Abstract
Purpose
To evaluate vitrectomy for diabetic macular edema (DME) in eyes with at least moderate vision loss and vitreomacular traction.
Design
Prospective cohort study
Participants
The primary cohort included 87 eyes with DME and vitreomacular traction based on investigator’s evaluation, visual acuity 20/63–20/400, optical coherence tomography (OCT) central subfield >300 microns and no concomitant cataract extraction at the time of vitrectomy.
Methods
Surgery was performed according to the investigator’s usual routine. Follow-up visits were performed after 3 months, 6 months (primary endpoint) and 1 year.
Main Outcome Measures
Visual acuity, OCT retinal thickening and surgical complications.
Results
At baseline, median visual acuity in the 87 eyes was 20/100 and median OCT thickness was 491 microns. During vitrectomy, additional procedures included epiretinal membrane peeling in 61%, internal limiting membrane peeling in 54%, panretinal photocoagulation in 40% and injection of corticosteroids at the close of the procedure in 64%. At 6 months, median OCT central subfield thickness decreased by 160 microns, with 43% having central subfield thickness <250 microns and 68% having at least a 50% reduction in thickening. Visual acuity improved by 10 or more letters in 38% (95% confidence interval 28% – 49%) and deteriorated by 10 or more letters in 22% (95% confidence interval 13% – 31%). Postoperative surgical complications through 6 months included vitreous hemorrhage (5 eyes), elevated intraocular pressure requiring treatment (7 eyes), retinal detachment (3 eyes) and endophthalmitis (1 eye). Little changes in results were noted between 6 months and one year.
Conclusion
Following vitrectomy performed for DME and vitreomacular traction, retinal thickening was reduced in most eyes. Between 28% and 49% of eyes with characteristics similar to those included in this study are likely to have improvement of visual acuity, while between 13% and 31% are likely to have worsening. The surgical complication rate is low and similar to what has been reported for this procedure. These data provide estimates of surgical outcomes and serve as a reference for future studies that might consider vitrectomy for DME in eyes with at least moderate vision loss and vitreomacular traction.
Introduction
Diabetic macular edema (DME) is a disorder of major and increasing public health importance throughout the world.1–4 The only proven effective therapy for DME at this time is focal/grid laser photocoagulation as performed in the Early Treatment Diabetic Retinopathy Study (ETDRS).5–7 However, even with photocoagulation, some eyes have persistent edema and visual loss. The Diabetic Retinopathy Clinical Research Network (DRCR.net) has shown that although approximately one-third of eyes treated with focal/grid photocoagulation improved by 10 or more letters at 2 years, approximately 20% lost 10 or more letters and approximately 50% still had evidence of central edema at 2 years.8 Other pharmacotherapeutic interventions are under investigation to determine if certain drugs, either alone, or in combination with focal/grid laser result in superior visual acuity outcomes compared with laser alone.
The vitreous has been implicated as a cause of macular edema in people with diabetes via several mechanical and physiologic mechanisms, all of which are postulated to lead to increased vascular permeability. 9–34 Suggested mechanisms include the following: (1) destabilization of the vitreous by abnormal glycation and crosslinking of vitreal collagen, leading to traction on the macula, (2) accumulation and concentration of factors causing vasopermeability in the premacular vitreous gel and (3) accumulation of chemoattractant factors in the vitreous, leading to cellular migration to the posterior hyaloid, contraction and macular traction. 9, 10, 12, 14–16, 19, 23, 24, 27, 28, 30 The observation that release of mechanical traction on the macula with subsequent reduction in DME, either by spontaneous posterior vitreous detachment or with vitrectomy, lends support to this line of reasoning. 11, 13, 14, 17, 20, 21, 25, 32 Furthermore, the evidence that vitrectomy produces improved retinal oxygenation,28, 29 taken together with the evidence that increased oxygenation can reduce DME,35 suggests an additional physiologic advantage, potentially conferred by vitrectomy.
A prospective observational protocol was developed by the DRCR.net to evaluate visual and anatomic outcomes following vitrectomy performed without concomitant cataract surgery in eyes with DME. A primary cohort was defined that included eyes that not only had vitreomacular traction based on clinical examination by their surgeon, but also had at least moderately impaired visual acuity and definite thickening within the central subfield on optical coherence tomography (OCT). This report describes the visual acuity and OCT outcomes in this primary cohort.
Methods
The study was conducted by the DRCR.net at 50 clinical sites in the United States. The protocol and HIPAA-compliant informed consent forms were approved by multiple institutional review boards. Each subject gave written informed consent to participate in the study. The study is listed on www.clinicaltrials.gov, under identifier NCT00709319 and the protocol is available on the DRCR.net website (www.drcr.net Accessed September 2, 2009). This paper reports data collected through the 6-month primary outcome phase of the protocol with additional safety data collected through the final follow-up at 1 year. A future report will evaluate factors associated with the outcome of vitrectomy in 241 eyes with DME, including the 87 eyes in the primary cohort described in this report.
Study Population
Eligible participants had to be at least 18 years old with type 1 or type 2 diabetes. Data were collected on 241 individuals who had a vitrectomy as treatment for DME. The current study included a predefined subset of eyes that met the following criteria for the primary analysis: (1) vitreomacular traction as the indication for vitrectomy based on investigator assessment, (2) best corrected visual acuity 20/63 to 20/400 (E-ETDRS letter score between 19 and 63), (3) retinal central subfield thickness >300 microns on Stratus OCT and (4) cataract extraction not performed in conjunction with vitrectomy. Major exclusion criteria included: (1) a history of macular photocoagulation, intravitreal corticosteroids or other treatment for DME within 3.5 months prior to enrollment, (2) peripheral scatter photocoagulation within 4 months prior to enrollment, (3) prior pars plana vitrectomy, (4) other major ocular surgery (including cataract extraction, scleral buckle or other intraocular surgery) within 6 months prior to enrollment or anticipated within the 6 months following enrollment or (5) YAG capsulotomy performed within 2 months prior to enrollment. Only one eye per participant could be enrolled.
Intervention
A standard pars plana vitrectomy was performed according to the investigator’s usual routine. General guidelines included: (1) three pars plana sclerotomies, (2) removal of the vitreous gel with peeling of the posterior hyaloid, if attached and removal of the peripheral vitreous leaving only a small residual vitreous skirt, (3) engagement and peeling of epiretinal membranes judged visually significant, (4) examination of the peripheral retina at the close of the procedure and treatment of peripheral breaks with laser or cryotherapy. Additional collected information included gauge of vitrectomy instrumentation and other maneuvers performed, such as removal of the internal limiting membrane, use of agents to improve visualization of membranes, use of corticosteroids at the close of the procedure and use of concomitant laser.
Follow-Up Visits
Follow-up visits were performed at 3 months, 6 months and 12 months within pre-specified time windows. At each visit, an interval history was elicited, which included medical and surgical treatment of the study eye. At baseline and at each follow-up visit, best-corrected visual acuity was measured at 3 meters by a certified tester using an electronic procedure based on the ETDRS method (E-ETDRS).36 OCT images were obtained through a dilated pupil by a certified operator using the Zeiss Stratus OCT (OCT3). OCT scans were 6 mm length and included the 6 radial line pattern (fast macular scan option with OCT3 (Carl Zeiss Meditec, Dublin, CA)) for quantitative measures and the cross hair pattern (6–12 to 9–3 o’clock) for qualitative assessment of retinal morphology. Seven-field fundus photographs were obtained at baseline, 6 months and 12 months.
OCT images and fundus photographs were sent to the DRCR.net Reading Center at the University of Wisconsin-Madison for grading. Fourteen percent of the 87 baseline scans and 27% of the 155 follow-up scans were judged by the Reading Center to have inaccurate automated central subfield thickness measurements. In these cases, center point thickness was measured manually and the resultant value used to impute a value for the central subfield thickness (based on a correlation of the two measures of 0.99) as previously published.7 Grading of fundus photographs for proliferative diabetic retinopathy (PDR) included both active neovascularization and prior panretinal photocoagulation (PRP) even without active neovascularization.
