Abstract
A number of methods have been developed to examine the morphologic, biochemical, and molecular changes that happen during the DNA damage response that may ultimately lead to death of cells through various mechanisms that include apoptosis. When cells are exposed to ionizing radiation or chemical DNA-damaging agents, double-stranded DNA breaks (DSB) are generated that rapidly result in the phosphorylation of histone variant H2AX. Because phosphorylation of H2AX at Ser 139 correlates well with each DSB, phospho-H2AX is a sensitive marker to used to examine the DNA damage and its repair. Apoptotic cells are characterized on the basis of their reduced DNA content and morphologic changes, including nuclear condensation, which can be detected by flow cytometry (sub-G1 DNA content), trypan blue, or Hoechst staining. The appearance of phosphatidylserine on the plasma membrane with annexin V–fluorochrome conjugates indicates the changes in plasma membrane composition and function. By combining it with propidium iodide staining, this method can also be used to distinguish early versus late apoptotic or necrotic events. The activation of caspases is another well-known biochemical marker of apoptosis. Finally, the Bcl-2 family of proteins and the mitochondria that play a critical role in DNA damage-induced apoptosis can be examined by translocation of Bax and cytochrome c in and out of mitochondria. In this chapter, we discuss the most commonly used techniques used in our laboratory for determining the DNA damage response leading to apoptosis.
1. Introduction
The cellular response to DNA damage has been an important component of many cytotoxic therapeutics commonly used in cancer therapy, as well as for physiologic processes that occur during DNA replication, DNA repair, and recombination. Among various DNA-damaging agents, ionizing radiation is a prototypical DNA-damaging agent that has been used in many laboratories to define our mechanistic understanding of the DNA damage response. DNA damage activates checkpoint mechanisms in mammalian cells that arrest cell cycle to allow time to repair the DNA damage, or if that is too severe to induce cell death, most commonly by apoptosis.
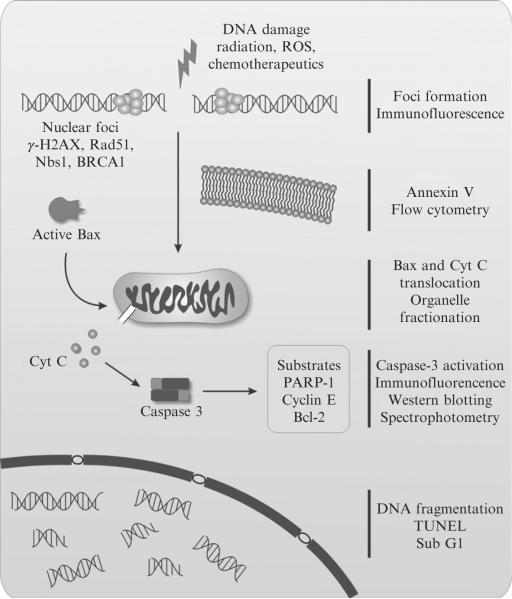
Formation of foci of different nuclear proteins is a widely used method for assessing DNA double-strand breaks (DSB) formation and their repair. Among these are 53BP1, Nbs1, Rad51, and BRCA1 (Paull et al., 2000), with γ-H2AX being the most frequently used (Fig. 6.1). Histone H2AX is phosphorylated in response to DNA damage on serine 139 (Rogakou et al., 1998) forming a nuclear focus. γ-H2AX foci serve as flags on the chromatin for further DNA repair. It has been shown that each γ-H2AX focus represents an individual DSB (Rothkamm and Lobrich, 2003). Quantification of γ-H2AX foci has become a standard in the detection and documentation of DNA damage and its repair.
Figure 6.1.
Schematic representation of the DNA damage and apoptotic responses. The most commonly used techniques for examining them are represented on the right side.
Apoptosis is a universal genetic program of cell death in higher eukaryotes that represents a basic process involved in cellular development and differentiation (Danial and Korsmeyer, 2004; vol. 322). Alternate models of programmed cell death (PCD) have been proposed, including necrosis, autophagy, paraptosis, mitotic catastrophe, and the descriptive model of apoptosis-like and necrosis-like PCD. Cell morphology still remains an important criterion for distinguishing these various forms of cell death from classical apoptosis, because, often, similar molecular mechanisms are involved in their execution.
