Abstract
The T-cell antigen receptor (TCR) α-chain (TCRα) is a type I integral membrane protein that becomes ubiquitinated and targeted to the endoplasmic reticulum (ER)-associated degradation (ERAD) pathway when it fails to assemble into the heteromeric TCR complex. Remarkably, TCRα has a cytosolic tail of only five amino acid residues (i.e. RLWSS), none of which is the conventional ubiquitin acceptor, lysine. Herein we report that substitution of two conserved serine residues in the cytosolic tail of TCRα to alanine decreased ubiquitination, whereas placement of additional serine residues enhanced it. Moreover, replacement of the cytosolic serine residues by other ubiquitinatable residues (i.e. cysteine, threonine, or lysine) allowed ubiquitination to take place. Serine-dependent ubiquitination perfectly correlated with targeting of TCRα for ERAD. We also found that this ubiquitination was mediated by the ER-localized ubiquitin ligase, HRD1. These findings indicate that serine-dependent, HRD1-mediated ubiquitination targets TCRα to the ERAD pathway.
Keywords: E3 Ubiquitin Ligase, Membrane Proteins, Protein Degradation, T-cell Receptor, Ubiquitination, ER-associated Degradation, HRD1 E3 Ligase, TCR, Non-lysine Ubiquitination
Introduction
Newly synthesized proteins that are translocated into the lumen of the endoplasmic reticulum (ER)2 or integrated into the ER membrane undergo a series of post-translational events, including covalent modifications, folding of their polypeptide chains and assembly into oligomeric complexes, prior to their export from the ER to the Golgi complex. Failure of any of these processes most often results in retention of the proteins in the ER, followed by their targeting to the ER-associated degradation (ERAD) pathway (1, 2). ERAD starts with the recognition of abnormal features of the proteins, which triggers their covalent modification with ubiquitin (Ub) and retrotranslocation through or dislocation from the ER membrane into the cytosol (3–5). The ubiquitinated proteins are subsequently degraded by the 26 S proteasome.
Protein ubiquitination is a widespread modification that regulates many cellular processes in addition to ERAD. The general process of ubiquitination involves a cascade of three types of enzyme (6). First, an ubiquitin-activating enzyme (E1) activates Ub by forming a thioester bond between an active site cysteine of the E1 and a C-terminal carboxyl group of Ub. Next, Ub is transferred to an active site cysteine of an ubiquitin-conjugating enzyme (E2). In mammals, three E2s, Ube2j1, and Ube2j2 (yeast Ubc6p homologs), and Ube2g2 (yeast Ubc7p homolog) have been implicated in ERAD (7). Finally, the charged E2 binds to an Ub ligase (E3) that facilitates transfer of Ub to a substrate by formation of an isopeptide bond between primary amine groups of lysine residues or the N terminus of the substrate and the C-terminal carboxyl group of Ub. In mammals, at least five E3s that are localized to the ER membrane (i.e. HRD1, GP78, TRC8, RMA1, and TEB4) have been implicated in ERAD of different substrates (7, 8). Ub itself can be ubiquitinated on several of its lysine residues, leading to the formation of polyubiquitin chains attached to the substrate (9, 10).
Recent studies have shown that Ub can be conjugated to cysteine, serine, and threonine residues in addition to lysine residues or the N termini of proteins (11, 12). Conjugation to cysteine involves the formation of a thioester bond between the cysteine sulfhydryl and the C terminus of Ub (11, 13). In the case of serine and threonine, a hydroxyester bond is formed between the amino acid hydroxyl and the C terminus of Ub (12, 14). For example, an N-terminal fragment of the cytosolic/mitochondrial protein Bid undergoes ubiquitination on cysteine and serine residues, leading to its proteasomal cleavage and consequent liberation of the proapoptotic Bid C-terminal fragment (15). The peroxisomal import receptor, Pex5p, is also ubiquitinated at a cysteine residue, a modification that in this case is required for its recycling from the peroxisome lumen to the cytosol (16, 17). Furthermore, the MIR1 E3 encoded by Kaposi's sarcoma-associated herpesvirus induces ubiquitination of cysteine and lysine residues in the cytosolic tail of the MHC class I (MHC-I) heavy chain, triggering its down-regulation through endocytosis from the plasma membrane (11, 18). Finally, the mK3 E3 ligase of murine herpesvirus MHV-68 mediates ubiquitination on serine, threonine, and lysine residues within the cytosolic tail of MHC-I heavy chain, targeting the protein for ERAD (12, 14). Despite the growing number of examples of non-lysine ubiquitination, it remains to be determined whether this modification is sufficient for ERAD targeting and whether there are cellular E3s that can mediate it.
The α-chain of the T-cell antigen receptor complex (TCRα) is one of the earliest identified and most thoroughly characterized ERAD substrates. TCRα is one of eight transmembrane proteins that make up the TCR complex (αβγδϵ2ζ2). This complex is assembled in the ER and subsequently transported to the cell surface where it plays a role in adaptive immune responses (19–21). Failure to assemble a complete complex results in ER retention and differential ERAD of the unassembled subunits (22–24). TCRα is particularly susceptible to ERAD and undergoes rapid degradation (t½ ∼1 h) when expressed alone in non-T-cells (22, 25, 26). TCRα is a variable ∼38-kDa glycoprotein composed of an extracellular immunoglobulin-like domain, a single transmembrane span, and a cytosolic tail of only five amino acid residues (Fig. 1A). An unusual feature of the TCRα transmembrane domain is the presence of two basic residues, arginine and lysine, which are conserved in different mammalian species (Fig. 1A and supplemental Fig. S1). These residues are important for assembly with other subunits of the TCR and for targeting of the unassembled TCRα to the ERAD pathway (27–30). Like other ERAD substrates, TCRα is polyubiquitinated (31, 32), and its degradation requires a functional ubiquitination machinery (33–36). However, the short TCRα tail does not contain any lysine residues (Fig. 1A). Moreover, mutation of all lysine residues in the extracellular domain of TCRα to arginine does not affect the degradation of the protein (26, 32). This suggests that TCRα ubiquitination and degradation might be dependent on residues other than lysine.
FIGURE 1.
