Abstract
GABAA receptors are composed predominantly of αβγ receptors, which mediate primarily synaptic inhibition, and αβδ receptors, which mediate primarily extrasynaptic inhibition. At saturating GABA concentrations, the barbiturate pentobarbital substantially increased the amplitude and desensitization of the α1β3δ receptor but not the α1β3γ2L receptor currents. To explore the structural domains of the δ subunit that are involved in pentobarbital potentiation and increased desensitization of α1β3δ currents, chimeric cDNAs were constructed by progressive replacement of γ2L subunit sequence with a δ subunit sequence or a δ subunit sequence with a γ2L subunit sequence, and HEK293T cells were co-transfected with α1 and β3 subunits or α1 and β3 subunits and a γ2L, δ, or chimeric subunit. Currents evoked by a saturating concentration of GABA or by co-application of GABA and pentobarbital were recorded using the patch clamp technique. By comparing the extent of enhancement and changes in kinetic properties produced by pentobarbital among chimeric and wild type receptors, we concluded that although potentiation of α1β3δ currents by pentobarbital required the δ subunit sequence from the N terminus to proline 241 in the first transmembrane domain (M1), increasing desensitization of α1β3δ currents required a δ subunit sequence from the N terminus to isoleucine 235 in M1. These findings suggest that the δ subunit N terminus and N-terminal portion of the M1 domain are, at least in part, involved in transduction of the allosteric effect of pentobarbital to enhance α1β3δ currents and that this effect involves a distinct but overlapping structural domain from that involved in altering desensitization.
Keywords: Allosteric Regulation, Biophysics, Drug Action, GABA Receptors, Ion Channels, Chimera, Deactivation, Desensitization, Pentobarbital, Potentiation
Introduction
γ-Aminobutyric acid, type A (GABAA)2 receptors, members of the Cys-loop receptor family, are heteropentameric ligand-gated chloride ion channels and play a critical role in mediating fast inhibition in the brain (1). Multiple GABAA receptor subunits as well as their subtypes have been identified (1, 2). Like the nicotinic cholinergic receptor, another member of the Cys-loop receptor family, each GABAA receptor subunit is thought to be composed of a large extracellular N terminus, four transmembrane domains (M1–M4), one extracellular M2–3 loop, two intracellular loops (M1–2 and M3–4), and an extracellular C terminus. Theoretically, an enormous number of receptors could be formed with different GABAA receptor subunit/subtype combinations. However, it has been proposed that αβγ and αβδ receptor isoforms are the predominant GABAA receptors in the brain (3). There is increasing evidence suggesting that αβγ GABAA receptors mainly locate within the synapses and mediate GABAergic phasic inhibition, whereas αβδ receptors are preferentially targeted to extra- or perisynaptic membranes and mediate tonic inhibition (4–6).
Barbiturates exert their effects in the brain by affecting GABAA receptor functions in a concentration-dependent manner. At lower concentrations, these compounds allosterically modulate GABAA receptors to potentiate GABAergic currents. At higher concentrations, they can directly activate GABAA receptors in the absence of GABA (2, 7, 8). It has been reported that the barbiturate pentobarbital substantially potentiated peak currents and increased desensitization of α1β3δ receptor currents evoked by saturating concentrations of GABA, but that these effects of pentobarbital were not observed with α1β3γ2L receptor currents at saturating GABA concentrations, although pentobarbital enhanced α1β3γ2L currents at subsaturating GABA concentrations (8), suggesting that the δ subunit rather than the γ2L subunit confers unique “modulatory potential” for this modulator at saturating GABA concentrations. The structural domains of the δ subunit that contribute to the potentiation and desensitization alterations by pentobarbital of currents evoked by saturating concentrations of GABA are currently unknown.
To explore this issue, we took advantage of the differential modulatory effects of pentobarbital on α1β3δ and α1β3γ2L receptors at a saturating GABA concentration to construct a series of chimeras by progressively replacing the rat γ2L subunit sequence with the corresponding rat δ subunit sequence or δ subunit sequence with the γ2L subunit sequence and co-transfected these chimeric subunits with wild type rat α1 and β3 subunits. Using an ultrafast drug delivery system and preapplication protocol, we performed whole cell patch clamp recordings of the currents evoked by a saturating concentration of GABA as well as by co-application of a saturating concentration of GABA and pentobarbital. By comparing the peak current amplitude enhancement and kinetic properties of chimeric receptors with those of the wild type α1β3δ and α1β3γ2L receptors, we explored the structural basis for the differential effects of pentobarbital on α1β3δ and α1β3γ2L receptor currents evoked by saturating concentrations of GABA.
EXPERIMENTAL PROCEDURES
Expression of Wild Type and Chimeric Recombinant GABAA Receptors
Chimeric subunits were constructed by gradual replacement of the GABAA receptor γ2L subunit sequence with the corresponding δ subunit sequence or vice versa from the N terminus toward the C terminus using the splice overhang extension method. The cDNAs encoding rat wild type α1, β3, γ2L, and δ subunits as well as the chimeric subunits were cloned using the expression vector pCMVneo. The sequence of all of the wild type and chimeric subunits was verified by DNA sequencing (Vanderbilt DNA Sequencing Facility, Nashville, TN). Three δ-γ2L chimeras were constructed by progressively replacing the γ2L subunit sequence with the δ subunit sequence from the N toward the C terminus: δ-γ2L(M1e) (δG232-γY235), δ-γ2L(M1pre-iso) (δY234-γT237), and δ-γ2L(M1p) (δP241-γC244). Three reverse γ2L-δ chimeras were constructed by progressively replacing the δ subunit sequence with the γ2L subunit sequence from the N toward the C terminus: γ2L-δ(M1e′) (γG234-δV233), γ2L-δ(M1p′) (γP243-δS242), and γ2L-δ(M1i′) (γI257-δS256).