Additional Treatment for DME
By protocol, injectable medications, focal laser or other treatments for DME were to be deferred until completion of the 6-month visit. Between 6 and 12 months, treatment of DME was at investigator discretion.
Statistical Methods
A sample size was planned to be approximately 100 eyes that met all the criteria for the primary cohort. This was a convenience sample based on the expected number of subjects to be enrolled in a given time period. However, based on actual recruitment, the 100 subject goal was not reached.
The main outcomes were best corrected visual acuity and OCT-measured central subfield thickeness at 6 months. The visual acuity letter score was used for analyses; but approximate Snellen equivalents are presented to facilitate interpretation. Signed rank tests were performed on changes in central subfield thickness from baseline to follow-up visits. Missed visits were excluded from the analysis. SAS version 9.1 (Cary, NC) was used for all analyses.
Results
Between 2005 and 2008, 87 subjects who met the primary cohort criteria for this study were enrolled at 35 sites. The baseline characteristics are presented in Table 1. The presence of vitreomacular traction, as identified by the investigator, was a requirement for inclusion in this cohort. However, epiretinal membranes (ERM) were only identified as “probably” or “definitely present” by the investigator in 71% of study eyes. Presumably the vitreomacular traction was not associated with a clinically apparent ERM in the other 29%. In 27 eyes (31%), the surgeon listed “unresponsive to other therapies” as an additional indication for the vitrectomy. Surgery characteristics are presented in Table 2. Visit completion was 95% at the 3-month visit, 93% at the 6-month visit, and 90% at the 12-month visit. Four subjects died (2 before and 2 after the 6-month visit) and four subjects dropped out of the study (2 before and 2 after the 6-month visit.
Table 1.
Baseline Characteristics (N=87)
| Gender: Women - n (%) | 39 (45%) |
| Age (yrs) - Median (25th, 75th percentile) | 66 (60, 72) |
| Race - n (%) | |
| White | 69 (79%) |
| African-American | 7 (8%) |
| Hispanic | 5 (6%) |
| Other | 6 (7%) |
| Diabetes Type - n (%) | |
| Type 1 | 14 (16%) |
| Type 2 | 73 (84%) |
| Duration of Diabetes (years) - | |
| Median (25th, 75th percentile) | 20 (12, 25) |
| HbA1c (%) - | |
| Median (25th, 75th percentile) | 7.1 (6.7, 7.9) |
| Prior treatment for DME* - n (%) | 51 (59%) |
| Macular photocoagulation | 38 (44%) |
| Intravitreal corticosteroid | 26 (30%) |
| Peribulbar corticosteroid | 3 (3%) |
| Other | 4 (5%) |
| E-ETDRS Visual Acuity letter score (Snellen equivalent) | |
| Median | 52 (20/100) |
| 75th, 25th percentile | 41, 58 (20/80, 20/160) |
| 63 to 54 (20/63 to 20/80) | 37 (43%) |
| 53 to 44 (20/100 to 20/125) | 25 (29%) |
| 43 to 19 (20/160 to 20/400) | 25 (29%) |
| Central Subfield Thickness† (microns) - | |
| Median (25th, 75th percentile) | 491 (356, 586) |
| 301 to <400 | 28 (34%) |
| 400 to <500 | 15 (18%) |
| 500 to <600 | 24 (29%) |
| ≥600 | 16 (19%) |
| Retinal Volume (mm3) - | |
| Median (25th, 75th percentile) | 9.2 (8.5, 11.8) |
| Retinopathy Severity‡ - n (%) | |
| Microaneurysms only | 1 (1%) |
| Mild/Moderate NPDR | 6 (8%) |
| Moderate Severe NPDR | 14 (18%) |
| Severe NPDR | 4 (5%) |
| PDR | 51 (67%) |
| Prior Scatter Photocoagulation - n (%) | 39 (45%) |
| Lens Status - n (%) | |
| Phakic | 37 (43%) |
| Pseudophakic/Aphakic | 50 (57%) |
| Epiretinal Membranes Present|| - n (%) | |
| No | 21 (24%) |
| Probable | 19 (22%) |
| Definite | 43 (49%) |
| Cannot determine | 4 (5%) |
| Status of vitreous|| - n (%) | |
| Attached | 49 (56%) |
| Partially attached | 28 (32%) |
| Detached | 5 (6%) |
| Uncertain | 5 (6%) |
| Reasons for Vitrectomy* - n (%) | |
| Vitreomacular interface abnormality | 87 (100%) |
| Unresponsive to other therapies | 27 (31%) |
Same subjects could be listed for multiple reasons
Missing for 4 eyes
Missing for 11 eyes
From investigator’s observations at enrollment
ETDRS= Early Treatment Diabetic Retinopathy Study, DME=Diabetic macular edema, NPDR = nonproliferative diabetic retinopathy, PDR -=proliferative diabetic retinopathy
Table 2.
Surgery Characteristics (N=87)
| Vitrectomy System - n (%) | |
| 19/20 gauge | 35 (40%) |
| 25 gauge | 43 (49%) |
| 23 gauge | 9 (10%) |
| Epiretinal Membrane Peeled - n (%) | 53 (61%) |
| Internal Limiting Membrane Removed - n (%) | 47 (54%) |
| Agents Used to Improve Visualization* - n (%) | 52 (60%) |
| Triamcinolone acetonide | 30 (34%) |
| Indocyanine Green | 24 (27%) |
| Trypan Blue | 2 (2%) |
| Laser Used*† - n (%) | 48 (55%) |
| Focal to break(s) | 14 (16%) |
| Panretinal photocoagulation, no prior PRP | 19 (22%) |
| Panretinal photocoagulation, prior PRP | 16 (18%) |
| Focal/grid to DME | 4 (5%) |
| With endoprobe | 21 (24%) |
| With laser indirect ophthalmoscope | 7 (8%) |
| Other** | 4 (5%) |
| Peripheral Cryotherapy Given - n (%) | |
| No | 80 (92%) |
| Yes, not treated for breaks | 6 (7%) |
| Yes, treated for breaks | 1 (1%) |
| Corticosteroids Used at Close* - n (%) | 56 (64%) |
| Intravitreal | 37 (43%) |
| Peribulbar | 4 (5%) |
| Subtenon’s | 13 (15%) |
| Subconjunctival | 18 (21%) |
| Lens Removed - n (%) | 0 |
| Posterior Capsulotomy Performed - n (%) | 7 (8%) |
| Epiretinal Membrane Present | |
| No | 31 (36%) |
| Probable | 13 (15%) |
| Definite | 43 (49%) |
| Status of Vitreous | |
| Attached | 59 (68%) |
| Partially attached | 22 (25%) |
| Detached | 5 (6%) |
| Uncertain | 1 (1%) |
| Complications from Vitrectomy - n (%) | 6 (7%) |
| Anesthesia complications | 0 |
| Surgical complications | 6 (7%) |
Same subjects could be listed for multiple categories
Laser technique was not recorded on the study data form in all cases
Includes scatter over peripheral schisis (N=1), barrier laser (N=3).
PRP= Panretinal photocoagulation
Visual Acuity
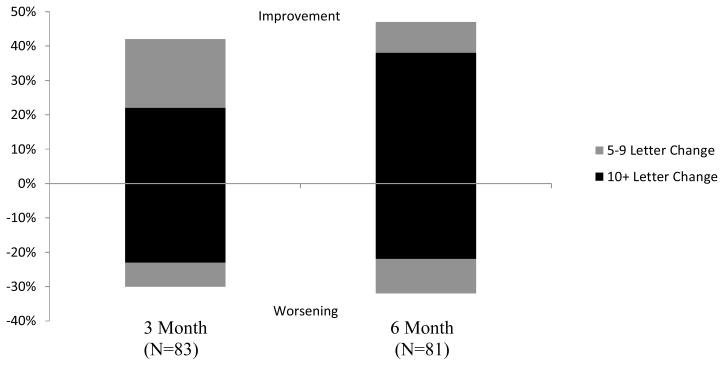
Median visual acuity was approximately 20/100 at baseline, 3 months, and 6 months. At 3 months, 22% of eyes had experienced an improvement of 10 or more letters from baseline (Figure 1). Conversely, 23% of the eyes had worsened 10 or more letters from baseline. At 6 months 38% of eyes were improved by 10 or more letters (95% confidence interval 28% to 49%) and 22% had worsened by 10 or more letters (95% confidence interval 13% to 31%). Among the 18 eyes that improved by 10 or more letters from baseline to 3 months, none had additional improvement of at least 10 or more letters from 3 to 6 months. One phakic eye lost 16 letters from 3 to 6 months. Among the 19 eyes that lost 10 or more letters from baseline to 3 months, 3 eyes (16%) lost an additional 10 or more letters from 3 to 6 months. All 3 of these eyes still were phakic at 6 months. Also, among the eyes that lost 10 or more letters from baseline to 3 months, 9 (47%) gained 10 or more letters from 3 to 6 months, including 3 eyes that had cataract surgery between 3 months and 6 months after vitrectomy.
