Abstract
Despite the therapeutic use and abuse potential of gamma-hydroxybutyrate (GHB or Xyrem), relatively few studies have examined the behavioral effects of GHB in humans under controlled laboratory conditions. Thus, this eight-session study examined in 10 non substance-abusing volunteers the behavioral effects of GHB at each of the following doses: 0, 0.32, 0.56, 0.75, 1.0, 1.8, 2.4, 3.2 g/70 kg, p.o.. Order of dose testing was random, except that the first two participants received active doses in ascending order and 2.4 g/70 kg was always tested before 3.2 g/70 kg. Prior to drug administration and at several post-drug time points, self-report, observer-report, physiological, and psychomotor performance measures were obtained. Analyses based on area under the curve showed that GHB produced dose-related increases in subjective ratings of sedative-like, stimulant-like, positive mood, and dissociative effects, but no changes in psychomotor performance measures or blood pressure. Analyses based on peak effects generally showed dose-related increases in ratings indicating sedative-like, dissociative, and drug liking, although some measures showed U-shaped dose-related changes. These initial findings suggest that GHB at doses of 0.32–3.2 g/70 kg produces dissociative, sedating and some stimulant-like effects in humans without a history of sedative abuse.
Keywords: Gamma-hydroxybutyric acid, Sodium oxybate, Human, Behavioral Pharmacology, Subjective effects
Introduction
Gamma-hydroxybutyric acid (GHB) is a recreational drug among typically young adults (e.g., Centers for Disease Control, 1997a,Centers for Disease Control, 1997b; Kam and Yoong, 1998; Maxwell and Spence, 2005; see review by Degenhardt et al., 2005) as well as gay and bisexual male club drug users (Halkitis and Palamar, 2006). The illicit use of GHB has historically been associated with coma, seizures, death and drug-facilitated sexual assault in the United States and other countries (e.g., Cameron, 2001; Centers for Disease Control, 1991, 1997a,1997b; Ferrara et al., 1995; Li et al., 1998; Stell and Ryan, 1996; Schwarz et al., 2000; Thomas et al., 1997), which resulted in its being classified as a controlled substance (see review by Carter et al., 2009a). Although legal restrictions on availability of GHB have been associated with decreased reported use and negative consequences (see Carter et al., 2009a), concerns regarding the availability and abuse of this agent still linger, particularly given that the US Food and Drug Administration approved the use of GHB to treat cataplexy in narcoleptic patients in 2002 under the name of Xyrem (Anon, 2002), and in 2005. extended the label to include excessive daytime sleepiness Be that as it may, there is a paucity of research investigating the behavioral effects of GHB, highlighting the importance of characterizing better the behavioral pharmacology of GHB in humans.
The pharmacology of GHB is complex. Several lines of evidence suggest that GHB, an endogenous compound in the human brain (Cash et al., 1979; Doherty et al., 1978) and peripheral tissues (Nelson et al., 1981) with GHB-specific binding sites (Benavides et al., 1982; Hosli and Hosli, 1983; Snead and Liu, 1984), is a neurotransmitter (e.g., Anderson et al., 1977; Benavides et al., 1982; Hosli and Hosli, 1983; Maitre and Mandel, 1982; Maitre et al., 1983; see Vayer et al., 1987). GHB also affects other neurotransmitter systems (Snead, 1977; Tunniclif, 1992), including gamma-aminobutyric acid (GABA), through weak affinity for GABA-B (Bernasconi et al., 1992; Ito et al., 1995; Marescaux et al., 1992; Mathivet et al., 1997), but not GABA-A (Serra et al., 1991; Snead and Liu, 1993) receptors in the brain. GHB produces a biphasic response on dopaminergic activity, inhibiting dopamine release at low doses and promoting release at high doses (Bustos et al., 1972; Bustos and Roth, 1972; Hechler et al., 1991, 1993). Moreover, GHB increases serotonin turnover in several brain regions without altering absolute concentration levels (Gobaille et al., 2002; Miguez et al., 1988), and inhibits norepinephrine release in the hypothalamus (Miguez et al., 1988 Szabo et al., 2004). GHB has also been shown to enhance the release of endogenous opioids by an apparently indirect mechanism at high doses (Greiner et al., 2003), as well as to stimulate growth hormone secretion (Blue-Pajot et al., 1978; Bluet-Pajot and Schaub, 1978; Gerra et al., 1994). Unlike most other known neurotransmitters, however, GHB administered systemically can cross the blood-brain barrier, resulting in CNS-mediated effects such as sedation, sleep, abnormal EEG, and anesthesia (see Cash, 1994). To date, no clear intrinsic physiological function for GHB has been defined.
The complexity of the actions of GHB is also highlighted by the myriad therapeutic roles for which it has been studied. For instance, GHB has been used as an intravenous anesthetic agent (Laborit, 1964) and in the treatment of sleep disorders (Mamelak et al., 1986). GHB has also been used to treat alcohol withdrawal (Addolorato et al., 1999a; Gallimberti et al., 1989), and alcohol dependence (Addolorato et al., 1997b, 2009; Gallimberti et al., 1992; Nava et al., 2007) in humans. In addition, GHB reportedly alleviates symptoms of opiate withdrawal in opioid-dependent humans (Gallimberti et al., 1993, 1994) as well as symptoms associated with fibromyalgia (Russell et al., 2009; Scharf et al., 2003).
Nevertheless, the therapeutic potential of GHB may be limited by its potential for abuse and dependence (e.g., Addolorato et al., 2009). Although generally not self-administered above rates seen with placebo in monkeys (Beardsley et al., 1996; Woolverton et al., 1999), GHB is self-administered in mice (Martellotta et al., 1998) and induces conditioned place preference in rats (Martellotta et al., 1997). Genetically bred ethanol-preferring rats have also shown greater GHB preference and intake than genetically bred ethanol-nonpreferring rats (Colombo et al., 1998). GHB has reportedly been used illicitly (e.g., Centers for Disease Control, 1997a,b; Galloway et al., 1997) and abused in alcohol treatment settings (Addolorato et al., 1996, 1997a,b). Prolonged GHB administration has also led to the development of physical dependence in nonhumans (e.g., Goodwin et al., 2006; Weerts et al., 2005) and humans (Addolorato et al., 1999b; Galloway et al., 1997; see review by van Noordan et al., 2009). These findings highlight the importance of identifying the behavioral effects of GHB more precisely in order to inform assessments of risks and benefits associated with the therapeutic use of this agent.
Several studies in humans have examined the behavioral effects of GHB under controlled laboratory conditions (e.g., Abanades et al., 2006, 2007; Carter et al., 2006, 2009a,b; Grove-White et al., 1971; Ferrara et al., 1999; Matilla et al., 1978; Metcalf et al., 1966; Rosen et al., 1997). Nevertheless, the behavioral pharmacology of GHB is yet to be characterized fully in humans. For instance, the majority of these studies have employed participants with recreational use or abuse histories (e.g., Abanades et al., 2006, 2007; Carter et al., 2006, 2009a,b; Rosen et al., 1997) and/or examined GHB at oral doses of 1.0 g or higher (e.g., Abanades et al., 2006, 2007; Carter et al., 2006, 2009a,b; Matilla et al., 1978; Metcalf et al., 1966; Rosen et al., 1997). Those studies that examined doses lower than 1 g, only examined one dose (e.g., 0.875 g/70 kg p.o., Ferrara et al., 1999; 0.7 g/70 kg i.v.,Grove-White et al., 1971). However, GHB is reportedly used recreationally at oral doses lower than 1.0 g (Erowid, 2009). Thus, the present study sought to clarify further the behavioral effects of GHB by testing doses across a wider - and lower - range than examined previously, in participants without extensive drug use histories; that is, at doses considered to be within “light” (0.5–1.5 g), “common” (1.0–2.5 g), and “strong” (2.0–4.5 g) oral dose ranges of GHB used recreationally (Erowid, 2009).
Methods
Participants
Seventeen healthy volunteers (71% female; 12 Caucasian, 4 African American, 1 Native American) with a mean age of 32.9 yrs (range: 19–47) were recruited for this study from the general West Haven-New Haven community via word-of-mouth, newspaper ads and flyers, and participated on an outpatient basis. Each subject's eligibility was ascertained through a comprehensive evaluation that included complete physical, neurological, and clinical examinations, laboratory chemistry tests, and electrocardiogram. In order to participate, subjects had to report ingesting >10 alcoholic drinks in their lifetime, score less than 4 on the Michigan Alcoholism Screening Test (MAST), have a negative pregnancy test and use effective birth control during study participation (for women), and submit a urine that tested negative for illicit drugs. Potential candidates could not participate if they had a major medical condition (e.g., major endocrine disorder), had a current diagnosis of drug or alcohol dependence or abuse (other than tobacco), had a history of organic psychiatric disorder (e.g., schizophrenia), reported recent use of over-the-counter or prescription psychoactive drugs, particularly any central nervous system depressant (Sanguineti et al., 1997; Steele & Watson, 1995), reported recent use of a drug that would have major interaction with GHB, or had a history of epilepsy or other seizure disorder. Inclusion and exclusion criteria were ascertained through comprehensive medical and psychiatric history, electrocardiogram, blood chemistries, urinalyses, urine toxicology screens and a physical exam by one of the study physicians. All participants gave written informed consent to participate in the present study and were compensated monetarily for their participation. The Yale Human Investigations Committee and the VA CT Healthcare System Human Subjects Subcommittee approved this protocol.
