Abstract
Colon transit (CT) measurements are used in the management of significant constipation. The radiopaque marker (ROM) method provides limited information. We proposed to validate wireless motility capsule (WMC), that measures pH, pressure and temperature, to ROM measurement of CT in patients with symptomatic constipation evaluated at multiple centers.
Of 208 patients recruited, 158 eligible patients underwent simultaneous measurement of CT time (CTT) using ROM (Metcalf method, cut off for delay >67 h), and WMC (cutoff for delay >59 h). The study was designed to demonstrate substantial equivalence, defined as diagnostic agreement >65% for patients who had normal or delayed ROM transit.
59/157 patients had delayed ROM CT. Transit results by the two methods differed: ROM median 55.0h [IQR 31.0–85.0] and WMC (43.5h [21.7–70.3], p<0.001. The positive percent agreement between WMC and ROM for delayed transit was ~80%; positive agreement in 47 by WMC/59 by ROM or 0.796 (95% CI = 0.67–0.98); agreement vs. null hypothesis (65%) p=0.01. The negative percent agreement (normal transit) was ~91%: 89 by WMC/98 by ROM or 0.908 (95% CI = 0.83–0.96); agreement vs null hypothesis (65%), p=0.00001. Overall device agreement was 87%. There were significant correlations (p<0.001) between ROM and WMC transit (CTT [r=0.707] and between ROM and combined small and large bowel transit [r=0.704]). There were no significant adverse events.
The 87% overall agreement (positive and negative) validates WMC relative to ROM in differentiating slow versus normal CT in a multicenter clinical study of constipation.
Keywords: positive agreement, negative agreement, correlation, radiopaque markers, wireless motility capsule, colonic transit time
INTRODUCTION
Chronic constipation is a common disorder and affects approximately 20% of the U.S. population (1). Systematic evaluation of the disorder includes detailed history, clinical evaluation and, in patients who do not respond to dietary changes and laxatives, objective assessment of colonic and anorectal function (2, 3).
In clinical practice, chronic functional constipation is defined by symptoms rather than specific abnormalities in physiology. Patients with chronic functional constipation may have a wide variety of symptoms including reduced stool frequency, hard stool consistency, straining, a sense of incomplete evacuation, and/or the need to use manual maneuvers to defecate. This diversity in clinical presentation is reflected in symptom-based diagnostic criteria (4, 5).
Because symptoms are poor predictors of underlying pathophysiology, physiological assessment of gastrointestinal (GI) tract transit is often indicated. In combination with measures of anorectal and pelvic floor function, transit assessments have been shown to facilitate a diagnosis of slow transit constipation and evacuation disorder although overlap exists among the conditions (3). In order to assess regional transit times, gastric emptying can be evaluated with scintigraphy using a technetium labeled egg sandwich meal and colonic transit time with radio opaque markers (6–8). Whole gut transit can be evaluated using scintigraphy (9–11), but this technique is expensive, involves radiation, and has limited clinical availability.
The wireless motility capsule (WMC) simultaneously measures the gastrointestinal and colonic pH, temperature and intraluminal pressure. The capsule can be used to measure whole gut transit and also regional GI transit, through identification of characteristic changes in pH profile down the GI tract, i.e. abrupt rise in pH on exiting the stomach, and rapid drop in pH from alkaline to mildly acid on passage through the ileocecal region. By utilizing this latter pH change as an indication of the onset of colonic transit, and also the temperature change associated with expulsion through defecation, Rao et al. (12) recently demonstrated the ability of the WMC to estimate colonic transit. The latter study also suggested that WMC may be able to assess colonic transit in patients with constipation.
The study hypothesis is that WMC provides a comparable evaluation of colonic and combined small intestinal and colonic transit to radiopaque markers (ROM) to identify slow transit in patients with constipation, as defined by Rome III criteria. The study was designed to compare simultaneously colonic transit time as measured by ROM and WMC in a multicenter study in patients with symptomatic chronic constipation. The primary objective of this trial was to demonstrate the statistical equivalence between the WMC and the current clinical standard, ROM.
METHODS
Study Design
This multicenter validation study (Protocol number120508) was designed to validate the WMC by measuring transit in patients with symptomatic chronic constipation and comparing to the widely used, quantitative segmental ROM protocol (8) in which 24 markers are ingested each day for three successive days with abdominal radiographs on the fourth and seventh day to count the number of ROM remaining in the abdomen. While performing the comparison of simultaneously measured colonic transit, the study served to establish the distribution of transit measurements in patients with chronic constipation relative to the normal data and threshold values (cut offs) previously established in healthy participants (12).
Participants
The study was designed to enroll 150 subjects with chronic functional constipation using criteria adapted from Rome III criteria with amendment to emphasize abnormal stool consistency. Eligibility criteria included: both genders between ages of 18–80 years with symptoms of chronic functional constipation for at least one year; self reported hard stool at least 25% of the time with at least one of the 6 symptoms of functional constipation as defined by Rome III criteria (such as self-reported bowel movement frequency of less than 3 bowel movements/week for at least 3 of the last 6 months [4]). Participating study centers, prohibited medications and comparator method (8) are included in the Appendix.
Conduct of the Study
Subjects participated in the study for approximately two weeks during which they attended the following study visits:
Visit 1 – screening – for approximately 1 hour
Visit 2 – WMC and ROM ingestion – for approximately 1 hour. Patients then took the ROM on day 2 and 3 on their own.
Visit 3 – abdominal radiograph on day 4 – for approximately 1 hour
Visit 4 – second abdominal radiograph, if necessary, on day 7 – for approximately 1 hour. The radiograph was not indicated if all the ROM and WMC had been expelled on the day 4 radiograph.
Throughout the study participants maintained a daily diary to record stool consistency by Bristol stool form scale (13); participants were encouraged to maintain the usual daily fiber intake and to follow their usual exercise routine.
