Abstract
It was reported recently that resveratrol could sensitize a number of cancer cell lines to the anticancer actions of several other cancer drugs, including paclitaxel. In the present study, we further investigated whether resveratrol could sensitize human breast cancer cells to paclitaxel-induced cell death. Unexpectedly, we found that resveratrol strongly diminished the susceptibility of MDA-MB-435s, MDA-MB-231 and SKBR-3 cells to paclitaxel-induced cell death in culture, although this effect was not observed in MCF-7 cells. A similar observation was made in athymic nude mice using MDA-MB-435s cells as a representative model. Mechanistically, the modulating effect of resveratrol was partially attributable to its inhibition of paclitaxel-induced G2/M cell cycle arrest, together with an accumulation of cells in the S-phase. In addition, resveratrol could suppress paclitaxel-induced accumulation of reactive oxygen species and subsequently the inactivation of anti-apoptotic Bcl-2 family proteins. These observations suggest that the strategy of concomitant use of resveratrol with paclitaxel is detrimental in certain types of human cancers. Given the widespread use of resveratrol among cancer patients, this study calls for more preclinical and clinical testing of the potential benefits and harms of using resveratrol as a dietary adjuvant in cancer patients.
Keywords: Resveratrol, Paclitaxel, Cell cycle arrest, Reactive oxygen species, Bcl-xL
1. INTRODUCTION
Resveratrol (trans-3,4’,5-trihydroxystilbene), a naturally-occurring polyphenolic compound, is highly enriched in a variety of food sources, such as grapes, peanuts, and red wine.1-3 A number of previous studies have investigated many of its unique beneficial effects, such as lifespan prolongation, cardiovascular protection, and anti-inflammation. In addition, studies have shown that resveratrol has a strong chemopreventive effect against the development of cancers of the skin, breast, prostate, and lung.4-6 The evidence for the cancer chemopreventive effect of resveratrol appeared rather convincing, because it was shown to prevent tumorigenesis in a number of animal models.6 In addition to these studies, it has also been reported that resveratrol can inhibit the growth of human cancer cells in vitro when it was present alone at rather high concentrations (usually >50 μM) or when it was used in combination with other anticancer drugs.7-19
Paclitaxel, one of most commonly-used chemotherapeutic agents, has clinical efficacy in a number of human cancers, such as cancer of the lung, ovary, and breast. Mechanistically, it is generally believed that paclitaxel disrupts the formation of normal spindles at the metaphase of cell division, resulting in G2/M or G1 cell cycle arrest and subsequently apoptotic cell death.20 Recently, it was reported that resveratrol could sensitize a number of cancer cell lines to the anticancer actions of several other cancer drugs, including paclitaxel.10,11,21,22 It was suggested that since resveratrol and paclitaxel can modify different regulatory proteins involved in apoptosis and cell cycle regulation, their combined use may yield synergistic anticancer activity.
In the present study, we investigated whether resveratrol could sensitize different human breast cancer cell lines (MDA-MB-435s, MDA-MB-231, SKBR-3, and MCF-7) to paclitaxel-induced cell death. Unexpectedly, we found that resveratrol strongly diminished the susceptibility of MDA-MB-435s, MDA-MB-231 and SKBR-3 cells to paclitaxel-induced cell death, although it did not have a similar effect in MCF-7 cells. This observation suggests that the combined use of resveratrol and paclitaxel may not be suitable for certain types of human cancers. In addition, we have also sought to determine the molecular mechanism(s) underlying resveratrol's effect by investigating the modulation of paclitaxel-induced cell cycle changes and reactive oxygen species (ROS) accumulation.
2. MATERIALS AND METHOD
2.1. Chemicals
Paclitaxel, resveratrol, 5-fluorouracil, etoposide, doxorubicin, the trypsin-EDTA mixture (containing 0.25% trypsin w/v and 0.02% EDTA w/v), and fetal bovine serum (FBS) were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO). Iscove's modified minimum essential medium was obtained from Life Technology (Rockville, MD). The antibiotics solution (containing 10,000 U/mL penicillin and 10 mg/mL streptomycin) was obtained from Invitrogen (Carlsbad, CA).
2.2. Cell culture conditions and assay of cell viability
MDA-MB-435s, MCF-7, HepG2, DU-145, MIA-PaCa-2, MDA-MB-231 and SKBR-3 cells were purchased from the American Type Culture Collection (ATCC; Manassas, VA). MDA-MB-435s cells were maintained in Iscove's modified minimum essential medium supplemented with 10% FBS v/v and 3.024 g/L NaHCO3, and incubated at 37°C under 5% CO2. Cells were subcultured every 3 to 4 days. The MCF-7, HepG2, DU-145, MIA-PaCa-2, MDA-MB-231, and SKBR-3 cells were maintained under vendor-recommended conditions.
The cells were seeded in 96-well plates at a density of 5,000 cells per well. The stock solution of anticancer drugs with or without resveratrol (dissolved in pure ethanol) was diluted in the culture medium immediately before addition to each well at the desired final concentration(s), and the treatment usually lasted for 2 to 3 days. For determining cell viability, the MTT assay was used. Ten μL of MTT (at 5 mg/mL) was added to each well at a final concentration of 500 μg/mL. After the mixture in each well was incubated for 1 h, it was removed and DMSO (100 μL) was added, and the absorbance was read with a UV max microplate reader (Molecular Device, Palo Alto, CA) at 560 nm. The relative cell viability was expressed as a percentage of the control well that was not treated with drugs.
2.3. Growth of human cancer cell xenografts in athymic nude mice
All procedures involving the use of live animals in this study were approved by the Institutional Animal Care and Use Committee of the University of Kansas Medical Center and strictly followed the NIH guidelines for humane treatment of animals. Six-week-old female athymic nu/nu mice (obtained from Harlan, Indianapolis, IN) were used in the present study. The animals were housed in sterilized cages with filtered air and under a 12-h light/12-dark dark cycle, and had free access to sterile water and animal feed. After approximately one week of acclimatization after arrival, the estrogen receptor-negative MDA-MB-435s cells (5 × 106 cells) were s.c. injected into the right and left flanks of each mouse. After the tumors were allowed to develop for 2 weeks, the animals were then randomly grouped (with 10 animals per group), and the animals received one of the following treatments: vehicle (2% ethanol v/v in PBS, i.p.), paclitaxel (10 mg/kg body weight per i.p. injection, once a week), resveratrol (16.5 mg/kg body weight per i.p. injection, three times a week), and resveratrol in combination with paclitaxel at the same doses. To estimate the tumor size, the maximum and minimam diameters of the tumors were measured twice a week using a slide caliper. Tumor volumes were calculated by assuming a spherical shape and using the following formula: volume = (mean of diameter)3 × π / 6. At the end of the experiment, the animals were euthanized with CO2 overdose followed by decapitation, and tumor tissues from each animal were removed, trimmed off excess collective tissues, and then weighed. The tumor tissue was then fixed in buffered formalin, embedded in paraffin, sectioned at 5-μm thickness, and mounted on glass slides. The tissue sections were processed for the following analyses: H/E staining, terminal deoxynucleotidyl transferase-mediated nick end labeling (TUNEL) analysis, and proliferating cell nuclear antigen (PCNA) immunohistochemical staining.
