Abstract
Autoregulation is one of the mechanisms of imparting feedback control on gene expression. Positive autoregulatory feedback results in induction of a gene, and negative feedback leads to its suppression. Here, we report an interesting mechanism of autoregulation operating on Drosophila Rel gene dorsal that can activate as well as repress its expression. Using biochemical and genetic approaches, we show that upon immune challenge Dorsal regulates its activation as well as repression by dynamically binding to two different κB motifs, κBI (intronic κB) and κBP (promoter κB), present in the dorsal gene. Although the κBI motif functions as an enhancer, the κBP motif acts as a transcriptional repressor. Interestingly, Dorsal binding to these two motifs is dynamic; immediately upon immune challenge, Dorsal binds to the κBI leading to auto-activation, whereas at the terminal phase of the immune response, it is removed from the κBI and repositioned at the κBP, resulting in its repression. Furthermore, we show that repression of Dorsal as well as its binding to the κBP depends on the transcription factor AP1. Depletion of AP1 by RNA interference resulted in constitutive expression of Dorsal. In conclusion, this study suggests that during acute phase response dorsal is regulated by following two subcircuits: (i) Dl-κBI for activation and (ii) Dl-AP1-κBP for repression. These two subcircuits are temporally delineated and bring about overall regulation of dorsal during immune response. These results suggest the presence of a previously unknown mechanism of Dorsal autoregulation in immune-challenged Drosophila.
Keywords: Antimicrobial Peptides, AP1 Transcription Factor, Chromatin Remodeling, Co-regulator Transcription, Drosophila, Gene Regulation, Gene Transcription, Innate Immunity
Introduction
Insects have evolved simple yet multipronged strategies to defend themselves against microbial invasion. The mechanisms that regulate the different arms of insect immunity have been well investigated in Drosophila melanogaster (1). To combat microbial challenge, Drosophila relies on multiple defense reactions, which partly resemble the innate immune response of higher organisms (2–5). Such a conserved innate immune pathway suggests ancient origin of immune response during metazoan evolution. Because of this evolutionary conservation, Drosophila has emerged as a model for studying common innate immune mechanisms in animals (5, 6). For example, homologues of the cell surface receptor protein Toll of Drosophila, and its downstream signaling pathway, are present in mammals as well. Activation of the Toll pathway leads to synthesis of antimicrobial peptides (AMPs)3 in both insects and mammals (3, 7). The hallmark of Drosophila immune defense is the infection-induced synthesis and secretion of a battery of AMPs into the hemolymph by the fat body (8–10). These AMPs are the downstream effector molecules of the two immune pathways, namely Toll and Imd (3, 8–12).
To understand induction of AMP genes upon activation of Toll/Imd pathway, regulatory elements in their promoters were analyzed and mapped. The analysis revealed the presence and requirement of DNA motifs resembling the κB motifs of mammals for inducibility of immune genes upon infection in Drosophila (13). Later, three NF-κB/Rel-like proteins were also identified in Drosophila (14). Two of these, Dorsal (Dl) and Dif, encoded by two clustered genes, are part of the Toll pathway signaling induced upon infection by Gram-positive bacteria or fungi (15, 16). Relish, the third member of this family, regulates induction of AMPs of the Imd pathway upon infection by Gram-negative bacteria (14, 17). Dif and Dorsal play redundant roles in regulating expression of drosomycin, a Toll pathway AMP gene, at the larval stage, whereas Dif alone mediates drosomycin expression in adults (15, 16). In Drosophila, activation of Toll upon microbial infection involves the recruitment of the adaptor protein Myd88, leading to the activation of the kinase Pelle and subsequent phosphorylation and degradation of Cactus, the cytoplasmic inhibitor of Dorsal and Dif, which brings about rapid nuclear translocation of these two transcription factors (17, 18). Dorsal also acts as a morphogen during embryonic development (19). In the early embryo, degradation of Cactus, upon developmental cues arising from activation of Toll, allows Dorsal to enter the nuclei along the dorso-ventral axis in a gradient. Formation of Dorsal gradient is important for the regulation of target gene expression involved in dorso-ventral patterning (19). However, the Toll signaling cascade controlling the AMP response differs from the dorso-ventral patterning pathway at the following two levels: (i) regulation by the serine proteases acting upstream of Spätzle in the signaling pathway, and (ii) use of Dif for immune response in the fat body, rather than Dorsal, which has a role in embryogenesis (8, 10, 17, 18).
Dorsal is a bifunctional transcription factor as it activates as well as suppresses transcription of target genes involved in embryonic development (20–22). For example, the twist enhancer region in Drosophila has multiple Dorsal-binding sites and is activated by Dorsal (23, 24), whereas Dorsal-binding sites in the zen promoter act as repressor elements (22). Point mutations in the Dorsal-binding motifs of the twist enhancer reduce ventral activation, whereas mutations in the Dorsal-binding sites in the zen silencer abolish ventral repression. These results suggest bi-functionality of Dorsal as a transcriptional activator as well as transcriptional repressor in vivo (20, 22, 25, 26).
Although there are many reports that emphasize regulation of Dorsal target genes, regulation of the dorsal (dl) gene itself has not been investigated thus far (27, 28). We are interested in understanding the molecular basis of sex-biased immune response in insects. While deciphering the molecular basis of the sex-biased immune response, we observed differential activation of Drosophila Rel proteins in the two sexes. We found that sex-differential activation of Rel proteins is modulated at different levels, including autoregulation (data not shown). In this study, we provide insights into the molecular mechanism underlying autoregulation of dl. We show that dl autoregulation is achieved by two different κB sites, a canonical κB motif (κBI) located in the first intron of dl and another functional but noncanonical κB motif (κBP) present upstream of the transcription start site (TSS). We show that the κBI motif acts as an enhancer, whereas the κBP motif is essential for the repression of dl at the termination of acute phase response. We found that Dorsal binding to the two motifs is dynamic and is temporally regulated. Immediately after immune challenge, Dorsal protein binds to the κBI motif, which results in immediate and strong expression of dl gene. However, later in acute phase response, Dorsal binding was located at the κBP motif and not at the κBI motif. Further analysis suggested that dl expression at the onset of acute phase response was regulated by Dorsal alone; however, its repression at the end of acute phase response required interaction with another transcription factor AP1. Drosophila AP1 is a homodimer or heterodimer of Jra (Jun-related antigen) and Fra (Fos-related antigen). Here, we show that Drosophila AP1 acts as a co-repressor in dl regulation.
EXPERIMENTAL PROCEDURES
Drosophila Stocks
w1118 flies were used as standard wild type strain. dl1 flies were obtained from Bloomington Stock Centre. All stocks were maintained and the experiments performed at 25 °C. Drs::gfp transgenic flies were provided by Bruno Lemaitre, CNRS, France.
Infection Experiments
Third instar wandering stage Drosophila larvae, maintained at 25 °C, were infected with the Gram-positive bacterium Micrococcus luteus by pricking with a sharp needle dipped in a bacterial pellet with absorbance of ∼100. Drosophila S2 cells were immune-challenged by adding 50 μg of lipopolysaccharide (LPS) (Sigma) and 50 μg of peptidoglycan (PGN) (Sigma) per well containing ∼1 × 106 cells.
