SUMMARY
Endothelin (Edn) signaling via the G-coupled, endothelin receptor type B (Ednrb) is essential for the development of melanocytes from the neural crest (NC) and has been associated with melanoma progression. Edn3 plays varying roles during melanocyte development, promoting the proliferation and self-renewal of NC-derived multi- and bipotential precursors as well as the survival, proliferation, differentiation and migration of committed melanocyte precursors. Melanocyte differentiation is achieved via the interaction of Ednrb and Kit signaling, with Ednrb being specifically required in the final differentiation step, rather than in the initial specification of melanocytic fate. Ednrb has also been implicated in the de-differentiation of mature melanocytes, a process that takes place during the malignant transformation of these cells. Ednrb was found to be upregulated in melanoma metastases and was shown to alter tumor-host interactions leading to melanoma progression. Antagonists to this receptor were shown to inhibit melanoma cell growth and increase the apoptotic rate of these cells, and to lead to disease stabilization in melanoma patients. Thus, endothelin signaling inhibition may prove useful in the treatment of certain types of melanoma.
Introduction
Amongst the major paracrine factors that are involved in melanocyte biology, the endothelins (Edns) play significant roles during the early development of these cells, in their response to ultraviolet radiation, and in pathological conditions including melanoma. Edns are signaling peptides composed of 21 amino acid residues. There are three endothelin peptides, Edn1, Edn2, and Edn3, produced from prepropolypeptide precursors which are first cleaved by prohormone processing enzymes like furin to yield biologically inactive intermediates, 38–41 amino acids long, known as big Edns. These intermediates are subsequently cleaved at the Trp-21-Val/Ile-22 site by endothelin converting enzyme (ECE-1 and ECE-2), to yield the active Edns (Emoto and Yanagisawa, 1995; Xu et al., 1994).
Edns act on two homologous G-protein coupled, heptahelical receptors, endothelin receptor type A (Ednra) and endothelin receptor type B (Ednrb) (reviewed Sakurai et al., 1992) (In this review we will use the official nomenclature for the murine endothelin receptor genes, Ednrb and Ednra, Ednrb and Ednra for the products of the genes, and for simplicity will apply it to all other animals except for humans, where it will be in capitals). A receptor closely related to Ednrb, Ednrb2, has been described only in avians (Lecoin et al., 1998) while a third receptor, endothelin receptor C, has been identified only in Xenopus (Karne et al., 1993). Ednra binds End1 and Edn2 with similar affinity and Edn3 with 1000 to 2000 fold lower affinity. Ednrb, on the other hand, binds all three isopeptides with equal affinity (reviewed in Sakurai et al., 1992). Binding of Edns to either receptor initiates a series of intracellular signal transduction events via heterotrimetic G proteins. Activated Ednra signals through Gq/G11 and G12/G13 while Ednrb signals through Gi/G0, Gq/G11 as well as G13 (reviewed in Kedzierski and Yanagisawa, 2001). The downstream events have been mostly characterized upon activation of Ednra in cell types such as smooth muscle and cardiomyocytes. These events lead to the activation of phospholipase Cβ, inhibition of adenyl cyclase, activation of plasma membrane Ca2+ channels, and activation of nonreceptor tyrosine kinases, among others (reviewed in Bouallegue et al., 2007; Sugden and Clerk, 2005). In melanocytes, binding of Edn1 or Edn3 to Ednrb causes the activation of the pathways that include protein kinase C (PKC), mitogen-activated protein kinase and Raf-1 as well as p90 ribosomal S6 kinase, cAMP response element binding protein (CREB) and cAMP-protein kinase A-CREB (Bohm et al., 1995; Imokawa et al., 1996, 1997; Sato-Jin et al., 2008).
Edn1 was the first member of the endothelin family to be described. It was first identified as a potent vasopressor derived from vascular endothelial cells (Yanagisawa et al., 1988). It was later shown to have a mitogenic and melanogenic effect on melanocytes (Imokawa et al., 1992, 1995; Yada et al., 1991). Of the three endothelin peptides, Edn3 was found to play a major role during the development of melanocytes from neural crest (NC) cell precursors. Mutations or deletions of the mouse genes Ednrb and Edn3 result in the piebald lethal (sl) and lethal spotting (ls) phenotypes, respectively (Fig. 1). Mice carrying the piebald lethal mutation are mostly white, except for spotted regions in the head and rump. They die as juveniles from megacolon due to the absence of enteric ganglia in the distal colon. The lethal spotting phenotype is similar to the piebald lethal phenotype (Baynash et al., 1994; Hosoda et al., 1994). EDNRB is mutated in a subset of cases of Hirschsprung disease and mutations in EDN3 have also been described that lead to gut aganglionosis associated with sensorineural deafness and pigmentary defects (reviewed in McCallion and Chakravarti, 2001). Edn1 and Edn3 have also been implicated in hyperpigmentation pathological conditions in mice (Fig. 1) and humans (Garcia et al., 2008; Kadono et al., 2001; Manaka et al., 2001; Okazaki et al., 2005) as well as in melanoma (reviewed in Lee et al., 2008). In this review, we will focus on the effects endothelin signaling via Ednrb has on the different stages of melanocyte development and melanoma progression.
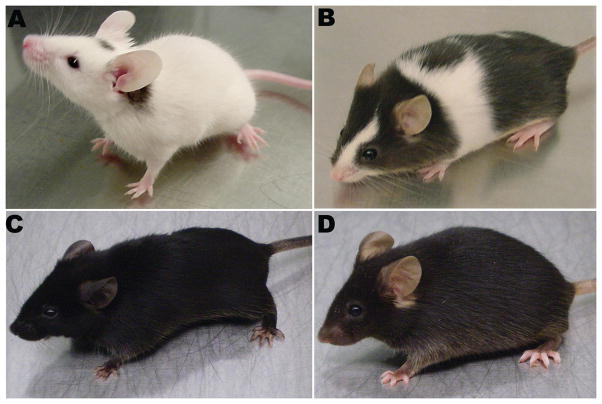
Figure 1.
Mouse mutants reveal the role of endothelin signaling in melanocytes. The spontaneous mutants (A) piebald lethal (Ednrbsl/sl) and (B) lethal spotting (Edn3ls/ls) display a hypopigmentation phenotype while (C) a transgenic mouse that over-expresses End3 under the keratin 5 promoter (K5-tTA; TRE-Edn3-lacZ : K5-Edn3) shows skin and coat hyperpigmentation when compared to (D) the wild type mouse (C57B6 background).
Endothelins and melanocyte development
Timing of action
Studies using the mouse as a model system have been instrumental in establishing the timing of action and the role of Ednrb signaling in melanocyte development. In homozygous piebald lethal mutants as well as in mice in which the LacZ gene was inserted downstream of the endogenous Ednrb promoter by homologous recombination there is a drastic reduction in the number of melanocyte precursors (melanoblasts) by embryonic day 12.5 (E12.5) (Lee et al., 2003; Pavan and Tilghman, 1994). This implies that Ednrb signaling affects melanocyte development prior to or at E12.5. In order to establish the time frame in which Ednrb signaling is required for melanocyte development Shin et al. (1999) used the tet-inducible system to temporally manipulate the expression of the Ednrb gene. This study demonstrated that Ednrb is required for the establishment of a wild type coat color pattern between E10 and E12.5, which corresponds to the period when melanoblasts have reached the migrating stage area (MSA). Since the Ednrb mutant phenotype could be rescued by activating the Ednrb gene as late as E10, it appears that Ednrb is not required for melanoblasts prior to reaching the MSA. The complete rescue of the hypopigmentation phenotype of piebald lethal mice by a transgene in which Ednrb was under the control of the Dopachrome tautomerase (Dct) promoter, further indicates that this pathway is not required before the precursors commit to the melanocytic fate (Ittah and Kos, unpublished observations). Additionally, the complete rescue of the wild type coat color phenotype through the expression of Ednrb until E12.5 and not thereafter, suggests that Ednrb is not required after melanoblasts migrate from the MSA (Shin et al., 1999).
The small numbers of melanobasts that are found in the Ednrb homozygous null mutants at E10-11.5 implies that Ednrb is not required for the initial specification of neural crest precursors into melanoblasts. The temporal requirement for Ednrb indicates that it may be required for the initiation of melanoblast migration from the MSA and/or for their survival during this migratory transition stage. A possible migratory function is supported by the observation that some of the remaining melanoblasts appear to accumulate in the MSA (Lee et al., 2003). This function has been more clearly shown in avian embryos where Ednrb2 along with the ephrin receptor EphB2 are essential for melanoblasts to choose and invade the dorsolateral pathway (Harris et al., 2008; Pla et al., 2005). Together, these receptors regulate a chemotactic response by allowing the melanoblasts to overcome repulsive cues present along the dorsolateral pathway (Harris et al., 2008).