2.4. Cell cycle analysis
After treatment with paclitaxel with or without resveratrol, cells were harvested by trypsinization and washed once with phosphate-buffered saline (PBS, pH 7.4). After centrifugation, cells were stained with propidium iodide (PI; Sigma) for analysis of cell cycles as described below. The cells were resuspended in 1 mL of 0.9 % NaCl w/v, and 2.5 mL of ice-cold 90% ethanol v/v were added. After incubation at room temperature for 30 min, cells were centrifuged and the supernatant was removed. The cells were then resuspended in 1 mL of PBS containing 50 μg/mL PI and 100 μg/mL ribonuclease A (Sigma), and incubated at 37°C for 30 min. After centrifugation, cells were resuspended in PBS, and analyzed using a flow cytometer (model BD LSR II, BD Bioscience, San Jose, CA).
2.5. Western blotting
For Western blotting, cells were washed first, and then were suspended in 100 μL lysis buffer (containing 20 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100 v/v, 10 mM NaF, 2 mM Na3VO4, and a protease inhibitor cocktail, pH 7.5). The amount of proteins was determined using the Bio-Rad protein assay (Bio-Rad, Hercules, CA). After an equal amount of proteins was loaded in each lane, they were separated by 10% SDS w/v -polyacrylamide gel electrophoresis (SDS-PAGE) and then electrically transferred to a polyvinylidene difluoride membrane (Bio-Rad). After blocking the membrane with 5% skim milk w/v, target proteins were immunodetected using specific antibodies. All primary antibodies were obtained from Cell Signaling Technology (Beverly, MA) and used 1: 1000 dilutions. Thereafter, the horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (Invitrogen) was applied as the secondary antibody, and the positive bands were detected using Amersham ECL Plus Western blotting detection reagents (GE Health care, Piscataway, NJ).
2.6. Measurement of reactive oxygen species
Reactive oxygen species (ROS) were detected using the 2’,7’-dichlorofluorescin diacetate (H2-DCF-DA) method. Cells were first cultured in 96-well plate and treated with paclitaxel and/or resveratrol for 24 h, and then 10 μM H2-DCF-DA was added to each well. After incubation for 10 min at 37°C, the liquid was removed and PBS was added. Intracellular ROS accumulation was observed and photographed under a fluorescence microscope (AXIO, Carl Zeiss Corporation, Germany).
2.7. Reproducibility of experiments and statistical analysis
For the in vitro cell culture study, each experiment was repeated at least three times. The data were presented as mean ± S.D. of multiple independent experiments. For the in vivo animal study, we have obtained similar results from two independent experiments, and only one set of the representative data was shown. Statistical significance was analyzed using the one-way ANOVA and Dunnett's test (SPSS software). A P value of less than 0.05 was considered statistically significant.
3. RESULTS
3.1. Resveratrol strongly diminishes paclitaxel's anticancer actions
3.1.1. In vitro study
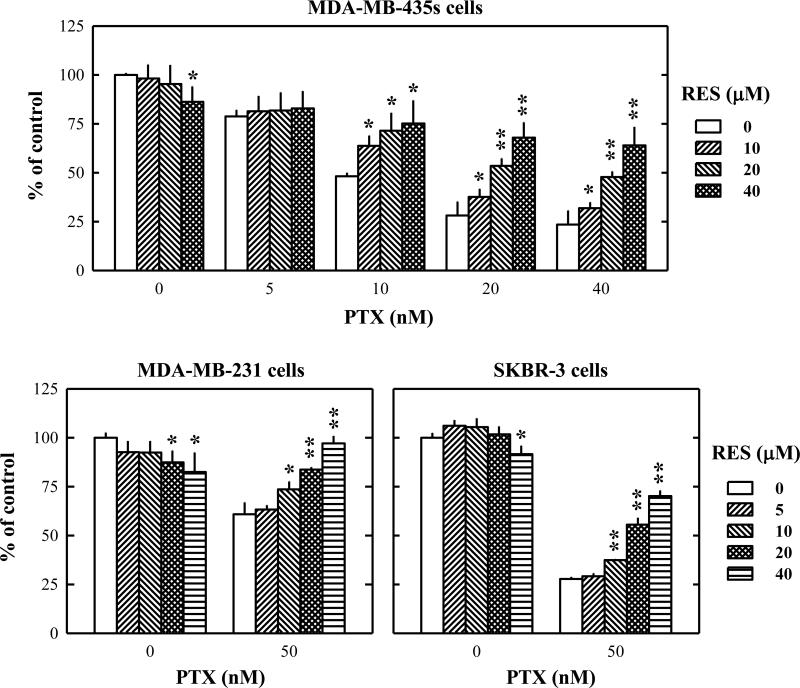
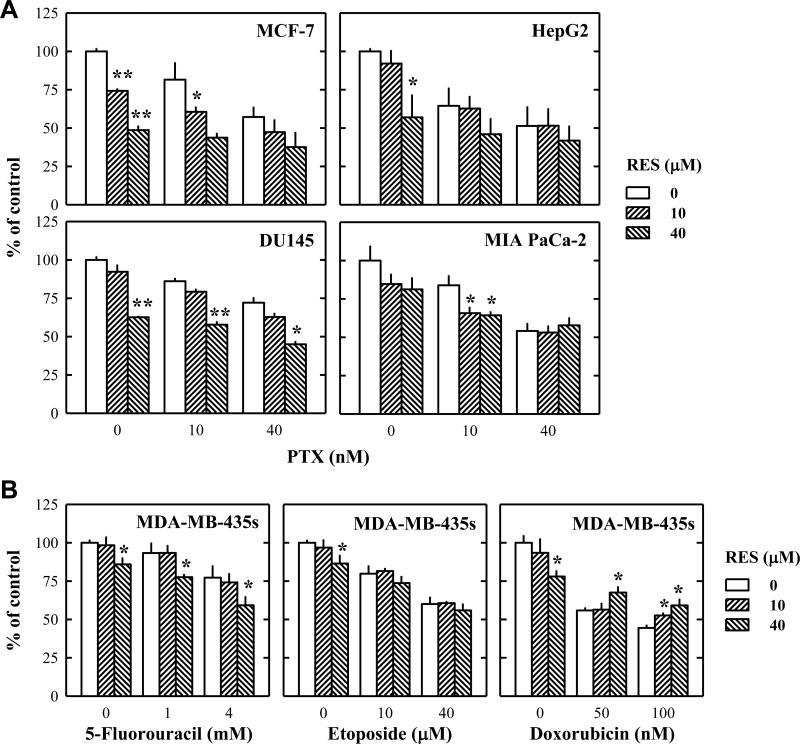