Electrophoretic Mobility Shift Assay
Embryonic nuclear extracts were prepared by homogenizing embryos (2–4-h-old) in extraction buffer (20 mm Hepes, pH 7.9, 5 mm MgCl2, 0.1 mm EGTA, 12.5% sucrose, 25% glycerol, 0.5 mm dithiothreitol, 0.5 mm phenylmethylsulfonyl fluoride and protease inhibitor mixture) using a Dounce homogenizer, followed by centrifugation at 3000 × g for 15 min at 4 °C. The precipitated nuclei were suspended in 1 ml of extraction buffer. For EMSA, 100 ng each of different double-stranded oligonucleotide probes was labeled with 2 μl of [γ-32P]ATP (5 × 105 cpm) and 1 μl of polynucleotide kinase (10 units/μl) in 1 μl of PNK buffer (New England Biolabs) for 1 h at 37 °C. The labeled DNA was purified, and binding reaction was performed for 45 min at room temperature by mixing 1 ng of purified 32P-labeled double-stranded synthetic oligonucleotide probe (4000 cpm/μl), 10 μl of nuclear extracts, and 300 ng of poly(dI-dC) in the presence of a protease inhibitor mixture (Sigma). Cold competition was performed by preincubating the extracts with a 50-fold excess of unlabeled oligonucleotide for 15 min at room temperature. For supershift experiment, anti-mouse Dorsal monoclonal antibody was added to the binding reaction for 30 min. The binding reaction was analyzed by electrophoresis on a 6% native polyacrylamide gel. The probe sequences are as follows: for κBP, ATGAGTCACAGAAAAACAAGAAAAACA; for mut-κB, ATGAGTCACAGAATAATCCAGAATAATCC, and for κBI, GGGAATTCCGGGAATTCCGGGAATTCC.
Immunodepletion
These experiments were performed essentially according to the protocols mentioned previously (29). Briefly, Dorsal was immunodepleted from the embryonic extract using anti-Dorsal monoclonal antibody obtained from Drosophila Studies Hybridoma Bank in a 100-μl final volume of buffer containing 30 mm Hepes-KOH, pH 7.4, 100 mm KOAc, 2 mm Mg(OAc)2, 2 mm dithiothreitol, and protease inhibitor mixture. Dorsal-depleted supernatant was collected and used immediately for coupled in vitro transcription and translation reaction.
Coupled in Vitro Transcription and Translation
Different luciferase constructs (1 μg each) were used for coupled in vitro transcription and translation. These plasmids were incubated with cell extracts prepared from LPS- and PGN-treated S2 cells. Additional supplements added in the reaction were RNase inhibitor, Mg2+, ATP, and amino acid mix as mentioned in Ref. 29. After adding all the components, the reaction was carried out for minimum of 2 h followed by Western analysis and/or luciferase assay.
Western Blot Analysis
Whole embryo extracts were prepared from 0- to 4-h-old dechorionated embryos in extraction buffer A (50 mm Tris, pH 7.5, 140 mm NaCl, 5 mm Mg(CH3COO)2, 0.05% Nonidet P-40, 1 mm phenylmethylsulfonyl fluoride, 10 mg of pepstatin A/ml, 10 mg of aprotinin/ml, and 1 mg of leupeptin/ml) at 40 °C.
Cell extracts containing 50 μg of protein were separated on a 10% SDS-polyacrylamide gel. The proteins were electrophoretically transferred to a Hybond-P membrane (Amersham Biosciences) using a Trans-blot cell (Bio-Rad), at 200 mA overnight at 4 °C. The blots were stained for total protein by Ponceau S (Sigma) and blocked in 10% nonfat dry milk in 0.5% Tween 20, 0.05% SDS in PBS (Blocking buffer). The blots were incubated for 6 h at room temperature in primary antibodies and then washed four times for 10 min in Tween 20 + PBS followed by a 2-h incubation at room temperature with the secondary antibody (Sigma). The blots were then washed three times for 30 min in Tween 20 + PBS and rinsed once in PBS. Anti-Dorsal monoclonal antibody in a 1:500 dilution was used for probing. The protein bands were detected using horseradish peroxidase-enhanced chemiluminescence (ECL, Amersham Biosciences).
Plasmid Constructs
The enhancer fragments were PCR-amplified from Drosophila genomic DNA with a 5′ primer containing a KpnI site and a 3′ primer containing an XhoI site and cloned into pGL3 Basic vector. (Primer information is provided as supplemental Information 1.) Cloned inserts were verified by restriction digestion and sequencing. P3 is a full-length promoter construct (1.25-kb-long region upstream to TSS) with three κB motifs. We also generated a full-length enhancer construct with both upstream and downstream regulatory regions. The plasmid P3-Ex1-In1-Ex2 contained the exon1, intron1, and part of exon2 apart from the 1.25-kb-long promoter region. To generate ΔκBP plasmid, the NsiI recognition sequence surrounding the κBP motif (AGAAAAACA) in the control P3-Ex1-In1-Ex2 plasmid was used to delete this motif by incubating with NsiI enzyme (New England Biolabs) followed by self-ligation. The same restriction site was used to insert the mutant κBP motif harboring NsiI recognition sequence on either side of the mutant kappaB motif sequence (AGAATAATC) in P3-Ex1-In1-Ex2 plasmid that generated the plasmid κBPmut. For motif swap experiment, the κBI motif was cloned into the NsiI sites surrounding the κBP motif. To generate κBImut plasmid, the mutant κBI motif, GGGAAATAC, was used to replace the wild type κBI (GGGAATTCC) by site-directed mutagenesis using QuickChange II site-directed mutagenesis kit (Stratagene) according to manufacturer's protocol. The mutated nucleotides of the κBI motif as part of the primer for site-directed mutagenesis are shown in boldface and underlined. To obtain ΔAP1 motif plasmid (AP1-del), the AP1 motif cluster in the control P3-Ex1-In1-Ex2 plasmid was deleted by restriction digestion with MmeI only or both MmeI and NaeI. All the plasmids were purified using Qiagen columns.
Luciferase Assay
Drosophila immune-competent Schneider (S2) cells were maintained at 25 °C in Schneider's Insect Cell Media (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen). For transfections, cells were seeded in 6-well plates at a density of 1 × 106 cells/ml. A day later, transfection was carried out using FuGENE transfection reagent (Roche Applied Science) without removing the plasmids. 12 h post-transfection, 50 μg of LPS (Sigma) and 50 μg of PGN (Sigma) were added per well, and the cells were harvested at different time points. Cell extracts were prepared in lysis buffer (Promega), and luciferase activity was measured according to manufacturer's instructions (Promega) on a luminometer. For luciferase assay, 100 μl of the reaction mixture/cell extract was added to 500 μl of luciferase reagent at room temperature. Luminometer was programmed to perform a 2-s measurement delay followed by a 10-s measurement.
RNAi Knockdown
RNAi strategy was used to knockdown Drosophila AP1. Both AP1 components jra and fra of Drosophila were targeted as they can act either as homodimer or heterodimer. 219-bp region of the jra and 239 bp of fra open reading frames were amplified and cloned into TOPOII PCR cloning vector (Invitrogen) (supplemental Information 1). Double strand RNA was synthesized by in vitro transcription using T7 and SP6 RNA polymerases and annealed after ethanol precipitation. For RNAi, 1 × 106 Drosophila S2 cells were plated, washed with serum-free medium, and incubated in the same medium for 5–6 h. 5 μg of dsRNA, at 25 μg/ml concentration, was used for transfection. All transfections were done using FuGENE transfection reagent (Roche Applied Science) according to manufacturer's protocol.
Chromatin Immunoprecipitation (ChIP)
The protocol followed for ChIP was essentially as mentioned on the flychip website with the following modifications. S2 cells were fixed by cross-linking with 1% formaldehyde followed by lysis in SDS lysis buffer and sonication. Fragmented chromatin was centrifuged at 13,000 rpm for 45 min; soluble fraction was collected and later used for immunoprecipitation performed with anti-Dorsal monoclonal antibody. DNA was purified using Qiagen columns and later used in PCR. All PCRs were done at 62 °C for 30 cycles. The following primers were used for ChIP assay: primers used for amplifying κBI motif, forward primer CAAAGAAAATGGAGGGCAGA and the reverse primer AAGAGAGAGTGGGCAAAGAGC. This primer pair amplifies 177-bp PCR product.
