Abstract
Fetal-neonatal iron deficiency alters hippocampal neuronal morphology, reduces its volume, and is associated with acute and long-term learning impairments. However, neither the effects of early-life iron deficiency anemia on growth, differentiation, and survival of hippocampal neurons nor regulation of the neurotrophic factors that mediate these processes has been investigated. We compared hippocampal expression of neurotrophic factors in male rats made iron deficient (ID) from gestational d 2 to postnatal d (P) 7 to iron-sufficient controls at P7, 15, and 30 with quantitative RT-PCR, Western analysis, and immunohistology. Iron deficiency downregulated brain-derived neurotrophic factor (BDNF) expression in the hippocampus without compensatory upregulation of its specific receptor, tyrosine-receptor kinase B. Consistent with low overall BDNF activity, we found lower expression of early-growth response gene-1 and -2, transcriptional targets of BDNF signaling. Doublecortin expression, a marker of differentiating neurons, was reduced during peak iron deficiency, suggesting impaired neuronal differentiation in the ID hippocampus. In contrast, iron deficiency upregulated hippocampal nerve growth factor, epidermal growth factor, and glial-derived neurotrophic factor accompanied by an increase in neurotrophic receptor p75 expression. Our findings suggest that fetal-neonatal iron deficiency lowers BDNF function and impairs neuronal differentiation in the hippocampus.
Introduction
Iron deficiency is one of the foremost early-life nutrient deficiencies, affecting ∼30–50% pregnancies worldwide, including an estimated 80% of pregnancies in developing countries (1). Late gestational and neonatal (perinatal) iron deficiency arises from 3 common maternal gestational conditions: severe iron deficiency anemia, placental vascular insufficiency resulting from maternal hypertension, and diabetes mellitus (2–4). In humans, neonatal iron deficiency causes deficits in cognitive function during the period of iron deficiency and poor school performance well after the period of iron deficiency (5,6). With early postnatal iron deficiency, although certain developmental deficits can be corrected with iron treatment, other neurological and cognitive developmental deficits persist up to 10 y after iron treatment (7–9). The neural basis of these developmental deficits continues to be investigated, with evidence from animal models suggesting that multiple developing brain processes, such as myelination, monoamine metabolism, energy metabolism, and dendritic arborization, might be affected (10–14). Based on the ontogeny of human brain development, perinatal iron deficiency may have large effects on rapidly differentiating regions such as the hippocampus (15). Consistent with this notion, peak iron import into the rat hippocampus occurs between postnatal d (P)8 5 and P15, just prior to maximal cellular differentiation, synaptogenesis, and dendritic growth and arborization (16).
Fetal-neonatal iron deficiency particularly affects the hippocampus as evidenced by decreased energy metabolism, impaired neuronal morphology and transmission, and increased susceptibility to infarction (13,14,17,18). A recent study profiling altered gene expression induced by perinatal iron deficiency identified alterations in salient molecular pathways involved in neuronal differentiation (19), most notably the mammalian target of rapamycin (mTOR) pathway, which integrates external stimuli such as nutrients and growth factors to regulate gene expression necessary for synaptic maturation and plasticity in the hippocampus (20–22). However, the specific effect of perinatal iron deficiency on the expression of neurotrophic growth factors critical for inducing and maintaining hippocampal neurogenesis, differentiation, and plasticity has not been investigated.
Among the known neurotrophic growth factors, brain-derived neurotrophic factor (BDNF) influences multiple aspects of hippocampal development and synaptic plasticity. BDNF regulates neurogenesis, survival, dendritic growth and branching, and plasticity across the life span (23–25). Induction of long-term potentiation (LTP), a cellular phenomenon associated with memory formation, in the rodent hippocampus rapidly increases BDNF transcript levels (26–28), whereas suppression of BDNF expression and genetic deletion of BDNF leads to impairment of learning, LTP formation, and affective behavior (29,30). BDNF signaling is mediated preferentially by tyrosine-receptor kinase B (TrkB) and neurotrophic receptor p75 (p75NTR) (31). Whereas BDNF/TrkB promotes neurogenesis, neurite outgrowth, and synaptic plasticity, BDNF/p75NTR facilitates long-term depression and reduces neurite outgrowth (32–34). Chronic stress, chronic antidepressant administration, learning, and LTP induction regulate both BDNF and TrkB expression in the hippocampus (35,36).
Based on our prior findings that perinatal iron deficiency induces defects in hippocampal dendritic morphology and neurotransmission, we hypothesized that iron deficiency would result in dysregulation of neurotrophic factors involved in neuronal differentiation and synaptic plasticity. Here, we present evidence that perinatal iron deficiency reduces BDNF activity and alters neuronal development in rat hippocampus.
Materials and Methods
Animals.
All animal experiments were conducted with the approval of the University of Minnesota Institutional Animal Care and Use Committee. Timed-pregnant Sprague-Dawley rats were purchased from Harlan. Fetal-neonatal iron deficiency was induced as previously described to achieve a 40% loss of total brain iron at P10 (12), a degree of brain iron deficiency equivalent to that in newborn humans (3). In this model, the hippocampus remains iron deficient (ID) through P30 (25% loss) and is iron sufficient (IS) by P56 (17). In brief, pregnant dams were maintained on an ID nonpurified diet from gestational d 2 to P7, after which time the nursing dams were given IS nonpurified diet (see below). Pups from dams given the IS diet served as IS controls. Litters were culled to 8 pups per litter. All pups were weaned at P21 and fed an IS diet for the duration of the experiment.
Diets.
IS control (198 mg/kg iron, Rx 241632) and ID (3 mg/kg iron, Rx 247497) nonpurified diets were purchased from Harlan Teklad. The composition of both the IS and ID diets has been described previously (12).
Tissue dissection and collection.
Male rats at P7, 15, and 30 were killed by an intraperitoneal injection of Beuthanasia (100 mg/kg). Specific postnatal ages were selected on the basis of the ontogeny of the hippocampal formation; P7 marks the end of the proliferative stage and P15 and P30 represent early and late differentiation, respectively (37,38). Brains were removed and bisected along the midline. Hippocampus was dissected, flash-frozen in liquid nitrogen, and stored at −80°C.