Initially, the experiments were planned to test whether resveratrol could sensitize MDA-MB-435s cells (a human breast cancer cell line) to the anticancer actions of paclitaxel in vitro. To our surprise, resveratrol did not enhance, but rather attenuated, the anticancer efficacy of paclitaxel in a concentration-dependent manner in cultured MDA-MB-435s cells (Fig. 1A). While only 20% of the cancer cells survived after treatment with 20 nM paclitaxel alone for 48 h, co-treatment with resveratrol markedly abrogated paclitaxel-induced reduction in cell viability. Following this observation, we then further determined whether the protective effect of resveratrol against paclitaxel-induced cell death was a general phenomenon for various types of cancer cells or a specific effect for certain types of cancer cells. As shown in Fig. 1B, resveratrol also exerted a protective effect against paclitaxel-induced cell death in other two human breast cancer cell lines, MDA-MB-231 and SKBR-3. However, when we treated MCF-7 human breast cancer cells, HepG2 human hepatocellular carcinoma cells, DU-145 human prostate carcinoma cells, and MIA-PaCa-2 human pancreas carcinoma cells with paclitaxel with or without resveratrol at varying concentrations for 48 h, resveratrol exerted no protective effect against paclitaxel-induced cell death (Fig. 2A). In addition, resveratrol did not exert a similar protective effect against the cell death induced by 5-fluorouracil or etoposide in MDA-MB-435s cells, but it slightly suppressed doxorubicin-induced cell death (Fig. 2B).
Figure 1. Resveratrol modulates the anticancer actions of paclitaxel in several human breast cancer cell lines in culture.
The human breast cancer cell lines (MDA-MB-435s, MDA-MB-231, and SKBR-3) were treated with resveratrol alone or in combination with paclitaxel at indicated concentrations for 48 h. After incubation, cell viability was determined by MTT assay as described in the Materials and Methods. Each data point is the mean ± S.D. of three independent experiments. * P < 0.05, ** P < 0.01 vs. anti-cancer drug alone treatment at each concentrations. PTX, paclitaxel; RES, resveratrol.
Figure 2. Resveratrol does not modulate the anti-cancer actions of paclitaxel in several human cancer cell lines in culture.
A. MCF-7, HepG2, Du145, and MIA PaCa-2 cells were treated with resveratrol alone or in combination with paclitaxel at indicated concentrations for 48 h. B. MDA-MB-435s cells were treated with resveratrol alone or in combination with 5-fluorouracil, etoposide, or doxorubicin at indicated concentrations for 48 h. After incubation, cell viability was determined by MTT assay as described in the Materials and Methods. Each data point is the mean ± S.D. of three independent experiments. * P < 0.05, ** P < 0.01 vs. anti-cancer drug alone treatment at each concentrations. PTX, paclitaxel; RES, resveratrol.
3.1.2 In vivo study
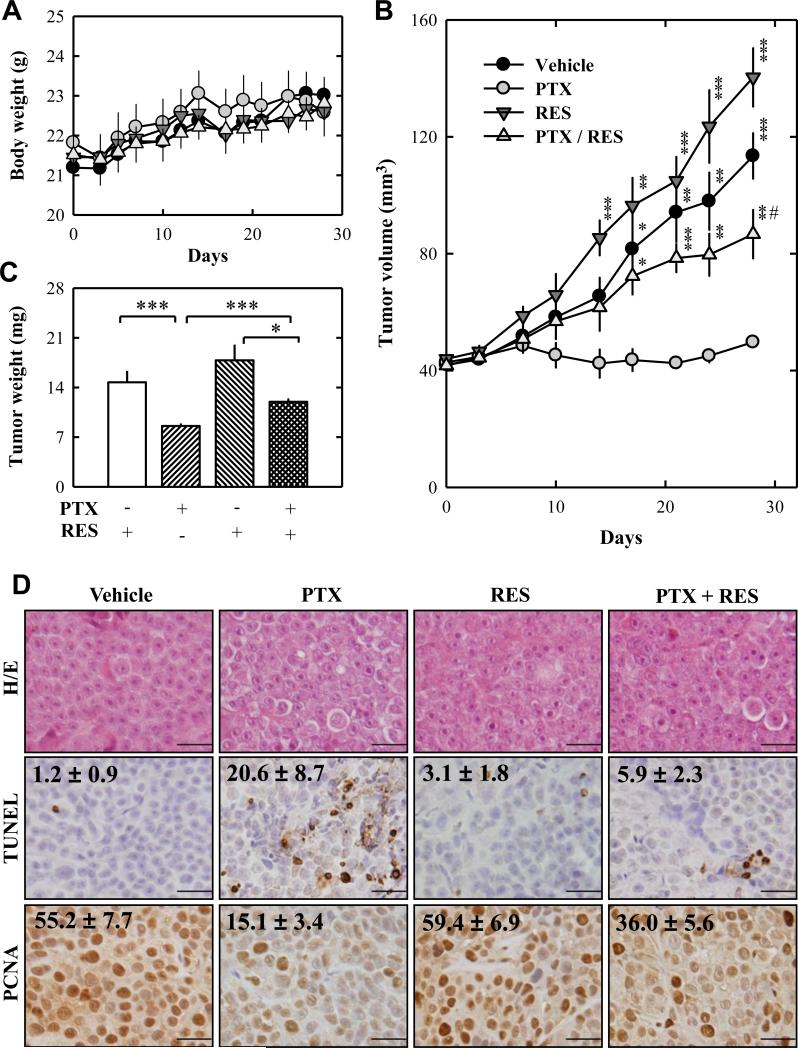
To evaluate the in vivo protective effect of resveratrol in combination with paclitaxel, we used the growth of MDA-MB-435s cell xenografts in female athymic nu/nu mice as an in vivo model. Ten animals per treatment group were used, and they were injected s.c. with MDA-MB-435s cells (at 5 × 106 cells/100 μL PBS) in the left and right flanks of the animals. Two weeks later, the animals were randomly grouped, and received one of following treatments: vehicle, paclitaxel (10 mg/kg, once per week, i.p.), resveratrol (16.5 mg/kg, three times per week, i.p.), and paclitaxel (once a week) in combination with resveratrol (three times per week) at the same doses. No significant difference was seen in the body weight changes among different treatment groups (Fig. 3A). While treatment with paclitaxel alone significantly suppressed tumor growth, combination of resveratrol and paclitaxel showed a markedly reduced growth inhibition of the tumor (Fig. 3B, 3C). Surprisingly, treatment with resveratrol alone slightly stimulated the growth of cancer xenograft compared to vehicle-treated animals (Fig. 3B, 3C).