WMC Method
The method for measuring gastric emptying, small bowel and colonic transit using the WMC has been described in detail in prior publications (12,14,15). The WMC incorporates sensors for pH, temperature and pressure and transmits sensed data at 434 MHz. The single use capsule measures pH from 0.5 to 9.0 pH units with an accuracy of ±0.5 pH units; pressure from 0 to 350 mmHg with an accuracy of ±5 mmHg up to 100 mmHg, and accuracy of ± 10% above 100 mmHg, and temperature from 25 to 49°C with an accuracy of ±1°C. The data are transmitted electronically and are recorded by a portable receiver worn by the participant. The receiver has rechargeable batteries with a life of around 6–7 days. Thus, pH, motility and temperature data are collected over time and exit from the body is signaled when the ambient, environmental temperature is sensed rather than body temperature. All data are initially downloaded from the receiver througha docking station via a USB connection to a Windows PC-compatiblelaptop computer, as previously described (15). The SmartPill GI Monitoring System Version 1.3.1 was used in this study.
Prior studies had established the cut off for delayed CTT was 59 hours (12); for combined small and large bowel transit (SLBTT), the cutoff was 65 hours (95th percentile for healthy participants, data on file, SmartPill Corporation).
The primary WMC parameter for comparison to ROM is colonic transit time (CTT). A secondary endpoint for this method is the combined small and large bowel transit time (SLBTT). This is used as the surrogate measure of colonic transit when CTT is not available. The literature demonstrates that emptying of a solid large particle from the stomach including this WMC usually required gastric migrating motor complex (MMC) activity (14) because of the sieving function of the pylorus (16); on the other hand, the small bowel transit time of such a large particle does not absolutely require MMCs (17) and, in general, large particles are able to traverse the ileocecal region with bolus movements that traverse this region (18). SLBTT also closely approximates CTT because the SBTT is generally 3 to 6 hours whereas colonic transit is generally 24 to 60 hours in asymptomatic people. The WMC estimates of CTT and SLBTT were calculated by a team overseen by one author (JS) all blinded to the ROM transit results. Data were centralized and statistical analysis completed by the statistician (GW) and first author (MC).
Adverse Events
Adverse events were collected using system organ class and preferred terms, and tabulated. All adverse events were recorded. PI for each study site determined classification severity. An independent medical safety monitor resolved any disputed adverse events.
Statistical Considerations
The primary objective of this trial was to demonstrate the equivalence between the diagnostic test under evaluation (WMC) and ROM colonic transit using the quantitative segmental Metcalf method (8) for distinguishing delayed and normal transit in patients with chronic constipation. Device agreement was assessed relative to the comparator method, ROM, which provides an indication of delayed or normal transit.
The primary endpoints of this trial were positive and negative percent agreements between WMC CTT and ROM colonic transit and between WMC SLBTT and ROM colonic transit. A positive and negative percent agreement of more than 0.65 was considered the minimum clinically acceptable level of agreement.
In the analysis, the WMC CTT cutoff [59 hours (12)] and the ROM quantitative segmental CTT cutoff used clinically [67 hours (8)] were applied to symptomatic patients who fulfilled eligibility criteria, and did not have device malfunction or were disqualified for non-compliance. Exact binomial tests were performed to evaluate both positive and negative percent agreement, each at the 0.0253 significance level in order to achieve an overall significance level of 0.05 (19). This design required a total of 150 patients in order to achieve approximately 0.83 power to detect differences of 10 percentage points (0.65 versus 0.75).
We used Wilcoxon signed rank test to compare results obtained by ROM for colonic transit, and for CTT and SLBTT by WMC. We explored further analyses including correlations between ROM and WMC measurements of CTT and SLBTT, and between CTT by WMC and stool frequency and stool consistency using the Bristol stool form scale (13).
We also described, as secondary objectives, the gastric emptying and small bowel transit times for the overall group and for the patients classified as STC or NTC by ROM or by WMC CTT.
RESULTS
Participants, Dispositions and Technical Considerations
Consort style flow chart demonstrates disposition of participants in Figure 1. Among the 158 patients whose data are used for comparison of WMC with ROM comparator method, 20 were male (13%) and 138 female (87%); age was 42.5 ± 12.2 years. The racial distribution was as follows: Caucasian 83%, Black 13%, Asian/Pacific Islanders 2%, Hispanic 1% and other 1%.
Figure 1.
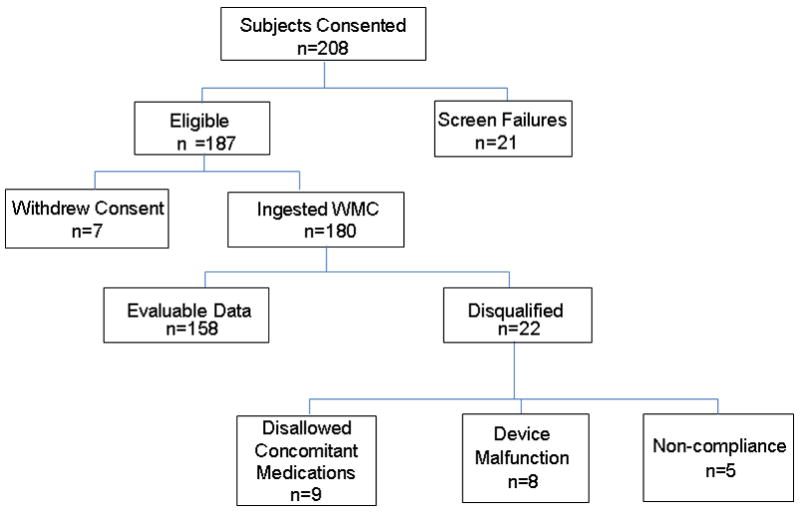
Consort flow chart of participants in study: 208 patients enrolled in the study with 158 successfully completing the study with analyzable data. The 5 subjects who were not compliant failed to attend the study site for the required abdominal radiograph for ROM transit measurement.
One hundred and eighty subjects participated in the study, ingesting WMC and ROM. Nine participants were excluded because of the intake of prohibited concomitant medications including prohibited antibiotics, laxatives, opiate medication and a proton pump inhibitor. Thirteen other participants were excluded because of either device malfunction or noncompliance (Figure 1).