The Bcl-2 family of proteins has been highly conserved during evolution; its members (24 to date) are critical regulators of apoptosis (Adams and Cory, 2007). Pro-apoptotic members of this family, such as Bax, after their activation, promote apoptosis by causing the release of cytochrome c from mitochondria into the cytosol. Cytochrome c acts as a cofactor to stimulate the complexing of Apaf-1 with caspase-9, which then initiates activation of the caspase cascade (Chen et al., 2000; Liu et al., 1996; Wang, 2001). Genotoxic stressors, such as ionizing radiation or topoisomerase inhibitors, induce Bax expression and activation, as well as its mitochondrial localization (Gong et al., 1999; Ray and Almasan, 2003).
Caspases are synthesized as inactive precursors, which are activated by proteolytic cleavage to generate active enzymes. The activation of caspases is a common and critical regulator of the execution phase of apoptosis, triggered by many factors, including treatment with radiotherapeutic and chemotherapeutic DNA-damaging agents (Chen et al., 2000; Gong et al., 1999; Mazumder et al., 2002; 2007; Ray and Almasan, 2003). They further proteolytically cleave proteins that are essential for maintenance of cellular cytoskeleton, DNA repair, signal transduction, and cell cycle control. More than 300 in vivo caspase substrates exist (Fischer et al., 2003); among them are poly (ADP-ribose) polymerase (PARP-1), ICAD/DFF45, Bcl-2, and cyclin E (Chen et al., 2000; Mazumder et al., 2002).
A number of methods have been developed to identify the DNA damage and subsequent apoptosis by their morphological, biochemical, and molecular alterations. γ-H2AX is the most recognized assay to measure the DNA damage, with two other methods, MTS and Hoechst staining, offering an initial indication for the occurrence of cell death. These initial observations need to be followed up by more specific assays. Changes in plasma membrane composition and function are detected by the appearance of phosphatidylserine, which reacts with annexin V–fluorochrome conjugates on the external plasma membrane surface. Apoptotic cells are recognized either on the basis of their reduced DNA-associated fluorescence as cells with diminished DNA content (sub-G1) or morphologic changes. Activation of caspases can be examined through a variety of methods: colorimetric, immunoblot, or immunohistochemical. Activated caspases cleave many cellular proteins, and the resulting fragments may also serve as useful biomarkers. This chapter provides a few standard protocols that we have successfully used in our laboratory for a number of experimental systems, including cells grown in culture (Chen et al., 2000; Mazumder et al., 2002; Ray and Almasan, 2003), xenografts (Ray and Almasan, 2003), or ex vivo grown specimens (blood or bone marrow) derived from patients with hematologic (Chen et al., 2001) or other malignancies (Masri et al., 2003). MTS and clonogenic assays provide a more general readout for the cumulative effect of various forms of cell death, as well as the ability of cells to proliferate (Brown and Attardi, 2005).
2. Methods
2.1. Treatment with DNA-damaging agents
The sources of ionizing radiation most commonly used to induce DNA damage are as follows:
A radioactive 137Cs γ-ray source, typically with a fixed dose rate of 2 to 3 Gy/min. We use a Shepherd Mark II Cesium-137 irradiator (Gong et al., 1999).
As X-ray source, we use a Pantak HF320 Cabinet X-ray irradiator (320kVP, 20 A, half-value layer 2 mm Cu, East Haven, CT) (Crosby et al., 2007).
As low-dose-rate irradiator (LDRI), an iridium-192 irradiator (Ir-192 has a half-life of ~74 days). Loss from radioactive decay is appreciable, amounting to approximately 1% per day.
Ionizing radiation mimetic drugs, such as bleomycin or its derivatives, peliomycin, talisomycin, and peplomycin, can also be used. If the DNA-damaging agent is a chemotherapeutic agent, such as an ionizing radiation mimetic drug, topoisomerase inhibitors (e.g., etoposide [VP16], camptothecin and its derivatives (e.g., CPT-11), or another therapeutic (e.g., fludarabine), this is added to the freshly replaced culture medium. If ionizing radiation is used to induce the DNA damage (for doses used see NOTE 1), the culture dishes may need to be transported to and/or from the irradiator and irradiated on ice to avoid phosphorylation of H2AX and other biochemical processes that do not take place at 4 °C.
2.2. DNA damage detection by use of γ-H2AX
We will only briefly describe one method used for assessing the DNA damage, because checkpoint responses and DNA repair have been recently covered in Volumes 408 and 409. Another method used for examining DNA damage and repair in single cells, the comet assay, has been also described (Piperakis et al., 1999). The detection of γ-H2AX foci can be performed by immunofluorescence (the most common method), Western blotting, or flow cytometry (for more detailed description, see Nakamura et al. [2006]).