Figure 3. Resveratrol strongly suppresses paclitaxel-induced death of MDA-MB-435s cancer cells grown in athymic nude mice as xenografts.
MDA-MB-435s cells (at 5 × 106 cells in 100 μL PBS) were injected s.c. into the right and left flanks of each athymic nude mouse. The animals then received vehicle (2% ethanol in PBS, i.p.), paclitaxel (10 mg/kg, once a week, i.p.), resveratrol (16.5 mg/kg, three times a week, i.p.), or combination of paclitaxel (10 mg/kg, once a week, i.p.) + resveratrol (16.5 mg/kg, three times a week, i.p.). The body weight change was measured three times a week (A), and tumor size of each mice was measured twice a week (B). At the end of the experiment, each tumor was removed, trimmed, and weighed (C). D. Tumor samples from each mouse were processed for regular H/E staining as well as for analysis of TUNEL- and PCNA-positive cells. Each value is the mean ± S.E. * P < 0.05, ** P < 0.01, and *** P < 0.001 vs. paclitaxel-treated group. # P < 0.01 vs. resveratrol-treated group. PTX, paclitaxel; RES, resveratrol.
Histological examination (H/E staining) of dissected tumor tissues revealed that the morphology and density of tumor cells were not significantly different (Fig. 3D; H/E staining). The data from the histochemical staining of TUNEL-positive cells (apoptotic cells) showed that the number of apoptotic cells was significantly increased by paclitaxel treatment, but it was significantly reduced by treatment with resveratrol + paclitaxel (Fig. 3D). Further immunohistochemical staining of PCNA-positive cells (proliferating cells) showed an inverse trend, i.e., while tumor cell growth was significantly suppressed in animals treated with paclitaxel alone, the suppression was markedly reduced in animals treated with resveratrol + paclitaxel (Fig. 3D). Collectively, these data unequivocally showed that resveratrol diminished the anticancer efficacy of paclitaxel in vivo.
3.2. Mechanism of the protective effect of resveratrol against paclitaxel-induced cell death
Because the protective effect of resveratrol on paclitaxel-induced cell death was rather strong, we also sought to determine the underlying mechanism(s) of its actions.
3.2.1 Modulation of paclitaxel-induced cell cycle change
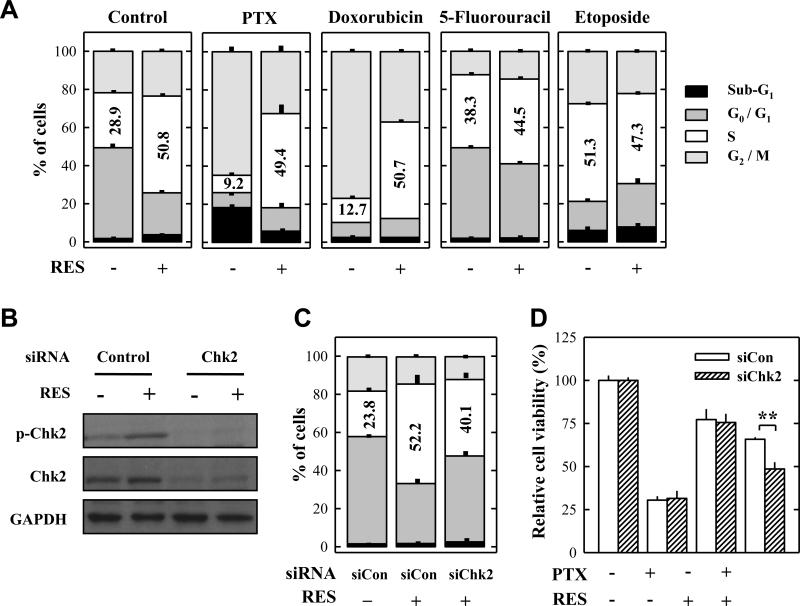
Paclitaxel, a microtuble-targeting agent, can induce G2/M cell cycle arrest and apoptosis.20 Therefore, we first determined the effect of paclitaxel, resveratrol, or their combination on cell cycle change. As shown in Fig. 4A, treatment with resveratrol alone induced S-phase arrest, whereas treatment with paclitaxel alone induced predominantly G2/M arrest. Combination of resveratrol and paclitaxel significantly decreased the population of cells in the G2/M phase compared with paclitaxel treatment alone, but increased the population in the S-phase. Similar changes were observed in doxorubicin-treated cells, but these changes were not observed when resveratrol was combined with 5-fluorouracil or etoposide.
Figure 4. Resveratrol affects the cell cycle change induced by paclitaxel and other anticancer drugs in cultured MDA-MB-435s cells.
A. Cell cycle analysis of MDA-MB-435s cells treated for 24 h with 10 nM paclitaxel, 50 nM doxorubicin, 50 μM 5-FU, or 40 μM etoposide in the absence or presence of 20 μM resveratrol. B. Western blot analysis of Chk2 phosphorylation in Chk2-knockdown MDA-MB-435s cells after treatment with 20 μM resveratrol for 24 h. Cell extracts were prepared and 10 μg of total proteins were subjected Western blot analysis. C. Cell cycle analysis of Chk2 siRNA-transfected cells after treatment with 20 μM resveratrol for 24 h. D. Cell viability (MTT assay) of Chk2 siRNA-transfected MDA-MB-435s cells after treatment with 10 nM paclitaxel with or without 20 μM resveratrol for 48 h. Each data resents the mean ± S.D. of three independent experiments. ** P < 0.01. PTX, paclitaxel; RES, resveratrol.
Recently, we reported that activation of the checkpoint kinase 2 (Chk2) contributes importantly to resveratrol-induced S-phase arrest in human hepatocellular carcinoma cells.12 Therefore, we investigated whether Chk2 also contributed to the protective effect of resveratrol against paclitaxel-induced cell death. To do so, Chk2 protein expression was knocked down using the Chk2-specific siRNA. As shown in Fig. 4B, resveratrol-induced increase in the levels of phosphorylated Chk2 was abolished in Chk2-knockdown cells. Also, the protective effect of resveratrol against paclitaxel was significantly weakened compared to the control (Fig. 4D), although the population of S-phase cells was only slightly decreased (Fig. 4C). These results indicate that resveratrol-induced Chk2 activation partially contributed to the attenuation of paclitaxel's efficacy, through the induction of S-phase arrest.
