Abstract
Ischemic heart disease remains one of the most common causes of mortality in developed countries. Recently, stem cell therapy is being considered for treating ischemic heart diseases. On the other hand, there has been evidence of chondro-osteogenic mass formation after stem cell injection in the heart. In a recent publication, Chiavegato et al. has suggested that amniotic fluid derived stem (AFS) cells cause chondro-osteogenic masses in the infarcted heart. The goal of the current study was to further examine the formation of such masses, specifically, the role of AFS cells in this process. Our results confirm the presence of similar bone-like masses in the left ventricular wall of infarcted rats; however, this phenomenon occurred independent of AFS cell injection into the myocardium. Moreover, AFS cell injection did not increase the presence of chondro-osteogenic masses. Echocardiographic analysis of large infarctions in rats frequently revealed the presence of echogenic structures in the left ventricular wall. We further demonstrated a significant relationship between the infarction size and chondro-osteogenic formation and subsequently decrease in cardiac function. Collectively, our study indicates that chondro-osteogenic differentiation can take place in infarcted rat heart independent of cell injection. These results have significant implications for future design and testing of stem cell therapy for treatment of cardiac muscle diseases.
Keywords: amniotic fluid stem cells, myocardial infarction, chondro-osteogenic, cell transplantation
INTRODUCTION
Acute Myocardial Infarction (AMI) is the loss of cardiomyocytes due to the blockage of coronary artery, and it remains one of the most severe health threats in the western world. Several different therapeutic modalities have been used to treat AMI; however, impaired heart function cannot be restored due to the low regenerative capability of cardiomyocytes. In the past decade, cell transplantation has emerged as a new option for treatment of AMI. A variety of stem and progenitor cells have shown the ability to differentiate into cardiac-like cells under different conditions in vitro 1–4, followed by in vivo experiments that confirmed an improvement of heart function after cell transplantation in animals 5–9 and in human patients 10–13.
Although there is a wealth of experimental data supporting the efficacy of stem cell therapy, there are several potential risks associated with stem cell transplantation due to their plasticity. Previous reports have specifically demonstrated that bone marrow derived- and other types of stem cells implanted in vivo form osteogenic masses 14–18. Recently, Chiavegato et al. has reported that amniotic fluid derived stem (AFS) cells caused chondro-osteogenic masses in the heart. Xenotransplantation of human (h) AFS cells into the infarcted rat hearts elicited severe inflammatory reaction in both immuno-deficient nude rats and immunocompetent Sprague–Dawley (SD) rats. The authors showed that the AFS cells formed bone-like cell aggregates using Von Kossa and alkaline phosphate staining (9). In the current study we performed similar experiments in order to further investigate the formation of chondro-osteogenic masses after hAFS cell transplantation. Although we confirmed the presence of such masses, we were able to show that chondro-osteogenic mass formation is independent of AFS cell injection and significantly correlated with infarct size. As expected, these chondro-osteogenic masses were significantly correlated with a decreased cardiac function. Taken together, as the use of cell transplantation is increasingly applied for ischemic heart disease, the choice of the animal model and careful analysis of the results are necessary to ensure safety for clinical translation.
METHODS
hAFS Cell Culture
A multipotent subpopulation of progenitor cells present in the human amniotic fluid was isolated as previously described under IRB approval 19. hAFS cells were plated on non-treated plastic dishes in culture medium consisting of minimum essential alpha-medium, embryonic stem cell certified fetal bovine serum (15% Hyclone, Inc. Logan, Utah), L-glutamine (1%), antibiotics (1%) (Invitrogen, Carlsbad, California), and Chang Medium® B (18%) and C (2%) (Irvine Scientific, Santa Ana, CA). Cells were labeled using an adenoviral vector using a cytomegalovirus (CMV) promoter encoding a bacterial LacZ reporter gene (generated at Harvard Gene Therapy Core Facility). Adenovirally labeled LacZ-hAFS cells were used for the cell transplantation studies (1,500–2,000 MOI).