Setting
This study was conducted at the Outpatient Behavioral Pharmacology Laboratory at the VA Connecticut Healthcare System, West Haven Campus. The laboratory consisted of a four-station experimental room with an adjacent lounge, where subjects could relax after the experimental portion of the session while waiting for drug effects to subside. A research nurse administered all medications and was present for the entire session. A physician was available on-site by pager during each session.
Study Design
Participants underwent eight experimental sessions in which GHB was administered in a double-blind manner at one of the following doses: 0, 0.32, 0.56, 0.75, 1.0, 1.8, 2.4, 3.2 g/70 kg. The study was a within-subject design, in that all subjects received all doses of GHB. Self-reported, observer-rated, physiological, and performance effects were assessed prior to and several time points following drug administration (see below).
Drugs
Orphan Medical supplied GHB and placebo in a liquid formula. The VA Research Pharmacist prepared the study medication. The volume of the liquid was held constant within subjects. For the first two participants active GHB was administered in ascending order of dose with placebo administered at some random point within the order, in order to ascertain the tolerability of these GHB doses. Thereafter GHB was administered double blind in a randomized dose sequence with the caveat that the 2.4 g/70 kg dose was always tested prior to the 3.2 g/70 kg dose. If, at any time, GHB produced significant behavioral effects (i.e., difficulty rousing from sleep), then the blind was broken for that session and higher doses were not tested. These doses of GHB generally were selected to provide a wide range of behavioral effects, from minimal effects at the lowest dose to maximal effect with little toxicity or behavioral impairment at the highest dose (Metcalf et al., 1966). In order to increase participant blindness, the subjects themselves were never told exactly what drug they would receive during the study, but were given a list of compounds (i.e., clonidine, dextromethorphan, gamma-hydroxybutyric acid, naloxone, triazolam, and placebo) with the instruction that they would receive 2–4 of the agents listed and never a drug that was not on the list.
Experimental Sessions
Because GHB has a short half-life of approximately 30 minutes (Ferrara et al., 1992; Palatini et al., 1993), sessions occurred up to 5 days/wk, depending upon subject availability. Each session began with collecting a urine sample to test for illicit drug use and a breath sample to analyze alcohol content. If the breathalyzer indicated recent alcohol use, the session was not conducted on that day and the subject was warned that another similar incident could mean discharge from the study. If the toxicology test came back positive for illicit drugs, the participant was counseled that further drug use could mean discharge from the study. Prior to each session, subjects passed a sobriety test (adapted from that used by law enforcement) that consisted of tests of balance, hand coordination and simple arithmetic, and completion of a pre-session form. The pre-session form consisted of questions related to daily routine in the last 24 hours such as hours of sleep, drugs ingested, unusual symptoms, food, caffeine and nicotine intake and, for women, whether they have begun their menstrual cycle. Any major departure from the subject's usual routine (e.g., having a cold or flu) was discussed and a decision made as to whether the subject participated on that day.
Otherwise, several pre-drug measures such as vital signs, body temperature, pulse oximetry, observer ratings, mood state, drug effects questionnaires and psychomotor performance were completed. Then liquid was administered. Subsequently, subjects underwent post-drug assessment cycles at 20, 40, 60, 90, 120, 150, 180, 210, 240, 270, 300, 330, and 360 minutes post-drug administration. During each cycle, subjects completed at least some of the following (see below and Table 1): mood state and drug effects questionnaires, symptoms list and a pharmacological drug class questionnaire. In addition, vital signs, body temperature, pulse oximetry, and observer ratings of the subject were taken. Between assessment cycles, participants were allowed to engage in quiet activities (e.g., reading, playing hand-held games, playing solitaire). After the 210-min post-drug time point, subjects entered an adjacent room where they were provided a light meal and engaged in sedentary activities for the next 2.5 hours with assessments made at half-hour intervals. At the 360-min post-drug time point, subjects underwent the sobriety test and psychomotor performance task to ensure scores returned to baseline performance and qualified for release from the laboratory.
Table 1.
Schedule of Assessments During an Experimental Session
| MINUTES | BEHAVIORAL PROCEDURE | PHYSIOLOGICAL PROCEDURE |
|---|---|---|
| −20 | Presession Assessments (e.g. sobriety test) | RR, Pulse Ox. |
| −15 | ARCI, Dysphoria Scale, Sedative Scale, Symptoms List, POMS, CADSS, DSST |
|
| −5 | RR, Pulse Ox. | |
| 0 | Vehicle Administration – liquid | BP, HR, RR, Temp., Pulse Ox., GCS |
| 20 | ARCI, Dysphoria Scale, Sedative Scale, Symptoms List, POMS, CADSS, VAS, DSST |
BP, HR, RR, Temp., Pulse Ox., GCS |
| 40 | ARCI, Dysphoria Scale, Sedative Scale, Symptoms List, POMS, CADSS, VAS, DSST |
BP, HR, RR, Temp., Pulse Ox., GCS |
| 60 | ARCI, Dysphoria Scale, Sedative Scale, Symptoms List, POMS, CADSS, VAS, DSST |
BP, HR, RR, Temp., Pulse Ox., GCS |
| 90 | ARCI, Dysphoria Scale, Sedative Scale, Symptoms List, POMS, CADSS, VAS, DSST |
BP, HR, RR, Temp., Pulse Ox., GCS |
| 120 | ARCI, Dysphoria Scale, Sedative Scale, Symptoms List, POMS, CADSS, VAS, DSST |
BP, HR, RR, Temp., Pulse Ox., GCS |
| 150 | ARCI, Dysphoria Scale, Sedative Scale, Symptoms List, POMS, CADSS, VAS, DSST |
BP, HR, RR, Temp., Pulse Ox., GCS |
| 180 | ARCI, Dysphoria Scale, Sedative Scale, Symptoms List, POMS, CADSS, VAS, DSST, Drug Class Questionnaire |
BP, HR, RR, Temp., Pulse Ox., GCS |
| 210 | Food Available | BP, HR, RR, Temp., Pulse Ox., GCS |
| 240 | BP, HR, RR, Temp., Pulse Ox., GCS |
|
| 270 | BP, HR, RR, Temp., Pulse Ox., GCS |
|
| 300 | BP, HR, RR, Temp., Pulse Ox., GCS |
|
| 330 | BP, HR, RR, Temp., Pulse Ox., GCS |
|
| 360 |
Assess for release from the laboratory DSST, Sobriety |
BP, RR, HR, Temp., Pulse Ox., GCS |
Dependent Measures
Self-report and observer-report were recorded via paper and pen. The psychomotor performance task was presented to subjects on a Macintosh computer.
Self-report measures
During experimental sessions (see table 1), subjects completed the following questionnaires: 1) the Addiction Research Center Inventory Short Form (ARCI), which lists 49 true/false questions that are scored as five subscales: morphine-benzedrine group (MBG), a measure of "euphoria"; pentobarbital-chlorpromazine-alcohol group (PCAG), a measure of "sedation"; lysergic acid diethyl amide (LSD), a measure of "dysphoria"; and the benzedrine (BG) and amphetamine (A) scales, which are sensitive to amphetamine-like effects (Jasinski, 1977; Martin et al., 1971); 2) the Profile of Mood States (POMS), which lists 72 adjectives that are scored on eight subscales: anxiety, depression, anger, vigor, fatigue, confusion, friendliness, and elation (McNair et al., 1981); 3) Visual Analog Scale Questionnaire (VAS), which consists of nine 100-mm lines anchored with "not at all" on one end and "extremely" on the other on which subjects report the extent to which they experienced the strength of the drug effect, drug-liking, "good" drug effects, "bad" drug effects, drug-induced "high," sedative effects, stimulant effects, relaxation effects, and nervousness/anxiety effects; 4) Dysphoria Scale, which contains nine items describing dysphoric effects (i.e., "disturbance in stomach", "working slowly", "anxious", "clumsy", "weird feeling", "body awareness", "weak feeling", "tingling", "shaky hands") that are rated on a 5-point scale ranging from 0 (Not at all) to 4 (Extremely); 5) Sedative Scale, which contains 16 items describing sedative-like effects (e.g., "spaced out", "difficulty walking", "dazed", and "relaxed") rated on a 5-point scale ranging from 0 (Not at all) to 4 (Extremely) (Bickel et al., 1989); 6) Symptoms List, which contains 17 items describing possible side effects of GHB (e.g., " trouble concentrating", "feeling "high"", "vision blurred", "headache") which are rated on a 5-point scale ranging from 0 (None) to 4 (Extremely) (Rosen et al., 1997); and 7) a Pharmacological Drug Class Questionnaire, adapted from that used in drug studies with experienced drug users (e.g., Preston et al., 1989a,b), which listed several classes of drugs (placebo (inactive medication), sedative, stimulant, alcohol, opiate, hallucinogen) from which subject selected one that most describes the effects experienced.