Primers for amplifying κBP motif forward primer TTGGTTACCATACAGTTGAATTCTCA and reverse primer AGGAATGCAGGCCAGTTGTT amplify a 196-bp PCR product. Both primer sets were standardized to amplify at Tm 62 °C and were thus used in multiplex PCR.
RESULTS
General Organization of Rel Promoters
Regulation of insect Rel genes is poorly understood. In silico analysis of regulatory sequences upstream of the transcription start sites of the three Drosophila Rel genes suggested the presence of putative binding sites for transcription factors like Dfd, Hb, Ftz, and BrCZ, but none of these is known to regulate immune response. However, one interesting prediction was the presence of κB motifs, which are known to be involved in regulation of immune response, in all three Rel gene promoters. GATA is another important regulator of immune response genes (30, 31). In a recent study, the existence and importance of a Rel-GATA module in the promoters of immune response genes, including AMP genes, was shown (28, 30). These GATA factors also impart tissue specificity and are known to modulate expression of AMP genes upon microbial infection (31). The presence of GATA motifs within 50 bp around the κB site, including their orientation with respect to the κB motif, was shown to be crucial for activation of AMP genes by Rel proteins upon immune challenge (28). The same Rel-GATA module was also found in the promoters of Dorsal target genes zen, rent, Ady, and fas3, which are expressed during embryonic stages, suggesting a common regulatory module in the Dorsal target genes (28). However, we did not find any Rel-GATA module either in the vicinity or far away from the κB motif in the three Rel gene promoters. Thus, absence of the Rel-GATA module is a major difference between regulation of Rel genes and Rel target genes. We also found that the three Rel genes lacked TATA elements in their promoters. On the contrary, Rel target immune response genes have TATA elements as their basal promoters. Furthermore, relish and DIF promoters have a single canonical κB motif in their promoters; however, the same is not true for the dl gene, which has multiple κB motifs (supplemental Information 2 and supplemental Fig. 1). In light of this observation, we were interested to know how Rel genes are regulated. Here, we investigated transcriptional regulation of dl expression during acute phase immune response, both in vitro and in vivo.
dl Gene Is Autoregulated
Dorsal, which is a maternally expressed gene product, plays an important role in dorso-ventral patterning of the early embryo and also regulates expression of the antibacterial gene drosomycin in the bacteria-challenged larvae of Drosophila. However, induction of drosomycin, upon microbial infection, is compromised in dl1 mutant flies indicating absence of functional Dorsal (10, 15, 18). dl1 mutant is a loss-of-function (amorphic) mutation and shows a dorsalized embryo phenotype that ranges from D0 (completely dorsalized) to D3 (weakly dorsalized) (19). In the D0 phenotype, the cuticles of embryos lack ventral denticle belts, Filzkörper, and consist only of a tube of dorsal epidermis.
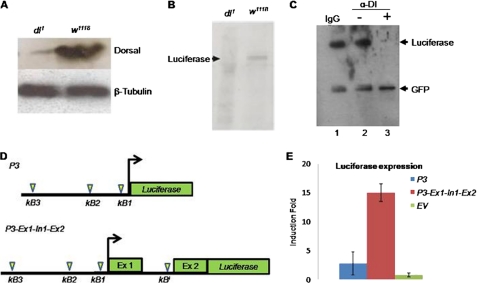
At the outset, we checked the status of Dorsal expression in dl1 flies. Interestingly, in comparison with w1118 flies, the level of Dorsal in extracts prepared from dl1 embryos was extremely low or absent (Fig. 1A). Absence of the Dorsal protein in dl1 mutant could be due to the following: (i) instability of dorsal transcript or (ii) lack of Dorsal expression. We tested these two possibilities, in vitro, by luciferase reporter assay. The luciferase reporter plasmid used in this study consists of the dl regulatory region until the second exon of dl. Coupled in vitro transcription and translation of this reporter led to synthesis of luciferase with w1118 embryonic extract but not with extracts from the mutant dl1 embryos (Fig. 1B). This result is consistent with the result shown in Fig. 1A where a negligible amount of Dorsal was present in the dl1 extract (Fig. 1, A and B) suggesting that dl promoter is not inducible in dl1 embryonic extract. This result also implies that absence of Dorsal in Fig. 1A and luciferase in Fig. 1B, both under the regulation of dl promoter, is due to lack of expression of respective genes. Thus, emphasizing that reason for lack of Dorsal in dl1 embryo is dl deregulation and not mRNA instability. Lack of Dorsal in the dl1 mutant can be explained if we assume that Dorsal regulates expression of its own gene, i.e. dl gene is autoregulated (Fig. 1, A and B).
FIGURE 1.
dl gene is autoregulated. A, absence of Dorsal in dl1 mutant flies compared with w1118, as seen in the Western blot, indicates deregulation of dl gene. Embryonic extracts from w1118 and dl1 mutant were transferred to a nylon membrane and probed with anti-Dorsal monoclonal antibody. Drosophila β-tubulin was used as loading control. B, to investigate regulation of dl promoter, a full-length regulatory region of dl was cloned upstream of luciferase reporter and subjected to coupled in vitro transcription and translation reaction. Western blot of the reaction product shows lack of luciferase synthesis in dl1 embryonic extract but not in w1118 extract when probed with anti-luciferase antibody. C, to confirm dl autoregulation, Dorsal protein from the wild type Drosophila was immunodepleted, and the Dorsal-depleted extract was used for coupled in vitro transcription and translation of the full-length reporter plasmid P3-Ex1-In1-Ex2::luciferase as shown in B. Immunodepletion of Dorsal (lane 3) from w1118 embryonic extract results in lack of luciferase synthesis. Luciferase synthesis was not affected with the embryonic extracts where either IgG was used (lane 1) or no antibody was added (lane 2). GFP synthesis was unaffected in all the three experiments. Control actin::GFP plasmid was simultaneously added along with P3-Ex1-In1-Ex2::luciferase plasmid in all three reactions. D, schematic showing location of putative κB motifs in different dl constructs. Plasmid P3 harbors three upstream κB motifs (κB1, κB2, and κB3), although the plasmid P3-Ex1-In1-Ex2 harbors additional κB motif (κBI) present in the first intron of the dl gene. E, luciferase induction upon PGN treatment was ∼15-fold with P3-Ex1-In1-Ex2 plasmid but only ∼3-fold with P3 plasmid suggesting that κBI motif has strong enhancer activity compared with the upstream three κB motifs.
If Dorsal regulates its own expression, then depleting wild type Dorsal from w1118 embryonic extract should also result in no synthesis of luciferase. To test this hypothesis, we performed an immunodepletion experiment where wild type Dorsal was depleted from the w1118 embryonic extracts using anti-Dorsal monoclonal antibody. The Dorsal-depleted w1118 embryonic extract failed to synthesize luciferase upon coupled in vitro transcription and translation from the P3-Ex1-In1-Ex2::luciferase reporter plasmid (Fig. 1C). Depletion of Dorsal, however, did not affect the coupled transcription and translation of GFP from an actin promoter-driven GFP construct, which was used as control plasmid, thus showing specificity of the immunodepletion reaction (Fig. 1C, lower band).