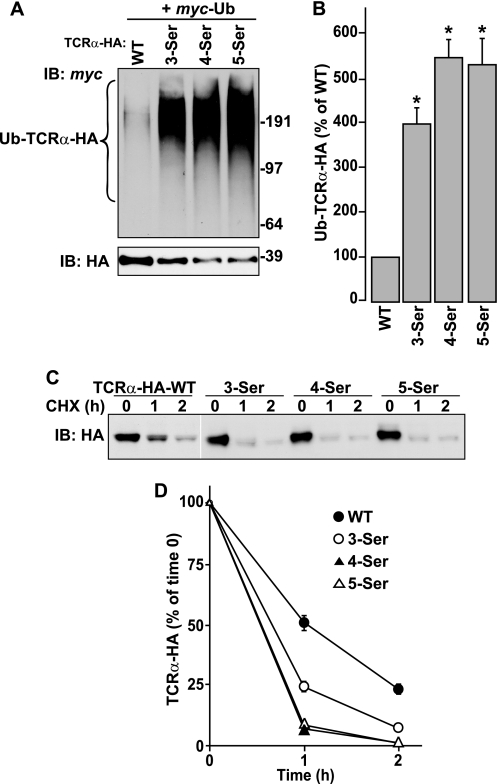
Mutation of two serine residues in the cytosolic tail of TCRα inhibits its ubiquitination and degradation. A, amino acid sequence of the transmembrane domain and cytosolic tail of WT TCRα and its mutants. A shortened HA tag (YPYDVPDYA) was appended to the C terminus of all constructs. Substituted residues are shown in red. B, HeLa cells transfected with plasmids encoding the indicated TCRα-HA constructs with or without Myc-Ub were left untreated or treated with 50 μm MG132 for 4 h at 37 °C. After lysis in RIPA buffer containing a protease inhibitors mixture, N-ethylmaleimide and iodoacetamide, TCRα-HA was immunoprecipitated with anti-HA monoclonal antibody. Immunoprecipitates were analyzed by SDS-PAGE and immunoblotting (IB) with anti-HA and anti-Myc polyclonal antibodies. Molecular masses of marker proteins (in kilodaltons) are indicated on the right. C, levels of ubiquitinated TCRα-HA were quantified by densitometry, normalized to levels of non-ubiquitinated TCRα-HA, and expressed as percent of WT TCRα-HA levels. Values represent the mean ± S.E. from three independent experiments. *, p < 0.05 versus WT. D, HeLa cells were transfected with plasmids encoding the indicated TCRα-HA constructs. After 24 h, the cells were left untreated or treated with 100 μg/ml CHX for the indicated time periods. Cell lysates were prepared in RIPA buffer, and analyzed by SDS-PAGE and immunoblotting with an anti-HA monoclonal antibody. E, TCRα-HA remaining at each time point was quantified and expressed as percent of TCRα present at time 0. Values represent the mean ± S.E. from three independent experiments.
We noticed that the cytosolic tail of TCRα has two serine residues that are conserved in different mammalian species (Fig. 1A and supplemental Fig. S1). Herein we report that these residues are critical for TCRα ubiquitination and degradation. In addition, we show that ubiquitination of TCRα is dependent on HRD1, an ER-localized E3. These observations constitute the first demonstration of the role of a cellular E3 in serine-dependent ubiquitination leading to ERAD.
EXPERIMENTAL PROCEDURES
Reagents and Constructs
Monoclonal and polyclonal antibodies to the HA epitope were from Covance (Berkeley, CA). Polyclonal antibody to the Myc epitope was from Cell Signaling (Danvers, MA). Polyclonal antibody to the FLAG epitope was from Sigma. Monoclonal antibody to calnexin was from BD Biosciences (San Jose, CA). MG132 was from Calbiochem (Gibbstown, NJ). Alexa Fluor 488 goat anti-rabbit IgG and Alexa Fluor 555 goat anti-mouse IgG antibodies were from Molecular Probes (Eugene, OR). ON-TARGETplus SMARTpool siRNA to human HRD1 (Cat. No. l-007090) or non-targeting siRNA control (Cat. No. d-001810) were from Dharmacon (Lafayette, CO).
To delete the last two residues (serine and leucine) from the HA tag sequence of the original construct, TCRα-HA was amplified by PCR using pcDNA3-TCRα-HA cDNA (a gift from Y. Ye, NIDDK, National Institutes of Health) as a template and subcloned into the KpnI and NotI sites of pcDNA 3.1(+). TCRα-HA mutants were made using the QuikChange site-directed mutagenesis kit (Stratagene, Cedar Creek, TX). Myc-tagged Ub (Myc-Ub) and FLAG-tagged Ub (FLAG-Ub) were subcloned into pCI-Neo and pcDNA 3.1(+), respectively. Wild-type and RING-domain mutant of Myc/His-tagged HRD1 were kind gifts from E. Wiertz (University Medical Center Utrecht, Utrecht, Netherlands). Rabbit polyclonal antibody to HRD1 was previously described (37, 38). All mutagenesis and subcloning products were verified by DNA sequencing.
Cell Culture and Transfection
HeLa cells (American Type Culture Collection, Manassas, VA) were grown in Dulbecco's modified Eagle's medium (DMEM; Mediatech, Manassas, VA) supplemented with 100 units/ml penicillin, 0.1 mg/ml streptomycin, 2 mm l-glutamine, and 10% (v/v) fetal bovine serum. Cells were transiently transfected with the indicated plasmids or siRNAs by using Lipofectamine 2000 or Oligofectamine, respectively (Invitrogen, Carlsbad, CA).
In Vivo Ubiquitination Assay
Cell extracts were prepared by incubating cells for 30 min at 4 °C in RIPA buffer supplemented with 5 mm N-ethylmaleimide (Calbiochem) and 10 mm α-iodoacetamide (Calbiochem) to minimize deubiquitination during extract preparation and with a protease inhibitors mixture. Cell lysates were spun for 15 min at 20,000 × g, and supernatants were recovered and mixed with an anti-HA monoclonal antibody bound to protein G-Sepharose (Amersham Biosciences). Immunoprecipitates were eluted by mixing with NuPAGE SDS sample buffer and heating at 85 °C for 10 min, and further analyzed by SDS-PAGE and immunoblotting.
