Abstract
Background
Depressive and anxiety symptoms tend to co-occur with heavy drinking. Specifically, their presence may exacerbate the severity and intractability of heavy drinking. Similarly, heavy drinking may increase the risk for and experience of depressive and anxiety symptoms. Although depressive and anxiety symptoms have been significantly correlated with alcohol craving in cue-exposure paradigms, physiological responses have not always mapped onto emotional responses. Therefore, this study sought to examine the role of depressive and anxiety symptoms using a more basic science approach, through examining functional brain changes.
Methods
Seventy nontreatment seeking, heavy drinking adults were recruited through a college campus (n = 45 men; mean age = 22.8). They completed measures of drinking, smoking, depressive symptoms, anxiety symptoms, and a functional magnetic resonance imaging (fMRI) cue-exposure paradigm.
Results
As hypothesized, depressive symptoms were positively correlated with activation during the alcohol (vs. appetitive control) cue in the insula, cingulate, ventral tegmentum, striatum, and thalamus (cluster-corrected p < 0.05, z = 2.3). Similarly, anxiety symptoms were positively correlated with activation during the alcohol (vs. appetitive control) cue in the striatum, thalamus, insula, and inferior frontal, mid-frontal, and cingulate gyri (cluster-corrected p < 0.05, z = 2.3).
Conclusions
Significant correlations were found between depressive symptoms, anxiety symptoms, and differential brain activation in response to an alcohol versus an appetitive control cue in an fMRI paradigm. Moreover, the pattern of activation mapped onto expected regions. This study strongly supports the posited relationships between depressive symptoms, anxiety symptoms, and differential brain activation in an alcohol cue-exposure paradigm with a sample of heavy drinking adults.
Keywords: Heavy Drinking, Depressive Symptoms, Anxiety Symptoms, Neuroimaging, Craving
Recent studies have supported an association between heavy drinking and depressive and anxiety symptoms (odds ratios of 1.2 to 2.2 and 1.2 to 3.0, respectively; Hasin et al., 2007). Heavy drinkers with co-occurring depressive and anxiety symptoms evidence heavier alcohol use (dependence vs. abuse), greater quantity of alcohol use, and an increased risk of relapse (e.g., Buckner et al., 2008; Grotheus et al., 2008; Kushner et al., 2005). These disorders have not only been found to be highly co-occurring, but the presence of depressive and anxiety symptoms may be associated with greater severity and intractability of heavy drinking. Similarly, heavy drinking may also increase the risk for and experience of depressive and anxiety symptoms.
Depressive and anxiety symptoms have also been examined in laboratory studies of cue-elicited craving. Cue-exposure paradigms are used to evaluate the relationship between alcohol cues, behavioral responses (e.g., subjective craving ratings), and physiological responses (e.g., heart rate, cortisol level). Previous studies have suggested that greater cue reactivity is associated with the risk of relapse (e.g., Cooney et al., 1997; Litt et al., 2000), although there has been debate about the strength of this relationship (Sayette et al., 2000; Tiffany, 1995). Regarding the association with alcohol cue reactivity, most studies have found a strong relationship between craving, depressive symptoms, and anxiety symptoms among heavy drinkers (Breese et al., 2005; Chiang et al., 2005; Cooney et al., 1997; Fox et al., 2007; Litt et al., 2000; Sinha, 2007; Sinha et al., 2008), although others have not (Jansma et al., 2000; Mason et al., 2008).
Several studies have successfully migrated the study of alcohol cue response to a neuroimaging (functional magnetic resonance imaging, fMRI) environment with gustatory (Filbey et al., 2008; Myrick et al., 2004), as well as visual alcohol cues (de Greck et al., 2008; Gilman and Hommer, 2008; Heinz et al., 2004). With a sample of heavy drinking adults, Filbey and colleagues (2008) found significant activation in mesocorticolimbic pathways (e.g., prefrontal cortex, striatum, ventral tegmental area [VTA]/substantia nigra) upon presentation of alcohol versus control appetitive gustatory cues. Moreover, this activation was significantly correlated with measures of alcohol dependence. Similarly, upon presentation of visual cues, de Greck and colleagues (2008) found that heavy drinking participants showed reduced signal changes in areas proximal to the VTA, as well as ventromedial prefrontal cortex during a reward task. Furthermore, Gilman and Hommer's (2008) research incorporated the role of depressive and anxiety symptoms with visual cues. Their work provides preliminary evidence for the role of emotional processing of alcohol cues for alcohol dependent, but not nondrinking, samples.
This study sought to extend these findings by evaluating the role of depressive and anxiety symptoms as potential comorbid risk factors (vs. trait emotionality or current stress) in functional brain changes that underlie the motivation to drink. It was hypothesized that in a sample of heavy drinking adults, individuals with greater co-occurring depressive symptoms would show greater differences in brain activation between alcohol and control cues. Similarly, it was hypothesized that individuals with greater co-occurring anxiety symptoms would also show greater differences in brain activation between alcohol and control cues.