Figure 1.
Distribution of Change in Visual Acuity from Baseline
Retinal Thickness
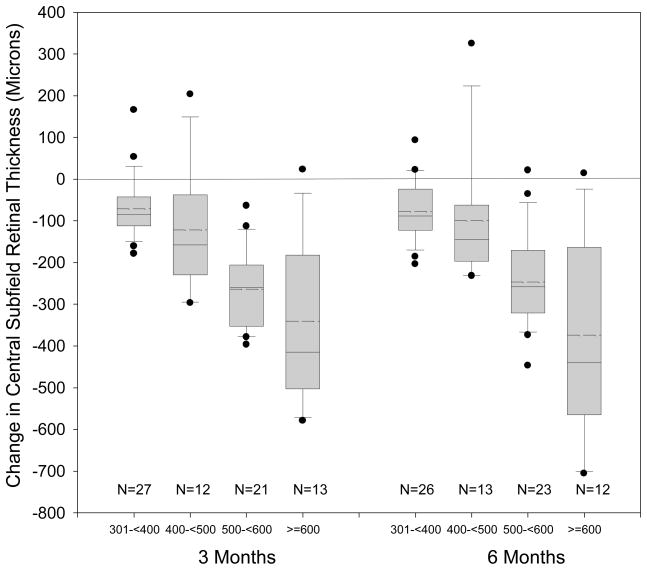
Median retinal central subfield thickness at baseline was 491 microns (interquartile range 356 to 586 microns). At both 3 and 6 months there was a median 160 micron decrease from baseline in the central subfield thickness (P<0.001, Figure 2). At 3 months, 82% and 68% had a decrease in thickness from baseline of 50 and 100 microns or more, respectively while only 3 (4%) eyes experienced an increase in thickness of at least 50 microns. At 6 months, the reduction in thickness from baseline of 50 and 100 microns or more were seen in 82% and 66% of the eyes, respectively, while 68% decreased in thickening by at least 50%. Reduction of central subfield thickness to less than 250 microns occurred in 33 eyes (43%). Eyes with greater central subfield thickness at baseline tended to have greater reduction in thickness after surgery (P<0.001). Results for OCT-measured retinal volume were similar to the central subfield results (data not shown).
Figure 2.
Distribution of Change in optical coherence tomography (OCT) Central Subfield Thickness in Categories According to Baseline Thickness
Box-whisker plot demonstrating mean (dashed horizontal line), median (solid horizontal line), 25 – 75th percentiles (extremes of the box), 10–90th percentiles (whiskers) and 5–95th percentiles (solid circles) of change in optical coherence tomography (OCT) central subfield.
From baseline to 3 months, 55 (81%) of 68 eyes with OCT measurements at baseline, 3 months and 6 months had a reduction in OCT central subfield of 50 microns or more, 10 (15%) changed by less than 50 microns and 3 (4%) worsened 50 microns or more. Among the 55 eyes that improved at least 50 microns from baseline to 3 months, 8 (15%) improved at least 50 more microns from 3 months to 6 months, 36 (65%) changed less than 50 microns and 11 (20%) worsened 50 microns or more. Among 10 eyes that changed less than 50 microns from baseline to 3 months, 8 changed less than 50 microns from 3 months to 6 months and 2 worsened 50 or more microns. Among 3 eyes that worsened 50 or more microns from baseline to 3 months, 2 improved 50 or more microns between 3 months and 6 months and 1 changed less than 50 microns.
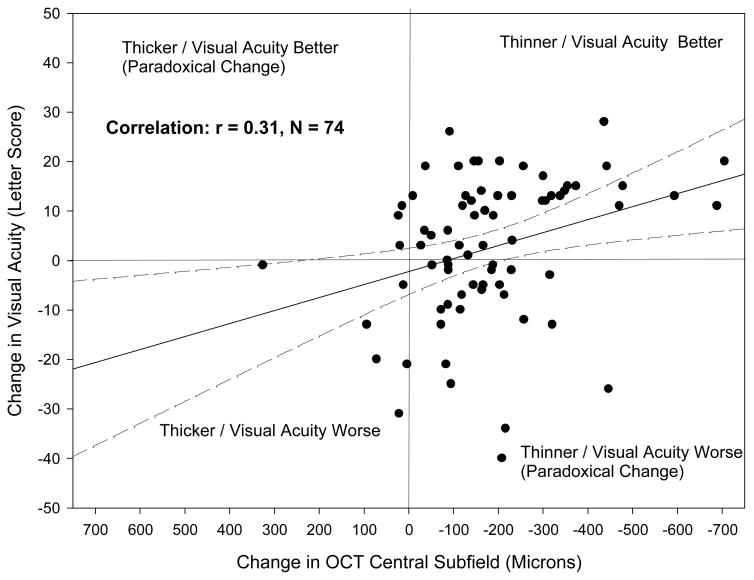
The correlation between changes in OCT central subfield thickness from baseline to 6 months and changes in visual acuity during this time was −0.31 (Figure 3). Except for cases with very large decreases in central subfield thickness (>350 microns), a given decrease in OCT was associated with a wide range of changes in visual acuity.
Figure 3.
Comparison of Change in Optical Coherence Tomography (OCT) Central Subfield and Change in Visual Acuity from Baseline to 6 Months. The solid line represents the regression line and the dotted lines represent the 95% confidence interval for the mean.
Postoperative Complications and Treatments
Of the 87 subjects, 16 (18%) experienced postoperative complications in the first 6 months (Table 3). Of greatest importance, 4 eyes developed a vitreous hemorrhage, 2 eyes developed a retinal detachment, 1 eye developed endophthalmitis and 1 eye developed vitreous hemorrhage and retinal detachment; 4 of these 8 eyes lost 10 or more letters from baseline to 6 months. One additional eye had a retinal detachment after the first 6 months.
Table 3.
Postoperative Complications (0 to 6 months, N=87)*
| Postoperative complications - N (%) | 16 (18%) |
| Vitreous hemorrhage | 5 (6%) |
| Development of additional vitreomacular interface abnormalities | 2 (2%) |
| Elevated IOP requiring treatment | 7 (8%) |
| Retinal detachment | 3 (3%) |
| Retinal tear | 0 |
| Endophthalmitis | 1 (1%) |
| Macular ischemia | 0 |
| Double vision | 2 (2%) |
| Lamella hole | 1 (1%) |
| Choroidal effusion | 1 (1%) |
| Other | 2 (2%) |
Same subject could have more than one complication
IOP=Intraocular pressure
Twenty eight (78%) of 36 eyes that were phakic at the time of vitrectomy (by definition, the cohort did not include eyes that had cataract surgery at the time of vitrectomy) and completed 6 month visit developed lens changes by 6 months based on investigator assessment, including 5 eyes that had cataract surgery by 6 months (Table 4). Cataract surgery was performed in 12 eyes between 6 and 12 months, for a total of 17 (46%) of the 37 eyes that were phakic prior to vitrectomy having cataract surgery within 12 months following vitrectomy.
Table 4.