Observer-report measures
The research nurse completed the Clinician Administered Dissociated States Scale (CADSS) and the Glasgow Coma Scale (GCS). The CADSS contains 23 questions answered by the subject and five questions completed by the rater that describe perceptual alterations that the subject may experience during the session and are rated on a 5-point scale ranging from 0 (Not at all) to 4 (Extremely). The GCS (Teasdale and Jennett, 1974) is the most widely-used scoring system for quantifying level of consciousness following traumatic brain injury. The scale consists of three tests: eye (scored from 1 to 4), verbal (scored from 1–5) and motor responses (scored from 1–6). The lowest possible total GCS score is 3 (deep coma or death) and the highest is 15 (fully awake person).
Psychomotor Performance
Psychomotor performance was assessed using an adapted, computerized version of the DSST (McLeod et al., 1982). During this test, randomly selected digits appeared on the center of the computer screen. Subjects responded on a numeric keypad to reproduce a geometric pattern associated with a digit according to the design presented continuously at the top of the screen. Subjects were instructed to complete as many patterns as possible while maintaining accuracy during the 90-sec presentation of the task. Data collected were the number of trials attempted and the number of correct trials completed.
Physiological Measures
Vital signs (blood pressure, heart rate, and respiration rate), pulse oximetry, and body temperature were measured immediately prior to drug administration, 20, 40, 60, 90, 120, 150, 180, 210, 240, 270, 300, 330, and 360 minutes post-drug administration. Blood pressure and heart rate were obtained using a Dinemapp. Body temperature was measured at the ear using a Thermoscan PR01 instant thermometer. The pulse oximetry was measured using the Nonin, Onyx Finger Pulse Oximeter, Model 9500).
Data Analyses
Data were entered into a SPSS data set using a double data entry procedure. Files were verified and any inconsistencies were corrected based upon the source document. GHB data were calculated as change from predrug scores. Several analyses were then performed as follows:
Area under the Curve
In order to determine whether there were any overall drug-induced changes during the course of the session, we used SPSS to calculate the area under the curve (AUC) for each outcome using a trapezoidal rule. This involves dividing each area into a number of strips of equal width. Then, the upper end was replaced by a chord forming a trapezium that approximates the area of each strip. The sum of these approximations gave the final numerical result of the AUC. These results were then entered into a one-way ANOVA with GHB dose (0, 0.32, 0.56, 0.72, 1.0, 1.8, 2.4, 3.2 g/kg) as the within-subject factor.
Peak Effects
In order to determine whether there were dose-related changes in the peak effects of GHB, data for each subject at each dose and time point (up to 2 hours post drug) were entered into an excel spreadsheet which calculated the peak change for each dose. These data were then entered into a one-way repeated-measures analysis of variance with GHB dose (0, 0.32, 0.56, 0.72, 1.0, 1.8, 2.4, 3.2 g/kg) as the factor. In order to discern any potential biphasic dose effects, orthogonal polynomials were used to partition the dose effect into linear, quadratic, cubic, etc., components (Winer, 1962), as dose represents a quantitative factor. Post hoc tests were performed contrasting placebo against each GHB dose in order to discern significant differences from placebo.
Time Course of Effects
Data for the GHB dose-effect curve determination were entered into a 7×8 repeated measures analysis of variance with GHB dose (0, 0.32, 0.56, 0.72, 1.0, 1.8, 2.4, 3.2 g/kg) and time point (i.e. postdrug1, postdrug2, etc.) as the within-subject factors. For each measure showing significant interactions, a series of pair-wise comparisons contrasting placebo with each of the dose levels were used to determine differences from placebo. For all analyses, a p value of less than or equal to 0.05 was used to infer statistical significance.
Results
Of the seventeen participants, seven (41%) dropped out or were discontinued for the following reasons: began a fulltime job (n=1); didn’t like the study (n=1); failed to show up for scheduled sessions and did not return phone calls (n=3); or were noncompliant with restriction on illegal drug use (n=2). Data for these individuals were not included in the analyses.
Of the other ten participants, one participant did not participate in one session at the 3.2 g/70 kg dose of GHB due to an adverse experience (e.g., transient nausea, vomiting, and sedation) during a previous session at the 2.4 g/70 kg dose, at which time the subject did not complete assessments at two time points. Another participant experienced a similar adverse effect (i.e., nausea and vomiting) during the session at the 3.2 g/70 kg dose of GHB and did not complete assessments at all time points. Data for these subjects were included in the analyses whenever possible.
The 10 participants (7 Caucasian, 2 African American, 1 Native American) were, on average, 34.3 (range: 19–47) yrs of age and had 14.2 (range: 12–17) yrs of formal education. All reported having used caffeinated and alcohol beverages in their lifetime, with eight and six, respectively, being current regular users. Only one participant reported being a current tobacco smoker. None reported current other drug use, with the exception that one participant reported using benzodiazepines as needed.
Area Under the Curve (AUC)
The significant differences in AUC following each dose of GHB relative to placebo are shown in Table 2. GHB at 0.32 g/70 kg decreased AUC on the POMS friendliness subscale relative to placebo. There were no significant changes on AUC at 0.56–1.0 g/70 kg doses of GHB for these measures. At the 1.8–3.2 mg/70 kg doses, significant increases in AUC occurred on the POMS confusion subscale, adjective ratings of sedative and dysphoric effects, and VAS drug strength, drug liking and high. At these doses, significant decreases in AUC occurred on POMS subscales for friendliness, elation and positive mood. No significant differences in AUC for DSST performance or physiological measures were observed at any dose of GHB.
Table 2.
Summary of AUC results. AUC contrasts between each dose and placebo that were significant (↑,↓) or marginally significant (⇑t, ⇓t) are presented. Each symbol indicates the direction of change relative to placebo.
| GHB Dose (g/70 kg) |
|||||||
|---|---|---|---|---|---|---|---|
| 0.32 | 0.56 | 0.72 | 1.0 | 1.8 | 2.4 | 3.2 | |
| Measure | |||||||
| POMS | |||||||
| Confusion | ↑ | ⇑t | |||||
| Friendliness | ↓ | ↓ | ⇓t | ||||
| Anxiety | ⇑t | ||||||
| Elation | ↓ | ||||||
| Positive Mood | ↓ | ||||||
| Arousal | ⇓t | ||||||
| Adjective Ratings | |||||||
| GHB Effects | ⇑t | ↑ | |||||
| Sedative Effects | ↑ | ||||||
| Dysphoric Effects | ↑ | ↑ | |||||
| ARCI | |||||||
| MBG Subscale | ⇑t | ||||||
| VAS | |||||||
| Any Drug Effect | ↑ | ↑ | ↑ | ||||
| Drug Liking | ⇑t | ↑ | ⇑t | ↑ | |||
| High | ↑ | ⇑t | ↑ | ||||
| Drowsy | ⇑t | ||||||
| DSST | |||||||
| Number Correct | ⇓t | ||||||
| Physiological Measures | |||||||
| Heart Rate | ⇓t | ||||||
Peak Effects
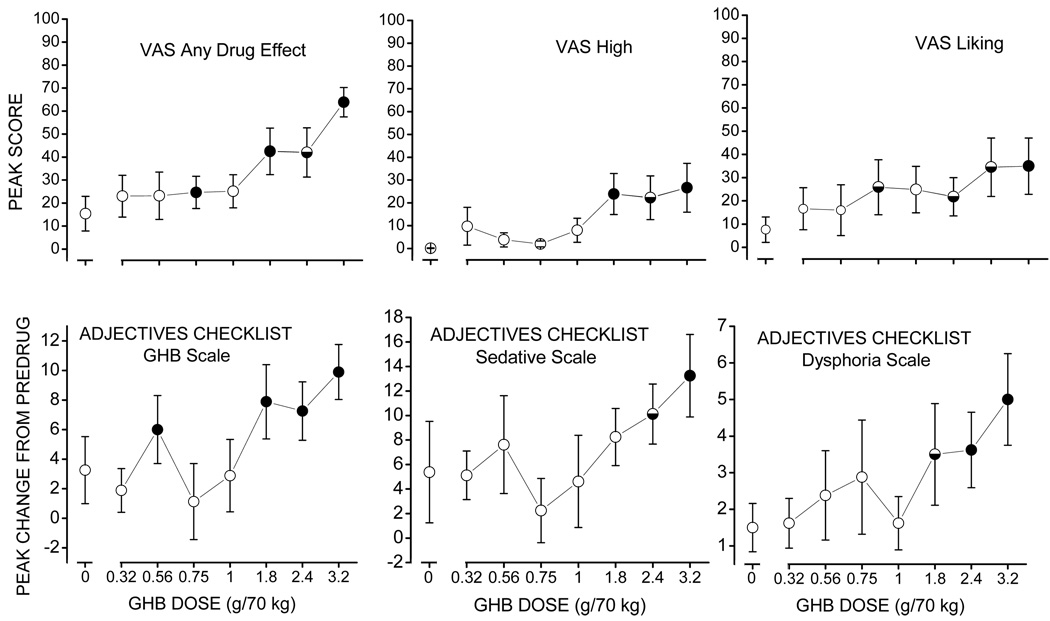
Measures that showed significant GHB-induced dose-related changes (p≤0.05) in peak effects are shown in Figures 1–2. GHB produced a dose-related increase in VAS ratings of “any drug effect” that approximated a quadratic shaped function (F(7,1)=16.9, p<0.005; Figure 1, upper left panel), with GHB-induced ratings at 0.75, 1.8, and 0.32 g/70 kg being significantly greater than placebo. GHB also produced linear dose-related increases in scores on high (F(7,1)=5.6, p<0.05; Figure 1, upper middle panel) and liking (F(7,1)=6.2, p<0.05; Figure 1, upper right panel) subscales of the VAS, with scores following GHB at 1.8 and/or 3.2 g/70 kg being significantly different from placebo.