The absolute time requirement for Ednrb during melanocyte development does not, however, preclude it from having an effect on melanocytes and their precursors at all stages. In fact, several in vitro studies suggest this to be the case. Experiments with NC explants and primary melanocyte cultures showed that Edn3 acts as a promoter of proliferation and self-renewal factor of a common melanocyte-glial precursor as well as a survival, mitogenic and differentiation factor for committed melanoblasts (Dupin et al., 2000, 2003; Hou et. al., 2004; Lavah et al., 1996, 1998; Opdecamp et al., 1998, Reid et al., 1996; Trentin et al., 2004).
Maintenance of undifferentiated state, promotion of survival and proliferation
In vitro studies provide evidence to support a role for Edn3 as an inhibitor of differentiation, survival and proliferation factor for NC cells in the avian system. Treatment of quail NC cultures with Edn3 was necessary for maintaining the viability of these cells and initially promoted their proliferation. In the absence of contact inhibition, cells treated with Edn3 were found to proliferate for two weeks without producing pigment (Lahav et al., 1996). Initial exposure of quail NC cells to Edn3 induced the expression of both Ednrb and Ednrb2 while prolonged exposure to Edn3 resulted in decreased Ednrb expression and increased Ednrb2 expression. This switch in receptor protein expression was accompanied by a gradual decrease in proliferation and an increase in the expression of melanocytic markers. These results indicate that in quail, initial exposure to Edn3 promotes NC cell proliferation via the activation of Ednrb, while prolonged exposure to Edn3 promotes melanocyte differentiation via the activation of Ednrb2 (Lahav et al., 1998).
In addition to promoting NC cell proliferation, Edn3 induces the proliferation and self-renewal of NC-derived multi- and bipotential precursors in avians. Treatment of NC clonal cultures with Edn3 promoted the survival and growth of a bipotent glia-melanocyte precursor (Lahav et al., 1998), which was shown to be dependent on Edn3 for self-renewal (Trentin et al., 2004). Edn3 treatment was found to stimulate the proliferation of clonal cultures of pigmented melanocytes (Dupin et al., 2000), favor their dedifferentiation into cells that re-express early NC markers such as Sox10, FoxD3, Pax3 and Slug (Real et al., 2006), and even lead them to transdifferentiate into glial marker-positive cells (Dupin et al., 2000). Edn3 increased the self-renewal capacity of multipotent glia-melanocyte, melanocyte-fibroblast, and glia-melanocyte-fibroblast precursors, derived from embryonic pigment cells. The heterogeneity of these clones was enhanced in the presence of Edn3, and continuous exposure to Edn3 was necessary for the maintenance of their multipotentiality. Edn3 was most effective in promoting the long-term propagation of glia-melanocyte precursor cells as opposed to the other precursors (Real et al., 2006). Whether the phenotypic reprogramming of differentiated melanocytes and the maintenance of multipotent precursors are simply in vitro phenomena or actually occur in vivo is not clear. They may, however, happen in situations of injury or pathological conditions such as tumorogenesis. For example, after sciatic nerve injury in mice, glial cells acquire a melanogenic phenotype unless they are suppressed by particular cytokines (Rizvi et al., 2002).
A role for Edn3 as a survival and proliferation factor for committed precursors was established in both the avian and mouse models. Treatment of quail NC clonal cultures with Edn3 promoted the survival and proliferation of unipotent melanocyte and glia precursors (Lahav et al., 1998). Exposure of murine NC cultures to Edn1 or Edn3 led to large numbers of Kit-positive melanoblasts via increased cell proliferation as measured by [3H]thymidine incorporation (Reid et al., 1996). Similarly, a proliferative effect of Edn3 was found in murine NC-derived melanoblasts, which express the tyrosine kinase receptor Kit, Dct, and the transcription factor Mitf. Initially, the number of Dct-positive cells in control cultures and in Edn3-supplemented cultures was similar. After the second day, however, the number of Dct-positive cells increased in the Edn3-supplemented cultures but decreased gradually in the control cultures. This increase in cell numbers in the treated cultures could not be accounted for solely by a proliferative effect, pointing to a possible role for Edn3 in the survival of melanoblasts from the NC (Opdecamp et al., 1998). These results suggest that Edn3 acts as a survival and proliferation factor for committed melanocyte precursors.
Differentiation
In vivo observations as well as in vitro studies have provided evidence to support a role for Ednrb signaling in melanocyte differentiation after the initial specification of NC cells into melanoblasts. In the avian system, although inhibition of differentiation upon initial exposure of NC cells to Edn3 was observed, prolonged treatment with Edn3 was shown to induce melanocyte differentiation as evidenced by the large increase in the number of pigmented cells (Lahav et al., 1996, 1998). Consistent with these observations in avians is the finding that in murine NC cultures Dct-positive cells are present in the absence of Edn3, but these cells fail to produce pigment unless they are treated with Edns or PKC activators (Opdecamp et al., 1998). Furthermore, Ednrb deficient melanoblasts are capable of generating Mitf, Sox10 and Kit-positive cells that do not progress to become tyrosinase-positive (Hou et al., 2004). The results of these studies imply that Ednrb is not necessary for the initial instruction of melanocyte development but is essential for the final differentiation of melanocytes, possibly via the regulation of melanogenic enzymes. Further support for a role in differentiation is provided by the conditions required to differentiate mouse and human embryonic stem cells into melanocytes (Motohashi et al., 2006). The differentiation of mouse embryonic stem cells co-cultured with a bone marrow –derived stroma cell line is greatly enhanced in the presence of Edn3 (Yamane et al., 1999). Induction of human embryonic stem cells with Edn3 in conjunction with Wnt3a and Kit-ligand (Kitl, SLF, SCF) give rise to cells that express melanogenic markers, such as Mitf and Sox10, develop melanosomes and produce melanin (Fang et al., 2006).
Edn3 acts together with Kitl, to promote the survival, proliferation and differentiation of melanoblasts. Similar to Edn3 and Ednrb mutations, Kitl and Kit receptor mutations result in a hypopigmentation phenotype. In vivo, the majority of melanoblasts are lost in the absence of either Ednrb or Kit signaling (Pavan and Tilghman, 1994; Steel et al., 1992; Wehrler-Haller and Weston, 1995), indicating that neither of these pathways can compensate for the loss of the other. However, not all melanoblasts and melanocytes seem to respond to these pathways in a similar manner. A recent study showed that non-cutaneous melanocytes found in the choroid, ciliary body, iris, cochlea and harderian gland are less dependent on Kit signaling than the follicular melanocytes. These non-cutaneous melanocytes along with dermal melanocytes grow and differentiate more effectively when exposed to exogenous End3 (Aoki et al, 2009).
In mouse NC cultures, the lack of either Edn3 or Kitl resulted in nearly a complete loss of melanoblasts. Melanoblast numbers increased in the presence of either factor; however, treatment with both Edn3 and Kitl led to a significant increase in the number of melanoblasts compared to either factor alone (Reid et al., 1996). Although the over-expression of Edn3 in Kit null mutant mice was capable of reducing white spotting (Aoki et al., 2005; Garcia et al., 2008), overactivation of Kit signaling by addition of Kitl to ES cells in which Ednrb signaling was completely disabled did not compensate for the failure of melanocyte development. This suggests that the survival of early melanocyte precursors is dependent upon the cooperative activity of these two pathways. A different scenario exists in avians, where Ednrb2 has been suggested to have a similar effect on melanoblasts as the sum of Ednrb and Kit signaling because Kit is produced after melanoblasts have begun their migration along the dorsolateral pathway and not while melanoblasts are in the MSA, as it occurs in mice (reviewed in Pla and Larue, 2003).
The treatment of mouse NC cells carrying Kitl mutations with Edn3 was not capable of generating Kit-positive and DOPA-positive cells, nor fully pigmented melanocytes. This indicates that in the absence of Kitl, Edn3 is not sufficient for the differentiation of NC cells into melanocytes. However, treating cultures with Kitl in the absence of Edn3 was sufficient for the generation of Kit-positive and DOPA-positive cells, although fully pigmented melanocytes only arose in the presence of both Edn3 and Kitl (Ono et al., 1998). Tyrosinase-positive cells can be generated when Ednrb null NC cells are co-cultured with wild typeneural tube explants, implying that wild type NC cells provide an exogenous signal capable of rescuing tyrosinase expression in cells lacking endogenous Ednrb. This cell non-autonomous action of Ednrb signaling was suggested to be mediated via Kit signaling, because in the absence of Ednrb signaling, the presence of Kitl is necessary and sufficient for the generation of tyrosinase-positive cells. This suggests that Ednrb may function to activate tyrosinase production indirectly by inducing the production or secretion of Kitl (Hou et al., 2004).
