Abstract
The mating systems of internal parasites are inherently difficult to investigate although they have important implications for the evolutionary biology of the species, disease epidemiology, and are important considerations for control measures. Using parentage analyses, three topics concerning the mating biology of Schistosoma mansoni were investigated: the number of mates per adult male and female, variance in reproductive success among individuals, and the potential role for sexual selection on male body size and also mate choice for genetically dissimilar individuals. Results indicated that schistosomes were mostly monogamous, and evidence of only one mate change occurred over a period of 5–6 weeks. One male was polygynous and contained 2 females in its gynecophoral canal although offspring were only detected for one of the females. Even though they were primarily monogamous and the sex ratio near even, reproductive success was highly variable, indicating a potential role for sexual selection. Male body size was positively related to reproductive success, consistent with sexual selection via male-male competition and female choice for large males. However, relatedness of pairs was not associated with their reproductive success. Finally, genetically identical individuals differed significantly in their reproductive output and identical males in their body size, indicating important partner and environmental effects on these traits.
Keywords: Schistosoma mansoni, Mating system, Sexual selection, Monogamy, Male-male competition, Mate choice, Genetic compatibility, Body size, Reproductive success, Relatedness
1. Introduction
The mating biology of parasitic organisms has traditionally been difficult to study because they live inside hosts and cannot be observed directly. Despite this difficulty, parasites are potentially excellent models for studying the evolutionary dynamics of mating systems because they live in discrete and alterable environments and because their interactions with host immune systems could place strong sexual selection on mate choice. Mating dynamics of parasites are also of epidemiological interest because they determine the effective size of a population and the alleles and allelic combinations that are transmitted across generations, important parameters for modeling the spread of novel traits such as drug resistance or virulence (Criscione and Blouin, 2006). Furthermore, for schistosome parasites, mating biology has another dimension of importance as it is the eggs, the product of reproduction, that cause the pathology associated with the disease schistosomiasis. Therefore, mating biology research offers opportunities for interruption of the schistosome life cycle and the prevention of human pathology (Haseeb et al., 2008). Through the use of molecular markers and parentage analysis, the mating systems of traditionally difficult systems can be investigated in vitro and evolutionary and epidemiological questions can be addressed.
Schistosoma mansoni is a trematode parasite that is dioecious and sexually dimorphic. Males are larger, more muscular, and have a ventral groove called the gynecophoral canal in which females reside for maturation, mating and egg production. The immature worms form mating pairs in the liver of their definitive host (typically humans) (Biolchini et al., 2006) and the male grasps the female with his gynecophoral canal. Females will not become sexually mature without male contact, which initiates gene expression to allow sexual maturation (Erasmus, 1973; Shaw et al., 1977; Popiel, 1986; Kunz et al., 1995; LoVerde et al., 2004). As a pair, they move from the liver, against blood flow, through the hepatic portal vein, and into the inferior mesenteric veins, a journey that females cannot undergo on their own (Standen, 1953; LoVerde et al., 2004). Females must release their eggs in the mesenteric veins so that they can reach the outside environment as the eggs move through the intestinal wall into the lumen and are excreted with the host’s feces. Larval stages hatch from the eggs in water, infect snails of the genus Biomphalaria and undergo a series of asexual reproducing generations which result in the production of several clonal larval stages that emerge from the snail and penetrate the skin of their definitive host (see Bayne and Grevelding, 2003; Yin et al., 2008 for a discussion on genetic similarity of clonal cercariae).
Organisms are rarely strictly monogamous because mating with additional partners often increases fitness for both sexes either through greater production of offspring or through genetic benefits (Bateman, 1948; Tregenza and Wedell, 2000; Zeh and Zeh, 2008). In fact, schistosomes are known to change mates in hosts that receive sequential infections of parasites (Tchuem Tchuente et al., 1996; Pica-Mattoccia et al., 2000; Beltran et al., 2008). In these studies worms were allowed to pair, and then new individuals of one sex were introduced to the system. Also, male schistosomes are capable of polygyny by holding multiple females in their gynecophoral canal, which has been observed in experimental infections (unpublished data) and reported for other species of schistosomes including Heterobilharzia americana (Armstrong, 1965) and Austrobilharzia variglandis (Chu and Cutress, 1954). The fitness outcome of polygyny has never been determined, but is predicted to increase male reproductive output if they are capable of maintaining and mating with multiple females. Female fitness will remain unaltered unless resource limitation reduces offspring production (Emlen and Oring, 1977).
Sexual selection promotes traits that enhance the number of successful offspring produced (reproductive success) through increased mate acquisition and the production of higher quality offspring (Arnold and Wade, 1984b); therefore reproductive success must be variable among individuals in order for sexual selection to occur. Both mate competition between males and mate choice of females are thought to drive sexual selection of S. mansoni. Male-male competition occurs in schistosomes and males can actively remove females from the gynecophoral canal of others. This process has been witnessed in vitro (Fig. 1; Supplementary Movie S1) and inferred from in vivo experiments (Tchuem Tchuente et al., 1995; Pica-Mattoccia et al., 2000). Recently, evidence for female choice for non-related mates has been shown in an experimental setting in which “divorce” or mate change of S. mansoni individuals was more frequent when less related males were introduced into an established infection than when more related males were introduced (Beltran et al., 2008). The dynamics of mate competition and mate choice of schistosomes are not well understood, but these studies indicate that sexual selection may be an important evolutionary force for this pathogen.
Fig. 1.
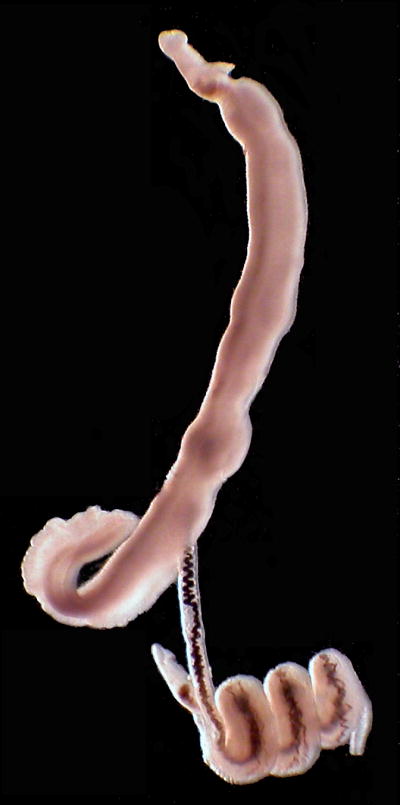
Photograph of putative male-male competition in Schistosoma mansoni in vitro from a previous study. A single male (bottom) is actively pulling a female out of the gynecophoral canal of her primary mate (top) by grasping her with his gynecophoral canal and coiling and twisting.