Quantitative RT- PCR.
Total RNA was isolated from dissected hippocampus using an RNA isolation kit (Stratagene) and concentrations were measured by absorbance at 260 nm (A260/280) using a NanoDrop ND-1000 (NanoDrop Technologies). Approximately 4μg of total RNA was used to generate cDNA by reverse transcription using SuperScript III (Invitrogen) and random hexamer primers per manufacturer recommendation. The resulting cDNA was diluted 7-fold to give a final volume of 140 μL. All quantitative RT-PCR experiments were performed with one-half the manufacturer's recommended volume (Applied Biosystems) consisting of 4 μL of diluted cDNA, 5 μL 2× Taqman quantitative RT-PCR Universal mix (No AmpErase), and 0.5 μL 20× Taqman Gene Expression Assay primer/probe mix. Thermocyling was conducted according to the manufacturer's protocol (ABI) using a MX3000P instrument (Stratagene). The transcripts that were analyzed are listed in Supplemental Table 1.
Western blot analysis.
Protein isolation was conducted as described previously (19). In brief, flash-frozen hippocampal tissues were lysed by sonication. Approximately 31 μg of total protein was loaded and separated in 10% SDS-PAGE gels. Protein was transferred onto Nitrocellulose membranes (Pierce) using semidry transfer (Bio-Rad). Membranes were blocked in 3% nonfat powder milk diluted in TBST (Tris buffer pH 7.4 + 0.1% Tween-20) for 1 h at room temperature. Membranes were incubated in primary antibody diluted in TBST containing 0.1% nonfat powder milk overnight at 4°C with rocking and rinsed in TBST (4×) to remove excess antibody. Membranes were then incubated in horseradish peroxidase-conjugated secondary antisera at room temperature for 2 h and excess antisera were removed with TBST washes (5×). The protein-antisera complex was detected using an ECL kit (GE Healthcare) and a darkroom equipped with a super-cooled charge coupled device camera (Bio-Rad). For quantification, integrated intensity of the protein of interest was standardized to actin, whose expression is not affected by iron deficiency and thus acts as an internal control. The primary antibodies included anti-TrkB (1:1000) rabbit polyclonal (Cell Signaling), anti-actin (1:500) mouse monoclonal (Sigma), and anti-p75NTR (1:1000) rabbit polyclonal (a generous gift from Dr. William Engeland, University of Minnesota).
Immunohistology.
Rats were deeply anesthetized with pentobarbital (100 mg/kg) and perfused transcardially with PBS and 10% formalin fixative. Brains were removed and further fixed in 10% formalin for 4–6 h at 4°C. Fixed brains were cryoprotected by immersion in 30% sucrose/PBS solution and embedded in frozen section medium (Neg-50, Richard-Allan Scientific). Twenty-μm coronal sections were obtained using a cryostat (Leica CM1900) and stored at −20°C. For fluorescence immunohistology, sections were equilibrated to room temperature and rehydrated in TBS. Antigen unmasking was performed by immersing sections in hot (95°C) 10 mmol/L Na citrate, pH 8.6, and then allowing them to cool to room temperature in a water bath. Sections were permeabilized in TBS + 0.2% Triton X-100 and incubated for 1 h in blocking solution (10 g/L bovine serum albumin diluted in TBS + 0.1% Tween-20) and then incubated in primary antibody overnight at 4°C. Excess antibody was removed with TBST washes. Sections were retreated with blocking solution, incubated in fluorescence-labeled secondary antibody overnight at 4°C, and washed with TBST. Finally, sections were mounted in aqueous mounting media with 4′,6-diamidino-phenylindole (Vector Laboratories). Antibodies included biotin-conjugated anti-BDNF (5 mg/L) chicken polyclonal (A&D System), anti-Neuronal Nuclei (1:200) raised in mouse (MAB377, Chemicon Internationals), anti-doublecortin (Dcx) (1:50) goat polyclonal (Santa Cruz Biotechnology), and anti-p75NTR (1:100) rabbit polyclonal. Fluorescence-labeled secondary antibodies were purchased from Molecular Probes (Invitrogen) and used according to the manufacturer's recommendation. Confocal images were captured with a Nikon Digital-Eclipse C1 microscope system.
Terminal deoxynucleotide transferase-mediated biotin-dUTP nick end labeling staining.
Detection of enzyme-mediated DNA fragmentation was carried out by terminal deoxynucleotide transferase (TdT)-mediated biotin-dUTP nick end labeling (TUNEL) staining (39) with modifications. In brief, following rehydration in TBS, 20-μm brain sections were permeabilized with TBS + 0.2% Triton X-100, rinsed in TdT buffer (30 mmol/L Tris, pH7.2, 0.02% CoCl2, 3% Na cacodylate), and incubated for 1 h at 37°C in terminal transferase solution (400 U TdT, 30 μmol biotin-dUTP, 70 μmol dUTP in TdT buffer). The reaction was terminated by incubation for 15 min in termination buffer (300 mmol/L NaCl, 30 mmol/L sodium citrate). Labeled DNA was detected by incubation in Cy3-conjugated strepavidin (1:100, Invitrogen) after blocking in 10% bovine serum albumin solution (Sigma). Fluorescence images were taken with a Nikon microscope (Eclipse E600) equipped with a charge coupled device camera.
Statistical methods.
Data for transcript levels were collected from 6 male rats per postnatal age for each dietary group. To assess neurotrophic factor expression during hippocampal differentiation, we compared transcript levels at P7, 15, and 30 in the developing IS rat hippocampus by 1-way ANOVA with post hoc between-time assessments by Bonferroni-corrected t tests. All expression data were normalized to the P7 IS group. Dietary group differences in transcript expression across time were assessed by 2-way ANOVA with Bonferroni-corrected t tests to assess differences between dietary groups at each age. Nonnormalized group data (i.e. protein data) were assessed using the Mann-Whitney U-test. Group data with significant differences in variance (i.e. TUNEL data) were assessed by unpaired t test with Welch's correction. Significance was set at an α of 0.05. Graphs and statistical calculations were performed with GraphPad Prism (GraphPad Software).