Identification of Autoregulatory Dorsal Enhancer Motif in dl Gene
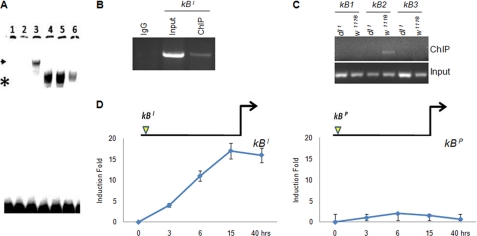
Dorsal, like other transcription factors of the Rel family, binds to a consensus DNA sequence GGGRNNYYCC called the κB motif. All the Dorsal target genes have this motif in their regulatory regions. If Dorsal regulates its own expression, then one such κB motif should also be present in the dl promoter. We surveyed 5 kb upstream of TSS and 2 kb downstream to identify putative κB motifs in dl. The search revealed three putative κB motifs upstream and one downstream of TSS in the first intron (supplemental Information 2 and supplemental Fig. 1). Because the dl gene has multiple κB sites, we set out to identify the functional Dorsal-binding motif(s) by luciferase reporter assay. We generated the following two plasmids: (i) a 1.25-kb-long reporter construct (P3) that harbors the three κB motifs present upstream of TSS, and (ii) P3-Ex1-In1-Ex2-luciferase plasmid, which also includes the intronic κB motif in addition to the three upstream κB motifs (Fig. 1D). Strong induction of luciferase was observed with the P3-Ex1-In1-Ex2 construct compared with the P3 plasmid upon immune challenge to S2 cells. Lack of luciferase induction from the P3 plasmid suggested that none of the three κB motifs upstream of TSS might cause auto-activation of dl. Strong luciferase induction from P3-Ex1-In1-Ex2 plasmid suggested that the functional Dorsal-binding motif was present downstream of TSS in the first intron (Fig. 1E). Next, the ability of Dorsal to interact physically with the intronic κB motif (GGGAATTCC), named κBI, was also checked by gel shift assay (Fig. 2A). Furthermore, supershift with anti-Dorsal monoclonal antibody confirmed that the DNA-protein complex retarded in other lanes indeed included Dorsal (Fig. 2A, lane 3). The results confirmed the physical interaction of Dorsal with the regulatory region in the dl itself upon immune challenge.
FIGURE 2.
Identification and characterization of functional κB motifs in the dl gene. A, dorsal-specific complex is retarded upon EMSA with κBI motif as probe (marked by asterisk, lanes 4 and 5), which was supershifted with the Dorsal antibody (marked by arrowhead, lane 3). Lane 1, free probe; lane 2, cold homologous competition; lane 3, supershift with Dorsal antibody; lane 4, nuclear extract isolated from S2 cells 15 h after PGN + LPS treatment; lane 5, embryonic extract from 4-h-old w1118 embryo; lane 6, nonspecific competition. B, ChIP performed with anti-Dorsal antibody resulted in enrichment of κBI motif. Chromatin for ChIP was precipitated from S2 cells 15 h post-LPS + PGN treatment. C, ChIP was also performed for the upstream Dorsal-binding motifs. Of the three κB motifs, only κB2 of the dl promoter was enriched. None of the κB motifs were enriched in the dl1 mutant. D, luciferase assay suggested that the κBI was inducible upon immune challenge but not the κBP. The schematics above the graphs show position and identity of κB motifs used in the dl promoter construct.
ChIP Experiment Identifies Two Dorsal-binding Sites in dl Gene
ChIP was performed to confirm in vivo interaction of Dorsal with its own gene in immune-challenged S2 cells. ChIP was performed on the nuclear extracts prepared from PGN + LPS-treated S2 cells 15 h post-infection (hpi). Enrichment of the intronic κB (κBI) motif upon ChIP confirmed in vivo interaction of Dorsal with the regulatory motif κBI (Fig. 2B). This is consistent with luciferase reporter assay and EMSA results (Figs. 1E and 2A). The three upstream κB motifs did not yield any PCR product when ChIP was performed with immune-challenged S2 cells 15 hpi. However, the second κB motif of the dl promoter, κB2, was enriched by PCR when ChIP was performed with the S2 cells 40 hpi (Fig. 2C). Because this was the only promoter κB motif that was precipitated upon ChIP, we named it κBP. These results suggested the presence of two functional Dorsal-binding κB motifs in the dl gene, one in the promoter, the κBP, and another one in the 1st intron, the κBI (Fig. 2C). Lack of enrichment of the other two promoter κB motifs suggested that they were probably not functional (Fig. 2C).
Because ChIP experiments identified two Dorsal-binding motifs in the dl gene, we next tested how these two motifs regulated dl expression. Although the κBI motif GGGAATTCC is a typical κB motif, the κBP motif AGAAAAACA is an atypical κB motif as it is significantly different from the consensus κB motif sequence GGGRNNYYCC. As the two motifs were enriched at different time points post-infection upon ChIP, we performed a time course of luciferase induction to elucidate the role of these two motifs in dl regulation. We checked the transcriptional activity of κBP and κBI individually in the same P3 promoter construct. For comparison, the κBP motif in the P3 plasmid was replaced with the κBI motif so that the two plasmids differed only in their Dorsal-binding sequences. Strong luciferase induction was observed with the P3-κBI plasmid but not with the P3-κBP plasmid under the same experimental conditions (Fig. 2D). In fact, luciferase induction with the P3-κBI promoter was comparable with that of the full-length construct P3-Ex1-In1-Ex2, suggesting the contribution of the κBI motif in Dorsal activation (Figs. 1E and 2D). If the κBP motif had no role in activation of dl, then what was the significance of its interaction with the Dorsal?
Dorsal Binding to κBP and κBI Motifs Is Dynamic and Temporally Regulated
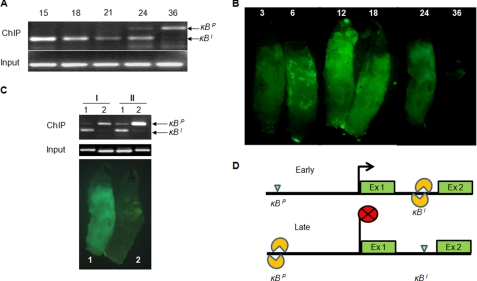
To understand how the two Dorsal-binding motifs together regulate dl expression, we performed ChIP at different time points after immune activation in S2 cells. We found that immediately after bacterial challenge Dorsal was bound only to the κBI motif, which explains Dorsal activation upon immune challenge (Fig. 3A). However, around 36 hpi, when the immune response had reached terminal stage, Dorsal binding was seen at the κBP motif but not at the κBI motif (Fig. 3A). This almost exclusive binding of Dorsal to two different κB motifs in the dl gene suggested that these two autoregulatory Dorsal-binding motifs independently regulate dl expression during the course of immune response. The critical feature of dl regulation by the two autoregulatory κB motifs is their temporal delineation in binding to Dorsal during acute phase response.
FIGURE 3.
Dorsal is repositioned to the κBP motif from the κBI motif during the course of immune response. A, ChIP suggests that Dorsal binds to the κBI motif at the onset of immune response and remains bound until ∼30 hpi in immune activated S2 cells. During this period, the κBP motif does not appear to be occupied by Dorsal. However, Dorsal binding to the κBP and not to the κBI motif is seen around 36 hpi. At 24 hpi, bands corresponding to κBP (196 bp) as well as κBI (177 bp) motifs are amplified indicating a transition stage when Dorsal is bound to both the motifs. Single band in the input panel corresponds to the κBI locus. B, time course of drosomycin expression in Drs::GFP larvae upon bacterial infection shows AMP expression profile during a typical acute phase response. C, repositioning of Dorsal from its binding site in the intron to the other binding site in the dl promoter was seen in Drosophila larvae too, as evidenced by ChIP assay. I and II represent two independent ChIP experiments performed in bacteria-challenged Drosophila larvae. Lane 1, chromatin precipitated 18 hpi when GFP expression was high; lane 2, chromatin isolated 48 hpi when GFP expression had died down signifying termination of immune response. D, schematic shows repositioning of Dorsal from the κBI to the κBP motif during immune response. Binding of Dorsal to the κBI is an early event (Early) that leads to induction of the dl. At a later stage of immune response (Late), Dorsal is removed from the κBI motif and repositioned at the κBP.