Rat Myocardial Infarction Model and hAFS Cell Transplantation
All study procedures and imaging protocols were approved by the Wake Forest University Health Sciences Animal Care and Use Committee. This study involved 8–10 week old male nude rats (Taconic, Rockville, MD), and 8–10 week old Sprague Dawley rats (Charles River, Wilmington, MA). Briefly, the rats were anesthetized with 2% isoflourane and intubated. The heart was visualized through a midline sternotomy, and the coronary artery was ligated using 6-0 prolene suture at the edge of the left atrium. Four groups were examined: Group 1, ligation only (n=10). Group 2, ligation plus injection of AFS cells (n=10). Group 3, no ligation, injection of AFS cells (n=5). For Group 2, immediately following ligation, 5 million cells were resuspended in 30μl PBS and injected into the ischemic heart in 3 different locations. For Group 3, cells were transplanted as described without the infarction injury. For Group 4, Sprague Dawley rats (n=5) underwent ligation only without cell transplantation. This group was performed in order to investigate if the chondro-osteogenic formation is related to the strain of the rats. Following injection of cells, all animals were recovered and monitored for 24 hours. The mortality rate of the animals was 20%. The n in each group reported is the animals that survived for the entire study.
Echocardiography Measurements
Transthoracic echocardiographic (TTE) measurements of myocardial structure and function were done on all rats at baseline – prior to myocardial infarction – and again at 1, 2, and 3 months post infarction. All procedures were done on anesthetized (2% isoflurane) rats lying in a left recumbent position. TTE (Siemens Sequoia 512) was performed using a 15-MHz linear array transducer (15L8, Acuson) and was used for all the animals. Two-dimensional left ventricular parasternal short-axis measurements were used to define internal diameters during systolic and diastolic time points. The left ventricular end systolic diameter (LVSD) and left ventricular end-diastolic diameter (LVDD) were measured according to the American Society for Echocardiography leading edge method from at least 3 consecutive cardiac cycles. M-mode echocardiography was used to define left ventricular diameters. LV fractional shortening was calculated from these measurements by LVDD-LVSD/LVDD. Based on the histological analysis if chondro-osteogenic masses were seen, the rats from Group 1 and Group 2 were divided into groups where chondro-osteogenic masses formed vs. no chondro-osteogenic masses formed, and the fractional shortenings of these animals were compared in these groups. Further analysis also included pooling the animals of both groups together that formed chondro-osteogenic masses and compared the fractional shortening of them to the animals that did not form chondro-osteogenic masses.
Histology
At 3 months after infarction, the rats were euthanized. The heart was removed, and rinsed in PBS. The heart was immediately cryofrozen using OCT Tissue-Tek medium (OCT compound; Miles, Elkhart, NJ). The heart was sliced transversely from the apex to the basal part of the left ventricle using a cryostat at 6-μm thickness (Leica RM 2145 microtome). All sections were mounted on glass slides and stained by Hematoxylin and Eosin staining, X-gal staining, Alizarin Red, Von-Kossa, and Movat’s Pentachrome stain following standard protocols.
For immunostaining, the tissues were fixed using 4% paraformaldehyde, permeabilized, blocked using Universal Protein Block (Dako Corp), and incubated with antibodies-β-galactosidase (Abcam, Cambridge, MA), osteocalcin, collagen type II (Santa-Cruz Biotechnology, Santa-Cruz, CA), and Matrix-Gla-Protein kindly provided by Dr. David Sane (11–13). Tissues were washed with PBS, and incubated with the appropriate fluorescein secondary antibodies (Jackson Immuno, West Grove, PA) for 45 minutes. Cells were mounted and viewed using multitrack high resolution confocal microscopy (Zeiss Axiovert 100M). Appropriate negative isotype controls were done in parallel.
For quantitative collagen analysis to determine the infarction size, Mason’s Trichrome staining was used. All histological sections were examined by a Zeiss Stemi 2000-C microscope. Images were captured with a dissecting scope using a Canon Powershot A620 at maximum zoom. Image J 1.34 software was used to measure the area of infarction (collagen blue stain). The infarct scar area and total area of the LV myocardium were traced manually in the digital images and measured automatically by the computer as performed previously 20. The infarct size, expressed as a percentage, was calculated by dividing the sum of all sections by the sum of LV areas from all sections and multiplying by 100.