Results
Increased neurotrophic factor expression during hippocampal differentiation.
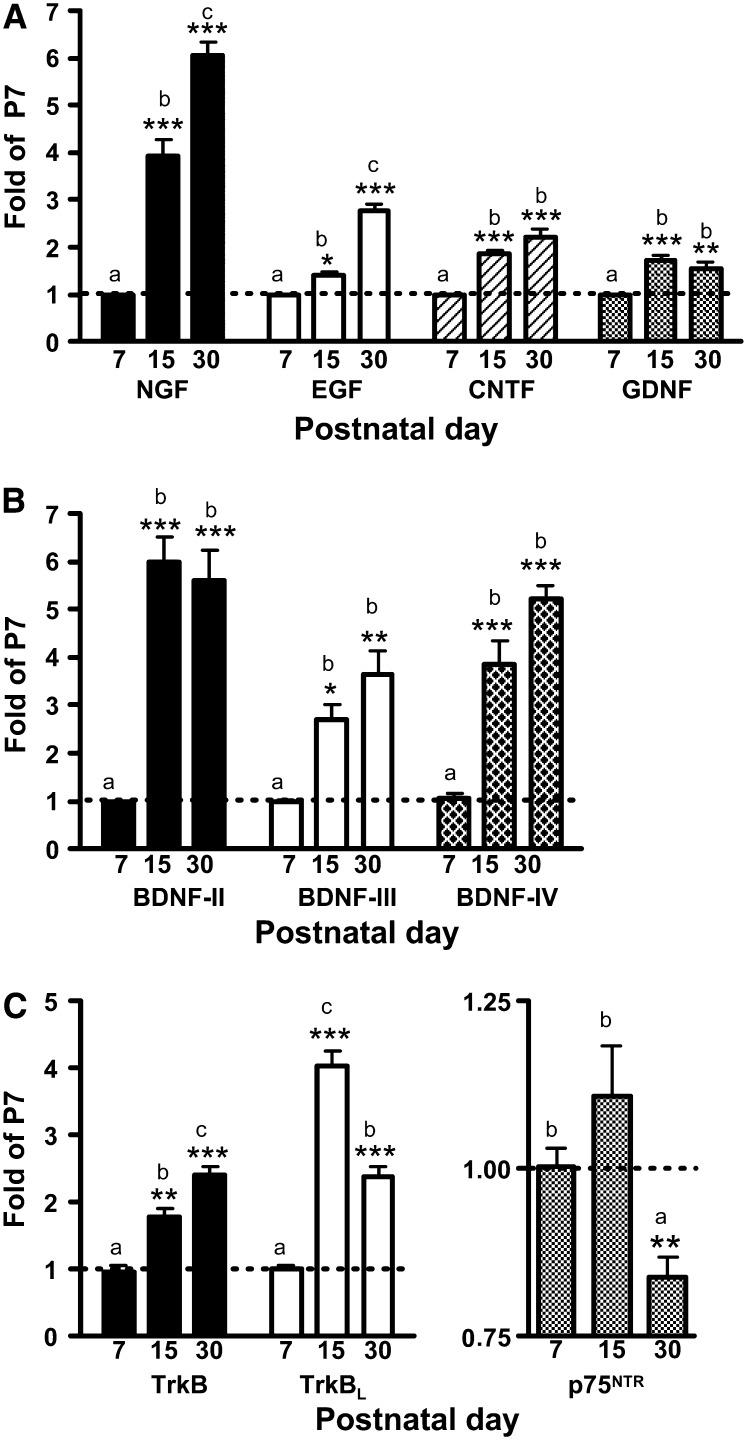
Nerve growth factor (NGF), epithelial growth factor (EGF), ciliary neurotrophic factor (CNTF), and glial-derived neurotrophic factor (GDNF) mRNA expression was higher at P15 and P30 compared with P7 (Fig. 1A). BDNF-II mRNA levels were negligible compared with BDNF-III and -IV and were not included in further analyses (data not shown). All 3 BDNF transcripts were greater at P15 and P30 compared with P7 (Fig. 1B). TrkBL and p75NTR peaked at P15 and total TrkB peaked at P30 (Fig. 1C).
FIGURE 1 .
Comparison of neurotrophic factor mRNA transcripts at P7, 15, and 30 in the hippocampus of male rats fed an IS diet. (A) NGF, EGF, CNTF, and GDNF mRNA. (B) BDNF-II, -III, and -IV mRNA. (C) Total TrkB, TrkBL, and p75NTR mRNA levels. Values are means ± SEM, n = 4–6. Asterisks indicate P-values as follows: *P < 0.05, **P < 0.01, ***P < 0.001. Bars without a common letter differ, P < 0.05.
Increased GDNF, EGF, and NGF and decreased BDNF expression in ID hippocampus.
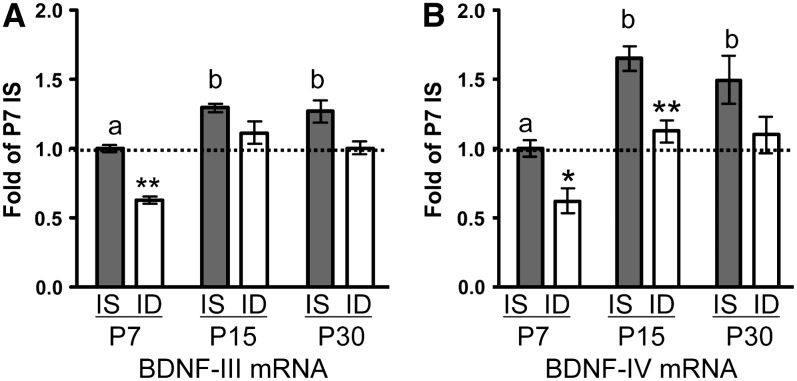
CNTF mRNA levels were unchanged by iron deficiency (Table 1). P7 ID GDNF mRNA levels were 80% greater than the P7 IS hippocampus levels; the difference was less at P15 and the groups did not differ at P30 (Table 1). EGF and NGF mRNA levels were greater in the ID rats compared with IS rats at P15 (Table 1). In contrast, iron deficiency decreased BDNF-III mRNA across development with a significant reduction at P7 but not at P15 or P30 (Fig. 2A). BDNF-IV mRNA was decreased at both P7 and P15 but not at P30 (Fig. 2B). In the hippocampal pyramidal cell layer, BDNF was localized in GFAP+ astrocytes, NeuN+ cells, and NeuN− cells with the highest expression in NeuN− cells (Supplemental Fig. 1).