Data from the 158 subjects were used for the assessment of device agreement between the ROM and WMC’s colonic transit time (CTT) and WMC’s small and large bowel transit time (SLBTT). In one subject, the value for CTT was missing due to the absence of the typical ileocecal junction pH change and consequent inability to identify the start of colonic transit. In 4 other subjects, SLBTT could not be estimated due to absence of the typical change in pH that is used to identify the time of exit of the capsule from the stomach.
Thus, assessment of colonic transit was based on comparisons between CTT by WMC and ROM in 157 patients, and comparison between SLBTT by WMC and ROM in 154 patients.
Comparison of WMC and ROM for Colonic Transit Time
Table I shows the positive percent agreement between the WMC colonic transit time cutoff of 59 hours and ROM’s quantitative segmental colonic transit time cutoff of 67 hours.
Table IA.
Number of Patients with Agreement between CTT by WMC and Day 4 + Day 7 ROM Colonic Transit
| D4+D7 ROM + | D4+D7 ROM − | Total | |
|---|---|---|---|
| WMC CTT+ | 47 | 9 | 56 |
| WMC CTT− | 12 | 89 | 101 |
| Total | 59 | 98 | 157 |
+=delay, − =normal transit
Forty-seven of the 59 patients with delayed colonic transit by ROM were also shown to have delayed colonic transit time by WMC. The positive percent agreement of WMC CTT and ROM was ~80% (47/59 = 0.796, 95% CI = 0.67–0.98), which is statistically significant vs the null hypothesis of 65% agreement, (p= 0.01). Similarly, the negative percent agreement is ~91% (89/98= 0.908, 95% CI = 0.83–0.96), which is statistically significant vs the null hypothesis of 65% agreement (p= 0.00001). Overall device agreement is 87% (that is [47+89]/[59+98], and 95% CI 0.80–0.92).
Comparison of WMC and ROM for Small Bowel and Colonic Transit Time
Table II shows the positive percent agreement between the WMC combined small and large bowel transit (SLBTT) cutoff of 65 hours and ROM’s quantitative segmental colonic transit time cutoff of 67 hours. Forty-six of the 58 subjects delayed by ROM were delayed by WMC SLBTT. The positive percent agreement for WMC SLBTT and ROM is ~80% (46/58 or 0.793, 95% CI 0.67– 0.89), which is statistically significant vs the null hypothesis of 65% agreement (p= 0.01). Similarly, the negative percent agreement is 91% (87/96 = 0.906, 95% CI, 0.83– 0.96), which is statistically significant vs the null hypothesis of 65% agreement (p= 0.00001). Overall device agreement is 86%.
Table II.
Median (25th, 75th percentile) Gastric Emptying and Small Bowel Transit Times in Minutes, as Determined by the WMC, in Patients Classified by both ROM and WMC
| Gastric emptying time | Small bowel transit time | Orocecal transit time | ||||
|---|---|---|---|---|---|---|
| Overall group | 185 (157, 248) | 234 (201, 293) | 437 (381, 531) | |||
| NTC | STC | NTC | STC | NTC | STC | |
| Classified by ROM | 179 (148, 244) | 196 (166, 259) | 234 (199, 285) | 233 (201, 315) | 429 (380, 528) | 479 (389, 622) |
| Classified by WMC | 179 (152, 243) | 197 (165, 292) | 232 (194, 285) | 236 (205, 322) | 425 (374, 528) | 505 (419, 618) |
NTC= normal transit constipation; STC=slow transit constipation
Gastric and Small Bowel Transit Times
Table II summarizes gastric emptying time and small bowel transit time for the overall group and for the patients classified as STC or NTC by ROM or by WMC CTT. The prevalence of WMC gastric emptying time >5 hours (cut-off used to suggest gastroparesis [15]) was 28/152 (18.3%) in these patients with constipation; 13 patients had slow colonic transit, and 15 had normal colonic transit.
Relationship between WMC and ROM Marker Estimates of Colonic Transit
Figure 2 shows a summary of the estimated CTT and SLBTT by WMC and colonic transit time by ROM. While there is clear overlap between groups, the paired analysis shows significant differences in the actual WMC estimates for CTT (43.5h [21.7–70.3], p<0.001) and SLBTT (median 47.0 h, [IQR 25.8–75.1] p=0.013, Wilcoxon Signed Rank Test) relative to the ROM estimated CTT (median 55.0h [IQR 31.0–85.0]).
Figure 2.
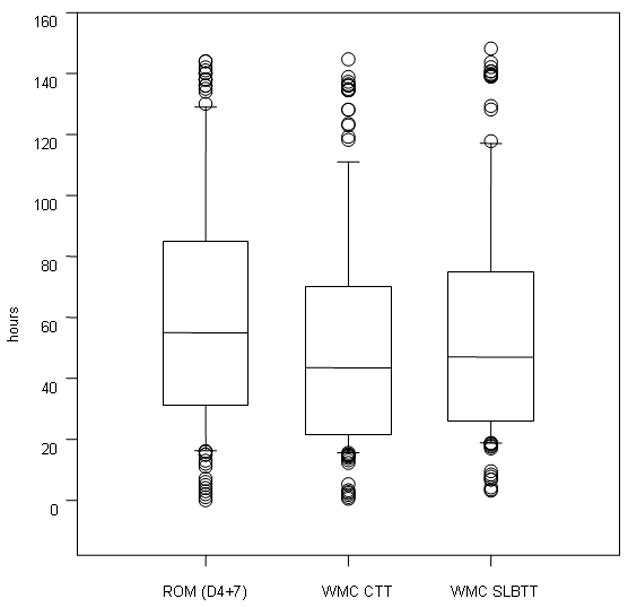
CTT and SLBTT by WMC and colonic transit time by ROM in the entire patient cohort with evaluable data. Data show median, interquartile range (box), 5–95 percentile (whiskers) and outliers as individual points. Note that, while there is clear overlap between the data by each method of transit estimation, the paired analysis shows significant differences in the WMC estimates for CTT (p<0.001) and SLBTT (p=0.013, Wilcoxon Signed Rank Test) relative to the ROM estimated colonic transit time. For reference purposes, note that 95th percentiles in healthy controls are 67 hours for ROM transit, 59 hours for CTT, and 65 hours for SLBTT.