Materials and Methods
Participants
As part of a larger study (Filbey et al., 2008), with institutional review board approval, 74 nontreatment seeking volunteers were recruited through college campus flyers and email listservs, and provided informed written consent to participate in a study evaluating response to taste cues in a neuroimaging paradigm (see Filbey et al., 2008). To participate, interested adults had to report heavy drinking, defined as drinking between 2 and 5 times per week, with a minimum of 2 (women) or 3 (men) drinks per drinking occasion, during 4 consecutive weeks prior to participation in the study. In addition, to participate, interested adults had to be between ages 21 and 40, demonstrate a breath alcohol level of 0.000, and be absent the following exclusion criteria: history of head injury with loss of consciousness greater than 30 minutes, current or prior history of central nervous system disease (e.g., stroke, epilepsy, seizure) or brain lesion, current or prior history of psychotic disorder in self or first degree relative (or use of antipsychotic medication), current or prior history of alcohol-induced seizures, current hypertension or diabetes, current pregnancy, current mental retardation, left handedness, current interest or receipt of psychotherapy, current use or abuse of cocaine, methamphetamine, or heroin, current suicidal ideation with significant plans/intent requiring hospitalization, and self-reported HIV+/AIDS.
Of the 74 subjects collected, 4 were excluded due to problems with the imaging data (e.g., motion >2 mm, incomplete scan), leaving a total sample size of 70. Participants included 45 men, 25 women, with an average age of 22.8 (SD = 2.42; range = 21 to 33). This sample self-identified as Caucasian (87.0%), Asian-American (2.9%), Latino (5.8%), and multiracial (4.3%). Most of the sample earned less than $20,000/y (<$9,999 = 55%, $10,000-$19,999 = 27.5%). All participants were right handed. Additional demographic characteristics are presented in Table 1.
Table 1.
Demographic Characteristics of Participating Sample (n = 70)
| Mean (SD) Male, n = 45 |
Mean (SD) Female, n = 25 |
Range | |
|---|---|---|---|
| Age (years) | 22.84 (2.49) | 22.76 (2.35) | 21–33 |
| Education (years completed) | 15.49 (1.26) | 16.30 (1.28) | 12–20 |
| Estimated verbal IQ (NAART) | 13.35 (6.08) | 15.18 (5.96) | 4–31 |
| Alcohol dependence (ADS) | 9.87 (4.05) | 9.52 (4.93) | 1–21 |
| Hazardous drinking (AUDIT) | 12.53 (4.99)* | 10.08 (4.41) | 4–24 |
| Average drinks per drinking day (past month) | 5.30 (2.31) | 4.52 (3.00) | 2–13 |
| Number of drinking days (past month) | 12.08 (4.65) | 11.68 (5.85) | 3–25 |
| Number of binge drinking days (past month) | 7.05 (4.32) | 6.02 (6.38) | 0–20 |
| Tobacco dependence (FTND) | 2.03 (2.16) | 2.40 (2.11) | 0–6 |
| Number of cigarettes smoked per day (past week) | 0.35 (0.95) | 0.71 (1.56) | 0–5 |
| Depressive symptoms (BDI) | 6.03 (4.82) | 8.00 (8.30) | 0–32 |
| Anxiety symptoms (BAI) | 6.64 (5.71) | 10.40 (9.86) | 0–38 |
Significant difference by gender, p < 0.05. NAART, North American Adult Reading Test; ADS, Alcohol Dependence Scale; AUDIT, Alcohol Use Disorders Identification Test; FTND, Fagerstrom Test for Nicotine Dependence; BDI, Beck Depression Inventory; BAI, Beck Anxiety Inventory.
Measures
Participants completed several questionnaires, including a demographic questionnaire, an estimation of right-handedness (The Edinburgh Handness Inventory; Oldfield, 1971), a brief measure of verbal intelligence (North American Adult Reading Test, NAART; Uttl, 2002), an evaluation of the quantity and frequency of drinking (Alcohol History Questionnaire as employed in Filbey et al., 2008; example items included, “In the past month, what is the average number of drinks you've had each time you've drank?”; “In the past month, on how many days did you have at least one alcoholic beverage?”; “In the past month, on how many days did you have 5 or more drinks?”), alcohol dependence symptoms (Alcohol Use Disorders Identification Test, AUDIT; Babor et al., 2006; Alcohol Dependence Scale, ADS; Skinner and Horn, 1984), current tobacco use (Smoking History Questionnaire; items included, “During the past week, on average, how many cigarettes have you had per day?”), tobacco dependence symptoms (Fagerstrom Test for Nicotine Dependence, FTND; Fagerstrom, 1978), depressive symptoms (Beck Depression Inventory-II, BDI-II; Beck et al., 1996), and anxiety symptoms (Beck Anxiety Inventory, BAI; Beck et al., 1988).
Procedures
All procedures followed those of Filbey and colleagues (2008). Before scanning, participants abstained from alcohol for 24 hours, from caffeine and cigarettes for the preceding 2 hours, and had a confirmed breath alcohol level of 0.000 at the start of their session. Participants received $120 in compensation for their participation.
Taste-Cue Paradigm
The taste-cue paradigm is similar to that described in Filbey and colleagues (2008). Briefly, participants received pseudorandom presentations of either an alcohol taste cue (their preferred alcoholic beverage) or an appetitive control taste cue that was selected to control for the appetitive and novel features of alcohol (litchi juice). Each of the 2 echo-planar imaging (EPI) runs consisted of 6 alcohol and 6 control cue trials. Each trial consisted of a 24-second taste delivery period of 1 ml of taste, followed by a washout period to allow the liquid taste to dissipate before the next trial. Notably, this small dose of alcohol (< 1 teaspoon per run) has been found to yield a valid, subject-specific cue that elicits craving during this paradigm (see Filbey et al., 2008). The washout period consisted of a 16-second rest period during which the word “REST” appeared on the screen; nothing was delivered during the rest period. The washout was followed by a 2-second urge question and a 2-second prompt screen. During the urge question, the subjects rated their urge to drink using a 4-point Likert scale.