Another study in which mouse NC cultures were treated with Edn3, Edn1, or Kitl showed an increase in the number of melanocyte progenitors; however, Kitl alone was not sufficient to induce the differentiation of melanocyte progenitors into mature melanocytes. Mature melanocytes were however observed, when treatment with Kitl was followed by Edn3 or Edn1 (Reid et al., 1996). As previously noted, although in the absence of Edn3, Kit-positive and DOPA-positive cells arose in mouse NC cultures, Ednrb signaling was required for the generation of fully pigmented melanocytes (Ono et al., 1998). These findings hint to a specific requirement for Ednrb signaling, independent of Kit signaling, in melanocyte differentiation. This requirement for Ednrb in the final phase of melanocyte differentiation may occur cell-autonomously, as suggested by the inability of Ednrb null cells to generate pigment even in the presence of Kitl (Hou et al., 2004). Together these findings point at a cooperative interaction between Kit and Ednrb signaling in melanocyte development, with Ednrb signaling being specifically required in the final differentiation step.
Endothelins and melanoma
Levels of expression
Cancer progression exhibits many of the characteristics seen during development. Such is the case of melanoma cells, which exhibit high proliferation and motility; attributes that are also present in melanocyte precursors during embryogenesis. Traditionally, the development of melanoma has been viewed as a linear process of abnormal differentiation resulting from the transformation of mature melanocytes (Clark et al., 1984), which gradually acquire mutations that impart them with the ability to self-renew (reviewed in Zabierowski and Herlyn, 2008). The traditional model of melanoma progression has been challenged by the cancer stem cell model, which proposes that the heterogeneity observed in many cancers arises as a result of epigenetic changes in cancer stem cells, analogous to the differentiation of normal stem cells, rather than as a result of progressive mutations. Under this model the majority of cells in a tumor are proposed to be non-tumorigenic as a result of epigenetic changes that lead to the loss of self-renewal capacity (Shackleton et al., 2009). Although the cell of origin in cancer does not need to be a normal stem cell, these cells already posses the capacity to continually self-renew, making it easier for them to accumulate transforming mutations (reviewed in Grichnik, 2008 and Reya et al., 2001). The recent recognition of a heterogeneous population of cells in melanoma and the characterization of a subset of stem cell marker-positive cells (Dou et al., 2007; Fang et al., 2005; Frank et al., 2005; Grichnik et al., 2006; Klein et al., 2007; Monzani et al., 2007; Schatton et al., 2008; Topczewska et al., 2006; Wang et al., 2006) has provided support for the stem cell model in melanoma progression. The stem cell marker-positive cells identified in melanomas were capable of self-renewal and of differentiating into various cell lineages. Furthermore, these cells were more tumorigenic when transplanted into immunocompromised NOD/SCID mice than their stem cell marker-negative counterparts. Together these findings imply that the traditional stepwise model of melanoma may not be sufficient to account for the development and progression of this disease (reviewed in Grichnik, 2008).
In spite of the evidence in favor of the stem cell model, support for the traditional stepwise model has been provided by studies in which differentiated melanocytes were shown to acquire a malignant phenotype when transformed with oncogenes (reviewed in Benjamin et al., 2007; Chudnovsky et al., 2005). Given that endothelin signaling has been implicated in the de-differentiation process of mature melanocytes (Dupin et al., 2000; Real et al., 2005), it may also be involved in this process in transformed cells. The placement of oncogenes under the control of melanocyte specific promoters has led to the development of melanoma in mice (Bradl et al., 1991; Broome-Powell et al., 1999; Chin et al., 1997; Kelsall and Mintz, 1998; Klein-Szanto et al., 1991, 1994; Powell et al., 1995; Wong and Chin, 2000; reviewed in Larue and Beermann, 2007), indicating that it is possible for melanoma to arise from a committed melanocyte precursor (reviewed in Grichnik, 2008). Recently, the finding that melanoma cell fractions transplanted into NOD/SCID IL2Rγnull mice are highly tumorigenic irrespective of whether or not they express the stem cell marker CD133, suggests that melanoma does not appear to follow the cancer stem cell model (Shackleton et al., 2009).
The increase in cell proliferation and inhibition of differentiation that occur during melanomagenesis and progression could be related to the activation of Ednrb which was shown to produce the same effect in cultured melanoblasts (Lahav et al., 1996, 1998; Reid et al., 1996). Although Ednrb downregulation has been reported in some human melanoma cell lines (Eberle et al., 1999), other studies found Ednrb to be upregulated in most melanoma cell lines (Bittner et al., 2000; Ross et al., 2000). Ednrb expression was found to be enhanced in melanoma metastases compared to primary tumors and thus it has been proposed as a marker for melanoma progression (Demunter et al., 2001). In addition to increased Ednrb levels, aberrant Edn3 expression was observed in metastatic melanoma cells in tissue biopsies and cell culture. These cells displayed increased survival in response to Edn3 in vitro (Tang et al., 2008). Interestingly, Ednrb non-synonymous mutations that result in impaired Ednrb function were detected in melanoma patients and it was suggested that the loss of Ednrb function could be associated with increased melanoma risk (Soufir et al., 2005, 2007). In contrast, activating mutations in GNAQ, a stimulatory Gq-protein that acts downstream of Ednrb, have been found in a large proportion of uveal melanoma tumors (Onken et al., 2008; Van Raamsdonk et al., 2009). This discrepancy in Ednrb activity levels in melanoma may be related to the specific type of tumor in question.
Ultraviolet (UV)-dependent effects
Endothelin signaling appears to interact with UV radiation in a complex manner to bring about both melanoma-preventing and melanoma-inducing changes. Edn1 secretion from keratinocytes increases after exposure to UV radiation (Ahn et al., 1998; Imokawa et al., 1992, 1997; Jamal and Schneider, 2002) and it was found to have an antiapoptotic effect on melanocytes (Kadekaro et al., 2005). This increase in Edn1 levels also leads to increased pigmentation (Imokawa et al., 1997) and the reduction of UV-induced DNA damage (Kadekaro et al., 2005) providing a protective effect on skin. On the other hand, in human skin grafted to severe combined immunodeficiency disease mice, the combination of UVB irradiation along with the cutaneous expression of Kitl, Edn3, and bFGF led to the development of severe pigmented lesions, which were histologically found to represent in situ and invasive melanomas. Combining UVB radiation with either factor alone did not lead to the development of melanoma phenotypes, implying that Kitl, bFGF and Edn3 exert synergistic effects on melanocytes. Moreover, in situ melanomas appeared in adult skin grafts, while invasive melanomas developed in newborn skin grafts indicating that the susceptibility of skin to environmental tumor promoters is dependent on age (Berking et al., 2004).
Tumor-host interactions
Edn1 and Edn3 acting through Ednrb activate signaling pathways that lead to the progression of cutaneous melanoma by altering tumor-host interactions. These factors were shown to induce the downregulation of E-cadherin and associated catenin adhesion proteins in melanocytes (Jamal and Schneider, 2002) and melanoma cells (Bagnato et al., 2004; Jamal and Schneider, 2002; Rosano et al., 2004). This event also takes place in melanoma progression and is commonly associated with its invasiveness (Hsu et al., 2000). Edn1 and Edn3 upregulated N-cadherin in human melanoma cell lines (Bagnato et al., 2004), a change that promotes loss of keratinocyte control on melanoma cells and the formation of heterotypic cell-cell interactions (reviewed in Li et al., 2002). Edn1 and Edn3 treatments also resulted in the impairment of intercellular communication through the phosphorylation of connexin 43, a gap junctional protein (Bagnato et al., 2004, Rivedal and Opsahl, 2001). Other Ednrb-mediated effects were the activation of the focal adhesion kinase and MAPK pathways, and the secretion and activation of matrix metalloproteinases (MMPs), which degrade the extracellular matrix, an event that is critical during tumor invasion. Additionally, Edn1 and Edn3 increased the expression of α2β1 and αvβ3 integrins (Bagnato et al., 2004), which play a major role in melanoma progression (Eguchi and Horikoshi, 1996; Natali et al., 1993, 1997). Edn1 was also shown to induce the secretion of the CXC chemokines CXCL1 and CXCL8, which are critical for melanoma metastasis (Mangahas et al., 2005). In A375 melanoma cells, Edn3 upregulated the expression of osteopontin (Chang-zheng et al., 2008), a glycophosphoprotein that acts downstream of PI3K in melanoma and whose expression is inversely correlated to that of the tumor suppressor PTEN (Packer et al., 2006). Osteopontin was found to induce melanoma cell migration in vitro and to promote lung and bone metastases of melanoma cells injected into nude mice (reviewed in Bellahcene et al., 2008).