We also validated these results in vivo by performing ChIP on nuclear extracts isolated from fat body of bacteria-challenged Drosophila larvae at 15 and 48 hpi (Fig. 3, B and C). In challenged larvae, Dorsal occupied the κBI motif at 15 hpi, and by 48 hpi, when the immune response had almost died down, Dorsal was found to be associated with the κBP motif (Fig. 3, B and C). These results also suggest that binding of Dorsal to the two autoregulatory kB motifs in the dl gene is a dynamic process as Dorsal initially binds to the κBI motif and later gets repositioned at the κBP motif (Fig. 3D).
Distinct Orientation of κBP and κBI Motifs Is Crucial for Dorsal Regulation
Although the κBP motif does not appear to control dl induction in vitro (Fig. 2D), its interaction with Dorsal in vivo only in the dying stages of acute phase response indicated its probable role in dl regulation. However, luciferase reporter experiment with individual κB motifs did not reveal any conclusive role of κBP in transcriptional regulation of dl (Fig. 2D). Hence, we set out to investigate the functional significance of Dorsal binding to the κBP motif (Fig. 2, C and D). Because Dorsal binding the two motifs is temporally exclusive, we decided to study its significance by sequentially replacing and swapping the two motifs in the full-length construct P3-Ex1-In1-Ex2.
P3-Ex1-In1-Ex2 plasmid was transfected into S2 cells, followed by immune induction by LPS + PGN treatment. A time course of luciferase expression revealed a response similar to acute phase response, where initially there was a continuous increase in luciferase expression in the first 18 h, and by 40 h luciferase expression reached control levels (Fig. 4A). For the next experiment, we generated a κBP-κBP construct, by replacing the κBI motif with the κBP, and found that this construct hardly showed any luciferase induction (Fig. 4B). In another experiment, the κBP motif in the control plasmid was replaced with the κBI; the resultant luciferase-reporter construct, harboring two κBI motifs (κBI-κBI), caused strong luciferase induction and remained constitutively active (Fig. 4C). These results demonstrated the enhancer property of κBI motif in the regulation of dl. Next, we swapped the two κB motifs; the resultant new plasmid had κBI motif in the promoter and κBP motif in the first intron (κBI-κBP plasmid). We found that κBI-κBP construct was constitutively active, and there was no decrease in luciferase synthesis by 40 hpi as observed with the control κBP-κBI construct (Fig. 4, A and D).
FIGURE 4.
Dorsal binding to the κBP and κBI motifs is distinctly regulated. A, luciferase expression construct mimicking the κBP-κBI organization in the dl gene shows rapid induction of luciferase expression followed by its repression by 40 hpi. B, replacing the κBI with the κBP resulted in κBP-κBP organization that was hardly inducible. C, reporter construct where the κBP was replaced with the κBI and thus had two κBI motifs (κBI-κBI) and remained constitutively active. D, when the κBP and κBI motifs in the reporter κBP-κBI plasmid were swapped, the resultant κBI-κBP plasmid remained constitutively active. These results suggest that κBP-κBI organization (A) controls time-dependent activation followed by repression of the dl gene during acute phase response. The order and type of κB motifs in the promoter constructs are shown above the respective graphs.
These results highlight distinct roles played by the two autoregulatory κB motifs in dl regulation. Although the κBI motif is responsible for initial activation of dl, the κBP motif is probably required for dl repression (Fig. 4, A–D). The motif-swapping experiment clearly suggests that the κBP motif lacks enhancer activity, which also explains the lack of luciferase induction as seen in Figs. 1E, 2D, and 4B.
The motif-swapping experiment further revealed that it is not only the presence of the two κB motifs but also their arrangement in the dl gene that is important for dl autoregulation during acute phase response. In other words, the κBP-κBI arrangement (where κBP is present upstream of TSS and κBI is present downstream) is required for initial activation and late repression of Dorsal in immune-challenged Drosophila. However, it was not clear why in the initial phase of immune challenge Dorsal bound only to the κBI and not to the κBP motif considering that an abundant amount of Dorsal protein was available. We speculate that the mere presence of Dorsal protein in abundance is not sufficient for its binding to the κBP motif, and possibly time-dependent recruitment of Dorsal to κBP requires participation of other proteins and/or chromatin changes.
Dorsal Recruitment to κBP Motif Requires AP1 as Co-regulator
The motif swap experiment suggested the requirement of κBP-κBI arrangement for control of the expression dynamics of dl, where κBP motif probably brings about the time-dependent repression of dl, although initial induction is controlled by the κBI motif. This raised the intriguing question of how Dorsal recruitment to κBP motif is temporally regulated and also how it brings about repression of dl transcription. One possibility worth investigating was the role of one or more co-regulators, if any, in the positioning of Dorsal at the κBP motif, as it is known that Dorsal interacts with co-regulators for effecting transcriptional repression of target genes (20, 21, 26).
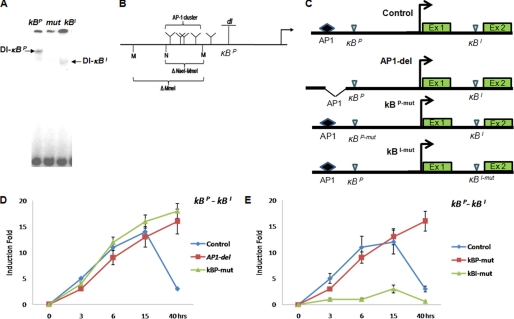
To find out if additional factors are involved in the recruitment of Dorsal to κBP or κBI motifs, we performed EMSA using κBP and κBI motifs as probes, which revealed retardation of complexes of different sizes (Fig. 5A). The protein complex recruited at the κBP motif was bigger in size compared with the complex retarded with the κBI motif, suggesting that one or more additional proteins were present in the Dorsal complex retarded with the κBP motif (Fig. 5A). Next, we investigated the following: (i) the identity of the co-regulatory protein(s) involved in Dorsal binding to the κBP motif, and (ii) whether the difference in protein-protein interaction can explain the difference in spatio-temporal regulation of dl by κBP and κBI motifs as seen in Figs. 3, A–C, and 4, A–D.
FIGURE 5.
Dorsal binding to the κBP motif is co-regulator-dependent. A, EMSA was performed with the κBI and κBP motifs as probes. κBP (AGAAAAACA) retards a larger complex (1st lane) compared with the κBI (GGGAATTCC) (lane 3). 2nd lane (mut) shows competition with mutant oligonucleotide (mut-AGAATAATCC) where no Dorsal complex is retarded. B, diagrammatic representation of clustered AP1-binding sites in the dl promoter upstream of κBP. Restriction enzymes used to delete the AP1 cluster are also shown. C, schematic representation of different reporter plasmids used for luciferase assay in experiments as explained in D and E. D, deletion of AP1 cluster in the dl promoter leads to constitutive expression of luciferase compared with control full-length reporter plasmid indicating the role of AP1 in dl repression. E, mutation in the κBP motif also leads to constitutive expression of luciferase suggesting that Dorsal binding to the κBP is also critical for dl repression. However, when the κBI was mutated, no induction of luciferase was seen suggesting that the κBI was required only for transcriptional activation of dl. The κBP-κBI promoter construct was used as the control plasmid.