Cycloheximide Chase
Cells transiently expressing the proteins of interest were left untreated or incubated in DMEM containing 100 μg/ml cycloheximide (CHX) (Sigma) for 1 or 2 h. Then the cells were washed with ice-cold PBS and lysed for 30 min at 4 °C in RIPA buffer (25 mm Tris-HCl, pH 7.5, containing 100 mm NaCl, 1% (v/v) Nonidet P-40, 1% (w/v) sodium deoxycholate and 0.1% (w/v) SDS) supplemented with a protease inhibitors mixture (Sigma). Cell lysates were spun for 15 min at 20,000 × g, and supernatants were recovered and mixed with NuPAGE SDS sample buffer (Invitrogen). Samples were heated at 85 °C for 10 min, and proteins resolved on NuPAGE Bis-Tris gels (Invitrogen). Immunoblot analysis was performed as previously described (39). Horseradish peroxidase-conjugated antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) were detected using Western Lightning Chemiluminescence Reagent (PerkinElmer, Boston, MA) and HyBlot CL autoradiography film (Denville Scientific, Metuchen, NJ). Data analysis was performed using the Image J software (NIH).
Endoglycosidase H (Endo H) and N-Glycosidase F (PNGase F) Digestion
Cells transiently expressing the proteins of interest were lysed in denaturing buffer (0.5% (w/v) SDS and 0.04 m dithiothreitol) and heated at 100 °C for 10 min. For Endo H digestion, samples were diluted with G5 buffer (New England Biolabs, Ipswich, MA) to a final concentration of 50 mm sodium citrate, pH 5.5, before incubation with 500 U Endo H at 37 °C overnight. For PNGase F digestion, samples were diluted with G7 buffer (New England Biolabs) to a final concentration of 50 mm sodium phosphate, pH 7.5, supplemented with 1% (v/v) Nonidet P-40, before incubation with 500 units of PNGase F at 37 °C overnight. Samples were mixed with NuPAGE SDS sample buffer and further analyzed by SDS-PAGE and immunoblotting as described above.
Cell Fractionation
Cells transiently expressing the proteins of interest were disrupted in homogenization buffer (10 mm Tris-HCl, pH 7.5, 250 mm sucrose with a protease inhibitors mixture) by 20 passages through a 23-gauge needle on ice. Samples were centrifuged at 500 × g for 10 min at 4 °C, and the resulting supernatants (postnuclear supernatant; PS) were subjected to ultracentrifugation at 105,000 × g for 1 h at 4 °C to obtain cytosolic (Cyt) and membrane (Memb) fractions. For extraction experiments, the membrane fraction was resuspended in 0.1 m Na2CO3 (pH 11.3) or lysis buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 5 mm EDTA, and 0.5% (v/v) Triton X-100) and then incubated for 1 h at 4 °C. After the incubation period, samples were centrifuged at 105,000 × g for 1 h, and supernatants were collected for further analysis by SDS-PAGE and immunoblotting.
Immunoprecipitation
Cell extracts were prepared by incubating cells for 30 min at 4 °C in lysis buffer (50 mm Tris-HCl, pH 7.5, containing 150 mm NaCl, 5 mm EDTA, and 0.5% (v/v) Triton X-100), supplemented with a protease inhibitors mixture. Cell lysates were spun for 15 min at 20,000 × g, and supernatants were recovered and mixed with an antibody to the HA epitope bound to protein G-Sepharose (Amersham Biosciences). Immunoprecipitates were eluted by mixing with NuPAGE SDS sample buffer and heating at 85 °C for 10 min, and further analyzed by SDS-PAGE and immunoblotting.
Immunofluorescence Microscopy
Cells grown on coverslips and transiently expressing the proteins of interest were fixed with 4% (v/v) paraformaldehyde in PBS for 30 min at room temperature. Fixed cells were permeabilized with 0.1% (v/v) Triton X-100 in PBS. Cells were incubated with primary and secondary antibodies in blocking solution (1% (w/v) bovine serum albumin in PBS). Coverslips were mounted on slides, and cells were analyzed with a Zeiss LSM 510 laser scanning confocal microscope.
Statistical Analysis
Statistical analyses were carried out using Prism 4.0 software (San Diego, CA). Groups were compared using analysis of variance with Tukey's post hoc analysis. p < 0.05 was considered statistically significant.
RESULTS
Serine Residues in the Cytosolic Tail Are Required for Ubiquitination and Degradation of TCRα
To analyze the mechanism of ubiquitination of unassembled TCRα, we established an in vivo system involving co-expression in HeLa cells of (i) TCRα appended at its C terminus with an abbreviated HA-tag (YPYDVPDYA) that does not contain any ubiquitinatable residues (TCRα-HA), and (ii) Ub tagged at its N terminus with the Myc epitope (Myc-Ub). Cells co-expressing these constructs were incubated with the proteasomal inhibitor MG132 to prevent degradation of ubiquitinated TCRα-HA, and solubilized with RIPA buffer containing the ionic detergents 0.1% (w/v) SDS and 1% (w/v) deoxycholate to solubilize TCRα-HA while releasing any associated proteins. Total TCRα-HA was isolated by immunoprecipitation with antibody to the HA epitope, and ubiquitinated TCRα-HA was detected by immunoblotting with antibody to the Myc epitope. Using this assay, ubiquitinated TCRα-HA appeared as a broad smear centered at ∼200 kDa on the immunoblots (Fig. 1B). The identity of this smear as TCRα-HA was confirmed by complete denaturation of cell lysate proteins at 100 °C in 1% (w/v) SDS and 10 mm dithiothreitol prior to immunoprecipitation and immunoblotting (data not shown). In all subsequent ubiquitination experiments, the amount of ubiquitinated TCRα-HA was related to that of non-ubiquitinated TCRα-HA (i.e. the major species) for comparison of different constructs and conditions.