Human embryonic kidney (HEK293T) cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen), supplemented with 10% fetal bovine serum (Invitrogen), 100 international units/ml penicillin and 100 μg/ml streptomycin (Invitrogen) in an incubator at 37 °C with 5% CO2 and 95% air. Cells were transfected with 2 μg of each cDNA encoding rat α1 and β3; α1, β3, and γ2L; α1, β3, and δ; or α1, β3, and chimeric GABAA receptor subunits using a modified calcium phosphate precipitation method (8). Two μg of pHOOK (Invitrogen) were co-transfected with the GABAA receptor subunits as a marker for the subsequent selection using an immunomagnetic bead separation method (9). Whole cell recordings were performed 24 h after the cells were selected.
Whole Cell Recordings
Whole cell currents were obtained using the patch clamp technique at room temperature. The external solution was composed of 142 mm NaCl, 1 mm CaCl2, 6 mm MgCl2, 8 mm KCl, 10 mm glucose, and 10 mm HEPES (pH 7.4, 323–329 mosmol). The recording electrodes were pulled from thin wall borosilicate glass tubes (World Precision Instruments, Sarasota, FL) on a P-87 Flaming Brown micropipette puller (Sutter Instruments, San Rafael, CA). The electrodes were fire-polished on an MF-9 microforge (Narishige, Tokyo, Japan). The resistances of the recording electrodes were 0.8–1.8 megohms after being filled with an internal solution consisting of 153 mm KCl, 1 mm MgCl2, 10 mm HEPES, 2 mm MgATP, and 5 mm EGTA (pH 7.3, 301–309 mosmol). Combination of the external and internal solutions produced a chloride equilibrium potential near 0 mV and a potassium equilibrium potential at −75 mV. Electrophysiological recordings were performed on either an Axopatch-1D or a 200A patch clamp amplifier (Molecular Devices, Union City, CA) and a Digidata 1200 series interface (Molecular Devices). Data were recorded on chart paper using a WR7400 arraycorder (Graphtec, Yokohama, Japan) as well as stored in a computer for offline analysis. All chemicals were purchased from Sigma-Aldrich. GABA and pentobarbital sodium salt were dissolved in water and prepared as stock solutions. Working solutions were made by diluting the stock solutions to desired concentrations with external solution on the day of the experiment. Drugs were applied by gravity via multibarrel tubes (two three-barrel square glass tubes glued together) connected to a Perfusion Fast-Step system (Warner Instruments, Hamden, CT). The 10–90% rise times of liquid junction currents were consistently <2 ms estimated by stepping a dilute external solution across an open electrode tip. GABA was applied for 4 s. The intervals between consecutive drug applications were at least 45 s to minimize desensitization accumulation.
Data Analysis
Whole cell currents were analyzed offline using Clampfit 8.1 (Molecular Devices). The peak currents were measured manually from the base line to the transient peak. The extent of enhancement of GABA currents by pentobarbital (IPB/ICONTROL) was calculated by dividing the peak current of co-application of GABA and pentobarbital by the peak current evoked by GABA alone. The extent of desensitization (% desensitization) was calculated by dividing the amount of current loss (peak current − current at the end of GABA/drug application) by peak current. Deactivation currents were fitted with one or two exponential components using the standard exponential Levenberg-Marquardt method in the form of Σanτn, where a denotes the relative amplitude of the exponential component, τ represents the time constant, and n is the number of exponential components. A weighted τ (a1·τ1 + a2·τ2)/(a1 + a2) was used to compare the rate of deactivation, where a1 and a2 are the relative amplitudes of the exponential components at time 0.
Data were reported as mean ± S.E. A paired Student's t test was used to compare the changes prior to and after pentobarbital treatment. One-way analysis of variance followed by Newman-Keuls multiple comparison test was utilized to analyze the differences among wild type and chimeric receptors. The difference was considered to be statistically significant if p < 0.05.
RESULTS
Pentobarbital Modulated Wild Type and δ-γ2L Chimeric GABAA Receptor Current Amplitudes
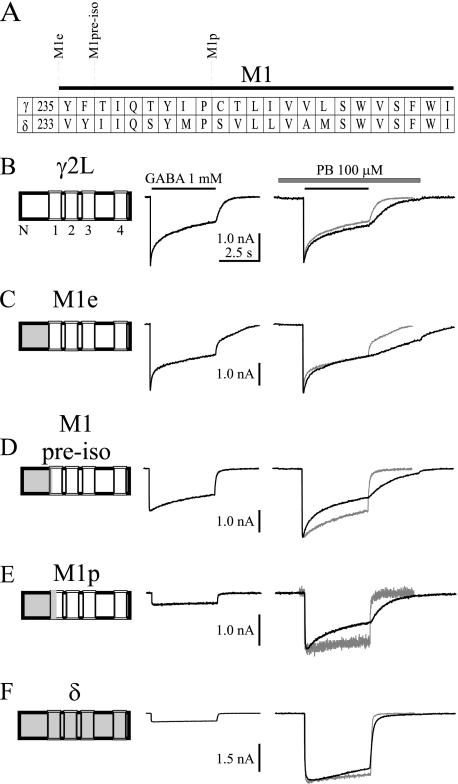