For the calcification index calculation, in all of the calcified samples, Hematoxylin and Eosin staining was performed at three slides at similar levels the basal, middle and apex regions of the left ventricle. Prior to staining all slides with Hematoxylin and Eosin, the calcification area was confirmed by Alizaren Red staining on multiple slides of in order to demonstrate that both staining techniques resulted in the same area of calcification. Subsequently, we chose to perform our study using the standard Hematoxylin and Eosin staining. Calcified area was calculated by Image J and total calcified area from all the slides was averaged. This calculation serves as an approximate index of calcification severity. We further analyzed the data by graphing the size of the calcification index of the two groups to determine if there are differences between them.
Statistical Analysis
All data are presented as means ± SEM. Echocardiographic parameters were measured using Two-Way Repeated Measures ANOVA. Volumes of the area of infarction comparisons and differences in calcification indexes and fractional shortenings were analyzed by Student’s t-test (Microsoft Excel). Linear regression analysis was performed to compare the calcification index and the infarction percentage. Data was analyzed using Graph Pad Prism 4. Data was considered significant at P<0.05.
RESULTS
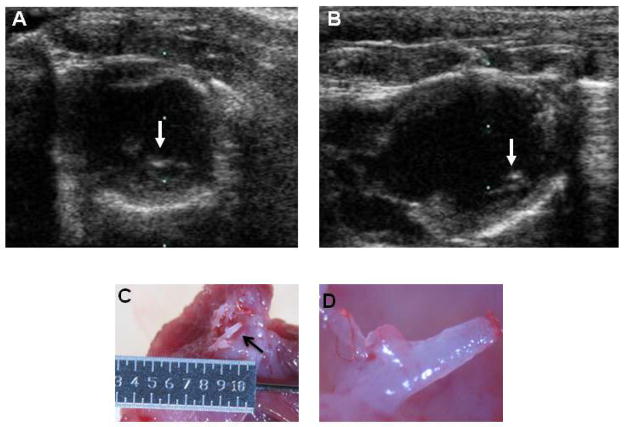
The goal of this study was to further investigate chondro-osteogenic differentiation in the pathological rat heart after hAFS cell injection. We studied 4 different groups of animals: Group 1-ligation only, Group 2-ligation plus injection of hAFS cells, Group 3-no ligation, injection of AFS cells and Group 4, ligation only without cell transplantation in Sprague Dawley rats. Representative transthoracic two-dimensional echocardiograms in the parasternal short-axis and long-axis views at 3 months are shown from a rat with a large myocardial infarction (Figure 1A, 1B). Large echogenic structures (bright spots) were observed during echocardiography in 50% of the animals (arrows). Upon sacrificing the rats, 3 months after surgery, these structures were confirmed to have a chondro-osteogenic appearance by gross analysis as indicated by the arrow (Figure 1C) and the close up image (Figure 1D).
Figure 1. Representative echocardiograms and gross morphology of chondro-osteogenic structures.
Representative parasternal short axis (A) and long axis (B) echocardiograms at 3 months post-infarction. Note the resemblance of echogenic structures (bright spots) indicated by the arrows clearly present in the infarcted heart wall. (C) and (D) representative gross morphology of the calcifications (arrow) in the infarcted heart wall.