Figure 1.
Effects of GHB on self-report measures at time of peak effect. Each point represents the mean +/− standard error in 8 participants. The horizontal axis represents GHB dose (g/70 kg). The vertical axis represents peak score (upper panels) or peak change from predrug measures (lower panels). Filled and half-filled circles represent significant (p≤0.05) and trends toward significant (0.05<p≤0.1) differences, respectively, relative to placebo. VAS – visual analog scale.
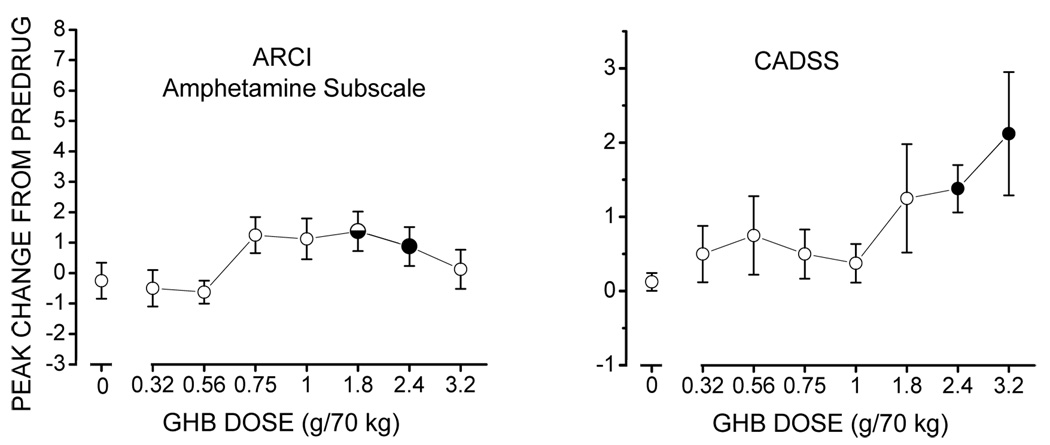
Figure 2.
Effects of GHB on self-report (left panel) and observer-rated (right panel) measures at time of peak effect. Each point represents the mean +/− standard error in 8 participants. The horizontal axis represents GHB dose (g/70 kg). The vertical axis represents peak change from predrug measures. Filled and half-filled circles represent significant (p≤0.05) and trends toward significant (0.05<p≤0.1) differences, respectively, relative to placebo. ARCI - Addiction Research Center Inventory Short Form; CADSS - Clinician Administered Dissociated States Scale.
The GHB-induced changes in ratings on the GHB subscale of the adjectives checklist were more complex, with increases in scores relative to placebo occurring at 0.56 g/70 kg as well as the 1.8, 2.4, and 3.2 g/70 kg doses of GHB (F(7,1)=18.9, p<0.005; Figure 1, lower left panel). GHB produced linear increases in ratings on the sedative (F(7,1)=17.5, p<0.005; Figure 1, lower middle panel) and dysphoria (F(7,1)=11.9, p<0.01; Figure 1, lower right panel) subscales of the adjectives checklist, with GHB-induced increases in scores at 2.4 and/or 3.2 g/70 kg being significantly different from placebo.
GHB produced significant linear increases in ratings on the A subscale of the ARCI (F(7,1)=8.9, p < 0.02; Figure 2, left panel) with the 2.4 g/70 kg dose being significantly greater than placebo. GHB also produced significant, dose-related, linear increases in ratings on the CADSS (F(7,1)=10.4, p<0.01; Figure 2, left panel), with GHB at 2.4 and 3.2 g/70 kg producing significantly greater scores than placebo. GHB produced no significant differences, relative to placebo, in performance on the DSST or cardiovascular measures (data not shown).
Time-Related Dose Effects
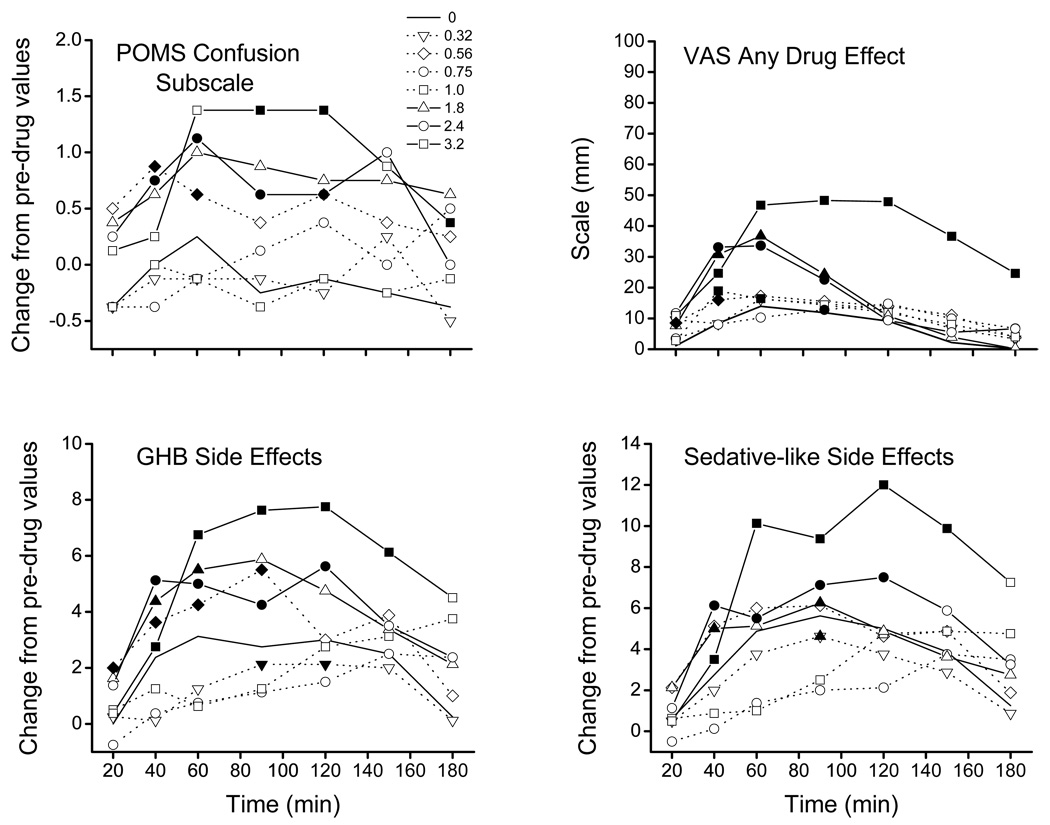
Selected measures showing a dose x time interaction are shown in Figure 3. GHB significantly increased scores, relative to placebo, on the confusion subscale of the POMS 40 and 60 min post-drug at 0.56 g/70 kg; 40, 60, 90, and 120 min post-drug at 2.4 g/70 kg; and 90, 120, and 180 min post-drug at 3.2 g/70 kg (overall F=8.0, p<0.05, Figure 3, upper left panel).
Figure 3.
Effects of GHB on selected self-report measures. Each point represents the mean +/− standard error in 8 participants. The horizontal axis represents time post drug administration. The vertical axis represents change from predrug measures (upper left and lower panels) or score (upper right panel). Filled and half-filled symbols represent significant (p≤0.05) and trends toward significant (0.05<p≤0.1) differences, respectively, relative to placebo at that time point. The legend indicating symbol association with GHB dose (g/70 kg) for all panels is shown in the upper left panel. POMS – Profile of Mood States; VAS – Visual Analog Scale.
GHB significantly increased scores, relative to placebo, on the “any drug effect” subscale of the VAS 20 and 40 min post-drug at 0.56 g/70 kg; 90 min post-drug at 0.75 g/70 kg; 40 and 60 min post-drug at 1.0 g/70 kg; 40, 60, and 90 min at 1.0 and 2.4 g/70 kg; and 40–180 min post-drug at 3.2 g/70 kg (overall F=18.8, p<0.005, Figure 3, upper right panel).