The modulatory effects of pentobarbital were determined by preapplying pentobarbital (100 μm) for 1.5 s prior to jumping into a saturating concentration of GABA (1 mm) and pentobarbital (100 μm) (see Fig. 1 and Fig. 3). Pentobarbital did not potentiate α1β3γ2L receptor peak currents (Figs. 1B and 2A) but did substantially enhance those for α1β3δ receptors (Figs. 1F and 2A), consistent with our previous report (8). Pentobarbital only slightly enhanced peak currents of α1β3 receptors evoked by a saturating concentration of GABA. Although the enhancement by pentobarbital of α1β3 receptor currents was not significantly different from that of α1β3γ2L receptor currents, it was, like α1β3γ2L receptors, significantly smaller than that of α1β3δ receptors (supplemental Fig. 1), suggesting that the dramatic enhancement of α1β3δ receptor currents by pentobarbital likely was conferred by incorporation of the δ subunit. Progressive replacement of the γ2L subunit N terminus and distal transmembrane M1 domain by the δ subunit sequence resulted in α1β3δ-γ2L chimeric receptors whose current enhancement was progressively increased by pentobarbital (Figs. 1, C–E, and 2A). Pentobarbital slightly potentiated the peak currents of α1β3δ-γ2L(M1e) receptors (n = 5), which contained the δ-γ2L(M1e) chimera with a δ subunit sequence in the entire extracellular N-terminal domain (Fig. 1A), but the extent of enhancement was not significantly different from α1β3γ2L receptors (Figs. 1C and 2A). For α1β3δ-γ2L(M1pre-iso) receptors (n = 7), which contained the δ-γ2L(M1pre-iso) chimera that advanced the δ subunit sequence just two amino acids into the M1 domain (Fig. 1A), the current enhancement by pentobarbital still was not significantly different from that of α1β3γ2L receptors (Figs. 1D and 2A). There was no significant difference in the current enhancements between α1β3δ-γ2L(M1pre-iso) and α1β3δ-γ2L(M1e) receptors (Fig. 2A). The current enhancement induced by pentobarbital for α1β3δ-γ2L(M1p) receptors (454.8 ± 78.6%, n = 7), which contained the δ-γ2L(M1p) chimera that advanced the δ subunit sequence seven more amino acids into the M1 domain (Fig. 1A), was greater than that of α1β3γ2L, α1β3δ-γ2L(M1e), or α1β3δ-γ2L(M1pre-iso) receptors (p < 0.01), and this enhancement was not significantly different from that of wild type α1β3δ receptors (582.6 ± 105.1%, n = 8) (Figs. 1, E and F, and 2A). Note that α1β3 currents evoked by a saturating concentration of GABA and pentobarbital exhibited multiphasic desensitization (supplemental Fig. 1, right trace). This was not observed with wild type α1β3γ2L and α1β3δ receptor as well as chimeric receptor currents (Figs. 1 and 3, right traces), indicating that the γ2L, δ, or chimeric subunit assembled with α1 and β3 subunits to form ternary receptors with negligible binary α1β3 receptors.
FIGURE 1.
Representative GABA current traces prior to and after pentobarbital modulation for wild type and δ-γ2L chimeric GABAA receptors. A, amino acid alignment for M1 domain of γ2L and δ subunits. δ-γ2L chimera splice sites were indicated by dashed lines. B–F, representative whole cell current traces evoked by a saturating concentration of GABA (1 mm) (left traces) as well as co-application of GABA (1 mm) and pentobarbital (100 μm) with pentobarbital (PB, 100 μm) preapplied for 1.5 s (right traces). The GABA control currents (gray traces) were normalized to the currents evoked by co-application of GABA and pentobarbital to show the alterations of desensitization and deactivation. A schematic of GABAA receptor wild type subunit γ2L (white shading), δ (gray shading), or a chimeric subunit is depicted before each set of traces. N represents the N terminus of the subunit, and numbers 1 to 4 denote M1–M4 of the subunit. The solid line above each current trace denotes the duration (4 s) of GABA application, and the hatched bar represents the duration of pentobarbital application. The horizontal time scale of C, D, E, and F is the same as that of B.
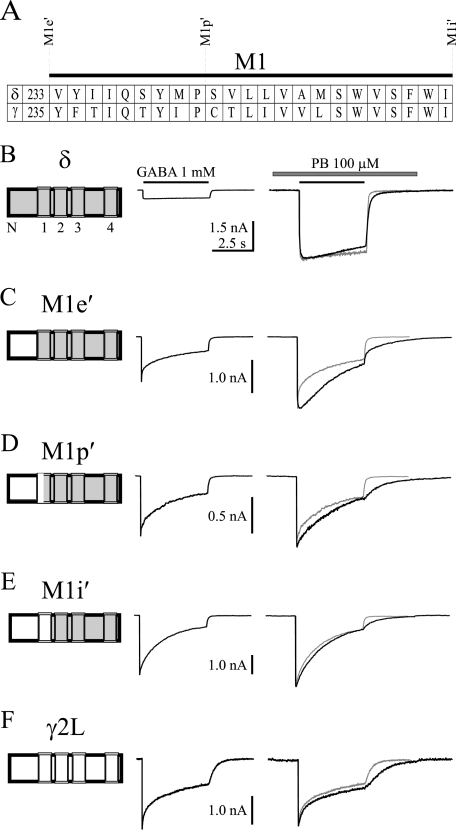
FIGURE 3.
Representative GABA current traces prior to and after pentobarbital modulation for wild type and γ2L-δ chimeric receptors. A, amino acid alignment for M1 domain of δ and γ2L subunits. γ2L-δ chimera splice sites were indicated by dashed lines. B–F, representative whole cell current traces evoked by a saturating concentration of GABA (1 mm) (left traces) as well as co-application of GABA (1 mm) and pentobarbital (PB, 100 μm) with pentobarbital (100 μm) preapplied for 1.5 s (right traces). The GABA control currents (gray traces) were normalized to the currents evoked by co-application of GABA and pentobarbital to show the alterations of desensitization and deactivation. A schematic of the GABAA receptor wild type subunit δ (gray shading), γ2L (white shading), or chimeric subunit was shown before each set of traces. N represents N terminus of the subunit, and numbers 1–4 denote M1–M4 of the subunit. The solid line above each current trace denotes the duration (4 s) of GABA application, and the hatched bar represents the duration of pentobarbital application. The horizontal time scale of C, D, E, and F is the same as that of B.
