Abstract
We examined the role of hematopoietic cell transplantation (HSCT) for patients aged ≤18 years with refractory or recurrent Burkitt (n=41), lymphoblastic (n=53), diffuse large B cell (n=52) and anaplastic large cell lymphoma (n=36), receiving autologous (n=90) or allogeneic (n=92 – 43 matched sibling and 49 unrelated donor) HSCT in 1990–2005. Risk factors affecting event-free survival (EFS) were evaluated using stratified Cox regression. Characteristics of allogeneic and autologous HSCT recipients were similar. Allogeneic donor HSCT was more likely to use irradiation-containing conditioning regimens, marrow stem cells, be performed in more recent years, and for lymphoblastic lymphoma. EFS rates were lower for patients not in complete remission at HSCT, regardless of donor type. After adjusting for disease status, 5-year EFS were similar after allogeneic and autologous HSCT for diffuse large B cell (50% vs. 52%), Burkitt (31% vs. 27%) and anaplastic large cell lymphoma (46% vs. 35%). However, EFS was higher for lymphoblastic lymphoma, after allogeneic HSCT (40% vs. 4%, p<0.01). Predictors of EFS for progressive or recurrent disease after HSCT included disease status at HSCT and use of allogeneic donor for lymphoblastic lymphoma. These data were unable to demonstrate a difference in outcome by donor type for the other histologic sub-types.
Keywords: Non-Hodgkin lymphoma, allogeneic HSCT, autologous HSCT
INTRODUCTION
More than 95% of pediatric non-Hodgkin lymphoma (NHL) is high-grade disease in contrast to adult NHL, where low grade and indolent disease predominate.(1) The four major histologic subtypes of NHL in children and adolescents are: Burkitt, lymphoblastic, diffuse large B cell and anaplastic large cell lymphoma.(1) Current results using intensive chemotherapy regimens are excellent even for patients with stages III/IV disease. Long-term event free survival (EFS) is between 60–90%, depending on histologic subtype. (2–9) For refractory or recurrent Burkitt, diffuse large cell and lymphoblastic lymphoma long-term survival is only 10–20%. (10–13) However, for refractory or recurrent anaplastic large cell lymphoma, up to 60% of patients may achieve long-term survival. (13, 14)
A Children’s Cancer Group (CCG) study for relapsed pediatric lymphoma demonstrated an EFS of 25% for all children with NHL, and for those with chemosensitive disease, EFS was similar with or without autologous hematopoietic stem cell transplantation (HSCT).(15) The French Society of Pediatric Oncology (SFOP) reported 3-year disease-free survival (DFS) of 45% for relapsed anaplastic large cell lymphoma with similar DFS after chemotherapy or autologous HSCT for patients in second complete remission (CR2).(14) In another report from the SFOP, patients with relapsed mature B-cell NHL (diffuse large B cell and Burkitt lymphoma) all succumbed to their disease without HSCT, and in those who underwent HSCT, the 3-year overall survival was only 27%.(16) Ladenstein and colleagues from the European Lymphoma Bone Marrow Transplantation Registry reported 5-year EFS of 40% for 89 pediatric patients with refractory/recurrent Burkitt or diffuse large B cell lymphoma who received autologous HSCT.(17) None of the patients with primary refractory disease or chemoresistant relapse survived. As their study spanned the period 1979–1991, only 10 patients received what would be considered modern first-line therapy, thereby raising concerns as to the applicability of their report in the current era. Several other reports on HSCT for pediatric NHL have included more than one histologic subtype, are limited to relatively small numbers of patients or have included children in a larger series that included adults. Consequently, the role of allogeneic HSCT compared to autologous HSCT for patients in CR2 or recurrent NHL is of interest to the pediatric oncologist.(13, 15, 18–24) The purpose of the current analyses were: a) to identify prognostic factors affecting outcomes and b) to assess the optimal donor source for children and adolescents treated with HSCT for refractory or recurrent NHL.
