Abstract
We have developed an instrumented pillbox, called a MedTracker, which allows monitoring of medication adherence on a continuous basis. This device improves on existing systems by providing mobility, frequent and automatic data collection, more detailed information about nonadherence and medication errors, and the familiar interface of a 7-day drug store pillbox. We report on the design of the MedTracker, and on the results of a field trial in 39 homes to evaluate the device.
I. Introduction
Poor medication adherence is one of the major causes of illness and of treatment failure in the USA. Bedell et. al. [1] found a 76% discrepancy rate between what medicines patients were prescribed, and what medicines (prescription and non-prescription) they actually took. Furthermore, clinical trials to assess the safety and efficacy of new drugs necessarily rely on proper medication adherence by study participants to obtain accurate data [2, 3]. Thus, accurate assessment of medication adherence is important to patients, caregivers, and researchers.
Existing methods of tracking medication adherence suffer from a number of drawbacks. Pill counts, the mostly commonly used method, overestimate adherence [4, 5]. So does self-report of adherence [5-7]. Existing electronic tracking systems such as the widely-used Medication Event Monitoring System [4] (MEMS, Aardex Ltd.) provide excellent information about adherence, but suffer from several drawbacks. First, the MEMS cap is difficult to open for arthritic hands [8, 9]. Second, MEMS does not report adherence in real time, so intervention cannot take place if medications are missed. Third, MEMS does not accommodate the use of pill boxes for sorting medications into daily doses, as are commonly used by the elderly [10] and when multiple drugs are taken [11, 12].
We have developed a simple electronic medication tracking device based on a standard multi-compartment pillbox that overcomes some of these limitations. In this paper we present an overview of the design of this system, followed by an evaluation of its use in monitoring adherence to a vitamin regimen among community-dwelling seniors. We conclude with a discussion of related work and potential enhancements to the device.
II. Design of the Adherence Monitoring Device
A. Requirements
We had four key requirements that drove the design of this device. First, we required that the device use a multi-compartment pillbox, such as is available at any pharmacy, for holding the medication. A number of studies have shown that multi-compartment pillboxes are widely used to manage medications [10-13]. These devices may aid adherence by providing visual cues as to whether or not medication has already been taken (pill remains in the compartment or not, if the lid left open to signal pill has been taken), and allow setup once weekly or once daily, reducing the risk of taking the wrong medication at the wrong time.
Second, we required that the device be portable, so that the user could move it from room to room, or even take it with them outside the home, as needed to maximize their likelihood of taking their medication on time. This meant that the system needed to run on batteries and had to be compact.
Third, we required that the system not require user interaction, beyond taking the medication itself. Our research is heavily focused on in-home assessment of community-dwelling seniors, including seniors who suffer from memory loss and early dementia. Thus, we wished to create a system that would not differ in use from the familiar drug-store model of a 7-day reminder pillbox.
Fourth, we required that the medication adherence data be available for review on demand, so that a failure to take medication could be detected immediately and intervention could be taken if appropriate. This requirement, coupled with the need for the device to be portable, led to a requirement for wireless transmission of data on a frequent or real-time basis.
B. Design Considerations
In order to simplify the prototype design and to make the use of the device as familiar as possible to the user, we chose to instrument an existing 7-day reminder pillbox, rather than design the pillbox itself from scratch. We required that the device record the time when each lid was opened or closed. We tested several designs for this feature: simple magnetic reed switches that were activated when the lid was closed; switches that would be activated by pressure from the lid; and a plunger which would be depressed by the lid and would contact a switch under the box on closure. While each of these approaches worked, we chose to build the device using the plunger for several reasons. The magnetic reed switches required that we glue a small magnet into the lids of the box, and there was therefore a risk that a magnet would detach and fall into the pill compartment and potentially be ingested. A switch placed into each compartment that would be activated when the lid was closed worked well, but the switch was exposed to dust from the pills in the compartment, and we felt this would be difficult to keep clean and working over time. The plunger was fitted through a plastic bushing inserted in each compartment. There was potential for pill dust to be trapped in the bushing as well; however, because of its size it was easy to clean, and the design ensured that all the electronic and precise mechanical parts were isolated from the pill chambers.