Figure 3 shows a significant correlation between WMC measurements and ROM markers for both CTT (r=0.707, p<0.001) and SLBTT (r=0.704, p<0.001).
Figure 3.
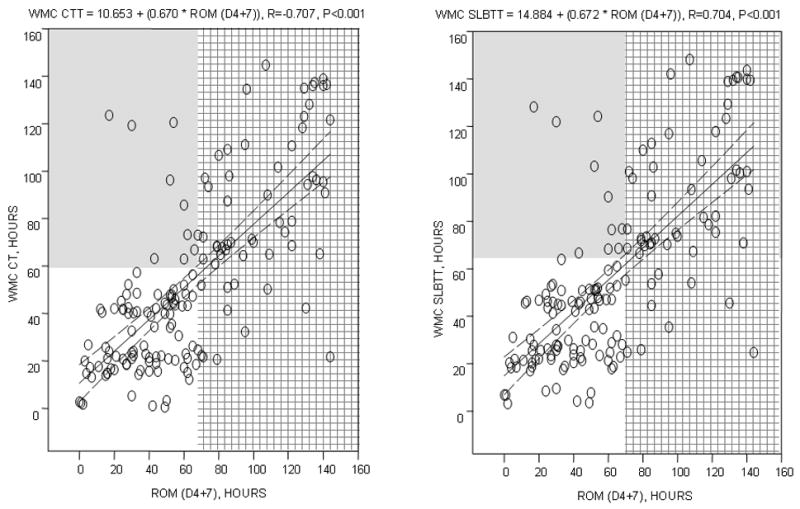
Relationship between CTT and SLBTT by WMC and colonic transit time by ROM (at day 4 plus 7) in the entire patient cohort with evaluable data. Note the significant correlations between WMC estimates and ROM transit time. Interrupted line shows the 95% CI around the regression line. The shaded areas show the values at and above the 95th percentiles for the different methods: 67 hours for ROM transit, 59 hours for CTT, and 65 hours for SLBTT.
Relationship between Wireless Capsule Estimates of Transit and Bowel Function
Spearman coefficients were used to explore the correlation between CTT and SLBTT by WMC and stool consistency using Bristol stool form scale and stool frequency. Data were complete from 154 of the 158 patients and show a significant, but only moderate, correlation of CTT and SLBTT with stool consistency (respectively r= −0.399 and r= −0.427, both p<.0001). In contrast the correlations with stool number per day (frequency) were non-significant (respectively, r=−0.015, p=0.85, and r=−0.023, p=0.78).
Adverse Events
No issues of safety were raised during the study and no serious adverse events were reported. In Table IV, all adverse events reported during the trial are listed by category. There were two females (38 and 50 years old) who were unable to swallow the capsule, and one female (47 years old) who experienced abdominal cramping (starting ~90 minutes after swallowing the capsule). These adverse events were classified as definitely related to WMC. Both subjects experiencing dysphagia ingested WMC after repeated attempts and completed the test without further incident. Symptoms in the patient with abdominal cramping abated after 4 hours. The subject reported no additional symptoms during the entire test and she completed the study without further incident. Abdominal radiographs at 4 and 7 days showed no features to suggest mechanical obstruction and confirmed that the capsule had exited the body by day 7. One case each of abdominal cramping, nausea and loose or soft stools were recorded as possibly related to the device. There were no serious adverse events and no incidents of capsule retention requiring intervention with colonoscopy or endoscopy.
DISCUSSION
This study has demonstrated that the colonic transit measured by WMC significantly correlated with the widely used comparator, ROM. Categorization of constipated subjects into slow or normal colonic transit based on WMC studies matched closely with ROM studies. Specifically, WMC estimate of colonic transit fulfills the expected concordance of at least 65% with ROM, validating WMC to determine whether colonic transit profile is normal or delayed.
There are numerical differences in the actual colonic transit estimates by the WMC and ROM techniques. This is not unexpected given the evidence that particle size influences the transit of solid particles in the small bowel and colon. For example, Stivland et al. observed differences between transit of 1 mm diameter pellets and ~4mm radiopaque markers (20). Van der Sijp et al. (21) documented faster transit of ROM relative to smaller radioisotopically labeled particles. Similarly, indigestible capsules travel more quickly through the colon than ROM, and capsule transit is faster than small dispersed particles (22,23).
ROM assesses whole gut transit as it assesses the location of the markers relative to the time of marker ingestion rather than the time of onset of colonic transit. Inclusion of gastric emptying and small bowel transit time to the transit estimate could account for 6 to 10 h difference between the colon transit time by ROM and the WMC technique. The absolute number estimate for colonic transit time is therefore less relevant than the correct classification of subjects having a normal or delayed transit and the sensitivity and specificity of the test.
The patients in this study represent the spectrum of colonic transit profiles usually encountered in clinical practice, with ~40% having objectively delayed colonic transit by the standard ROM method. This multicenter cohort of patients with constipation reflects the experience of documented slow transit in 38–80% of patients with constipation in other studies (3,24) adding to the generalizability of the data in this study to clinical practice.