To determine if the five residues that make up the TCRα cytosolic tail (RLWSS, residues 264–268, Fig. 1A) were required for ubiquitination, we mutated each of them to alanine in the context of the TCRα-HA construct and tested them in the in vivo ubiquitination assay. We observed that mutation of Arg-264 (R264A mutant) had no effect, whereas mutation of Leu-265 or Trp-266 (L265A and W266A mutants, respectively) increased TCRα-HA ubiquitination by ∼3-fold (Fig. 1, B and C). In contrast, mutation of Ser-267 or Ser-268 (S267A and S268A mutants, respectively) decreased TCRα ubiquitination to ∼50% (Fig. 1, B and C). The effects of the serine mutations were additive, as their combined substitution (SS-AA mutant) further reduced TCRα-HA ubiquitination to ∼30% relative to the wild-type (WT) control (Fig. 1, B and C). Thus, the identity of cytosolic tail residues determines the extent of TCRα-HA ubiquitination, with the two serine residues being required for the bulk of the ubiquitination. Next, we examined the degradation of TCRα-HA variants in transfected HeLa cells by performing cycloheximide chase experiments. In agreement with previous studies using untagged TCRα-HA (22, 25, 26), we observed that WT TCRα-HA was degraded with t½ ∼1 h (Fig. 1, D and E). Importantly, the mutants that were more ubiquitinated (L265A and W266A) were degraded faster, whereas those that were less ubiquitinated (S267A and S268A) were degraded more slowly than WT TCRα-HA (Fig. 1, D and E). Combined mutation of both S267A and S268A abrogated virtually all TCRα-HA degradation over the time course of the experiment (Fig. 1, D and E). These results indicated that ubiquitination dependent on cytosolic tail residues determines the degradation rate of TCRα-HA.
Although combined mutation of Ser-267 and Ser-268 abolished TCRα-HA degradation, the mutant protein was nonetheless retained in the ER as a fully glycosylated species bearing high mannose N-linked chains, as evidenced by its sensitivity to endoglycosidase H (Endo H) (supplemental Fig. S1B) and co-localization with the ER-resident protein, calnexin (supplemental Fig. S1, C–H). Moreover, the mutant protein remained integrally associated with the ER membrane, as shown by its resistance to extraction with 0.1 m Na2CO3, pH 11.3 (supplemental Fig. S1I). These findings indicated that TCRα-HA has additional determinants of ER retention and that the normal topology of the protein is not altered by inhibition of ubiquitination and degradation.
Placement of Additional Cytosolic Serine Residues Enhances TCRα Ubiquitination and Degradation
The experiments described above revealed a requirement of the cytosolic Ser-267 and Ser-268 residues for ubiquitination and degradation of TCRα-HA. To further examine the dependence of these processes on cytosolic serine residues, we placed one to three additional serine residues by stepwise substitution of Trp-266, Leu-265, and Arg-264, resulting in mutants bearing three to five cytosolic serine residues (3-Ser, 4-Ser, and 5-Ser; Fig. 1A). We observed that substitution of serine for Trp-266 (3-Ser mutant) greatly increased TCRα-HA ubiquitination (Fig. 2, A and B) and degradation (Fig. 2, C and D). Additional substitution of serine for Leu-265 (4-Ser mutant) yielded a modest increase in TCRα-HA ubiquitination (Fig. 2, A and B) and degradation (Fig. 2, C and D), whereas placement of an additional serine for Arg-264 (5-Ser mutant) had no further effect (Fig. 2, A–D).
FIGURE 2.
Addition of serine residues to the cytosolic tail of TCRα enhances its ubiquitination and degradation. A and B, ubiquitination of WT TCRα and serine-addition mutants of TCRα (Fig. 1A) was determined as described in the legend to Fig. 1, B and C. *, p < 0.05 versus WT. C and D, CHX chase experiments and quantification were performed as described in the legend to Fig. 1, D and E.
In additional experiments, we inserted a single serine residue in place of Arg-264, Leu-265, or Trp-266 in the context of a construct with both Ser-267 and Ser-268 mutated to alanine (R264S/SS-AA, L265S/SS-AA, and W266S/SS-AA; Fig. 1A). We observed that R264S/SS-AA was more ubiquitinated than SS-AA, indicating that serine residue at 264 can mediate ubiquitination independently of Ser-267 and Ser-268. Moreover, L265S/SS-AA and W266S/SS-AA were more ubiquitinated than not only SS-AA, but also S267A and S268A (Fig. 3, A and B). This indicated that placement of a single serine at position 265 or 266 results in even better ubiquitination of TCRα. As in the previous experiments, there was a perfect correlation between the extent of ubiquitination and the degradation rates of the serine insertion mutants (Fig. 3, C and D). The dependence of ubiquitination on the number and position of serine residues strongly suggests that the serine residues themselves are ubiquitinated. Alternatively, the cytosolic serine residues could positively regulate ubiquitination of other residues on TCRα. In any case, it is clear that this serine-dependent modification determines targeting of TCRα-HA for degradation.
FIGURE 3.
Ubiquitination and degradation of TCRα mutants bearing single serine residues at different positions within the cytosolic tail. A and B, ubiquitination of WT TCRα and single-serine insertion mutants of TCRα (Fig. 1A) was determined as described in the legend to Fig. 1, B and C. *, p < 0.05 versus SS-AA mutant. C and D, CHX chase experiments and quantification were performed as described in the legend to Fig. 1, D and E.
Cytosolic Cysteine, Threonine, and Lysine Can Substitute for Serine Residues in Mediating Ubiquitination and Degradation of TCRα
In addition to serine residues, cysteine, threonine, and lysine residues can also act as Ub-acceptor sites (11, 12). To test whether these alternative residues could functionally replace serine in our system, we substituted cysteine, threonine, or lysine pairs for both Ser-267 and Ser-268 (SS-CC, SS-TT, and SS-KK mutants, respectively; Fig. 1A), and examined the effect of these substitutions on TCRα-HA ubiquitination and degradation. We found that the SS-CC and SS-TT mutants were ubiquitinated (Fig. 4, A and B) and degraded (Fig. 4, C and D) to the same extent as WT TCRα-HA. The SS-KK mutant was ubiquitinated (Fig. 4, A and B) and degraded (Fig. 4, C and D) even more efficiently. The fact that cysteine can substitute for serine residues further supports the inference that ubiquitination rather than some other modification (e.g. serine phosphorylation) mediates TCRα-HA targeting for ERAD.
FIGURE 4.
Ubiquitination and degradation of TCRα bearing cysteine, threonine, or lysine residues in place of serine residues in the cytosolic tail. A and B, ubiquitination of WT TCRα and cysteine, threonine, or lysine substitution mutants of TCRα (Fig. 1A) was determined as described in the legend to Fig. 1, B and C. *, p < 0.05 versus WT. C and D, CHX chase experiments and quantification were performed as described in the legend to Fig. 1, D and E.