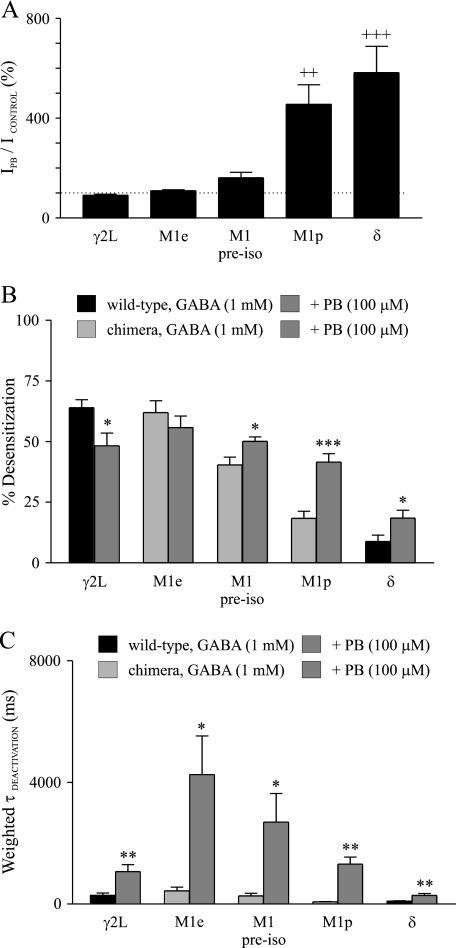
FIGURE 2.
Pentobarbital modulation of peak currents, desensitization, and deactivation of wild type and δ-γ2L chimeric receptors. A, shown is the mean extent of enhancement by pentobarbital (PB) of wild type and δ-γ2L chimeric receptors. The dashed line indicates 100%. B, shown is a comparison of the mean desensitization prior to and after pentobarbital treatment among wild type and δ-γ2L chimeric receptors. C, the mean deactivation time constant was greater after pentobarbital treatment as compared with GABA control for wild type and δ-γ2L chimeric receptors. ++, significantly different from γ2L, M1e, or M1pre-iso at p < 0.01; +++, p < 0.001 (one-way analysis of variance followed by Newman-Keuls multiple comparison test); *, significantly different from GABA control at p < 0.05; **, p < 0.01; ***, p < 0.001 (paired Student's t test).
Pentobarbital Modulated the Desensitization and Deactivation of Wild Type and δ-γ2L Chimeric GABAA Receptors
As reported previously (10), wild type and chimeric GABAA receptor currents evoked by a saturating concentration of GABA exhibited different extents of desensitization (Fig. 1, B–F, left traces). Mean % desensitization of α1β3γ2L or α1β3δ-γ2L(M1e) currents was greater than that of α1β3δ-γ2L(M1pre-iso), α1β3δ-γ2L(M1p), or α1β3δ currents (Fig. 2B; p < 0.001). Desensitization of α1β3δ-γ2L(M1pre-iso) currents was greater than that of α1β3δ-γ2L(M1p) or α1β3δ currents (p < 0.001), and desensitization of α1β3δ-γ2L(M1p) currents was greater than that of α1β3δ currents (p < 0.05) (Fig. 2B). Desensitization was not significantly different between α1β3γ2L and α1β3δ-γ2L(M1e) currents.
Consistent with our previous report (8), pentobarbital significantly decreased the desensitization of α1β3γ2L currents but increased that of α1β3δ currents evoked by a saturating concentration of GABA (Figs. 1, B and F, and 2B). Desensitization of α1β3δ-γ2L(M1e) currents was not significantly altered by pentobarbital (Figs. 1C and 2B). However, pentobarbital increased the desensitization of α1β3δ-γ2L(M1pre-iso) currents (40.4 ± 3.3% versus 50.1 ± 1.8%) (p < 0.05) (Figs. 1D and 2B). Desensitization of α1β3δ-γ2L(M1p) currents was increased by pentobarbital from 18.4 ± 2.9% to 41.5 ± 3.6% (p < 0.001) (Figs. 1E and 2B).
Currents evoked by a saturating concentration of GABA also deactivated differently among wild type and δ-γ2L chimeric receptors (Fig. 1, B--F, left traces). The mean deactivation time constant of α1β3δ-γ2L(M1e) currents (438.9 ± 118.3 ms) was greater than that of α1β3δ-γ2L(M1p) (77.6 ± 9.0 ms) or α1β3δ (95.7 ± 12.6 ms) currents (p < 0.01), although it was not significantly different from that of α1β3γ2L (292.9 ± 69.4 ms) or α1β3δ-γ2L(M1pre-iso) (269.7 ± 86.0 ms) currents. The deactivation time constant was not significantly different among α1β3γ2L, α1β3δ-γ2L(M1pre-iso), α1β3δ-γ2L(M1p), and α1β3δ currents (Fig. 2C).
Pentobarbital significantly prolonged the deactivation of all of the wild type and δ-γ2L chimeric receptor currents (Fig. 2C). The deactivation time constant of α1β3γ2L currents was increased by pentobarbital to 1072.5 ± 220.9 ms (p < 0.01) (Figs. 1B and 2C). Pentobarbital increased the deactivation time constant of α1β3δ-γ2L(M1e) currents to 4253.9 ± 1274.9 ms (p < 0.05) (Figs. 1C and 2C). An increase in deactivation time constant also was observed for α1β3δ-γ2L(M1pre-iso) (2698.6 ± 935.0 ms) (p < 0.05), α1β3δ-γ2L(M1p) (1315.0 ± 225.0 ms) (p < 0.01), and α1β3δ (286.3 ± 62.1 ms) (p < 0.01) currents (Figs. 1, D–F, and 2C).