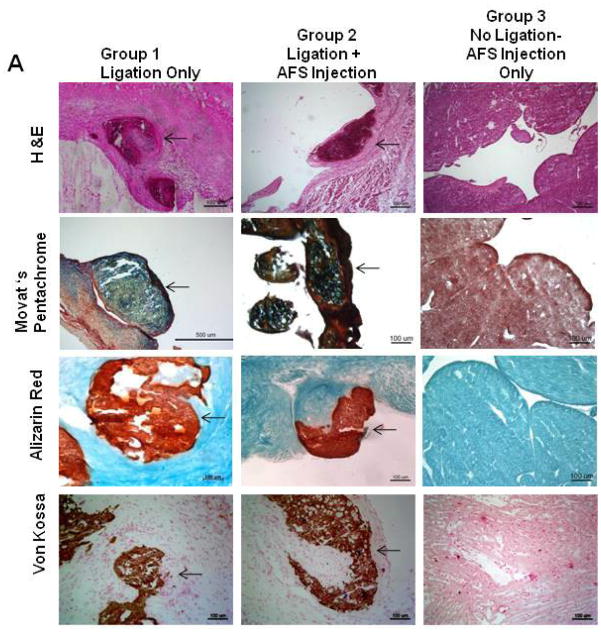
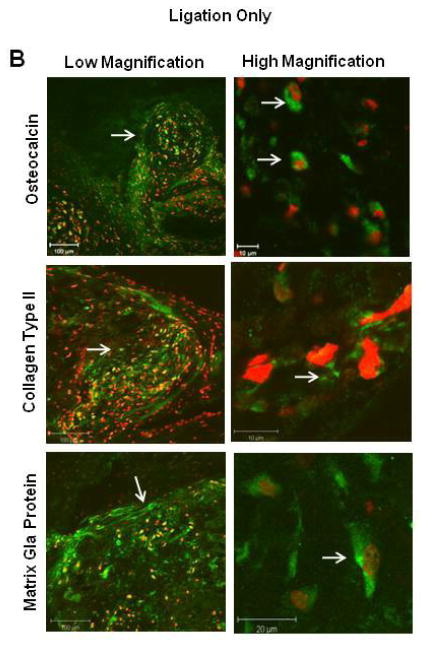
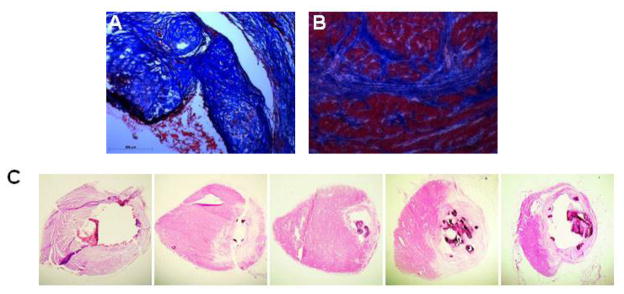
We further characterized the chondro-osteogenic masses in the different groups. We found these masses in the subendocardial border of the infarcted heart in Groups 1, 2, and 4. No masses were seen in Group 3 (no ligation with injection of AFS cells). Representative heart tissue sections of Group 1 and 2 rats are shown in Figure 2A, and the staining characteristics were similar for all the groups. Hematoxylin and Eosin staining demonstrated that the cells were arranged in a random manner with osteogenic and chondrogenic tissue morphologies. Movat-Pentachrome staining demonstrated that the masses had collagen and bone (yellow), mucins (blue) that were inside the left ventricular cardiac muscle (red). Alizarin red staining demonstrated the presence of calcium deposition in the mass. Calcium deposition was further confirmed using Von Kossa stain. Further analysis was done by immunostaining for osteocalcin, Type II collagen, and Matrix Gla Protein (Figure 2B). The masses stained positive for a noncollagenous protein found in bone, osteocalcin, Type II collagen, as well as Matrix-Gla-Protein (MGP), a protein most abundantly found in bone and cartilage. Similar results were obtained when hAFS cells were injected into the heart of immune competent Sprague-Dawley rats that had ligation-induced infarction, indicating that this phenomenon is not due to lack of normal immune system.
Figure 2. Characterization of Chondro-Osteogenic Masses.
(A) Representative histological examination after 3 months of Group 1 (ligation only) vs. Group 2 (ligation plus AFS injection). Both groups revealed similar chondro-osteogenic masses in the subendocardial border of the infarcted heart. Hematoxylin and Eosin staining demonstrated that the cells were arranged in a random manner with osteogenic and chondrogenic cell morphologies The masses expressed bone (yellow), collagens (blue), and mucins (red) demonstrated by Movat’s Pentachrome stain. Alizarin Red and Von Kossa assays confirmed production of calcium. (B) Immunohistochemical analysis of representative sections from Group 1 of the chondro-osteogenic masses formed in the infarcted rat hearts revealed osteocalcin, collagen type II, and Matrix-Gla-Protein staining.