TABLE 1.
Comparison of neurotrophic factor and receptor expression in the developing IS and ID rat hippocampus1
| Fold of P7 IS |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| P7 |
P15 |
P30 |
2-Way ANOVA P-values |
||||||
| Transcript | IS | ID | IS | ID | IS | ID | Iron status | Postnatal age | Interaction |
| CNTF | 1.0 ± 0.0a | 1.3 ± 0.1 | 1.9 ± 0.1b | 2.1 ± 0.2 | 2.2 ± 0.2b | 2.2 ± 0.2 | 0.11 | <0.01 | 0.65 |
| GDNF | 1.0 ± 0.0a | 1.8 ± 0.1* | 1.7 ± 0.1b | 2.4 ± 0.2* | 1.5 ± 0.2b | 1.6 ± 0.2 | <0.01 | <0.01 | 0.01 |
| EGF | 1.0 ± 0.1a | 1.2 ± 0.1 | 1.4 ± 0.2b | 2.2 ± 0.2* | 2.8 ± 0.33c | 2.9 ± 0.4 | <0.01 | <0.01 | <0.01 |
| NGF | 1.0 ± 0.0a | 1.0 ± 0.1 | 3.8 ± 0.4b | 5.2 ± 0.5* | 5.8 ± 0.3c | 6.5 ± 0.3 | <0.01 | <0.01 | 0.07 |
| TrkB | 1.0 ± 0.1a | 1.0 ± 0.0 | 2.3 ± 0.3b | 2.2 ± 0.3 | 3.0 ± 0.2c | 2.8 ± 0.3 | 0.38 | <0.01 | 0.91 |
| TrkBL | 1.0 ± 0.0a | 1.2 ± 0.1 | 4.0 ± 0.6c | 3.7 ± 0.2 | 2.4 ± 0.2b | 2.9 ± 0.2 | 0.22 | <0.01 | 0.14 |
| p75 | 1.0 ± 0.0b | 1.6 ± 0.2* | 1.1 ± 0.1b | 1.9 ± 0.1* | 0.8 ± 0.0a | 0.9 ± 0.1 | <0.01 | <0.01 | <0.01 |
| Egr-1 | 1.0 ± 0.1a | 1.0 ± 0.1 | 9.2 ± 0.4c | 4.9 ± 0.5* | 5.8 ± 0.4b | 7.1 ± 1.0 | 0.05 | <0.01 | <0.01 |
| Egr-2 | 1.0 ± 0.0a | 1.4 ± 0.1 | 7.0 ± 1.3c | 3.8 ± 0.5* | 2.6 ± 0.2b | 4.1 ± 0.3 | 0.30 | <0.01 | <0.01 |
Values are means ± SEM, n = 4–6. Means in a row with superscripts without a common letter differ, P < 0.05. *Different from IS at that time, P < 0.05.
FIGURE 2 .
BDNF-III (A) and BDNF-IV (B) hippocampal mRNA levels in male IS and ID rats at P7, 15, and 30. Values are means ± SEM, n = 4–6/group. *Different from IS at that age, *P < 0.05. IS bars without a common letter differ, P < 0.05.
Absence of TrkB upregulation in ID hippocampus.
Lower BDNF expression in the ID hippocampus could induce a compensatory increase in TrkB expression. Instead, TrkB mRNA transcripts did not significantly differ between the ID and IS groups at P7, 15, and 30 (Table 1). The ratio of total TrkB:BDNF was used to assess the relationship between supply and demand and was similar for both IS and ID rats at the 3 ages (P = 0.34). However, the specific TrkBL:BDNF ratio was higher in the ID group than in the IS group (P < 0.01). Consistent with the mRNA transcript level, Western blot analysis showed similar TrkB protein levels in the 2 groups at P15 (Supplemental Fig. 2A).
Acute upregulation of p75NTR receptor in ID hippocampus.
ID rats had 90% greater p75NTR mRNA expression at P15 compared with IS rats (Table 1) with a corresponding increase in protein level (Supplemental Fig. 2B). Levels of p75NTR mRNA normalized at P30 in the ID group (Table 1). The p75NTR protein was localized primarily in the neurites of stratum oriens and stratum lucidum (Supplemental Fig. 3). In the pyramidal cell layer, p75NTR was localized to the cellular membrane and was enriched in cells with lower NeuN expression (Supplemental Fig. 3). Moreover, p75NTR and BDNF were not extensively colocalized in the developing hippocampus of either the IS or ID group (Supplemental Fig. 3).
Reduced early growth response gene-1 and -2 expression and decreased neuronal death in ID hippocampus.