In primary and metastatic melanoma cell lines, Edn1 and Edn3 were found to upregulate vascular endothelial growth factor (VEGF) via Ednrb-induced upregulation of the transcription factor HIF-1α (Spinella et al., 2007). HIF-1α plays a major role in the adaptation of cells to hypoxic conditions, which may arise in the tumor microenvironment, and is associated with increased VEGF expression and thus angiogenesis. High VEGF levels are in turn associated with poor prognosis in human melanoma tumors (Giatromanolaki et al., 2003). The endothelin pathway also acts with HIF-1α in stimulating cyclooxygenase (COX-1/COX-2) protein expression, COX-2 transcriptional activity, prostaglandin E2 (PGE2) production, and metalloproteinase (MMP) activity (Spinella et al., 2007). The COX-1 and COX-2 enzymes are involved in prostaglandin synthesis and have been implicated in tumor progression in several solid tumors (reviewed in Jeon and Song, 2006; Zha et al., 2004). Inhibitors against these enzymes can block PGE2 and VEGF secretion, MMP activation and cell invasion induced by Edn3 (Spinella et al., 2007).
Therapeutic value of Ednrb antagonists
Ednrb small interfering RNA (siRNA) and the Ednrb antagonist BQ788 are able to block endothelin-induced effects that can lead to melanoma progression (Spinella et al., 2007). Other studies using the same antagonist have reported an inhibition of melanoma growth (Baganato et al., 2004; Lahav et al., 1999, 2004,). BQ788 was found to inhibit the growth of seven melanoma cell lines, causing an increase in pigmentation and dendritic shape, which characterizes mature melanocytes. This inhibitor was also capable of increasing cell death in culture and reducing human melanoma tumor growth in nude mice (Lahav et al., 1999). A subsequent study found that BQ788 is most effective at inducing apoptosis in metastatic melanoma cells (Lahav et al., 2004). Another Ednrb antagonist, the small molecule A-192621, was also found to significantly inhibit melanoma cell growth in nude mice (Bagnato et al., 2004; Rosano et al., 2004).
Recent studies using the Ednrb antagonist bonsentan suggest that the use of Ednrb antagonists may prove useful for the treatment of melanoma. In human melanoma cell lines, bonsentan treatment induced apoptosis and resulted in decreased viability and DNA synthesis. Furthermore, the combination of bosentan and alkylating agents, which are commonly used in melanoma treatment, had an additive effect in promoting apoptosis in melanoma cells (Berger et al., 2006). Bosentan has also been tested in a phase II study as a single therapeutic agent for stage IV metastatic melanoma. Disease stabilization without unanticipated adverse effects, was observed in 6 of 35 patients at 12 weeks of treatment and in 5 of 35 patients at 24 weeks suggesting that Ednrb antagonists may prove useful in metastatic disease stabilization (Kefford et al., 2007) particularly when used in combination with other anticancer drugs (Berger et al., 2006; Kefford et al., 2007,).
Perspectives
The endothelin signaling pathway is involved in various aspects of melanocyte biology, from the very early developmental stages to the final differentiation steps (Fig. 2), as well as during transformation and progression of malignant state (Fig. 3). Some of the effects the Edns have on melanocytes appear antagonistic such as the maintenance of undifferentiated state in early precursors and the promotion of the final steps of differentiation by the regulation of tyrosinase expression and activity. This also applies within the melanoma context where Edn1, released by keratinocytes in response to UV, increases melanization and decreases DNA damage, thus, exerting a protective effect, while Edn1 and Edn3 cause changes in the tumor environment that contribute to malignancy.
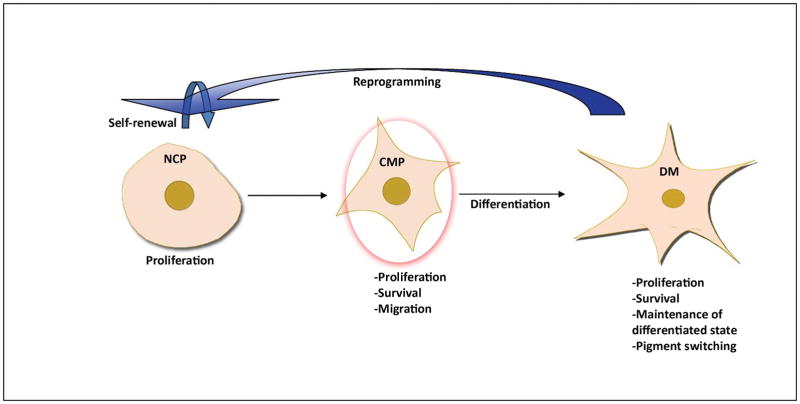
Figure 2.
Edn3/Ednrb affect melanocytes at all developmental stages. Red circle indicates the absolute time requirement for the generation of a normal pigmentation phenotype. NCP: neural crest progenitor (multipotent or glia/melanocyte bipotent cell), CMP: committed melanocyte precursor, DM: differentiated melanocyte.
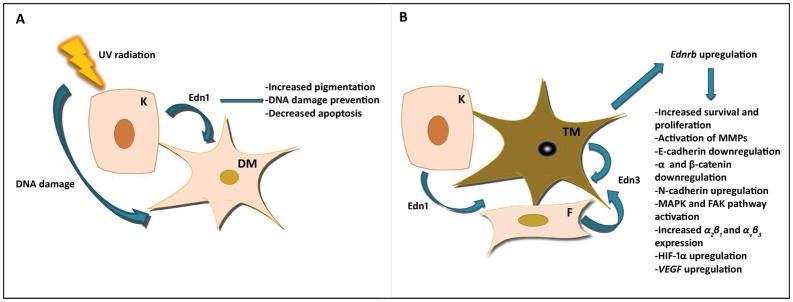
Figure 3.
Edns/Ednrb affect melanocytes after UV exposure and transformation. A. Effect of UV-induced Edn1 secretion on melanocytes. B. Effects of Edn1 and Edn3 on transformed melanocytes. K: keratinocyte, F: fibroblast, DM: differentiated melanocyte, TM: transformed melanocyte.
The development of melanocytes is affected by Ednrb signaling via Gq/G11 and their hypermorphic alleles result in hyperpigmentation of the skin in mice (Van Raamsdonk et al., 2004). Interestingly, NC-specific deletions of these G-proteins or G12/G13 do not generate pigmentation phenotypes in mice (Dettlaff-Swiercz et al., 2005), suggesting that Ednrb signaling in melanocytes may involve other G-proteins. The downstream events triggered by the activation of the G-proteins upon binding of Edn1 or Edn3 to Ednrb in melanocytes have been identified and these lead to the regulation of Mitf both at the transcriptional and translational levels (Sato-Jin et al., 2008). Although some of the many effects Edns have on melanoblasts, melanocytes, and melanoma cells may be mediated by the activation of Mitf, other downstream effectors might be involved and should be investigated. For example, the mechanisms employed by the endothelin pathway in the regulation of melanoblast migration and melanoma invasion and metastasis are not clear and, most likely, are not Mitf-dependent. The interactions of the Ednrb pathway with other pathways that participate in melanocyte development and transformation are also critical for defining the cellular outcomes at particular times. As discussed, much has already been shown about the requirement for a cooperative interaction between the Edn3 and Kitl pathways, but its association with others, such as the Wnt pathway in the early events of melanoblast commitment (Dunn et al., 2000), the ephrin pathway during migration (Harris et al., 2008), or the α-MSH pathway later in differentiation in the control of tyrosinase levels (Garcia et al., 2008) needs to be further explored.
The capacity of Edn3 to maintain the multipotentiality and self-renewal of avian NC precursors has not been demonstrated in mammalian cells. Since these are critical properties for any factor being considered as a potential agent for therapeutic purposes in regenerative medicine, they should be researched in mammalian cells. This should, however, be done in the light that Edn3 along with other factors has been shown to differentiate mammalian embryonic stem cells preferentially into melanocytes (Aoki et al., 2005; Fang et al., 2006).
Little is known about the mechanisms underlying the regulation of Ednrb expression in melanocytes. This is especially relevant given the upregulation of Ednrb throughout melanoma progression. During the development of murine enteric neurons, Sox10 regulates Ednrb by binding to a specific enhancer that does not appear to be critical for melanocytes (Zhu et al., 2004). Genetic studies have also suggested that Sox10 and Ednrb do not interact during murine melanocyte development (Hakami et al., 2006). However, in human melanocyte and melanoma cell lines, SOX10, alone or in combination with the transcription factor Sp1, was shown to transactivate the EDNRB promoter through three cis-acting elements (Yokoyama et al., 2006). In human melanocytes, EDNRB also seems to be regulated by MITF in a feedback pathway manner (McGill et al., 2002; Sato-Jin et al., 2008). A clarification of whether the putative activation of Ednrb by Sox10 in melanocytes is temporal and/or species-specific is needed as well as the identification of other transcription factors that may be involved in this process. Recently, hypoxia response element sites, that are required for the binding of HIF-1α, were described in the proximal promoter of the rat Ednrb gene (Yeligar et al., 2009). A reciprocal relationship between Edns, the expression level of endothelin receptors, and HIF-1α activity has been demonstrated in various cancers (reviewed in Grimshaw, 2007) and should be more thoroughly investigated during melanoma progression. The presence of other regulatory elements upstream of Ednrb remains to be explored. This information will be critical for elucidating the mechanism of Ednrb upregulation during melanoma progression and thus for the development of new molecular targets for intervention in the treatment of this disease.