The pH change as the capsule traverses from the ileum to the colon determines the time of onset of colon transit with WMC,. The pH in the cecum is more acidic than that of the ileum because of the fermentation of digestive residue by colonic anerobic flora and the nature and concentration of the colonic flora (25,26). This pH drop at the ileocecal junction is well documented in the medical literature (26–28) with the use of ingestible radiotelemetry capsules initially in healthy volunteers and subsequently in patients with a variety of diseases including inflammatory bowel disease (29) and adenomas in the colon, and even in children (26). Overall, pH profiles in the GI tract are characterized by an abrupt rise in pH between the stomach and duodenum, a slow continued rise in pH through the small bowel until reaching the cecum where pH decreases about 1 unit, and subsequently, there is a slow rise in pH through the colon. In general, these changes in pH differed slightly in health and disease, for example, pH decrease was greater in the healthy subjects (7.4 to 5.8) than in the Crohn’s disease patients [7.3 to 6.7 (29)], but the observed decrease in both populations was sufficient to identify transition from ileum to cecum. A recent scintigraphic study has validated this pH change at the ileocolonic junction and has shown that the fall in pH observed with WMC corresponds to the time of arrival of the WMC (labeled with a radioisotope) into the cecum or ascending colon [the anatomy having been outlined with use of a different radioisotope (30)]. In this validation study, we did not assess the effect of different amounts or types of fiber intake, the effect of vigorous exercise, or the potential influence of significant sigmoid diverticulosis and hypertrophy of the muscularis propria. These conditions might alter the pH profile at the ileocecal region or the propulsion or retention of the capsule through the distal colon. Formal prospective studies will be required to address these questions.
Occasionally, the pH drop at the ileocecal junction is not clearly discernible, as occurred in 1 participant in the current study. Reasons for the lack of the pH drop are not understood but may be related to the bacteria in the cecum, and previous food intake. In 4 participants, we could not clearly identify the rise in pH between the stomach and duodenum and this compromised assessment of the SLBTT. Therefore, there might be difficulty in interpreting the test in <1% for CTT and 3–4% for SLBTT, consistent with previous studies (12,15).
The study also showed the relationship between CTT and SLBTT measured by the WMC and stool consistency measured by Bristol stool form scale, rather than stool frequency, confirming a prior study (31). This is consistent with the significant relationship between colonic transit by scintigraphy and stool form in pharmacodynamic studies of renzapride or linaclotide (32,33). In 10 healthy volunteers, there was significant correlation between overall, gastric and colonic transit measured by scintigraphy and by WMC (34).
This study has therefore provided validation of the WMC to estimate colonic transit, by showing good agreement between the WMC and ROM method. The agreement and correlation in this study are higher than the study of Rao et al. that used a simplified ROM method to assess colonic transit with a single abdominal radiograph taken five days after marker ingestion (12). It is relevant to note that relative to ROM, the WMC provides a 20% misdiagnosis in slow transit constipation and a false positive rate of 9% in normal transit constipation. However, this assumes that ROM is a “gold standard”; whereas, it should be termed a non-reference standard. The WMC is able to characterize pressure activity in the colon in health and disease states (35). Studies are now under way to determine whether additional information of clinical relevance is provided by measurement of colonic contractile functions. The ability to measure transit and pressures has the potential to enhance the ability of gastroenterologists and surgeons in practice to assess patients with suspected motility disorders such as gastroparesis and slow transit constipation. This general availability of a technique with standardized and automated analysis contrasts with the lack of general applicability or availability of whole gut scintigraphy and intubated intraluminal manometry available at tertiary referral centers (36–38). Capsule based methods do not expose patients to radiation, in contrast to radioscintigraphy and radiopaque marker methods, the latter requiring multiple fluoroscopic or radiologic images (39).
Other capsule techniques are reported to measure gastrointestinal motility noninvasively, without radiation exposure, using very different techniques and measuring different dimensions of motor function. A magnet tracking analyses the origin, direction, amplitude and velocity of movements of a magnetic capsule relative to space-time plots detected through a detection matrix (4 × 4 magnetic field sensors) and dedicated software implanted in a laptop computer (40). At present, this is a research technique in non-ambulant subjects. Image analysis with capsule endoscopy detects contractile patterns (phasic luminal closure and radial wrinkles by wall texture analysis), non-contractile patterns, intestinal content, and endoluminal motion (41). It was used in patients with small intestinal motility disorders and in healthy volunteers exposed to glucagon (42).
There are potential pitfalls with using all capsules to measure gut transit including technical failures, inability to swallow the capsule, the potential for non-passage of or intestinal obstruction by the capsule in stenosing gut disorders, and greater cost relative to the radiopaque marker transit method. Application of the WMC is contraindicated in patients with known esophageal or intestinal strictures, and children under 18 years of age, in whom validation studies have not yet been completed.
In conclusion, the WMC provides a clinically relevant estimate of colonic transit that is able to differentiate slow from normal transit constipation. Wireless motility capsule technology has the potential to bring valid, noninvasive motility measurements to the practice of gastroenterology in the community.
Table IB.
Number of Patients with Agreement between Small and Large Bowel TT by WMC and Day 4 + Day 7 ROM Colonic Transit
| D4+ D7 ROM + | D4+ D7 ROM − | Total | |
|---|---|---|---|
| WMC SLBTT+ | 46 | 9 | 55 |
| WMC SLBTT− | 12 | 87 | 99 |
| Total | 58 | 96 | 154 |
+ = delay, − = normal transit
Table III.