GHB at 0.32 g/70 kg produced decreases, relative to placebo, in scores 90 and 120 min post-drug administration on the GHB side effects subscale of the adjectives checklist (overall F= 1.69, p< 0.01, Figure 3, lower left panel). In contrast, GHB increased scores relative to placebo 20–90 min post-drug at 0.56 g/70 kg; 40–60 min post-drug at 1.8 g/70 kg; 40–120 min post-drug at 2.4 g/70 kg; and 40–150 min post-drug at 3.2 g/70 kg.
On the sedative subscale of the adjective checklist, GHB decreased scores relative to placebo 90 min post-drug at 0.32 g/70 kg. GHB increased scores relative to placebo 40 and 90 min post-drug at 1.8 g/70 kg, 40–120 min post-drug at 2.4 g/70 kg, and 40–150 min post-drug at 3.2 g/70 kg (overall F= 1.82, p< 0.002, Figure 3, lower right panel).
Drug Class Questionnaire
Placebo was identified as placebo by 8 of 10 participants (80%). GHB at 0.32 g/70 kg was identified as placebo by 3 participants (30%), sedative by 6 participants (60%), and alcohol by one participant (10%). There was no consensus on the identification of GHB at 0.56–1.8 g/70 kg, in that the majority of participants did not identify GHB as coming from any particular drug class. GHB at the highest doses tested (i.e., 2.4 and 3.2 g/70 kg) was identified as sedative by 9 of 10 participants (90%) and 8 of 9 participants (88.9%), respectively.
Discussion
This study of the behavioral effects of GHB in humans without sedative abuse histories found that GHB produced dose-related increases in self-reported sedative-like and dissociative-like effects, and ratings of drug liking and high, without impairing psychomotor performance or altering cardiovascular measures. Moreover, GHB produced a U-shaped dose effect function on a few measures, in that low (e.g., 0.32–0.56 g/70 kg) and high (1.8–3.2 g/70 kg) doses of GHB demonstrated behavioral activity on the drug class questionnaire and a few self-report ratings. In addition, the onset and duration of GHB-induced effects were dose dependent, with lower doses producing more short-lived effects but these effects were noted more quickly with lower than higher doses.
GHB typically produced minimal self-reported effects except at the highest doses (1.8–3.2 g/70 kg), although behavioral effects appeared to be discernible on a few measures at the 0.32–0.75 g/70 kg doses. This U-shaped pattern of subject effects is quite unique and the reasons for this pattern are unclear. GHB has been considered to produce dose-dependent, biphasic behavioral effects, with low doses producing motor stimulation and high doses sedation and anesthesia (Maitre, 1997). However, a closer examination of the adjectives on those subscales where GHB-induced changes were reported at low doses showed that scores increased on such items as “drowsy”, “sleepy”, and “relaxed” following GHB at the lowest doses, suggesting that these effects were not experienced as stimulant-like (data not shown). In addition, scores on measures specifically describing stimulant-like effects were not significantly increased at these lower doses, even though scores for one measure, the A subscale of the ARCI, were significantly increased at higher doses. Thus, these results deserve replication in order to ascertain the reliability and significance of these effects.
Nevertheless, that GHB at doses producing increases in sedation and dissociation also increased ratings on the amphetamine subscale of the ARCI indicates a mixture of excitability and sedation at higher doses. This mixture of stimulant- and sedative-like effects is consistent with previous reports for GHB (e.g., Abanades et al, 2006, 2007), alcohol (e.g., Holdstock and de Wit, 1998; Pierucci-Lagha et al., 2006) and ketamine (Krystal et al., 2006), suggesting that a shared mechanism for these three agents may be blockade of the NMDA glutamate receptor function (e.g., Akk et al., 2008; Krystal et al., 2003).
The findings of the present study extend those of prior studies in participants without extensive drug histories to doses lower than 1.0 g. For instance, in healthy non substance using volunteers, GHB (1–2 g) has been shown to produce no adverse effects on driving skills, few self-reported effects, and no significant increases in the effects of alcohol (Matilla et al., 1978). GHB (0.875 and 1.75 g/70 kg) has also been shown to have no effects on attention, alertness, short-term memory or psychomotor coordination relative to placebo (Ferrara et al., 1999). GHB-induced subjective effects were also minimal at these doses, with GHB producing subjective feelings of dizziness and dullness, which disappeared 30–60 minutes after drug administration. Otherwise, self-reported calmness increased following the lower dose and contentedness decreased after both doses, relative to placebo (Ferrara et al., 1999). The findings of the present study indicate that GHB at doses as low as 0.32 g/70 kg is behaviorally active.
The onset of GHB-induced behavioral effects varied from 20–90 min and effects were dose dependent, with higher doses typically producing a longer time to onset of effect and longer duration of action. These findings are consistent with reported nonlinear pharmacokinetics of GHB, which may be a possibly important factor in the development of acute GHB intoxication (Abanades et al., 2006). Interestingly, GHB at the 0.32 g/70 kg dose increased scores on the GHB side effects scale and the sedative-like scale at the 90+ min post-drug time point, which is inconsistent with a fast onset and short duration of effect at this dose. Moreover, a closer examination of the effects of this dose on AUC for the friendliness subscale of the POMS showed that scores also increased at later than expected post-drug assessment time points (data not shown). These findings suggest that the effects of these lower doses may be due, at least in part, to some sort of rebound effect, after effects of the doses that may not have been detected with our assessments have worn off. The significance of this phenomenon is unknown at this time.
Several limitations of the study should be noted. For instance, the sample size was relatively small, in part due to missing data in a couple of subjects from the lack of tolerability to GHB at the highest doses. Thus, potential GHB-induced effects may not have been revealed, which limits our conclusions regarding, for instance, the lack of impairment on the psychomotor performance task. Moreover, due to the in-depth study of GHB at multiple doses, other agents were not examined to allow for more direct comparisons with GHB. In addition, the precise doses of GHB used recreationally have not been not well documented. For instance, GHB has been reportedly administered one or more times per episode in differing volumes (e.g., teaspoons, vials, capfuls, etc.) with concentrations that often vary or are unknown (e.g., Barker et al., 2007; Centers for Disease Control, 1991; Lee and Levounis, 2008; Miotto et al., 2001). Thus, the significance of these findings in relation to the real world context is difficult to discern at this time. Nevertheless, these findings do extend the literature on the behavioral effects of GHB across a wider – and lower – dose range than examined previously, providing more information on quantity and quality of effects of GHB in a non-substance using population at doses that are reported to be used recreationally (Erowid, 2009). These findings suggest that GHB at doses lower than those examined more in-depth to date are behaviorally active, which is consistent with reports of recreational use at these lower doses (Erowid, 2009). More work is necessary to determine the reliability and clinical significance of GHB effects at low (i.e., 0.32–0.56 g/70 kg) doses.
Table 3.
Summary of Drug Class Questionnaire results.