Investigator assessment of lens changes from baseline to 6 months in eyes phakic at baseline* (N=36**)
| 6 Month |
||||
|---|---|---|---|---|
| Baseline | Absent | Present <standard | Present >=standard | Cataract Extraction |
| Nuclear Sclerosis (N=36) | ||||
| Absent | 1 (3%) | 1 (3%) | 0 | 1 (3%) |
| Present < standard | 0 | 13 (36%) | 8 (22%) | 4 (11%) |
| Present >= standard | 0 | 2 (6%) | 6 (17%) | 0 |
| Posterior Subcapsular Cataract (N=36) | ||||
| Absent | 15 (42%) | 6 (17%) | 3 (8%) | 4 (11%) |
| Present < standard | 1 (3%) | 4 (11%) | 1 (3%) | 1 (3%) |
| Present >= standard | 0 | 0 | 1 (3%) | 0 |
| Cortical Cataract (N=36) | ||||
| Absent | 12 (33%) | 6 (17%) | 0 | 3 (8%) |
| Present < standard | 4 (11%) | 7 (19%) | 2 (6%) | 2 (6%) |
| Present >= standard | 0 | 0 | 0 | 0 |
| Highest grade among all 3 types of lens opacity | ||||
| Absent | 1 (3%) | 0 | 0 | 1 (3%) |
| Present < standard | 0 | 14 (39%) | 8 (22%) | 4 (11%) |
| Present >= standard | 0 | 2 (6%) | 6 (17%) | 0 |
Bold cells represent cataract progression. By definition of the cohort, no eyes had cataract extraction at the time of vitrectomy.
One eye that was phakic at baseline did not complete 6 month visit.
Postoperatively, no eye had PRP performed, 4 eyes had macular laser performed, 2 eyes had intravitreal injections of corticosteroid and 2 eyes received injections of anti vascular endothelial growth factor within the first 6 months.
12-month Outcomes
Between 6 months and 12 months, 20 (26%) of 78 study eyes that completed 12 month visit received some form of treatment for DME and 58 (74%) did not, including 10 eyes that received laser (including 1 eye listed below that also received intravitreal corticosteroids), 8 eyes that received intravitreal corticosteroids (including 1 eye that also received laser listed above and 1 eye that also received peribulbar steroids listed below), 2 eyes that received peribulbar steroids (including 1 eye noted above that also received intravitreal corticosteroids and 1 eye that listed below that also received intravitreal bevacizumab), and 3 eyes that received intravitreal bevacizumab (including 1 eye noted above that also received peribulbar steroids).
At one year, median visual acuity was approximately 20/80 (interquartile range 20/50 to 20/160), with 30 (38%) of the 78 eyes having improved 10 or more letters from the preoperative visual acuity and 20 (26%) having worsened 10 or more letters. Of the 29 eyes that had improved by 10 or more letters from baseline at 6 months, 22 still had 10 more letter improvement from baseline to 1 year and only 1 worsened 10 or more letters from baseline to 1 year. Of the 17 eyes that lost 10 or more letters from baseline at 6 months, 12 still had lost 10 or more letters from baseline at 1 year and only 1 had improved 10 or more letters form baseline to 1 year.
Median OCT central subfield thickness at 12 months was 256 microns (interquartile range 205 to 340 microns), with the median change from the preoperative OCT measurement being a decrease in thickness of 153 microns (interquartile range 286 to 61 microns). In 33 (47%) of the 70 eyes with a 12-month OCT, central subfield thickness was <250 microns.
Discussion
In this prospective study of 87 eyes undergoing vitrectomy for DME associated with at least moderate visual loss and investigator-determined vitreomacular traction, the median change in visual acuity at 6 months was an improvement of 3 letters, with visual acuity improving 10 or more letters from baseline to 6 months in 38% (95% confidence interval 28% to 49%) and worsening 10 or more letters in 22% (95% confidence interval 13% to 31%). Reduction in OCT central subfield thickness to <250 microns occurred in almost half, and most eyes had a reduction of thickening of at least 50%. As one might expect, eyes with greater retinal thickness at baseline tended to have greater reduction in retinal thickness following surgery likely reflecting, at least in part, a floor effect on the amount of thickness reduction that can occur when the macula is only mildly thickened. Little changes in results were noted between 6 months and one year even though additional procedures to treat DME were performed in 20 subjects and cataract surgery in 12 subjects.
With respect to safety, the surgical complication rate, including vitreous hemorrhage, retinal detachment or other serious adverse events, was similar to what has been reported for this procedure.9, 10, 12–19, 22–27, 30–34, 37 Most phakic eyes developed lens changes by 6 months after vitrectomy, which may account for some decrease in visual acuity between 3 and 6 months.
The surgical techniques recorded appear to mirror recent vitreoretinal surgical practice trends in North America,38 characterized by the increased use of smaller gauge vitrectomy systems, injection of triamcinolone acetate and other agents to aid in intraoperative visualization of membranes and widespread use of epiretinal and internal limiting membrane peeling in the treatment of patients with macular disorders. However, the relative benefits or risks of these preferences on visual acuity outcomes remain unknown.
There are several strengths to this study, including the prospective collection of visual acuity and anatomic outcomes following vitrectomy for DME in the presence of vitreoretinal traction. This is also the first large surgical series to have OCT measurements at baseline and during follow-up. Other strengths of this study include uniform entry criteria and greater than 90% follow-up through 6 months. Although this study did not mandate standardizing the precise surgical maneuvers used, data regarding the details of surgery were acquired in a standardized fashion.
A potential study weakness is that the assessment of the presence or absence of vitreomacular traction was made by the individual investigators based on their clinical judgment, without standardized criteria and without central reading center assessment or independent confirmation. However, the lack of centralized assessment may have more generalizability when applying these results to clinical practice, where there generally is no independent confirmation of vitreomacular traction.
The lack of a concurrent control group also is a study weakness. Our study was designed as a prospective cohort investigation rather than as a randomized trial of vitrectomy versus laser or observation because of a lack of equipoise on the part of the participating surgeons, who were uncomfortable randomizing eyes with traction and decreased vision to a non-vitrectomy trial arm.
The study protocol to defer macular photocoagulation until after 6 months may also have affected the visual acuity and retinal thickness outcomes. In addition, approximately two-thirds of these cases had PDR. It is possible that the visual outcome for cases with this degree of vascular compromise or retinal ischemia associated with PDR is worse than DME treatments in the absence of PDR. Also, many of these eyes may have had limited potential for improvement and a relatively high chance of losing vision due to complications of PDR or associated capillary non-perfusion in the macula. Even though approximately 40% of the study eyes received PRP intraoperatively, which in the setting of pre-existing DME might have exacerbated the macular edema; very few eyes had an increase in edema. Among the eyes that received PRP, the average change in OCT from baseline to 6 months appeared similar to the average change in eyes that did not receive PRP. An additional consideration may be that eyes in the study had relatively poor visual acuity (median 20/100) and a relatively thickened macula (median central subfield thickness 491 microns) at the time of vitrectomy. Perhaps earlier intervention in these compromised eyes would have improved visual results.
There are few reports in the literature with which to compare these DRCR.net results. Hikichi et al reported a series of 53 consecutive non-diabetic eyes followed with vitreomacular traction in the pre-OCT era,39 64% of which lost 2 or more lines of vision over the course of follow-up. In the absence of a spontaneous peripheral vascular disease in this series, 87% lost vision at the final visit. Since the report by Smiddy et al in 1988 describing successful surgery for non-diabetic eyes with macular traction and visual decrease,40 many surgeons have elected to operate rather than to follow patients with evident vitreomacular traction and significant visual impairment. The report by Lewis et al in 1992 and subsequent series extended this surgical indication to include diabetic patients with evidence of tangential hyaloidal traction.17 This study did not examine the impact of vitrectomy for DME when traction was not identified by the investigator. Reports in the literature on vitrectomy for such eyes, in the absence of traction, include mixed visual acuity results. While some studies suggested positive outcomes,9, 14, 16, 30, 33 recent studies have shown anatomical but not visual improvement after surgery.10, 19, 24
In summary, this report adds prospective visual acuity and OCT data to our understanding of the effect of vitrectomy on DME in eyes with visual acuity 20/63 to 20/400 in the presence of vitreomacular traction and central subfield thickening confirmed on OCT, when cataract surgery is not performed at vitrectomy. Vitrectomy performed for this indication with the techniques reported herein usually resulted in a reduction in macular thickening. Visual acuity results were less consistent with some eyes improving (estimated between 28% to 49%) and some eyes worsening (estimated between 13% to 31%). Whether vitrectomy provides an improvement over other therapies or over the natural history of DME in this setting requires further investigation.