Adverse Events
| AE Relationship to the Study Device | ||||||
|---|---|---|---|---|---|---|
| Not Related | Probably Not Related | Possibly Related | Probably Related | Definitely Related | ||
| N | N | N | N | N | ||
| System Organ Class | Preferred Term | |||||
| Gastrointestinal Disorders | Abdominal Pain | 0 | 1 | 1 | 0 | 1 |
| Diarrhea | 0 | 0 | 1 | 0 | 0 | |
| Dysphagia | 0 | 0 | 0 | 0 | 2 | |
| Frequent bowel movements | 0 | 2 | 0 | 0 | 0 | |
| Gastrointestinal Pain | 0 | 1 | 0 | 0 | 0 | |
| Nausea | 1 | 2 | 2 | 0 | 0 | |
| Vomiting | 1 | 2 | 0 | 0 | 0 | |
| General disorders and administrative site conditions | Pyrexia | 0 | 1 | 0 | 0 | 0 |
| Sluggishness | 1 | 0 | 0 | 0 | 0 | |
| Infections and Infestations | Bronchitis | 1 | 0 | 0 | 0 | 0 |
| Cystitis | 1 | 0 | 0 | 0 | 0 | |
| Ear Infection | 1 | 0 | 0 | 0 | 0 | |
| Pharyngitis streptococcal | 1 | 0 | 0 | 0 | 0 | |
| Tooth abscess | 1 | 0 | 0 | 0 | 0 | |
| Upper Respiratory Tract Infection | 1 | 0 | 0 | 0 | 0 | |
| Injury, poisoning and procedural complications | Post procedural complication | 1 | 0 | 0 | 0 | 0 |
| Musculoskeletal and connective tissue disorders | Muscle spasms | 1 | 0 | 0 | 0 | 0 |
| Nervous System disorders | Headache | 1 | 1 | 0 | 0 | 0 |
| Migraine | 1 | 0 | 0 | 0 | 0 | |
| Respiratory, thoracic and mediastinal disorders | Asthma | 1 | 0 | 0 | 0 | 0 |
| Total Number of Adverse Events | 14 | 10 | 4 | 0 | 3 | |
Acknowledgments
The study was supported by a “New York State Foundation for Science, Technology and Innovation Grant”. Funding grant support was received by all study centers from the SmartPill Corporation, Buffalo, New York. Dr. Camilleri is supported in part by grant RO1-DK-54681 from National Institutes of Health. The authors acknowledge the excellent contribution of gastroenterology colleagues at the different medical centers who helped conduct this study: Ashok Attaluri, MD, University of Iowa; Shanthini Daniel, MD, Jasper Clinic; Javier Gomez, MD, Temple University; Otto Linet, MD, Jasper Clinic Sahar D. Mohammed, MD, Queen Mary University, London; Suwebatu Odunsi-Shiyanbade, MD, Mayo Clinic; Irene Sarosiek, MD, Texas Tech University
APPENDIX
Participating Study Centers
The following is the number of eligible subjects at the investigative sites: Mayo Clinic Rochester (n=30), University of Iowa (n=28), Wake Forest University (n=28), Jasper Clinic, Inc., Kalamazoo, Michigan (n=19), University of North Carolina (n=18), University of Michigan (n=15), University of Buffalo VAMC (n=10), Kansas University (n=12), Massachusetts General Hospital (n=8), Queen Mary University, London, United Kingdom (n=8), Cedars-Sinai Medical Center Los Angeles (n=3), and Temple University (n=1).
Prohibited Medications
Two main categories of medication were prohibited prior to or during the studies:
Medications which alter gastric pH, including proton pump inhibitors for 7 days prior and including the day of WMC ingestion; H2 blockers for 3 days including the day of WMC ingestion, and antacids for 1 day prior to ingestion.
Medications that affect gastrointestinal motility, including prokinetics, antiemetics, narcotic analgesics, anticholinergic agents, medications for constipation, 5-HT3 antagonists, antidiarrheal agents, opiates used to treat diarrhea and NSAIDs.
Comparator Method
The Metcalf method involves ingestion of a capsule containing 24 radiopaque markers on three successive days Abdominal x-rays are taken on day 4 (72 hours after the ingestion of the first 24 markers) and day 7 (144 hours after the ingestion of the first 24 markers) and the number and distribution of the markers present in the colon is counted. Colonic transit time is calculated by summing the number of markers visualized on the day 4 and day 7 x-rays and equating 1 marker to 1 hour of colonic transit time. Colonic transit time of greater than 67 hours with this ROM method is considered delayed and is derived from the 95th percentile of colonic transit time of healthy subjects as reported by Metcalf et al. (8). The colonic transit times were reported by the investigators at each study center.
Footnotes
Authors’ Contributions: Dr. Camilleri: study conceptualization, study center PI, data interpretation, writing protocol and paper;
Dr. Rao: study conceptualization, study center PI, writing protocol and paper;
Dr. Parkman: study conceptualization, study center PI, writing protocol and paper;
Dr. Kuo: study conceptualization, study center PI, writing protocol and paper;
Dr. Semler: study conceptualization, writing protocol and paper;
Dr. Wilding: study conceptualization, statistical analyses, writing protocol and paper
Dr. Hasler: study conceptualization, study design and implementation, and writing paper,
Dr. Gupta: study conceptualization, study center PI, and writing paper
Dr. McCallum: study conceptualization, study center PI, and writing paper
Dr. Thorne: study center PI, and writing paper
Dr. Scott: study center PI, and writing paper
Dr. Soffer: study center PI, and writing paper
Dr. Ringel: study center PI, and writing paper
Dr. Esfandyari: study center PI, and writing paper
Clinical Trials.gov identifier: NCT00857363
Conflict of Interest: Dr. Camilleri serves as a consultant to SmartPill Corporation, with compensation to Mayo Clinic, not to him. He received grant support to participate in this study.
Dr. Rao, Dr. Hasler, Dr. Kuo, Dr. McCallum, Dr. Parkman, Dr. Scott, Dr. Wilding serve as speakers, consultants, or advisory board members for the SmartPill Corporation. These authors and Dr. Soffer have received research funding for other research studies from the SmartPill Corporation.
Dr. Semler is an employee of the SmartPill Corporation and owns stock in the SmartPill Corporation.