Whenever the user opened a subcompartment lid on the pillbox, the plunger would release a switch inside the device, sending a signal to the microcontroller indicating that the door was open. The status of each lid was checked once a second, and any change in status was time-stamped using a 32kHz watch crystal and saved to a circular RAM buffer in the 18LF252 PIC microcontroller. Timestamps reflected the number of seconds since the device was last reset, and were stored as 3-byte values. Given the capacity of the memory and the amount of data required by each event (4 bytes), the memory can store up to 256 events.
The need to collect the data from the device on a frequent basis, and for the device to be portable, required that we provide wireless connectivity. We chose to use Bluetooth for this connectivity for a number of reasons. Bluetooth provides a well-defined protocol stack that operates in an unlicensed ISM band (2.45GHz). The range is appropriate for these studies (100m specification, 30m achieved in a home). It provides both voice and high-speed two-way data transfer, making it able to support future modifications to the device that may require these features. Bluetooth is readily supported with commercially available devices and computers, and there is a strong Open Source code base should we need to modify the Bluetooth stack. We used the BlueStamp BR-SC30A module (BlueRadios Inc., Englewood, CO) in our design.
The need to provide portability (and therefore battery power) and the need for frequent data access are clearly in contention. It would be ideal to have the device transfer data on demand, but this would require that the Bluetooth radio remain on continuously, which is incompatible with long battery life. The BlueStamp radio is low power (40mA receive, 120mA transmit), but would drain a 9V battery in less than 10 hours if left on continuously. Since we did not require real-time data (users were taking pills only a few times each day), we compromised by having the Bluetooth link controlled by the PIC controller on the board, which woke up every two hours to send data and battery voltage over the Bluetooth link. Data was sent at 9600 baud in groups of 100 bytes followed by 100ms delay before the next 100 bytes. The Bluetooth protocol includes error checking and packet retransmission for reliable communication. We chose not to do additional error checking and transfer confirmation during communication, which would have required leaving the radio on longer, and could potentially have resulted in battery drain if there were difficulties in confirming successful data transfer. Rather, the most recent 100 events were sent in each transmission, so that if a transmission failed, events would still be transferred at a later point in time. In addition, a DIP switch allowed us to force the radio on continuously for testing or manual downloading of data. With this periodic data transfer protocol, battery life (using a 9V battery) was about 8 weeks.
C. Prototype Device
Figure 1 shows the outside and inside of the final prototype device, called a MedTracker. The initial device deployed in the field trials described below was susceptible to electrostatic discharge into the PIC reset pin, causing an SCR latchup on the PIC. When this happened, the PIC continued to run, but the battery would drain, and the Bluetooth pairing would sometimes be lost. This problem was corrected in the MedTracker II by adding a 10k resistor and a 0.1μF capacitor at the reset pin. In the MedTracker II we also added a DIP switch for forcing the radio on for initial setup.
Figure 1.
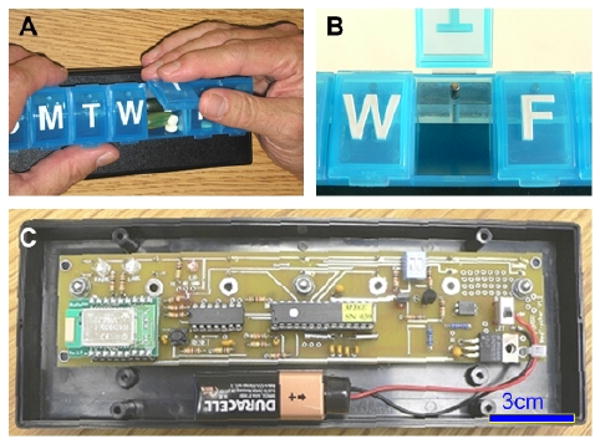
The MedTracker device. A: outside of the device. B: closeup of the plunger that activates the switch to record door openings; C: internals.
