Abstract
Background
Heterogeneous results for research investigating health-related quality of life (HRQL) in patients undergoing sphincter-ablating procedures for rectal cancer are likely due to single institution experiences and measurement of HRQL. To address this heterogeneity, we evaluated HRQL in patients with rectal cancer by type of surgery, location of tumor and receipt of adjuvant therapy using an HRQL instrument that has not been used to address rectal cancer patients in a population-based sample over time.
Methods
The Functional Assessment of Cancer Therapy-Colorectal instrument (FACT-C) instrument was administered at 9 and 19 months after diagnosis to a consecutive sample of 160 patients in Northern California identified by the California Cancer Registry. A broad multidimensional interpretation of HRQL was used to examine the impact of tumor location and treatment status, stage of disease, age and gender.
Results
In general, men had lower social well-being scores and younger patients had lower physical and emotional well-being scores and colorectal concerns scores. We found no differences in HRQL by either tumor location or type of surgery, at either 9 or 19 months after diagnosis. Lower physical well-being and greater adverse colorectal concerns were reported at 9 months among patients who received adjuvant therapy; however, only adverse colorectal concerns persisted over time.
Conclusions
This study provides additional evidence that sphincter-ablating procedures do not necessarily reduce quality of life in patients with rectal cancer. Distinctive features of this study include a broad multidimensional interpretation of HRQL, the 19 months of longitudinal follow-up, and a prospective population-based study design.
Keywords: rectal cancer oncology quality-of-life
Introduction
Postoperative health-related quality of life (HRQL) is an important outcome for the approximately 35,000 patients who undergo surgery for rectal cancer each year in the US [1-3]. Abdominoperineal resection (APR) is historically the preferred surgical procedure when the tumor is close to the anus [3,4], although it results in a permanent colostomy with its obvious shortcomings. Sphincter-sparing techniques such as low anterior resection (LAR) can avoid a colostomy but may contribute to pain, nerve damage, sexual dysfunction, bowel and urinary problems [5,6]. Research on the impact of location of anastomosis on fecal continence has generated conflicting results [7,8] and the impact on overall HRQL is uncertain.
Most studies evaluating post-operative HRQL in patients with rectal cancer hypothesize that sphincter ablating surgery is associated with worse HRQL than sphincter-sparing surgery. Although older studies confirm this hypothesis, more recent research findings are conflicting [9-12]. These differences have resulted in the search for tools to assess function and well-being after surgery for rectal cancer as an active area of investigation [13-14]. The Functional Assessment of Cancer Therapy-Colorectal (FACT-C) was developed to measure HRQL in patients with cancer of the colon or rectum [15]. We are not aware of research using the FACT-C to address the association of HRQL, treatment and tumor characteristics in rectal cancer patients exclusively. Older studies had methodological shortcomings [15], but in more recent studies evaluating HRQL with comprehensive, validated instruments, conflicting associations may be due to patients selected at specific institutions receiving differing quality of follow-up care and differences in the interpretation of HRQL.
Health-related quality of life is the patient's multidimensional and subjective appraisal and satisfaction with their current level of functioning as compared to what they perceive to be ideal [17,18]. A recent review by Pachler et al. and a meta-analysis by Cornish et al. challenge the assumption that APR impairs patients' HRQL and conclude that more research is needed [19,20]. Since recommended adjuvant therapy [21-28] can decrease HRQL due to side effects [29,30] but also improve HRQL due to better expected health outcomes [31], adjuvant therapy must also be considered. The objective of this study is to investigate rectal cancer patients' postoperative HRQL based on a broad multidimensional interpretation of HRQL using the FACT-C in a prospective population-based cohort. Specifically, we will assess whether HRQL differs by tumor location, type of surgery (APR. vs. LAR) and receipt of adjuvant therapy.
Materials and Methods
Eligible patients included incident cases of rectal cancer in persons aged 40-84 years of age who were diagnosed between April 1, 1999 and June 30, 2000 in nine Northern California counties and reported to the California Cancer Registry (CCR). Incident cases were collected via rapid case ascertainment within 3 months of diagnosis. Only participants with adenocarcinoma who underwent segmental resection (i.e. anterior resection, low anterior resection) or total proctectomy (i.e. APR) procedures were included. Subjects who did not speak English or Spanish were excluded.
After informed consent was obtained, trained interviewers administered a telephone survey to patients as previously described [32,33]. The first set of interviews was conducted an average of 9 months after diagnosis and a follow-up survey was administered an average of 19 months after diagnosis. Surveys were mailed to patients who did not respond after 15 phone contacts or who specified such a preference. Patients were offered a $20 stipend for completing the survey. Institutional review boards of the Public Health Institute, California Department of Health Services, Northern California Cancer Center, and Harvard Medical School approved the study protocol.
The registry collects demographic, clinical, and treatment information from medical facilities. Treatment variables included type of surgical procedure, reason for no surgery, receipt of radiation therapy, sequence of radiation relative to surgery, and receipt of chemotherapy. Information on the anatomic location of tumor was obtained from a text abstract that summarizes information from patient admission, surgery, radiology, endoscopy and pathology reports found in the medical chart. Although this information is not required, it is present in about 80% of registry records. The presence of this data is not dependent on the patient, it is dependent on the completeness of the medical records and abstraction. Other researchers have utilized location of tumor data from the registry [34]. The location was categorized as the lower rectum (0-5 cm from anal verge), the middle rectum (6-10 cm from the anal verge) and the upper rectum (11-15 cm from the anal verge)[34]. Stage of disease was categorized before treatment using the staging criteria of the American Joint Committee on Cancer converted from SEER Extent of Disease [35].
Patients were surveyed with the FACT-C, a general HRQL instrument combined with a colorectal subscale targeting colorectal cancer patients receiving treatment [15]. The subscales have been validated independent of the total score [15,36]. The five FACT-C subscales include physical well-being, functional well-being, emotional well-being, social/family well-being and colorectal cancer specific concerns. The physical well-being subscale includes questions that reveal how a patient feels (energy levels, pain, side effects and ability to meet their needs). The social/family well-being subscale deals with issues involving family and friends and includes an item on satisfaction with sexual issues. The emotional well-being subscale deals with fear, sadness, nervousness, hope and coping abilities. The functional well-being subscale involves enjoyment with life, recreation and work and sleeping patterns. The colorectal concerns subscale includes questions about bowel function, digestion, appetite, and body appearance.