PATIENTS AND METHODS
Data collection
Data on patients undergoing HSCT were obtained from the Statistical Center of the Center for International Blood and Marrow Transplant Research (CIBMTR). The CIBMTR is a voluntary working group of more than 500 transplant centers worldwide that report patient, disease and transplant characteristics and outcome data on consecutive transplants to a Statistical Center at the Medical College of Wisconsin. The Institutional Review Board of the Medical College of Wisconsin approved the study.
Inclusion criteria
Included are patients aged ≤18 years in second or subsequent complete remission (complete disappearance of all known disease for ≥4 weeks) and refractory/recurrent NHL (relapse or progression defined as increase in size of sites of disease [≥ 25% increase in largest diameter] and/or new disease sites and/or histological evidence of disease NHL who received an autologous or allogeneic HSCT as their first transplant. Patients who had received an autologous transplant prior to allogeneic transplantation were excluded (n=13). All patients received myeloablative transplant conditioning regimens and were transplanted between 1990 and 2005. Ninety autologous transplant recipients and 92 allogeneic transplant recipients were eligible.
Endpoints
Neutrophil recovery was defined as achieving an absolute neutrophil count (ANC) of ≥ 0.5 × 109/L for three consecutive days and platelet recovery, unsupported platelet count > 20 × 109/L for 7 days. Incidences of grades 2, 3 and 4 acute graft-versus-host disease (GVHD)(25) and chronic GVHD(26) were determined for allogeneic HSCT. Deaths occurring in continuous remission were defined as transplant-related mortality. Progression was defined as progressive disease (≥ 28 days from HCST) or recurrent disease in patients who achieved remission post transplant. Progression could follow a period of “stable” disease post transplant, or partial remission. Progression/recurrence represented an increase in size of sites of disease (≥25% increase in largest diameter) and/or new disease sites and/or histological evidence of disease. EFS was defined as survival without recurrent or progression of lymphoma.
Statistical analysis
The probability of EFS was calculated with the Kaplan-Meier estimator.(27) For analysis of EFS, relapse or progression of disease or death from any cause was considered an event and surviving patients censored at last follow-up. The incidence of neutrophil and platelet recovery, acute and chronic GVHD, transplant-related mortality and relapse/progression were calculated with the use of the cumulative-incidence-function method.(27) For hematopoietic recovery and GVHD, death without the event was the competing event. For transplant-related mortality, relapse/progression was the competing event and for relapse/progression, transplant-related mortality, the competing event. Confidence intervals (CI) were calculated with the use of log-transformation.
Stratified Cox regression models were built for analysis of risk factors for relapse/progression and EFS.(28) Models were stratified by disease (Burkitt, lymphoblastic, diffuse large B cell and anaplastic large cell lymphoma) and donor type (autologous, allogeneic). Multivariate models were built using stepwise forward selection, with a p-value of 0.05 or less considered to indicate statistical significance. Our primary objective was to determine whether there was an advantage to offering an allogeneic HSCT compared to autologous HSCT for refractory/recurrent NHL. Other variables considered were age (≤10 vs. 11–18 years), sex, performance score (90–100 vs. < 90), interval from diagnosis to HSCT (< 6 vs. 6–12 vs. >12 months), disease status (≥2 complete remission vs. not in remission) graft type (bone marrow vs. peripheral blood) and year of HSCT (1990–1994 vs.1995–1999 vs. 2000–2005). Allogeneic transplants were grouped together regardless of donor type. Prior to combining HLA-matched sibling and unrelated donor transplant recipients, analyses were preformed to detect differences in transplant-outcome and found none except for a higher incidence of acute grade 2–4 GVHD in unrelated donor transplant recipients (data not shown). There was no difference in survival rates between HLA-matched sibling and unrelated donor transplants. We tested for an effect of transplant center on EFS and found none.(29) P-values are two-sided. Analyses were performed using SAS software, version 9.1 (SAS Institute, Cary, NC).