3.2.2. Modulation of paclitaxel-induced ROS accumulation
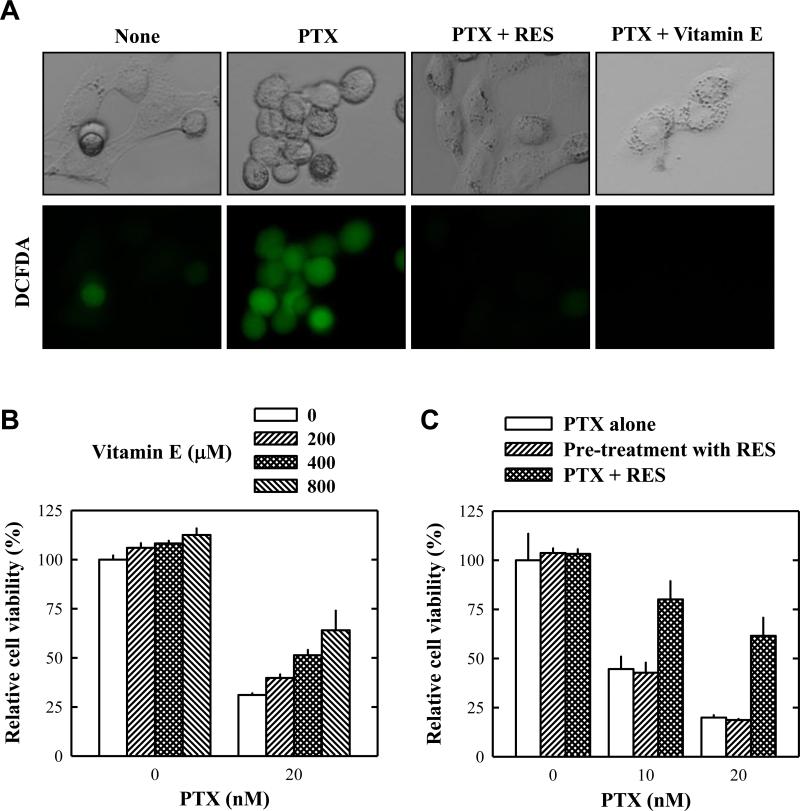
Recent studies showed that paclitaxel-induced ROS formation in cancer cells contributes importantly to its anticancer action.23,24 Therefore, we examined whether alteration of the intracellular ROS accumulation contributed to resveratrol's effect. As shown in Fig. 5, we confirmed that paclitaxel treatment induced intracellular ROS accumulation (shown as green fluorescence). Combined use of resveratrol + paclitaxel significantly reduced intracellular ROS accumulation. In this study, we also investigated whether another antioxidant α-tocopherol (vitamin E) had a similar effect as resveratrol. As shown in Fig. 5A, pre-treatment of cells with α-tocopherol for 2 h completely abrogated paclitaxel-induced intracellular ROS accumulation and cell morphological change. In addition, it also abrogated paclitaxel-induced cell death in a concentration-dependent manner (Fig. 5B).
Figure 5. Antioxidants abrogate paclitaxel-induced intracellular ROS accumulation and cell death.
A. Intracellular ROS accumulation in MDA-MB-435s cells (using the H2-DCF-DA method) that were pre-treated with 20 μM resveratrol or 500 μM vitamin E for 2 h and then co-incubated with 10 nM paclitaxel for 24 h. B. Viability of cells (MTT assay) treated with paclitaxel alone or in combination with vitamin E at indicated concentrations for 48 h. C. Viability of cells that were treated with resveratrol in three different ways: The open column shows cells that were treated with paclitaxel alone at indicated concentrations for 48 h. The hatched column shows cells that were pre-treated with 20 μM resveratrol for the first 8 h (resveratrol was washed out afterwards) and then treated with paclitaxel alone at indicated concentrations for 48 h. The cross-hatched column shows cells that were treated with 20 μM resveratrol + pclitaxel at indicated concentrations together for 48 h. Cell viability was determined by MTT assay. Each data point is the mean ± S.D. of three independent experiments. PTX, paclitaxel; RES, resveratrol.
A recent study showed that pretreatment of mouse hippocampal cells in vitro with resveratrol for a few hours exerted a prolonged antioxidant effect (after the chemical was removed) through the prior induction of a mitochondrial antioxidant enzyme.25 This possibility was also examined in this study. As shown in Fig. 5C, we found that pre-treatment of MDA-MB-435s cells with resveratrol for 8 h did not exert a significant protective effect when the chemical was removed. These results indicate that the direct antioxidant activity of resveratrol contributed predominantly to the suppression of paclitaxel's anticancer activity.
3.2.3 Modulation of paclitaxel-induced Bcl-xL phosphorylation
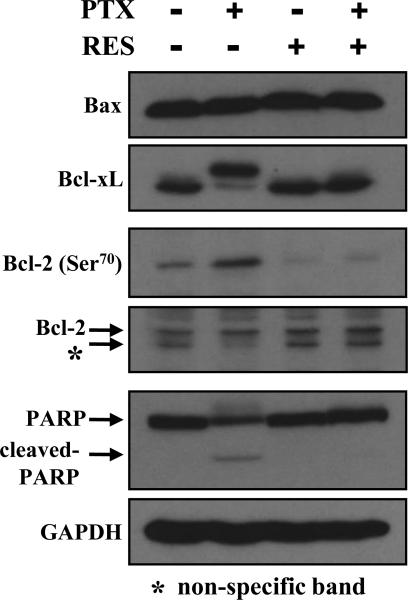
Recent studies reported that the microtubule-targeting agents could inactivate the anti-apoptotic Bcl-2 family proteins (Bcl-2 and Bcl-xL) in cancer cells.26,27 In this study, we examined whether resveratrol modulated the phosphorylation of Bcl-2 and Bcl-xL induced by paclitaxel. As shown in Fig. 6, treatment with paclitaxel or resveratrol alone or in combination did not affect the levels of Bax (a major pro-apoptotic Bcl-2 family protein). However, the presence of paclitaxel alone increased the inactivation (phosphorylation) of the anti-apoptotic Bcl-2 family proteins Bcl-xL and Bcl-2, and co-presence of resveratrol significantly suppressed their inactivation. Consistent with this observation, poly (ADP-ribose) polymerase (PARP) cleavage (one of the indicators of apoptotic cell death) was induced by paclitaxel treatment, but it was inhibited by the combined treatment. Taken together, these data showed that resveratrol strongly inhibited paclitaxel-induced Bcl-2 and Bcl-xL inactivation and, subsequently, the apoptotic cell death.