| Drug Class | ||||||
|---|---|---|---|---|---|---|
| GHB dose (g/70 kg) |
Placebo | Opiate | Sedative | Hallucinogen | Stimulant | Alcohol |
| 0.00 | 8 | 0 | 2 | 0 | 0 | 0 |
| 0.32 | 3 | 0 | 6 | 0 | 0 | 1 |
| 0.56 | 5 | 1 | 2 | 0 | 2 | 0 |
| 0.75 | 2 | 1 | 4 | 0 | 2 | 1 |
| 1.00 | 1 | 1 | 4 | 0 | 2 | 2 |
| 1.80 | 0 | 1 | 5 | 1 | 1 | 2 |
| 2.40 | 0 | 0 | 9 | 0 | 1 | 0 |
| 3.20 | 0 | 1 | 8 | 0 | 0 | 0 |
Acknowledgements
This study was supported by grant DA14388 from the National Institute on Drug Abuse. Orphan Medical, Inc generously donated GHB and vehicle solutions. The authors thank Dr. Craig Rush for providing a peak effects data management program and Dr. Lawrence Carter for his comments regarding the manuscript. A preliminary report of this work was presented at the 2004 Annual Meeting of the College on Problems of Drug Dependence, San Juan, PR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abanades S, Farré M, Segura M, Pichini S, Barral D, Pacifici R, Pellegrini M, Fonseca F, Langohr K, De La Torre R. Gamma-hydroxybutyrate (GHB) in humans: pharmacodynamics and pharmacokinetics. Ann NY Acad Sci. 2006;1074:559–576. doi: 10.1196/annals.1369.065. [DOI] [PubMed] [Google Scholar]
- Abanades S, Farré M, Barral D, Torrens M, Closas N, Langohr K, Pastor A, de la Torre R. Relative abuse liability of gammahydroxybutyric acid, flunitrazepam, and ethanol in club drug users. J Clin Psychopharmacol. 2007;27:625–638. doi: 10.1097/jcp.0b013e31815a2542. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Castelli E, Stefanini GF, Casella G, Caputo, Marsigli L, Bernardi M, Gasbarrini G. An open multicentric study evaluating 4-hydroxybutyric acid sodium salt in the medium-term treatment of 179 alcohol dependent subjects: GHB Study Group. Alcohol and Alcoholism. 1996;31:341–345. doi: 10.1093/oxfordjournals.alcalc.a008160. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Caputo F, Stefanini GF, Gasbarrini G. Gamma-hydroxybutyric acid in the treatment of alcohol dependence: possible craving development for the drug. Addiction. 1997a;92:1035–1036. doi: 10.1111/j.1360-0443.1997.tb02984.x. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Stefanini GF, Gasbarrini G. Manageability and tolerability of gamma-hydroxybutyric acid in the medium-term outpatient treatment of alcoholism. Alcoholism: Clinical and Experimental Research. 1997b;31:380. doi: 10.1111/j.1530-0277.1997.tb03777.x. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Balducci G, Capristo E, Attilia ML, Taggi F, Gasbarrini G, Ceccanti M. Gamma-hydroxybutyric acid (GHB) in the treatment of alcohol withdrawal syndrome: a randomized comparative study versus benzodiazepine. Alcoholism: Clinical and Experimental Research. 1999a;23:1596–1604. [PubMed] [Google Scholar]
- Addolorato G, Caputo F, Capristo E, Bernardi M, Stefanini GF, Gasbarrini G. A case of gamma-hydroxybutyric acid withdrawal syndrome during alcohol addiction treatment: utility of diazepam administration. Clinical Neuropharmacology. 1999b;22:60–62. doi: 10.1097/00002826-199901000-00011. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Leggio L, Ferrulli A, Caputo F, Gasbarrini A. The therapeutic potential of gamma-hydroxybutyric acid for alcohol dependence: balancing the risks and benefits. A focus on clinical data. Expert Opinion on Investigational Drugs. 2009;18(5):675–686. doi: 10.1517/13543780902905855. [DOI] [PubMed] [Google Scholar]
- Akk G, Mennerick S, Steinbach JH. Actions of anesthetics on excitatory transmitter-gated channels. Handbook of Experimental Pharmacology. 2008;182:53–84. doi: 10.1007/978-3-540-74806-9_3. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Ritzmann RF, Tabakoff B. Formation of gamma-hydroxybutyrate in brain. Journal of Neurochemistry. 1977;28:633–639. doi: 10.1111/j.1471-4159.1977.tb10435.x. [DOI] [PubMed] [Google Scholar]
- Anonymous Xyrem approved for muscle problems in narcolepsy. FDA Consumer. 2002;36(5):7. [PubMed] [Google Scholar]
- Barker JC, Harris SL, Dyer JE. Experiences of gammahydroxybutyrate (GHB) ingestion: a focus group study. J. Psychoactive Drugs. 2007;39:115–129. doi: 10.1080/02791072.2007.10399870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Balster RL, Harris LS. Evaluation of the discriminative stimulus and reinforcing effects of gammahydroxybutyrate (GHB) Psychopharmacology. 1996;127:315–322. doi: 10.1007/s002130050092. [DOI] [PubMed] [Google Scholar]
- Benavides J, Rumigny JF, Bourguignon JJ, Cash C, Wermuth CG, Mandel P, Vincendon G, Maitre E. High affinity binding site for gamma-hydroxybutryic acid in rat brain. Life Science. 1982;30:953–961. doi: 10.1016/0024-3205(82)90624-5. [DOI] [PubMed] [Google Scholar]
- Bernasconi R, Lauber J, Marescaux C, Vergnes M, Martin P, Rubio V, Leonhardt T, Reymann N, Bittiger H. Experimental absence seizures: potential role of gamma-hydroxybutyric acid and GABAB receptors. Journal of Neural Transmission. Supplementum. 1992;35:155–177. doi: 10.1007/978-3-7091-9206-1_11. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Bigelow GE, Preston KL, Liebson IA. Opioid drug discrimination in humans: Stability, specificity and relation to self-reported drug effect. J Pharmacol Exp Ther. 1989;251:1053–1063. [PubMed] [Google Scholar]
- Bluet-Pajot MT, Schaub C. The effect of anesthetics on the basal secretion of immunoreactive growth hormone in rats (in French) Comptes Rendus Hebdomadaires des Seances de l'Academie des Sciences - D: Sciences Naturelles. 1978;286:1707–1710. [PubMed] [Google Scholar]
- Bluet-Pajot MT, Schaub C, Nasiet J. Growth hormone response to hypoglycemia under gamma-hydroxybutyrate narco-analgesia in the rat. Neuroendocrinology. 1978;26:141–149. doi: 10.1159/000122776. [DOI] [PubMed] [Google Scholar]
- Bustos G, Roth RH. Release of monoamines from the striatum and hypothalamus: effect of gamma-hydroxybutyrate. British Journal of Pharmacology. 1972;46:101–115. doi: 10.1111/j.1476-5381.1972.tb06853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustos G, Kuhar MJ, Roth RH. Effect of gamma-hydroxybutyrate and gamma-butyrolactone on dopamine synthesis and uptake by rat striatum. Biochemical Pharmacology. 1972;21:2649–2652. doi: 10.1016/0006-2952(72)90233-x. [DOI] [PubMed] [Google Scholar]
- Cameron S. Toxicity, gamma-hydrobutrate. eMedicine Journal. 2001;2:1–7. [Google Scholar]
- Carter LP, Pardi D, Gorslinec J, Griffiths RR. Illicit gamma-hydroxybutyrate (GHB) and pharmaceutical sodium oxybate (Xyrem®): Differences in characteristics and misuse. Drug and Alcohol Dependence. 2009a;104:1–10. doi: 10.1016/j.drugalcdep.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, Richards BD, Mintzer MZ, Griffiths RR. Relative abuse liability of GHB in humans: A comparison of psychomotor, subjective, and cognitive effects of supratherapeutic doses of triazolam, pentobarbital, and GHB. Neuropsychopharmacology. 2006;31:2537–2551. doi: 10.1038/sj.npp.1301146. [DOI] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR, Mintzer MZ. Cognitive, psychomotor, and subjective effects of sodium oxybate and triazolam in healthy volunteers. Psychopharmacology. 2009b doi: 10.1007/s00213-009-1589-1. published online 20, June 2009b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash CD. Gamma-hydroxybutyrate: an overview of the pros and cons for it being a neurotransmitter and/or a useful therapeutic agent. Neurosci Biobehav Rev. 1994;18:291–304. doi: 10.1016/0149-7634(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Cash CD, Maitre M, Mandel P. Purification from human brain and some properties of two NADPH-linked aldehyde reductases which reduce succinic semialdehyde to 4-hydroxybutyrate. Journal of Neurochemistry. 1979;33:1169–1175. doi: 10.1111/j.1471-4159.1979.tb05261.x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control. Multistate outbreak of poisonings associated with the illicit use of gamma hydroxybutyrate. Journal of the American Medical Association. 1991;265:620–627. [PubMed] [Google Scholar]
- Centers for Disease Control. Gamma hydroxybutyrate use. MMWR Morb Mortal Wkly Report. 1997a;46:281–283. [Google Scholar]
- Centers for Disease Control. Gamma hydroxybutyrate use in New York and Texas. Journal of the American Medical Association. 1997b;277:1511. [Google Scholar]
- Colombo G, Agabio R, Diaz G, Fa M, Lobina C, Reali R, Gessa GL. Gamma-hydroxybutyric acid intake in ethanol-preferring sP and -nonprefering sNP rats. Physiology and Behavior. 1998;64:197–202. doi: 10.1016/s0031-9384(98)00033-x. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Copeland J, Dillon P. Recent Trends in the Use of “Club Drugs”: An Australian Review. Substance Use & Misuse. 2005;40:1241–1256. doi: 10.1081/JA-200066777. [DOI] [PubMed] [Google Scholar]
- Doherty JD, Hattox SE, Snead OC, Roth RH. Identification of endogenous gamma-hydroxybutyrate in human and bovine brain and its regional distribution in human, guinea pig and rhesus monkey brain. Journal of Pharmacology and Experimental Therapeutics. 1978;207:130–139. [PubMed] [Google Scholar]
- Erowid GHB Dosage. Erowid.org. 2009 April 21; Retrieved September 19, 2009, from < http://www.erowid.org/chemicals/ghb/ghb_dose.shtml>.