III. Field Trials
A. Subjects
39 elderly subjects (aged 82.4 ± 5.8 years, 13 men, 26 women) participated in a field study in which we assessed their adherence to a twice daily vitamin regimen using the MedTracker. Subjects were also asked to complete a questionnaire about the usability of the device. All subjects were living independently in townhomes and apartments.
B. Methods
Subjects were asked to take a single low-dose (250mg) vitamin C tablet twice daily (once in the morning, once in the afternoon) for five weeks, using the MedTracker. Subjects loaded the MedTracker once a week. Data were uploaded via the Bluetooth link from the MedTracker to a computer placed in the home. Collected data were transferred nightly to a central computer through a dial-up connection.
Whenever a subcompartment door changed state, the MedTracker stored the door id, the time since last reset, the current battery level, the number of registered events, the number of successful Bluetooth connections since the last event, the number of failed Bluetooth connections since the last event, and a sequence number. Thus a number of measures of interest could be derived from these data. The time when the device was loaded was determined by identifying those periods in which all 7 doors were opened at least once in a 10 minute period. Single door openings were considered pill-taking events. Opening of the wrong compartment (i.e. the wrong day) was flagged as an error, although as long as at least 1 door was opened at the correct time, we considered a pill to have been taken. We examined three adherence-related measures: percentage of days in which both vitamins were taken; percentage of doses in which vitamins were taken within 1 hour before or 2 hours after the prescribed time (dose time adherence); and number of errors in which the wrong door was opened, indicating confusion about the date. In addition, we recorded the number of failed and successful attempts to transfer data from the device via the Bluetooth link, days of data lost due to MedTracker problems, and difficulties the users had using the device.
C. Results
38 subjects successfully used the device throughout the 5-week period. The final subject loaded the device once but then forgot to use it thereafter, although she continued to take the vitamins from the bottle, hence her adherence data were excluded from analysis. Two subjects took the device on vacation with them (1 week); when they returned, connection to the computer was automatically reestablished and the data for that week was successfully uploaded. Table 1 shows the results for each of our measures.
Table 1. Measures of device behavior and adherence.
| Device measures | Mean | S.D. | Range |
|---|---|---|---|
| Transfer success rate | 90.9% | 14.6% | 46.3 - 100% |
| Successful transfers | 373.8 | 125.3 | 105 - 495 |
| Failed Transfers | 34.7 | 63.0 | 0 - 250 |
| Data loss due to device issues | 0.8 | 3.0 | 0 - 15 |
| Adherence measures | Mean | S.D. | Range |
| Total adherence | 79.4% | 29.6% | 8.6 – 100% |
| Dose time adherence | 71.0% | 24.2% | 20.4 - 100% |
| Door errors/confusion | 3.6 | 6.6 | 0 - 33 |
1) Device reliability
Overall, the MedTracker performed well in the field. Data was successfully transferred every two hours 90.9±14.6% of the time. The first 9 subjects used the MedTracker 1 version of the device, which was susceptible to power surges due to static discharge, resulting in a loss of pairing with the computer and a failure to transfer data. The MedTracker 2 version of the device did not suffer from this problem. Failed transfers accounted for 18.5% of transfer attempts among MedTracker 1 users, versus 4.8% among MedTracker 2 users.
Two devices were not retrieved within 8 weeks from the start of their deployment, and ran out of batteries. Also, in the case where pairing of the device with the computer was lost, it was possible to lose data due to a filling of the circular RAM buffer. This occurred in only 2 cases. Out of a total 1330 days of data collected, 29 days of data were lost due to connection failure and filling of the buffer, or to power failure.
2) Usability of the device
All but 1 subject reported that the MedTracker was easy to use, although one additional subject reported difficulty in opening the compartments. 17% of the subjects used a similar pillbox for their own medications. 6 people reported that the size of the device was problematic, as they would have liked to take it with them in their pockets when they were not going to be home to take their vitamin.
3) Adherence to the vitamin regimen
Total adherence was about 79% overall. Pill counts at the end of the study confirmed these estimates. In contrast, subjects' self-report indicated far better adherence (93% or better on average) than the objective data provided by the MedTracker. Also, 12% of subjects believed they never missed a dose (versus the 5.3% who actually never did).