Acknowledgments
Supported through a cooperative agreement from the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Department of Health and Human Services EY14231, EY14269, EY14229
Appendix
Writing Committee: Lead Authors: Julia A. Haller, M.D.,1 Haijing Qin, M.S.,2 Additional Writing Committee Member (Alphabetical) Rajendra S. Apte, M.D.,3 Roy R. Beck, M.D.,2 Neil M. Bressler, M.D.,4 David J. Browning, M.D.,5 Ronald P. Danis, M.D.,6 Adam R. Glassman, M.S,2 Joseph M. Googe, M.D.,7 Craig Kollman, Ph.D.,2 Andreas K. Lauer M. D.,8 Mark A. Peters M. D.,9 Margaret E. Stockman,10
Wills Eye Institute,1 Jaeb Center for Health Research,2 Barnes Retina Institute,3 Wilmer Eye Institute,4 Charlotte Eye Ear Nose and Throat Assoc., PA 5 University of Wisconsin Dept. of Ophthalmology & Visual Sciences,6 Southeastern Retina Associates, PC,7 Oregon Health & Science University,8 Retina Northwest, PC,9 Joslin Diabetes Center,10
Diabetic Retinopathy Clinical Research Network Clinical Sites that participated on this protocol
Sites are listed in order by number of subjects enrolled into the study. The number of subjects enrolled is noted in parenthesis preceded by the site location and the site name. Personnel are listed as (I) for Investigator, (C) for Coordinator, (V) for Visual Acuity Tester and (P) for Photographer.
Portland, OR Casey Eye Institute (27): Andreas K. Lauer(I); David J. Wilson(I); Christina J Flaxel(I); Susan I. Pope (C,V); Ann D. Lundquist(V); Maureen D. Toomey(V); Shirley D. Ira(V); Debora R. Vahrenwald(V); Susan K. Nolte(V); Peter N. Steinkamp(P); Joseph Cilio Rossi(P); Kelly L. West(P); Scott R. Pickell(P); Patrick B. Rice(P); Chris S Howell(P); Jessica M Gaultney(P); Patrick R. Wallace(P) Jacksonville, FL University of Florida College of Med., Jacksonville Health Science Cent (18): Kakarla V. Chalam(I); Shailesh K. Gupta(I); Sandeep Grover(I); Ravi Keshavamurthy (C,V); Tamil M Singh(C); Sadiq N. Syed(C); Vikram S. Brar(P); John R. Carpentier(P) Knoxville, TN Southeastern Retina Associates, P.C. (18): Joseph Googe(I); Stephen L. Perkins(I); Tod A. McMillan(I); Christina T. Higdon (C,V); Stephanie Evans(C); Charity D. Morris(C); Mary M. Johnson(V); Vicky L. Seitz(V); Cecile Hunt(V); Ann Arnold(V); Jerry K. Whetstone(P); David J. Cimino(P); Paul A. Blais(P); Michael Jacobus(P) Boston, MA Joslin Diabetes Center (14): George S. Sharuk(I); Jennifer K. Sun(I); Timothy J. Murtha(I); Deborah K. Schlossman(I); Lloyd Paul Aiello(I); Paul G. Arrigg(I); Sabera T. Shah(I); Ann Kopple(C); Margaret E Stockman (C,P,V); Richard M. Calderon(V); Elizabeth S. Weimann(V); Jerry D. Cavallerano(V); Leila Bestourous(V); James Strong(P); Robert W. Cavicchi(P); Ellen L. Casazza(P) Lakeland, FL Central Florida Retina Institute (12): Scott M. Friedman(I); Oren Zev Plous(I); Kelly A. Blackmer(C); Jolleen S. Key (C,P,V); Steve Carlton (C,P); Jessica Maldonado(V); Yvette Fraser-Neumann(V); Virginia Gregory(V); Karen Sjoblom(V); Katie Gostischa(P); Sheila Walters-Treon(P); Allen McKinney(P) St. Louis, MO Barnes Retina Institute (12): Rajendra S. Apte(I); Nicholas E Engelbrecht(I); Prabakar Kumar Rao(I); Kevin J. Blinder(I); Ginny S. Nobel(C); Pamela A. Light(C); Rhonda F. Weeks(C); Lynda K. Boyd(V); Carolyn L. Walters(V); Merrilee K. Sgorlon(V); Tammy J Ressel(V); Annette M. Vaughn(P); Timothy L Wright(P); Dana L Gabel(P); Matt L. Raeber(P); Jarrod Wehmeier(P) Charlotte, NC Charlotte Eye, Ear, Nose and Throat Assoc., PA (9):David Browning(I); Andrew N. Antoszyk(I); Danielle R. Brooks (C,V); Jennifer V. Helms (C,V); Angela K. Price (C,V); Melissa K. Cowen (C,V); Angella S. Karow(V); Wayne Lail(V); Heather L. Murphy(V); Rachel E. Pierce(V); Sarah A. Ennis(V); Roderick O. Walker(V); Michele E. Powers(P); Jennifer A. Ballard(P); Linda M Davis(P); Richard J. George(P); Uma M. Balasubramaniam(P); Donna McClain(P); Michael D. McOwen(P); Loraine M. Clark(P) Portland, OR Retina Northwest, PC (9): Mark A. Peters(I); Irvin L. Handelman(I); Stephen Hobbs (C,P,V); Dawn A. Hudson (C,V); Sennie M. Kramer(V); Marcia Kopfer(V); Joe Logan(P); Howard Daniel(P); Christine Hoerner(P); Harry Wohlsein(P) New Albany, IN John-Kenyon American Eye Institute (8): Howard S. Lazarus(I); Debra Paige Bunch (C,V); Angela D. Ridge(C); Kelly Booth(V); Liana C. Davis(V); Margaret Trimble(P); Jay Moore(P) Baltimore, MD Wilmer Eye Institute at Johns Hopkins (7): Sharon D. Solomon(I); Daniel Finkelstein(I); Neil M. Bressler(I); Mary Frey (C,V); Sandra West (C,V); Warren Doll (C,V); Vanessa Kellner(V); Deborah Donohue(V); Dennis Cain(P); Syed M. Shah (P); David Emmert(P); Rachel Falk(P); Janis Graul(P); Jacquelyn McDonald(P); Charles Herring(P) Salt Lake City, UT Rocky Mountain Retina Consultants (7): David W. Faber(I); Douglas S. Mehr(I); Roy A. Goodart(I); Hollie J. Murphy(C); Trisha L. Perkins(P); Richard W. Osguthorpe(P); Donna Knight(P) Slingerlands, NY Retina Consultants, PLLC (7): Paul M. Beer(I); Naomi Falk(I); Eugenia Olmeda (C,V); Amy L. Newton (V); Charisse Whitney(V); Lisa A. Faulhammer(P); Joe Fischer(P) Rapid City, SD Black Hills Regional Eye Institute (6): Prema Abraham(I); Kristi Livermont(C); Buffi L. Green(C); Kayla Riley(V); Deborah Stewart(V); Erin Robbins(V); Teresa A. Frisk(V); Dan Parks(P) Detroit, MI Henry Ford Health System, Dept of Ophthalmology and Eye Care Services (5): Paul Andrew Edwards(I); Michael D. Ober(I); Janet Murphy (C,V); Damon Q. Fletcher (C,V); Sheila M Rock (C,V); Mary K. Monk (C,V); Bradley