fMRI Data Acquisition
A volume selective z-shim EPI technique was used to acquire the functional images (Du et al., 2007). We acquired whole-brain fMRI scans with 29 slice locations using a repetition time (TR) of 2 s. Z-shim compensation was applied in 5 of the 29 slice locations, at the region including and immediately above the orbitofrontal cortex (OFC). Other parameters of the EPI data acquisition were: echo time = 26 ms, flip angle = 771, field of view (FOV) = 22 cm, matrix size = 64_64, slice thickness = 4 mm without inter-slice gap. As the effective TR was 1 s in the z-shim slices, a lower flip angle of 621 was used to maximize the image signal intensity in these slices. For a 2-stage registration of the EPI images, high-resolution T1-weighted FLAIR part-head images (29 axial slices of part head, matrix = 56 × 192) were acquired using the same slice angles, thickness, and gap as the EPI images. Another high-resolution full-head 3D structural image was collected in coronal plane using an inversion-recovery spoiled gradient echo (SPGR) sequence (TI = 500 ms, flip angle = 101, slice thickness = 1.4 mm, 256 × 256 matrix, 220 × 220 mm FOV, bandwidth = 15.6 kHz, 124 slices).
fMRI Data Preprocessing
Before statistical analysis, the first 7 volumes of each EPI run were discarded to allow the MR signal to reach steady state. The remaining volumes in each participant's time series were motion corrected using FSL's (FMRIB's Software Library, http://www.fmrib.ox.ac.uk/fsl) McFLIRT Version 5.0 (Motion Correction using FMRIB's Linear Image Registration Tool; Jenkinson et al., 2002). All participants had minimal head movement (<2 mm within a run).
fMRI data analyses employed FMRI Expert Analysis Tool (FEAT) Version 5.63, part of FSL (FMRIB's Software Library, http://www.fmrib.ox.ac, Oxford, UK) used the following prestatistics processing: nonbrain tissue/skull removal using Brain Extraction Tool (BET; Smith, 2002); spatial smoothing using a Gaussian kernel of FWHM 8 mm; mean-based intensity normalization of all volumes by the same factor; highpass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma = 50.0 s). Time-series statistical analysis employed FMRIB's Improved Linear Model (FILM) with local autocorrelation correction (Woolrich et al., 2001). The analyses modeled the activation of the mesocorticolimbic structures after the initial swallow prompt until the end of the washout period (see Filbey et al., 2008). Explanatory variables (e.g., taste and baseline periods for alcohol and control trials, separately) were created by convolving the stimulus timing files with a double gamma hemodynamic response function in FEAT. A multiple linear regression analysis was performed to estimate the hemodynamic parameters for the different explanatory variables and a corresponding t-statistic indicated the significance of the activation of the stimulus. Contrast maps were created by contrasting alcohol taste versus control taste, alcohol taste versus baseline, and control taste versus baseline conditions. Statistical maps were then registered to the Montreal Neurological Institute (MNI) template with a 2-step process. First, EPI images were registered to the part-head high-resolution T1-weighted image acquired in the same plane as the EPI images. The part-head anatomical image was then registered to the high-resolution full-head image, which was subsequently registered to the 152 brain average MNI template. Registration was performed using FMRIB's Linear Image Registration Tool (FLIRT; Jenkinson et al., 2002; Jenkinson and Smith, 2001).
Group analyses were carried out using a mixed effects analysis with FLAME (Beckmann et al., 2003; Woolrich et al., 2004). In 2 separate analyses, the additional effect of depressive and anxiety symptoms on the blood oxygen level dependent (BOLD) response to alcohol taste cues was carried out by entering the orthogonalized total BDI and BAI scores as regressors. To control for multiple comparisons, we used a cluster-threshold of p < 0.05, z = 2.3. Specifically, a Z-statistic threshold was first used to define contiguous clusters (e.g., z > 2.3). Then, each cluster's estimated significance level (from Gaussian random field-theory) was compared with the cluster probability threshold (e.g., p < 0.05; see Smith, 2004). Notably, a height threshold of z = 2.3 (or 1-tailed p < 0.01) was applied simply to determine contiguous clusters (e.g., clusters also had to be compared with the cluster probability threshold). For visualization and display of significant activation, the z-maps were overlaid on the T1 canonical MNI template using MRIcro visualization software (Rorden and Brett, 2000).
Results
Psychosocial Variables: Drinking, Smoking, Depressive Symptoms, and Anxiety Symptoms
Drinking and Smoking
Consistent with study aims, all participants were heavy drinkers (see Table 1). Their designation as a heavy drinker was supported by several measures, including their average quantity of use per drinking occasion, their scores on the ADS (mean = 9.74, SD = 4.35; note: total score ≥9 is predictive of alcohol dependence; n = 39 met or surpassed this cutscore), and the AUDIT (mean = 11.66, SD = 4.91; note: total score >8 is indicative of harmful or hazardous alcohol use). This sample had a past month average of 11.93 drinking days (SD = 5.08), with an average of 5.00 (SD = 2.58) drinks per drinking day and 6.67 (SD = 5.14) binge drinking days. Relevant for the cue-exposure paradigm, most (79.7%) participants' preferred beverage was beer, followed by mixed drinks (10.1%), wine (8.7%), and 1 preferred hard alcohol without a mixer (1.5%). In addition, the 57 current smokers in this sample had relatively low levels of tobacco use, with all reporting smoking less than 5 cigarettes per day in the pervious week (mean = 0.48/day; SD = 1.20), with an average FTND score of 2.18 (SD = 2.13; see Table 1). Comparing alcohol and tobacco use variables by gender revealed no significant differences by ADS, drinks per drinking day, number of drinking days, frequency of binge drinking, or either of the smoking variables. However, a significant difference emerged for the AUDIT, with men having significantly higher AUDIT scores t(68)=2.05, p = 0.044 (see Table 1).