This study addresses the mating systems and reproductive success of schistosome parasites by using parentage analysis. This is the first study of schistosomes to use this technique to track the reproduction of individuals within an infection, which allows mating systems to be investigated without the use of inbred strains or sequential infections. Also, it allows the measurement of reproductive success, which is an important parameter for mating systems research as sexual selection acts on traits that enhance reproductive success through increased mate acquisition and the production of higher quality offspring (Arnold and Wade, 1984b). The first goal was to determine the number of mates per individual after a primary infection without the addition of new competitors, and to determine whether reproduction with multiple partners was due to polygyny or to mate change. Second, the potential for sexual selection via male-male competition and mate choice was investigated by measuring the reproductive success of males and females and relating it to male body size and genetic relatedness of pairs. It is expected that larger males will produce more offspring because they will be better competitors and will be able to obtain and retain more or higher quality females and thus produce more successful offspring. It is also expected that less genetically related individuals will produce more successful offspring due to genetic benefits or by avoiding potential negative effects of inbreeding. Also, the presence of genetic clones was used to assess the variance in reproductive success of female clones and body size variance in male clones.
2. Materials and methods
2.1. Mouse infections and recovery of adults and offspring
Schistosoma mansoni was originally obtained from three human fecal samples derived from the Lake Victoria region of Kenya and had been passaged in the laboratory for one to three generations in snails (Biomphalaria sudanica) and out-bred mice. For this experiment, snails were infected by exposing each to three to five miracidia in a 24-well plate. Cercariae were obtained from the snails 30 days post exposure by isolating the snails in 24-well plates and exposing them to bright light. Cercariae from 14 snails were used to infect two mice, which were infected by placing them in a 10 cm fingerbowl together with 12 cercariae from each snail for 30 min. Worms were removed from the mice 80–81 days post exposure using a modified version of the technique described by Smithers and Terry (1965). Pairs and single worms were immediately placed in separate wells of a 24-well plate, in order to accurately record their pairing relationships. All worms for which mates were available were paired, indicating that pairing was maintained throughout the perfusion process. Male worms were photographed with the aid of a dissecting microscope, and the body size was measured as the area of each worm using AxioVision 4.7.1 (Carl Zeiss Imaging Solutions GmbH). One male in each mouse was severed at the anterior end and could not be measured accurately. For molecular analysis, males and females were lysed using the HotSHOT procedure described by Truett et al. (2000). Because S. mansoni from Kenya becomes reproductively mature between 36–38 days (Fallon et al., 1997), we estimate that egg production occurred for 40–45 days during the experiment; however pairing of schistosomes is initiated early in development before reproductive maturity is attained (LoVerde et al., 2004).
Offspring were obtained from fecal material in the intestine of each mouse and from their livers at a single time point. Eggs accumulate in the liver over time and are a record of the mating interactions throughout the course of the experiment, while eggs in the feces are produced over a shorter period of time. The rate of egg destruction in mouse tissues is slow (26 weeks) and presumed to have little influence on the outcome of this study (Cheever et al., 1992). The liver or fecal material was blended in 0.9% saline and rinsed by sedimentation in an Erlenmeyer flask. To hatch the eggs, distilled water was added to the flask, and it was exposed to light for 30 min. The flask was then covered, leaving the top portion exposed to a bright light to attract the free swimming larvae, miracidia. Miracidia were isolated by pouring off the top portion of the flask into a Petri dish and individually pipetting them into a 96-well plate in 3 ul of water. This process was repeated until no miracidia hatched. Miracidia were lysed using the HotSHOT procedure (Truett et al., 2000) with modifications described by Steinauer et al. (2008a).
2.2. Molecular methods and data analysis
For adults, 21 previously published microsatellite loci (Durand et al., 2000; Blair et al., 2001; Curtis et al., 2001; Rodrigues et al., 2002a; Rodrigues et al., 2002b; Silva et al., 2006) were amplified in four multiplex PCR reactions using the following panels of loci as described by Steinauer et al. (2008a): P17, P22, P23 and P24. Only 20 of the loci were used in the analyses after determining that locus X77211 suffered from high error rates and potentially null alleles (Steinauer et al., 2008a) and that locus SMC1 (Curtis et al., 2001) is the same as locus SMMS16 (Silva et al., 2006), but reported as independent loci in the literature. Locus SMC1 was removed. Also, genotypes of the multiplexed PCR products were assessed using an ABI3100 automated sequencer (Applied Biosystems) and analyzed with GeneMapper® v. 4.0 (Applied Biosystems) software. All genotype calls were verified manually.
For offspring, one to four panels of microsatellite loci were genotyped using the same procedure. For most individuals, a single panel of seven loci was able to distinguish parentage unambiguously. Parentage of the offspring was assessed by eye following Mendelian inheritance of alleles as described by Steinauer et al. (2008a) and by using Parentage v. 1.0 (Emery et al., 2001) (http://www.mas.ncl.ac.uk/~nijw/), which uses a Markov chain Monte Carlo (MCMC) Bayesian inference technique to estimate parentage. The analysis was performed with the parental genotypes and 3,000 samples with 400 iterations between each sample were collected from the chains after a burn-in of 15,000 iterations.
2.3. Mating system and reproductive success
The number of mates for each male and female was determined by comparing the pairing relationships of the adults with the sired offspring. If offspring were found to have parents that differed from the mating pairs observed, then a mate change had occurred. Reproductive success was measured by the number of larvae produced by each female, each male and each mated pair, and the standardized variance in reproductive success was calculated as the variance divided by the square of the mean (Arnold and Wade, 1984a). Chi-squared goodness of fit tests were used to determine whether pairs differed in their reproductive success (only pairs were tested since the worms turned out to be primarily monogamous). Chi-squared tests were also used to determine whether reproductive success differed among individuals that were clonal genotypes. Also, the reproductive output of individuals was investigated based on whether the eggs were collected from the liver or from the feces. Although the sample sizes were not adequate to test egg habitat as a main effect, the data were explored to determine whether it was biased based on the egg location. Therefore, the samples were analyzed as “total” eggs, “fecal” eggs and “liver” eggs with separate Chi-squared tests for each set of clones. It was also noted whether any differences in reproductive output were consistent throughout habitat samples so as to determine whether individuals that produced a majority of eggs in the liver sample also did so in the fecal sample, or if their contributions differed among the samples.