References
- 1.Higgins DR, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004;99:750–759. doi: 10.1111/j.1572-0241.2004.04114.x. [DOI] [PubMed] [Google Scholar]
- 2.Lembo A, Camilleri M. Chronic constipation. N Engl J Med. 2003;349:1360–1368. doi: 10.1056/NEJMra020995. [DOI] [PubMed] [Google Scholar]
- 3.Rao SS, Ozturk R, Laine L. Clinical utility of diagnostic tests for constipation in adults: a systematic review. Am J Gastroenterol. 2005;100:1605–1615. doi: 10.1111/j.1572-0241.2005.41845.x. [DOI] [PubMed] [Google Scholar]
- 4.Rome III [The Rome Foundationhttp://www.romecriteria.org/questionnaires ])
- 5.Brandt LJ, Prather CM, Eamonn MM, et al. Systematic review on the management of chronic constipation in North America. Am J Gastroenerol. 2005;100(S1):S5–S21. doi: 10.1111/j.1572-0241.2005.50613_2.x. [DOI] [PubMed] [Google Scholar]
- 6.Hinton JM, Lennard-Jones JE, Young AC. A new method for studying gut transit times using radioopaque markers. Gut. 1969;10:842–847. doi: 10.1136/gut.10.10.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arhan P, Devroede G, Jehannin B, Lanza M, Faverdin C, Dornic C, Persoz B, Tétreault L, Perey B, Pellerin D. Segmental colonic transit time. Dis Colon Rectum. 1981;24:625–629. doi: 10.1007/BF02605761. [DOI] [PubMed] [Google Scholar]
- 8.Metcalf AM, Phillips SF, Zinsmeister AR, MacCarty RL, Beart RW, Wolff BG. Simplified assessment of segmental colonic transit. Gastroenterology. 1987;92:40–47. doi: 10.1016/0016-5085(87)90837-7. [DOI] [PubMed] [Google Scholar]
- 9.Camilleri M, Colemont LJ, Phillips SF, Brown ML, Thomforde GM, Chapman N, Zinsmeister AR. Human gastric emptying and colonic filling of solids characterized by a new method. Am J Physiol. 1989;257:G284–G290. doi: 10.1152/ajpgi.1989.257.2.G284. [DOI] [PubMed] [Google Scholar]
- 10.Camilleri M, Zinsmeister AR. Towards a relatively inexpensive, noninvasive, accurate test for colonic motility disorders. Gastroenterology. 1992;103:36–42. doi: 10.1016/0016-5085(92)91092-i. [DOI] [PubMed] [Google Scholar]
- 11.Bonapace ES, Maurer AH, Davidoff S, Krevsky B, Fisher RS, Parkman HP. Whole gut transit scintigraphy in the clinical evaluation of patient with upper and lower gastrointestinal symptoms. Am J Gastroenterol. 2000;95:2338–2347. doi: 10.1111/j.1572-0241.2000.03195.x. [DOI] [PubMed] [Google Scholar]
- 12.Rao SS, Kuo B, McCallum RW, Chey WD, DiBaise JK, Hasler WL, Koch KL, Lackner JM, Miller C, Saad R, Semler JR, Sitrin MD, Wilding GE, Parkman HP. Investigation of colonic and whole-gut transit with wireless motility capsule and radiopaque markers in constipation. Clin Gastroenterol Hepatol. 2009;7:537–544. doi: 10.1016/j.cgh.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Heaton KW, Radvan J, Cripps H, Mountford RA, Braddon FE, Hughes AO. Defecation frequency and timing, and stool form in the general population: a prospective study. Gut. 1992;33:818–824. doi: 10.1136/gut.33.6.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cassilly D, Kantor S, Knight LC, Maurer AH, Fisher RS, Semler J, Parkman HP. Gastric emptying of a non-digestible solid: assessment with simultaneous SmartPill pH and pressure capsule, antroduodenal manometry, gastric emptying scintigraphy. Neurogastroenterol Motil. 2008;20:311–319. doi: 10.1111/j.1365-2982.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- 15.Kuo B, McCallum RW, Koch KL, Sitrin MD, Wo JM, Chey WD, Hasler WL, Lackner JM, Katz LA, Semler JR, Wilding GE, Parkman HP. Comparison of gastric emptying of a nondigestible capsule to a radio-labelled meal in healthy and gastroparetic subjects. Aliment Pharmacol Ther. 2008;27:186–196. doi: 10.1111/j.1365-2036.2007.03564.x. [DOI] [PubMed] [Google Scholar]
- 16.Meyer JH, Thomson JB, Cohen MB, Shadchehr A, Mandiola SA. Sieving of solid food by the canine stomach and sieving after gastric surgery. Gastroenterology. 1979;76:804–813. [PubMed] [Google Scholar]
- 17.Sarr MG, Kelly KA. Patterns of movement of liquids and solids through canine jejunum. Am J Physiol. 1980;239:G497–503. doi: 10.1152/ajpgi.1980.239.6.G497. [DOI] [PubMed] [Google Scholar]
- 18.Phillips SF, Quigley EM, Kumar D, Kamath PS. Motility of the ileocolonic junction. Gut. 1988;29:390–406. doi: 10.1136/gut.29.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pepe MS. The Statistical Evaluation of Medical Tests for Classification and Prediction. Oxford University Press; New York: 2003. [Google Scholar]
- 20.Stivland T, Camilleri M, Vassallo M, Proano M, Rath D, Brown M, Thomforde G, Pemberton J, Phillips S. Scintigraphic measurement of regional gut transit in idiopathic constipation. Gastroenterology. 1991;101:107–115. doi: 10.1016/0016-5085(91)90466-x. [DOI] [PubMed] [Google Scholar]