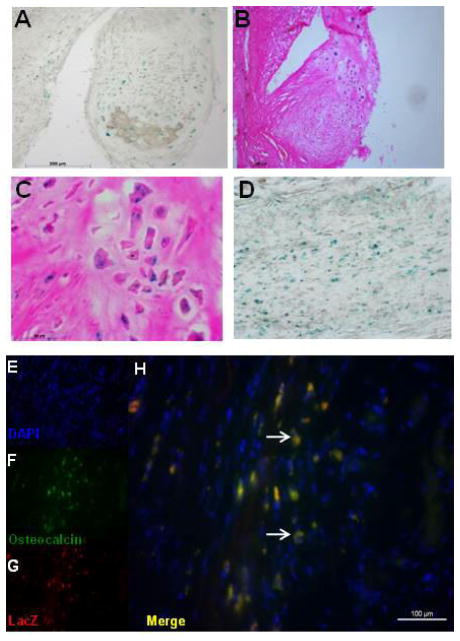
Human AFS cells were labeled with LacZ by adenoviral infection in order to identify them histologically after harvesting the heart. X-gal staining of rat hearts that received cell injection identified hAFS cells integrated in these masses (Figure 3A, B, C), as well as in the infarcted border zone of the ventricular wall (Figure 3D). These results were confirmed by immunohistological analysis of β-galactosidase expression. Co-immunostaining showed LacZ positive cells that express osteocalcin, suggesting that hAFS cells develop an osteogenic morphology in vivo after injury (Figure 3E–H). No β-galactosidase positive hAFS were found in the masses of rats that were not ligated (Group 3) suggesting injury is needed to permit survival of hAFS cells in the tissue.
Figure 3. Engraftment of AFS cells in Infarcted Heart.
(A–D) X-gal staining of Group 2 revealed engraftment of AFS cells in the chondro-osteogenic masses as well as in the infarcted heart wall. (E-H) Co-staining of osteocalcin and β-galactosidase revealed AFS cells express osteocalcin in the infarcted heart wall.
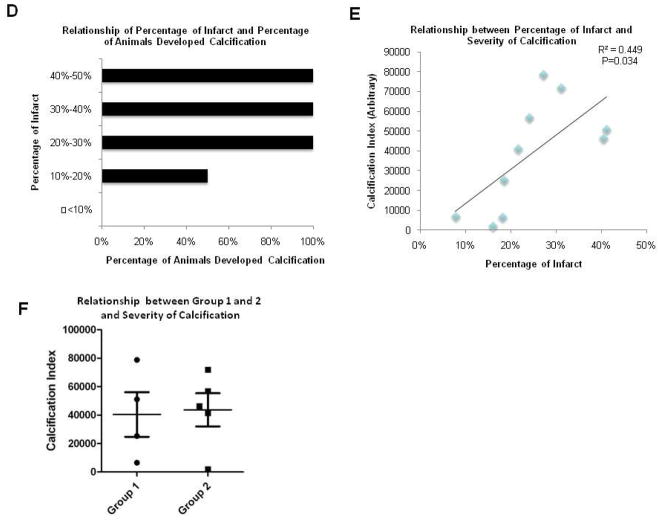
In order to determine if the degree of infarction size contributes to the formation of chondro-osteogenic masses we compared the infarction sizes of rats that developed masses versus rats without masses. To do this, we measured the infarction size after trichrome staining using Image J analysis. The infarct size, expressed as a percentage, was calculated by dividing the sum of the infarcted area in all sections by the sum of LV areas from all sections. Two representative trichrome stainings of the calcification area and the infarcted border are shown in Figure 4A and 4B, respectively. Representative serial sections of a heart stained by H&E displaying chondro-osteogenic masses are shown in Figure 4C. The H&E staining results identify similar calcified areas as the standard Alizarin Red and Von Kossa techniques, as shown in Figure 2. Comparison of infarction sizes of rats that had chondro-osteogenic masses to rats with no masses showed a significant difference between the two groups. As the infarction size measured by Mason’s Trichrome increased, the percentage of animals that displayed calcifications as seen histologically also increased (Figure 4D). Next, we graphed the infarction size against the calcification index. The calcification index, an index of severity of calcification, was performed on 10 rats that demonstrated chondro-osteogenic masses as seen by Hematoxylin and Eosin staining. The calcification index was calculated by averaging the total calcified area in the basal, middle and apex regions of the left ventricle. The results showed a significant linear relationship between the calcified index and the infarction size (r2=0.4134 and p value=0.034) (Figure 4E) confirming that the amount of calcification correlates with infarction size. Thus, as the percentage of infarction increases, the severity of calcification also increased. Next, we plotted the calcification indexes between the two individual groups, Group 1 and Group 2, and plotted the animals that showed chondro-osteogenic masses histologically to determine if there were any differences in the severity of calcification (Figure 4F). The data suggests there is no significant difference between the calcification index of the two groups (p-value= 0.86)
Figure 4. The Formation of Chondro-Osteogenic Masses are Related to Increased Infarction Size.