As expected, dose time adherence was much lower (about 71%), with lower adherence for the evening dose (67.4 ± 30.3%) than the morning dose (78.3±24.9%). Furthermore, in 27 cases, the pills were taken from the wrong subcompartment or multiple compartments were opened at the dose time in at least one instance outside of the loading of the device, suggesting some confusion about the day of week or about whether or not previous doses had been taken. Thus the MedTracker provided more detailed analysis of adherence issues than would be possible with existing devices.
IV. Discussion
A number of researchers have proposed devices that will improve or assess medication adherence [14, 15]. Wan [14] proposed the “Magic Medicine Cabinet” which used RFID to identify which medications were taken out of a cabinet, face recognition to identify who approached the device, and a broadband connection to be able to provide an integrated “situation health portal”. Thus, users were required to use the medicine cabinet and to store all medications in separate bottles that could be RFID tagged. Fishkin et al. [15] proposed a lighter-weight version of the medicine cabinet, in which RFID tagged bottles were kept on a monitoring pad that incorporated an RFID tag reader and a scale, for determining how much medication had been taken. These comprehensive systems provide for much richer interaction with the user than our MedTracker, but lack portability and the ability to take advantage of the adherence cues of dose-specific compartments.
Our current work has a different goal, namely to enable improved assessment of adherence without requiring the user to modify how they take their medications, and to provide a solution that is particularly effective for seniors, who frequently have trouble opening standard pill containers. The portability of the MedTracker is an important feature, since subjects were able to take the device with them when they were traveling, allowing collection of adherence data even when they were away. This is particularly important for use in clinical trials, where accurate adherence information throughout the trial is vital to assessing efficacy and side effects of new drugs. The MedTracker also allows the collection of key information about adherence behavior as compared with existing devices. For example, it was possible to identify when incorrect compartments were opened, which could be extremely important in cases where different medications were to be taken on different days.
Clearly, the current MedTracker is not optimized for size, and could be made much smaller. In addition, other form factors could be instrumented in a similar way (such as single-day multi-compartment pillbox, or single week twice-daily pillbox). The immediate availability of data opens the possibility of prompting the user to take medication specifically when they have missed a dose, although it requires that a computer be available in the home to collect and process the data in real time. We are currently working with researchers in Intel Corporation's Digital Health Group who are developing a context-aware medication prompting system based on a device derived from our prototype.
In summary, the MedTracker provides an easy-to-use alternative to the MEMS cap currently used to assess adherence in many studies of drug and therapy efficacy, and adds some of the features available in more complex research solutions. We anticipate that the MedTracker will be a valuable tool for use in studies of treatment effects, as well as in our own studies of medication management in the elderly.
Acknowledgments
The authors would like to thank Nicole Baggette of the Department of Biomedical Engineering for her work on building the devices and working with subjects; Janna Kimel of Intel Corporation for her help working with subjects; Sarah Prewitt and John Larson of Pacific Retirement Services for their support in recruiting and enabling the field study; and the residents of Holladay Park Plaza and Rogue Valley Manor who participated in this study.
This work was supported in part by a National Institute on Aging Grant P30 AG024978, and by the OGI School of Science and Engineering Bioengineering and Information Sciences Research Awards Program.
Contributor Information
Tamara. L. Hayes, Email: hayest@bme.ogi.edu, Department of Biomedical Engineering, Oregon Health and Science University, Portland, OR, USA (phone: 503-748-7372; fax: 503-748-7038).
John M. Hunt, Email: johnhunt@csee.ogi.edu, Departments of Biomedical Engineering and of Computer Science and Electrical Engineering, Oregon Health and Science University, Portland, OR, USA.
Andre Adami, Email: adami@bme.ogi.edu, Department of Biomedical Engineering, Oregon Health and Science University, Portland, OR, USA.
Jeffrey A. Kaye, Email: kaye@ohsu.edu, Department of Neurology, Oregon Health and Science University, Portland, OR, USA.