Two Basic Residues in the Transmembrane Domain of TCRα Are Required for Degradation Though Not Through Their Effect on Ubiquitination
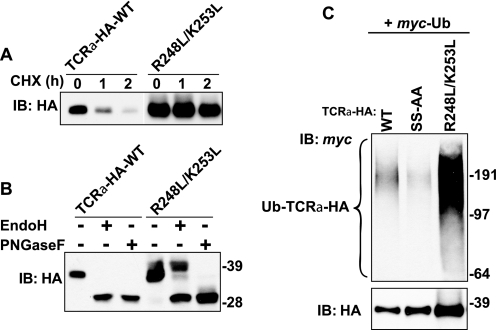
Targeting of unassembled TCRα for degradation has long been known to depend on two basic residues, Arg-248 and Lys-253, in the transmembrane domain of the protein (27, 28). To determine whether this dependence reflects a requirement of these residues for ubiquitination, we changed both Arg-248 and Lys-253 to leucine (R248L/K253L mutant; Fig. 1A) and examined the ubiquitination status of the mutant protein. As expected, this double mutation abolished degradation of TCRα-HA (Fig. 5A). Most of the mutant protein remained in the ER, although a fraction moved to the Golgi complex and underwent processing to complex oligosaccharides, as revealed by treatment with Endo H (Fig. 5B). Thus, structural determinants other than the basic transmembrane residues (perhaps the unassembled luminal domain) also contribute to TCRα-HA retention in the ER. Remarkably, mutation of the basic transmembrane residues did not prevent ubiquitination; rather, the recovery of ubiquitinated species was greatly increased, mainly owing to the inhibition of degradation and higher steady-state levels of the mutant protein (Fig. 5C). Therefore, the two transmembrane charged residues are not required for recruitment of the ubiquitination machinery but must be involved in another step of ERAD such as dislocation from the membrane. An important corollary of these experiments is that ubiquitination of TCRα is not sufficient for targeting to ERAD.
FIGURE 5.
Ubiquitination is independent of two basic residues in the transmembrane domain of TCRα. A, CHX chase experiments were performed as described in the legend to Fig. 1D. B, lysates from HeLa cells transfected with plasmids encoding WT TCRα-HA or R248L/K253L mutant were left untreated or digested with Endo H or PNGase F before immunoblotting with anti-HA monoclonal antibody. C, ubiquitination of WT and mutant TCRα constructs (Fig. 1A) was determined as described in the legend to Fig. 1B. Molecular mass markers (in kilodaltons) are indicated on the right.
The HRD1 E3 Ub Ligase Mediates TCRα Ubiquitination
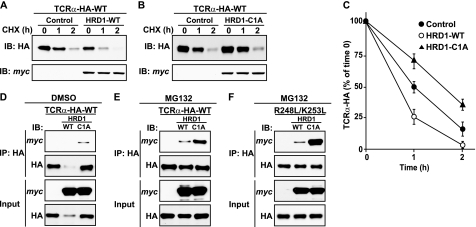
What Ub ligase recognizes TCRα as a substrate, leading to serine-dependent ubiquitination? The RING-type E3, HRD1, was previously shown to mediate ERAD of TCRα (33, 34). In accordance with this proposal, we observed that overexpression of WT HRD1 accelerated, whereas overexpression of an inactive, RING domain (C1A) mutant, of HRD1, slowed degradation of TCRα-HA (Fig. 6, A–C). These observations indicated that HRD1 promotes TCRα-HA degradation by virtue of its Ub-ligase activity. The C1A mutant of HRD1, but not WT HRD1, coprecipitated with TCRα-HA (Fig. 6D). This difference could be due to the disparate levels of TCRα-HA (Fig. 6D) resulting from the opposite effects of C1A and WT HRD1 on degradation (Fig. 6, A–C). Inhibition of degradation with MG132 increased TCRα-HA coprecipitation with C1A HRD1 and revealed some coprecipitation with WT HRD1, but did not completely eliminate the different levels of coprecipitation (Fig. 6E). Thus, HRD1 interacts with TCRα-HA in a manner such that Ub-ligase activity promotes dissociation of the complex.
FIGURE 6.
HRD1 Ub ligase interacts with TCRα and promotes its degradation. A–C, HeLa cells were transfected with plasmids encoding WT TCRα-HA with or without WT or C1A mutant HRD1. CHX chase experiments and quantification were performed as described in the legend to Fig. 1, D and E. D–F, HeLa cells transfected with plasmids encoding WT TCRα-HA or R248L/K253L mutant with or without WT or C1A mutant HRD1 were left untreated or treated with 50 μm MG132 for 4 h at 37 °C. After lysis, TCRα-HA was immunoprecipitated with anti-HA monoclonal antibody. Lysates (5% input) and immunoprecipitates (IP) were analyzed by SDS-PAGE and immunoblotting (IB) with anti-HA and anti-Myc polyclonal antibodies.
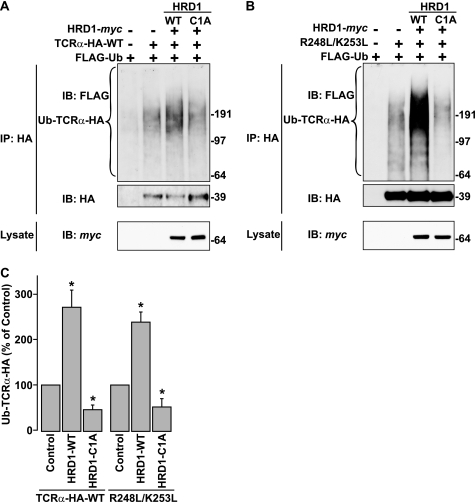
Despite the known role of HRD1 in TCRα degradation, prior to our work there was no evidence that HRD1 mediated TCRα ubiquitination. To address this issue, we performed in vivo ubiquitination experiments in which WT or mutant TCRα-HA, Myc-tagged WT or C1A HRD1, and FLAG-tagged Ub were co-expressed in HeLa cells. In preliminary experiments, we confirmed that FLAG-tagged Ub behaved as Myc-tagged Ub in the in vivo assay, with the added advantage that the FLAG tag allowed greater sensitivity of detection (data not shown). Using this experimental setup, we observed that WT HRD1 expression increased, whereas C1A HRD1 decreased, ubiquitination of WT TCRα-HA relative to non-ubiquitinated TCRα-HA (Fig. 7, A and C), consistent with a role of HRD1 in TCRα ubiquitination. Similarly, ubiquitination of the R248L/K253L mutant was enhanced by WT HRD1 and inhibited by C1A HRD1 (Fig. 7, B and C). Co-precipitation experiments showed that the R248L/K253L double mutant interacted with the C1A and, to a lesser extent, WT forms of HRD1 (Fig. 6F), indicating that the two basic residues in the transmembrane domain are not required for recognition of TCRα by HRD1.