- Ferrara SD, Zotti S, Tedeschi L, Frison G, Castagna F, Gallimberti L, Gessa GL, Palatini P. Pharmacokinetics of gamma-hydroxybutyric acid in alcohol dependent patients after single and repeated oral doses. British Journal of Clinical Pharmacology. 1992;34:231–235. doi: 10.1111/j.1365-2125.1992.tb04129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara SD, Tedeschi L, Frison G, Rossi A. Fatality due to gamma hydroxy butyric acid (GHB) and heroin intoxication. Journal of Forensic Sciences. 1995;40:501–504. [PubMed] [Google Scholar]
- Ferrara SD, Giorgetti R, Zancaner S, Orlando R, Tagliabracci A, Cavarzeran F, Palatini P. Effects of single dose of gamma-hydroxybutric acid and lorazepam on psychomotor performance and subjective feelings in healthy volunteers. European Journal of Clinical Pharmacology. 1999;54:821–827. doi: 10.1007/s002280050560. [DOI] [PubMed] [Google Scholar]
- Gallimberti L, Canton G, Gentile N, Ferri M, Cibin M, Ferrara SD, Fadda F, Gessa GL. Gamma-hydroxybutyric acid for treatment of alcohol withdrawal syndrome. Lancet. 1989;2:787–789. doi: 10.1016/s0140-6736(89)90842-8. [DOI] [PubMed] [Google Scholar]
- Gallimberti L, Ferri M, Ferrara SD, Fadda F, Gessa GL. Gamma-hydroxybutyric acid in the treatment of alcohol dependence: a double-blind study. Alcoholism: Clinical and Experimental Research. 1992;16:673–676. doi: 10.1111/j.1530-0277.1992.tb00658.x. [DOI] [PubMed] [Google Scholar]
- Gallimberti L, Cibin M, Pagnin P, Sabbion R, Pani PO, Pirastu R, Ferrara SD, Gessa GL. Gamma-hydroxybutyric acid for treatment of opiate withdrawal syndrome. Neuropsychopharmacology. 1993;9:77–81. doi: 10.1038/npp.1993.45. [DOI] [PubMed] [Google Scholar]
- Gallimberti L, Schifano F, Forza G, Miconi L, Ferrara SD. Clinical efficacy of gamma-hydroxybutyric acid in treatment of opiate withdrawal. European Archives of Psychiatry Clinical Neuroscience. 1994;244:113–114. doi: 10.1007/BF02191883. [DOI] [PubMed] [Google Scholar]
- Galloway GP, Frederick SL, Staggers FK, Gonzales M, Stalcup SA, Smith DE. Gamma-hydroxybutyrate: an emerging drug of abuse that causes physical dependence. Addiction. 1997;92:89–96. [PubMed] [Google Scholar]
- Gerra G, Caccavari R, Fontanesi B, Marcato A, Fertonani Affini G, Maestri D, Avanzini P, Lecchini R, Delsignore R, Mutti A. Flumazenil effects on growth hormone response to gamma-hydroxybutyric acid. Int Clin Psychopharmacol. 1994;9:211–215. doi: 10.1097/00004850-199409000-00011. [DOI] [PubMed] [Google Scholar]
- Gobaille S, Schleef C, Hechler V, Viry S, Aunis D, Maitre M. Gamma-hydroxybutyrate increases tryptophan availability and potentiates serotonin turnover in rat brain. Life Sciences. 2002;70:2101–2112. doi: 10.1016/s0024-3205(01)01526-0. [DOI] [PubMed] [Google Scholar]
- Goodwin AK, Griffiths RR, Brown PR, Froestl W, Jakobs C, Gibson KM, Weerts EM. Chronic intragastric administration of gamma-butyrolactone produces physical dependence in baboons. Psychopharmacology. 2006;189:71–82. doi: 10.1007/s00213-006-0534-9. [DOI] [PubMed] [Google Scholar]
- Greiner CC, Röhl JE, Ali-Gorji, Wassmann H, Speckmann EJ. Different actions of γ-hydroxybutyrate: a critical outlook. Neurol Res. 2003;25:759–763. doi: 10.1179/016164103101202138. [DOI] [PubMed] [Google Scholar]
- Grove-White IG, Kelman GR. Effect of methohexitone, diazepam and sodium 4-hydroxybutyrate on short-term memory. British Journal of Anaesthesia. 1971;43:113–116. doi: 10.1093/bja/43.2.113. [DOI] [PubMed] [Google Scholar]
- Halkitis PN, Palamar JJ. GHB use among gay and bisexual men. Addictive Behaviors. 2006;31:2135–2139. doi: 10.1016/j.addbeh.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Hechler V, Gobaille S, Bourguignon JJ, Maitre M. Extracellular events induced by gamma-hydroxybutyrate in striatum: a microdialysis study. Journal of Neurochemistry. 1991;56:938–944. doi: 10.1111/j.1471-4159.1991.tb02012.x. [DOI] [PubMed] [Google Scholar]
- Hechler V, Peter P, Gobaille S, Bourguignon J, Schmitt M, Ehrhardt J, Mark J, Maire M. Gamma-hydroxybutyrate ligands possess antidopmainergic and neuroleptic-like activities. Journal of Pharmacology and Experimental Therapeutics. 1993;264:1406–1414. [PubMed] [Google Scholar]
- Holdstock L, de Wit H. Individual differences in the biphasic effects of ethanol. Alcoholism: Clinical and Experimental Research. 1998;22:1903–1911. [PubMed] [Google Scholar]
- Hosli E, Hosli L. Binding sites for (3H) gamma-hydroxybutyrate on cultured neurons of rat cerebellum and spinal cord. Neuroscience Letters. 1983;42:145–148. doi: 10.1016/0304-3940(83)90397-x. [DOI] [PubMed] [Google Scholar]
- Ito Y, Ishige K, Zaitsu E, Anzai K, Fukuda H. Gamma-hydroxybutyric acid increases intracellular Ca2+ concentration and nuclear cyclic AMP-responsive element- and activator protein 1 DNA- binding activities through GABA receptor in cultured cerebellar granule cells. Journal of Neurochemistry. 1995;65:75–83. doi: 10.1046/j.1471-4159.1995.65010075.x. [DOI] [PubMed] [Google Scholar]
- Jasinski DR. Assessment of the abuse potential of morphine-like drugs (methods used in man) In: Martin WR, editor. Drug Addiction I. New York: Springer-Verlag; 1977. pp. 197–258. [Google Scholar]
- Kam PCA, Yoong FFY. Gamma-hydroxybutyric acid: an emerging recreational drug. Anaesthesia. 1998;53:1195–1198. doi: 10.1046/j.1365-2044.1998.00603.x. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Mason G, D’Souza DC. NMDA glutamate receptors and alco-holism: reward, dependence, treatment and vulnerability. Pharmacol Therapeut. 2003;99:79–94. doi: 10.1016/s0163-7258(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Madonick S, Perry E, Gueorguieva R, Brush L, Wray Y, Belger A, D-Souza DC. Potentiation of low dose ketamine effects by naltrexone: Potential implications for the pharmacology of alcoholism. Neuropsychopharmacology. 2006;31:1793–1800. doi: 10.1038/sj.npp.1300994. [DOI] [PubMed] [Google Scholar]
- Laborit H. Sodium 4-hydroxybutyrate. International J Neuropharmacology. 1964;43:433–452. doi: 10.1016/0028-3908(64)90074-7. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Levounis P. Gamma Hydroxybutyrate: An Ethnographic Study of Recreational Use and Abuse. J Psychoactive Drugs. 2008;40:245–253. doi: 10.1080/02791072.2008.10400639. [DOI] [PubMed] [Google Scholar]
- Li J, Stokes SA, Woeckener A. A tale of novel intoxication: a review of the effects of gamma-hydroxybutyric acid with recommendations for management. Annals of Emergency Medicine. 1998;31:729–736. [PubMed] [Google Scholar]
- Maitre M. The gamma-hydroxybutyrate signalling system in brain: organization and functional implications. Progress in Neurobiology. 1997;51:337–361. doi: 10.1016/s0301-0082(96)00064-0. [DOI] [PubMed] [Google Scholar]
- Maitre M, Mandel P. Calcium-dependent liberation of gamma-hydroxybutyrate after depolarization of rat brain slices. (in French) Comptes Rendus des Seances de l Academie des Sciences - Serie Iii, Sciences de la Vie. 1982;295:741–743. [PubMed] [Google Scholar]
- Maitre M, Cash C, Weissmann-Nanopoulos D, Mandel P. Depolarization-evoked release of gamma-hydroxybutyrate from rat brain slices. Journal of Neurochemistry. 1983;41:287–290. doi: 10.1111/j.1471-4159.1983.tb11843.x. [DOI] [PubMed] [Google Scholar]
- Mamelak M, Scharf MB, Woods M. Treatment of narcolepsy with gamma-hydroxybutyrate: a review of clinical and sleep laboratory findings. Sleep. 1986;9:285–289. doi: 10.1093/sleep/9.1.285. [DOI] [PubMed] [Google Scholar]
- Marescaux C, Vergnes M, Bernasconi R. GABAB receptor antagonists: potential new anti-absence drugs. J Neural Transm Suppl. 1992;35:179–188. doi: 10.1007/978-3-7091-9206-1_12. [DOI] [PubMed] [Google Scholar]
- Martellotta MC, Fattore L, Cossu G, Fratta W. Rewarding properties of gamma-hydroxybutyric acid: an evaluation through place preference paradigm. Psychopharmacology. 1997;132:1–5. doi: 10.1007/s002130050312. [DOI] [PubMed] [Google Scholar]