RESULTS
Patient, disease and transplant characteristics by donor type are shown in Table 1. Patients with lymphoblastic lymphoma were more likely to receive allogeneic HSCT compared to other histologic subtypes. Allogeneic HSCT recipients were more likely to receive irradiation containing conditioning regimen, bone marrow graft and be transplanted in more recent years. Sixteen patients received HSCT for primary induction failure (14 received autologous HSCT). Approximately 65% of autologous and allogeneic transplant recipients received 1–2 lines of therapy prior to transplantation and 35%, 3–5 lines of therapy prior to transplantation. Over half of allogeneic recipients received grafts from an unrelated donor, the majority of which were HLA mismatched. Twenty percent of allogeneic transplant recipients received anti-thymocyte globulin as part of transplant conditioning. The median follow up of surviving patients is approximately 6 years after autologous HSCT and 4.5 years after allogeneic HSCT.
Table 1.
Patient, disease and transplant characteristics
| Characteristics of patients | Autologous, N (%) | Allogeneic, N (%) | P-value |
|---|---|---|---|
| Number of patients | 90 | 92 | |
| Age at transplant, years | |||
| ≤ 10 | 26 (29) | 29 (32) | |
| 11 – 18 | 64 (71) | 63 (68) | |
| Performance score prior to TX | 0.12 | ||
| < 90% | 17 (19) | 29 (32) | |
| ≥ 90% | 70 (78) | 60 (65) | |
| Unknown | 3 ( 3) | 3 ( 3) | |
| Disease | <0.001 | ||
| Diffuse large cell | 35 (38) | 17 (19) | |
| Lymphoblastic | 14 (16) | 39 (42) | |
| Burkitt | 17 (19) | 24 (26) | |
| Anaplastic | 24 (27) | 12 (13) | |
| Interval diagnosis to transplant | 0.54 | ||
| < 6 months | 9 (10) | 5 ( 5) | |
| 6 – 12 months | 30 (33) | 33 (36) | |
| ≥ 12 months | 51 (57) | 54 (59) | |
| Disease status prior to transplant | 0.65 | ||
| ≥ second complete remission | 43 (48) | 47 (51) | |
| Relapse or Progression | 47 (52) | 45 (49) | |
| Interval from diagnosis to 1st relapse1 | 0.18 | ||
| < 6 months | 15(20) | 29 (32) | |
| 6 – 12 months | 23 (30) | 21 (23) | |
| > 12 months | 38 (50) | 40 (44) | |
| Conditioning regimen | <0.001 | ||
| Total body irradiation + cyclophosphamide | 25 (28) | 74 (80) | |
| Total body irradiation + other agents | 10 (11) | 5 ( 6) | |
| Busulfan + cyclophosphamide | 4 ( 5) | 13 ( 2) | |
| Cyclophosphamide + etoposide | 26 (29) | 0 | |
| Other | 25 (27) | 0 | |
| Graft type | <0.001 | ||
| Bone marrow | 39 (43) | 75 (82) | |
| Peripheral blood | 51 (57) | 17 (18) | |
| Year of transplant | < 0.001 | ||
| 1990–1994 | 47 (52) | 12 (13) | |
| 1995–2005 | 43 (48) | 80 (87) | |
| Type of Donor | |||
| HLA-identical sibling | NA | 43 (47) | |
| Unrelated 2 | 49 (53) | ||
| Median (range) follow-up, months |
71 (2 – 142) | 43 (2 – 157) | |
Excludes patients who did not achieve first CR; n = 2 allogeneic transplant recipients and n = 14 autologous transplant recipients had progressive disease with front-line therapy and proceeded to transplantation without achieving CR
Matched URD, n=9; Mismatched URD, n= 40
Hematopoietic recovery
The incidence of neutrophil recovery (day +28) after allogeneic and autologous HSCT were 87% (95% CI 73–96) and 74% (95% CI 62–85), respectively. Corresponding incidence at day +100 were 93% (95% CI 77–100) and 90% (95% CI 75–99). The incidence of platelet recovery (day +100) following allogeneic and autologous HSCT were 67% (95% CI 54–78) and 76% (95% CI 62–88), respectively. In 47 patients, neutrophil and/or platelet recovery was not achieved either due to disease progression or death from a transplant-related complication.