References
- 1.Bedell SE, Jabbour S, Goldberg R, Glaser H, Gobble S, Young-Xu Y, Graboys TB, Ravid S. Discrepancies in the use of medications: their extent and predictors in an outpatient practice. Arch Intern Med. 2000;160:2129–34. doi: 10.1001/archinte.160.14.2129. [DOI] [PubMed] [Google Scholar]
- 2.Gorkin L, Goldstein MG, Follick MJ, Lefrbvre RC. Strategies for enhancing adherence in clinical trials. In: Ockene JK, editor. Handbook of Health Behavior Change. New York: Springer; 1990. pp. 361–375. [Google Scholar]
- 3.Dunbar-Jacob J, Burke LE, Puczynski S. Clinical assessment and management of adherence to medical regimens. In: Smith TW, editor. Managing Chronic Illness. Washington, DC: American Psychological Association; 1995. pp. 313–350. [Google Scholar]
- 4.Cramer JA, Mattson RH, Prevey ML, Scheyer RD, Ouellette VL. How often is medication taken as prescribed? A novel assessment technique. Jama. 1989;261:3273–7. [PubMed] [Google Scholar]
- 5.Grosset KA, Bone I, Reid JL, Grosset D. Measuring therapy adherence in Parkinson's disease: a comparison of methods. J Neurol Neurosurg Psychiatry. 2006;77:249–51. doi: 10.1136/jnnp.2005.064709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olivieri NF, Matsui D, Hermann C, Koren G. Compliance assessed by the Medication Event Monitoring System. Arch Dis Child. 1991;66:1399–402. doi: 10.1136/adc.66.12.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shalansky SJ, Levy AR, Ignaszewski AP. Self-reported Morisky score for identifying nonadherence with cardiovascular medications. Ann Pharmacother. 2004;38:1363–8. doi: 10.1345/aph.1E071. [DOI] [PubMed] [Google Scholar]
- 8.Keram S, Williams ME. Quantifying the ease or difficulty older persons experience in opening medication containers. J Am Geriatr Soc. 1988;36:198–201. doi: 10.1111/j.1532-5415.1988.tb01800.x. [DOI] [PubMed] [Google Scholar]
- 9.Atkin PA, Finnegan TP, Ogle SJ, Shenfield GM. Functional ability of patients to manage medication packaging: a survey of geriatric inpatients. Age Ageing. 1994;23:113–6. doi: 10.1093/ageing/23.2.113. [DOI] [PubMed] [Google Scholar]
- 10.Branin JJ. The role of memory strategies in medication adherence among the elderly. Home Health Care Serv Q. 2001;20:1–16. doi: 10.1300/J027v20n02_01. [DOI] [PubMed] [Google Scholar]
- 11.Wendel CS, Mohler MJ, Kroesen K, Ampel NM, Gifford AL, Coons SJ. Barriers to use of electronic adherence monitoring in an HIV clinic. Ann Pharmacother. 2001;35:1010–5. doi: 10.1345/aph.10349. [DOI] [PubMed] [Google Scholar]
- 12.Kalichman SC, Cain D, Cherry C, Kalichman M, Pope H. Pillboxes and antiretroviral adherence: prevalence of use, perceived benefits, and implications for electronic medication monitoring devices. AIDS Patient Care STDS. 2005;19:833–9. doi: 10.1089/apc.2005.19.833. [DOI] [PubMed] [Google Scholar]
- 13.Connor J, Rafter N, Rodgers A. Do fixed-dose combination pills or unit-of-use packaging improve adherence? A systematic review. Bull World Health Organ. 2004;12:935–939. [PMC free article] [PubMed] [Google Scholar]
- 14.Wan D. Magic Medicine Cabinet: a situated portal for consumer healthcare. Presented at Proceedings of HUC99: International Symposium on Handheld and Ubiquitous Computing 1999; 27-29 Sept. 1999; Karlsruhe, Germany. 1999. [Google Scholar]
- 15.Fishkin K, Wang M, Borriello G. A ubiquitous system for medication monitoring. Presented at Pervasive 2004; April 18-23; Vienna, Austria. 2004. [Google Scholar]