Transcriptional regulation of Dorsal target genes is sometimes modulated by other proteins; Groucho acts as co-repressor for Dorsal target genes along dorso-ventral axis (19), whereas GATA factors co-regulate AMP gene expression (30, 31). Because GATA-binding motifs are not present in the dl promoter (supplemental Information 2), and Groucho is not known to have a role in the immune response, we excluded these two proteins as candidates for modulation of dl expression. One potential candidate as co-regulator of Dorsal binding to the κBP motif that we came across after bioinformatic analysis of the dl promoter was AP1. A cluster of multiple AP1-binding sites is present just upstream of the κBP motif in the dl promoter (Fig. 5B and supplemental Information 2). To test if the AP1 and Dorsal-binding motifs interacted in cis, we generated different reporter plasmids with mutation in the two Dorsal-binding motifs and deletion of AP1-binding cluster (Fig. 5C). Because multiple AP1-binding motifs are clustered in the dl promoter, for functional analysis we deleted the AP1 cluster by restriction digestion in the full-length P3-Ex1-In1-Ex2 plasmid. Thus, generated AP1-del luciferase reporter plasmid was constitutively active, whereas luciferase expression from the control P3-Ex1-In1-Ex2 plasmid underwent time-dependent repression (Fig. 5D). To confirm the probable cross-talk between AP1 and Dorsal, we mutated the κBP motif in the P3-Ex1-In1-Ex2 plasmid to generate κBP-mut plasmid. Luciferase expression from the κBP-mut construct was also constitutive similar to that of AP1-del construct (Fig. 5D). Constitutive expression of luciferase upon deletion of the AP1 cluster or mutation of the κBP motif suggests that both κBP and AP1-binding elements may be required for time-dependent dl repression during acute phase response (Fig. 5D). On the other hand, when the κBI was mutated (κBI-mut plasmid), no significant luciferase induction was observed at any time point, suggesting that the κBI motif is required for the dl activation (Fig. 5E). These results are consistent with motif swap experiments where replacing the κBP motif with the κBI also resulted in constitutive expression of luciferase (Fig. 4D). Thus, our data confirm that the κBI is an enhancer motif and is required for the initial induction of dl (Figs. 5D and 3, A–D). On the other hand, the κBP motif, occupied by Dorsal at the terminal stage of the acute phase response (Fig. 4, A–D), controls dl repression (Fig. 5, C and D). These data emphasize the presence of two temporally delineated Dorsal modules involved in dl autoregulation.
Assembly of Dorsal-AP1 Complex at κBP Motif Leads to dl Repression
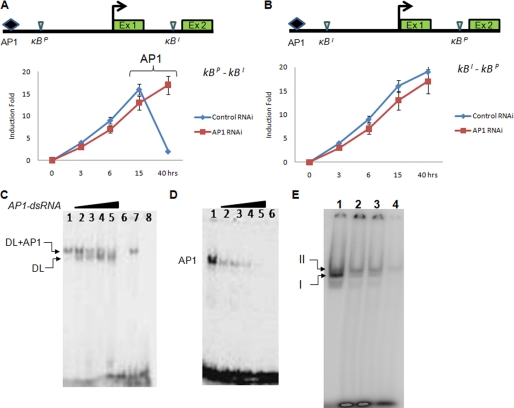
To further dissect the role of AP1-Dorsal interaction in dl regulation, we took to the RNAi approach. The κBP-κBI reporter construct was co-transfected with AP1-dsRNA construct into S2 cells, and luciferase expression was quantitated at different time points after LPS + PGN treatment. We found that depletion of AP1 by RNAi resulted in loss of repression of dl with respect to control RNAi (Fig. 6A). However, when κBI-κBP plasmid was used for reporter assay in the presence of AP1-dsRNA, there was no repression of luciferase activity (Fig. 6B). These results further suggest the following: (i) κBI is an enhancer motif, and (ii) κBP functions as a repressor motif but only in the presence of AP1.
FIGURE 6.
Recruitment of AP1-Dorsal complex on the κBP motif controls dl repression. A, depletion of AP1 by RNAi abolishes dl repression with κBP-κBI construct but does not affect its activation. Effect of depletion of AP1 on dl repression is seen only in the later phase of immune response, although AP1-dsRNA is present throughout the experiment. B, no effect of AP1 depletion on dl repression was seen with κBI-κBP plasmid (where the two motifs have been swapped) suggesting specific requirement of the κBP in the vicinity of the AP1-binding motif in the dl promoter. C, depletion of AP1 by RNAi resolves the larger complex of Dorsal (DL) and AP1 (lane 1) into smaller one of Dorsal only (lanes 2–5), suggesting assembly of both Dorsal and AP1 proteins as a complex on the κBP motif. Lane 1, mock transfection; lanes 2–5, increasing amount of AP1-dsRNA; lane 6, cold competition; lane 7, nontarget RNAi; lane 8, mutant κBP oligonucleotide. D, specific depletion of AP1 by RNAi was confirmed by EMSA. Nuclear extracts used in lanes 1–6 are the same as in C. E, dorsal interaction with AP1 and their recruitment on the κBP motif is time-dependent as seen in EMSA. I and II denote two different sized complexes (complex I = Dorsal alone and complex II = Dorsal-AP1). Nuclear extracts prepared at different time points post-PGN + LPS treatment of S2 cells were incubated with radiolabeled κBP and κBI probes together and resolved by EMSA. Numbers above each lane indicate nuclear extracts isolated at four different time points from immune-challenged S2 cells. Lanes 1, 15 hpi; lane 2, 30 hpi; lane 3, 36 hpi; lane 4, 40 hpi.
The RNAi data indicated that Dorsal-AP1 interaction may be responsible for repression of dl at the end of acute phase response. EMSA results have clearly shown retardation of a larger Dorsal-DNA complex with the κBP motif with respect to κBI motif (Fig. 5A). We speculated that the larger Dorsal-κBP complex probably also contained AP1 proteins apart from Dorsal. To test such a possibility, we performed EMSA using κBP as probe with whole and AP1-depleted nuclear extracts. A clear shift in gel retardation was seen between control (Fig. 6C, lane 1) and AP1-depleted nuclear extracts (Fig. 6C, lanes 2–5). Depletion of AP1 resolves the control band (Fig. 6C, lane 1) into two (lanes 2–5) suggesting that AP1 interacts with the Dorsal-κBP complex (Fig. 6, A and B). Depletion of AP1 by RNAi was also verified by EMSA, which showed progressive loss of AP1-specific band (Fig. 6D).
ChIP results and RNAi data together suggest that AP1 action is seen after 30 hpi (Figs. 6A, 2C, and 3, A and C). This may explain the recruitment of Dorsal to the κBP motif late in immune response but not in early stages. To follow the time-dependent assembly of the Dorsal-AP1 complex on the κBP motif, we performed competitive EMSA where the κBP and κBI motif probes were added together in equal concentration for the binding reaction. The mixture of the two probes was incubated with the nuclear extracts isolated at different time points from PGN + LPS-treated S2 cells so that the two complexes of Dorsal with κBP and κBI could be resolved in the same lane. Fig. 6E, lane 1 (where nuclear extract isolated at 15 hpi was used), shows a strong retardation of a smaller complex of Dorsal bound to κBI. However, with nuclear extracts isolated 24, 30, and 40 hpi (Fig. 6E, lanes 2–4), retardation of a higher size complex corresponding to Dorsal bound to κBP is also seen. Results of competitive EMSA further emphasize that interaction of Dorsal with the two κB motifs is dynamic and temporally regulated.