2.4. Male body size
To determine whether male body size was associated with the number of offspring they sired, an Analysis of Covariance (ANCOVA) [define] was performed with SYSTAT 11 (Systat Software, Inc.). Worm body size, mouse and the interaction were the independent variables, and the percentages of offspring produced were dependent variables. Body size and percent of offspring produced were natural log transformed to meet the assumptions of normality and homogeneity of variance. It should be noted that worm combinations that were represented by multiple identical pairs (two cases), were removed from the analysis because the clones varied in body size and there was no way to determine how many offspring each produced since they were genetically identical. To test whether the body sizes of clonal males were less variable than the variance amongst clones, a nested analysis of variance was performed on the natural log transformed body size and nesting clones within mouse categories. It should also be noted that the male that could not be genotyped was removed from the body size and fecundity analysis. This male could be one for which offspring were not detected, or it could be a clone of one of four different males that were paired up with the same clonal female genotype, in which case the offspring production of this pair would be overestimated in the analysis.
2.5. Relatedness
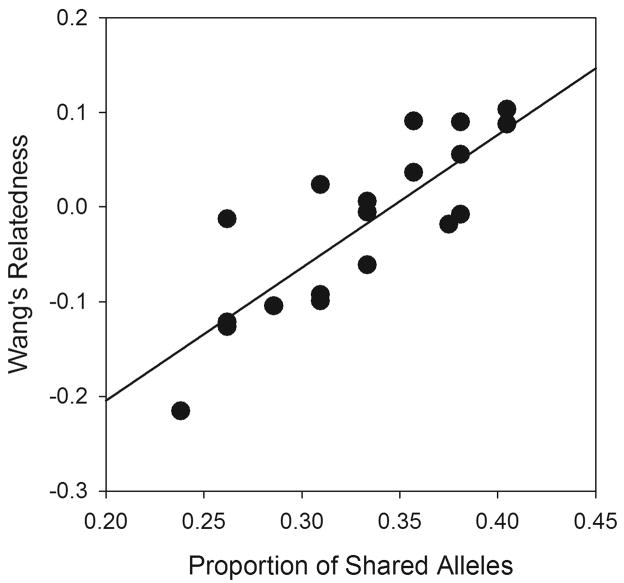
We tested whether relatedness between mates was associated with success in terms of production of successful offspring. Because there is evidence that females may choose males that are less related (Beltran et al., 2008), the hypothesis was that relatedness and reproductive success are negatively associated. Using SYSTAT 11, we tested this as with an ANCOVA using percent of offspring produced as the dependent variable, relatedness as the covariate, and mouse as the categorical variable. The interaction was pooled with the error because the F ratio was low (F < 0.9). Relatedness was calculated using two methods. First Wang’s estimator, r, (Wang, 2004) was calculated using the software package SPAGeDi v. 1.2 (Hardy and Vekemans, 2002) using all the adult worms in the experiment to calculate background allele frequencies. Because this type of estimator relies on knowledge of the background allele frequencies in the population, which were unknown, we also calculated relatedness as the proportion of alleles shared between partners using the Excel Microsatellite Toolkit (http://animalgenomics.ucd.ie/sdepark/ms-toolkit/). Both of these estimators were similar in terms of their relationship to each other (Fig. 2) (Linear regression F1,22 = 38.96, P < 0.001, r2 = 0.639), and did not change the results of the analyses.
Fig. 2.
Regression between two relatedness estimators, Wang’s r and proportion of shared alleles, used to measure relatedness between pairs of schistosomes. Linear regression: F1,22 = 38.96, P < 0.001, r2 = 0.639.
The methods used in this project was approved by the University of New Mexico’s Institutional Animal Care and Use Committee (IACUC), the Scientific Steering Committee of the Kenya Medical Research Institute and the KEMRI/National Ethics Review Board of Kenya. All investigators/assistants in this study attained animal use certification regarding the ethical treatment of animals.
3. Results
3.1. Adult populations
The worm infra-population of mouse 1 consisted of 13 males and 14 females, all of which were paired, including one male that paired with two females. The males comprised nine unique genotypes while the females comprised five. In this mouse, one male could not be successfully genotyped; therefore, its identity was undetermined. Mouse 2 contained 11 males and nine females, and the two excess males were unpaired. The males comprised six unique genotypes while the females comprised five. Both mice contained genetically identical pairs of worms.
3.2. Number of mates and reproductive variance
All offspring could be assigned to adults present in the population. Most offspring were sired by parents that were paired at the termination of the experiment with two exceptions. Firstly, the mating trio of one male and two females did not appear to be viable because offspring for only one female were detected. However, because offspring were not exhaustively sampled throughout the entire course of infection, offspring from the other female could have been missed if those represented a small proportion of total offspring. Second, no offspring were detected from one pair in mouse 2, but several offspring were detected for the same female and a male that was unpaired at the termination of the experiment, indicating that a mate change had occurred.
Within each mouse, pairs differed in their reproductive success (Mouse 1: χ211df = 72.63, P < 0.0005, n = 207 offspring; Mouse 2: χ27df = 45.86, P < 0.0005, n = 142 offspring). Because the sex ratios were almost even, these numbers also reflect differences in male and pair reproductive success, which varied greatly (Fig. 3). The skew in reproductive success was similar between mice: 41% and 43% of the offspring from mouse 1 and mouse 2, respectively, were produced by 23% and 22% of the total pairs of adults, and 75% of the offspring from both mouse infections were produced by 54% and 56% of the pairs in mouse 1 and mouse 2, respectively. The standardized reproductive variances for both sexes were similar between mice and was greater for the more abundant sex due to lack of reproduction of unpaired individuals (Mouse 1: males = 24.15, females = 27.84; Mouse 2: males = 27.94, females = 22.7).
Fig. 3.