Patients rated each item on a scale from 0 (not at all) to 4 (very much) according to how much that item pertained to them in the past 7 days. Questions were scored so that a higher score always corresponds to better HRQL. The questions in each subscale were summed to create a final score for each domain, if more than 50 percent of the items comprising the subscale were answered. Missing data were imputed from the average of all the completed items in that subscale if more than half of the items within the subscale were answered [37]. Possible scores for the physical, social/family, functional, and colorectal concerns subscales range from 0 to 28 and possible scores for the emotional subscale range from 0 to 24. Higher subscale scores indicate better well-being.
The FACT-C showed good internal consistency reliability as assessed using inter-item and corrected item subscale correlations and Cronbach's alpha [15]. The FACT-C also showed good concurrent validity as assessed by comparison with the Functional Living Index-Cancer, Brief Profile of Mood States, and Marlowe-Crowne Social Desirability Scale, Profile of Mood States, the Eastern Cooperative Oncology Group Performance Status Rating, and Neuroticism Scale in the Eysenck Personality Questionnaire [15,38]. Also the FACT-C was able to distinguish between groups based on functional status, change in functional status and extent of disease [15]. The Spanish version of the FACT-C is reliable and valid for use in research with colorectal cancer patients [15]. Additionally, the FACT-C showed some advantages over the QLQ-CR38 in a French study of patients with colorectal cancer [39].
The overall HRQL of these patients was modeled with multivariate analysis of variance (MANOVA), addressing all 5 correlated subscales simultaneously with Wilks' Lambda multivariate significance test of mean differences. MANOVA allowed us to account for correlations among a linear combination of all five HRQL subscales and to investigate the effects of our variables (surgery type, location of tumor, receipt of adjuvant treatment, stage of disease, age group and gender). This method helps identify stronger relationships than are observable from any single HRQL variable alone [37]. Specifically, it reduces the number of post-hoc analyses and reduces the probability of a Type I error; MANOVA controls the experiment-wide error rate. It acts as a gateway though the Wilks Lambda test statistic. If the Wilks's Lambda test statistic showed significant differences, post-hoc ANOVA comparisons were conducted to assess the HRQL subscales individually using Bonferroni's adjustment for multiple comparisons and a type I error rate of 0.05. We also conducted repeated measures analysis of variance over time using data from both the initial response and follow-up survey to assess the significance of change over time in HRQL scores. Chi Square analysis was used to assess the potential bias in our main hypotheses due to non-response (completers vs non-completers). Kolmogorov-Smirnov was used to assess normality of scores. All analyses were conducted in SAS [40].
Stage IV patients were excluded from analyses of the association between HRQL and adjuvant treatment because they are receiving palliative care and are not being treated for cure. Stage I patients were included, although this treatment approach does not meet NIH guidelines, because these patients presumably had tumors that were aggressive in a way other than stage (i.e. poorly differentiated) and they were being treated for cure. Stage II and III were grouped together since NIH recommends similar treatments. HRQL varies by stage of disease [41], therefore, we stratified our analysis by stage.
A sample size calculation was conducted at the proposal stage of this project [42]. Given that average values for subscales are in the range of 19-25 and standard deviations are 6.0 or smaller [15], a power calculation using an alpha error level of 0.05 and a beta error level of 0.2, our subgroup analyses have sufficient power to detect statistically significant differences.
Results
A total of 216 rectal cancer patients were eligible for the initial survey. 160 patients responded to the initial survey so the response rate was 74 percent. Due to resource constraints, only 123 of the 160 patients who responded to the first survey were invited to participate in the follow-up survey. Out of 123 patients, 89 completed the follow-up survey resulting in a 72 percent response rate. In the follow-up survey, 15 of the original responders to the first survey could not be contacted and 17 refused to participate in the follow-up survey.
To assess potential bias when testing our main hypothesis, we compare the characteristics of completers and non-completers of the first and follow-up (second) surveys in Table 1. Of the completers, total-completers responded to all surveys (the first and follow-up surveys). First-survey completers are those that completed only the first survey. The non-completers include total non-completers (those that did not respond to any survey) and second-survey non-completers (those that responded to the first survey and were invited to the follow-up survey but did not respond to the second survey) Although first-survey completers were slightly younger than total non-completers (p=0.003) and total-completers were more likely to be female (48 vs. 39 percent) and to have been diagnosed at Stage I (38 vs. 27 percent) compared to first-survey completers, there were no other statistically significant differences between completers and non-completers to the initial survey.
Table 1.
Characteristics of rectal cancer patients who completed and did not complete the HRQL questionnaire
| Completers | Non-completers | |||
|---|---|---|---|---|
| Characteristic | TOTAL COMPLETERS1 N=89 (%) | FIRST-SURVEY COMPLETER2 N=71 (%) | TOTAL NON-COMPLETER3 N=56 (%) | SECOND SURVEY NON- COMPLETER4 N=34 (%) |
| Age (yr) mean | 65.0 (10.2) | 63.6 (11.8) | 68.0 (10.4) | 62.7 (12.9) |
| Gender | ||||
| Male | 46 (52%) | 43 (61%) | 36 (64%) | 20 (59%) |
| Female | 43 (48%) | 28 (39%) | 20 (36%) | 14 (41%) |
| Race | ||||
| Non-Hispanic White | 72 (81%) | 53 (75%) | 36 (64%) | 26 (76%) |
| Hispanic | 2 (2%) | 6 (5%) | 4 (7%) | 3 (9%) |
| Non-Hispanic Black | 7 (8%) | 4 (5%) | 7 (13%) | 1 (3%) |
| Asian/Pacific Islander | 8 (9%) | 8 (11%) | 9 (16%) | 4 (12%) |
| AJCC stage | ||||
| I | 34 (38%) | 19 (27%) | 18 (32%) | 10 (29%) |
| II | 22 (25%) | 19 (27%) | 17 (30%) | 9 (27%) |
| III | 28 (31%) | 28 (39%) | 15 (27%) | 13 (38%) |
| IV | 5 (6%) | 5 (7%) | 6 (11%) | 2 (6%) |
| Location in rectum | ||||
| Upper | 22 (25%) | 15 (21%) | Unknown6 | 4 (12%) |
| Middle | 28 (31%) | 25 (35%) | Unknown | 17 (50%) |
| Lower | 20 (22%) | 16 (23%) | Unknown | 6 (18%) |
| Unknown5 | 19 (21%) | 15 (21%) | Unknown | 7 (20%) |
| Surgery | ||||
| Sphincter-sparing | 62 (70%) | 48 (68%) | 43 (77%) | 23 (68%) |
| Sphincter-ablating | 27 (30%) | 23 (32%) | 13 (23%) | 11 (32%) |
| Adjuvant treatment | ||||
| Pre-operative radiation +chemotherapy | 18 (20%) | 10 (14%) | 6 (11%) | 5 (15%) |
| Post-operative radiation +chemotherapy | 31 (35%) | 29 (41%) | 17 (30%) | 12 (35%) |
| Radiation only | 1 (1%) | 4 (6%) | 0 (0%) | 3 (9%) |
| Chemotherapy only | 3 (4%) | 2 (3%) | 4 (7%) | 1 (3%) |
| None | 36 (40%) | 26 (37%) | 29 (52%) | 13 (38%) |
TOTAL COMPLETERS: Completed the first and follow-up surveys
FIRST-SURVEY COMPLETER Completed the first survey only (includes those who were invited and not invited to complete the second survey)
TOTAL NON-COMPLETER Did not complete any survey
SECOND SURVEY NON- COMPLETER Was invited to complete the follow-up survey but did not participate
Although a majority CCR text records have location of tumor data, this data is not required by the CCR.