Acute and chronic GVHD
Amongst allogeneic HSCT recipients, the incidence at day +100 of grade 2–4 acute GVHD was 43% (95% CI 33–54); grade 2 (n=22), grade 3 (n=11) and grade 4 (n=5).. Fourteen patients developed chronic GVHD; the 5-year incidence of chronic GVHD was 16% (95% CI 9–25).
Transplant-related mortality
The 1 and 5-year transplant-related mortality (TRM) rates were similar after autologous and allogeneic HSCT: 14% (95% CI 7–22) and 24% (95% CI 16–34) (p=0.08) and 17% (95% CI 9–25) and 25% (95% CI 17–35) (p=0.15) at 1 and 5-years after autologous and allogeneic HSCT, respectively.
Relapse or progression
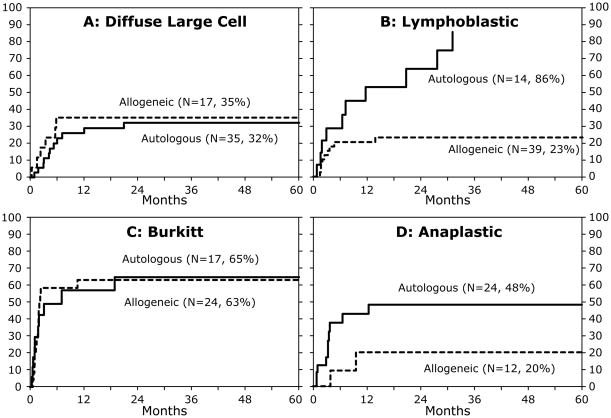
Relapse or progression of disease occurred in 42 patients after autologous and 32 patients after allogeneic HSCT. The risk of recurrent or progressive disease was higher in patients who were not in CR at transplantation (RR 2.46, 95% CI 1.50– 4.04. p<0.01) regardless of donor type. Of the 90 patients (allogeneic and autologous recipients) transplanted in CR, 77 patients were in second CR and 13 patients in third CR. Given the very few patients transplanted in third CR we are unable to provide relapse rates separately for patients in second and third CR. There were no significant differences in the 5-year probabilities of relapse/progression by donor type for diffuse large B cell, Burkitt lymphoma and anaplastic large cell lymphoma (Figure 1A, 1C, 1D). The apparent decrease in progression found in patients with anaplastic large cell lymphoma receiving allogeneic grafts did not reach statistical significance. For patients with lymphoblastic lymphoma, the 5-year probabilities of relapse/progression were lower after allogeneic compared to autologous HSCT (Figure 1B).
Figure 1.
The probability of recurrent or progressive disease by NHL subtype and donor
Event-free survival
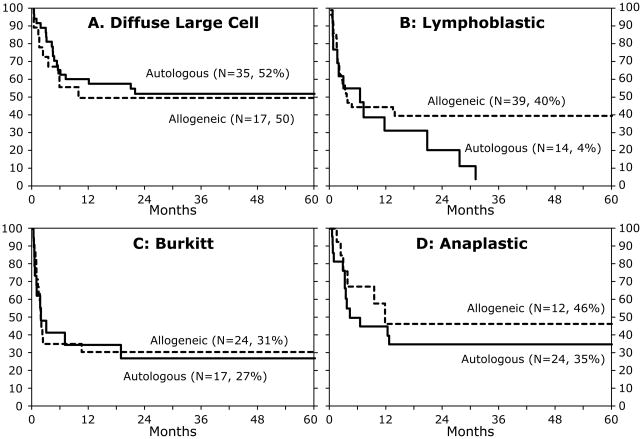
In multivariate analysis, risks of treatment failure (relapse/progression or death, inverse of EFS) were higher for patients who were not in CR at transplantation (RR 2.42, 95% CI 1.62–3.62, p<0.01) regardless of donor type with a 5 year EFS of 28% for autologous and 20% for allogeneic HSCT. The corresponding probabilities for patients transplanted in second or third CR were 40% and 57%, respectively. No effect of duration of first CR on EFS was found (RR 0.80, 95% CI 0.58 – 1.10, p=0.16 in patients with CR1 duration >12 months compared to ≤12 months). The 5-year probabilities of EFS by donor type for Burkitt, diffuse large B cell and anaplastic large cell lymphoma were similar (Figure 2A, 2C, 2D). The 5-year probability of EFS was higher after allogeneic HSCT compared to autologous for patients with lymphoblastic lymphoma (Figure 2B). Recurrent or progressive disease was the most frequent cause of death after autologous (34 of 48; 70%) compared to allogeneic transplantation (29 of 50; 58%). Death from transplant-related complications were higher after allogeneic HSCT (infection [n=8 vs. 3], interstitial pneunonitis/adult respiratory syndrome [n=4 vs. 6], GVHD [n=1 vs. 0], organ failure [n=6 vs. 3] and other causes [n=3 vs. 2].