Acknowledgments
The authors acknowledge funding from NIH/NIGMS R25 GM061347 (ACS) and 5 NIH/SC2 CA138175-02 (LK).
References
- Ahn GY, Butt KI, Jindo T, Yaguchi H, Tsuboi R, Ogawa H. The expression of endothelin-1 and its binding sites in mouse skin increased after ultraviolet B irradiation or local injection of tumor necrosis factor alpha. J Dermatol. 1998;25:78–84. doi: 10.1111/j.1346-8138.1998.tb02354.x. [DOI] [PubMed] [Google Scholar]
- Aoki H, Motohashi T, Yoshimura N, Yamazaki H, Yamane T, Panthier JJ, Kunisada T. Cooperative and indispensable roles of endothelin 3 and KIT signalings in melanocyte development. Dev Dyn. 2005;233:407–17. doi: 10.1002/dvdy.20340. [DOI] [PubMed] [Google Scholar]
- Aoki H, Yamada Y, Hara A, Kunisada T. Two distinct types of mouse melanocyte: differential signaling requirement for the maintenance of non-cutaneous and dermal versus epidermal melanocytes. Development. 2009;136:2511–21. doi: 10.1242/dev.037168. [DOI] [PubMed] [Google Scholar]
- Bagnato A, Rosano L, Spinella F, Di Castro V, Tecce R, Natali PG. Endothelin B receptor blockade inhibits dynamics of cell interactions and communications in melanoma cell progression. Cancer Res. 2004;64:1436–43. doi: 10.1158/0008-5472.can-03-2344. [DOI] [PubMed] [Google Scholar]
- Baynash AG, Hosoda K, Giaid A, Richardson JA, Emoto N, Hammer RE, Yanagisawa M. Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell. 1994;79:1277–85. doi: 10.1016/0092-8674(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bellahcene A, Castronovo V, Ogbureke KU, Fisher LW, Fedarko NS. Small integrin-binding ligand N-linked glycoproteins (SIBLINGs): multifunctional proteins in cancer. Nat Rev Cancer. 2008;8:212–26. doi: 10.1038/nrc2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin CL, Melnikova VO, Ananthaswamy HN. Models and mechanisms in malignant melanoma. Mol Carcinog. 2007;46:671–8. doi: 10.1002/mc.20353. [DOI] [PubMed] [Google Scholar]
- Berger Y, Bernasconi CC, Juillerat-Jeanneret L. Targeting the endothelin axis in human melanoma: combination of endothelin receptor antagonism and alkylating agents. Exp Biol Med (Maywood) 2006;231:1111–9. [PubMed] [Google Scholar]
- Berking C, Takemoto R, Satyamoorthy K, Shirakawa T, Eskandarpour M, Hansson J, Vanbelle PA, Elder DE, Herlyn M. Induction of melanoma phenotypes in human skin by growth factors and ultraviolet B. Cancer Res. 2004;64:807–11. doi: 10.1158/0008-5472.can-03-3438. [DOI] [PubMed] [Google Scholar]
- Bittner M, Meltzer P, Chen Y, Jiang Y, Seftor E, Hendrix M, Radmacher M, Simon R, Yakhini Z, Ben-Dor A, et al. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000;406:536–40. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]
- Bohm M, Moellmann G, Cheng E, Alvarez-Franco M, Wagner S, Sassone-Corsi P, Halaban R. Identification of p90RSK as the probable CREB-Ser133 kinase in human melanocytes. Cell Growth Differ. 1995;6:291–302. [PubMed] [Google Scholar]
- Bouallegue A, Daou GB, Srivastava AK. Endothelin-1-induced signaling pathways in vascular smooth muscle cells. Curr Vasc Pharmacol. 2007;5:45–52. doi: 10.2174/157016107779317161. [DOI] [PubMed] [Google Scholar]
- Bradl M, Klein-Szanto A, Porter S, Mintz B. Malignant melanoma in transgenic mice. Proc Natl Acad Sci USA. 1991;88:164–8. doi: 10.1073/pnas.88.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome Powell M, Gause PR, Hyman P, Gregus J, Lluria-Prevatt M, Nagle R, Bowden GT. Induction of melanoma in TPras transgenic mice. Carcinogenesis. 1999;20:1747–53. doi: 10.1093/carcin/20.9.1747. [DOI] [PubMed] [Google Scholar]
- Chang-Zheng H, Jin T, Juan T, Ye-Qiang L, Yan L, Ling-Yun Y, Jing Z, Yan-Qiu L, Si-Yuan C, Neng-Xing L, et al. Endothelin signaling axis activates osteopontin expression through PI3 kinase pathway in A375 melanoma cells. J Dermatol Sci. 2008;52:130–2. doi: 10.1016/j.jdermsci.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Chin L, Pomerantz J, Polsky D, Jacobson M, Cohen C, Cordon-Cardo C, Horner JW, 2nd, Depinho RA. Cooperative effects of INK4a and ras in melanoma susceptibility in vivo. Genes Dev. 1997;11:2822–34. doi: 10.1101/gad.11.21.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudnovsky Y, Khavari PA, Adams AE. Melanoma genetics and the development of rational therapeutics. J Clin Invest. 2005;115:813–24. doi: 10.1172/JCI24808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark WH, Jr, Elder DE, Guerry DT, Epstein MN, Greene MH, Van Horn M. A study of tumor progression: the precursor lesions of superficial spreading and nodular melanoma. Hum Pathol. 1984;15:1147–65. doi: 10.1016/s0046-8177(84)80310-x. [DOI] [PubMed] [Google Scholar]
- Demunter A, De Wolf-Peeters C, Degreef H, Stas M, Van Den Oord JJ. Expression of the endothelin-B receptor in pigment cell lesions of the skin. Evidence for its role as tumor progression marker in malignant melanoma. Virchows Arch. 2001;438:485–91. doi: 10.1007/s004280000362. [DOI] [PubMed] [Google Scholar]
- Dettlaff-Swiercz DA, Wettschureck N, Moers A, Huber K, Offermanns S. Characteristic defects in neural crest cell-specific Galphaq/Galpha11- and Galpha12/Galpha13-deficient mice. Dev Biol. 2005;282:174–82. doi: 10.1016/j.ydbio.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Dou J, Pan M, Wen P, Li Y, Tang Q, Chu L, Zhao F, Jiang C, Hu W, Hu K, et al. Isolation and identification of cancer stem-like cells from murine melanoma cell lines. Cell Mol Immunol. 2007;4:467–72. [PubMed] [Google Scholar]
- Dunn KJ, Williams BO, Li Y, Pavan WJ. Neural crest-directed gene transfer demonstrates Wnt1 role in melanocyte expansion and differentiation during mouse development. Proc Natl Acad Sci USA. 2000;97:10050–5. doi: 10.1073/pnas.97.18.10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupin E, Real C, Glavieux-Pardanaud C, Vaigot P, Le Douarin NM. Reversal of developmental restrictions in neural crest lineages: transition from Schwann cells to glial-melanocytic precursors in vitro. Proc Natl Acad Sci USA. 2003;100:5229–33. doi: 10.1073/pnas.0831229100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupin E, Glavieux C, Vaigot P, Le Douarin NM. Endothelin 3 induces the reversion of melanocytes to glia through a neural crest-derived glial-melanocytic progenitor. Proc Natl Acad Sci USA. 2000;97:7882–7. doi: 10.1073/pnas.97.14.7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle J, Fecker LF, Orfanos CE, Geilen CC. Endothelin-1 decreases basic apoptotic rates in human melanoma cell lines. J Invest Dermatol. 2002;119:549–55. doi: 10.1046/j.1523-1747.2002.01848.x. [DOI] [PubMed] [Google Scholar]
- Eberle J, Weitmann S, Thieck O, Pech H, Paul M, Orfanos CE. Downregulation of endothelin B receptor in human melanoma cell lines parallel to differentiation genes. J Invest Dermatol. 1999;112:925–32. doi: 10.1046/j.1523-1747.1999.00598.x. [DOI] [PubMed] [Google Scholar]
- Eguchi H, Horikoshi T. The expression of integrin alpha 2 beta 1 and attachment to type I collagen of melanoma cells are preferentially induced by tumour promoter, TPA (12-O-tetradecanoyl phorbol-13-acetate) Br J Dermatol. 1996;134:33–9. [PubMed] [Google Scholar]
- Emoto N, Yanagisawa M. Endothelin-converting enzyme-2 is a membrane-bound, phosphoramidon-sensitive metalloprotease with acidic pH optimum. J Biol Chem. 1995;270:15262–8. doi: 10.1074/jbc.270.25.15262. [DOI] [PubMed] [Google Scholar]