We did not have identifiers for participants who did not respond to the survey.
LAR was performed in 95 percent of patients with tumors in the upper rectum, 72 percent of those with tumors in the middle rectum and 36 percent of those with tumors in the lower rectum (p<0.001). Receipt of any type of adjuvant treatment (p=0.50) and stage of disease did not vary by location of tumor (p=0.50). Twenty percent of stage I, 82 percent of stage II patients and 80 percent of stage III patients received both chemotherapy and radiation therapy. All but two participants had begun adjuvant therapy by the initial survey date; 88 percent had completed radiation treatment and 55 percent had completed chemotherapy.
The mean age at diagnosis did not vary by type of surgery (p=0.48), but those receiving adjuvant therapy were significantly younger (p<0.02). Participants with tumors high in the rectum were younger than those with tumors low in the rectum (p=0.01). There was no significant difference in the gender of participants by type of surgery or receipt of adjuvant therapy.
Initial Survey Response
The scores were in the normal range, so scores were not transformed. Table 2 shows the means and standard deviations of FACT-C scores by gender, race/ethnicity, stage, location, surgery type, receipt of chemotherapy and radiation treatment for participants completing the first survey.
Table 2.
Mean Initial FACT-C Scores (standard deviation) and MANOVA test statistic, Wilks Lambda P-value.
| P-value1 | N2 | Physical well-being | Social/Family well-being | Emotional well-being | Functional well-being | Colorectal concerns | |
|---|---|---|---|---|---|---|---|
| Age | 0.005 | ||||||
| <=65 | 78 | 20.4 (6.1) | 22.4 (4.5) | 18.9 (4.9) | 18.9 (6.3) | 19.9 (4.9) | |
| >65 | 82 | 23.4 (4.3) | 22.7 (4.6) | 20.7 (3.1) | 19.9 (5.4) | 21.7 (4.4) | |
| Gender | 0.009 | ||||||
| Male | 89 | 22.2 (5.2) | 21.7 (5.0) | 20.0 (3.8) | 19.0 (5.8) | 21.2 (4.6) | |
| Female | 71 | 21.6 (5.7) | 23.7 (3.6) | 19.7 (4.7) | 20.0 (6.0) | 20.3 (4.9) | |
| Race/Ethnicity | 0.19 | ||||||
| White | 125 | 22.3 (5.1) | 22.6 (4.3) | 19.9 (3.9) | 19.4 (5.7) | 20.7 (4.6) | |
| Black | 8 | 20.8 (6.9) | 22.3 (4.2) | 21.0 (4.0) | 20.1 (5.0) | 20.0 (7.1) | |
| Hispanic | 11 | 21.1 (6.3) | 23.3 (4.9) | 20.1 (3.8) | 19.3 (7.2) | 23.5 (3.5) | |
| Asian/PI | 16 | 20.0 (7.1) | 22.3 (6.1) | 18.7 (6.7) | 19.1 (7.4) | 20.3 (5.0) | |
| AJCC stage | 0.0003 | ||||||
| I | 53 | 23.9 (4.0) | 21.8 (5.6) | 21.0 (3.8) | 20.8 (5.7) | 22.1 (3.7) | |
| II | 41 | 21.8 (5.7) | 23.3 (3.5) | 20.1 (4.8) | 19.5 (5.9) | 20.2 (4.9) | |
| III | 56 | 20.9 (5.6) | 23.0 (4.2) | 19.4 (3.8) | 18.3 (5.9) | 20.1 (5.1) | |
| IV | 10 | 17.8 (5.8) | 21.9 (3.1) | 15.0 (5.5) | 18.5 (5.8) | 20.9 (5.5) | |
| Tumor location | 0.51 | ||||||
| Upper | 37 | 21.3 (5.8) | 22.6 (5.3) | 19.6 (4.9) | 19.5 (6.2) | 20.6 (5.7) | |
| Middle | 53 | 21.6 (5.8) | 22.7 (3.7) | 19.7 (3.8) | 18.1 (6.6) | 20.1 (5.0) | |
| Lower | 36 | 20.0 (5.3) | 21.9 (4.6) | 19.6 (4.0) | 19.9 (8.2) | 20.7 (3.8) | |
| Unknown | 34 | 23.0 (4.7) | 23.3 (4.7) | 20.6 (4.3) | 20.9 (5.2) | 22.3 (3.9) | |
| Surgery | 0.96 | ||||||
| LAR3 | 110 | 22.1 (5.2) | 22.8 (4.6) | 20.0 (4.4) | 19.6 (6.0) | 20.9 (4.8) | |
| APR4 | 50 | 21.5 (6.0) | 22.1 (4.3) | 19.5 (3.8) | 19.1 (5.6) | 20.6 (4.6) | |
| Adjuvant Therapy | 0.004 | ||||||
| None | 62 | 24.0 (4.4) | 22.6 (4.8) | 20.9 (3.9) | 20.6 (5.9) | 21.8 (4.3) | |
| Both | 88 | 20.1 (5.7) | 22.6 (4.4) | 19.1 (4.2) | 18.3 (5.8) | 19.9 (5.0) | |
| Some5 | 10 | 24.9 (2.3) | 22.6 (4.1) | 20.1 (4.6) | 21.9 (4.9) | 23.4 (3.0) |
P-value for Wilks' Lambda test statistic of multivariate mean differences for the MANOVA. Significant p-values lead to post-hoc analysis (ANOVA and repeated measures)
Initial respondents include the 71 patients who completed only the initial survey and the 89 patients who completed both initial and follow-up surveys, for a total of 160.