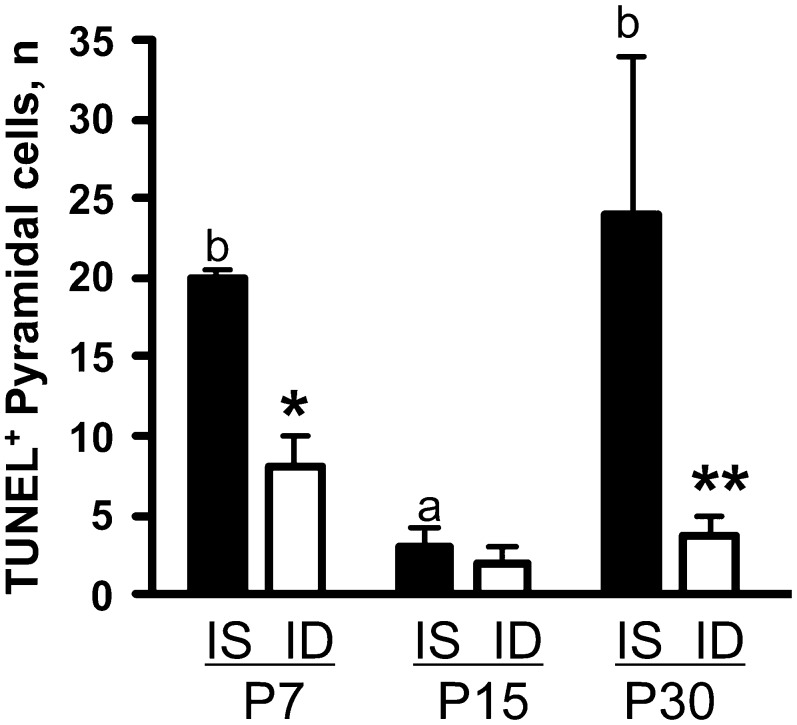
To assess whether lower BDNF expression in the developing ID hippocampus would lead to decreased BDNF activity, mRNA transcript levels of early growth response gene-1 (Egr-1) and Egr-2, 2 known transcriptional targets of BDNF signaling (40,41), were examined in the IS and ID hippocampus. Egr-1 and -2 were downregulated by 50% at P15 and recovered by P30 (Table 1). Egr-1 has been implicated in promoting cellular apoptosis (42,43). Apoptotic cell death was diminished in the dorsal ID hippocampus compared with the IS hippocampus (Fig. 3).
FIGURE 3 .
Number of TUNEL positive pyramidal cells in dorsal hippocampus of IS and ID rats. Values are means ± SEM, n = 4/group. *Different from IS at that age, *P < 0.05. IS bars without a common letter differ, P < 0.05.
Decreased Dcx expression associated with delayed NeuN-nuclear accumulation in ID hippocampus.
We assessed the expression of Dcx, a microtubule-associated protein expressed in differentiating neurons (44), to further define how iron deficiency affects neural differentiation. At P30, the IS and ID groups did not differ, but at P15, Dcx protein in the ID group was 0.6 that of the IS group (P < 0.05). Dcx was histologically localized to CA3 pyramidal cells and to dentate granular and hilus cells of P15 and P30 hippocampi (Supplemental Fig. 4). NeuN accumulation in pyramidal cell nuclei occurred by P30 in IS rats but was absent in ID rats (Supplemental Fig. 4).
Discussion
We and others have established neurological and behavioral deficits associated with fetal-neonatal iron deficiency anemia, many of which last beyond the period of iron deficiency (1,8). Fetal-neonatal iron deficiency affects structure and function of the hippocampus among other brain regions responsible for learning and memory formation (15), yet little is known about the cellular mechanism that may be responsible for this altered development. This study demonstrates that early-life iron deficiency decreases BDNF levels and increases GDNF, EGF, and NGF levels in the developing rat hippocampus. Compensatory TrkB expression does not occur despite reduced BDNF expression and is accompanied by downregulation of BDNF target genes and altered neuronal differentiation. The upregulation of GDNF, EGF, and NGF as well as p75NTR suggests utilization of alternate signaling pathways to compensate for the abnormalities in BDNF-driven pathways. These findings suggest that iron homeostasis is critical for proper neurotrophic factor expression during early life and provide a possible molecular basis for the neuro-morphologic and behavioral deficits in perinatal iron deficiency (8).
Our previous study showed that iron deficiency downregulates mRNA and protein levels of factors important for synaptic structure and plasticity, including calmodulin-regulated kinase-IIα and postsynaptic density 95 (19). The translation of these factors at the synapse is in turn regulated by mTOR, a basic intracellular signaling pathway that integrates stimuli, including growth factors, nutrient and energy availability, and oxidative stress, to regulate protein translation and cellular growth (22,45,46). Iron deficiency significantly alters components of the mTOR signaling pathway (19). In turn, BDNF modulates the activity of critical components of the mTOR signaling pathway, including Akt (22,45). We speculate that decreased BDNF, coupled with altered components of the mTOR signaling cascade, serve as the basis for the reduced expression of calmodulin-regulated kinase-IIα and postsynaptic density 95, ultimately leading to the documented impairment of synaptic plasticity in the ID rat (47,48).
The reduced BDNF levels in the current study during periods of neural proliferation and early differentiation, P7 and P15, respectively (37,38), may underlie compromised cell number and dendritic complexity in the ID hippocampus. Either or both might account for the smaller hippocampal size (R. Rao and M. K. Georgieff, unpublished observation) and abnormal Cornu Ammonis area 1 dendritic structure in ID rats (13). The effect of iron deficiency on proliferation and cell number remains unresolved. Determining the consequences of perinatal iron deficiency on cell proliferation will be valuable in understanding how BDNF participates in modulating early hippocampal development. Downregulation of BDNF target genes (Egr-1 and Egr-2), which also promote neuronal differentiation (49), together with decreased Dcx expression and delayed nuclear accumulation of NeuN provide evidence for impaired neuronal differentiation in the ID hippocampus during a period of normally rapid dendtritogenesis and synaptogenesis. These data suggest that iron deficiency places a brake on the developing hippocampus. It would be of interest to determine whether promotion of BDNF expression by alternative means such as exposure to an enriched environment or antidepressant drugs (50,51) would reverse or prevent the neural differentiation defects of perinatal iron deficiency.
In contrast to a model of chronic placental insufficiency, which had a decreased BDNF transcript level accompanied by increased TrkB expression (52,53), we found no compensatory expression of total TrkB mRNA levels in ID rats. However, the finding of a greater TrkBL:BDNF ratio suggests an increased availability of this TrkB isoform for BDNF binding in ID hippocampus. We predict that intracellular signaling effectors of BDNF (i.e. P-Mek, P-Erk) would be lowered in the ID group, because the TrkBL isoform contains the intracellular signaling domain. These findings may be important in terms of understanding the early antecedents of adult neurological disorders characterized by reduced hippocampal function or early hippocampal degeneration, including Alzheimer and Parkinson's diseases. Both are characterized by reduced BDNF levels without compensatory increased TrkB expression (25,54). The altered expression of genes involved in the pathogenesis of Alzheimer's disease as well as elevated susceptibility of brain injury in this same model that we have demonstrated in our previous studies supports this possibility (18,19).