Pentobarbital Modulated the Current Amplitudes of γ2L-δ Chimeric GABAA Receptors
The results obtained using δ-γ2L chimeras suggested that the N terminus as well as the N-terminal portions of the M1 domain of the δ subunit were critical for pentobarbital modulation. However, all of the constructs contained the δ subunit sequence in the N terminus, so it was not possible to isolate the role of the M1 domain. Is this domain sufficient to support δ subunit-like modulation by pentobarbital? One way to address this issue is to examine reverse γ2L-δ chimeras.
The M1e′ γ2L-δ chimera contained the γ2L subunit sequence in the entire extracellular N-terminal domain (Fig. 3A). Pentobarbital slightly enhanced peak currents of the α1β3γ2L-δ(M1e′) receptors (130.4 ± 14.1%, n = 6), but this enhancement was substantially smaller than that of α1β3δ receptors (p < 0.001) (Figs. 3, B and C, and 4A). The M1p′ chimera advanced the γ2L subunit sequence nine amino acids into the M1 domain (Fig. 3A). The α1β3γ2L-δ(M1p′) currents (n = 8) were slightly enhanced by pentobarbital, whose enhancement was much smaller than that of α1β3δ receptors (p < 0.001) (Figs. 3, B and D, and 4A). The M1i′ chimera advanced the γ2L subunit sequence fourteen more amino acids into the M1 domain (Fig. 3A), and pentobarbital minimally enhanced α1β3γ2L-δ(M1i′) currents (n = 7) (Figs. 3E and 4A). The current enhancement evoked by pentobarbital of α1β3γ2L-δ(M1e′), α1β3γ2L-δ(M1p′), or α1β3γ2L-δ(M1i′) receptors was not significantly different from that of α1β3γ2L receptors (Fig. 4A).
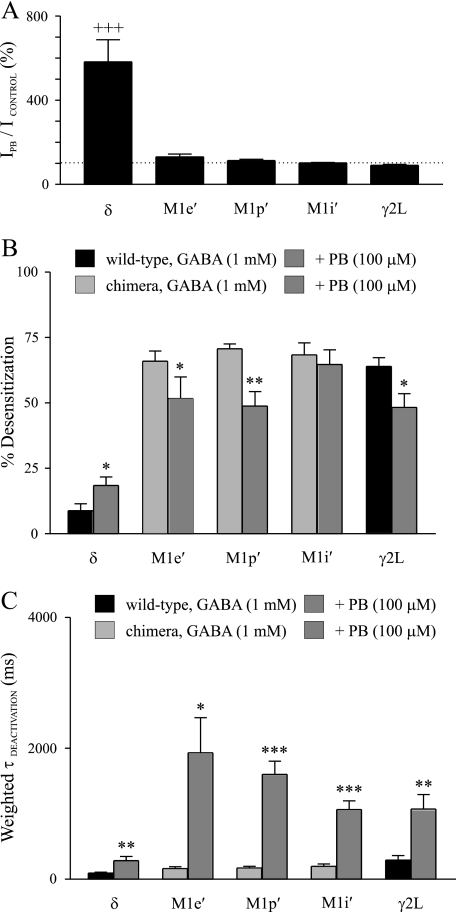
FIGURE 4.
Pentobarbital modulation of peak currents, desensitization and deactivation of wild type and γ2L-δ chimeric receptors. A, shown is the mean extent of enhancement by pentobarbital (PB) of wild type and γ2L-δ chimeric receptors. The dashed line indicates 100%. B, shown is the comparison of the mean desensitization prior to and after pentobarbital treatment among wild type and γ2L-δ chimeric receptors. C, the mean deactivation time constant was greater after pentobarbital treatment as compared with GABA control for wild type and γ2L-δ chimeric receptors. +++, significantly different from M1e′, M1p′, M1i′, and γ2L at p < 0.001 (one-way analysis of variance followed by Newman-Keuls multiple comparison test); *, significantly different from GABA control at p < 0.05; **, p < 0.01; ***, p < 0.001 (paired Student's t test).
Pentobarbital Modulated the Desensitization and Deactivation of γ2L-δ Chimeric GABAA Receptors
The currents of α1β3γ2L-δ(M1e′), α1β3γ2L-δ(M1p′), and α1β3γ2L-δ(M1i′) receptors evoked by 1 mm GABA exhibited substantial desensitization, which was quite different from that of α1β3δ receptors (Fig. 3, B–E, left traces). Instead, the desensitization of these receptor currents was similar to that of wild type α1β3γ2L receptors (Fig. 3, C--F, left traces), and the mean desensitization of these receptors was not significantly different from one another (Fig. 4B). Pentobarbital decreased the desensitization of both α1β3γ2L-δ(M1e′) (66.0 ± 3.9% versus 51.7 ± 8.3%) (p < 0.05) and α1β3γ2L-δ(M1p′) (70.7 ± 1.9% versus 48.8 ± 5.6%) (p < 0.01) currents, as was observed for α1β3γ2L currents (Fig. 4B). However, the desensitization of α1β3γ2L-δ(M1i′) currents was not altered significantly by pentobarbital (Fig. 4B).
Deactivation of α1β3γ2L-δ(M1e′), α1β3γ2L-δ(M1p′), or α1β3γ2L-δ(M1i′) currents was similar (Fig. 3, C–E, left traces), and the mean deactivation time constants of these receptors were not significantly different from either α1β3δ or α1β3γ2L receptors (Fig. 4C). Pentobarbital significantly prolonged deactivation of the α1β3γ2L-δ(M1e′), α1β3γ2L-δ(M1p′), and α1β3γ2L-δ(M1i′) currents (Fig. 4C). The deactivation time constant of α1β3γ2L-δ(M1e′) currents was increased from 160.5 ± 32.2 ms to 1932.6 ± 534.7 ms (p < 0.05) by pentobarbital (Figs. 3C and 4C). Pentobarbital increased the deactivation time constant of α1β3γ2L-δ(M1p′) currents from 171.8 ± 25.5 ms to 1600.8 ± 203.5 ms (p < 0.001) (Figs. 3D and 4C). The deactivation time constant of α1β3γ2L-δ(M1i′) currents in the presence of pentobarbital was increased to 1065.4 ± 134.3 ms from GABA control of 199.8 ± 35.0 ms (p < 0.001) (Figs. 3E and 4C).