Figure 6. Resveratrol alters paclitaxel-induced change in Bcl-2 family protein expression and phosphorylation (inactivation).
MDA-MB-435s cells were treated with 10 nM paclitaxel alone or in combination with 20 μM resveratrol for 24 h. Cell extracts were subjected to SDS-PAGE separation and then immunoblotted with antibodies specific for Bcl-2 family proteins. Membranes were stripped and re-probed for GAPDH as a loading control. Two experiments were conducted, and shown are results from a representative experiment. PTX, paclitaxel; RES, resveratrol.
4. DISCUSSION
In this study, we demonstrate that resveratrol can strongly attenuate the anticancer actions of paclitaxel in several human breast cancer cell lines (MDA-MB-435s, MDA-MB-231 and SKBR-3) in culture. A similar observation was also made in athymic nude mice using MDA-MB-435s cells as a representative model. This undesirable effect of resveratrol appears to be both cell type-dependent as well as anticancer drug-dependent. To understand the mechanism(s) of resveratrol's opposing actions, we sought to determine the contribution of its antioxidant activity to the observed effect, because recent studies showed that reactive oxygen and nitrogen species play a critical role in paclitaxel-induced anticancer activity.23,24,28 We found that co-presence of resveratrol or vitamin E almost completely abrogated paclitaxel-induced intracellular ROS accumulation, morphological change and loss of viability (Fig. 5A, 5B). This observation suggests that resveratrol's antioxidant activity contributes importantly to its suppression of paclitaxel's anticancer action. In addition, earlier studies showed that paclitaxel and 2-methoxyestradiol, two microtubule-targeting agents, can inactivate (through phosphorylation) the anti-apoptotic Bcl-2 family proteins Bcl-2 and Bcl-xL in human cancer cell lines, and this inactivation plays an important role in the induction of apoptosis in these cells.26,27 Our results show that paclitaxel and resveratrol do not affect the pro-apoptotic protein Bax expression level. However, paclitaxel increases the phosphorylation of Bcl-2 and Bcl-xL, and their phosphorylation is almost completely abrogated when resveratrol was co-presence (Fig. 6). Earlier studies have shown that paclitaxel or 2-methoxyestradiol-induced phosphorylation of Bcl-xL and Bcl-2 is regulated by the activity of the c-jun N-terminal kinase (JNK),26,27 which is activated by ROS through thioredoxin and apoptosis signal-regulating kinase 1.29,30 Also, it was shown that inhibition of JNK activity can effectively protect cell against apoptotic cell death induced by these agents.26,27 Taken together, these data collectively suggest that resveratrol exerts its strong opposing effect against paclitaxel's anticancer actions partly through suppression of paclitaxel-induced ROS accumulation and subsequently the inactivation of anti-apoptotic Bcl-2 family proteins.
In this study, we have also studied the modulating effect of resveratrol on paclitaxel-induced cell cycle changes. It is known that paclitaxel, a microtuble-targeting agent, can strongly induce G2/M cell cycle arrest as well as apoptosis,20 and these characteristic changes are confirmed in this study. Our recent study showed that the presence of resveratrol alone at relatively low concentrations induced a reversible, non-cytotoxic S-phase arrest.12 Therefore, we tested whether resveratrol-induced S-phase arrest contributes to the suppression of paclitaxel-induced G2/M cell cycle arrest and subsequently cell death. We found that when resveratrol is used in combination with paclitaxel, it reduces the population of G2/M phase cells but increases the population of S-phase cells. Similar observations are made with another anticancer drug doxorubicin when it is used in combination with resveratrol. Since resveratrol-induced S-phase arrest is associated with Chk2 activation,12 we probed the role of Chk2 activation in mediating resveratrol's modulating effect. We found that down-regulation of Chk2 significantly reduces the effect of resveratrol against paclitaxel-induced cell death, although it only slightly reduces resveratrol-induced S-phase cell cycle arrest (Fig. 4C and 4D). These results indicate that activation of Chk2 by resveratrol partly contributes to its modulation of paclitaxel-induced cell cell changes and apoptosis.
The HER-2 proto-oncogene encodes a receptor-like transmembrane protein with homology to the epidermal growth factor receptor31 and overexpression of HER-2 has been observed in approximately 25-30% of human breast cancers.32,33 Among the breast cancer cell lines tested, HER-2 expression levels are significantly higher in SKBR-3, MDA-MB-435s, and MDA-MB-231 cells than in MCF-7 cells.34-37 It appears that the levels of HER-2 expression are correlated with resveratrol's protective effect against paclitaxel-induced cell death in cultured human breast cancer cells. An earlier study showed that paclitaxel-induced apoptosis is suppressed in HER-2-overexpressing cells.38 Mechanistically, it is known that HER-2 overexpression would up-regulate p21, which, in turn, would suppress paclitaxel-induced Cdc2 activation and delay the entry into the G2/M phase, thereby inhibiting paclitaxel-induced apoptosis. Since resveratrol can also inhibit paclitaxel-induced G2/M arrest in HER-2-overexpressing cells, it is possible that resveratrol's protective effect may be partially related to the HER-2 signaling pathway. However, this suggestion is weakened by the observation that DU145 and MIA PaCa-2 cells, two cell lines that also express HER-239-41, do not respond to the growth-modulating effect of resveratrol in a similar manner. More studies are needed to determine the potential role, if any, of the HER-2 signaling pathway in mediating resveratrol's opposing actions against paclitaxel-induced cell cycle change and apoptosis.
Lastly, it is also worth a brief note concerning the lineage controversy over MDA-MB-435s cells. This cell line was originally reported to be derived from the pleural effusion of a female patient with breast cancer,42 and has been widely used as an in vitro model in studying human breast cancer. However, analysis of gene expression patterns of this cell line has revealed its unique resemblance to melanoma cells.43-46 These features are very distinct from other human breast cancer cell lines, including MCF-7 cells.44 It will be of interest to determine whether any of these known unique molecular features of MDA-MB-435s cells specifically determine their sensitivity to resveratrol's actions.
In summary, the results of our present study indicate that resveratrol can significantly attenuate the efficacy of paclitaxel's anticancer actions in certain human breast cancer cell lines both in vitro and in vivo. This effect is caused by two different mechanisms: one is through the inhibition of paclitaxel-induced G2/M cell cycle arrest, and the other one is through the suppression of paclitaxel-induced ROS accumulation and subsequently the inactivation of anti-apoptotic Bcl-2 family proteins. Although it has been suggested that addition of resveratrol may enhance the anticancer efficacy of paclitaxel in some human cancers, this strategy may be detrimental in certain types of cancers. Given that resveratrol at present is commonly used among cancer patients as a healthy dietary supplement, the results of our present study are very timely, and call for more preclinical and clinical testing of the potential benefits and harms of using resveratrol as an anticancer adjuvant in cancer patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was supported, in part, by a grant from the National Institutes of Health (grant No. ES015242).