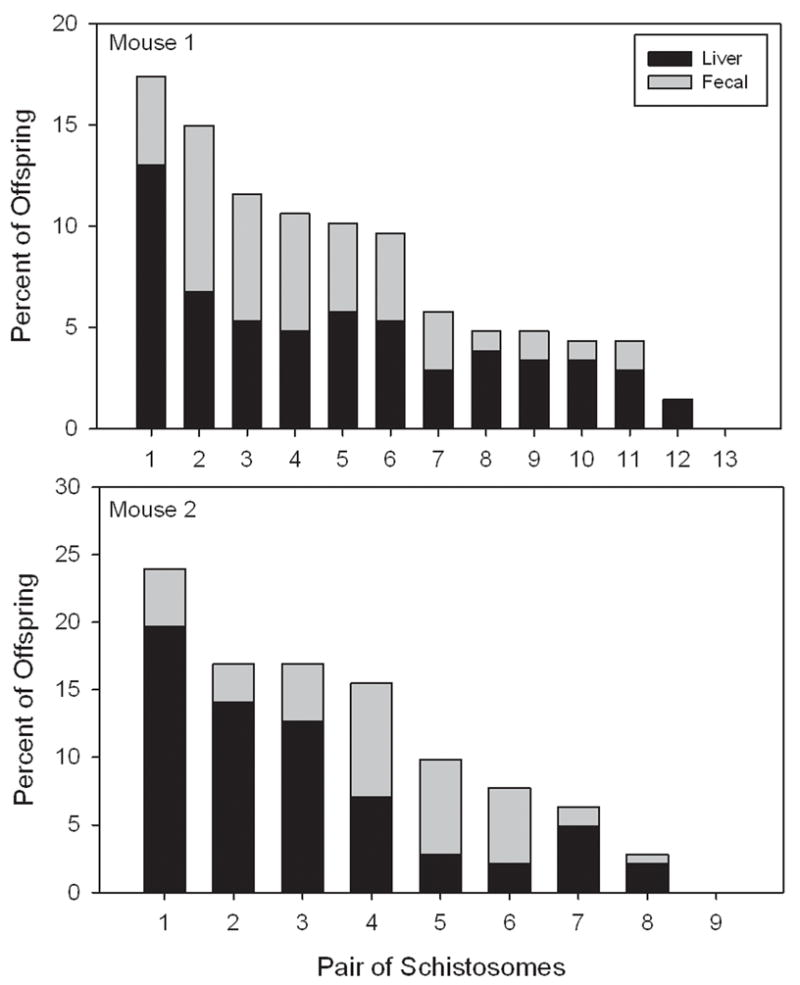
Reproductive success of pairs of schistosomes from experimental infections of mice. Pair “4” in mouse 1 and mouse 2 were each represented by two identical pairs. All other bars represent a single pair.
Reproductive success was different among six of the eight sets of genetic clones when eggs from both liver and fecal material were combined (Table 1). Statistically, skewed reproductive success was greater on eggs collected from the liver than the fecal samples; however, sample sizes of fecal eggs were generally smaller than eggs obtained from the liver. This difference is expected because liver eggs are accumulated over time while fecal eggs represent a short time period. Liver eggs should show cumulative reproductive differences that may not be detected in fecal eggs if reproduction fluctuates daily and if maturation times differ among the worms. Importantly, for six of the sets of clones reproduction was skewed in the same direction in the fecal and liver egg samples regardless of statistical significance. For example, individuals that produced larger numbers of eggs than expected in the liver sample, also did so in the fecal sample and vice versa (Table 1).
Table 1.
Skew in the reproductive success of genetically individual Schistosoma mansoni.
| ID | Sex | Host | n | Total | Fecal | Liver | Deviation |
|---|---|---|---|---|---|---|---|
| 1f | f | 1 | 3 | <0.001 (56) | <0.05 (14) | <0.001 (42) | Yes |
| 2f | f | 1 | 4 | <0.05 (65) | <0.05 (30) | NS (35) | Yes |
| 2f | f | 2 | 3 | <0.01 (44) | NS (14) | <0.001 (30) | No |
| 3f | f | 1 | 4 | <0.001 (74) | <0.1 (36) | <0.01 (38) | Yes |
| 3f | f | 2 | 2 | <0.001 (28) | <0.1 (07) | <0.001 (21) | Yes |
| 1m | m | 1 | 3 | NS (28) | NS (08) | NS (20) | Yes |
| 2m | m | 1 | 2 | <0.001 (48) | NS (15) | <0.001 (33) | Yes |
| 3m | m | 2 | 4 | <0.05 (60) | NS (28) | <0.001 (32) | No |
The identification (ID) of each clonal genotype, sex, host and the number of parental individuals per genotype (n) are given. Clonal individuals share the same ID. Data were analyzed by chi-squared goodness of fit tests for total offspring (Total), offspring derived from fecal material (Fecal), and from the liver (Liver) for each parental genotype. The probability values below 0.1 are given and “NS” indicates no statistical significance. The sample size of offspring in each category is given in parentheses. Deviation indicates whether individuals deviated from expected fecundity in the same direction for both the fecal and liver samples.
3.3. Male body size
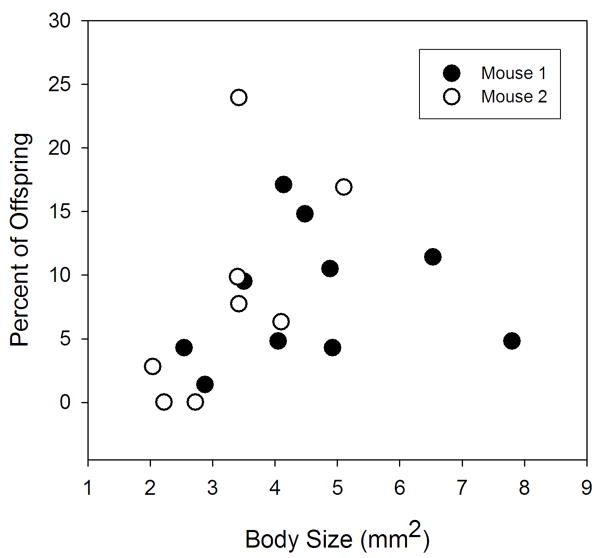
Male body size was positively related to the number of offspring sired (Body Size F1,14 = 8.261, P = 0.012; Mouse F1,14 = 2.914, P = 0.110; Interaction F1,14 = 3.446, P = 0.085; r2 = 0.429) (Fig. 4). This relationship was significant regardless of whether only fecal eggs, liver eggs, or all eggs were pooled together (data not shown). Two statistical outliers were identified by the analysis, but were not removed (removal greatly increased the significance of body size, P = 0.007). One was an individual that was large and produced few offspring; the other was relatively small and produced a large number of offspring. This second outlier was the male involved in the mate change and was not in copula during the entire infection period. Males that were genetic clones varied in body size as much as the different clones compared with each other (clones nested within Mouse F3,9 = 0.001, P = 0.986; Mouse F1,9 = 5.449, P = 0.044).
Fig. 4.