Depressive and Anxiety Symptoms
In terms of symptoms, the majority of this sample showed mild through moderate depressive and anxiety symptoms, with an average score of 6.74 (SD = 6.30) on the BDI (note: range for mild to moderate depression = 10 to 18; moderate to severe depression = 19 to 29; severe ≥30) and an average score of 7.99 (SD = 7.61) on the BAI (note: range for mild to moderate anxiety = 8 to 15; moderate anxiety = 16 to 25; severe ≥26), see Table 1.
Correlations Between Drinking, Smoking, Depressive Symptoms, Anxiety Symptoms, and Demographic Variables
As anticipated, several significant correlations emerged when evaluating the relationships between psychosocial variables. First, as could be expected, BDI and BAI were significantly correlated (r = 0.616, p < 0.001). In addition, BDI and BAI were both significantly positively correlated with number of cigarettes smoked per day (BDI: r = 0.320, p = 0.015; BAI: r = 0.567, p < 0.001). And, supporting the literature (e.g., Chaplin et al., 2008; Nesic and Duka, 2006), BAI was significantly correlated with gender (r = 0.238, p = 0.049). Notably, no significant correlations emerged between depressive, anxiety, and alcohol or tobacco dependence symptoms (ADS, AUDIT, FTND).
fMRI Results
Main Effects of Alcohol Cues
We replicated the previously reported effects of alcohol taste cues on BOLD response in mesocorticolimbic areas (Filbey et al., 2008). Specifically, the alcohol versus control (litchi) contrast showed greater activation in structures within the reward-craving pathway such as the striatum, OFC, ventromedial, prefrontal cortex (PFC), inferior frontal gyrus (IFG), insula, and anterior cingulate gyrus (ACG) (cluster-corrected p < 0.05, z = 2.3).
Depressive Symptoms (BDI) With Alcohol Versus Control
There were widespread areas of significantly positive correlations between BDI scores and BOLD response to alcohol cues, such that the higher the BDI score, the greater the activation during the alcohol (vs. litchi control) cue in the insula, cingulate, striatum (caudate and lentiform nucleus), thalamus, and VTA (cluster-corrected p < 0.05, z = 2.3, Table 2; see Fig. 1A).
Table 2.
Maximum Loci of Activation for BDI Correlations With BOLD Response to Alcohol Taste Cues (vs. Control Cues) (Cluster-Corrected p < 0.05, z = 2.3)
| BDI–BOLD response | |||||
|---|---|---|---|---|---|
| Localization | BA | x | y | z | Max Z |
| R Thalamus | 27 | 14 | −13 | 9 | 5.03 |
| R Superior Temporal Gyrus | 41 | 42 | −35 | 13 | 4.32 |
| L Thalamus | 27 | −16 | −17 | 11 | 4.28 |
| R Precentral Gyrus | 44 | 50 | 5 | 13 | 4.16 |
| R Postcentral Gyrus | 43 | 64 | −11 | 17 | 3.94 |
| R Midbrain | 27 | 6 | −29 | −9 | 3.90 |
| R Cingulate Gyrus | 24 | 2 | 9 | 35 | 3.87 |
| R Middle Frontal Gyrus | 6 | 36 | −1 | 41 | 3.59 |
| R Insula | 22 | 32 | 13 | 3 | 3.49 |
| L Postcentral Gyrus | 2 | −56 | −19 | 33 | 3.30 |
| L Inferior Parietal Lobule | 40 | −38 | −39 | 29 | 3.26 |
| R Inferior Parietal Lobule | 40 | 36 | −45 | 39 | 3.22 |
| L Claustrum | 5 | −30 | 11 | −1 | 3.20 |
| L Precentral Gyrus | 44 | −48 | −1 | 11 | 3.16 |
| L Inferior Parietal Lobule | 40 | −48 | −43 | 43 | 3.15 |
| L Lateral Ventricle | 24 | −10 | 21 | 3 | 2.97 |
| L Lateral Ventricle | 19 | −34 | −43 | −1 | 2.80 |
BDI, Beck Depression Inventory; R, right; L, left.
Fig. 1.
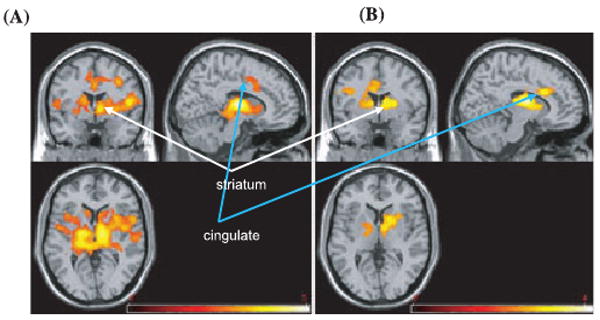
Areas of significantly positive correlations between (A) total BDI (B) and total BAI scores and BOLD response to alcohol taste cues (vs. control cues) (cluster-corrected p < 0.05, z = 2.3). Right side represents right hemisphere activations.