- Fang D, Leishear K, Nguyen TK, Finko R, Cai K, Fukunaga M, Li L, Brafford PA, Kulp AN, Xu X, et al. Defining the conditions for the generation of melanocytes from human embryonic stem cells. Stem Cells. 2006;24:1668–77. doi: 10.1634/stemcells.2005-0414. [DOI] [PubMed] [Google Scholar]
- Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE, Herlyn M. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328–37. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- Frank NY, Margaryan A, Huang Y, Schatton T, Waaga-Gasser AM, Gasser M, Sayegh MH, Sadee W, Frank MH. ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res. 2005;65:4320–33. doi: 10.1158/0008-5472.CAN-04-3327. [DOI] [PubMed] [Google Scholar]
- Garcia RJ, Ittah A, Mirabal S, Figueroa J, Lopez L, Glick AB, Kos L. Endothelin 3 induces skin pigmentation in a keratin-driven inducible mouse model. J Invest Dermatol. 2008;128:131–42. doi: 10.1038/sj.jid.5700948. [DOI] [PubMed] [Google Scholar]
- Giatromanolaki A, Sivridis E, Kouskoukis C, Gatter KC, Harris AL, Koukourakis MI. Hypoxia-inducible factors 1alpha and 2alpha are related to vascular endothelial growth factor expression and a poorer prognosis in nodular malignant melanomas of the skin. Melanoma Res. 2003;13:493–501. doi: 10.1097/00008390-200310000-00008. [DOI] [PubMed] [Google Scholar]
- Grichnik JM, Burch JA, Schulteis RD, Shan S, Liu J, Darrow TL, Vervaert CE, Seigler HF. Melanoma, a tumor based on a mutant stem cell? J Invest Dermatol. 2006;126:142–53. doi: 10.1038/sj.jid.5700017. [DOI] [PubMed] [Google Scholar]
- Grichnik JM. Melanoma, nevogenesis, and stem cell biology. J Invest Dermatol. 2008;128:2365–80. doi: 10.1038/jid.2008.166. [DOI] [PubMed] [Google Scholar]
- Grimshaw MJ. Endothelins and hypoxia-inducible factor in cancer. Endocr Relat Cancer. 2007;14:233–44. doi: 10.1677/ERC-07-0057. [DOI] [PubMed] [Google Scholar]
- Hakami RM, Hou L, Baxter LL, Loftus SK, Southard-Smith EM, Incao A, Cheng J, Pavan WJ. Genetic evidence does not support direct regulation of EDNRB by SOX10 in migratory neural crest and the melanocyte lineage. Mech Dev. 2006;123:124–34. doi: 10.1016/j.mod.2005.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris ML, Hall R, Erickson CA. Directing pathfinding along the dorsolateral path - the role of EDNRB2 and EphB2 in overcoming inhibition. Development. 2008;135:4113–22. doi: 10.1242/dev.023119. [DOI] [PubMed] [Google Scholar]
- Hosoda K, Hammer RE, Richardson JA, Baynash AG, Cheung JC, Giaid A, Yanagisawa M. Targeted and natural (piebald-lethal) mutations of endothelin-B receptor gene produce megacolon associated with spotted coat color in mice. Cell. 1994;79:1267–76. doi: 10.1016/0092-8674(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Hou L, Pavan WJ, Shin MK, Arnheiter H. Cell-autonomous and cell non-autonomous signaling through endothelin receptor B during melanocyte development. Development. 2004;131:3239–47. doi: 10.1242/dev.01193. [DOI] [PubMed] [Google Scholar]
- Hsu MY, Meier FE, Nesbit M, Hsu JY, Van Belle P, Elder DE, Herlyn M. E-cadherin expression in melanoma cells restores keratinocyte-mediated growth control and down-regulates expression of invasion-related adhesion receptors. Am J Pathol. 2000;156:1515–25. doi: 10.1016/S0002-9440(10)65023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imokawa G, Kobayashi T, Miyagishi M, Higashi K, Yada Y. The role of endothelin-1 in epidermal hyperpigmentation and signaling mechanisms of mitogenesis and melanogenesis. Pigment Cell Res. 1997;10:218–28. doi: 10.1111/j.1600-0749.1997.tb00488.x. [DOI] [PubMed] [Google Scholar]
- Imokawa G, Yada Y, Kimura M. Signalling mechanisms of endothelin-induced mitogenesis and melanogenesis in human melanocytes. Biochem J. 1996;314(Pt 1):305–12. doi: 10.1042/bj3140305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imokawa G, Miyagishi M, Yada Y. Endothelin-1 as a new melanogen: coordinated expression of its gene and the tyrosinase gene in UVB-exposed human epidermis. J Invest Dermatol. 1995;105:32–7. doi: 10.1111/1523-1747.ep12312500. [DOI] [PubMed] [Google Scholar]
- Imokawa G, Yada Y, Miyagishi M. Endothelins secreted from human keratinocytes are intrinsic mitogens for human melanocytes. J Biol Chem. 1992;267:24675–80. [PubMed] [Google Scholar]
- Jamal S, Schneider RJ. UV-induction of keratinocyte endothelin-1 downregulates E-cadherin in melanocytes and melanoma cells. J Clin Invest. 2002;110:443–52. doi: 10.1172/JCI13729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon YT, Song YS. Cyclooxygenases in cancer: chemoprevention and sensitization to conventional therapies. Mini Rev Med Chem. 2006;6:827–33. doi: 10.2174/138955706777698660. [DOI] [PubMed] [Google Scholar]
- Kadekaro AL, Kavanagh R, Kanto H, Terzieva S, Hauser J, Kobayashi N, Schwemberger S, Cornelius J, Babcock G, Shertzer HG, et al. alpha-Melanocortin and endothelin-1 activate antiapoptotic pathways and reduce DNA damage in human melanocytes. Cancer Res. 2005;65:4292–9. doi: 10.1158/0008-5472.CAN-04-4535. [DOI] [PubMed] [Google Scholar]
- Kadono S, Manaka I, Kawashima M, Kobayashi T, Imokawa G. The role of the epidermal endothelin cascade in the hyperpigmentation mechanism of lentigo senilis. J Invest Dermatol. 2001;116:571–7. doi: 10.1046/j.1523-1747.2001.01296.x. [DOI] [PubMed] [Google Scholar]
- Karne S, Jayawickreme CK, Lerner MR. Cloning and characterization of an endothelin-3 specific receptor (ETC receptor) from Xenopus laevis dermal melanophores. J Biol Chem. 1993;268:19126–33. [PubMed] [Google Scholar]
- Kedzierski RM, Yanagisawa M. Endothelin system: the double-edged sword in health and disease. Annu Rev Pharmacol Toxicol. 2001;41:851–76. doi: 10.1146/annurev.pharmtox.41.1.851. [DOI] [PubMed] [Google Scholar]
- Kefford R, Beith JM, Van Hazel GA, Millward M, Trotter JM, Wyld DK, Kusic R, Shreeniwas R, Morganti A, Ballmer A, et al. A phase II study of bosentan, a dual endothelin receptor antagonist, as monotherapy in patients with stage IV metastatic melanoma. Invest New Drugs. 2007;25:247–52. doi: 10.1007/s10637-006-9014-7. [DOI] [PubMed] [Google Scholar]
- Kelsall SR, Mintz B. Metastatic cutaneous melanoma promoted by ultraviolet radiation in mice with transgene-initiated low melanoma susceptibility. Cancer Res. 1998;58:4061–5. [PubMed] [Google Scholar]
- Klein WM, Wu BP, Zhao S, Wu H, Klein-Szanto AJ, Tahan SR. Increased expression of stem cell markers in malignant melanoma. Mod Pathol. 2007;20:102–7. doi: 10.1038/modpathol.3800720. [DOI] [PubMed] [Google Scholar]
- Klein-Szanto AJ, Silvers WK, Mintz B. Ultraviolet radiation-induced malignant skin melanoma in melanoma-susceptible transgenic mice. Cancer Res. 1994;54:4569–72. [PubMed] [Google Scholar]
- Klein-Szanto A, Bradl M, Porter S, Mintz B. Melanosis and associated tumors in transgenic mice. Proc Natl Acad Sci USA. 1991;88:169–73. doi: 10.1073/pnas.88.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahav R, Suva ML, Rimoldi D, Patterson PH, Stamenkovic I. Endothelin receptor B inhibition triggers apoptosis and enhances angiogenesis in melanomas. Cancer Res. 2004;64:8945–53. doi: 10.1158/0008-5472.CAN-04-1510. [DOI] [PubMed] [Google Scholar]