Figure 2.
The probability of EFS by NHL subtype and donor after adjustment for disease status at transplantation
DISCUSSION
The purpose of this study was to identify prognostic factors, including the optimal donor source, for children and adolescents with refractory/recurrent NHL undergoing HSCT. With the current up-front chemotherapy regimens, outcome for pediatric NHL is excellent even for those with advanced (stage III or IV) disease.(2–9) However, salvage of patients with refractory or recurrent disease remains very poor.(10–14) Since data on front-line therapies for this cohort was not available, patients receiving transplant prior to 1990 were excluded, the era prior to the advent of more aggressive front-line therapies. EFS was worse for patients not in CR at transplantation independent of donor type and EFS was superior for lymphoblastic lymphoma after allogeneic HSCT. Our report confirms and extends the observation of several others. First, salvage rates are higher for large cell lymphoma (diffuse large B cell or anaplastic) compared to small cell lymphoma Burkitt and lymphoblastic) and second, disease status at transplantation is a strong prognostic indicator.(16–18, 20, 24) Of note, for patients, who were not in CR at HSCT, there appears to be no differences in 5 year EFS after allogeneic and autologous transplants (20% vs. 28%). Our report also illustrates the complexities of extrapolating results from reports on HSCT for adults with NHL. Our findings differ from a report by Levine and colleagues comparing allogeneic and autologous HSCT for lymphoblastic lymphoma.(21) In their report, fewer than 20% of the study population were ≤18 years.(21) Consequently, the higher TRM after allogeneic HSCT and the absence of a significant difference in EFS after autologous and allogeneic HSCT in the report by Levine and colleagues is expected and explained by the inclusion of mostly older patients.
While a significantly lower progression rate and improved EFS was seen in patients with lymphoblastic lymphoma after allogeneic HSCT, we were unable to demonstrate superiority of donor type (autologous vs. allogeneic) for Burkitt, diffuse large B cell and anaplastic large cell lymphoma. It is tempting to speculate that a graft-versus-lymphoma (GVL) effect exists in lymphoblastic lymphoma and perhaps in anaplastic large cell lymphoma. However, in our cohort, numbers were insufficient to correlate EFS or progression rate with GVHD or allow examination of differences in EFS for related versus unrelated donor transplant recipients. A recent Berlin-Frankfurt-Munster (BFM) report suggests a benefit of allogeneic HSCT for high risk anaplastic large cell lymphoma, especially in patients relapsing following autologous HSCT.(22) Small numbers prevented us examining for an effect of autologous HSCT prior to allogeneic HSCT.
There are inherent weaknesses in all studies that use data collected by transplant registries. The outcome of patients not receiving HSCT during the time period studied is unknown and the decision to offer HSCT or donor choice not reported. Patients were more likely to receive autologous HSCT in the early time period. This is likely explained by a bias that developed over time that allogeneic HSCT is superior for pediatric relapsed/refractory NHL. While we observed no differences in numbers of lines of therapy for autologous and allogeneic transplant recipients, in the absence of detailed information on front-line therapies it is conceivable that results for HSCT in this cohort are biased as patients may have received front-lines therapies of varying intensity which may influence EFS after HSCT. One could also speculate outcome for allogeneic HSCT was superior in the later time period due to improved donor-recipient HLA matching and improved supportive care resulting in lower transplant related mortality. Though numbers are small, no differences in outcome over time were observed in the current analysis. Achieving disease control can be very difficult and short-lived, especially in relapsed Burkitt or lymphoblastic lymphoma and may influence donor selection. There may exist, a bias favoring allogeneic HSCT, as unrelated donor HSCT (50% of allogeneic HSCT) was likely only pursued in patients with more responsive disease and durable remissions. However, we observed no difference in time to transplant between autologous and allogeneic HSCT in this cohort. Disease characteristics such as involvement of site(s) at diagnosis and relapse and their impact on HSCT outcome were not examined as these data are not adequately captured.