Increased EGF, GDNF, NGF, and p75NTR expression in the ID hippocampus suggest alternate signaling pathways in lieu of lower BDNF expression. GDNF has been shown to synergize with BDNF in promoting neuronal survival (55). Increased GDNF transcript levels in P7 and P15 ID hippocampus could minimize the deleterious effects of reduced BDNF by facilitating the survival of hippocampal neurons. Given that GDNF and BDNF are synthesized by astrocytes, but have opposing expression levels in the ID hippocampus, it is likely that BDNF was also up-regulated in astrocytes but was insufficient to compensate for its overall decrease in the ID hippocampus. Additional study is needed to elucidate these differential outcomes and whether the upregulation of GDNF is sufficient to mitigate the effects of lowered BDNF, awaiting genetic models where iron deficiency could be targeted in neurons or astrocytes. Likewise, the upregulation of EGF in P15 ID hippocampus could promote survival at the expense of differentiation as suggested by the reduced Dcx expression in P15 ID hippocampus. Increased EGF levels may also affect astrocytic regulation of glutamine synthase activity, which would be consistent with our prior finding of increased glutamine levels in the ID hippocampus (12,56).
It is less clear what effects the upregulation of NGF and p75NTR may have in the ID hippocampus. ProNGF/p75NTR signaling induces neuronal apoptosis, in contrast to the survival effect of the mature-NGF/TrkA signaling system (57,58). Protein levels of proNGF and TrkA were not determined in this study; however, the elevated NGF and p75NTR mRNA transcripts and corresponding p75NTR protein levels in the ID hippocampus would have predicted an increase in neuronal cell death. Instead, the reduced apoptosis in the ID hippocampus suggests that these increases in NGF and P75NTR likely mediate survival, as proposed in other studies (59,60). p75NTR promotes high-affinity ligand/receptor binding (e.g. BDNF/TrkB) as well as ligand/receptor retrograde transport (31). The upregulation of p75NTR in the ID hippocampus might act to maximize the activity of available BDNF/TrkB, which has been shown to promote spine formation and LTP (33,61,62). Conversely, increased p75NTR expression in the ID hippocampus might also increase BDNF/p75NTR signaling, which reduces dendritic branching and synaptic spine formation (32,34). Our findings revealed little if any p75NTR and BDNF colocalization in the developing hippocampus, making it unlikely that BDNF/p75NTR signaling is a major contributing effector in the ID hippocampus.
In summary, our findings suggest that perinatal iron deficiency lowers BDNF activity associated with delayed neural differentiation in the developing hippocampus. These defects might serve as the basis for the morphologic and functional deficits during the period of iron deficiency. It remains unknown if these alterations are consequences of the lack of neuronal iron per se or of other confounding factors in this anemia model, including hypoxia at systemic and/or cellular levels. Which factors drive neuronal differentiation in the ID hippocampus following iron treatment and, more broadly, how iron deficiency affects the development of glial cells remains to be determined. The current study also suggests the possibility that interventions that enhance BDNF activity may work as a therapeutic approach to mitigate against acute and long-term effects of perinatal iron deficiency.
Supplementary Material
Acknowledgments
We thank Dr. Raghavendra Rao for reviewing the manuscript and Heather McLaughlin for providing editorial assistance.
Supported by National Institute of Child Health and Development RO1 HD29421 to M.K.G., National Institute of Mental Health training grant T32MH073129 to P.V.T., and National Institute of Neurological Disorders and Stroke NRSA F31NS04876 to E.S.C.
Supplemental Figures 1–4 and Supplemental Table 1 are available with the online posting of this paper at jn.nutrition.org.
Author disclosures: P. Tran, E. Carlson, S. Fretham, and M. Georgieff, no conflicts of interest.
Abbreviations used: BDNF, brain-derived neurotrophic factor; CNTF, ciliary neurotrophic factor; Dcx, doublecortin; Egr, early-growth response gene; EGF, epidermal growth factor; GDNF, glial-derived neurotrophic factor; ID, iron deficient; IS, iron sufficient; LTP, long-term potentiation; mTOR, mammalian target of rapamycin; NGF, nerve growth factor; p75NTR, neurotrophic receptor p75; P, postnatal day; TdT, terminal deoxynucleotide transferase; TrkB, tyrosine-receptor kinase B; TUNEL, terminal deoxynucleotide transferase-mediated biotin-dUTP nick end labeling.