Depending on the cell type (cell size and doubling time), cells are plated 24 h before the treatment on coverslips in 6-well plates or 60-mm dishes at a density that would reach 50 to 75% confluency at the time of treatment. Cells are maintained in a tissue culture incubator at 37 °C, 5% CO2 overnight.
At the end of the incubation period cells are washed three times with PBS (phosphate-buffered saline) at room temperature. TBS (Tris-buffered saline) may also be used instead of PBS. Fix the cells at room temperature with 4% paraformaldehyde in PBS solution for 10 to 30 min depending on the cell line. Wash again three times with PBS, then permeabilize with ice-cold 0.5% Triton X-100 in PBS for 5 min. After permeabilization, wash three times for 5 min with PBS. Block by incubating the cells in a solution of 3% BSA (bovine serum albumin) in PBS for at least 30 min.
Primary anti-γ-H2AX antibody is prepared in 1% BSA in PBS. One of the most commonly used antibodies for detection of γ-H2AX is the mouse monoclonal antiphospho-histone γ-H2AX (Ser139) raised against the synthetic peptide CKATQA[pS]QEY available from Upstate (Temecula, CA, cat.#05–636). The antibody is specific for human origin material but has also been used successfully in other species. Typically, 1:100 dilution of anti-γ-H2AX should be sufficient, but this may be variable depending on the cell type or temperature of incubation. Place the coverslips on a paper towel and add 200 μl of the diluted primary antibody. Coverslips are covered with the lids of the 60-mm dishes and incubated overnight at 4 °C. Covering them with the lids makes sure that the solution will not evaporate, thus leaving the coverslips dry. After the incubation, place the coverslips back in the 60-mm culture dishes and wash three times for 5 min with PBS.
A solution of secondary antibody is prepared by diluting 1:500 to 1:1000 anti-mouse Alexa fluor 488 (Molecular Probes; cat.#A11029) or anti-mouse Alexa fluor 594 (cat.#A11032) in 1% BSA in PBS. Add 200 μl of the secondary antibody solution to the coverslips, as in the preceding, and incubate for 1 h at room temperature. At the end of the incubation time wash the cells three times for 5 min with PBS.
Coverslips are counterstained with 4′,6′-diamidino-2-phenylindole hydrochloride (DAPI)–containing mounting medium Vectashield (Vector Laboratories, Burlingame, CA, cat.#H-1200) and placed upside down on slides. The edges may be sealed with nail polish to make the storage easier. The slides can now be stored at −20°C until visualization by microscopy.
2.3. MTS assay as an easy, first-hand method for assessing viability and cell proliferation
MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay is a colorimetric method used to determine the number of viable cells in proliferation or chemosensitivity assays. MTS (or as an alternative MTT) is a tetrazolium compound that is bioreduced by the cells into formazan, and the quantity of formazan produced is directly proportional to the number of living cells in culture. The absorbance of formazan at 490 nm can be measured directly in 96-well plates. Although very easy and amenable to larger screens, the MTS data obtained alone are not a proof for apoptosis, because they measures changes in metabolic activity that could be also caused by a change in cell proliferation. To assess long-term outcome, clonogenic assays have been commonly used (see NOTE 2).
A 96-well plate is used that contains cells cultured in a 100 μl volume of media.
Seed cells at a concentration of 2 to 3 × 103 to 104 cells/ml, depending on cell type and growth characteristics, in 100 μl medium.
Add 20 μl MTS solution (CellTiter 96® Aqueous One Solution Reagent; Promega) to each well. MTT (Promega) can be used as an alternative.
Incubate the plate for 1 to 4 h in a humidified 5% CO2 atmosphere.
Record the absorbance at 490-nm on an ELISA plate reader.
2.4. Hoechst staining
Illuminating DNA-bound Hoechst results in blue-light fluorescence. Apoptotic cells appear first as brightly stained with condensed chromatin that are later partitioned into blue beads in fragmented nuclei. This assay allows examination of chromatin condensation of the apoptotic nuclei, as a quick and easy way to differentiate between normal and apoptotic cells on the basis of their fluorescence (UV-excitation < 350 nm).
All cells (untreated and treated) are pooled by centrifugation and washed one time with PBS.
The PBS is decanted, and the cells are mixed and incubated with the Hoechst 33258 dye (1 μg/ml; Sigma) for 5 min.
The fluorescence of the apoptotic cells is determined with a UV-equipped fluorescence microscope.
The number of apoptotic cells is scored in several fields (minimum 200 cells/field should be scored).