Anxiety Symptoms (BAI) With Alcohol Versus Control
Similarly, there were significantly positive correlations between BAI scores and BOLD response to alcohol cues such that the higher the BAI score, the greater the activation during the alcohol (vs. litchi control) cue in the striatum (caudate and lentiform nucleus), thalamus, insula, cingulate, mid-frontal gyrus, and IFG (cluster-corrected p < 0.05, z = 2.3, Table 3; see Fig. 1B).
Table 3.
Maximum Loci of Activation for BAI Correlations With BOLD Response to Alcohol Taste Cues (vs. Control Cues) (Cluster-Corrected p < 0.05, z = 2.3)
| BAI–BOLD response | |||||
|---|---|---|---|---|---|
| Localization | BA | x | y | z | Max Z |
| R Caudate | – | 10 | −7 | 15 | 4.17 |
| L Thalamus | 28 | −16 | −15 | −13 | 3.75 |
| L Inferior Frontal Gyrus | 9 | −52 | 21 | 27 | 3.47 |
| L Cingulate Gyrus | 32 | −8 | 21 | 29 | 3.42 |
| R Anterior Cingulate | 32 | 12 | 19 | 27 | 3.42 |
| L Middle Frontal Gyrus | 46 | −36 | 33 | 23 | 3.35 |
| R Superior Frontal Gyrus | 9 | 32 | 35 | 37 | 2.84 |
| L Insula | 13 | −36 | 13 | 13 | 2.81 |
| L Precentral Gyrus | 6 | −36 | −1 | 29 | 2.77 |
| L Cingulate Gyrus | 31 | −12 | −33 | 37 | 2.64 |
| L Precentral Gyrus | 6 | −61 | 1 | 27 | 2.54 |
BAI, Beck Anxiety Inventory; R, right; L, left.
Individual Differences in Drinking and Smoking Variables With Alcohol Versus Control
To rule out the potentially confounding effects of individual differences on the observed brain changes, we analyzed the alcohol variables, smoking variables, and gender. In these analyses, 4 significant findings emerged (cluster-corrected p < 0.05, z = 2.3). In the female sample only, average number of drinks per drinking day and number of binge drinking days showed significant activation in the precuneus. In the male sample only, the total score on the ADS was associated with activation in the striatum, ACG, and OFC. Finally, across both genders, number of cigarettes per day was associated with significant activation in the VTA, hippocampus, and inferior temporal gyrus.
In addition, as gender and BAI were correlated (r = 0.238, p = 0.049), we evaluated the potential influence of gender in the correlation analyses between BAI and BOLD response by including it as a covariate in the BAI analyses. No significant activation emerged suggesting that gender did not influence the correlations between BAI and BOLD response to alcohol taste cues. We also evaluated the role of gender in brain response to alcohol versus control cues. No significant differences emerged indicating that men and women in this sample responded similarly to the alcohol compared with the control cues.
Manipulation Check
To evaluate whether craving was successfully induced, subjective craving for the alcohol and control (litchi) beverages were measured at 5 time points; prior to the administration of alcohol, prior to the administration of litchi, following the administration of alcohol, following the administration of litchi, and after removal from the scanner. Comparing the mean subjective craving following presentation of each beverage yielded a significant difference [t(48) = 2.27, p = 0.028] highlighting comparatively greater craving for alcohol. Comparison of the final urge rating (after removal from the scanner) yielded an even more impressive effect in favor of craving for alcohol: t(48) = 7.31, p < 0.000. Together, these data support successful induction of craving for alcohol, which substantially surpassed the craving for the appetitive control cue.
In addition, to determine the strength of the above correlations, we also evaluated the correlations between BDI and alcohol > baseline, BDI and litchi > baseline, BAI and alcohol > baseline, and BAI and litchi > baseline. The results revealed no areas of significantly positive correlations, indicating that the alcohol versus control (litchi) correlations in the observed areas were specific to alcohol, and above and beyond the response to an appetitive cue.
Discussion
Building upon the work of previous studies (e.g., Breese et al., 2005; Chiang et al., 2005; Cooney et al., 1997; Fox et al., 2007; Litt et al., 2000; Sinha, 2007; Sinha et al., 2008) examining the relationship between depressive and anxiety symptoms with alcohol cue response, as well as neuroimaging investigations of alcohol cue reactivity (e.g., de Greck et al., 2008; Filbey et al., 2008; Gilman and Hommer, 2008; Heinz et al., 2004; Myrick et al., 2004), the findings from this study highlighted significant correlations between depressive symptoms, anxiety symptoms, and significant differential BOLD activation in response to a gustatory alcohol versus an appetitive control cue. Similar to the observed relationship between cue-exposure, depressive symptoms, and anxiety by Gilman and Hommer (2008), the pattern of activation found within this study mapped onto the expected regions (e.g., de Greck et al., 2008; Filbey et al., 2008). Integrating facets of previous work, this study supported the posited relationships (e.g., Sinha et al., 2008) between depressive symptoms, anxiety symptoms, and differential brain activity in response to an alcohol versus control cue in a sample of heavy drinking adults.