Relationship between body size of male schistosomes and the number of offspring sired from two populations in mice. Analysis of Covariance: Body Size F1,14 = 8.261, P = 0.012; Mouse F1,14 = 2.914, P = 0.110; Interaction F1,14 = 3.446, P = 0.085; r2 = 0.429.
3.4. Relatedness
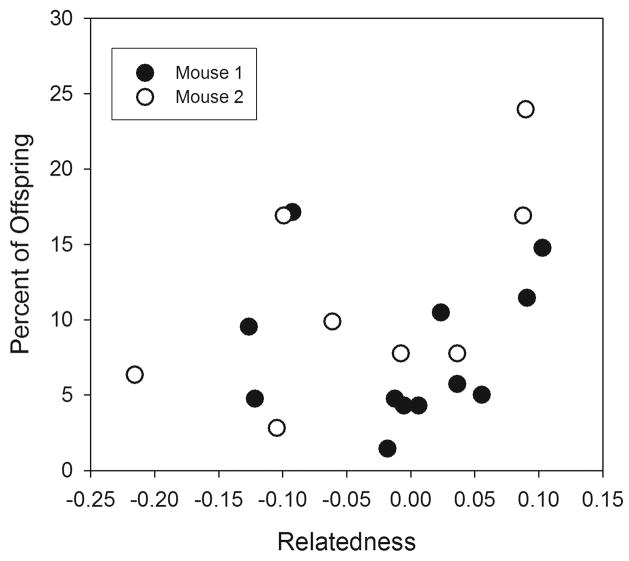
There was no significant association between the relatedness of mating pairs and the number of offspring sired regardless of Wang’s relatedness estimator or proportional allele sharing methods of relatedness were applied (Wang: Relatedness F1,17 = 1.321, P = 0.266; Mouse F1,17 = 0.470, P = 0.502; Proportion of Alleles: Relatedness F1,17 = 0.758, P = 0.396; Mouse F1,17 = 0.143, P = 0.710) (Fig. 5). This relationship was the same whether analyzed using only eggs from feces, eggs from liver, or all eggs.
Fig. 5.
Relationship between relatedness of mating schistosomes and their fecundity from two populations in mice.
4. Discussion
The majority of schistosomes were strictly monogamous over the course of the experiment. Most offspring in the sample were sired by pairs that were detected in the mice, indicating that most mating pairs were monogamous over the course of the infections. The majority of worms only paired with one partner and produced offspring with one partner. However, one mate change was detected by the presence of offspring that were sired by a male and a female that were not paired within the mouse. The male was found unpaired while the female was paired with a different male. Interestingly, no offspring were found for the female and her new partner and it cannot be determined whether this mate change lead to the production of offspring or not. If so, offspring may not have been detected if the change was recent and the offspring were in low proportion. The change would have to be very recent because offspring were detected in high proportions in both the liver and fecal material (30% of offspring sampled from liver and 12% from feces). While the ages of eggs in the liver may vary because they become trapped in tissue, eggs in feces are relatively recent as they move from the mesenteric veins to the lumen of the intestine and are excreted with the feces, a process that takes about 6 days in mice (Maldonado, 1959). Therefore, the mate change would have occurred within 6 days of perfusion. An alternative explanation worth further investigation is that post-copulatory mechanisms of sexual selection are involved. Females have a seminal receptacle and are capable of storing sperm (Neves et al., 2005), thus sperm competition (e.g. Parker, 1970) or cryptic female choice (e.g. Thornhill, 1983) could mediate the outcome of offspring production. In either case, I believe this is the first report of a mate change for S. mansoni in a primary infection, without the introduction of additional individuals through a sequential exposure.
In the female-biased infection, one male was paired with two females simultaneously, suggesting polygyny. However, the male appeared to be successfully mating with only one female. From the second female, there may have been too few offspring to detect as the male itself produced few offspring (1.4% of total offspring). Low fecundity could be characteristic of that particular male, but could also suggest that mating with more than one female simultaneously can actually be detrimental to reproductive success. Even in the absence of competitors, males could have difficulty maintaining and mating with multiple females because males play a crucial role in female growth and development (Erasmus, 1973; Shaw et al., 1977; Popiel, 1986; LoVerde et al., 2004). This limitation would favor the evolution of a monogamous mating system (Emlen and Oring, 1977; Wittenberger and Tilson, 1980). Unfortunately, polygynous males are relatively rare in experimental infections and a larger sample size of polygamous males, perhaps induced by female-biased sex ratios, is necessary to investigate this question further.
The variance in reproductive success of individuals in a population indicates the potential for sexual selection, because selection will act on individuals that mate with partners that yield the highest reproductive success (Wade, 1979). Due to the nearly even sex ratio and lack of polygamy in this study, reproductive variance was similar among male and female worms; however, variance was skewed among pairs. In each mouse, two to three worm pairs (22%) sired over 40% of the offspring. This variance likely was due to differences in egg production or fecundity as well as differential hatching success. Variable fecundity of schistosome pairs in vitro or in infections of a single pair has been reported previously (Cheever et al., 1994; ElRidi et al., 1997), although neither of these studies accounts for host effects and intra-specific competition. Interestingly, females that were genetically identical but paired with different males also varied in their reproductive success, indicating an important role for male contribution and possibly environmental effects in offspring production and success.
Male body size was positively associated with reproductive success. Large body size of males is commonly selected for in systems that involve male-male competition because of the advantage in obtaining mates and producing more offspring (Darwin, 1871; Blanckenhorn, 2000; Kingsolver and Pfennig, 2004). Because the males primarily mated with one female over a 40–45 day period, a size advantage would manifest itself in the acquisition of higher quality rather than quantity of mates, suggesting a mechanism of male choice and competition for high quality females. Although mate choice is paradigmatically considered a female role due to the investment in gametes, male mate choice is an important consideration, especially in systems where male investment is high such as a monogamous system (Andersson, 1994; Bonduriansky, 2001; Lihoreau et al., 2008). Female choice for large males could also be operating if they have an active role in partnering, if body size is an honest indicator of good genes, and if body size at the end of the experiment reflects size at the time of partnering. Because male body size was variable among genetically identical males, this may not seem like a good indicator. However, if indicators are always honest, female choice would drive fixation of the trait. Environmental dependent variance is a mechanism by which variability of indicator traits such as body size can be maintained and function in female choice (Andersson and Simmons, 2006; Bussière et al., 2008). There are several non-exclusive alternative hypotheses for the relationship between male body size and fecundity. One possibility is that larger males stimulate greater egg production from females directly through nutrition or other factors. Males stimulate female maturation and could potentially drive egg production through similar mechanisms (Erasmus, 1973; Shaw et al., 1977; Popiel, 1986; Kunz et al., 1995; LoVerde et al., 2004). Also, males play a role in the nutrition of females (Cornford and Fitzpatrick, 1985; Gupta and Basch, 1987), and it is possible that larger males are able to provide higher nutrient uptake. Another possibility is that larger males obtain higher quality territories that increase female fecundity. A final explanation is that larger males produce higher quantity or quality sperm than smaller males, requiring that reproduction in schistosomes be limited by sperm production. Further studies are necessary to distinguish among these hypotheses.