DISCUSSION
Pentobarbital Required the N Terminus and N-terminal Portion of the δ Subunit M1 Domain from Valine 233 to Proline 241 for Potentiation of α1β3δ Receptor Currents
A large body of literature demonstrates that αβδ receptors are selectively enhanced by a variety of structurally different compounds as compared with αβγ receptors (8, 11–20). Currents evoked by a saturating concentration of GABA were consistently smaller for α1β3δ receptors than for α1β3γ2L receptors (8, 15, 19, 21). Single channel recordings found that αβδ currents exhibited brief openings, whereas αβγ currents displayed bursting openings with longer mean open duration (8, 15, 18, 22–24). In addition, 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol and pentobarbital evoked greater currents than GABA from αβδ receptors (8, 12, 18). These studies strongly suggest that GABA is a partial agonist for αβδ receptors such that it leaves substantial “modulatory capacity” for many allosteric modulators (16).
Several modulators, including pentobarbital, enhanced αβδ currents evoked by a saturating concentration of GABA mainly by increasing channel gating efficacy (8, 15, 18, 20). The present study sought to determine the structural domains of the δ subunit involved in transduction of the allosteric effect of pentobarbital to current enhancement and alteration of desensitization. We observed that the enhancement by pentobarbital of currents evoked by a saturating concentration of GABA for α1β3δ-γ2L(M1p) receptors was not different from that for α1β3δ receptors, suggesting that the δ subunit domain from the beginning of the N terminus to proline 241 in M1 is sufficient for maximal potentiation by pentobarbital. The enhancement of the reversed chimeric α1β3γ2L-δ(M1p′) receptor currents by pentobarbital was not different from α1β3γ2L receptors, supporting the idea that the δ subunit domain from the beginning of N terminus to the proline 241 in M1 is necessary and sufficient for maximal potentiation by pentobarbital. The enhancement by pentobarbital of α1β3γ2L-δ(M1e′) currents was <25% of α1β3δ receptors, implying that the δ subunit N terminus plays a critical role in pentobarbital enhancement. However, α1β3δ-γ2L(M1e) currents were only slightly potentiated by pentobarbital. These observations lead to the conclusion that both the N terminus and M1 domain from valine 233 to proline 241 of the δ subunit are necessary and sufficient for pentobarbital potentiation of α1β3δ currents evoked by saturating concentrations of GABA.
Both GABAA and nicotinic cholinergic receptors are members of the Cys-loop receptor family and are proposed to share similar topological structures. It was reported that some of the residues in the N-terminal portion of M1 domain of the nicotinic cholinergic receptor were exposed in the channel lining and might be involved in gating (25). An invariant proline residue in M1 domain of nicotinic cholinergic receptor subunits is critically coupled with ligand binding and channel gating (26). Mutation of the M1 proline (equivalent to proline 241 in the δ subunit) in α1 or β1 subunits reduced the enhancement by barbiturates of submaximal GABA-evoked currents in α1β1γ2 receptors (27), suggesting that the M1 proline is involved in transduction of allosteric effect of barbiturates. An invariant glycine residue (equivalent to glycine 232 in the δ subunit) at the entrance to M1 of GABAA receptors also might be involved in transduction of allosteric effect of anesthetics including pentobarbital (28). The present finding that the M1 domain from valine 233 to proline 241 of the δ subunit contributes to the pentobarbital potentiation of α1β3δ currents implies that some of these residues may be directly involved in the transduction of the pentobarbital allosteric effect to channel gating. Furthermore, in addition to the M1 domain residues, we also demonstrated that the N terminus of the δ subunit contributed to the pentobarbital potentiation of α1β3δ currents. Multiple residues in the GABAA receptor N terminus were reported to couple with the M2–3 linker to affect channel gating (29). The finding that the structural domains of the δ subunit beyond M1 may not be critically involved in transduction of the pentobarbital allosteric effect suggests that the δ subunit N terminus may be able to interact with either the δ or γ2L subunit M2–3 linker. We cannot, however, rule out an interaction between these two domains that occurs via residues that are conserved between the δ and γ2L subunits.
Like the nicotinic cholinergic receptor (30–34), the M2 domains form the ion conduction pathway and the channel gate of GABAA receptors. Pentobarbital at modulating concentrations did not seem to interfere with the gate via this domain because the M2 domain of δ subunit was not required for potentiation of α1β3δ currents evoked by a saturating concentration of GABA. Some residues in transmembrane regions (including M2 domain) of α and β subunits have been reported to be involved in anesthetic modulation (35–39). These studies suggested that allosteric modulation of GABAA receptors by anesthetics is subunit-dependent and that different anesthetics may have different actions on GABAA receptors.
It is possible that the domain from the beginning of the N terminus to M1 proline 241 might only confer α1β3δ receptors with a “partial agonist” property, such that these residues were not necessarily involved in transduction of pentobarbital allosteric effects. If this was the case, the similar structural domain is likely to be involved in the enhancement of α1β3δ receptor currents by different modulators. However, we found that the neurosteroid tetrahydrodeoxycorticosterone required structural domains beyond M2 for full potentiation of α1β3δ currents induced by a saturating concentration of GABA.3 In addition, mutation of the M1 proline in α1 or β1 of the GABAA receptor only reduced the enhancement by barbiturates without interfering with that by neurosteroids (27). Therefore, this required domain from the beginning of the N terminus to M1 proline 241 is to some extent “specific” for pentobarbital modulation. These residues are unlikely to contribute to the formation of a barbiturate binding site as previous studies in δ subunit knock-out mice suggest that general anesthetics like barbiturates, etomidate, and propofol are not selective for αβδ receptors, although there may be some δ subunit selectivity for neurosteroids (40, 41).