A,B. Trichrome stainings of the calcification area and the infarcted border, respectively. C. Representative serial sections of a heart stained by H&E displaying chondro-osteogenic masses. D. Comparison of infarction sizes of rats that showed chondro-osteogenic masses to rats with no masses showed a significant difference between the two groups. As the infarction percentage increased, the percentage of animals that developed calcifications also increased. E. Calcification index as a function of the infarction percentage. There is a significant linear relationship between the calcified index and the infarction percentage (r2=0.4134 and p value=0.034). F. Calcification indexes of Group 1-ligation only and Group 2-ligation plus injection of hAFS cells suggest that there is no significant difference between the calcification index of the two groups (p-value= 0.86).
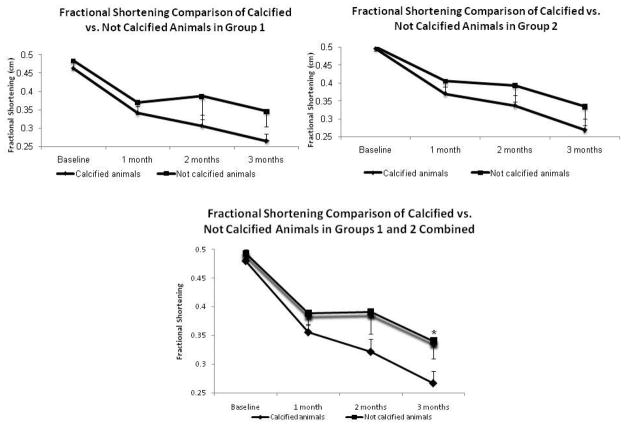
In order to test if the presence of chondro-osteogenic masses has an effect on cardiac function, we compared the fractional shortening of the rats from Groups 1 (ligation only) and Groups 2 (ligation and AFS cell injection). In each Group we compared the calcified animals vs. the noncalcified animals, as well as pooling the groups together for comparisons. It can be clearly seen that in Group 1 and 2 there is a decreased fractional shortening in the animals that had calcifications (Figure 5A and 5B). When pooling the animals in the groups together, the results demonstrate that there was a significant difference in fractional shortening between the calcified and non-calcified group’s fractional shortenings at 3 months. (p=0.031) (Figure 5C). This data suggests that rats that that developed calcifications also had a decreased cardiac function.
Figure 5. The Presence of Chondro-Osteogenic Mass Formation in the Heart is related to a Decrease Cardiac Function.
Fractional Shortening Comparison of Calcified vs. Not Calcified. Animals in Fractional shortening analysis of the rats from Group 1 (A, ligation only) and Group 2 (B, ligation and AFS cell injection) show decreased fractional shortening in the animals that had calcifications. When pooling the animals in the groups together (C), the results demonstrate that there is a significant difference between the calcified and non-calcified group’s fractional shortenings at 3 months. (p=0.031). This data suggests that rats that had presence of calcifications also had a decreased functional improvement.
DISCUSSION
The main goal of the current study was to assess the role of stem cell injection in the formation of chondro-osteogenic masses, as previously reported 21. Our data indicates that these chondro-osteogenic masses are present independently of cell injection into the myocardium. Our data indicates a positive relationship between the decreased cardiac function, increased infarction size and formation of chondro-osteogenic masses. Using multiple complementary methods of histology and immunostaining, we characterized these masses and show that they are of osteogenic and chondrogenic origin. Taken together, these results indicate that chondro-osteogenic masses can be formed in experimental animals as a result of the severity of the infarct, not due to the stem cell injection. Since animal models of cardiac infarct are being extensively used to determine the effects of stem cell injection for cardiac muscle repair, the current study underscores the need for careful examination of the tissue damage because it may have a significant effect on the experimental outcome.