There was no relationship between genetic relatedness and reproductive success even though a previous study showed that relatedness of schistosomes influences pairing choices (Beltran et al., 2008). If sexual selection were driving this choice, it would be expected that non-related pairs would be more successful. Mate choice for genetic compatibility can be manifested as a choice for dissimilar alleles to increase diversity in offspring or as inbreeding avoidance (Andersson and Simmons, 2006). In either case, it may be that large differences in relatedness are necessary to have an effect on either mate choice or reproduction. The differences in relatedness values of possible partners in our study (max = 0.31) were not nearly as large as those from the study by Beltran and colleagues (2008) (max = 0.88) although these values are not directly comparable. One hypothesis is that with differences in relatedness of 0.88 (1 is identical) the results of the previous study were due to inbreeding avoidance, which may not be relevant to the current study. Another possibility is that the fitness benefits from genetic compatibility are manifested later, perhaps when the offspring interact with the immune system of the snail or vertebrate host. Although relatedness values are difficult to measure without error, this does not seem a likely explanation for the lack of correlation since the data showed a non-significant trend that indicated a positive relationship between relatedness and fecundity, the opposite of what was expected.
Where do mating interactions occur and who initiates those? Initial pairing of immature worms occurs in the liver, the dynamics of which are not well understood. Males are thought to play a primary role in this interaction due to their greater musculature and mobility; however, females may have other mechanisms that influence pairing such as chemical signaling. After the worms have moved to egg laying sites, the mesenteric veins, mate change likely is initiated by single males that migrate from the liver (site of development) to the mesenteric veins (site of reproduction). Single females are incapable of this journey and therefore not likely initiators. Paired individuals are also unlikely to willingly separate unless a superior mate is readily available. If females separate, they will not only be carried to the liver away from egg laying sites, but they will also developmentally regress into an immature state and cease egg production, a seemingly high fitness cost. Similarly, males will have to seek a new mate either in the liver or from the gynecophoral canal of a competitor, also a fitness cost. In a species with a heavily male-biased sex ratio, 2.36 males to females in Kenya (Steinauer et al., 2008b), mate change could be a common occurrence (Emlen and Oring, 1977; Morand and Muller-Graf, 2000). In fact, sequential infections indicated that mate change occurred more frequently in male-biased compared with female-biased experimental infections (Beltran et al., 2008). Also, hosts with repeated contact with contaminated water can constantly recruit new worms, which can greatly impact the mating dynamics.
This study demonstrated the utility of parentage analyses to investigate the mating systems of internal parasites and uncovered some intriguing patterns in the mating dynamics of schistosomes. This technique differs from traditional methods in that it does not rely on the use of clonal strains and sequential infections to determine mating dynamics. Also, this method measures fecundity and reproductive success so that questions of mate competition, mate choice and post-copulatory mechanisms of sexual selection can be addressed. With a modest sample size, this technique determined that in a primary infection schistosomes were mostly monogamous although both mate change and polygyny were observed. Also, reproductive success was highly variable among pairs and correlated positively with male body size. This technique also allowed the investigation of reproductive and body size variation among genetically identical individuals. Expansion of this methodology can be applied to investigate mating systems over time, under different environmental circumstances such as with skewed sex ratios, and when relatives are present.
Supplementary Material
Acknowledgments
Funding was provided by the U.S. National Institutes of Health, Grant AI044913. I thank Eric S. Loker, G. M. Mkoji, and Elizabeth Hatton for their assistance on this project, and Ben Hanelt and Sara V. Brant for comments on the manuscript. I would also like to thank Diana Karanja, Ibrahim N. Mwangi, Geoffrey M. Maina, Joseph M. Kinuthia, Martin W. Mutuku, Ben Mungai and Boniface Mualuko for their assistance in obtaining parasites. Also, Sandra Melman found and aided in the photography of the mating worms in Fig. 1. Support was also obtained from the Kenya Medical Research Institute, the University of New Mexico Center for Evolutionary and Theoretical Immunology (CETI), and the UNM Molecular Biology Facility (NIH Grant Number 1P20RR18754 from the Institute Development Award (IDeA) Program of the National Center for Research Resources).