The first finding that emerged was the relationship between depressive symptoms and differential BOLD activation. Participants with higher depressive symptoms (BDI scores) evidenced greater activation in the insula, cingulate, VTA, striatum, and thalamus following presentation of the alcohol versus control appetitive cue. These findings build upon previous literature, which have identified several key neural substrates, but have not yet explicitly addressed the potential role of depressive symptoms in the activation of these substrates. The activation of the insula and cingulate highlight the involvement of the limbic system, responsible for sensory and emotional information integration, during this task. Moreover, the activation of the VTA, which has strong dopaminergic function, is likely to play a strong role in emotion, reward perception, and motivation. The striatum has been implicated in planned and executed behavior, both in terms of movement, as well as in executive control. Finally, the thalamus is believed to be involved in the processing and communication of sensory information throughout the cerebral cortex. Together, these findings highlight that the observed relationship between depressive symptoms and differential BOLD activation in these areas indicate the role of perception, processing, and evaluation of emotionally laden and potentially rewarding information, as well as potential decision-making around the perception of that information. This is important, as these data highlight the utility of investigating the role of co-occurring depressive affect and anxiety in understanding the risk that heavy drinking adults may face when presented with alcohol cues. Notably, this sample had minimal levels of depressive symptoms; the majority was under the cutpoint for moderate depression. Thus, a critical next step will be to evaluate these results with a sample that meets clinical (DSM-IV-TR) criteria for depressive disorders.
Our second finding was that participants with greater levels of anxiety symptoms evidenced greater activation in the striatum, thalamus, insula, and unique to this analysis, IFG. These findings build upon previously observed relationships between anxiety symptoms and differential BOLD activation in mesocorticolimbic circuits after alcohol cue exposure (e.g., Chiang et al., 2005). In addition, the consistency of the activation between the depressive and the anxiety symptoms suggest that experiencing depressive and anxiety symptoms may engage similar neurocircuitry in terms of response to alcohol cues, reward perception, and decisions to drink. In terms the findings with the IFG, this is consonant with the work of Karch and colleagues (2008), who found greater activation of the IFG with alcohol dependent participants who had greater levels of co-occurring anxiety symptoms. In addition, despite our significant findings, this sample's level of anxiety symptoms was mostly below clinical cutpoints; it will be highly beneficial for future studies to evaluate this relationship with a sample that meets clinical (DSM-IV-TR) criteria for anxiety disorders.
On a more general level, the results address the posited relationship between depressive symptoms, anxiety symptoms, and risk for relapse (e.g., Yoon et al., 2006; Zywiak et al., 2006). Over the past 2 decades, many psychosocial alcohol interventions have explicitly included content addressing management of depressive and/or anxiety symptoms (e.g., Kavanagh et al., 2006; Marlatt, 1996). Our findings suggest that there may indeed be a neural basis for these observed behavioral relationships. This is critical in considering how best to target prevention and intervention efforts to reduce hazardous drinking; this study suggests that helping heavy drinkers understand that they might be more sensitive to alcohol, neurologically, as well as potentially emotionally and physically, when feeling even modestly depressed or anxious, may help guide their decision-making around whether or not to consume alcohol in the context of feeling even mildly depressed or anxious.
While we can only speculate regarding the reason for the relationship between greater activation in these regions with depressive and anxiety symptoms, we have several ideas. First, we believe that this relationship may stem from differences in the incentive/reward value of alcohol. It is possible that alcohol may be employed to mitigate the experience of depressive and anxiety symptoms. With time, a person may come to rely upon alcohol more to ameliorate these symptoms. As a person has greater reliance upon alcohol, they may subsequently experience greater anticipation of reward, as well as greater positive expectancies of the power of alcohol to reduce depressive and anxiety symptoms. Across the 2 symptom groups (BDI and BAI), the areas of activation that emerged in this study supports this hypothesis. Specifically, the involvement of the striatum, thalamus, and VTA (depressive symptoms only), indicates the sensitivity to and/or anticipation of reward. Moreover, the involvement of the anterior insula and dorsal anterior cingulate indicates the potential involvement of cognitive processes, such as subjective feeling of motivation, maintaining goals (Craig, 2009), and conflict detection (Matthews et al., 2004). Finally, the involvement of the mid-frontal gyrus and the IFG (with the anxiety symptoms only) indicates more focused attention.
To exclude the obvious hypothesis that the association between depressive and anxiety symptoms and the alcohol versus control contrast was driven by the appetitive control condition (rather than the alcohol condition), we explicitly evaluated the strength of the aforementioned contrasts by comparing the activation with the cues against baseline (alcohol > baseline; litchi > baseline). These analyses support that the observed results with depressive and anxiety symptoms were unique to alcohol. This indicates that participants with higher BDI and BAI scores were not simply showing an overall reduced activation to the control cue, but instead showed differentially greater activation to the alcohol versus the control cue.
In addition, we evaluated gender as a correlate in the imaging analyses. We found no significant gender differences in overall response to alcohol versus control cues, indicating that men and women, in general, responded to the alcohol cue task similarly. However, despite a lack of group mean differences across drinking and smoking variables (with the exception of the AUDIT), several significant gender differences in activation emerged when examining the drinking and smoking variables. Specifically, among the women only, those with greater quantity/frequency of drinking (e.g., number of drinks per drinking day and number of binge drinking days) evidenced significantly greater activation in the precuneus. In contrast, with the men only, a significant relationship emerged between those with greater scores on the ADS and activation in the striatum, ACG, and OFC. Finally, across both genders, only one smoking variable was associated with significant activation; those who had greater number of cigarettes per day, evidenced activation in the VTA, hippocampus, and inferior temporal gyrus. The pattern that emerged from the male sample directly maps onto recent research, with greater alcohol dependence symptoms corresponding to greater mesocorticolimbic activation (e.g., Filbey et al., 2008). In contrast, for women, the compelling outcome was not for alcohol dependence symptoms, but for actual alcohol use, highlighting the importance of looking at quantity of frequency of use with female participants. Together, these findings provide preliminary evidence for the importance of examining neural outcomes by gender in alcohol and tobacco research.