Cell transplantation has emerged as a potential method for treating ischemic heart disease as reviewed by Anversa et al. 22. As in the current study, studies using stem cells in ischemic injury models have also shown similar bone formation in the heart. One study concluded that the engrafted stem cells were responsible for the formation of the bone in the myocardial heart 14. Another recent study using bone marrow stem cells by Breitbach et al. also showed that both BM cells and mesenchymal stem cells (MSCs) show evidence of bone formation after direct injection into the injured myocardium. In their study, the groups receiving the vehicle, fibroblasts, or hematopoietic progenitor cells did not form these masses 15. Other investigators have been searching for the mechanism underlying this phenomenon which has also been shown to occur in other organ systems 16–18. For example, Kan et al. demonstrated that dysregulation of local stem/progenitor cells could be a common cellular mechanism for calcification in skeletal muscle 17. Further, Collet et. al summarized in a review that angiogenesis is potentially associated with ectopic calcification. The review highlighted on the evidence that osteoprogenitor cells could be the pericytes that are present in the new blood vessels indicating a potential link between ectopic calcification and angiogenesis 23.
There are limited reports of myocardial calcification occurring in animals. Several studies in mice have shown myocardial calcification after ischemia is exhibited in certain strains of mice 24, and have linked it to the causal gene Dyscalc1 25, 26 trying to elucidate the mechanism behind dystrophic cardiac calcifications in humans. One study by Fitzpatrick et al. showed the presence of chondrocytes in aged rat hearts, and they concluded that many of the proteins that are associated with calcification in bone are present in the cartilage and the vascular tissue, suggesting that endochondral calcification is another possible mechanism by which calcification of vascular tissue may occur 27.
Recently, Chiavegato et al. published a study reporting that AFS cells caused chondro-osteogenic masses in the heart 21. The authors claimed that AFS cells were responsible for the formation of bone-like cell aggregates. However, the authors did not mention if control animals exhibited the same phenomena. The results of our study indicate that these unusual masses can form in the absence of AFS cell injection and that the injection does not increase the chances of obtaining chondro-osteogenic masses in the infracted heart. Indeed, AFS cells have the potential to differentiate into osteogenic cells and form bone in vitro and in vivo under the proper conditions as published by De Coppi et al. 19, which potentially could suggest that AFS cells stain positive for osteocalcin. Collectively, our data together with previous studies with AFS cells suggest that a host microenvironment favoring bone differentiation factors, as in the injured heart, induce AFS cells to osteogenic and chondrogenic lineage.
It is possible that the presence of chondro-osteogenic masses in the heart could affect the cardiac function analysis. Since we were able to identify rats that showed masses in the heart without harvesting the hearts we compared the cardiac function between rats with such masses to rats that did not develop masses. We performed clinical assessment of cardiac function, using echocardiography, and showed significant correlation between the infarction size and reduced fractional shortening, and the chondro-osteogenic mass formation. This data suggests that a decrease in cardiac function could potentially result in chondro-osteogenic mass formation. Currently, we do not completely understand the mechanism behind this phenomenon, but importantly, we observed similar chondro-osteogenic formation in rats that underwent ligation without cell transplantation. As expected, we did not see any chondro-osteogenic masses following injection of AFS cells into the uninjured hearts, nor could we identify β–galactosidase positive cells in the uninjured hearts at 3 months. We demonstrated that it is not the cell transplantation that caused the chondro-osteogenic masses, but the degree of myocardial infarction and fractional shortening. Further, our data supports a recently published paper by Ribeiro et al. in 2006, in which they showed that chondro-osteogenic differentiation can take place in the Wistar rat heart independent of animal treatment with bone marrow cells 28.
In conclusion, our study clearly indicates that chondro-osteogenic differentiation can take place in the pathological rat heart independent of amniotic fluid stem cell transplantation in the heart. We demonstrated by several analyses, that the formation of chondro-osteogenic masses is dependent on, and significantly correlated with, the infarction size and fractional shortening. This finding warrants careful evaluation of the outcome after experimental myocardial infarction when investigating stem cell therapy for treatment of cardiovascular disease.