A. Stern(P); Lisa M. Schillace(P); Mark Croswell(P); Tracy A. Troszak(P); Steven F. Ogilvy(P) Providence, RI Retina Consultants (5): Caldwell W. Smith(I); Harold A. Woodcome(I); Robert H. Janigian(I); Collin L. DuCoty(C); Emiliya German(C); Sylvia Varadian(C); Erika Banalewicz(V); Claudia Salinas(V); Mark Hamel(P) Syracuse, NY Retina-Vitreous Surgeons of Central New York, PC (5): G. Robert Hampton(I); Paul F. Torrisi(I); Bryan K. Rutledge(I); Cindy J. Grinnell(C); Fayth M. DiSano (C,V); Lynn M. Kwasniewski(V); Tanya C. Czajak(V); Peter B. Hay(P); Lynn A. Capone(P); Bob Corey(P); Kelly M. Harrison(P) Columbia, SC Carolina Retina Center (4): Jeffrey G. Gross(I); Barron C. Fishburne(I); Amy M. Flowers (C,V); Peggy W. Cummings(C); Kayla L. Henry (C,V); Regina A. Gabriel(V); Heidi K. Lovit(V); Kristin K. Bland(V); Randall L. Price(P); Chris N. Mallet(P); Rick Christoff(P) Loma Linda, CA Loma Linda University Health Care, Department of Ophthalmology (4): Joseph T. Fan(I); Kara E. Rollins (C,P,V); Carrousel J. Corliss (C,P,V); William H. Kiernan(V); Johnathan D. Cloud(P); Gene Saldana(P); William Milam(P) Abilene, TX West Texas Retina Consultants P.A. (3): Sunil S. Patel(I); Yvone Flores(C); Kristen L. Garcia (C,P); Brenda K. Arrington(P); Misty Dawn McArthur(P); Tamara A. Bartlett(P) Austin, TX Retina Research Center (3): Brian B. Berger(I); Ginger J. Manhart(C); Renee Morris(C); Linda N. Nguyen (C,V); Telisa L. Clevenger-Smith(C); Erin N. Scrivner (C,V); Elisabeth A. Durham(C); Melissa A. Talbert(V); Nicole Callen(V); Yong Ren(P); Ben Ostrander(P) Baltimore, MD Elman Retina Group, P.A. (3): Michael J. Elman(I); Michelle D. Sloan(C); JoAnn Starr (C,V); Pamela V. Singletary (C,V); Theresa M. Butcher(C); Dena Y. Salfer-Firestone(V); Teresa Coffey(V); Giorya Andreani(P); Terri Cain(P); Peter Sotirakos(P) Lubbock, TX Texas Retina Associates (3): Michel Shami(I); Carrie L. Tarter (C,V); Phyllis Pusser(C); Linda Squires(V); Thom F. Wentlandt(P) McAllen, TX Valley Retina Institute (3): Victor Hugo Gonzalez(I); Vincent R. Vann(I); Yu-Tang James Su(I); ReAnna L. McNames(C); Jessica Herrera (C,P); Marlene Lopez(V); Maria M. Martinez(V); Maria G. Trevino(V); Alma Herrera(V); Cassandra Garza(P); Daniel Cuellar(P); Rosie M. Corona(P) Minneapolis, MN University of Minnesota (3): Todd Robert Klesert(I); Timothy W. Olsen(I); Jamie Marie Walski(C); Sally Cook(C); Dave Philiph(V); Sabrina M. Rolfer(V); Pamela K. Patterson(V); Pat Stanaitis Harvey(P); Mark J. Cohen(P) Minneapolis, MN Retina Center, PA (3): Abdhish Bhavsar(I); Geoffrey G. Emerson(I); Vu T. Huynh(C); Jennifer Ries(C); Tanya M Olson(C); Craig H. Hager(V); Dwight L. Selders(V); Melinda E. Spike(V); Christopher M. Smith(P); William B. Carli(P); Laura Taylor-Reetz(P); Jessica A. Kells(P); Carmen Chan-Tram(P) Salisbury, MD Retina Consultants of Delmarva, P.A. (3): Jeffrey D. Benner(I); John W. Butler(I); Hannah Scott (C,V); Jennifer M. McCrorey(V); Cristy Carbaugh(P); Robin L. Hurley(P) Sarasota, FL Sarasota Retina Institute (3): Melvin Chen(I); John H. Niffenegger(I); Keye L. Wong(I); Christine Holland(C); Karen Hagin(V); Hasseema R Shelton(V); Rosa Miller(P); Mark Sneath(P) Seattle, WA University of Washington Medical Center (3): James L. Kinyoun(I); Susan A. Rath (C,V); Patricia K. Ernst(V); James D. Leslie(P); Chuck Stephens(P); Brad C. Clifton(P) West Columbia, SC Palmetto Retina Center (3): W. Lloyd Clark(I); Marcia D. Gridine (C,V); Cassie P. Cahill (C,V); Peggy D. McDougal(V); Robbin Spivey(P); Melissa L. Henderson(P); Amy B. Hickman(P) Aiea, HI The Retina Center at Pali Momi (2): Gregg T. Kokame(I); Jacqueline Shen(C); Andrew Yuen(P) Charlotte, NC Horizon Eye Care, PA (2): Miriam E. Ridley(I); April E. Glessner(C); Mara-Leigh Schafer(C); Dali Munoz(V); Amy A. Brogdon(V); Jeanine K. Cisco(P); Viktor Kummer(P); David C. Peterson(P) Dallas, TX Texas Retina Associates (2): Robert C. Wang(I); Jean Arnwine(C); Brenda Sanchez(V); Diana Jaramillo(P); Hank Aguado(P); Kimberly Cummings(P) Grand Rapids, MI Associated Retinal Consultants (2): Thomas M. Aaberg(I); Debra Markus (C,P); Sarita Scott (C,V); Sandy Kronlein (C,V); Sandra Lewis(P) Houston, TX Retina and Vitreous of Texas (2): H. Michael Lambert(I); Mikki R. O’Neal (C,V); Susan K. Busch (C,P,V); Allison W. Schmidt(P); Joseph A. Morales(P) Indianapolis, IN Raj K. Maturi, M.D., P.C. (2): Raj K. Maturi(I); Laura A. Bleau (C,P,V); Michelle Storie(V); Carolee K. Novak(V); Thomas Steele(P); Jama L. Poston(P); Abby Maple(P) Madison, WI University of Wisconsin-Madison, Dept of Ophthalmology/Retina Service (2): Michael M. Altaweel(I); Michael S. Ip(I); Kathryn F. Burke(C); Barbara H. Soderling (C,V); Angela M. Wealti(V); Guy F. Somers(V); Denise A. Krolnik(P); Gene E. Knutson(P); John C. Peterson(P) Nashville, TN Vanderbilt University Medical Center (2):Franco Maria Recchia(I); Christine C. Franklin(C); Kamila M. Kinder (C,V); Sandy Owings (C,V); Mary B. Taylor-Ward(V); Tony Adkins(P) Palm Springs, CA Southern California Desert Retina Consultants, MC (2): Clement K Chan(I); David M. Salib(I); Steven G Lin(I); Asha S.D. Nuthi(I); Kimberly S. Walther(C); Isela Aldana(C); Teri A. Andresen(C); Tina B Wiskirchen(C); Sara Warren(V); Kara Rollins(V); Sandra U. Castillo(V); Donna J. Chesbrough(P); Kenneth M Huff(P) San Francisco, CA West Coast Retina Medical Group, Inc. (2): J. Michael Jumper(I); H. Richard McDonald(I); Margaret Stolarczuk(C); Paula Fiermonte(C); Silvia C. Linares(V); Jeremy Miller(P); Dawn