholar]
- 22.Seol J. H., Shevchenko A., Deshaies R. J. (2001) Nat. Cell Biol. 3, 384–391 [DOI] [PubMed] [Google Scholar]
- 23.Smardon A. M., Tarsio M., Kane P. M. (2002) J. Biol. Chem. 277, 13831–13839 [DOI] [PubMed] [Google Scholar]
- 24.Nelson H., Nelson N. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 3503–3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munn A. L., Riezman H. (1994) J. Cell Biol. 127, 373–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plant P. J., Manolson M. F., Grinstein S., Demaurex N. (1999) J. Biol. Chem. 274, 37270–37279 [DOI] [PubMed] [Google Scholar]
- 27.Padilla-López S., Pearce D. A. (2006) J. Biol. Chem. 281, 10273–10280 [DOI] [PubMed] [Google Scholar]
- 28.Sherman F. (1991) Methods Enzymol. 194, 3–21 [DOI] [PubMed] [Google Scholar]
- 29.Ali R., Brett C. L., Mukherjee S., Rao R. (2004) J. Biol. Chem. 279, 4498–4506 [DOI] [PubMed] [Google Scholar]
- 30.Brett C. L., Tukaye D. N., Mukherjee S., Rao R. (2005) Mol. Biol. Cell 16, 1396–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts C. J., Raymond C. K., Yamashiro C. T., Stevens T. H. (1991) Methods Enzymol. 194, 644–661 [DOI] [PubMed] [Google Scholar]
- 32.Kane P. M. (1995) J. Biol. Chem. 270, 17025–17032 [PubMed] [Google Scholar]
- 33.Liu M., Tarsio M., Charsky C. M., Kane P. M. (2005) J. Biol. Chem. 280, 36978–36985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smardon A. M., Kane P. M. (2007) J. Biol. Chem. 282, 26185–26194 [DOI] [PubMed] [Google Scholar]
- 35.Uchida E., Ohsumi Y., Anraku Y. (1985) J. Biol. Chem. 260, 1090–1095 [PubMed] [Google Scholar]
- 36.Kane P. M., Kuehn M. C., Howald-Stevenson I., Stevens T. H. (1992) J. Biol. Chem. 267, 447–454 [PubMed] [Google Scholar]
- 37.Platara M., Ruiz A., Serrano R., Palomino A., Moreno F., Ariño J. (2006) J. Biol. Chem. 281, 36632–36642 [DOI] [PubMed] [Google Scholar]
- 38.Fernandes A. R., Durão P. J., Santos P. M., Sá-Correia I. (2003) Biochem. Biophys. Res. Commun. 312, 1317–1324 [DOI] [PubMed] [Google Scholar]
- 39.Parra K. J., Kane P. M. (1996) J. Biol. Chem. 271, 19592–19598 [DOI] [PubMed] [Google Scholar]
- 40.Hurtado-Lorenzo A., Skinner M., El Annan J., Futai M., Sun-Wada G. H., Bourgoin S., Casanova J., Wildeman A., Bechoua S., Ausiello D. A., Brown D., Marshansky V. (2006) Nat. Cell Biol. 8, 124–136 [DOI] [PubMed] [Google Scholar]
- 41.Serrano R., Bernal D., Simón E., Ariño J. (2004) J. Biol. Chem. 279, 19698–19704 [DOI] [PubMed] [Google Scholar]
- 42.Sambade M., Alba M., Smardon A. M., West R. W., Kane P. M. (2005) Genetics 170, 1539–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong S. P., Carlson M. (2007) J. Biol. Chem. 282, 16838–16845 [DOI] [PubMed] [Google Scholar]
- 44.Parra K. J., Kane P. M. (1998) Mol. Cell. Biol. 18, 7064–7074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blank L. M., Sauer U. (2004) Microbiology 150, 1085–1093 [DOI] [PubMed] [Google Scholar]
- 46.Burtner C. R., Murakami C. J., Kennedy B. K., Kaeberlein M. (2009) Cell Cycle 8, 1256–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]