FIGURE 7.
HRD1 Ub ligase mediates TCRα ubiquitination. A and B, HeLa cells were transfected with the indicated constructs. Ubiquitination of TCRα-HA was determined in the absence of MG132 as described in the legend to Fig. 1B with the exception of using anti-FLAG antibody instead of anti-Myc polyclonal antibody to detect ubiquitinated TCRα-HA. C, ubiquitinated TCRα-HA levels were normalized to non-ubiquitinated TCRα-HA levels. Values represent the mean ± S.E. from three independent experiments. *, p < 0.05 versus untreated with HRD1 cDNA (Control).
To confirm the involvement of HRD1 in TCRα ubiquitination, we tested the effect of HRD1 knockdown by siRNA. Treatment with HRD1 siRNA resulted in ∼60% decrease in HRD1 levels. We observed that ubiquitination (in relation to the non-ubiquitinated protein) and degradation of WT TCRα-HA were inhibited by knockdown of HRD1 (Figs. 8, A–D). Taken together, these experiments indicated that HRD1 participates in ubiquitination of TCRα to target the protein to ERAD.
FIGURE 8.
HRD1 knockdown inhibits degradation and ubiquitination of TCRα. A and B, HeLa cells were left untreated (UnTransfect) or treated with non-targeting siRNA (siControl) or HRD1 siRNA (siHRD1). After 72 h, cells were transfected with WT TCRα-HA and then CHX chase experiments and quantification were performed as described in the legend to Fig. 1, D and E. Expression of endogeneous HRD1 was determined by immunoblotting with anti-HRD1 polyclonal antibody. C, HeLa cells treated with the indicated siRNA were transfected with FLAG-Ub, and WT TCRα-HA or R248L/K253L mutant. Ubiquitination of TCRα-HA was determined in the absence of MG132 as described in the legend to Fig. 7. Endogeneous HRD1 in the lysate was detected as described above. D, ubiquitinated TCRα-HA levels were normalized to non-ubiquitinated TCRα-HA levels. Values represent the mean ± S.E. from three independent experiments. *, p < 0.05 versus treated with non-targeting siRNA (siControl).
DISCUSSION
The results presented here show that TCRα ubiquitination and ERAD targeting are dependent on two serine residues, Ser-267 and Ser-268, in the cytosolic tail of TCRα. Substitution of these serine residues to alanine decreased ubiquitination of TCRα (Fig. 1), whereas placement of additional serine residues enhanced it (Figs. 2 and 3). Moreover, substitution of the serine residues by other ubiquitinatable residues (i.e. cysteine, threonine, or lysine) allowed ubiquitination to take place (Fig. 4). These findings suggest that the serine residues themselves act as the main ubiquitin acceptors. The fact that ∼30% of ubiquitinated TCRα remains after mutation of both Ser-267 and Ser-268 to alanines indicates that other residues, most likely in the luminal domain, are also ubiquitinated. An alternative explanation for the requirement of Ser-267 and Ser-268 would be that these residues promote ubiquitination of residues elsewhere in the protein. Distinguishing between these possibilities will require the development of methods to detect ubiquitinated serine species, which up until now have not been reported for all other cases of presumed serine-ubiquitination (11, 12, 16). In any case, the perfect correlation of serine-dependent ubiquitination with degradation of the various TCRα mutants suggests that this particular modification is the one that mediates ERAD of TCRα.
The two serine residues are conserved in the cytosolic tails of TCRα from all mammalian species, and none of these tails contain any lysine residues (supplemental Fig. S1A). Thus, serine-dependent ubiquitination is likely a conserved mechanism for ERAD of unassembled TCRα among mammals. The β-subunit of the TCR (TCRβ) is also degraded when it cannot assemble with other TCR subunits (27, 40). TCRβ from different mammalian species has a cytosolic tail of 5–7 amino acid residues that contains 2–3 lysine residues (supplemental Fig. S1A). Therefore, unlike TCRα, TCRβ might rely on conventional lysine-ubiquitination for targeting to ERAD. The conservation of ubiquitinatable residues in the cytosolic tails of TCRα and TCRβ ensures the disposal of these proteins when other subunits of the TCR complex are missing. Assembly of the complete TCR complex likely masks these residues, resulting in stabilization of the complex and its ensuing transport out of the ER and to the plasma membrane (27).
The proteins Pex5p and Bid are two examples of proteins for which non-lysine ubiquitination is the key modification leading to transport and degradation, respectively (15–17). MHC-I heavy chains are also ubiquitinated on non-lysine residues in the cytosolic tail by the action of the viral MIR1 and mK3 E3s (11, 12). However, MHC-I heavy chains also contain lysine residues in their cytosolic tails, and the individual contribution of each type of residue to ubiquitination has not been assessed. Recently, Ube2j2 was identified as the primary cellular E2 recruited by the mK3 E3 ligase (14). The Ube2j2-mK3 pair preferentially promotes ubiquitination of serine and threonine residues even when lysine residues are present on the tail of MHC- I heavy chain (14). Moreover, the E2 is the enzyme that determines non-lysine versus lysine ubiquitination (14), the length and topology of ubiquitination chains and the processivity of the chain assembly reaction (41). Both Ube2g2 (36) and Ube2j2 (35) have been previously implicated in the degradation of TCRα, and might therefore be the E2s that catalyze serine-dependent ubiquitination of TCRα for ERAD targeting.