M. Ryan(P); Rona Lyn Esquejo(P); Sarah Huggans(P) Beachwood, OH Retina Associates of Cleveland, Inc. (1): David G. Miller(I); Elizabeth McNamara (C,V); Trina M. Nitzsche(V); Sheila K. Smith-Brewer(P); Larraine Stone (C) Hermitage, PA Vitreo-Retinal Consultants, Inc. (1): Stephen R. Kaufman(I); Robert E. Wenz(I); Kathleen A. Huff(C); Denise M. Williams(P) Honolulu, HI Retina Associates of Hawaii, Inc. (1): John H. Drouilhet(I); Susan Pelke(C); Teresa Bell(V); Deborah Nobler(P) Houston, TX Charles A. Garcia, P.A and Associates (1): Charles A. Garcia(I); Edgardo Santisbon (C,V); Penelope Reyes Villeda (C,V); Emma M. Lessieur (C,V); Cecilia Vi Nguyen(V); Juan P. Montoya(V); Cary A. Stoever(P); Luis R. Salinas(P); Sindya M. Cerda(P) Irvine, CA University of California, Irvine (1): Baruch D. Kuppermann(I); Jeff Grijalva(C); Rosie Magallon(V); Bret Trump(P) Kingsport, TN Southeastern Retina Associates, PC (1): Howard L. Cummings(I); D. Allan Couch(I); Deanna Jo Long (C,P); Gail Darnell(C); Stacy Carpenter(V) Oklahoma City, OK Dean A. McGee Eye Institute (1): Robert E. Leonard(I); Misty D. Youngberg(C); Jesse S. Hart(V); Connie J. Dwiggins(V); Russ Burris(P); William R. Richmond(P) Paducah, KY Paducah Retinal Center (1): Carl W. Baker(I); Tracey M. Caldwell (C,P); Tracey R. Martin(V); Lynnette F. Lambert(V); Dawn D. Darden(P); Alecia B. Travis(P) Toms River, NJ Retina Vitreous Center, PA (1): Daniel B Roth(I); Thea L Tantum (C,V); Finn Anderson(P) Walnut Creek, CA Bay Area Retina Associates (1): Stewart A. Daniels(I); Maria Carmencita Aguilos(C); Nicole Hom(V); William M. Combs(V); Sean M. Teshima-McCormick(V); Fred Hanamoto(P) Winter Haven, FL Center for Retina and Macular Disease (1): Michael Tolentino(I); Richard S. Hamilton(I); Suk Jin Moon(I); Janelle L. Davies Anderson(C); Shelly L. Cooper(V); Cindy Williams(V); Donald Trueman(V); Vera Dilts(P)
DRCR.net Coordinating Center- Jaeb Center for Health Research, Tampa, FL (staff as of 3/31/09): Roy W. Beck (Director), Adam R. Glassman (Assistant Director), Brian B. Dale, Alyssa Baptista, Sharon R. Constantine, Simone S. Dupre, Allison R. Edwards, Meagan Hutton, Paula A. Johnson, Craig Kollman, Lee Anne Lester, Brenda L. Loggins, Shannon L. McClellan, Lina Caicedo, Pamela S. Moke, Haijing Qin, Rosa Pritchard, Hiram Ramirez, Eureca Scott, Cynthia R. Stockdale
Fundus Photograph Reading Center – University of Wisconsin-Madison, Madison, WI (staff as of 5/1/08): Matthew D. Davis (Director Emeritus), Ronald P. Danis (Director), Larry Hubbard (Associate Director), James Reimers (Lead Color Photography Evaluator), Pamela Vargo (Lead Photographer), Ericka Moeller (Digital Imaging Specialist), Dawn Myers (Lead OCT Evaluator), Michael Daywalt (Project Manager)
DRCR.net Chair: Neil M. Bressler – Baltimore, MD (2006-current), Lloyd Paul Aiello – Boston, MA (2002 – 2005)
National Eye Institute: Donald F. Everett, Päivi H. Miskala (2007)
Steering Committee: Alexander J. Brucker (2005–2008), David Browning (2005–2008), Steve Carlton (2006 – 2007) Emily Y. Chew (2005–2008), Ronald P. Danis (2003–2008), Julia A. Haller (2005–2008), Lloyd P. Aiello (2003–2008), Carl W. Baker (2007 –2008), Neil M. Bressler (2005–2008), Debra Paige Bunch (2007–2008), Donald F. Everett (2006–2008), Frederick Ferris (2005–2008), Don S. Fong (2003–2007), Adam R. Glassman (2005–2008), Jeffrey G. Gross (2006 – 2007), Helen K. Li (2006 – 2007), Dennis M. Marcus (2007 – 2008), Päivi Miskala, Ph.D. (2005–2007).
Executive Committee: Michael J. Elman (Chair 2009- present), Neil M. Bressler (Chair 2006–2009), Lloyd Paul Aiello (2002-present; Chair 2002 – 2005), Andrew N. Antoszyk (2009), Roy W. Beck (2002-present), Susan B. Bressler (2009-Present), Alexander J. Brucker (2009-present), Ronald P. Danis (2004-present), Matthew D. Davis (2002-present),, Donald F. Everett (2002-present), Frederick L. Ferris(2002-present), Joan Fish (2008 - present), Scott Friedman (2007 – present), Adam R. Glassman (2002-present) Joseph Googe (2009-present), Raj K. Maturi (2009-present), Ingrid U. Scott (2009-Present). Prior Members: Abdhish Bhavsar (2007 – 2008), David M. Brown (2006–2007), David J. Browning (2005–2006), Andreas Lauer (2007–2008) Kim McLeod (2002–2006), Päivi H. Miskala(2005–2007), Cynthia J. Grinnell (2006–2007).
Footnotes
An address for reprints will not be provided.
Conflicts of interest statement: A complete list of all DRCR.net investigator financial disclosures can be found at www.drcr.net.
Dr. Neil M. Bressler Finacial relationships: Grants to investigators at The Johns Hopkins University are negotiated and administered by the institution which receives the grants, typically through the office of Research Administration. Individual investigators who participate in the sponsored project(s) are not directly compensated by the sponsor, but may receive salary or other support from the institution to support their effort on the project(s).
Dr. Neil Bressler is Principal Investigator for the following: Allergan, Bausch & Lomb, Bristol-Meyers Squibb, Carl Zeiss Meditec, EMMES Corporation, Genentech, Lumenis, Notal Vision, Novartis, Othera, QLT, Regeneron, Steba Biotech.
Dr. Susan Bressler (spouse) is Co-Investigator for the following: Genentech, Inc. Dr. Susan Bressler has been or is presently Consultant for the following: AstraZeneca, Genentech, Notal Vision, Pfizer.
But we do not believe that any of these constitutes a financial conflict of interest for this manuscript.