- 21.van der Sijp JR, Kamm MA, Nightingale JM, Britton KE, Mather SJ, Morris GP, Akkermans LM, Lennard-Jones JE. Radioisotope determination of regional colonic transit in severe constipation: comparison with radio opaque markers. Gut. 1993;34:402–408. doi: 10.1136/gut.34.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holdstock DJ, Misiewicz JJ, Smith T, Rowlands EN. Propulsion (mass movements) in the human colon and its relationship to meals and somatic activity. Gut. 1970;11:91–99. doi: 10.1136/gut.11.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hebden JM, Gilchrist PJ, Blackshaw E, Frier ME, Perkins AC, Wilson CG, Spiller RC. Night-time quiescence and morning activation in the human colon: effect on transit of dispersed and large single unit formulations. Eur J Gastroenterol Hepatol. 1999;11:1379–1385. doi: 10.1097/00042737-199912000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Ravi K, Bharucha AE, Camilleri M, Rhoten D, Bakken T, Zinsmeister AR. Phenotypic variation of colonic motor functions in chronic constipation. Gastroenterology. 2009 Aug 4; doi: 10.1053/j.gastro.2009.07.057. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fallingborg J, Christensen LA, Ingeman-Nielsen M, Jacobsen BA, Abildgaard K, Rasmussen HH, Rasmussen SN. Measurement of gastrointestinal pH and regional transit times in normal children. J Pediatr Gastroenterol Nutr. 1990;11:211–214. doi: 10.1097/00005176-199008000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Fallingborg J, Christensen LA, Ingeman-Nielsen M, Jacobsen BA, Abildgaard K, Rasmussen HH, Rasmussen SN. Gastrointestinal pH and transit times in healthy subjects with ileostomy. Aliment Pharmacol Ther. 1990;4:247–253. doi: 10.1111/j.1365-2036.1990.tb00469.x. [DOI] [PubMed] [Google Scholar]
- 27.Evans DF, Pye G, Bramley R, Clark AG, Dyson TJ, Hardcastle JD. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut. 1988;29:1035–1041. doi: 10.1136/gut.29.8.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watson BW, Meldrum SJ, Riddle HC, Brown RL, Sladen GE. pH profile of gut as measured by radiotelemetry capsule. Br Med J. 1972;2:104–106. doi: 10.1136/bmj.2.5805.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fallingborg J, Pedersen P, Jacobsen BA. Small intestinal transit time and intraluminal pH in ileocecal resected patients with Crohn’s disease. Dig Dis Sci. 1998;43:702–705. doi: 10.1023/a:1018893409596. [DOI] [PubMed] [Google Scholar]
- 30.Zarate N, Mohammed S, O’Shaughnessy E, Newell M, Yazaki E, Semler J, Scott SM. Accurate localisation of a fall in pH within the ileo-caecal region. Neurogastroenterol Motil. 2009;21(Suppl 1):43. (abstract) [Google Scholar]
- 31.Saad RJ, Chey WD, Rao SS, Hasler WL, Katz LA, Koch KL, Kuo B, Lackner JM, Parkman HP, McCallum R, Miller C, Sarosiek I, Semler J, Sitrin MD, Wilding GE. Reduced stool frequency does not predict colonic and whole gut transit in constipated patients. Gastroenterology. 2009;136(Suppl 1):M1209. (abstract) [Google Scholar]
- 32.Camilleri M, McKinzie S, Fox J, Foxx-Orenstein A, Burton D, Thomforde G, Baxter K, Zinsmeister AR. Effect of renzapride on transit in constipation-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2004;2:895–904. doi: 10.1016/s1542-3565(04)00391-x. [DOI] [PubMed] [Google Scholar]
- 33.Andresen V, Camilleri M, Busciglio I, Grudell A, Burton D, McKinzie S, Foxx-Orenstein A, Kurtz CB, Sharma V, Johnston JM, Currie MG, Zinsmeister AR. Effect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome. Gastroenterology. 2007;133:761–768. doi: 10.1053/j.gastro.2007.06.067. [DOI] [PubMed] [Google Scholar]
- 34.Maqbool S, Parkman HP, Friedenberg FK. Wireless capsule motility: comparison of the SmartPill((R)) GI Monitoring System with scintigraphy for measuring whole gut transit. Dig Dis Sci. 2009;54:2167–2174. doi: 10.1007/s10620-009-0899-9. [DOI] [PubMed] [Google Scholar]
- 35.Hasler WL, Saad RJ, Rao SR, Wilding GE, Parkman HP, Koch KL, McCallum RW, Kuo B, Sarosiek I, Sitrin MD, Semler JR, Chey WD. Heightened coln motor activity measured by a wireless capsule in patients with constipation: Relation to colon transit and IBS. Am J Physiol Gastrointest Liver Physiol. 2009 Oct 1; doi: 10.1152/ajpgi.00136.2009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Charles F, Camilleri M, Phillips SF, Thomforde GM, Forstrom LA. Scintigraphy of the whole gut: clinical evaluation of transit disorders. Mayo Clin Proc. 1995;70:113–118. doi: 10.4065/70.2.113. [DOI] [PubMed] [Google Scholar]
- 37.Bonapace ES, Maurer AH, Davidoff S, Krevsky B, Fisher RS, Parkman HP. Whole gut transit scintigraphy in the clinical evaluation of patients with upper and lower gastrointestinal symptoms. Am J Gastroenterol. 2000;95:2838–2847. doi: 10.1111/j.1572-0241.2000.03195.x. [DOI] [PubMed] [Google Scholar]
- 38.Dinning PG, Bampton PA, Andre J, Kennedy ML, Lubowski DZ, King DW, Cook IJ. Abnormal predefecatory colonic motor patterns define constipation in obstructed defecation. Gastroenterology. 2004;127:49–56. doi: 10.1053/j.gastro.2004.03.066. [DOI] [PubMed] [Google Scholar]
- 39.Sadik R, Stotzer PO, Simrén M, Abrahamsson H. Gastrointestinal transit abnormalities are frequently detected in patients with unexplained GI symptoms at a tertiary centre. Neurogastroenterol Motil. 2008;20:197–205. doi: 10.1111/j.1365-2982.2007.01025.x. [DOI] [PubMed] [Google Scholar]
- 40.Hiroz P, Schlageter V, Givel JC, Kucera P. Colonic movements in healthy subjects as monitored by a magnet tracking system. Neurogastroenterol Motil. 2009;21:838–857. doi: 10.1111/j.1365-2982.2009.01298.x. [DOI] [PubMed] [Google Scholar]
- 41.Malagelada C, De Iorio F, Azpiroz F, Accarino A, Segui S, Radeva P, Malagelada JR. New insight into intestinal motor function via noninvasive endoluminal image analysis. Gastroenterology. 2008;135:1155–1162. doi: 10.1053/j.gastro.2008.06.084. [DOI] [PubMed] [Google Scholar]
- 42.de Iorio F, Malagelada C, Azpiroz F, Maluenda M, Violanti C, Igual L, Vitrià J, Malagelada JR. Intestinal motor activity, endoluminal motion and transit. Neurogastroenterol Motil. 2009 Jul 10; doi: 10.1111/j.1365-2982.2009.01363.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