LAR=low anterior resection
APR= abdominoperineal resection
Either chemotherapy or radiation therapy, but not both.
Except for social/family well-being, scores were highest for patients with Stage I cancer and declined slightly with increasing stage. The P-value for the Wilks Lambda test by predictor variables are shown in Table 2. Neither location of tumor nor surgery type alone nor both variables together were significantly associated with any of the five domains of HRQL in the MANOVA analysis. Gender, age and adjuvant therapy were statically significant in the MANOVA (p<0.009). Post-hoc analysis showed males had significantly lower social well-being scores (p=0.004) than females. The other domains did not show gender differences. Patients aged 65 years or younger had significantly lower scores on physical well-being (p=0.0004), emotional well-being (p=0.006), and colorectal concerns (p=0.02) than older patients.
For adjuvant therapy, we grouped radiation and chemotherapy together, excluding the ten patients who received either therapy alone. Post-hoc analysis showed patients who received adjuvant therapy had lower physical (p<0.001) and emotional well-being scores (p=0.02) than patients who did not receive adjuvant treatment (Table 2).
Participants who had completed chemotherapy had significantly better scores in all subscales except social/family well-being than those who had not completed chemotherapy. Table 3 presents the post-hoc results by receipt and completion of chemotherapy.
Table 3.
Comparison of mean initial FACT-C scores (standard deviations) for participants who had and had not completed chemotherapy at the time of the first survey
| Physical well-being | Social/family well-being | Emotional well-being | Functional well-being | Colorectal concerns | |
|---|---|---|---|---|---|
| Did not receive chemotherapy (n=67) | 24.1 | 22.7 | 20.8 | 20.7 | 21.8 |
| Completed chemotherapy | |||||
| Yes (n=51) | 21.1 | 22.8 | 20.0 | 19.7 | 20.4 |
| No (n=39) | 18.9 | 22.0 | 18.1 | 16.9 | 19.4 |
| P-value | <0.0001 | 0.6480 | 0.0065 | 0.0050 | 0.0346 |
* P values describe the statistical significance of differences in HRQL outcomes between the 39 patients who had completed chemotherapy and the 51 who were receiving chemotherapy, but had not yet completed it.
Follow-up Survey
There were no significant effects of surgery type or location of tumor on any subscale. While participants receiving adjuvant therapy had statistically significant lower follow-up physical well being scores than those not receiving adjuvant therapy (p=0.003), their scores improved significantly from the initial to follow-up surveys (p=0.006; Figure 1). However, the colorectal concerns score was significantly higher at the initial survey for patients not receiving adjuvant therapy (2.4 points, p=0.009), and this difference persisted despite increased scores in both groups (p=0.04; Figure 2). There were no statistically significant associations between receipt of adjuvant therapy and the other scales.
Figure 1.
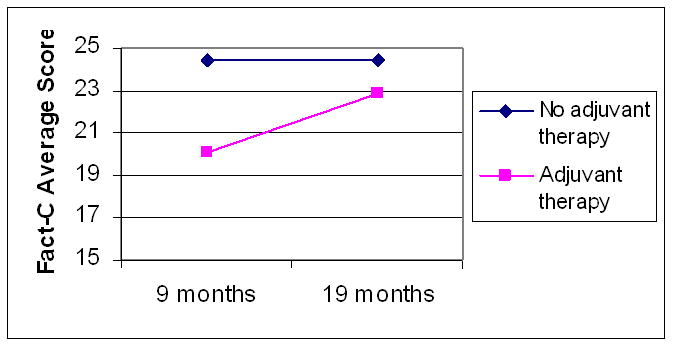
FACT-C physical well being scores over time by receipt of adjuvant therapy
Figure 2.
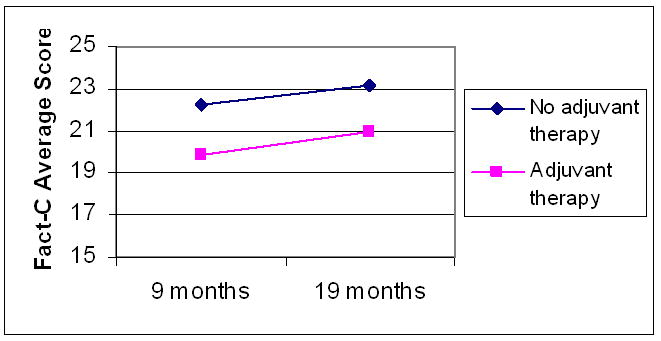
FACT-C colorectal concerns subscale scores over time by receipt of adjuvant therapy
Discussion
This study demonstrated that patients undergoing APR for rectal cancer did not have worse HRQL than patients undergoing LAR and HRQL was not affected by the anatomic location of the rectal tumor. Younger patients in general had lower HRQL than older patients. This finding is similar ash other research in examining colorectal cancer patients [43,44], rectal cancer patients [45,46], breast cancer patients [47]. Since younger patients may be more active, they may perceive cancer and surgery to be a larger burden than older patients. Of those receiving adjuvant therapy, only older patients with stage I disease had lower HRQL. Patients with stage I cancer may perceive adjuvant therapy to be a larger burden than those with higher stages of disease because they are not as ill. Older patients may perceive adjuvant therapy to be a larger burden than younger patients if health status at diagnosis is worse.
Consistent with our findings, several prior studies have found that patients have better or at least similar HRQL after organ-ablating surgery than after organ-sparing surgery [48-52]. Our results are also similar to those reported from four Scottish hospitals that assessed 106 rectal cancer patients using the EORTC QLQ-C30 and QLQ-CR38 [53]. HRQL is often independent of physical symptoms; indeed, patients with major physical limitations often have high HRQL [54]. Additionally, an individual's expectation of symptoms and limitations affects the degree of distress experienced [55]. For example, patients who expect to have a well-functioning sphincter, but do not, may have poorer HRQL than patients who have a well-functioning stoma [6]. A well-designed, single institution study found that colorectal cancer patients with stomas had lower HRQL than those without stomas, but having realistic goals and expectations appeared to have an important effect on HRQL [56]. Stoma patients may have better or similar HRQL because they receive detailed information on self-care for treatment-related problems, which sphincter-sparing patients do not receive [57]. For example, Engle et al. found that patients who underwent APR had lower HRQL than patients who underwent LAR or high anterior resection but APR patients were poorly informed about stoma irrigation [9]. In the larger cohort of both colon and rectal cancer patients from which our study sample was drawn, the presence of a stoma and crude tumor location (colon vs. rectum) did not predict HRQL [33].