Overall, our data suggest that the κBI motif controls the induction of dl seen in immune-challenged Drosophila, whereas the κBP motif brings about the repression of dl observed at the end of the acute phase response. The fact that Dorsal binding at these two motifs is time-dependent is suggestive of dynamic interaction of Dorsal with the two κB motifs. During the late phase of immune response, Dorsal is removed from the κBI motif (Figs. 4, A–D, 5D, and 6D) and is repositioned at the κBP motif. We have shown that the κBP motif on its own is not a repressor motif (Figs. 2D and 3B), but it is the binding of Dorsal-AP1 complex to this motif that leads to repression of the downstream gene (Fig. 6, A–E). From the promoter swap experiments, we have shown that dl expression requires both κB motifs in a κBP-κBI orientation (Fig. 3, A–D). The requirements of κBP-κBI orientation and dynamic relocation of Dorsal from one motif to another imply a possible role of chromatin dynamics in the regulation of dl.
DISCUSSION
Conventional knowledge of autoregulation suggests that the gene product causes either auto-activation or auto-repression of its own gene. Here, while studying dl regulation, we have deciphered a novel mechanism of autoregulation where the gene product controls activation as well as repression of its own gene. In the study reported here, the following points have emerged. (i) dl autoregulation is mediated by two distinct κB motifs in the dl gene as follows: an enhancer motif present in the first intron, and a repressor motif in the promoter. (ii) The two motifs act independently to control overall regulation (initial activation and late repression) of dl during the course of acute phase response. (iii) dl activation appears to be independent of co-factor requirement; however, dl repression requires a co-repressor, identified here as AP1. (iv) Both AP1 and Dorsal proteins are required for the repression of dl at the terminal phase of acute phase response; in the absence of AP1 or its binding motif, Dorsal bound to the κBP motif did not function as either activator or repressor. (v) Dorsal repositioning at these two motifs is temporally regulated and probably involves chromatin alteration.
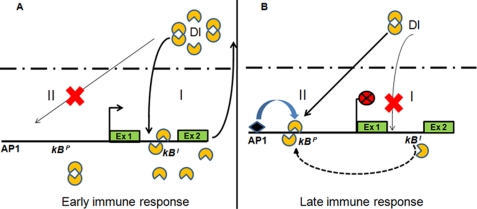
Dorsal is a transcription factor and orchestrates many events, including embryonic development and immune response in Drosophila (32–35). We have shown that Dorsal acts during immune response via two subcircuits that dynamically interact with each other. The first subcircuit activates the dl gene with binding of Dorsal to the enhancer motif,κBI. This subcircuit gets established just after immune challenge and ensures supply of Dorsal during acute phase response. This positive feedback leads to accumulation of Dorsal protein during the post-infection period (Fig. 7A). But once the bacterial infection is cleared, the cell needs to come back to normal state, which requires shutting off the AMP genes, for which Dorsal availability has to be retrenched. At this point, as we have shown in this study, the second subcircuit involving AP1-Dorsal-κBP gets activated and causes removal of Dorsal from the κBI motif and its recruitment to the κBP motif.
FIGURE 7.
Model of Dorsal autoregulation. Infection by Gram-positive bacteria or fungi activates the Toll-Dorsal circuit that leads to nuclear localization of transcription factor Dorsal. A, once inside the nucleus, Dorsal binds to the enhancer κBI motif of the dl gene leading to induction of Dorsal synthesis thus establishing a positive feedback loop that constitutes subcircuit I (thick arrow). B, termination of immune response is marked by repositioning of Dorsal at repressor κBP motif (dashed arrow). Binding of Dorsal to the κBP motif is facilitated by its interaction with co-regulator AP1. Assembly of Dorsal-AP1 complex at κBP shuts dl expression thus marking the termination of acute phase response. This constitutes subcircuit II of the autoregulatory loop. It is to be noted that dl activation is independent of any co-regulator. In contrast, dl repression is co-regulator-dependent (AP1 shown as diamond). Thus, both activation and repression of dl are autoregulated by Dorsal in a modular fashion and are temporally regulated.
Our data indicate that Dorsal bound to the κBI motif activates its own expression possibly by directly interacting with transcriptional machinery (Fig. 7). However, in later stage of acute phase response, binding of AP1 to κBP motif might cause localized chromatin changes facilitating its interaction with Dorsal but at the same time preventing interaction of Dorsal with transcriptional machinery to turn off dl transcription.
We believe that this repositioning of Dorsal is facilitated by localized chromatin changes in the dl gene region that lead to opening of the chromatin near the κBP motif in the promoter and contraction of chromatin near the κBI motif in the first intron. As a result, the κBI motif would become inaccessible to Dorsal. Simultaneous opening of chromatin in the promoter region may allow Dorsal to bind the κBP motif. Our results that Dorsal auto-activation is independent of co-regulator and auto-repression is dependent on its interaction with a co-repressor, AP1, support the previous findings that the Dorsal, by default, is an activator and to function as repressor it needs to interact with a co-repressor (26). To account for overall Dorsal regulation presented here, a mechanism explaining Dorsal acting as an auto-activator versus auto-repressor is warranted. On the basis of the data presented in this study, we propose that the distinction between auto-activator and auto-repressor Dorsal lies in its ability to interact with co-regulators, which also probably involves chromatin changes.
Any gene regulation mechanism that employs general factors for regulation must involve specific transcriptional regulators for spatio-temporal specificity. In yeast, for example, tissue-specific repression of α2 promoter is regulated by its own gene in the presence of a general factor SIN4 (36). Being a chromatin modifier, SIN4 acts as a general factor, but spatial specificity is imparted by the tissue-specific transcription factor α2. Furthermore, GATA factors have been shown to impart tissue specificity in the expression of AMP genes (30, 31). Our result that AP1 not only acts as co-repressor of Dorsal but also imparts temporal specificity in binding of Dorsal to the κBP is consistent with these findings that gene expression is regulated by a general factor in combination with a specific factor. Hence, we propose that Rel proteins act as general transcription factors during immune response, and spatio-temporal specificity of Rel-mediated gene expression is imparted by other regulators like AP1 and GATA.
AP1-binding region in the dl promoter is A-T-rich. Similar A-T-rich sequence is also present upstream of the Dorsal-binding site in the zen promoter (37). Deletion or point mutation in the A-rich sequence of the zen promoter turns Dorsal into an activator. Although the nature of the putative co-repressor has remained uncharacterized, its physical interaction with Dorsal was established (37). Together with the findings of Kirov et al. (37), the results presented here highlight the role of cis-motifs proximal to Dorsal-binding sites in co-regulation of Dorsal.
Our results demonstrate that the κBI motif is a general enhancer motif. However, dl autorepression requires not only binding of Dorsal to the κBP but also its interaction with AP1 (Fig. 6). The motif-swapping experiments demonstrate that AP1 interaction was specific for the κBP motif (Figs. 4–6). It raises the following question: how does only the κBP motif facilitate cross-talk between Dorsal and AP1 but not the κBI motif? It is pertinent to note that the repressor motifs κBP of dl (AGAAAAACA) and zen promoter (GGAAAATCC) have an A-rich core (37). It is known that a continuous stretch of four or more “A” nucleotides induces bending in the DNA (38). Furthermore, biophysical analyses of different κB motifs suggest that A-tract imparts a flexible conformation that may favor Dorsal-co-regulator interaction compared with typical κB motifs.4 This may explain why the AP1-κBP motif in dl promoter interacts in cis but not when the κBI motif is placed next to AP1 motif in the same dl promoter (Figs. 4–6).