Our studies have uncovered two other interesting properties of serine-dependent ubiquitination. The first one is that residues neighboring the serines have an effect on the efficiency of the reaction. Indeed, substitution of the non-ubiquitinatable cytosolic tail residues Leu-265 and Trp-266 by alanine enhanced the ubiquitination and degradation of TCRα (Fig. 1). E3s are thought to act as scaffolds to bring Ub-charged E2 and substrate into close proximity (42). The bulky hydrophobic side chains of Leu-265 and Trp-266 might therefore hinder access of the E2-E3 complex to substrate serine residues. The second interesting property of serine-dependent ubiquitination is its relative independence of the exact position of the serine residues (Figs. 2 and 3), as is also the case for lysine-ubiquitination (43–45).
Another important finding in our study is that ubiquitination of TCRα is promoted by HRD1. This represents the first identification of a cellular E3 that can promote non-lysine-dependent ubiquitination. HRD1 is a human homologue of Saccharomyces cerevisiae Hrd1p (34), which was originally identified as an E3 that is responsible for sterol-regulated degradation of Hmg2p, a yeast homologue of HMG-CoA reductase (46). HRD1/Hrd1p is a multispanning membrane protein localized to the ER, consisting of an N-terminal domain with six transmembrane spans and a C-terminal cytosolic domain with a RING-H2 domain responsible for the transfer of Ub from the E2 to the substrate (34, 47). In addition to Hmg2p, many other proteins have been identified as substrates of HRD1/Hrd1p, including mutants of carboxypeptidase Y (48), Ig-μ (33), huntingtin (49), and the Pael receptor (50). Although HRD1 has been shown to be capable of targeting TCRα for degradation, there was no evidence that it promoted ubiquitination of this protein. In this study, we showed that expression of WT HRD1 increased whereas RING-domain mutant of HRD1 decreased the ubiquitination of TCRα (Fig. 7). Taken together, these observations suggest that HRD1 promotes serine-dependent ubiquitination of TCRα, targeting the protein to ERAD. The effects of manipulating HRD1 on TCRα ubiquitination, however, were not complete. It thus remains to be determined whether HRD1 is uniquely and directly responsible for serine-dependent ubiquitination of TCRα. In this regard, the gp78 Ub ligase has also been shown to participate in TCRα ERAD (37). Evidence of cooperation among E3s has been reported for ubiquitination of other ERAD substrates (51).
Recently, Hrd1p was shown to recognize a misfolded protein by virtue of its transmembrane domain (52). Since the two basic residues in the transmembrane domain of TCRα, Arg-248 and Lys-253, are required for degradation of the protein (27, 28), we hypothesized that these residues could be recognized by the transmembrane domain of HRD1. However, mutations of Arg-248 and Lys-253 to leucine did not inhibit the ubiquitination of TCRα (Fig. 5C). Furthermore, overexpression of HRD1 increased whereas overexpression of the RING-domain mutant of HRD1 decreased the ubiquitination of the R248L/K253L mutant (Fig. 7B). Thus, the two basic residues in the transmembrane domain of TCRα are not required for recognition by HRD1; rather, they might be important for dislocation of the protein from the membrane, perhaps by loosening interactions with the lipid bilayer or allowing interaction with a putative retrotranslocation channel.
Supplementary Material
Acknowledgments
We thank X. Zhu and N. Tsai for expert technical assistance, Y. Ye and E. Wiertz for kind gifts of reagents, and M. Yang and J. Magadán for helpful discussions.
This work was supported, in whole or in part, by the National Institutes of Health Intramural AIDS Targeted Antiviral Program (IATAP) and the Intramural Programs of NICHD and NCI.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- ER
- endoplasmic reticulum
- CHX
- cycloheximide
- Endo H
- Endoglycosidase H
- ERAD
- ER-associated degradation
- MHC
- major histocompatibility complex
- PNGase F
- N-glycosidase F
- TCR
- T-cell antigen receptor
- TCRα
- α-chain of the TCR
- TCRβ
- β-chain of the TCR
- Ub
- ubiquitin
- WT
- wild-type
- HA
- hemagglutinin
- RIPA
- radioimmune precipitation assay buffer
- PBS
- phosphate-buffered saline.
REFERENCES
- 1.Ellgaard L., Helenius A. (2003) Nat. Rev. Mol. Cell Biol. 4, 181–191 [DOI] [PubMed] [Google Scholar]
- 2.Kleizen B., Braakman I. (2004) Curr. Opin. Cell Biol. 16, 343–349 [DOI] [PubMed] [Google Scholar]
- 3.Kostova Z., Wolf D. H. (2003) EMBO J. 22, 2309–2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meusser B., Hirsch C., Jarosch E., Sommer T. (2005) Nat. Cell Biol. 7, 766–772 [DOI] [PubMed] [Google Scholar]
- 5.Vembar S. S., Brodsky J. L. (2008) Nat. Rev. Mol. Cell Biol. 9, 944–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang S., Weissman A. M. (2004) Cell Mol. Life Sci. 61, 1546–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kostova Z., Tsai Y. C., Weissman A. M. (2007) Semin Cell Dev. Biol. 18, 770–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirsch C., Gauss R., Horn S. C., Neuber O., Sommer T. (2009) Nature 458, 453–460 [DOI] [PubMed] [Google Scholar]
- 9.Ciechanover A. (1998) EMBO J. 17, 7151–7160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komander D. (2009) Biochem. Soc. Trans. 37, 937–953 [DOI] [PubMed] [Google Scholar]
- 11.Cadwell K., Coscoy L. (2005) Science 309, 127–130 [DOI] [PubMed] [Google Scholar]