To our knowledge, this is the first study to examine the impact of location of tumor, type of surgery and adjuvant treatment for rectal cancer on HRQL using the FACT-C in a population-based cohort. The FACT-C is important because it is designed to detect clinically meaningful changes in scores in addition to clinically meaningful differences between groups of patients [58]. Other research examining postoperative HRQL found that sphincter-ablating surgery was associated with worse HRQL than sphincter-sparing surgery [6,56, 59-69]. However, these studies were drawn from convenience samples at single institutions and most did not use validated, comprehensive HRQL instruments. Only one other study [9] examined outcomes longer than 19 months after diagnosis. Patients selected in convenience series are vulnerable to the potential bias that physicians may only have invited ‘well-adjusted’ patients to participate [70]. In addition, the results may not apply to other centers with different surgeons, and may not generalize to broader populations.
The majority of recent studies have measured HRQL with the European Organization for Research and Treatment of Cancer (EORTC) Core (C)-30 and Colorectal (CR)-38. The results of these studies are conflicting [9-12,56,69,71]. Some researchers have questioned the sensitivity of EORTC-30 and CR-38 to detect differences in bowel function [13]. To overcome limitations of interpreting HRQL based on a total score as was the focus in previous studies, we examined multiple domains simultaneously using MANOVA to account for correlations among the subscales, and we adjusted for multiple comparisons to reduce our type 1 error rate. Consequently, through our analysis using MANOVA, we defined quality of life in a broad, multidimensional manner, instead of focusing individual symptoms or total scores.
Other researchers have used instruments of questionable sensitivity in rectal cancer patients, such as the Short Form-36 Medical Outcomes Survey (SF-36) and the Gastrointestinal Quality of Life Index [14]. General HRQL instruments, such as the SF-36, RAND's adaptation of the SF-36, and the Nottingham Health Profile, are designed to compare patients across many disease categories and may lead to equivocal results when a single disease is studied [69,72].
Patients with higher stage of disease had lower HRQL in physical and functional scales [42]. When we stratified our results by patient characteristics, there were no differences in HRQL by gender. Older patients with Stage I cancers receiving adjuvant treatment had worse physical well-being, emotional well-being, and colorectal concerns scores than 23 similar patients who were not receiving adjuvant therapy. These differences exceeded the threshold for a clinically important difference for FACT-C subscales, which has been estimated as 2-3 points [73,74]. Since adjuvant treatment is not generally recommended for Stage I patients [28], it is possible that those who received adjuvant treatment had some indication such as tumor behavior, which placed them at higher risk.
Receipt of chemotherapy was associated with lower HRQL, but we found that HRQL scores improved by approximately 19 months after diagnosis in patients completing both surveys, confirming others' finding that the adverse effects of adjuvant therapy on quality of life are relatively short-lived [9,72-75]. Although tumor location was predictive of type of surgery in the present study, it was not associated with HRQL. Tumor location is a very important variable that is rarely available in population-based studies. It has been postulated to confound the relationship between type of surgery and quality of life in previous research [76], but our study did not confirm this hypothesis.
In conclusion, we used a validated, multi-dimensional, cancer-specific instrument to assess postoperative HRQL in a population-based sample of patients with rectal cancer, and found neither statistically significant nor clinically meaningful differences based on tumor location or sphincter-sparing surgery. Future large studies should also assess the relation of HRQL to follow-up treatment, comorbid conditions, perceived quality of care, and evolving surgical techniques such as total mesorectal excision [77-80]. Further research is also needed to address sexual functioning, because this domain is addressed with only one item in the FACT-C. These studies can help patients and physicians to choose between different treatment regimens, especially when the more aggressive approach does not have a clear survival advantage [81]. Although this study does not report on data before patients were diagnosed with cancer, this research controls for stage of disease at diagnosis in the MANOVA, which should account for some of the unknown differences in HRQL due to disease rather than treatment. Studies of HRQL will help physicians and their patients to make more informed decisions regarding choice of treatment, based on realistic patient expectations of symptoms and limitations.
Acknowledgments
The authors appreciate the efforts of Mark Allen (CCR), Scott Riddle (CSP) and Gerri Poindexter (NCCC) for preparing the dataset, David Cella, PhD, for authorizing the use of the FACT-C, Dee West, William Wright, Alan Zaslavsky, Edward Guadagnoli and Charles Fuchs for survey and study design.
This research was supported by grants from the Centers for Disease Control and Prevention, National Program of Cancer Registries, U75-CCU910677, the National Cancer Institute Surveillance, Epidemiology and End Results Special Study program (NO1-PC-65107), the Agency for Healthcare Research and Quality (R01 HS09869), and the National Cancer Institute (U01 CA93324).
The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC- 35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred.
Footnotes
Competing interests: The authors declare that they have no competing interests.
Authors' contributions: JSG conducted the data analysis and prepared a draft of the manuscript. JZA designed the study and collected the data. RDC and CMD supervised the design of the study and the data analysis. PSR and KJY participated in the preparation and revision of the manuscript. All authors participated in the preparation and revision of the manuscript, read, and approved the final manuscript.