Conflict of interest statement
None to declare.
REFERECES
- 1.Roldan A, Palacios V, Caro I, Perez L. Resveratrol content of Palomino fino grapes: influence of vintage and fungal infection. J Agric Food Chem. 2003;51:1464–8. doi: 10.1021/jf020774u. (2003) [DOI] [PubMed] [Google Scholar]
- 2.Kopp P. Resveratrol, a phytoestrogen found in red wine. A possible explanation for the conundrum of the ‘French paradox’? Eur J Endocrinol. 1998;138:619–20. doi: 10.1530/eje.0.1380619. [DOI] [PubMed] [Google Scholar]
- 3.Sobolev VS, Cole RJ. Trans-resveratrol content in commercial peanuts and peanut products. J Agric Food Chem. 1999;47:1435–9. doi: 10.1021/jf9809885. [DOI] [PubMed] [Google Scholar]
- 4.Kundu JK, Surh YJ. Cancer chemopreventive and therapeutic potential of resveratrol: mechanistic perspectives. Cancer Lett. 2008;269:243–61. doi: 10.1016/j.canlet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 5.Goswami SK, Das DK. Resveratrol and chemoprevention. Cancer Lett. 2009;284:1–6. doi: 10.1016/j.canlet.2009.01.041. [DOI] [PubMed] [Google Scholar]
- 6.Bishayee A. Cancer prevention and treatment with resveratrol: from rodent studies to clinical trials. Cancer Prev Res. 2009;2:409–18. doi: 10.1158/1940-6207.CAPR-08-0160. [DOI] [PubMed] [Google Scholar]
- 7.Nakagawa H, Kiyozuka Y, Uemura Y, et al. Resveratrol inhibits human breast cancer cells growth and may mitigate the effect of linoleic acid, a potent breast cancer cell stimulator. J Cancer Res Clin Oncol. 2001;127:258–64. doi: 10.1007/s004320000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alkhalaf M. Resveratrol-induced growth inhibition in MDA-MB-231 breast cancer cells is associated with mitogen-activated protein kinase signaling and protein translation. Eur J Cancer Prev. 2007;16:334–41. doi: 10.1097/01.cej.0000228413.06471.4c. [DOI] [PubMed] [Google Scholar]
- 9.Fulda S, Debatin KM. Sensitization for tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by the chemopreventive agent resveratrol. Cancer Res. 2004;64:337–46. doi: 10.1158/0008-5472.can-03-1656. [DOI] [PubMed] [Google Scholar]
- 10.Gill C, Walsh SE, Morrissey C, Fitzpatrick JM, Watson RWG. Resveratrol sensitizes androgen independent prostate cancer cells to death-receptor mediated apoptosis through multiple mechanisms. Prostate. 2007;67:1641–53. doi: 10.1002/pros.20653. [DOI] [PubMed] [Google Scholar]
- 11.Fulda S, Debatin KM. Sensitization for anticancer drug-induced apoptosis by the chemopreventive agent resveratrol. Oncogene. 2004;23:6702–11. doi: 10.1038/sj.onc.1207630. [DOI] [PubMed] [Google Scholar]
- 12.Zhou R, Fukui M, Choi HJ, Zhu BT. Induction of a reversible, non-cytotoxic S-phase delay by resveratrol: implications for a mechanism of lifespan prolongation and cancer protection. Br J Pharmacol. 2009;158:462–74. doi: 10.1111/j.1476-5381.2009.00268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.She QB, Bode AM, Ma WY, Chen NY, Dong Z. Resveratrol-induced activation of p53 and apoptosis is mediated by extracellular-signal-regulated protein kinase and p38 kinase. Cancer Res. 2001;61:1604–10. [PubMed] [Google Scholar]
- 14.Su JL, Lin MT, Hong CC, et al. Resveratrol induces FasL-related apoptosis through Cdc42 activation of ASK1/JNK-dependent signaling pathway in human keukemia HL-60 cells. Carcinogenesis. 2005;26:1–10. doi: 10.1093/carcin/bgh220. [DOI] [PubMed] [Google Scholar]
- 15.Aziz MH, Nihal M, Fu VX, Jarrard DF, Ahmad N. Resveratrol-caused apoptosis of human prostate carcinoma LNCaP cells in mediated via modulation of phosphatidilinositol 3’-kinase/Akt pathway and Bcl-2 family proteins. Mol. Cancer Ther. 2006;5:1335–41. doi: 10.1158/1535-7163.MCT-05-0526. [DOI] [PubMed] [Google Scholar]
- 16.She QB, Huang C, Zhang Y, Dong Z. Involvement of c-jun NH2-terminal kinases in resveratrol-induced activation of p53 and apoptosis. Mol Carcinogen. 2002;33:244–50. doi: 10.1002/mc.10041. [DOI] [PubMed] [Google Scholar]
- 17.Opipari AW, Jr, Tan L, Boitano AE, Sorenson DR, Aurora A, Liu JR. Resveratrol-induced autophagocytosis in ovarian cancer cells. Cancer Res. 2004;64:696–703. doi: 10.1158/0008-5472.can-03-2404. [DOI] [PubMed] [Google Scholar]
- 18.Pozo-Guisado E, Merino JM, Mulero-Navarro S, et al. Resveratrol-induced apoptosis in MCF-7 human breast cancer cells involves a caspase-independent mechanism with downregulation of Bcl-2 and NF-kappaB. Int J Cancer. 2005;115:74–84. doi: 10.1002/ijc.20856. [DOI] [PubMed] [Google Scholar]
- 19.Benitez DA, Pozo-Guisado E, Alvarez-Barrientos A, Fernandez-Salguero PM, Castellón EA. J Androl. 2007;28:282–93. doi: 10.2164/jandrol.106.000968. [DOI] [PubMed] [Google Scholar]
- 20.Woods CM, Zhu J, McQueney PA, Bollag D, Lazarides E. Taxol-induced mitotic block triggers rapid onset of a p53-independent apoptotic pathway. Mol Med. 1995;1:506–26. [PMC free article] [PubMed] [Google Scholar]
- 21.Kubota T, Uemura Y, Kobayashi M, Taguchi H. Combined effects o resveratrol and paclitaxel on lung cancer cells. Anticancer Res. 2003;23:4039–46. [PubMed] [Google Scholar]
- 22.Jazirehi AR, Bonavida B. Resveratrol modifies the expression o apoptotic regulatory proteins and sensitizes non-Hodgkin's lymphoma and multiple myeloma cell lines to paclitaxel-induced apoptosis. Mol Cancer Ther. 2004;3:71–84. [PubMed] [Google Scholar]