2.5. Plasma membrane changes detected by annexin-V staining
During the early phases of apoptosis, phosphatidylserine (PS), a protein usually located on the inner leaflet of the plasma membrane in healthy cells, translocates to the outer layer, where it is exposed on the external surface of the cells. Annexin-V has a high affinity for PS, and fluorochrome-tagged annexin-V staining is used as an indicator of apoptosis. The staining is done in combination with PI, a nucleic acid–specific stain that is excluded from live and early apoptotic or necrotic cells but stains DNA and RNA once the plasma membrane is disrupted in these cells. Therefore, it is possible to distinguish live, healthy cells (negative for both annexin-V and PI) from early apoptotic cells (annexin-V positive/PI negative) and late apoptotic or necrotic cells (positive for both annexin-V and PI) by flow cytometry.
Remove medium and rinse cells with PBS.
Trypsinize attached cells by use of trypsin/EDTA. Only mild trypsinization should be used to get a single cell suspension, because trypsin may damage the PS on the plasma membranes. Alternative methods should be used (e.g., EDTA w/o trypsin) whenever these are effective in dissociating attached cells.
Wash cells twice with cold PBS and resuspend 1 × 106 cells in 1 ml of 1 × binding buffer (10 mM HEPES/NaOH, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2).
Transfer 100 μl of the solution (1 × 105 cells) to a 5-ml polystyrene tube.
Add 5 μl of annexin-V (fluorescein or other fluorophore-conjugated annexin-V) and 10 μl of PI (propidium iodide; Sigma): 50 μg/ml stock solution of PI in PBS after which gently vortex the cells.
Incubate at room temperature for 15 min in the dark.
Add 300 μl of 1 × binding buffer to each tube.
Analyze by flow cytometry within 1 h.
2.6. Flow cytometry-based assays for cellular morphology and DNA fragmentation
The sub-G1 method for detecting cell death relies on the principle that after DNA endonucleolytic cleavage, the fragmented low-molecular-weight DNA is released from cells during prolonged fixation. That will yield a population of cells that binds a quantitative DNA stain, PI, to a lesser extent than what is characteristic for G1 cells; G1 represents the longest phase of the cell cycle and, therefore, the largest fraction of cells are typically in G1. As a result, there will be a population of cells that appears to the left of the G1 peak (see NOTE 3).
Flow cytometry has the advantage of rapidly examining a large cell population. An analysis of the forward and side light scatter signals of the cells provides an additional method for identification of apoptotic cells on the basis of their physical properties. Thus, for example, as cells shrink during the early stages of apoptosis, the intensity of light that is scattered by these cells in a forward direction along the laser beam will also decrease. As chromatin condenses and apoptotic bodies are formed during the later stages of apoptosis, these cells reflect and refract more light, which can be determined by the increase in the intensity of light scattered at a 90°C angle (side scatter).
Collect 1 × 106 cells by centrifugation at 500g.
Prepare a second set of 1-ml centrifuge tubes containing 900 μl of 100% absolute methanol. Methanol needs to be chilled to −20°C.
Resuspend the cells in 100 μl of PBS.
Add the cells drop wise by use of a Pasteur pipette to the tubes containing the 100% methanol, while gently vortexing to ensure that the cells are in a single-cell suspension.
Place the tubes in a −20°C freezer and allow fixation to proceed for at least 12 h.
Centrifuge the cells at 1000g and aspirate the methanol.
Wash the fixed cells two times with 1 ml of cold PBS, again centrifuging at 1000g and being careful to not aspirate cells.
Treat the cells with RNase A (100 μg/ml, Gentra Systems) for 20 min at 37°C.
Turn off the lights and stain cells with 50 μg/ml per sample of PI for at least 30 min at room temperature or 15 min at 37°C.
Keep the samples in the dark and run the samples on the flow cytometer, measuring the emission wavelength (617 nm) with a 600- or 610-nm filter.
2.7. Immunocytochemistry to detect active bax/bak, caspase-3, and cytochrome c
Whenever Bax and Bak get activated, there is a conformation change that takes place when an internal epitope is exposed, which can be detected by specific antibodies. Although active Bax can be also detected by its mitochondrial translocation, Bak is present mostly in the mitochondria so this is the only way to examine its activation. After their activation, Bax and Bak permeabilize the mitochondrial outer membrane thus facilitating the release of cytochrome c into the cytosol.
For plating the cells we use glass coverslips (sterilized by dipping in ethanol and passing through flame) that are placed into 6-well plates. Cells (1 × 105 cells/well) are seeded and grown overnight.
Remove media and rinse cells with PBS warmed up to 37°C.