References
- 1.Engstrom P. Colorectal Cancer. In: Lenhard R, Osteen RT, Gansler T, editors. The American Cancer Society's Clinical Oncology. Atlanta, GA: American Cancer Society; 2001. pp. 361–371. [Google Scholar]
- 2.Venook A, Goodnight J, Kumar S, et al. Practice Guidelines for Colorectal Cancer. Cancer. J Sci Am. 1996;2(3A):S23. [PubMed] [Google Scholar]
- 3.Gordon P, Nivatvongs S. Principles and Practice of Surgery for the Colon, Rectum and Anus. Second. St. Louis, MO: Quality Medical Publisher; 1999. pp. 55–780. [Google Scholar]
- 4.Schroen AT, Cress RD. Use of surgical procedures and adjuvant therapy in rectal cancer treatment: a population-based study. Ann Surg. 2001;234:641–51. doi: 10.1097/00000658-200111000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corman M. Colon and Rectal Surgery. 4th. Lippincott-Raven; NY: 1998. pp. 905–1041. [Google Scholar]
- 6.Sprangers M, Taal BG, Aaronson NK, et al. Quality of life in colorectal cancer: stoma vs. nonstoma patients. Dis Colon Rectum. 1995;38:361–369. doi: 10.1007/BF02054222. [DOI] [PubMed] [Google Scholar]
- 7.Lewis WG, Holdsworth PJ, Stephenson BM, et al. Role of the rectum in the physiological and clinical results of coloanal and colorectal anastomosis after anterior resection for rectal carcinoma. Br J Surg. 1992;79:1082–6. doi: 10.1002/bjs.1800791032. [DOI] [PubMed] [Google Scholar]
- 8.Joo JS, Latulippe JF, Alabaz O, et al. Long-term functional evaluation of straight coloanal anastomosis and colonic J-pouch: is the functional superiority of colonic J-pouch sustained. Dis Colon Rectum. 1998;41:740–6. doi: 10.1007/BF02236262. [DOI] [PubMed] [Google Scholar]
- 9.Engel J, Kerr J, Schlesinger-Raab A, et al. Quality of life in rectal cancer patients: a four-year prospective study. Ann Surg. 2003;2:203–13. doi: 10.1097/01.sla.0000080823.38569.b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grumann M, Noack EM, Hoffman IA, et al. Comparison of quality of life in patients undergoing abdominoperineal extirpation or anterior resection for rectal cancer. Ann Surg. 2001;233:149–156. doi: 10.1097/00000658-200102000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guren MG, Eriksen MT, Wiig JN, et al. Quality of life and functional outcome following anterior or abdominoperineal resection for rectal cancer. Eur J Surg Oncol. 2005;31:735–42. doi: 10.1016/j.ejso.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Sideris L, Zenasni F, Vernerey D, et al. Quality of life of patients operated on for low rectal cancer: impact of the type of surgery and patients' characteristics. Dis Colon Rectum. 2005;48:2180–91. doi: 10.1007/s10350-005-0155-0. [DOI] [PubMed] [Google Scholar]
- 13.Neuman HB, Schrag D, Cabral C, et al. Can differences in bowel function after surgery for rectal cancer be identified by the European Organization for Research and Treatment of Cancer quality of life instrument. Ann Surg Oncol. 2007;14:1727–34. doi: 10.1245/s10434-006-9283-6. [DOI] [PubMed] [Google Scholar]
- 14.Siassi M, Hohenberger W, Lösel F, et al. Quality of life and patient's expectations after closure of a temporary stoma. Int J Colorectal Dis. 2008;23:1207–12. doi: 10.1007/s00384-008-0549-2. [DOI] [PubMed] [Google Scholar]
- 15.Ward WL, Hahn EA, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) quality of life instrument. Qual Life Res. 1999;8:181–95. doi: 10.1023/a:1008821826499. [DOI] [PubMed] [Google Scholar]
- 16.Camilleri-Brennan J, Steele RJ. Quality of life after treatment for rectal cancer. Br J Surg. 1998;85:1036–43. doi: 10.1046/j.1365-2168.1998.00808.x. [DOI] [PubMed] [Google Scholar]
- 17.Yost K, Cella D. Health-related quality of life and colorectal cancer. Colorectal Cancer Index Rev. 2003;4:4–7. [Google Scholar]
- 18.Cella DF, Cherin EA. Quality of life during and after cancer treatment. Compr Ther. 1988;14:69–75. [PubMed] [Google Scholar]
- 19.Pachler J, Wille-Jorgensen P. Quality of life after rectal resection for cancer, with or without permanent colostomy. Cochrane Database Syst Rev. 2005;18:CD004323. doi: 10.1002/14651858.CD004323.pub3. [DOI] [PubMed] [Google Scholar]
- 20.Cornish JA, Tilney HS, Heriot AG, et al. A meta-analysis of quality of life for abdominoperineal excision of rectum versus anterior resection for rectal cancer. Ann Surg Oncol. 2007;14:2056–68. doi: 10.1245/s10434-007-9402-z. [DOI] [PubMed] [Google Scholar]
- 21.Gastrointestinal Tumor Study Group. Prolongation of the disease-free interval in surgically treated rectal carcinoma. N Engl J Med. 1985;312:1465–72. doi: 10.1056/NEJM198506063122301. [DOI] [PubMed] [Google Scholar]
- 22.Diaz-Canton EA, Pazdur R. Adjuvant medical therapy for colorectal cancer. Surg Clin North Am. 1997;77:211–28. doi: 10.1016/s0039-6109(05)70540-5. [DOI] [PubMed] [Google Scholar]
- 23.Fleshman JW, Myerson RJ. Adjuvant radiation therapy for adenocarcinoma of the rectum. Surg Clin North Am. 1997;77:15–25. doi: 10.1016/s0039-6109(05)70530-2. [DOI] [PubMed] [Google Scholar]
- 24.Stockholm Rectal Cancer Study Group. Preoperative short-term radiation therapy in operable rectal carcinoma. A prospective randomized trial. Cancer. 1990;66:49–55. doi: 10.1002/1097-0142(19900701)66:1<49::aid-cncr2820660111>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 25.Swedish Rectal Cancer Trial. Local recurrence rate in a randomised multicentre trial of preoperative radiotherapy compared with operation alone in resectable rectal carcinoma. Eur J Surg. 1996;162:397–402. [PubMed] [Google Scholar]
- 26.Gerard A, Buyse M, Nordlinger B, et al. Preoperative radiotherapy as adjuvant treatment in rectal cancer. Final results of a randomized study of the European Organization for Research and Treatment of Cancer (EORTC) Ann Surg. 1988;208:606–14. doi: 10.1097/00000658-198811000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709–15. doi: 10.1056/NEJM199103143241101. [DOI] [PubMed] [Google Scholar]