- 12.Wang X., Herr R. A., Chua W. J., Lybarger L., Wiertz E. J., Hansen T. H. (2007) J. Cell Biol. 177, 613–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grou C. P., Carvalho A. F., Pinto M. P., Huybrechts S. J., Sá-Miranda C., Fransen M., Azevedo J. E. (2009) J. Biol. Chem. 284, 10504–10513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X., Herr R. A., Rabelink M., Hoeben R. C., Wiertz E. J., Hansen T. H. (2009) J. Cell Biol. 187, 655–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tait S. W., de Vries E., Maas C., Keller A. M., D'Santos C. S., Borst J. (2007) J. Cell Biol. 179, 1453–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carvalho A. F., Pinto M. P., Grou C. P., Alencastre I. S., Fransen M., Sá-Miranda C., Azevedo J. E. (2007) J. Biol. Chem. 282, 31267–31272 [DOI] [PubMed] [Google Scholar]
- 17.Williams C., van den Berg M., Sprenger R. R., Distel B. (2007) J. Biol. Chem. 282, 22534–22543 [DOI] [PubMed] [Google Scholar]
- 18.Cadwell K., Coscoy L. (2008) J. Virol. 82, 4184–4189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alarcón B., Gil D., Delgado P., Schamel W. W. (2003) Immunol. Rev. 191, 38–46 [DOI] [PubMed] [Google Scholar]
- 20.Call M. E., Wucherpfennig K. W. (2007) Nat. Rev. Immunol. 7, 841–850 [DOI] [PubMed] [Google Scholar]
- 21.Klausner R. D., Lippincott-Schwartz J., Bonifacino J. S. (1990) Annu. Rev. Cell Biol. 6, 403–431 [DOI] [PubMed] [Google Scholar]
- 22.Bonifacino J. S., Suzuki C. K., Lippincott-Schwartz J., Weissman A. M., Klausner R. D. (1989) J. Cell Biol. 109, 73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen C., Bonifacino J. S., Yuan L. C., Klausner R. D. (1988) J. Cell Biol. 107, 2149–2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lippincott-Schwartz J., Bonifacino J. S., Yuan L. C., Klausner R. D. (1988) Cell 54, 209–220 [DOI] [PubMed] [Google Scholar]
- 25.Huppa J. B., Ploegh H. L. (1997) Immunity 7, 113–122 [DOI] [PubMed] [Google Scholar]
- 26.Yu H., Kaung G., Kobayashi S., Kopito R. R. (1997) J. Biol. Chem. 272, 20800–20804 [DOI] [PubMed] [Google Scholar]
- 27.Bonifacino J. S., Cosson P., Klausner R. D. (1990) Cell 63, 503–513 [DOI] [PubMed] [Google Scholar]
- 28.Bonifacino J. S., Cosson P., Shah N., Klausner R. D. (1991) EMBO J. 10, 2783–2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonifacino J. S., Suzuki C. K., Klausner R. D. (1990) Science 247, 79–82 [DOI] [PubMed] [Google Scholar]
- 30.Manolios N., Bonifacino J. S., Klausner R. D. (1990) Science 249, 274–277 [DOI] [PubMed] [Google Scholar]
- 31.Yang M., Omura S., Bonifacino J. S., Weissman A. M. (1998) J. Exp. Med. 187, 835–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu H., Kopito R. R. (1999) J. Biol. Chem. 274, 36852–36858 [DOI] [PubMed] [Google Scholar]
- 33.Cattaneo M., Otsu M., Fagioli C., Martino S., Lotti L. V., Sitia R., Biunno I. (2008) J. Cell. Physiol. 215, 794–802 [DOI] [PubMed] [Google Scholar]
- 34.Kikkert M., Doolman R., Dai M., Avner R., Hassink G., van Voorden S., Thanedar S., Roitelman J., Chau V., Wiertz E. (2004) J. Biol. Chem. 279, 3525–3534 [DOI] [PubMed] [Google Scholar]
- 35.Lenk U., Yu H., Walter J., Gelman M. S., Hartmann E., Kopito R. R., Sommer T. (2002) J. Cell Sci. 115, 3007–3014 [DOI] [PubMed] [Google Scholar]
- 36.Tiwari S., Weissman A. M. (2001) J. Biol. Chem. 276, 16193–16200 [DOI] [PubMed] [Google Scholar]
- 37.Chen B., Mariano J., Tsai Y. C., Chan A. H., Cohen M., Weissman A. M. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 341–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai Y. C., Mendoza A., Mariano J. M., Zhou M., Kostova Z., Chen B., Veenstra T., Hewitt S. M., Helman L. J., Khanna C., Weissman A. M. (2007) Nat. Med. 13, 1504–1509 [DOI] [PubMed] [Google Scholar]
- 39.Mardones G. A., Burgos P. V., Brooks D. A., Parkinson-Lawrence E., Mattera R., Bonifacino J. S. (2007) Mol. Biol. Cell 18, 3486–3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee S. J. (1998) Exp. Mol. Med. 30, 159–164 [DOI] [PubMed] [Google Scholar]
- 41.Ye Y., Rape M. (2009) Nat. Rev. Mol. Cell Biol. 10, 755–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Passmore L. A., Barford D. (2004) Biochem. J. 379, 513–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hou D., Cenciarelli C., Jensen J. P., Nguygen H. B., Weissman A. M. (1994) J. Biol. Chem. 269, 14244–14247 [PubMed] [Google Scholar]
- 44.King R. W., Glotzer M., Kirschner M. W. (1996) Mol. Biol. Cell 7, 1343–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu G., Xu G., Schulman B. A., Jeffrey P. D., Harper J. W., Pavletich N. P. (2003) Mol. Cell 11, 1445–1456 [DOI] [PubMed] [Google Scholar]
- 46.Hampton R. Y., Gardner R. G., Rine J. (1996) Mol. Biol. Cell 7, 2029–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bays N. W., Gardner R. G., Seelig L. P., Joazeiro C. A., Hampton R. Y. (2001) Nat. Cell Biol. 3, 24–29 [DOI] [PubMed] [Google Scholar]
- 48.Bordallo J., Plemper R. K., Finger A., Wolf D. H. (1998) Mol. Biol. Cell 9, 209–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang H., Zhong X., Ballar P., Luo S., Shen Y., Rubinsztein D. C., Monteiro M. J., Fang S. (2007) Exp. Cell Res. 313, 538–550 [DOI] [PubMed] [Google Scholar]
- 50.Omura T., Kaneko M., Okuma Y., Orba Y., Nagashima K., Takahashi R., Fujitani N., Matsumura S., Hata A., Kubota K., Murahashi K., Uehara T., Nomura Y. (2006) J. Neurochem. 99, 1456–1469 [DOI] [PubMed] [Google Scholar]
- 51.Morito D., Hirao K., Oda Y., Hosokawa N., Tokunaga F., Cyr D. M., Tanaka K., Iwai K., Nagata K. (2008) Mol. Biol. Cell 19, 1328–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sato B. K., Schulz D., Do P. H., Hampton R. Y. (2009) Mol. Cell 34, 212–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.