To fix the cells, add 1 to 2 ml of 4% formaldehyde to each well. Leave cells to fix for 20 min at room temperature.
Wash with PBS for 5 min, three times, each well.
Incubate cells in blocking buffer for 5 to 10 min at room temperature.
Dilute the primary antibody (two primary antibodies could be added at the same time, but they need to be of different origin (e.g., one rabbit and the other mouse) in 100 to 200 μl blocking buffer, according to the recommended dilution (Primary antibodies: active-Bax, cytochrome c [BD Pharmingen], active-Bak [Calbiochem], active Caspase-3 [Cell Signalling]).
Use a different 6-well dish to incubate the antibodies. Soak filter paper (3-cm diameter circles) in PBS and place them into the wells; this is necessary to maintain the humidity in the chamber. Place the coverslips on top of the soaked filter paper. Add the primary antibody carefully to cover the entire coverslip. Incubate at room temperature for 1 to 2 h.
Wash with PBS for 5 min, three times, each well.
Add the secondary antibody (fluorochrome conjugated) in blocking buffer and incubate for 30 to 45 min at room temperature in the dark. For dual staining, the fluorophores need to have different emissions spectra for each individual antibody (e.g., FITC at 525 nM and Phycoerythrin at 578 nM). A set of very sensitive and stable Alexa dyes are available (Molecular Probes, now part of Invitrogen); consult The Handbook—A Guide to Fluorescent Probes and Labeling Technologies for a comprehensive resource for fluorescence technology and its applications (http://probes.invitrogen.com/handbook/).
Wash with PBS for 5 min, three times, each well.
Pick up coverslips with a forceps and drain away excess PBS.
For mounting, add a drop of Vectashield to a clean microscope slide and gently lay the coverslip on top.
Remove excess Vectashield (mounting medium for fluorescence [Vector Laboratories, Inc.[), with or without DAPI by blotting with Kim wipe and seal with nail polish.
After adding the secondary antibody, it is important to keep slides in the dark at all times. A similar protocol can be used for tissue sections (see NOTE 4).
Store slides in a −20°C freezer.
2.8. Caspase-3 activity determination: Colorimetric assay
A simple colorimetric assay can measure the release of the chromogenic group from the synthetic substrate, most commonly p-nitroanilide (pNA) by activated caspases. Ac-DEVD-pNA is most frequently used, with the cleaved pNA being monitored colorimetrically through its absorbance at 405 to 410 nm. Although DEVD-based substrates are called caspase-3-specific, they are in fact cleaved by most caspases, with caspase-3 being the most efficient. In vitro titration experiments and/or use of specific inhibitors may be required to distinguish the activity of various caspases (Gong et al., 1999; vol. 322).
Wash cells (1 × 106) with cold PBS and resuspend them in 50 μl of cold lysis HEPES (pH 7.5), 4 mM EDTA. Just before use, add the following protease inhibitors: aprotinin (10 μg/ml), leupeptin (10 μg/ml), pepstatin (10 μg/ml), and PMSF (1 mM), vortex, and keep on ice for 30 min.
Centrifuge the cell lysates at 12,000g for 10 min at 4 °C, collect the supernatant in fresh tubes, and assay the protein concentration for each sample. Keep on ice.
To a 96-well plate add reaction buffer (100 mM HEPES [pH 7.5], 20% v/v glycerol, 5 mM dithiothreitol (DTT), 0.5 mM EDTA), caspase substrate (100 μM final concentration; Ac-DEVD-pNA, Calbiochem), 20 mM stock in DMSO, and 20 to 50 μg cell lysates for a final 200-μl reaction volume.
Incubate samples at 37 °C for 1 to 2 h and monitor the enzyme-catalyzed release of pNA at 405 nm with a microtiter plate reader.
2.9. Immunoblot detection of active bax and caspase-3
In case of Bax-mediated apoptosis, active Bax can be also detected by immunoblot by use of either active Bax-specific antibody or by applying crosslinking agents. Moreover, in most cases, pro-caspase (inactive caspase)-3 is processed into active caspase-3 that can be similarly detected by Western blot analyses. One of its many cellular substrates is PARP-1. In apoptotic cells, PARP-1 (116-kDa) is cleaved into two fragments, most frequently the 86-kDa fragment being detected by the available commercial antibodies.
2.10. Immunoblot analyses
Collect the treated and untreated cells (1 × 106) by centrifugation (500g for 5 min). Decant the medium and resuspend the cell pellet in cold PBS very gently and spin it down (500g for 5 min). Decant the supernatant and repeat the process one more time. Remove the PBS carefully without disturbing the cell pellet.