Despite these limitations, the study population herein represents the largest pediatric NHL cohort to have received HSCT as salvage therapy. It is unlikely that a randomized study will ever be done to assess the benefit of autologous versus allogeneic HSCT in pediatric NHL, due to the relatively small numbers of patients and the inherent problems of donor availability when considering allogeneic HSCT. Therefore, using data collected by transplant registries offer an alternative for studying treatment choices though this is not ideal. We demonstrate that both autologous and allogeneic HSCT can be effective in salvaging children and adolescents with refractory or recurrent NHL and results are superior if CR can be achieved prior to HSCT. Additionally, the data suggest an allogeneic donor is preferred for patients with lymphoblastic lymphoma.
Acknowledgments
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from AABB; Aetna; American Society for Blood and Marrow Transplantation; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US, Inc.; Baxter International, Inc.; Bayer HealthCare Pharmaceuticals; Be the Match Foundation; Biogen IDEC; BioMarin Pharmaceutical, Inc.; Biovitrum AB; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Canadian Blood and Marrow Transplant Group; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Centers for Disease Control and Prevention; Children’s Leukemia Research Association; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Cubist Pharmaceuticals; Cylex Inc.; CytoTherm; DOR BioPharma, Inc.; Dynal Biotech, an Invitrogen Company; Eisai, Inc.; Enzon Pharmaceuticals, Inc.; European Group for Blood and Marrow Transplantation; Gamida Cell, Ltd.; GE Healthcare; Genentech, Inc.; Genzyme Corporation; Histogenetics, Inc.; HKS Medical Information Systems; Hospira, Inc.; Infectious Diseases Society of America; Kiadis Pharma; Kirin Brewery Co., Ltd.; The Leukemia & Lymphoma Society; Merck & Company; The Medical College of Wisconsin; MGI Pharma, Inc.; Michigan Community Blood Centers; Millennium Pharmaceuticals, Inc.; Miller Pharmacal Group; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Nature Publishing Group; New York Blood Center; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Pall Life Sciences; Pfizer Inc; Saladax Biomedical, Inc.; Schering Corporation; Society for Healthcare Epidemiology of America; StemCyte, Inc.; StemSoft Software, Inc.; Sysmex America, Inc.; Teva Pharmaceutical Industries;; THERAKOS, Inc.; Thermogenesis Corporation; Vidacare Corporation; Vion Pharmaceuticals, Inc.; ViraCor Laboratories; ViroPharma, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sandlund JT, Downing JR, Crist WM. Non-Hodgkin's lymphoma in childhood. N Engl J Med. 1996;334:1238–1248. doi: 10.1056/NEJM199605093341906. [DOI] [PubMed] [Google Scholar]
- 2.Link MP, Shuster JJ, Donaldson SS, Berard CW, Murphy SB. Treatment of children and young adults with early-stage non-Hodgkin's lymphoma. N Engl J Med. 1997;337:1259–1266. doi: 10.1056/NEJM199710303371802. [DOI] [PubMed] [Google Scholar]
- 3.Gerrard M, Cairo MS, Weston C, et al. Excellent survival following two courses of COPAD chemotherapy in children and adolescents with resected localized B-cell non-Hodgkin's lymphoma: results of the FAB/LMB 96 international study. Br J Haematol. 2008;141:840–847. doi: 10.1111/j.1365-2141.2008.07144.x. [DOI] [PubMed] [Google Scholar]