- Lahav R, Heffner G, Patterson PH. An endothelin receptor B antagonist inhibits growth and induces cell death in human melanoma cells in vitro and in vivo. Proc Natl Acad Sci USA. 1999;96:11496–500. doi: 10.1073/pnas.96.20.11496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahav R, Dupin E, Lecoin L, Glavieux C, Champeval D, Ziller C, Le Douarin NM. Endothelin 3 selectively promotes survival and proliferation of neural crest-derived glial and melanocytic precursors in vitro. Proc Natl Acad Sci USA. 1998;95:14214–9. doi: 10.1073/pnas.95.24.14214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahav R, Ziller C, Dupin E, Le Douarin NM. Endothelin 3 promotes neural crest cell proliferation and mediates a vast increase in melanocyte number in culture. Proc Natl Acad Sci USA. 1996;93:3892–7. doi: 10.1073/pnas.93.9.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larue L, Beermann F. Cutaneous melanoma in genetically modified animals. Pigment Cell Res. 2007;20:485–97. doi: 10.1111/j.1600-0749.2007.00411.x. [DOI] [PubMed] [Google Scholar]
- Lecoin L, Sakurai T, Ngo MT, Abe Y, Yanagisawa M, Le Douarin NM. Cloning and characterization of a novel endothelin receptor subtype in the avian class. Proc Natl Acad Sci USA. 1998;95:3024–9. doi: 10.1073/pnas.95.6.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HO, Levorse JM, Shin MK. The endothelin receptor-B is required for the migration of neural crest-derived melanocyte and enteric neuron precursors. Dev Biol. 2003;259:162–75. doi: 10.1016/s0012-1606(03)00160-x. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Wall B, Chen S. G-protein-coupled receptors and melanoma. Pigment Cell Melanoma Res. 2008;21:415–28. doi: 10.1111/j.1755-148X.2008.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Satyamoorthy K, Herlyn M. Dynamics of cell interactions and communications during melanoma development. Crit Rev Oral Biol Med. 2002;13:62–70. doi: 10.1177/154411130201300107. [DOI] [PubMed] [Google Scholar]
- Manaka L, Kadono S, Kawashima M, Kobayashi T, Imokawa G. The mechanism of hyperpigmentation in seborrhoeic keratosis involves the high expression of endothelin-converting enzyme-1alpha and TNF-alpha, which stimulate secretion of endothelin 1. Br J Dermatol. 2001;145:895–903. doi: 10.1046/j.1365-2133.2001.04521.x. [DOI] [PubMed] [Google Scholar]
- Mangahas CR, Dela Cruz GV, Friedman-Jimenez G, Jamal S. Endothelin-1 induces CXCL1 and CXCL8 secretion in human melanoma cells. J Invest Dermatol. 2005;125:307–11. doi: 10.1111/j.0022-202X.2005.23820.x. [DOI] [PubMed] [Google Scholar]
- Mccallion AS, Chakravarti A. EDNRB/EDN3 and Hirschsprung disease type II. Pigment Cell Res. 2001;14:161–9. doi: 10.1034/j.1600-0749.2001.140305.x. [DOI] [PubMed] [Google Scholar]
- Mcgill GG, Horstmann M, Widlund HR, Du J, Motyckova G, Nishimura EK, Lin YL, Ramaswamy S, Avery W, Ding HF, et al. Bcl2 regulation by the melanocyte master regulator Mitf modulates lineage survival and melanoma cell viability. Cell. 2002;109:707–18. doi: 10.1016/s0092-8674(02)00762-6. [DOI] [PubMed] [Google Scholar]
- Monzani E, Facchetti F, Galmozzi E, Corsini E, Benetti A, Cavazzin C, Gritti A, Piccinini A, Porro D, Santinami M, et al. Melanoma contains CD133 and ABCG2 positive cells with enhanced tumourigenic potential. Eur J Cancer. 2007;43:935–46. doi: 10.1016/j.ejca.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Motohashi T, Aoki H, Yoshimura N, Kunisada T. Induction of melanocytes from embryonic stem cells and their therapeutic potential. Pigment Cell Res. 2006;19:284–9. doi: 10.1111/j.1600-0749.2006.00317.x. [DOI] [PubMed] [Google Scholar]
- Natali PG, Hamby CV, Felding-Habermann B, Liang B, Nicotra MR, Di Filippo F, Giannarelli D, Temponi M, Ferrone S. Clinical significance of alpha(v)beta3 integrin and intercellular adhesion molecule-1 expression in cutaneous malignant melanoma lesions. Cancer Res. 1997;57:1554–60. [PubMed] [Google Scholar]
- Natali PG, Nicotra MR, Bartolazzi A, Cavaliere R, Bigotti A. Integrin expression in cutaneous malignant melanoma: association of the alpha 3/beta 1 heterodimer with tumor progression. Int J Cancer. 1993;54:68–72. doi: 10.1002/ijc.2910540112. [DOI] [PubMed] [Google Scholar]
- Okazaki M, Yoshimura K, Uchida G, Suzuki Y, Kitano Y, Harii K. Epidermal hyperpigmentation in non-syndromic solitary cafe-au-lait macules may be associated with increased secretion of endothelin-1 by lesional keratinocytes. Scand J Plast Reconstr Surg Hand Surg. 2005;39:213–7. doi: 10.1080/02844310510006303. [DOI] [PubMed] [Google Scholar]
- Onken MD, Worley LA, Long MD, Duan S, Council ML, Bowcock AM, Harbour JW. Oncogenic mutations in GNAQ occur early in uveal melanoma. Invest Ophthalmol Vis Sci. 2008;49:5230–4. doi: 10.1167/iovs.08-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono H, Kawa Y, Asano M, Ito M, Takano A, Kubota Y, Matsumoto J, Mizoguchi M. Development of melanocyte progenitors in murine Steel mutant neural crest explants cultured with stem cell factor, endothelin-3, or TPA. Pigment Cell Res. 1998;11:291–8. doi: 10.1111/j.1600-0749.1998.tb00738.x. [DOI] [PubMed] [Google Scholar]
- Opdecamp K, Kos L, Arnheiter H, Pavan WJ. Endothelin signalling in the development of neural crest-derived melanocytes. Biochem Cell Biol. 1998;76:1093–9. [PubMed] [Google Scholar]
- Packer L, Pavey S, Parker A, Stark M, Johansson P, Clarke B, Pollock P, Ringner M, Hayward N. Osteopontin is a downstream effector of the PI3-kinase pathway in melanomas that is inversely correlated with functional PTEN. Carcinogenesis. 2006;27:1778–86. doi: 10.1093/carcin/bgl016. [DOI] [PubMed] [Google Scholar]
- Pavan WJ, Tilghman SM. Piebald lethal (sl) acts early to disrupt the development of neural crest-derived melanocytes. Proc Natl Acad Sci USA. 1994;91:7159–63. doi: 10.1073/pnas.91.15.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pla P, Larue L. Involvement of endothelin receptors in normal and pathological development of neural crest cells. Int J Dev Biol. 2003;47:315–25. [PubMed] [Google Scholar]
- Pla P, Alberti C, Solov’eva O, Pasdar M, Kunisada T, Larue L. Ednrb2 orients cell migration towards the dorsolateral neural crest pathway and promotes melanocyte differentiation. Pigment Cell Res. 2005;18:181–7. doi: 10.1111/j.1600-0749.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- Powell MB, Hyman P, Bell OD, Balmain A, Brown K, Alberts D, Bowden GT. Hyperpigmentation and melanocytic hyperplasia in transgenic mice expressing the human T24 Ha-ras gene regulated by a mouse tyrosinase promoter. Mol Carcinog. 1995;12:82–90. doi: 10.1002/mc.2940120205. [DOI] [PubMed] [Google Scholar]
- Real C, Glavieux-Pardanaud C, Le Douarin NM, Dupin E. Clonally cultured differentiated pigment cells can dedifferentiate and generate multipotent progenitors with self-renewing potential. Dev Biol. 2006;300:656–69. doi: 10.1016/j.ydbio.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Reid K, Turnley AM, Maxwell GD, Kurihara Y, Kurihara H, Bartlett PF, Murphy M. Multiple roles for endothelin in melanocyte development: regulation of progenitor number and stimulation of differentiation. Development. 1996;122:3911–9. doi: 10.1242/dev.122.12.3911. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Rivedal E, Opsahl H. Role of PKC and MAP kinase in EGF- and TPA-induced connexin43 phosphorylation and inhibition of gap junction intercellular communication in rat liver epithelial cells. Carcinogenesis. 2001;22:1543–50. doi: 10.1093/carcin/22.9.1543. [DOI] [PubMed] [Google Scholar]