- 23.Alexandre J, Batteux F, Nicco C, et al. Accumulation of hydrogen peroxide is an early and crucial step for paclitaxel-induced cancer cell death both in vitro and in vivo. Int J Cancer. 2006;119:41–8. doi: 10.1002/ijc.21685. [DOI] [PubMed] [Google Scholar]
- 24.Alexandre J, Hu Y, Lu W, Pelicano H, Huang P. Novel action of paclitaxel against cancer cells: bystander effect mediated by reactive oxygen species. Cancer Res. 2007;67:3512–7. doi: 10.1158/0008-5472.CAN-06-3914. [DOI] [PubMed] [Google Scholar]
- 25.Fukui M, Choi HJ, Zhu BT. Mechanism for the protective effect of resveratrol against glutamate-induced neuronal death in HT22 cells. Free Radical Biol Med. 2010 doi: 10.1016/j.freeradbiomed.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basu A, Haldar S. Identification of a novel Bcl-xL phosphorylation site regulating the sensitivity of taxol- or 2-methoxyestradiol-induced apoptosis. FEBS Lett. 2003;538:41–7. doi: 10.1016/s0014-5793(03)00131-5. [DOI] [PubMed] [Google Scholar]
- 27.Fukui M, Zhu BT. Mechanism of 2-methoxyestradiol-induced apoptosis and growth arrest in human breast cancer cells. Mol Carcinog. 2009;48:66–78. doi: 10.1002/mc.20458. [DOI] [PubMed] [Google Scholar]
- 28.Ramanathan B, Jan KY, Chen CH, Hour TC, Yu HJ, Pu YS. Resistance to paclitaxel is proportional to cellular total antioxidant capacity. Cancer Res. 2005;65:8455–60. doi: 10.1158/0008-5472.CAN-05-1162. [DOI] [PubMed] [Google Scholar]
- 29.Ichijo H, Nishida E, Irie K, et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–4. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 30.Saitoh M, Nishitoh H, Fujii M, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bargmann CI, Hung MC, Weinberg RA. The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature. 1986;319:226–30. doi: 10.1038/319226a0. [DOI] [PubMed] [Google Scholar]
- 32.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 33.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–12. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 34.Tari AM, Hung MC, Li K, Lopez-Berestein G. Growth inhibition of breast cancer cells by Grb2 downregulation is correlated with inactivation of mitogen-activated protein kinase in EGFR, but not in ErbB2, cells. Oncogene. 1999;18:1325–32. doi: 10.1038/sj.onc.1202422. [DOI] [PubMed] [Google Scholar]
- 35.Simeone AM, Broemeling LD, Rosenblum J, Tari AM. HER2/neu reduces the apoptotic effects of N-(4-hydroxyphenyl)retinamide (4-HPR) in breast cancer cells by decreasing nitric oxide production. Oncogene. 2003;22:6739–47. doi: 10.1038/sj.onc.1206786. [DOI] [PubMed] [Google Scholar]
- 36.Meric F, Lee WP, Sahin A, Zhang H, Kung HJ, Hung MC. Expression profile of tyrosine kinases in breast cancer. Clin Cancer Res. 2002;8:361–7. [PubMed] [Google Scholar]
- 37.Rait AS, Pirollo KF, Rait V, Krygier JE, Xiang L, Chang EH. Inhibitory effects of the combination of HER-2 antisense oligonucleotide and chemotherapeutic agents used for the treatment of human breast cancer. Cancer Gene Ther. 2001;8:728–39. doi: 10.1038/sj.cgt.7700359. [DOI] [PubMed] [Google Scholar]
- 38.Yu D, Jing T, Liu B, et al. Overexpression of ErbB2 blocks Taxol-induced apoptosis by upregulation of p21Cip1, which inhibits p34Cdc2 kinase. Mol Cell. 1998;2:581–91. doi: 10.1016/s1097-2765(00)80157-4. [DOI] [PubMed] [Google Scholar]
- 39.Tatebe H, Shimizu M, Shirakami Y, Tsurumi H, Moriwaki H. Synergistic growth inhibition by 9-cis-retinoic acid plus trastuzumab in human hepatocellular carcinoma cells. Clin Cancer Res. 2008;14:2806–12. doi: 10.1158/1078-0432.CCR-07-4708. [DOI] [PubMed] [Google Scholar]
- 40.Kominsky SL, Hobeika AC, Lake FA, Torres BA, Johnson HM. Down-regulation of neu/HER-2 by interferon-gamma in prostate cancer cells. Cancer Res. 2000;15:3904–8. [PubMed] [Google Scholar]
- 41.DeArmond D, Brattain MG, Jessup JM, et al. Autocrine-mediated ErbB-2 kinase activation of STAT3 is required for growth factor independence of pancreatic cancer cell lines. Oncogene. 2003;22:7781–95. doi: 10.1038/sj.onc.1206966. [DOI] [PubMed] [Google Scholar]
- 42.Cailleau R, Olivé M, Cruciger QV. Long-term human breast carcinoma cell lines of metastatic origin: preliminary characterization. In Vitro. 1978;14:911–15. doi: 10.1007/BF02616120. [DOI] [PubMed] [Google Scholar]
- 43.Chambers AF. MDA-MB-435 and M14 cell lines: identical but not M14 melanoma? Cancer Res. 2009;69:5292–3. doi: 10.1158/0008-5472.CAN-09-1528. [DOI] [PubMed] [Google Scholar]
- 44.Ross DT, Scherf U, Eisen MB, et al. Systematic variation in gene expression patterns in human cancer cell lines. Nature Genet. 2000;24:227–35. doi: 10.1038/73432. [DOI] [PubMed] [Google Scholar]
- 45.Sellappan S, Grijalva R, Zhou X, et al. Lineage infidelity of MDA-MB-435 cells: expression of melanocyte proteins in a breast cancer cell line. Cancer Res. 2004;15:3479–85. doi: 10.1158/0008-5472.CAN-3299-2. [DOI] [PubMed] [Google Scholar]
- 46.Ellison G, Klinowska T, Westwood RF, Docter E, French T, Fox JC. Further evidence to support the melanocytic origin of MDA-MB-435. Mol Pathol. 2002;55:294–9. doi: 10.1136/mp.55.5.294. [DOI] [PMC free article] [PubMed] [Google Scholar]