In summary, this investigation sought to evaluate the relationship between depressive symptoms, anxiety symptoms, and neural response after alcohol cue exposure. The results indicate that co-occurring depressive symptoms and anxiety symptoms are associated with significant differential activation in key neurobiological regions in response to alcohol versus appetitive control cues with heavy drinking adults. This study has important implications for both research and clinical applications. Specifically, this study indicates that depressive or anxiety symptoms may increase the salience of alcohol cues, increase the perception of the positive aspects of alcohol consumption, and reduce attention to the negative consequences of alcohol use (e.g., Monti et al., 2000). Similarly, it is equally possible that heavy drinking may increase the risk for and experience of depressive and anxiety symptoms.
Results of this study should be considered in light of the following limitations. First, this was a preliminary study evaluating the role of depressive and anxiety symptoms upon cue-elicited craving in a neuroimaging paradigm. This sample was a heavy drinking, young adult sample, with a relatively mild level of depressive and anxiety symptoms. Subsequently, these results should only be generalized to a similar population. And, replication with more severe samples (in terms of anxiety, depressive, and alcohol dependence symptoms) is necessary before results can be generalized to clinical populations. In addition, this sample did not include a comparison group of nondrinking adults with depressive and anxiety symptoms. Replication with a control nondrinking clinical sample would also yield beneficial comparison data. As heavy drinking is likely to influence the development of depressive and anxiety symptoms, future studies would benefit from specifically investigating the role of alcohol abuse and dependence on increased depressive and anxiety symptoms, and subsequent cue-elicited brain response. In addition, while this study examined current depressive and anxiety symptoms in relationship to differential activation, future studies examining depressive and anxiety manipulations will help illuminate the nature of the observed relationship. Finally, this study takes a step toward evaluating the underlying biological substrates behind the relationship between depressive and anxiety symptoms, however, evaluation of the results in terms of potential underlying genetic risk factors (e.g., Hutchison et al., 2008) will be important in future work. Together, we believe that these steps will help improve the formulation of targeted efficacious treatments for heavy drinking, and ultimately, alcohol dependent adults.
Acknowledgments
We would like to thank Amy Audette and Yiping Du for their assistance with this study.
Sources of Support: This research was supported by a grant (R01AA012238-07) from the National Institute on Alcohol Abuse and Alcoholism.
Footnotes
Financial Disclosures: The authors declare that they have no competing financial or other conflicts of interest relating to the data included in the manuscript.
References
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. AUDIT: The Alcohol Use Disorders Indentification Test: Guidelines for Use in Primary Care. 2nd. The World Health Organization; Geneva, Switzerland: 2006. [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–63. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Breese GR, Chu K, Dayas CV, Funk D, Knapp DJ, Koob GF, Le DA, O'Dell LE, Overstreet DH, Roberts AJ, Sinha R, Valdez GR, Weiss F. Stress enhancement of craving during sobriety: a risk for relapse. Alcohol Clin Exp Res. 2005;29:185–195. doi: 10.1097/01.alc.0000153544.83656.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Timpano KR, Zvolensky MJ, Sachs-Ericsson N, Schmidt NB. Implications of comorbid alcohol dependence among individuals with social anxiety disorder. Depress Anxiety. 2008;25:1028–1037. doi: 10.1002/da.20442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin TM, Hong K, Bergquist K, Sinha R. Gender differences in response to emotional stress: an assessment across subjective, behavioral, and physiological domains and relations to alcohol craving. Alcohol Clin Exp Res. 2008;32:1242–1250. doi: 10.1111/j.1530-0277.2008.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang SS, Schuetz C, Soyka M. Effects of cue exposure on the subjective perception of alcohol dependents with different types of cue reactivity. J Neural Transm. 2005;112:1275–1278. doi: 10.1007/s00702-005-0355-8. [DOI] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. J Abnorm Psychol. 1997;106:243–250. doi: 10.1037//0021-843x.106.2.243. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Du YP, Dalwani M, Wylie K, Claus E, Tregellas JR. Reducing susceptibility artifacts in fMRI using volume-selective z-shim compensation. Magn Reson Med. 2007;57:396–404. doi: 10.1002/mrm.21150. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Claus E, Audette AR, Niculescu M, Banich MT, Tanabe J, Du YP, Hutchison KE. Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology. 2008;33:1391–1401. doi: 10.1038/sj.npp.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Bergquist KL, Hong Kl, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcohol Clin Exp Res. 2007;31:395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Hommer DW. Modulation of brain response to emotional images by alcohol cues in alcohol-dependent patients. Addict Biol. 2008;13:423–434. doi: 10.1111/j.1369-1600.2008.00111.x. [DOI] [PubMed] [Google Scholar]