- Rizvi TA, Huang Y, Sidani A, Atit R, Largaespada DA, Boissy RE, Ratner N. A novel cytokine pathway suppresses glial cell melanogenesis after injury to adult nerve. J Neurosci. 2002;22:9831–40. doi: 10.1523/JNEUROSCI.22-22-09831.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano L, Spinella F, Genovesi G, Di Castro V, Natali PG, Bagnato A. Endothelin-B receptor blockade inhibits molecular effectors of melanoma cell progression. J Cardiovasc Pharmacol. 2004;44(Suppl 1):S136–9. doi: 10.1097/01.fjc.0000166247.35992.dd. [DOI] [PubMed] [Google Scholar]
- Ross DT, Scherf U, Eisen MB, Perou CM, Rees C, Spellman P, Iyer V, Jeffrey SS, Van De Rijn M, Waltham M, et al. Systematic variation in gene expression patterns in human cancer cell lines. Nat Genet. 2000;24:227–35. doi: 10.1038/73432. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Yanagisawa M, Masaki T. Molecular characterization of endothelin receptors. Trends Pharmacol Sci. 1992;13:103–8. doi: 10.1016/0165-6147(92)90038-8. [DOI] [PubMed] [Google Scholar]
- Sato-Jin K, Nishimura EK, Akasaka E, Huber W, Nakano H, Miller A, Du J, Wu M, Hanada K, Sawamura D, et al. Epistatic connections between microphthalmia-associated transcription factor and endothelin signaling in Waardenburg syndrome and other pigmentary disorders. FASEB J. 2008;22:1155–68. doi: 10.1096/fj.07-9080com. [DOI] [PubMed] [Google Scholar]
- Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM, Weishaupt C, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–9. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138:822–9. doi: 10.1016/j.cell.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Shin MK, Levorse JM, Ingram RS, Tilghman SM. The temporal requirement for endothelin receptor-B signalling during neural crest development. Nature. 1999;402:496–501. doi: 10.1038/990040. [DOI] [PubMed] [Google Scholar]
- Soufir N, Ollivaud L, Bertrand G, Lacapere JJ, Descamps V, Vitoux D, Lebbe C, Wolkenstein P, Dupin N, Saiag P, et al. A French CDK4-positive melanoma family with a co-inherited EDNRB mutation. J Dermatol Sci. 2007;46:61–4. doi: 10.1016/j.jdermsci.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Soufir N, Meziani R, Lacapere JJ, Bertrand G, Fumeron F, Bourillon A, Gerard B, Descamps V, Crickx B, Ollivaud L, et al. Association between endothelin receptor B nonsynonymous variants and melanoma risk. J Natl Cancer Inst. 2005;97:1297–301. doi: 10.1093/jnci/dji253. [DOI] [PubMed] [Google Scholar]
- Spinella F, Rosano L, Di Castro V, Decandia S, Nicotra MR, Natali PG, Bagnato A. Endothelin-1 and endothelin-3 promote invasive behavior via hypoxia-inducible factor-1alpha in human melanoma cells. Cancer Res. 2007;67:1725–34. doi: 10.1158/0008-5472.CAN-06-2606. [DOI] [PubMed] [Google Scholar]
- Steel KP, Davidson DR, Jackson IJ. TRP-2/DT, a new early melanoblast marker, shows that steel growth factor (c-kit ligand) is a survival factor. Development. 1992;115:1111–9. doi: 10.1242/dev.115.4.1111. [DOI] [PubMed] [Google Scholar]
- Sugden PH, Clerk A. Endothelin signalling in the cardiac myocyte and its pathophysiological relevance. Curr Vasc Pharmacol. 2005;3:343–51. doi: 10.2174/157016105774329390. [DOI] [PubMed] [Google Scholar]
- Tang L, Su M, Zhang Y, Ip W, Martinka M, Huang C, Zhou Y. Endothelin-3 is produced by metastatic melanoma cells and promotes melanoma cell survival. J Cutan Med Surg. 2008;12:64–70. doi: 10.2310/7750.2008.06164. [DOI] [PubMed] [Google Scholar]
- Topczewska JM, Postovit LM, Margaryan NV, Sam A, Hess AR, Wheaton WW, Nickoloff BJ, Topczewski J, Hendrix MJ. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat Med. 2006;12:925–32. doi: 10.1038/nm1448. [DOI] [PubMed] [Google Scholar]
- Trentin A, Glavieux-Pardanaud C, Le Douarin NM, Dupin E. Self-renewal capacity is a widespread property of various types of neural crest precursor cells. Proc Natl Acad Sci USA. 2004;101:4495–500. doi: 10.1073/pnas.0400629101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk CD, Bezrookove V, Green G, Bauer J, Gaugler L, O’brien JM, Simpson EM, Barsh GS, Bastian BC. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457:599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk CD, Fitch KR, Fuchs H, De Angelis MH, Barsh GS. Effects of G-protein mutations on skin color. Nat Genet. 2004;36:961–8. doi: 10.1038/ng1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E, Voiculescu S, Le Poole IC, El-Gamil M, Li X, Sabatino M, Robbins PF, Nickoloff BJ, Marincola FM. Clonal persistence and evolution during a decade of recurrent melanoma. J Invest Dermatol. 2006;126:1372–7. doi: 10.1038/sj.jid.5700193. [DOI] [PubMed] [Google Scholar]
- Wehrle-Haller B, Weston JA. Soluble and cell-bound forms of steel factor activity play distinct roles in melanocyte precursor dispersal and survival on the lateral neural crest migration pathway. Development. 1995;121:731–42. doi: 10.1242/dev.121.3.731. [DOI] [PubMed] [Google Scholar]
- Wong AK, Chin L. An inducible melanoma model implicates a role for RAS in tumor maintenance and angiogenesis. Cancer Metastasis Rev. 2000;19:121–9. doi: 10.1023/a:1026537423753. [DOI] [PubMed] [Google Scholar]
- Xu D, Emoto N, Giaid A, Slaughter C, Kaw S, Dewit D, Yanagisawa M. ECE-1: a membrane-bound metalloprotease that catalyzes the proteolytic activation of big endothelin-1. Cell. 1994;78:473–85. doi: 10.1016/0092-8674(94)90425-1. [DOI] [PubMed] [Google Scholar]
- Yada Y, Higuchi K, Imokawa G. Effects of endothelins on signal transduction and proliferation in human melanocytes. J Biol Chem. 1991;266:18352–7. [PubMed] [Google Scholar]
- Yamane T, Hayashi S, Mizoguchi M, Yamazaki H, Kunisada T. Derivation of melanocytes from embryonic stem cells in culture. Dev Dyn. 1999;216:450–8. doi: 10.1002/(SICI)1097-0177(199912)216:4/5<450::AID-DVDY13>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–5. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Yeligar S, Tsukamoto H, Kalra VK. Ethanol-induced expression of ET-1 and ET-BR in liver sinusoidal endothelial cells and human endothelial cells involves hypoxia-inducible factor-1alpha and microrNA-199. J Immunol. 2009;183:5232–43. doi: 10.4049/jimmunol.0901084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S, Takeda K, Shibahara S. SOX10, in combination with Sp1, regulates the endothelin receptor type B gene in human melanocyte lineage cells. FEBS J. 2006;273:1805–20. doi: 10.1111/j.1742-4658.2006.05200.x. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Kunisada T, Kusakabe M, Nishikawa S, Nishikawa SI. Distinct stages of melanocyte differentiation revealed by analysis of nonuniform pigmentation patterns. Development. 1996;122:1207–14. doi: 10.1242/dev.122.4.1207. [DOI] [PubMed] [Google Scholar]
- Zabierowski SE, Herlyn M. Melanoma stem cells: the dark seed of melanoma. J Clin Oncol. 2008;26:2890–93. doi: 10.1200/JCO.2007.15.5465. [DOI] [PubMed] [Google Scholar]
- Zha S, Yegnasubramanian V, Nelson WG, Isaacs WB, De Marzo AM. Cyclooxygenases in cancer: progress and perspective. Cancer Lett. 2004;215:1–20. doi: 10.1016/j.canlet.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Zhu L, Lee HO, Jordan CS, Cantrell VA, Southard-Smith EM, Shin MK. Spatiotemporal regulation of endothelin receptor-B by SOX10 in neural crest-derived enteric neuron precursors. Nat Genet. 2004;36:732–7. doi: 10.1038/ng1371. [DOI] [PubMed] [Google Scholar]