References
- 1.Rao R, Georgieff MK. Iron in fetal and neonatal nutrition. Semin Fetal Neonatal Med. 2007;12:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Georgieff MK, Wewerka SW, Nelson CA, Deregnier RA. Iron status at 9 months of infants with low iron stores at birth. J Pediatr. 2002;141:405–9. [DOI] [PubMed] [Google Scholar]
- 3.Petry CD, Eaton MA, Wobken JD, Mills MM, Johnson DE, Georgieff MK. Iron deficiency of liver, heart, and brain in newborn infants of diabetic mothers. J Pediatr. 1992;121:109–14. [DOI] [PubMed] [Google Scholar]
- 4.Chockalingam UM, Murphy E, Ophoven JC, Weisdorf SA, Georgieff MK. Cord transferrin and ferritin values in newborn infants at risk for prenatal uteroplacental insufficiency and chronic hypoxia. J Pediatr. 1987;111:283–6. [DOI] [PubMed] [Google Scholar]
- 5.Siddappa AM, Georgieff MK, Wewerka S, Worwa C, Nelson CA, Deregnier RA. Iron deficiency alters auditory recognition memory in newborn infants of diabetic mothers. Pediatr Res. 2004;55:1034–41. [DOI] [PubMed] [Google Scholar]
- 6.Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13:158–65. [DOI] [PubMed] [Google Scholar]
- 7.Tamura T, Goldenberg RL, Hou J, Johnston KE, Cliver SP, Ramey SL, Nelson KG. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. J Pediatr. 2002;140:165–70. [DOI] [PubMed] [Google Scholar]
- 8.Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64:S34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felt BT, Beard JL, Schallert T, Shao J, Aldridge JW, Connor JR, Georgieff MK, Lozoff B. Persistent neurochemical and behavioral abnormalities in adulthood despite early iron supplementation for perinatal iron deficiency anemia in rats. Behav Brain Res. 2006;171:261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connor JR, Menzies SL. Relationship of iron to oligodendrocytes and myelination. Glia. 1996;17:83–93. [DOI] [PubMed] [Google Scholar]
- 11.Beard J, Erikson KM, Jones BC. Neonatal iron deficiency results in irreversible changes in dopamine function in rats. J Nutr. 2003;133:1174–9. [DOI] [PubMed] [Google Scholar]
- 12.Rao R, Tkac I, Townsend EL, Gruetter R, Georgieff MK. Perinatal iron deficiency alters the neurochemical profile of the developing rat hippocampus. J Nutr. 2003;133:3215–21. [DOI] [PubMed] [Google Scholar]
- 13.Jorgenson LA, Wobken JD, Georgieff MK. Perinatal iron deficiency alters apical dendritic growth in hippocampal CA1 pyramidal neurons. Dev Neurosci. 2003;25:412–20. [DOI] [PubMed] [Google Scholar]
- 14.deUngria M, Rao R, Wobken JD, Luciana M, Nelson CA, Georgieff MK. Perinatal iron deficiency decreases cytochrome c oxidase (CytOx) activity in selected regions of neonatal rat brain. Pediatr Res. 2000;48:169–76. [DOI] [PubMed] [Google Scholar]
- 15.Georgieff MK. Nutrition and the developing brain: nutrient priorities and measurement. Am J Clin Nutr. 2007;85:S614–20. [DOI] [PubMed] [Google Scholar]
- 16.Siddappa AJ, Rao RB, Wobken JD, Leibold EA, Connor JR, Georgieff MK. Developmental changes in the expression of iron regulatory proteins and iron transport proteins in the perinatal rat brain. J Neurosci Res. 2002;68:761–75. [DOI] [PubMed] [Google Scholar]
- 17.Jorgenson LA, Sun M, O'Connor M, Georgieff MK. Fetal iron deficiency disrupts the maturation of synaptic function and efficacy in area CA1 of the developing rat hippocampus. Hippocampus. 2005;15:1094–102. [DOI] [PubMed] [Google Scholar]
- 18.Rao R, Tkac I, Townsend EL, Ennis K, Gruetter R, Georgieff MK. Perinatal iron deficiency predisposes the developing rat hippocampus to greater injury from mild to moderate hypoxia-ischemia. J Cereb Blood Flow Metab. 2007;27:729–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carlson ES, Stead JD, Neal CR, Petryk A, Georgieff MK. Perinatal iron deficiency results in altered developmental expression of genes mediating energy metabolism and neuronal morphogenesis in hippocampus. Hippocampus. 2007;17:679–91. [DOI] [PubMed] [Google Scholar]
- 20.Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci USA. 2002;99:467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cammalleri M, Lutjens R, Berton F, King AR, Simpson C, Francesconi W, Sanna PP. Time-restricted role for dendritic activation of the mTOR-p70S6K pathway in the induction of late-phase long-term potentiation in the CA1. Proc Natl Acad Sci USA. 2003;100:14368–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schratt GM, Nigh EA, Chen WG, Hu L, Greenberg ME. BDNF regulates the translation of a select group of mRNAs by a mammalian target of rapamycin-phosphatidylinositol 3-kinase-dependent pathway during neuronal development. J Neurosci. 2004;24:7366–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000;3:533–5. [DOI] [PubMed] [Google Scholar]
- 24.Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH, Kuhn HG. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003;17:2042–6. [DOI] [PubMed] [Google Scholar]
- 25.Tapia-Arancibia L, Rage F, Givalois L, Arancibia S. Physiology of BDNF: focus on hypothalamic function. Front Neuroendocrinol. 2004;25:77–107. [DOI] [PubMed] [Google Scholar]
- 26.Patterson SL, Grover LM, Schwartzkroin PA, Bothwell M. Neurotrophin expression in rat hippocampal slices: a stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT-3 mRNAs. Neuron. 1992;9:1081–8. [DOI] [PubMed] [Google Scholar]
- 27.Castren E, Pitkanen M, Sirvio J, Parsadanian A, Lindholm D, Thoenen H, Riekkinen PJ. The induction of LTP increases BDNF and NGF mRNA but decreases NT-3 mRNA in the dentate gyrus. Neuroreport. 1993;4:895–8. [DOI] [PubMed] [Google Scholar]
- 28.Kovalchuk Y, Hanse E, Kafitz KW, Konnerth A. Postsynaptic induction of BDNF-mediated long-term potentiation. Science. 2002;295:1729–34. [DOI] [PubMed] [Google Scholar]
- 29.Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci USA. 1995;92:8856–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12:656–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. [DOI] [PubMed] [Google Scholar]
- 32.Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, Hempstead BL, Lu B. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci. 2005;8:1069–77. [DOI] [PubMed] [Google Scholar]