Footnotes
Note: Supplementary data included with this submission
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson M. Sexual Selection. Princeton University Press; Princeton: 1994. [Google Scholar]
- Andersson M, Simmons LW. Sexual selection and mate choice. Trends Ecol Evol. 2006;21:296–302. doi: 10.1016/j.tree.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Armstrong JC. Mating behavior and development of schistosomes in the mouse. J Parasitol. 1965;51:605–616. [PubMed] [Google Scholar]
- Arnold SJ, Wade MJ. On the measurement of natural and sexual selection: Theory. Evolution. 1984a;38:709–719. doi: 10.1111/j.1558-5646.1984.tb00344.x. [DOI] [PubMed] [Google Scholar]
- Arnold SJ, Wade MJ. On the measurement of natural and sexual selection: Applications. Evolution. 1984b;38:720–734. doi: 10.1111/j.1558-5646.1984.tb00345.x. [DOI] [PubMed] [Google Scholar]
- Bateman AJ. Intra-sexual selection in Drosophila. Heredity. 1948;2:349–368. doi: 10.1038/hdy.1948.21. [DOI] [PubMed] [Google Scholar]
- Bayne CJ, Grevelding CG. Cloning of Schistosoma mansoni sporocysts in vitro and detection of genetic heterogeneity among individuals within clones. J Parasitol. 2003;89:1056–1060. doi: 10.1645/GE-3186RN. [DOI] [PubMed] [Google Scholar]
- Beltran S, Cezilly F, Boissier J. Genetic dissimilarity between mates, but not male heterozygosity influences divorce in schistosomes. PLoS One. 2008;3:1–6. doi: 10.1371/journal.pone.0003328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biolchini CD, Neves RH, Hulstijn M, Gomes DC, Machado-Silva JR. Development of Schistosoma mansoni worms in mice analyzed by bright field and confocal microscopy. Mem Inst Oswaldo Cruz. 2006;101:261–265. doi: 10.1590/s0074-02762006000900040. [DOI] [PubMed] [Google Scholar]
- Blair L, Webster JP, Barker GC. Isolation and characterization of polymorphic microsatellite markers in Schistosoma mansoni from Africa. Mol Ecol Notes. 2001;1:93–95. [Google Scholar]
- Blanckenhorn WU. The evolution of body size: What keeps organisms small? Q Rev Biol. 2000;75:385–407. doi: 10.1086/393620. [DOI] [PubMed] [Google Scholar]
- Bonduriansky R. The evolution of male mate choice in insects: a synthesis of ideas and evidence. Biol Rev. 2001;76:305–339. doi: 10.1017/s1464793101005693. [DOI] [PubMed] [Google Scholar]
- Bussière L, Hunt J, Stölting K, Jennions M, Brooks R. Mate choice for genetic quality when environments vary: Suggestions for empirical progress. Genetica. 2008;134:69–78. doi: 10.1007/s10709-007-9220-z. [DOI] [PubMed] [Google Scholar]
- Cheever AW, Macedonia JG, Erik SD, Cheever EA, Mosimann JE. Persistence of eggs and hepatic fibrosis after treatment of Schistosoma mansoni infected mice. Am J Trop Med Hyg. 1992;46:752–758. doi: 10.4269/ajtmh.1992.46.752. [DOI] [PubMed] [Google Scholar]
- Cheever AW, Macedonia JG, Mosimann JE, Cheever EA. Kinetics of egg production and egg excretion by Schistosoma mansoni and Schistosoma japonicum in mice infected with a single pair of worms. Am J Trop Med Hyg. 1994;50:281–295. doi: 10.4269/ajtmh.1994.50.281. [DOI] [PubMed] [Google Scholar]
- Chu GWTC, Cutress CE. Austrobilharzia variglandis (Miller and Northup, 1926) Penner, 1953, (Trematoda: Schistosomatidae) in Hawaii with notes on Its biology. J Parasitol. 1954;40:515–524. [PubMed] [Google Scholar]
- Cornford EM, Fitzpatrick AM. The mechanism and rate of glucose transfer from male to female schistosomes. Mol Biochem Parasitol. 1985;17:131–141. doi: 10.1016/0166-6851(85)90012-x. [DOI] [PubMed] [Google Scholar]
- Criscione CD, Blouin MS. Minimal selfing, few clones, and no among-host genetic structure in a hermaphroditic parasite with asexual larval propagation. Evolution. 2006;60:553–562. [PubMed] [Google Scholar]
- Curtis J, Sorensen RE, Page LK, Minchella DJ. Microsatellite loci in the human blood fluke Schistosoma mansoni and their utility for other schistosome species. Mol Ecol Notes. 2001;1:143–145. [Google Scholar]
- Darwin CR. The Descent of Man, and Selection in Relation to Sex. John Murray; London: 1871. [Google Scholar]
- Durand P, Sire C, Théron A. Isolation of microsatellite markers in the digenetic trematode Schistosoma mansoni from Guadeloupe island. Mol Ecol. 2000;9:997–998. doi: 10.1046/j.1365-294x.2000.00939-4.x. [DOI] [PubMed] [Google Scholar]
- ElRidi R, Ozaki T, Inaba T, Ito M, Kamiya H. Schistosoma mansoni oviposition in vitro reflects worm fecundity in vivo: Individual, parasite age, and host-dependent variations. Int J Parasitol. 1997;27:381–387. doi: 10.1016/s0020-7519(96)00191-9. [DOI] [PubMed] [Google Scholar]
- Emery AM, Wilson IJ, Craig S, Boyle PR, Noble LR. Assignment of paternity groups without access to parental genotypes: multiple mating and developmental plasticity in squid. Mol Ecol. 2001;10:1265–1278. doi: 10.1046/j.1365-294x.2001.01258.x. [DOI] [PubMed] [Google Scholar]
- Emlen ST, Oring LW. Ecology, sexual selection, and evolution of mating systems. Science. 1977;197:215–223. doi: 10.1126/science.327542. [DOI] [PubMed] [Google Scholar]
- Erasmus DA. Comparative study of reproductive system of mature, immature and unisexual female Schistosoma mansoni. Parasitology. 1973;67:165–183. doi: 10.1017/s0031182000046394. [DOI] [PubMed] [Google Scholar]
- Fallon PG, Mubarak JS, Fookes RE, Niang M, Butterworth AE, Sturrock RF, Doenhoff MJ. Schistosoma mansoni: Maturation rate and drug susceptibility of different geographic isolates. Exp Parasitol. 1997;86:29–36. doi: 10.1006/expr.1997.4149. [DOI] [PubMed] [Google Scholar]
- Gupta BC, Basch PF. Evidence for transfer of a glycoprotein from male to female Schistosoma mansoni during pairing. J Parasitol. 1987;73:674–675. [PubMed] [Google Scholar]
- Hardy OJ, Vekemans X. SPAGeDi: a versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol Ecol Notes. 2002;2:618–620. [Google Scholar]
- Haseeb MA, Thors C, Linder E, Eveland LK. Schistosoma mansoni: Chemoreception through n-acetyl D-galactosamine containing receptors in females offers insight into increased severity of schistosomiasis in individuals with blood group A. Exp Parasitol. 2008;119:67–73. doi: 10.1016/j.exppara.2007.12.016. [DOI] [PubMed] [Google Scholar]