Interestingly, it has been reported recently that general anesthetics like propofol, etomidate, and tetrahydrodeoxycorticosterone enhanced both α4β3 and α4β3δ receptors to the same extent when applied with saturating concentrations of GABA (42), indicating that it is the α4 instead of the δ subunit that confers current enhancement by general anesthetics. However, in the current study, we demonstrated that δ subunits play an important role in pentobarbital potentiation of α1β3δ currents, as pentobarbital greatly enhanced α1β3δ currents but only slightly enhanced α1β3 currents. Therefore, α subunits may play a role in modulation of αβδ receptors by anesthetics.
Pentobarbital Required the N Terminus and N-terminal Portion of the δ Subunit M1 Domain from Valine 233 to Isoleucine 235 to Alter α1β3δ Current Desensitization
Pentobarbital decreased the desensitization of α1β3γ2L currents but increased that of α1β3δ currents induced by a saturating concentration of GABA (8, this study). Pentobarbital did not alter desensitization of α1β3δ-γ2L(M1e) receptor currents and decreased desensitization of most α1β3γ2L-δ chimeric receptor currents, suggesting that the N terminus of the δ subunit may be involved in the pentobarbital-induced increase of desensitization. Some residues in the δ subunit M1 domain may also cause the desensitization increase since an increase in desensitization by pentobarbital was observed for α1β3δ-γ2L(M1pre-iso) and α1β3δ-γ2L(M1p) receptor currents. These data imply that δ subunit residues from the beginning of the N terminus to M1 isoleucine 235 are a requirement for pentobarbital to increase α1β3δ current desensitization. Our previous study (10) and the current study (Fig. 1D, left trace) showed that these δ subunit residues were required to abolish the fast desensitization of currents evoked by a saturating concentration of GABA. One possibility is that pentobarbital might modulate the rate constants into and out of the desensitized states to increase the occupancy of the desensitized states. It was reported that the desensitized state of nicotinic cholinergic receptors was conferred by some residues lining the inside of the ion conduction pathway and that the desensitized state was structurally different from other states (43). We demonstrated here that the structural domain of the δ subunit involved in increasing desensitization overlapped with but was distinct from that involved in transduction of the allosteric effect of pentobarbital.
Pentobarbital Prolonged Deactivation of All Wild Type and Chimeric Receptor Currents
Pentobarbital was reported to prolong the deactivation of α1β3δ and α1β3γ2L receptor currents evoked by a saturating concentration of GABA (8). It was proposed that prolongation of deactivation is “coupled” with increased desensitization (23, 44). Consistent with this idea, pentobarbital prolonged deactivation and enhanced desensitization of α1β3δ receptors as well as most of the δ-γ2L chimeric receptors. However, “uncoupling” of desensitization and deactivation was also observed. Pentobarbital decreased the desensitization of α1β3γ2L receptors as well as most of the α1β3γ2L-δ chimeric receptors and did not modify the desensitization of α1β3δ-γ2L(M1e) receptors, although deactivation of these receptors was prolonged by pentobarbital. These data further support the idea that other factors in addition to desensitization affect deactivation. Deactivation has been demonstrated to be prolonged by increased gating efficacy and agonist affinity (45). Interestingly, we observed that pentobarbital dramatically prolonged deactivation of α1β3δ-γ2L(M1e) receptor currents, but that peak currents and current desensitization were not modulated dramatically by pentobarbital. One possible explanation may be that pentobarbital slowed GABA unbinding, which has been reported for another general anesthetic drug, halothane (46).
Supplementary Material
Acknowledgments
We thank Luyan Song for excellent technical assistance in preparing the GABAA receptor subunit and chimeric cDNAs. We also appreciate Drs. Matt Bianchi, Dorothy Jones-Davis, and Martin Gallagher for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant NSR0I 33300 (to R. L. M.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
M. T. Bianchi and R. L. Macdonald, unpublished observations.
- GABAA
- γ-aminobutyric acid, type A
- M
- transmembrane domain
- HEK293T
- human embryonic kidney.