References
- 1.Aiello LP, Bursell SE, Clermont A, et al. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes. 1997;46:1473–80. doi: 10.2337/diab.46.9.1473. [DOI] [PubMed] [Google Scholar]
- 2.Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin epidemiologic study of diabetic retinopathy. XV. The long-term incidence of macular edema. Ophthalmology. 1995;102:7–16. doi: 10.1016/s0161-6420(95)31052-4. [DOI] [PubMed] [Google Scholar]
- 3.Moss SE, Klein R, Klein BE. Ten-year incidence of visual loss in a diabetic population. Ophthalmology. 1994;101:1061–70. doi: 10.1016/s0161-6420(94)31217-6. [DOI] [PubMed] [Google Scholar]
- 4.Moss S, Klein R, Klein B. The 14-year incidence of visual loss in a diabetic population. Ophthalmology. 1998;105:998–1003. doi: 10.1016/S0161-6420(98)96025-0. [DOI] [PubMed] [Google Scholar]
- 5.Diabetic Retinopathy Clinical Research Network. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115:1447–9. 1449, e1–10. doi: 10.1016/j.ophtha.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103:1796–806. [PubMed] [Google Scholar]
- 7.Writing Committee for the Diabetic Retinopathy Clinical Research Network. Fong DS, Strauber SF, et al. Comparison of the modified Early Treatment Diabetic Retinopathy Study and mild macular grid laser photocoagulation strategies for diabetic macular edema. Arch Ophthalmol. 2007;125:469–80. doi: 10.1001/archopht.125.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ip MS, Bressler SB, Antoszyk AN, et al. A randomized trial comparing intravitreal triamcinolone and laser photocoagulation for diabetic macular edema: Baseline features. Retina. 2008;28:919–30. doi: 10.1097/IAE.0b013e31818144a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dillinger P, Mester U. Vitrectomy with removal of the internal limiting membrane in chronic diabetic macular oedema. Graefes Arch Clin Exp Ophthalmol. 2004;242:630–7. doi: 10.1007/s00417-003-0849-8. [DOI] [PubMed] [Google Scholar]
- 10.Figueroa MS, Contreras I, Noval S. Surgical and anatomical outcomes of pars plana vitrectomy of diffuse nontractional diabetic macular edema. Retina. 2008;28:420–6. doi: 10.1097/IAE.0b013e318159e7d2. [DOI] [PubMed] [Google Scholar]
- 11.Foos RY, Kreiger AE, Forsythe AB, Zakka KA. Posterior vitreous detachment in diabetic subjects. Ophthalmology. 1980;87:122–8. doi: 10.1016/s0161-6420(80)35269-x. [DOI] [PubMed] [Google Scholar]
- 12.Gandorfer A, Messmer EM, Ulbig MW, Kampik A. Resolution of diabetic macular edema after surgical removal of the posterior hyaloid and the inner limiting membrane. Retina. 2000;20:126–33. [PubMed] [Google Scholar]
- 13.Harbour JW, Smiddy WE, Flynn HW, Jr, Rubsamen PE. Vitrectomy for diabetic macular edema associated with a thickened and taut posterior hyaloid membrane. Am J Ophthalmol. 1996;121:405–13. doi: 10.1016/s0002-9394(14)70437-4. [DOI] [PubMed] [Google Scholar]
- 14.Ikeda T, Sato K, Katano T, Hayashi Y. Vitrectomy for cystoid macular edema with attached posterior hyaloid membrane in patients with diabetes. Br J Ophthalmol. 1999;83:12–4. doi: 10.1136/bjo.83.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhn F, Kiss G, Mester V, et al. Vitrectomy with internal limiting membrane removal for clinically significant macular oedema. Graefes Arch Clin Exp Ophthalmol. 2004;242:402–8. doi: 10.1007/s00417-004-0876-0. [DOI] [PubMed] [Google Scholar]
- 16.La Heij EC, Hendrikse F, Kessels AGH, Derhaag PJFM. Vitrectomy results in diabetic macular oedema without evident vitreomacular traction. Graefes Arch Clin Exp Ophthalmol. 2001;239:264–70. doi: 10.1007/s004170000251. [DOI] [PubMed] [Google Scholar]
- 17.Lewis H, Abrams GW, Blumenkranz MS, Campo RV. Vitrectomy for diabetic macular traction and edema associated with posterior hyaloidal traction. Ophthalmology. 1992;99:753–9. doi: 10.1016/s0161-6420(92)31901-3. [DOI] [PubMed] [Google Scholar]
- 18.Micelli Ferrari T, Cardascia N, Durante G, et al. Pars plana vitrectomy in diabetic macular edema. Documenta Ophthalmologica. 1999;97:471–4. doi: 10.1023/a:1002464307469. [DOI] [PubMed] [Google Scholar]
- 19.Mochizuki Y, Hata Y, Enaida H, et al. Evaluating adjunctive surgical procedures during vitrectomy for diabetic macular edema. Retina. 2006;26:143–8. doi: 10.1097/00006982-200602000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Nasrallah FP, Jalkh AE, Van Coppenolle F, et al. The role of the vitreous in diabetic macular edema. Ophthalmology. 1988;95:1335–9. doi: 10.1016/s0161-6420(88)33004-6. [DOI] [PubMed] [Google Scholar]
- 21.Nasrallah FP, Van De Velde F, Jalkh AE, et al. Importance of the vitreous in young diabetics with macular edema. Ophthalmology. 1989;96:1511–6. doi: 10.1016/s0161-6420(89)32698-4. [DOI] [PubMed] [Google Scholar]
- 22.Otani T, Kishi S. Tomographic assessment of vitreous surgery for diabetic macular edema. Am J Ophthalmol. 2000;129:487–94. doi: 10.1016/s0002-9394(99)00409-2. [DOI] [PubMed] [Google Scholar]
- 23.Otani T, Kishi S. A Controlled study of vitrectomy for diabetic macular edema. Am J Ophthalmol. 2002;134:214–9. doi: 10.1016/s0002-9394(02)01548-9. [DOI] [PubMed] [Google Scholar]
- 24.Patel JI, Hykin PG, Schadt M, et al. Pars plana vitrectomy with and without peeling of the inner limiting membrane for diabetic macular edema. Retina. 2006;26:5–13. doi: 10.1097/00006982-200601000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Pendergast SD, Hassan TS, Williams GA, et al. Vitrectomy for diffuse diabetic macular edema associated with a taut premacular posterior hyaloid. Am J Ophthalmol. 2000;130:178–86. doi: 10.1016/s0002-9394(00)00472-4. [DOI] [PubMed] [Google Scholar]
- 26.Radetzky S, Walter P, Fauser S, et al. Visual outcome of patients with macular edema after pars plana vitrectomy and indocyanine green assisted peeling of the internal limiting membrane. Graefes Arch Clin Exp Ophthalmol. 2004;242:273–8. doi: 10.1007/s00417-003-0731-8. [DOI] [PubMed] [Google Scholar]
- 27.Sato Y, Lee Z, Shimada H. Vitrectomy for diabetic cystoid macular edema. Jpn J Ophthalmol. 2002;46:315–22. doi: 10.1016/s0021-5155(02)00471-9. [DOI] [PubMed] [Google Scholar]
- 28.Stefansson E, Landers MBI, Wolbarsht ML. Increased retinal oxygen supply following pan-retinal photocoagulation and vitrectomy and lensectomy. Tr Am Ophth Soc. 1981;79:307–34. [PMC free article] [PubMed] [Google Scholar]
- 29.Stefansson E, Novack RL, Hatchell D. Vitrectomy prevents retinal hypoxia in branch retinal vein occlusion. Invest Ophthalmol Vis Sci. 1990;31:284–9. [PubMed] [Google Scholar]
- 30.Tachi N, Ogino N. Vitrectomy for diffuse macular edema in cases of diabetic retinopathy. Am J Ophthalmol. 1996;122:258–60. doi: 10.1016/s0002-9394(14)72018-5. [DOI] [PubMed] [Google Scholar]
- 31.Takagi H, Otani A, Kiryu J, Ogura Y. New surgical approach for removing massive foveal hard exudates in diabetic macular edema. Ophthalmology. 1999;106:249–57. doi: 10.1016/S0161-6420(99)90054-4. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto T, Akabane N, Takeuchi S. Vitrectomy for diabetic macular edema: The role of posterior vitreous detachment and epimacular membrane. Am J Ophthalmol. 2001;132:369–77. doi: 10.1016/s0002-9394(01)01050-9. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto T, Hitani K, Tsukahara I, et al. Early postoperative retinal thickness changes and complications after vitrectomy for diabetic macular edema. Am J Ophthalmol. 2003;135:14–9. doi: 10.1016/s0002-9394(02)01819-6. [DOI] [PubMed] [Google Scholar]
- 34.Yang CM. Surgical treatment for severe diabetic macular edema with massive hard exudates. Retina. 2000;20:121–5. [PubMed] [Google Scholar]
- 35.Nguyen QD, Shah SM, Van Anden E, et al. Supplemental oxygen improves diabetic macular edema: A pilot study. Invest Ophthalmol Vis Sci. 2004;45:617–24. doi: 10.1167/iovs.03-0557. [DOI] [PubMed] [Google Scholar]
- 36.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003;135:194–205. doi: 10.1016/s0002-9394(02)01825-1. [DOI] [PubMed] [Google Scholar]
- 37.Kumagai K, Furukawa M, Ogino N, et al. Long-term follow-up of vitrectomy for diffuse nontractional diabetic macular edema. Retina. 2009;29:464–72. doi: 10.1097/IAE.0b013e31819c632f. [DOI] [PubMed] [Google Scholar]
- 38.American Society of Retina Specialist. PAT Survey. 2008 [Google Scholar]
- 39.Hikichi T, Yoshida A, Trempe CL. Course of vitreomacular traction syndrome. Am J Ophthalmol. 1995;119:55–61. doi: 10.1016/s0002-9394(14)73813-9. [DOI] [PubMed] [Google Scholar]
- 40.Smiddy WE, Michels RG, Glaser BM, deBustros S. Vitrectomy for macular traction caused by incomplete vitreous separation. Arch Ophthalmol. 1988;106:624–8. doi: 10.1001/archopht.1988.01060130678025. [DOI] [PubMed] [Google Scholar]