- de Greck M, Supady A, Thiemann R, Tempelmann C, Bogerts B, Forschner L, Ploetz KV, Northoff G. Decreased neural activity in reward circuitry during personal reference in abstinent alcoholics-A fMRI study. Hum Brain Mapp. 2008;30:1691–1704. doi: 10.1002/hbm.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotheus JM, Bischof G, Reinhardt S, Meyer C, John U, Rumpf HJ. Effectiveness of brief alcohol interventions for general practice patients with problematic drinking behavior and comorbid anxiety or depressive disorders. Drug Alcohol Depend. 2008;94:214–220. doi: 10.1016/j.drugalcdep.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Heinz A, Seissmeier T, Wrase J, Hermann D, Klein S, Grusser SM, Flor H, Braus DF, Buchholz HG, Grunder G, Schreckenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P. Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry. 2004;161:1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Haughey HM, Niculescu M, Schact J, Kaiser A, Stitzel J, Horton WJ, Filbey FM. The incentive salience of alcohol: translating the effects of genetic variant in CNR1. Arch Gen Psychiatry. 2008;65:841–850. doi: 10.1001/archpsyc.65.7.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansma A, Breteler MH, Schippers GM, de Jong CA, Van Der Staak CF. No effect of negative mood on the alcohol cue reactivity of in-patient alcoholics. Addict Behav. 2000;25:619–624. doi: 10.1016/s0306-4603(99)00037-4. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Karch S, Jager L, Karamatskos E, Graz C, Stammel A, Flatz W, Lutz J, Holtschmidt-Täschner B, Genius J, Leicht G, Pogarell O, Born C, Möller HJ, Hegerl U, Reiser M, Soyka M, Mulert C. Influence of trait anxiety on inhibitory control in alcohol-dependent patients: simultaneous acquisition of ERPs and BOLD responses. J Psychiatr Res. 2008;42:734–745. doi: 10.1016/j.jpsychires.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Kavanagh DJ, Sitharthan G, Young RM, Thiagarajan S, Sitharthan T, Saunders JB, Shockley N, Giannopoulos V. Addiction of cue exposure to cognitive-behaviour therapy for alcohol misuse: a randomized trial with dysphoric drinkers. Addiction. 2006;101:1106–1116. doi: 10.1111/j.1360-0443.2006.01488.x. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Abrams K, Thuras P, Hanson KL, Brekke M, Sletten S. Follow-up study of anxiety disorder and alcohol dependence in comorbid alcoholism treatment patients. Alcohol Clin Exp Res. 2005;29:1432–1443. doi: 10.1097/01.alc.0000175072.17623.f8. [DOI] [PubMed] [Google Scholar]
- Litt MD, Cooney NL, Morse P. Reactivity to alcohol-related stimuli in the laboratory and in the field: predictors of craving in treated alcoholics. Addiction. 2000;95:889–900. doi: 10.1046/j.1360-0443.2000.9568896.x. [DOI] [PubMed] [Google Scholar]
- Marlatt GA. Taxonomy of high-risk situations for alcohol relapse: evolution and development of a cognitive-behavioral model. Addiction. 1996;91:S37–S49. [PubMed] [Google Scholar]
- Mason BJ, Light JM, Escher T, Drobes DJ. Effect of positive and negative affective stimuli and beverage cues on measures of craving in non treatment-seeking alcoholics. Psychopharmacology. 2008;200:141–150. doi: 10.1007/s00213-008-1192-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews SC, Paulus MP, Simmons AN, Nelesen RA, Dimsdale JE. Functional subdivisions within anterior cingulate cortex and their relationship to autonomic nervous system function. Neuroimage. 2004;22:1151–1156. doi: 10.1016/j.neuroimage.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Hutchison KE. Toward bridging the gap between biological, psychobiological and psychosocial models of alcohol craving. Addiction. 2000;95(Suppl 2):S229–S236. doi: 10.1080/09652140050111799. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, George MS. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- Nesic J, Duka T. Gender specific effects of a mild stressor on alcohol cue reactivity in heavy social drinkers. Pharmacol Biochem Behav. 2006;83:239–248. doi: 10.1016/j.pbb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12(4):191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Shiffman S, Tiffany ST, Niaura RS, Martin CS, Shadel WG. The measurement of drug craving. Addiction. 2000;95:S189–S210. doi: 10.1080/09652140050111762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. The role of stress in addiction relapse. Curr Psychiatry Rep. 2007;9:388–395. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz K. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2008;34:1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Horn JL. Alcohol Dependence Scale (ADS) Users Guide. Addiction Research Foundation; Toronto: 1984. [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Overview of fMRI analysis. Br J Radiol. 2004;77:S167–175. doi: 10.1259/bjr/33553595. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. The role of cognitive factors in reactivity to drug cues. In: Drummond D, Tiffany S, Glautier S, Remington B, editors. Addictive Behaviour: Cue Exposure Theory and Practice. Vol. 7. Wiley; New York, NY: 1995. pp. 137–165. [Google Scholar]
- Uttl B. North American Adult Reading Test: age norms, reliability, and validity. J Clin Exp Neuropsychol. 2002;24:1123–1137. doi: 10.1076/jcen.24.8.1123.8375. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modeling for fMRI group analysis using Bayesian inferecence. Neuroimage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–86. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Yoon G, Kim SW, Thuras P, Grant JE, Westermeyer J. Alcohol craving in outpatients with alcohol dependence: rate and clinical correlates. J Stud Alcohol. 2006;67:770–777. doi: 10.15288/jsa.2006.67.770. [DOI] [PubMed] [Google Scholar]
- Zywiak WH, Stout RL, Trefry WB, Glasser I, Connors GJ, Maisto SA, Westerberg VS. Alcohol relapse repetition, gender, and predictive validity. J Subst Abuse Treat. 2006;30:349–353. doi: 10.1016/j.jsat.2006.03.004. [DOI] [PubMed] [Google Scholar]