Lyse the cells in a buffer (20 mM HEPES, pH 7.5, 1 mM EDTA, 150 mM NaCl, 1% NP-40, 1 mM DTT with protease inhibitors (1 mM PMSF, 10 μg/ml leupeptin), and incubate for 30 min on ice with occasional vortexing.
Centrifuge the cells for 15 min at 15,000g and collect the supernatants.
The protein estimation of these samples will be performed by use of a spectrophotometric method with the Bio Rad Protein Assay reagent (working solution, 1:10 dilution) at 595 nm (1 to 2 μl of the sample will be mixed with 1 ml of diluted Bio-Rad protein assay reagent), and the concentration of unknown samples will be measured from the BSA standard curve (the curve can be drawn from the spectrophotometric readings of known concentrations of BSA).
Load 50 to 100 μg protein with SDS-sample buffer (finally 1×) containing beta-mercaptoethanol (as well as the protein standard marker on a 8 to 15% SDS-PAGE to separate the proteins under denaturing conditions.
Transfer the proteins to a nitrocellulose membrane either by the wet or semidry transfer method.
Block the membrane with 5% milk for 1 h at room temperature or overnight at 4 °C.
Incubate the membrane with primary antibodies (PARP-1, active Bax, or active caspase-3) for 2 h at room temperature or overnight at 4 °C (following the company's recommended dilution; PARP-1, active caspase-3 (Cell Signalling), or active Bax (6A7; Pharmingen), antibodies; see NOTE 5).
Wash the blot three times with PBST (PBS with 0.1% Tween 20) at room temperature at 10-min intervals.
Add the appropriate secondary antibody (anti-mouse or anti-rabbit depending on the primary antibody) with a 1:2000 dilution to the blot and incubate for 1 to 1.5 h at room temperature.
Wash the blot five times with PBST at room temperature at 10 min intervals.
Wash the blot with double-distilled water for very short time to get rid of Tween 20 and develop it by use of chemiluminescent reagents, such as Lumiglo or ECL, following the company's suggested protocol.
2.11. Cell fractionation to detect protein translocation to and from mitochondria
During apoptosis, Bax is activated and translocates to the mitochondria. At the same time, cytochrome c is released from the mitochondria into the cytosol. The detection of these and other apoptosis-relevant proteins (Danial and Korsmeyer, 2004; vol. 322) in various subcellular compartments can be used as an important indication of apoptosis.
5 × 107 cells (untreated and treated) are washed first with cold medium (without FBS), then, with ice-cold mitochondria isolation buffer (20 mM Hepes-KOH, pH 7.2, 10 mM KCl, 1.5 mM MgCl2,1 mM EGTA, 1 mM EDTA, 250 mM sucrose, 1 mM EDTA, 1 mM EGTA, with protease inhibitors (1 mM PMSF, 10 μg/ml aprotinin, 10 μg/ml, leupeptin, and 10 μg/ml pepstatin).
Five volumes of the preceding buffer are added to the cell pellet and incubated on ice for 30 min. The cell suspension is then homogenized gently with a Dounce homogenizer, with the extent of lysis being determined by trypan blue exclusion (80 to 90% of cells should be lysed; see NOTE 6). By this procedure, no or minimal cytochrome c should be present in the cytosolic extracts of healthy, untreated cells.
The homogenate is centrifuged at 750g for 10 min to remove unbroken cells, large debris, and nuclei.
The supernatant is again centrifuged at 10,000g for 15 min.
The pellet containing mitochondria, designated as P10, is used for further experiments.
The supernatant is subjected to ultracentrifugation at 100,000g for 30 min.
The resulting pellet, designated as P100, represents the cellular membranes.
The supernatant consists of the cytosolic fraction, designated as S100.
The proteins in the cell lysates are separated by SDS-PAGE and transferred to a nitrocellulose membrane.
Western blot analysis is performed with primary anti-cytochrome c (Pharmingen) and Bax (Santa Cruz), antibodies. As controls, cytochrome c oxidase I or VDAC (Molecular Probes) are used as mitochondrial markers, with β-actin (Sigma) as a marker for the cytosol to indicate any possible mitochondrial or cytoplasmic contamination in the cellular fractions.
ACKNOWLEDGMENTS
We thank previous members of our laboratory, particularly Drs. Meredith Crosby and Marcela Oancea, for development of some of the methods presented. The work described in this article was supported by a research grant from the US National Institutes of Health (CA81504).