- Kingsolver JG, Pfennig DW. Individual level selection as a cause of Cope’s rule of phyletic size increase. Evolution. 2004;58:1608–1612. doi: 10.1111/j.0014-3820.2004.tb01740.x. [DOI] [PubMed] [Google Scholar]
- Kunz W, Gohr L, Grevelding C, Schussler P, Sommer G, Menrath M, Michel A. Schistosoma mansoni: Control of female fertility by the male. Mem Inst Oswaldo Cruz. 1995;90:185–189. doi: 10.1590/s0074-02761995000200010. [DOI] [PubMed] [Google Scholar]
- Lihoreau M, Zimmer C, Rivault C. Mutual mate choice: When it pays both sexes to avoid inbreeding. PLoS One. 2008;3:1–7. doi: 10.1371/journal.pone.0003365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoVerde PT, Niles EG, Osman A, Wu WJ. Schistosoma mansoni male-female interactions. Can J Zool. 2004;82:357–374. [Google Scholar]
- Maldonado JF. The longevity of the unhatched miracidium of Schistosoma mansoni in the tissues of mice. Am J Trop Med Hyg. 1959;8:16–19. doi: 10.4269/ajtmh.1959.8.16. [DOI] [PubMed] [Google Scholar]
- Morand S, Muller-Graf CDM. Muscles or testes? Comparative evidence for sexual competition among dioecious blood parasites (Schistosomatidae) of vertebrates. Parasitology. 2000;120:45–56. doi: 10.1017/s0031182099005235. [DOI] [PubMed] [Google Scholar]
- Neves R, Biolchini CD, Machado-Silva JR, Carvalho JJ, Branquinho TB, Lenzi HL, Hulstijn M, Gomes DC. A new description of the reproductive system of Schistosoma mansoni (Trematoda: Schistosomatidae) analyzed by confocal laser scanning microscopy. Parasitol Res. 2005;95:43–49. doi: 10.1007/s00436-004-1241-2. [DOI] [PubMed] [Google Scholar]
- Parker GA. Sperm competition and its evolutionary consequences in insects. Biol Rev Camb Philos Soc. 1970;45:525–567. [Google Scholar]
- Pica-Mattoccia L, Moroni R, Tchuente LAT, Southgate VR, Cioli D. Changes of mate occur in Schistosoma mansoni. Parasitology. 2000;120:495–500. doi: 10.1017/s0031182099005685. [DOI] [PubMed] [Google Scholar]
- Popiel I. Male stimulated female maturation in Schistosoma: a review. J Chem Ecol. 1986;12:1745–1754. doi: 10.1007/BF01022380. [DOI] [PubMed] [Google Scholar]
- Rodrigues NB, Coura P, de Souza CP, Passos LKJ, Dias-Neto E, Romanha AJ. Populational structure of Schistosoma mansoni assessed by DNA microsatellites. Int J Parasitol. 2002a;32:843–851. doi: 10.1016/s0020-7519(02)00031-0. [DOI] [PubMed] [Google Scholar]
- Rodrigues NB, LoVerde PT, Romanha AJ, Oliveira G. Characterization of new Schistosoma mansoni microsatellite loci in sequences obtained from public DNA databases and microsatellite enriched genomic libraries. Mem Inst Oswaldo Cruz. 2002b;97:71–75. doi: 10.1590/s0074-02762002000900015. [DOI] [PubMed] [Google Scholar]
- Shaw JR, Marshall I, Erasmus DA. Schistosoma mansoni: in vitro stimulation of vitelline cell development by extracts of male worms. Exp Parasitol. 1977;42:14–20. doi: 10.1016/0014-4894(77)90056-x. [DOI] [PubMed] [Google Scholar]
- Silva L, Liu S, Blanton RE. Microsatellite analysis of pooled Schistosoma mansoni DNA: an approach for studies of parasite populations. Parasitology. 2006;132:331–338. doi: 10.1017/S0031182005009066. [DOI] [PubMed] [Google Scholar]
- Smithers SR, Terry RS. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology. 1965;55:695–700. doi: 10.1017/s0031182000086248. [DOI] [PubMed] [Google Scholar]
- Standen OD. The relationship of sex of Schistosoma mansoni to migration within the hepatic portal system of experimentally infected mice. Ann Trop Med Parasitol. 1953;47:139–145. doi: 10.1080/00034983.1953.11685555. [DOI] [PubMed] [Google Scholar]
- Steinauer ML, Agola LE, Mwangi IN, Mkoji GM, Loker ES. Molecular epidemiology of Schistosoma mansoni: a robust, high-throughput method to assess multiple microsatellite markers from individual miracidia. Infect Genet Evol. 2008a;8:68–73. doi: 10.1016/j.meegid.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinauer ML, Mwangi IN, Maina GM, Kinuthia JM, Mutuku MW, Agola LE, Mungai BN, Mkoji GM, Loker ES. Interactions between natural populations of human and rodent schistosomes in the Lake Victoria region of Kenya: a molecular epidemiological approach. PLoS Negl Trop Dis. 2008b;2:1–11. doi: 10.1371/journal.pntd.0000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchuem Tchuente LA, Southgate VR, Imbertestablet D, Jourdane J. Change of mate and mating competition between Males of Schistosoma intercalatum and Schistosoma mansoni. Parasitology. 1995;110:45–52. doi: 10.1017/s0031182000081038. [DOI] [PubMed] [Google Scholar]
- Tchuem Tchuente LAT, Southgate VR, Combes C, Jourdane J. Mating behaviour in schistosomes: Are paired worms always faithful? Parasitol Today. 1996;12:231–236. doi: 10.1016/0169-4758(96)10020-x. [DOI] [PubMed] [Google Scholar]
- Thornhill R. Cryptic female choice and its implications in the scorpionfly Harpobittacus nigricepts. Am Nat. 1983;122:765–788. [Google Scholar]
- Tregenza T, Wedell N. Genetic compatibility, mate choice and patterns of parentage: Invited review. Mol Ecol. 2000;9:1013–1027. doi: 10.1046/j.1365-294x.2000.00964.x. [DOI] [PubMed] [Google Scholar]
- Truett GE, Heeger P, Mynatt RL, Truett AA, Walker JA, Warman ML. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT) BioTechniques. 2000;29:52–53. doi: 10.2144/00291bm09. [DOI] [PubMed] [Google Scholar]
- Wade MJ. Sexual selection and variance in reproductive success. Am Nat. 1979;114:742–747. doi: 10.1086/424531. [DOI] [PubMed] [Google Scholar]
- Wang J. Estimating pairwise relatedness from dominant genetic markers. Mol Ecol. 2004;13:3169–3178. doi: 10.1111/j.1365-294X.2004.02298.x. [DOI] [PubMed] [Google Scholar]
- Wittenberger JF, Tilson RL. The evolution of monogamy: Hypotheses and evidence. Annu Rev Ecol Syst. 1980;11:197–232. [Google Scholar]
- Yin MB, Hu W, Mo XJ, Wang SY, Brindley PJ, McManus DP, Davis GM, Feng Z, Blair D. Multiple near identical genotypes of Schistosoma japonicum can occur in snails and have implications for population-genetic analyses. Int J Parasitol. 2008;38:1681–1691. doi: 10.1016/j.ijpara.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeh J, Zeh D. Maternal inheritance, epigenetics and the evolution of polyandry. Genetica. 2008;134:45–54. doi: 10.1007/s10709-007-9192-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.