Another example of tissue-specific regulation by an atypical A-rich kB motif comes from the regulation of iNOS gene by NFκB protein (39). Human iNOS gene has multiple κB motifs, and the one present 6.4 kb upstream has an A-rich core (GGAAAAACC), similar to the κBP motif. The GGAAAAACC motif of iNOS was functional in A549 cells but not in AKN-1 cells unlike the other four κB motifs that were functional in both the cell types (39). The tissue-specific activation/silencing of GGAAAAACC motif of iNOS may be due to interaction of NFκB with a co-regulator that may be present in one cell type but not in the other. Taken together, these results prompt us to hypothesize that A-rich κB motifs might be more amenable to facilitating Rel interactions with co-regulators. In conclusion, our study demonstrates that Dorsal autoregulation constitutes a regulatory feedback loop through two subcircuits during immune response. Currently, we are testing this model of dl regulation in early embryonic development. However, it remains to be investigated whether the kinetics of activation of the two subcircuits also depends on the amount of Dorsal. Importantly, we have shown context-dependent repositioning of one regulator to two different cis-elements in the same gene leading to different phenotypes. Our study suggests that autoregulation can be a dynamic process that allows the regulator to interact with co-regulator(s) as well as different cis-elements, separated in space and time, leading to distinct phenotypes.
Supplementary Material
Acknowledgments
We thank Bruno Lemaitre (CNRS, France) for providing the Drs::GFP reporter line. We also thank L. S. Shashidhara, Centre for Cellular and Molecular Biology, India, for providing Drosophila stocks.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Information 1 and 2 and Fig. 1.
N. Mrinal, A. Tomar, and J. Nagaraju, submitted for publication.
- AMP
- anti-microbial protein/peptide
- TSS
- transcription start site
- LPS
- lipopolysaccharide
- PGN
- peptidoglycan
- hpi
- hours post-infection
- ChIP
- chromatin immunoprecipitation
- EMSA
- electrophoretic mobility shift assay
- RNAi
- RNA interference
- PBS
- phosphate-buffered saline
- GFP
- green fluorescent protein
- dsRNA
- double strand RNA.
REFERENCES
- 1.Tzou P., De Gregorio E., Lemaitre B. (2002) Curr. Opin. Microbiol. 5, 102–110 [DOI] [PubMed] [Google Scholar]
- 2.Brennan C. A., Anderson K. V. (2004) Annu. Rev. Immunol. 22, 457–483 [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann J. A. (2003) Nature 426, 33–38 [DOI] [PubMed] [Google Scholar]
- 4.Hultmark D. (2003) Curr. Opin. Immunol. 15, 12–19 [DOI] [PubMed] [Google Scholar]
- 5.Dushay M. S., Eldon E. D. (1998) Am. J. Hum. Genet. 62, 10–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belvin M. P., Anderson K. V. (1996) Annu. Rev. Cell Dev. Biol. 12, 393–416 [DOI] [PubMed] [Google Scholar]
- 7.Lemaitre B. (2004) Nat. Rev. Immunol. 4, 521–527 [DOI] [PubMed] [Google Scholar]
- 8.Lemaitre B., Hoffmann J. (2007) Annu. Rev. Immunol. 25, 697–743 [DOI] [PubMed] [Google Scholar]
- 9.Uvell H., Engström Y. A. (2007) Trends Genet. 23, 342–349 [DOI] [PubMed] [Google Scholar]
- 10.Engström Y. (1999) Dev. Comp. Immunol. 23, 345–358 [DOI] [PubMed] [Google Scholar]
- 11.Lemaitre B., Kromer-Metzger E., Michaut L., Nicolas E., Meister M., Georgel P., Reichhart J. M., Hoffmann J. A. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 9465–9469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levashina E. A., Ohresser S., Lemaitre B., Imler J. L. (1998) J. Mol. Biol. 278, 515–527 [DOI] [PubMed] [Google Scholar]
- 13.Engström Y., Kadalayil L., Sun S. C., Samakovlis C., Hultmark D., Faye I. (1993) J. Mol. Biol. 232, 327–333 [DOI] [PubMed] [Google Scholar]
- 14.Rushlow C., Warrior R. (1992) BioEssays 14, 89–95 [DOI] [PubMed] [Google Scholar]
- 15.Manfruelli P., Reichhart J. M., Steward R., Hoffmann J. A., Lemaitre B. (1999) EMBO J. 18, 3380–3391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng X., Khanuja B. S., Ip Y. T. (1999) Genes Dev. 13, 792–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dushay M. S., Asling B., Hultmark D. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 10343–10347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemaitre B., Nicolas E., Michaut L., Reichhart J. M., Hoffmann J. A. (1996) Cell 86, 973–983 [DOI] [PubMed] [Google Scholar]
- 19.Govind S., Steward R. (1991) Trends Genet. 7, 119–125 [DOI] [PubMed] [Google Scholar]
- 20.Ratnaparkhi G. S., Jia S., Courey A. J. (2006) Development 133, 4409–4414 [DOI] [PubMed] [Google Scholar]
- 21.Pan D., Courey A. J. (1992) EMBO J. 11, 1837–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ip Y. T., Park R. E., Kosman D., Yazdanbakhsh K., Levine M. (1992) Genes Dev. 6, 1518–1530 [DOI] [PubMed] [Google Scholar]
- 23.Ip Y. T., Kraut R., Levine M., Rushlow C. A. (1991) Cell 64, 439–446 [DOI] [PubMed] [Google Scholar]
- 24.Jiang J., Kosman D., Ip Y. T., Levine M. (1991) Genes Dev. 5, 1881–1891 [DOI] [PubMed] [Google Scholar]
- 25.Jiang J., Rushlow C. A., Zhou Q., Small S., Levine M. (1992) EMBO J. 11, 3147–3154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang J., Cai H., Zhou Q., Levine M. (1993) EMBO J. 12, 3201–3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biemar F., Nix D. A., Piel J., Peterson B., Ronshaugen M., Sementchenko V., Bell I., Manak J. R., Levine M. S. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 12763–12768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Senger K., Armstrong G. W., Rowell W. J., Kwan J. M., Markstein M., Levine M. (2004) Mol. Cell 13, 19–32 [DOI] [PubMed] [Google Scholar]
- 29.Mrinal N., Nagaraju J. (2008) J. Biol. Chem. 283, 23376–23387 [DOI] [PubMed] [Google Scholar]
- 30.Tingvall T. O., Roos E., Engström Y. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 3884–3888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Senger K., Harris K., Levine M. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 15957–159562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong J. W., Hendrix D. A., Papatsenko D., Levine M. S. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 20072–20076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minakhina S., Steward R. (2006) Oncogene 25, 6749–6757 [DOI] [PubMed] [Google Scholar]
- 34.Moussian B., Roth S. (2005) Curr. Biol. 15, R887–899 [DOI] [PubMed] [Google Scholar]
- 35.Stathopoulos A., Levine M. (2002) Dev. Biol. 246, 57–67 [DOI] [PubMed] [Google Scholar]
- 36.Chen S., West R. W., Jr., Johnson S. L., Gans H., Kruger B., Ma J. (1993) Mol. Cell. Biol. 13, 831–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirov N., Zhelnin L., Shah J., Rushlow C. (1993) EMBO J. 12, 3193–3199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hizver J., Rozenberg H., Frolow F., Rabinovich D., Shakked Z. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 8490–8495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor B. S., de Vera M. E., Ganster R. W., Wang Q., Shapiro R. A., Morris S. M., Jr., Billiar T. R., Geller D. A. (1998) J. Biol. Chem. 273, 15148–15156 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.