- 4.Patte C, Auperin A, Gerrard M, et al. Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents: it is possible to reduce treatment for the early responding patients. Blood. 2007;109:2773–2780. doi: 10.1182/blood-2006-07-036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cairo MS, Gerrard M, Sposto R, et al. Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood. 2007;109:2736–2743. doi: 10.1182/blood-2006-07-036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burkhardt B, Woessmann W, Zimmermann M, et al. Impact of cranial radiotherapy on central nervous system prophylaxis in children and adolescents with central nervous system-negative stage III or IV lymphoblastic lymphoma. J Clin Oncol. 2006;24:491–499. doi: 10.1200/JCO.2005.02.2707. [DOI] [PubMed] [Google Scholar]
- 7.Seidemann K, Tiemann M, Schrappe M, et al. Short-pulse B-non-Hodgkin lymphoma-type chemotherapy is efficacious treatment for pediatric anaplastic large cell lymphoma: a report of the Berlin-Frankfurt-Munster Group Trial NHL-BFM 90. Blood. 2001;97:3699–3706. doi: 10.1182/blood.v97.12.3699. [DOI] [PubMed] [Google Scholar]
- 8.Laver JH, Kraveka JM, Hutchison RE, et al. Advanced-stage large-cell lymphoma in children and adolescents: results of a randomized trial incorporating intermediate-dose methotrexate and high-dose cytarabine in the maintenance phase of the APO regimen: a Pediatric Oncology Group phase III trial. J Clin Oncol. 2005;23:541–547. doi: 10.1200/JCO.2005.11.075. [DOI] [PubMed] [Google Scholar]
- 9.Brugieres L, Le Deley MC, Rosolen A, et al. Impact of the methotrexate administration dose on the need for intrathecal treatment in children and adolescents with anaplastic large-cell lymphoma: results of a randomized trial of the EICNHL Group. J Clin Oncol. 2009;27:897–903. doi: 10.1200/JCO.2008.18.1487. [DOI] [PubMed] [Google Scholar]
- 10.Cairo MS, Sposto R, Hoover-Regan M, et al. Childhood and adolescent large-cell lymphoma (LCL): a review of the Children's Cancer Group experience. Am J Hematol. 2003;72:53–63. doi: 10.1002/ajh.10262. [DOI] [PubMed] [Google Scholar]
- 11.Cairo MS, Sposto R, Perkins SL, et al. Burkitt's and Burkitt-like lymphoma in children and adolescents: a review of the Children's Cancer Group experience. Br J Haematol. 2003;120:660–670. doi: 10.1046/j.1365-2141.2003.04134.x. [DOI] [PubMed] [Google Scholar]
- 12.Atra A, Gerrard M, Hobson R, Imeson JD, Hann IM, Pinkerton CR. Outcome of relapsed or refractory childhood B-cell acute lymphoblastic leukaemia and B-cell non-Hodgkin's lymphoma treated with the UKCCSG 9003/9002 protocols. Br J Haematol. 2001;112:965–968. doi: 10.1046/j.1365-2141.2001.02647.x. [DOI] [PubMed] [Google Scholar]
- 13.Attarbaschi A, Dworzak M, Steiner M, et al. Outcome of children with primary resistant or relapsed non-Hodgkin lymphoma and mature B-cell leukemia after intensive first-line treatment: a population-based analysis of the Austrian Cooperative Study Group. Pediatr Blood Cancer. 2005;44:70–76. doi: 10.1002/pbc.20121. [DOI] [PubMed] [Google Scholar]
- 14.Brugieres L, Quartier P, Le Deley MC, et al. Relapses of childhood anaplastic large-cell lymphoma: treatment results in a series of 41 children--a report from the French Society of Pediatric Oncology. Ann Oncol. 2000;11:53–58. doi: 10.1023/a:1008352726155. [DOI] [PubMed] [Google Scholar]
- 15.Kobrinsky NL, Sposto R, Shah NR, et al. Outcomes of treatment of children and adolescents with recurrent non-Hodgkin's lymphoma and Hodgkin's disease with dexamethasone, etoposide, cisplatin, cytarabine, and l-asparaginase, maintenance chemotherapy, and transplantation: Children's Cancer Group Study CCG-5912. J Clin Oncol. 2001;19:2390–2396. doi: 10.1200/JCO.2001.19.9.2390. [DOI] [PubMed] [Google Scholar]