- 33.Xu B, Gottschalk W, Chow A, Wilson RI, Schnell E, Zang K, Wang D, Nicoll RA, Lu B, et al. The role of brain-derived neurotrophic factor receptors in the mature hippocampus: modulation of long-term potentiation through a presynaptic mechanism involving TrkB. J Neurosci. 2000;20:6888–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zagrebelsky M, Holz A, Dechant G, Barde YA, Bonhoeffer T, Korte M. The p75 neurotrophin receptor negatively modulates dendrite complexity and spine density in hippocampal neurons. J Neurosci. 2005;25:9989–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nibuya M, Takahashi M, Russell DS, Duman RS. Repeated stress increases catalytic TrkB mRNA in rat hippocampus. Neurosci Lett. 1999;267:81–4. [DOI] [PubMed] [Google Scholar]
- 36.Croll SD, Ip NY, Lindsay RM, Wiegand SJ. Expression of BDNF and trkB as a function of age and cognitive performance. Brain Res. 1998;812:200–8. [DOI] [PubMed] [Google Scholar]
- 37.Bayer SA. Development of the hippocampal region in the rat. I. Neurogenesis examined with 3H-thymidine autoradiography. J Comp Neurol. 1980;190:87–114. [DOI] [PubMed] [Google Scholar]
- 38.Stead JD, Neal C, Meng F, Wang Y, Evans S, Vazquez DM, Watson SJ, Akil H. Transcriptional profiling of the developing rat brain reveals that the most dramatic regional differentiation in gene expression occurs postpartum. J Neurosci. 2006;26:345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calella AM, Nerlov C, Lopez RG, Sciarretta C, von Bohlen Und Halbach O, Bereshchenko O, Minichiello L. Neurotrophin/Trk receptor signaling mediates C/EBPalpha, -beta and NeuroD recruitment to immediate-early gene promoters in neuronal cells and requires C/EBPs to induce immediate-early gene transcription. Neural Develop. 2007;2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rossler OG, Thiel G. Brain-derived neurotrophic factor-, epidermal growth factor-, or A-Raf-induced growth of HaCaT keratinocytes requires extracellular signal-regulated kinase. Am J Physiol Cell Physiol. 2004;286:C1118–29. [DOI] [PubMed] [Google Scholar]
- 42.Cho SJ, Kang MJ, Homer RJ, Kang HR, Zhang X, Lee PJ, Elias JA, Lee CG. Role of early growth response-1 (Egr-1) in interleukin-13-induced inflammation and remodeling. J Biol Chem. 2006;281:8161–8. [DOI] [PubMed] [Google Scholar]
- 43.Choi SY, Hwang JJ, Koh JY. NR2A induction and NMDA receptor-dependent neuronal death by neurotrophin-4/5 in cortical cell culture. J Neurochem. 2004;88:708–16. [DOI] [PubMed] [Google Scholar]
- 44.Francis F, Koulakoff A, Boucher D, Chafey P, Schaar B, Vinet MC, Friocourt G, McDonnell N, Reiner O, et al. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron. 1999;23:247–56. [DOI] [PubMed] [Google Scholar]
- 45.Takei N, Inamura N, Kawamura M, Namba H, Hara K, Yonezawa K, Nawa H. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J Neurosci. 2004;24:9760–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology (Bethesda). 2006;21:362–9. [DOI] [PubMed] [Google Scholar]
- 48.Vaynman S, Ying Z, Wu A, Gomez-Pinilla F. Coupling energy metabolism with a mechanism to support brain-derived neurotrophic factor-mediated synaptic plasticity. Neuroscience. 2006;139:1221–34. [DOI] [PubMed] [Google Scholar]
- 49.Harada T, Morooka T, Ogawa S, Nishida E. ERK induces p35, a neuron-specific activator of Cdk5, through induction of Egr1. Nat Cell Biol. 2001;3:453–9. [DOI] [PubMed] [Google Scholar]
- 50.Falkenberg T, Mohammed AK, Henriksson B, Persson H, Winblad B, Lindefors N. Increased expression of brain-derived neurotrophic factor mRNA in rat hippocampus is associated with improved spatial memory and enriched environment. Neurosci Lett. 1992;138:153–6. [DOI] [PubMed] [Google Scholar]
- 51.Russo-Neustadt AA, Beard RC, Huang YM, Cotman CW. Physical activity and antidepressant treatment potentiate the expression of specific brain-derived neurotrophic factor transcripts in the rat hippocampus. Neuroscience. 2000;101:305–12. [DOI] [PubMed] [Google Scholar]
- 52.Dieni S, Rees S. BDNF and TrkB protein expression is altered in the fetal hippocampus but not cerebellum after chronic prenatal compromise. Exp Neurol. 2005;192:265–73. [DOI] [PubMed] [Google Scholar]
- 53.Gomez-Pinilla F, Vaynman SA. A “deficient environment” in prenatal life may compromise systems important for cognitive function by affecting BDNF in the hippocampus. Exp Neurol. 2005;192:235–43. [DOI] [PubMed] [Google Scholar]
- 54.Siegel GJ, Chauhan NB. Neurotrophic factors in Alzheimer's and Parkinson's disease brain. Brain Res Brain Res Rev. 2000;33:199–227. [DOI] [PubMed] [Google Scholar]
- 55.Erickson JT, Brosenitsch TA, Katz DM. Brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor are required simultaneously for survival of dopaminergic primary sensory neurons in vivo. J Neurosci. 2001;21:581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamada M, Ikeuchi T, Hatanaka H. The neurotrophic action and signalling of epidermal growth factor. Prog Neurobiol. 1997;51:19–37. [DOI] [PubMed] [Google Scholar]
- 57.Friedman WJ. Neurotrophins induce death of hippocampal neurons via the p75 receptor. J Neurosci. 2000;20:6340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–8. [DOI] [PubMed] [Google Scholar]
- 59.Bui NT, Konig HG, Culmsee C, Bauerbach E, Poppe M, Krieglstein J, Prehn JH. p75 Neurotrophin receptor is required for constitutive and NGF-induced survival signalling in PC12 cells and rat hippocampal neurones. J Neurochem. 2002;81:594–605. [DOI] [PubMed] [Google Scholar]
- 60.Culmsee C, Gerling N, Lehmann M, Nikolova-Karakashian M, Prehn JH, Mattson MP, Krieglstein J. Nerve growth factor survival signaling in cultured hippocampal neurons is mediated through TrkA and requires the common neurotrophin receptor P75. Neuroscience. 2002;115:1089–108. [DOI] [PubMed] [Google Scholar]
- 61.Tyler WJ, Pozzo-Miller LD. BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J Neurosci. 2001;21:4249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Minichiello L, Calella AM, Medina DL, Bonhoeffer T, Klein R, Korte M. Mechanism of TrkB-mediated hippocampal long-term potentiation. Neuron. 2002;36:121–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.