REFERENCES
- 1.Olsen R. W., Macdonald R. L. (2002) Glutamate and GABA Receptors and Transporters: Structure, Function, and Pharmacology, pp. 202–235, Taylor & Francis, London [Google Scholar]
- 2.Hevers W., Lüddens H. (1998) Mol. Neurobiol. 18, 35–86 [DOI] [PubMed] [Google Scholar]
- 3.McKernan R. M., Whiting P. J. (1996) Trends Neurosci. 19, 139–143 [DOI] [PubMed] [Google Scholar]
- 4.Mody I., Pearce R. A. (2004) Trends Neurosci. 27, 569–575 [DOI] [PubMed] [Google Scholar]
- 5.Farrant M., Nusser Z. (2005) Nat. Rev. Neurosci. 6, 215–229 [DOI] [PubMed] [Google Scholar]
- 6.Belelli D., Harrison N. L., Maguire J., Macdonald R. L., Walker M. C., Cope D. W. (2009) J. Neurosci. 29, 12757–12763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulz D. W., Macdonald R. L. (1981) Brain Res. 209, 177–188 [DOI] [PubMed] [Google Scholar]
- 8.Feng H. J., Bianchi M. T., Macdonald R. L. (2004) Mol. Pharmacol. 66, 988–1003 [DOI] [PubMed] [Google Scholar]
- 9.Greenfield L. J., Jr., Sun F., Neelands T. R., Burgard E. C., Donnelly J. L., MacDonald R. L. (1997) Neuropharmacology 36, 63–73 [DOI] [PubMed] [Google Scholar]
- 10.Bianchi M. T., Haas K. F., Macdonald R. L. (2001) J. Neurosci. 21, 1127–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lees G., Edwards M. D. (1998) Anesthesiology 88, 206–217 [DOI] [PubMed] [Google Scholar]
- 12.Adkins C. E., Pillai G. V., Kerby J., Bonnert T. P., Haldon C., McKernan R. M., Gonzalez J. E., Oades K., Whiting P. J., Simpson P. B. (2001) J. Biol. Chem. 276, 38934–38939 [DOI] [PubMed] [Google Scholar]
- 13.Sundstrom-Poromaa I., Smith D. H., Gong Q. H., Sabado T. N., Li X., Light A., Wiedmann M., Williams K., Smith S. S. (2002) Nat. Neurosci. 5, 721–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson S. A., Wingrove P. B., Connelly L., Whiting P. J., Wafford K. A. (2002) Mol. Pharmacol. 61, 861–869 [DOI] [PubMed] [Google Scholar]
- 15.Wohlfarth K. M., Bianchi M. T., Macdonald R. L. (2002) J. Neurosci. 22, 1541–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bianchi M. T., Macdonald R. L. (2003) J. Neurosci. 23, 10934–10943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallner M., Hanchar H. J., Olsen R. W. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 15218–15223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akk G., Bracamontes J., Steinbach J. H. (2004) J. Physiol. 556, 387–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng H. J., Macdonald R. L. (2004) Mol. Pharmacol. 66, 1517–1524 [DOI] [PubMed] [Google Scholar]
- 20.Feng H. J., Macdonald R. L. (2004) J. Neurophysiol. 92, 1577–1585 [DOI] [PubMed] [Google Scholar]
- 21.Saxena N. C., Macdonald R. L. (1994) J. Neurosci. 14, 7077–7086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher J. L., Macdonald R. L. (1997) J. Physiol. 505, 283–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haas K. F., Macdonald R. L. (1999) J. Physiol. 514, 27–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinbach J. H., Akk G. (2001) J. Physiol. 537, 715–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akabas M. H., Karlin A. (1995) Biochemistry 34, 12496–12500 [DOI] [PubMed] [Google Scholar]
- 26.England P. M., Zhang Y., Dougherty D. A., Lester H. A. (1999) Cell 96, 89–98 [DOI] [PubMed] [Google Scholar]
- 27.Greenfield L. J., Jr., Zaman S. H., Sutherland M. L., Lummis S. C., Niemeyer M. I., Barnard E. A., Macdonald R. L. (2002) Neuropharmacology 42, 502–521 [DOI] [PubMed] [Google Scholar]
- 28.Carlson B. X., Engblom A. C., Kristiansen U., Schousboe A., Olsen R. W. (2000) Mol. Pharmacol. 57, 474–484 [DOI] [PubMed] [Google Scholar]
- 29.Kash T. L., Jenkins A., Kelley J. C., Trudell J. R., Harrison N. L. (2003) Nature 421, 272–275 [DOI] [PubMed] [Google Scholar]
- 30.Filatov G. N., White M. M. (1995) Mol. Pharmacol. 48, 379–384 [PubMed] [Google Scholar]
- 31.Labarca C., Nowak M. W., Zhang H., Tang L., Deshpande P., Lester H. A. (1995) Nature 376, 514–516 [DOI] [PubMed] [Google Scholar]
- 32.Wilson G. G., Karlin A. (1998) Neuron 20, 1269–1281 [DOI] [PubMed] [Google Scholar]
- 33.Zhang H., Karlin A. (1998) Biochemistry 37, 7952–7964 [DOI] [PubMed] [Google Scholar]
- 34.Miyazawa A., Fujiyoshi Y., Unwin N. (2003) Nature 423, 949–955 [DOI] [PubMed] [Google Scholar]
- 35.Nishikawa K., Harrison N. L. (2003) Anesthesiology 99, 678–684 [DOI] [PubMed] [Google Scholar]
- 36.Li G. D., Chiara D. C., Sawyer G. W., Husain S. S., Olsen R. W., Cohen J. B. (2006) J. Neurosci. 26, 11599–11605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campagna-Slater V., Weaver D. F. (2007) Neurosci. Lett. 418, 28–33 [DOI] [PubMed] [Google Scholar]
- 38.Richardson J. E., Garcia P. S., O'Toole K. K., Derry J. M., Bell S. V., Jenkins A. (2007) Anesthesiology 107, 412–418 [DOI] [PubMed] [Google Scholar]
- 39.Stewart D., Desai R., Cheng Q., Liu A., Forman S. A. (2008) Mol. Pharmacol. 74, 1687–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mihalek R. M., Banerjee P. K., Korpi E. R., Quinlan J. J., Firestone L. L., Mi Z. P., Lagenaur C., Tretter V., Sieghart W., Anagnostaras S. G., Sage J. R., Fanselow M. S., Guidotti A., Spigelman I., Li Z., DeLorey T. M., Olsen R. W., Homanics G. E. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 12905–12910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stell B. M., Brickley S. G., Tang C. Y., Farrant M., Mody I. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 14439–14444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meera P., Olsen R. W., Otis T. S., Wallner M. (2009) Neuropharmacology 56, 155–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson G., Karlin A. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 1241–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones M. V., Westbrook G. L. (1995) Neuron 15, 181–191 [DOI] [PubMed] [Google Scholar]
- 45.Bianchi M. T., Botzolakis E. J., Haas K. F., Fisher J. L., Macdonald R. L. (2007) J. Physiol. 584, 769–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X., Pearce R. A. (2000) J. Neurosci. 20, 899–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.