- 28.National Institute of Health. NIH Consensus Conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA. 1990;264:1444–50. [PubMed] [Google Scholar]
- 29.Zaheer S, Pemberton JH, Farouk R, et al. Surgical treatment of adenocarcinoma of the rectum. Ann Surg. 1998;227:800–811. doi: 10.1097/00000658-199806000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murata A, Brown CJ, Raval M, et al. Impact of short-course radiotherapy and low anterior resection on quality of life and bowel function in primary rectal cancer. Am J Surg. 2008;195:611–5. doi: 10.1016/j.amjsurg.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 31.Osoba DL. Lessons learned from measuring health-related quality of life in oncology. J Clin Oncol. 1994;12:608–616. doi: 10.1200/JCO.1994.12.3.608. [DOI] [PubMed] [Google Scholar]
- 32.Ayanian JZ, Zaslavsky AM, Guadagnoli E, et al. Patients' perceptions of quality of care for colorectal cancer by race, ethnicity, and language. J Clin Oncol. 2005;23:6576–86. doi: 10.1200/JCO.2005.06.102. [DOI] [PubMed] [Google Scholar]
- 33.Yost KJ, Hahn EP, Zaslavsky, et al. Predictors of health-related quality of life in patients with colorectal cancer. Health and Quality of Life Outcomes. 2008;6:66. doi: 10.1186/1477-7525-6-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schroen AT, Cress RD. Use of surgical procedures and adjuvant therapy in rectal cancer treatment: a population-based study. Ann Surg. 2001;234:641–51. doi: 10.1097/00000658-200111000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.American Joint Committee on Cancer. The Manual for Staging of Cancer. Philadelphia: JB Lippincott; 1998. [Google Scholar]
- 36.Fairclough DL, Cella DF. Functional Assessment of Cancer Therapy (FACT-G): non-response to individual questions. Qual Life Res. 1996;5:321–9. doi: 10.1007/BF00433916. [DOI] [PubMed] [Google Scholar]
- 37.Harris R. A Primer of Multivariate Statistics. Third. Mahwah, NJ: Lawrence Erlbaum Associates; 2001. pp. 210–654. [Google Scholar]
- 38.Yoo HJ, Kim JC, Eremenco S, Han OS. Quality of life in colorectal cancer patients with colectomy and the validation of the Functional Assessment of Cancer Therapy-Colorectal (FACT-C), Version 4. J Pain Symptom Manage. 2005 Jul;30(1):24–32. doi: 10.1016/j.jpainsymman.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 39.Rotonda C, Conroy T, Mercier M, Bonnetain F, Uwer L, Miny J, Montcuquet P, Léonard I, Adenis A, Breysacher G, Guillemin F. Validation of the French version of the colorectal-specific quality-of-life questionnaires EORTC QLQ-CR38 and FACT-C. Qual Life Res. 2008 Apr;17(3):437–45. doi: 10.1007/s11136-008-9322-9. [DOI] [PubMed] [Google Scholar]
- 40.SAS Institute. SAS Computer software. Version 8; Cary, NC: 2001. [Google Scholar]
- 41.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 42.Smith Gagen J. Ph D dissertation. University of California; Davis: 2004. Patterns of care for rectal cancer patients: Quality-of-life, survival and physician's decision on choice of treatment; pp. 1–111. [Google Scholar]
- 43.Arndt V, Merx H, et al. Quality of life in patients with colorectal cancer 1 year after diagnosis compared with the general population: a population-based study. J Clin Oncol. 2004;22:4829–36. doi: 10.1200/JCO.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 44.Klemm P, Miller MA, Fernsler J. Demands of illness in people treated for colorectal cancer. Oncol Nurs Forum. 2000;27:633–9. [PubMed] [Google Scholar]
- 45.Schmidt CE, Bestmann B, et al. Impact of age on quality of life in patients with rectal cancer. World J Surg. 2005;29(2):190–7. doi: 10.1007/s00268-004-7556-4. [DOI] [PubMed] [Google Scholar]
- 46.MacDonald LD, Anderson HR. Stigma in patients with rectal cancer: a community study. Journal of Epidemiology and Community Health. 1984;38:284–290. doi: 10.1136/jech.38.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wenzel L, Fairclough D, et al. Age-related differences in quality of life of breast carcinoma patients after treatment. Cancer. 1999;86:1768–74. [PubMed] [Google Scholar]
- 48.Weddington WW, Jr, Segraves KB, Simon MA. Psychological outcome of extremity sarcoma survivors undergoing amputation or limb salvage. J Clin Oncol. 1985;3:1393–9. doi: 10.1200/JCO.1985.3.10.1393. [DOI] [PubMed] [Google Scholar]
- 49.Sugarbaker PH, Barofsky I, Rosenberg SA, et al. Quality of life assessment of patients in extremity sarcoma clinical trials. Surgery. 1982;91:17–23. [PubMed] [Google Scholar]
- 50.Kiebert GM, de Haes JC, van de Velde CJ. The impact of breast-conserving treatment and mastectomy on the quality of life of early-stage breast cancer patients: a review. J Clin Oncol. 1991;9:1059–70. doi: 10.1200/JCO.1991.9.6.1059. [DOI] [PubMed] [Google Scholar]
- 51.Camilleri-Brennan J, Steele RJ. Objective assessment of quality of life following panproctocolectomy and ileostomy for ulcerative colitis. Ann R Coll Surg Engl. 2001;83:321–4. [PMC free article] [PubMed] [Google Scholar]
- 52.Guren MG, Wiig JN, Dueland S, et al. Quality of life in patients with urinary diversion after operation for locally advanced rectal cancer. Eur J Surg Oncol. 2001;27:645–51. doi: 10.1053/ejso.2001.1195. [DOI] [PubMed] [Google Scholar]
- 53.Camilleri-Brennan J, Steele RJC. Objective assessment of morbidity and quality of life after surgery for low rectal cancer. Colorectal Dis. 2002;4:61–66. doi: 10.1046/j.1463-1318.2002.00300.x. [DOI] [PubMed] [Google Scholar]
- 54.Albrecht G, Devlieger P. The disability paradox: High quality of life against all odds. Soc Sci Med. 1999;48:977–988. doi: 10.1016/s0277-9536(98)00411-0. [DOI] [PubMed] [Google Scholar]
- 55.Gotay CC. Trial-related quality of life: using quality-of-life assessment to distinguish among cancer therapies. J Natl Cancer Inst Monogr. 1996:1–6. [PubMed] [Google Scholar]