Footnotes
For hematopoietic cells, 1 to 4 Gy induces DNA damage that leads to apoptosis, whereas for epithelial cells, 5 to 10 Gy is the usual dose used. Ionizing radiation is administered in the clinic not only as external beam radiation (γ- and X-rays) but also as brachytherapy, which can be mimicked by use of low-dose rate irradiators (up to 10 to 50 cGy per h). The dose of ionizing radiation required to induce DNA damage in model organisms (e.g., C. elegans, yeast) is quite high compared with mammalian cells.
Clonogenic assays provide a more general readout for the cumulative effect of various forms of cell death, as well as the ability of cells to proliferate (Gupta et al., 2008; Morrison et al., 2002; Ray et al., 2007). This assay has been extremely useful for epithelial cells and fibroblasts for which it could well predict the clinical tumor response (Brown and Attardi, 2005). There has been one notable exception in lymphoma cells (Schmitt et al., 2000). Less suitability of hematopoietic cells for this assay could be because their ability to proliferate or even to survive very much depends on cell density; a low density may result in low efficiency of plating (below the 10%, a limit under which this assay is not recommended) (Brown and Attardi, 2005). A lack of a difference in clonogenic survival could mean that compensatory mechanisms have shifted the cellular response from apoptosis to necrosis, autophagy, or another form of cell death. Although the outcome may seem at a first glance to be the same by this assay, how the cells die is important, particularly in vivo, because diverting cells from apoptotic to necrotic cell death profoundly alters tumor microenvironment, producing inflammatory cell infiltration and cytokine signaling (Degenhardt et al., 2006). This inflammatory response could extend well beyond the tumor that is targeted by radiation therapy.
If cells enter apoptosis at phases other than G1, or if aneuploidy is present, there may not be a sub-G1 peak. In addition, microscopic examination should discern among debris, intact single cells, or doublets. Unless otherwise mentioned, keep the samples on ice throughout staining for flow cytometric studies. In addition, sometimes a sub-G2 DNA content can be detected that could be misinterpreted as S-phase content by PI staining, but which becomes clear once it is shown that cells are negative for BrdU incorporation (Crosby et al., 2007).
Formalin-fixed and paraffin-embedded mouse (Ray and Almasan, 2003) or patient-derived human (Masri et al., 2003) tissue sections can be also examined. The slides are deparaffinized with xylene and graded alcohol and treated with citrate buffer (pH 6) for 20 min for antigen retrieval before incubation with primary antibodies. Sections are counterstained with hematoxylin before being examined under the microscope. Immunohistochemistry for caspase-3 can be combined with the in situ detection of apoptotic cells by terminal deoxynucleotide transferase-mediated dUTP nick-end labeling (TUNEL) (Masri et al., 2003). TUNEL or Comet (Piperakis et al., 1999) assays are methods that detect DNA strand breaks that are associated with apoptosis. For TUNEL, the samples are first immunostained for caspase-3 and, after washing in PBS, with a horseradish peroxidase (HRP)-linked secondary antibody. Immunoreactivity is visualized by a 10-min incubation with the HRP substrate diaminobenzidine. After staining for caspase-3, the same slides are then processed for in situ detection and localization of apoptosis at the level of single cells. Sections are then stained with anti-fluorescein antibodies linked with alkaline phosphatase, developed with Fast Red substrate and counterstained with hematoxylin.
The molecular weight of native PARP-1 is 116 kDa and that of cleaved PARP-1 is of 86 kDa. The molecular weight of pro caspase-3 is 32-kDa, whereas active caspase-3 migrates at 17, as well as 12 kDa. Some antibodies recognize only the pro-form of caspase-3, some recognize only the active form, and some can recognize both. The primary antibodies can be reused a couple of times if they are stored at 4 °C in the presence of sodium azide (0.01%, w/v).
For cell fractionation protocols, kits from different companies (e.g., Qiagen, Pierce) are available that are practical and work well for separation of proteins from the various subcellular compartments. Optimal conditions for cell homogenization will depend on the cell type and Dounce homogenizer used. Therefore, the number of strokes required to detect cytochrome c in the cytosol of apoptotic but not in control cells will need to be determined.