- Martellotta MC, Cossu G, Fattore L, Gessa GL, Fratta W. Intravenous self-administration of gamma-hydroxybutyric acid in drug-naive mice. European Neuropsychopharmacology. 1998;8:293–296. doi: 10.1016/s0924-977x(97)00087-4. [DOI] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapiro JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- Mathivet P, Bernasconi R, De Barry J, Marescaux C, Bittiger H. Binding characteristics of gamma-hydroxybutyric acid as a weak but selective GABAB receptor agonist. European Journal of Pharmacology. 1997;321(1):67–75. doi: 10.1016/s0014-2999(96)00916-8. [DOI] [PubMed] [Google Scholar]
- Mattila MJ, Palva E, Seppala T, Ostrovskaya RU. Actions and interactions with alcohol of drugs on psychomotor skills: comparison of diazepam and gamma-hydroxybutyric acid. Archives of International Pharmacodynamics. 1978;234:236–246. [PubMed] [Google Scholar]
- Maxwell JC, Spence RT. Profiles of club drug users in treatment. Substance Use & Misuse. 2005;40:1409–1426. doi: 10.1081/JA-200066968. [DOI] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling J. An automated version of the digit symbol substitution test (DSST) Behav Res Meth Instrum. 1982;14:463–466. [Google Scholar]
- McNair DM, Lorr M, Droppelman LF. Manual for the Profile of Mood States. San Diego: Educational and Industrial Testing Services; 1981. [Google Scholar]
- Metcalf DR, Emde RN, Stripe JT. An EEG-behavioral study of sodium hydroxybutyrate in humans. Electroencephalography and Clinical Neuropsychology. 1966;20:506–512. doi: 10.1016/0013-4694(66)90107-6. [DOI] [PubMed] [Google Scholar]
- Miguez I, Aldegunde M, Duran R, Veira JA. Effect of low doses of gamma-hydroxybutyric acid on serotonin, noradrenaline, and dopamine concentrations in rat brain areas. Neurochemistry Research. 1988;13:531–533. doi: 10.1007/BF00973292. [DOI] [PubMed] [Google Scholar]
- Miotto K, Darakjian J, Basch J, Murray S, Zogg J, Rawson R. Gamma-Hydroxybutyric Acid: Patterns of Use, Effects and Withdrawal. The American Journal on Addictions. 2001;10:232–241. doi: 10.1080/105504901750532111. [DOI] [PubMed] [Google Scholar]
- Nava F, Premi S, Manzato E, Campagnola W, Lucchini A, Gessa GL. Gamma-hydroxybutyrate reduces both withdrawal syndrome and hypercortisolism in severe abstinent alcoholics: an open study vs. diazepam. American Journal of Drug & Alcohol Abuse. 2007;33:379–392. doi: 10.1080/00952990701315046. [DOI] [PubMed] [Google Scholar]
- Nelson T, Kaufman E, Kline J, Sokoloff L. The extraneural distribution of gamma-hydroxybutyrate. Journal of Neurochemistry. 1981;37:1345–1348. doi: 10.1111/j.1471-4159.1981.tb04689.x. [DOI] [PubMed] [Google Scholar]
- Palatini P, Tedeschi L, Frison G, Padrini R, Zordan R, Orlando R, Gallimberti L, Gessa GL, Ferrara SD. Dose-dependent absorption and elimination of gamma-hydroxybutyric acid in health volunteers. European Journal of Clinical Pharmacology. 1993;45:353–356. doi: 10.1007/BF00265954. [DOI] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, Khisti RT, Morrow AL, Marx CE, Shampine LJ, Kranzler HR. Subjective effects and changes in steroid hormone concentrations in humans following acute consumption of alcohol. Psychopharmacology. 2006;186:451–461. doi: 10.1007/s00213-005-0231-0. [DOI] [PubMed] [Google Scholar]
- Preston KL, Bigelow GE, Bickel WK, Liebson IA. Drug discrimination in human post-addicts: Agonist-Antagonist opioids. J Pharmacol Exper Ther. 1989a;250:184–196. [PubMed] [Google Scholar]
- Preston KL, Bigelow GE, Liebson IA. Antagonist effects of nalbuphine in opioid-dependent human volunteers. J Pharmacol Exper Ther. 1989b;248:929–937. [PubMed] [Google Scholar]
- Rosen MI, Pearsall HR, Woods SW, Kosten TR. Effects of gamma-hydroxybutyric acid (GHB) in opioid-dependent patients. Journal of Substance Abuse Treatment. 1997;14:149–154. doi: 10.1016/s0740-5472(96)00157-2. [DOI] [PubMed] [Google Scholar]
- Roth RH, Giarman NJ. Conversion of in vivo gamma aminobutyric acid to gamma butyric acid in the rat. Biochemical Pharmacology. 1969;18:247–250. doi: 10.1016/0006-2952(69)90032-x. [DOI] [PubMed] [Google Scholar]
- Russell IJ, Perkins AT, Michalek JE, Oxybate SXB-26 Fibromyalgia Syndrome Study Group Sodium oxybate relieves pain and improves function in fibromyalgia syndrome: a randomized, double-blind, placebo-controlled, multicenter clinical trial. Arthritis & Rheumatism. 2009;60(1):299–309. doi: 10.1002/art.24142. [DOI] [PubMed] [Google Scholar]
- Sanguineti VR, Angelo A, Frank MR. GHB a home brew. Am J Drug Alcohol Abuse. 1997;23:637–642. doi: 10.3109/00952999709016901. [DOI] [PubMed] [Google Scholar]
- Scharf MB, Baumann M, Berkowitz DV. The effects of sodium oxybate on clinical symptoms and sleep patterns in patients with fibromyalgia. J Rheumatology. 2003;30:1070–1074. [PubMed] [Google Scholar]
- Schwartz RH, Milteer R, Lebeau MA. Drug-facilitated sexual assault (‘date rape’) South Med J. 2000;93:558–561. [PubMed] [Google Scholar]
- Serra M, Sanna E, Foddi C. Failure of gamma-hydroxybutyrate to alter the function of the GABAA receptor complex in the rat cerebral cortex. Psychopharmacology. 1991;104:351–355. doi: 10.1007/BF02246035. [DOI] [PubMed] [Google Scholar]
- Snead OC., III Minireview: gamma hydroxybutyrate. Life Sciences. 1977;20:1935–1944. doi: 10.1016/0024-3205(77)90171-0. [DOI] [PubMed] [Google Scholar]
- Snead OC, III, Liu CC. Gamma-hydroxybutyric acid binding sites in rat and human brain synaptosomal membranes. Biochemistry & Pharmacology. 1984;33:2587–2590. doi: 10.1016/0006-2952(84)90629-4. [DOI] [PubMed] [Google Scholar]
- Snead OC, III, Liu CC. GABAA receptor function in the gamma-hydroxybutyrate model of generalized absence seizures. Neuropharmacology. 1993;32:401–409. doi: 10.1016/0028-3908(93)90163-w. [DOI] [PubMed] [Google Scholar]
- Steele MT, Watson WA. Acute poisoning from gamma hydroxybutyrate (GHB) Missouri Medicine. 1995;92:354–357. [PubMed] [Google Scholar]
- Stell JM, Ryan JM. Ecstasy and neurodegeneration. gamma-Hydroxybutyrate is a new recreational drug that may lead to loss of consciousness. British Medical Journal. 1996;313:424. doi: 10.1136/bmj.313.7054.424a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo ST, Gold MS, Goldberger BA, Blier P. Effects of sustained gamma-hydroxybutyrate treatments on spontaneous and evoked firing activity of locus coeruleus norepinephrine neurons. Biological Psychiatry. 2004;55:934–939. doi: 10.1016/j.biopsych.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Thomas G, Bonner S, Gascoigne A. Coma induced by abuse of gamma-hydroxybutyrate (GHB or liquid ecstasy): a case report. British Medical Journal. 1997;314:35–36. doi: 10.1136/bmj.314.7073.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunnicliff G. Significance of gamma-hydroxybutyric acid in the brain. General Pharmacology. 1992;23:1027–1034. doi: 10.1016/0306-3623(92)90282-o. [DOI] [PubMed] [Google Scholar]
- van Noorden MS, van Dongen LC, Zitman FG, Vergouwen TA. Gamma-hydroxybutyrate withdrawal syndrome: dangerous but not well-known. General Hospital Psychiatry. 2009;31:394–396. doi: 10.1016/j.genhosppsych.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Vayer P, Mandel M, Maitre M. Gamma-hydroxybutyrate, a possible neurotransmitter. Life Sci. 1987;41:1547–1557. doi: 10.1016/0024-3205(87)90721-1. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Goodwin AK, Griffiths RR, Brown PR, Froestl W, Jakobs C, Gibson KM. Spontaneous and precipitated withdrawal after chronic intragastric administration of gamma-hydroxybutyrate (GHB) in baboons. Psychopharmacology. 2005;179:678–687. doi: 10.1007/s00213-004-2079-0. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Rowlett JK, Winger G, Woods JH, Gerak LR, France CP. Evaluation of the reinforcing and discriminative stimulus effects of gamma-hydroxybutyrate in rhesus monkeys. Drug & Alcohol Dependence. 1999;54:137–143. doi: 10.1016/s0376-8716(98)00153-7. [DOI] [PubMed] [Google Scholar]