- 16.Philip T, Hartmann O, Pinkerton R, et al. Curability of relapsed childhood B-cell non-Hodgkin's lymphoma after intensive first line therapy: a report from the Societe Francaise d'Oncologie Pediatrique. Blood. 1993;81:2003–2006. [PubMed] [Google Scholar]
- 17.Ladenstein R, Pearce R, Hartmann O, Patte C, Goldstone T, Philip T. High-dose chemotherapy with autologous bone marrow rescue in children with poor-risk Burkitt's lymphoma: a report from the European Lymphoma Bone Marrow Transplantation Registry. Blood. 1997;90:2921–2930. [PubMed] [Google Scholar]
- 18.Sandlund JT, Bowman L, Heslop HE, et al. Intensive chemotherapy with hematopoietic stem-cell support for children with recurrent or refractory NHL. Cytotherapy. 2002;4:253–258. doi: 10.1080/146532402320219763. [DOI] [PubMed] [Google Scholar]
- 19.Won SC, Han JW, Kwon SY, et al. Autologous peripheral blood stem cell transplantation in children with non-Hodgkin's lymphoma: A report from the Korean society of pediatric hematology-oncology. Ann Hematol. 2006;85:787–794. doi: 10.1007/s00277-006-0169-2. [DOI] [PubMed] [Google Scholar]
- 20.Bureo E, Ortega JJ, Munoz A, et al. Bone marrow transplantation in 46 pediatric patients with non-Hodgkin's lymphoma. Spanish Working Party for Bone Marrow Transplantation in Children. Bone Marrow Transplant. 1995;15:353–359. [PubMed] [Google Scholar]
- 21.Levine JE, Harris RE, Loberiza FR, Jr, et al. A comparison of allogeneic and autologous bone marrow transplantation for lymphoblastic lymphoma. Blood. 2003;101:2476–2482. doi: 10.1182/blood-2002-05-1483. [DOI] [PubMed] [Google Scholar]
- 22.Woessmann W, Peters C, Lenhard M, et al. Allogeneic haematopoietic stem cell transplantation in relapsed or refractory anaplastic large cell lymphoma of children and adolescents--a Berlin-Frankfurt-Munster group report. Br J Haematol. 2006;133:176–182. doi: 10.1111/j.1365-2141.2006.06004.x. [DOI] [PubMed] [Google Scholar]
- 23.Gordon BG, Warkentin PI, Weisenburger DD, et al. Bone marrow transplantation for peripheral T-cell lymphoma in children and adolescents. Blood. 1992;80:2938–2942. [PubMed] [Google Scholar]
- 24.Fujita N, Mori T, Mitsui T, Inada H, Horibe K, Tsurusawa M. The role of hematopoietic stem cell transplantation with relapsed or primary refractory childhood B-cell non-Hodgkin lymphoma and mature B-cell leukemia: a retrospective analysis of enrolled cases in Japan. Pediatr Blood Cancer. 2008;51:188–192. doi: 10.1002/pbc.21585. [DOI] [PubMed] [Google Scholar]
- 25.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 26.Flowers ME, Kansu E, Sullivan KM. Pathophysiology and treatment of graft-versus-host disease. Hematol Oncol Clin North Am. 1999;13:1091–1112. viii–ix. doi: 10.1016/s0889-8588(05)70111-8. [DOI] [PubMed] [Google Scholar]
- 27.Klein JP, Moeschberger ML. Survival Analysis: techniques of censored and truncated data. New York, NY: Springer-Verlag; 2003. [Google Scholar]
- 28.Cox DR. Regression models an dlife tables. J R Stat Soc B. 1972;34:187–202. [Google Scholar]
- 29.Andersen PK, Klein JP, Zhang MJ. Testing for centre effects in multi-centre survival studies: a Monte Carlo comparison of fixed and random effects tests. Stat Med. 1999;18:1489–1500. doi: 10.1002/(sici)1097-0258(19990630)18:12<1489::aid-sim140>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]