REFERENCES
- Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, Attardi LD. The role of apoptosis in cancer development and treatment response. Nat. Rev. Cancer. 2005;5:231–237. doi: 10.1038/nrc1560. [DOI] [PubMed] [Google Scholar]
- Chen Q, Gong B, Almasan A. Distinct stages of cytochrome c release from mitochondria: Evidence for a feedback amplification loop linking caspase activation to mitochondrial dysfunction in genotoxic stress induced apoptosis. Cell Death Differ. 2000;7:227–233. doi: 10.1038/sj.cdd.4400629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Gong B, Mahmoud-Ahmed A, Zhou A, Hsi ED, Hussein M, Almasan A. Apo2L/TRAIL and Bcl-2-related proteins regulate type I interferon- induced apoptosis in multiple myeloma. Blood. 2001;98:2183–2192. doi: 10.1182/blood.v98.7.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby ME, Jacobberger J, Gupta D, Macklis RM, Almasan A. E2F4 regulates a stable G(2) arrest response to genotoxic stress in prostate carcinoma. Oncogene. 2007;26:1897–1909. doi: 10.1038/sj.onc.1209998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death. Critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, Mukherjee C, Shi Y, Gelinas C, Fan Y, Nelson DA, Jin S, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumor-igenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer U, Janicke RU, Schulze-Osthoff K. Many cuts to ruin: A comprehensive update of caspase substrates. Cell Death Differ. 2003;10:76–100. doi: 10.1038/sj.cdd.4401160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong B, Chen Q, Endlich B, Mazumder S, Almasan A. Ionizing radiation-induced, Bax-mediated cell death is dependent on activation of serine and cysteine proteases. Cell Growth Diff. 1999;10:491–502. [PubMed] [Google Scholar]
- Gupta D, Crosby ME, Almasan A, Macklis RM. Regulation of CD20 expression by radiation-induced changes in intracellular redox milieau. Free Radic. Biol. Med. 2008;44:614–623. doi: 10.1016/j.freeradbiomed.2007.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- Masri SC, Yamani MH, Russell MA, Ratliff NB, Yang J, Almasan A, Apperson-Hansen C, Li J, Starling RC, McCarthy P, Young JB, Bond M. Sustained apoptosis in human cardiac allografts despite histologic resolution of rejection. Transplantation. 2003;76:859–864. doi: 10.1097/01.TP.0000084824.70320.DA. [DOI] [PubMed] [Google Scholar]
- Mazumder S, Chen Q, Gong B, Drazba JA, Buchsbaum JC, Almasan A. Proteolytic cleavage of cyclin E leads to inactivation of associated kinase activity and amplification of apoptosis in hematopoietic cells. Mol. Cell. Biol. 2002;22:2398–2409. doi: 10.1128/MCB.22.7.2398-2409.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder S, Plesca D, Kintner M, Almasan A. Interaction of a Cyclin E fragment with Ku70 regulates Bax-mediated apoptosis in hematopoietic cells. Mol. Cell Biol. 2007;27:3511–3520. doi: 10.1128/MCB.01448-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison BH, Bauer JA, Hu J, Grane RW, Ozdemir AM, Chawla-Sarkar M, Gong B, Almasan A, Kalvakolanu DV, Lindner DJ. Inositol hexakisphosphate kinase 2 sensitizes ovarian carcinoma cells to multiple cancer therapeutics. Oncogene. 2002;21:1882–1889. doi: 10.1038/sj/onc/1205265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Sedelnikova OA, Redon C, Pilch DR, Sinogeeva NI, Shroff R, Lichten M, Bonner WM. Techniques for gamma-H2AX detection. Methods Enzymol. 2006;409:236–250. doi: 10.1016/S0076-6879(05)09014-2. [DOI] [PubMed] [Google Scholar]
- Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. 2000;10:886–895. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- Piperakis SM, Visvardis EE, Tassiou AM. Comet assay for nuclear DNA damage. Methods Enzymol. 1999;300:184–194. doi: 10.1016/s0076-6879(99)00125-1. [DOI] [PubMed] [Google Scholar]
- Ray S, Almasan A. Apoptosis induction in prostate cancer cells and xenografts by combined treatment with Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand and CPT-11. Cancer Res. 2003;63:4713–4723. [PubMed] [Google Scholar]
- Ray S, Shyam S, Fraizer GC, Almasan A. S-phase checkpoints regulate Apo2 ligand/TRAIL and CPT-11-induced apoptosis of prostate cancer cells. Mol. Cancer Ther. 2007;6:1368–1378. doi: 10.1158/1535-7163.MCT-05-0414. [DOI] [PubMed] [Google Scholar]
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- Rothkamm K, Lobrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc. Natl. Acad. Sci. USA. 2003;100:5057–5062. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt CA, Rosenthal CT, Lowe SW. Genetic analysis of chemoresistance in primary murine lymphomas. Nat. Med. 2000;6:1029–1035. doi: 10.1038/79542. [DOI] [PubMed] [Google Scholar]
- Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]