- 56.Wilson TR, Alexander DJ. Clinical and non-clinical factors influencing postoperative health-related quality of life in patients with colorectal cancer. Br J Surg. 2008;95:1408–15. doi: 10.1002/bjs.6376. [DOI] [PubMed] [Google Scholar]
- 57.Caffo O, Amichetti M, Romano M, et al. Evaluation of toxicity and quality of life using a diary card during postoperative radiotherapy for rectal cancer. Dis Colon Rectum. 2002;45:459–65. doi: 10.1007/s10350-004-6220-2. [DOI] [PubMed] [Google Scholar]
- 58.Cella D, Hahn EA, Dineen K. Meaningful change in cancer specific quality of life score differences between improvement and worsening. Qual Life Res. 2002;3:207–21. doi: 10.1023/a:1015276414526. [DOI] [PubMed] [Google Scholar]
- 59.Balslev I, Harling H. Sexual dysfunction following operation for carcinoma of the rectum. Dis Colon Rectum. 1983;26:785–788. doi: 10.1007/BF02554748. [DOI] [PubMed] [Google Scholar]
- 60.Havenga K, Enker WE, McDermott K, et al. Male and female sexual and urinary function after total mesorectal excision with autonomic nerve preservation for carcinoma of the rectum. J Am Coll Surg. 1996;182:495–502. [PubMed] [Google Scholar]
- 61.Danzi M, Ferulano GP, Abate S, et al. Male sexual function after abdominoperineal resection for rectal cancer. Dis Colon Rectum. 1983;26:665–8. doi: 10.1007/BF02553339. [DOI] [PubMed] [Google Scholar]
- 62.Kinn AC, Ohman U. Bladder and sexual function after surgery for rectal cancer. Dis Colon Rectum. 1986;29:43–8. doi: 10.1007/BF02555287. [DOI] [PubMed] [Google Scholar]
- 63.La Monica G, Audisio RA, Tamburini M, et al. Incidence of sexual dysfunction in male patients treated surgically for rectal malignancy. Dis Colon Rectum. 1985;28:937–40. doi: 10.1007/BF02554311. [DOI] [PubMed] [Google Scholar]
- 64.MacDonald LD, Anderson HR. Stigma in patients with rectal cancer: a community study. J Epidemiol Community Health. 1984;38:284–90. doi: 10.1136/jech.38.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Santangelo ML, Romano G, Sassaroli C. Sexual function after resection for rectal cancer. Am J Surg. 1987;154:502–4. doi: 10.1016/0002-9610(87)90264-9. [DOI] [PubMed] [Google Scholar]
- 66.Fazio VW, Fletcher J, Montague D. Prospective study of the effect of resection of the rectum on male sexual function. World J Surg. 1980;4:149–52. doi: 10.1007/BF02393562. [DOI] [PubMed] [Google Scholar]
- 67.Weinstein M, Roberts M. Sexual potency following surgery for rectal carcinoma. A followup of 44 patients. Ann Surg. 1977;185:295–300. doi: 10.1097/00000658-197703000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Williams NS, Johnston D. The quality of life after rectal excision for low rectal cancer. Br J Surg. 1983;70:460–2. doi: 10.1002/bjs.1800700805. [DOI] [PubMed] [Google Scholar]
- 69.Fucini C, Gattai R, Urena C, et al. Quality of life among five-year survivors after treatment for very low rectal cancer with or without a permanent abdominal stoma. Ann Surg Oncol. 2008;15:1099–106. doi: 10.1245/s10434-007-9748-2. [DOI] [PubMed] [Google Scholar]
- 70.Gotay CC, Korn EL, McCabe MS, et al. Quality-of-life assessment in cancer treatment protocols: research issues in protocol development. J Natl Cancer Inst. 1992;84:575–9. doi: 10.1093/jnci/84.8.575. [DOI] [PubMed] [Google Scholar]
- 71.Pucciarelli S, Del Bianco P, Toppan P, et al. Health-related quality of life outcomes in disease-free survivors of mid-low rectal cancer after curative surgery. Ann Surg Oncol. 2008;15:1846–54. doi: 10.1245/s10434-008-9923-0. [DOI] [PubMed] [Google Scholar]
- 72.Mannaerts GH, Rutten HJ, Martijn H, et al. Effects on functional outcome after IORT-containing multimodality treatment for locally advanced primary and locally recurrent rectal cancer. Int J Radiat Oncol Biol Phys. 2002;54:1082–8. doi: 10.1016/s0360-3016(02)03012-2. [DOI] [PubMed] [Google Scholar]
- 73.Yost KJ, Cella D, Chawla A, et al. Minimally important differences were estimated for the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) instrument using a combination of distribution- and anchor-based approaches. J Clin Epidemiol. 2005;58:1241–51. doi: 10.1016/j.jclinepi.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 74.Yost KJ, Eton DT. Combining distribution- and anchor-based approaches to determine minimally important differences: the FACIT experience. Eval Health Prof. 2005;28:172–91. doi: 10.1177/0163278705275340. [DOI] [PubMed] [Google Scholar]
- 75.Guren MG, Dueland S, Skovlund E, et al. Quality of life during radiotherapy for rectal cancer. Eur J Cancer. 2003;39:587–94. doi: 10.1016/s0959-8049(02)00741-4. [DOI] [PubMed] [Google Scholar]
- 76.Vironen JH, Kairaluoma M, Aalto AM, et al. Impact of functional results on quality of life after rectal cancer surgery. Dis Colon Rectum. 2006;49:1–11. doi: 10.1007/s10350-006-0513-6. [DOI] [PubMed] [Google Scholar]
- 77.Ayanian JZ, Weissman JS. Teaching hospitals and quality of care: a review of the literature. Milbank Q. 2002;80:569–93. doi: 10.1111/1468-0009.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Porter G, Soskolne CL, Yakimets WW, et al. Surgeon-related factors and outcome in rectal cancer. Ann Surg. 1998;227:157–167. doi: 10.1097/00000658-199802000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ayanian JZ, Chrischilles EA, Wallace RB, et al. Understanding cancer treatment and outcomes; the Cancer Care outcomes research and surveillance consortium. J Clin Oncol. 2004;15:2992–2996. doi: 10.1200/JCO.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 80.Law WL, Chu KW. Impact of total mesorectal excision on the results of surgery of distal rectal cancer. Br J Surg. 2001;88:1607–12. doi: 10.1046/j.0007-1323.2001.01929.x. [DOI] [PubMed] [Google Scholar]
- 81.Bowling A. Measuring disease: a review of disease specific quality of life measurement scales. Philadelphia: Open University Press; 1995. [Google Scholar]