Acknowledgments
We would like to thank Dr. Mark Willingham and Ken Grant in the Microscopy Core for their assistance.
SOURCES OF FUNDING
This research was supported, in part, by a NIH Ruth L. Kirschstein National Research Service Award Individual Fellowship (F31) (#AA016056-01) (DD), Supported by China Scholarship Council (XG), and Wake Forest Institute for Regenerative Medicine internal department funds.
Footnotes
DISCLOSURES
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dawn M. Delo, Email: dawn.delo@gmail.com, Wake Forest Institute for Regenerative Medicine, Wake Forest University Health Sciences, Medical Center Blvd, Winston-Salem, NC 27157.
Xuan Guan, Email: xguan@wfubmc.edu, Wake Forest Institute for Regenerative Medicine, Wake Forest University Health Sciences, Medical Center Blvd, Winston-Salem, NC 27157.
Zhan Wang, Email: zhawang@wfubmc.edu, Wake Forest Institute for Regenerative Medicine, Wake Forest University Health Sciences, Medical Center Blvd, Winston-Salem, NC 27157.
Leanne Groban, Email: lgroban@wfubmc.edu, Department of Anesthesiology, Wake Forest University Health Sciences, Medical Center Blvd, Winston-Salem, NC 27157.
Michael Callahan, Email: Callahan@wfubmc.edu, Department of Orthopaedic Surgery, Wake Forest University Health Sciences, Medical Center Blvd, Winston-Salem, NC 27157.
Tom Smith, Email: tsmith@wfubmc.edu, Department of Orthopaedic Surgery, Wake Forest University Health Sciences, Medical Center Blvd, Winston-Salem, NC 27157.
David Sane, Email: dsane@wfubmc.edu, Department of Cardiology, Wake Forest University Health Sciences, Medical Center Blvd, Winston-Salem, NC 27157.
R. Mark Payne, Email: Rpayne@iupui.edu, Riley Heart Research Center, Wells Center for Pediatric Research, Department of Medicine, Indiana University School of Medicine, 1044 West Walnut, R4, Room 402, Indianapolis, IN 46202.
Anthony Atala, Email: aatala@wfubmc.edu, Wake Forest Institute for Regenerative Medicine, Wake Forest University Health Sciences, Medical Center Blvd, Winston-Salem, NC 27157.
Shay Soker, Email: ssoker@wfubmc.edu, Wake Forest Institute for Regenerative Medicine, Wake Forest University Health Sciences, Medical Center Blvd, Winston-Salem, NC 27157.
References
- 1.Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: the scientific foundations of cardiac repair. J ClinInvest. 2005;115:572–83. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laflamme MA, Murry CE. Regenerating the heart. Nat Biotech. 2005;23:845–56. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 3.Reinecke H, Minami E, Zhu WZ, Laflamme MA. Cardiogenic differentiation and transdifferentiation of progenitor cells. Circ Res. 2008;103:1058–71. doi: 10.1161/CIRCRESAHA.108.180588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–42. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 5.Leor J, Guetta E, Feinberg MS, Galski H, Bar I, Holbova R, et al. Human umbilical cord blood-derived CD133+ cells enhance function and repair of the infarcted myocardium. Stem Cells. 2006;24:772–80. doi: 10.1634/stemcells.2005-0212. [DOI] [PubMed] [Google Scholar]
- 6.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–73. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 7.Kofidis T, Lebl DR, Swijnenburg RJ, Greeve JM, Klima U, Robbins RC. Allopurinol/uricase and ibuprofen enhance engraftment of cardiomyocyte-enriched human embryonic stem cells and improve cardiac function following myocardial injury. Eur J Cardiothorac Surg. 2006;29:50–5. doi: 10.1016/j.ejcts.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–24. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 9.Gandia C, Arminan A, Garcia-Verdugo JM, Lledo E, Ruiz A, Minana MD, et al. Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells. 2008;26:638–45. doi: 10.1634/stemcells.2007-0484. [DOI] [PubMed] [Google Scholar]
- 10.Brasselet C, Morichetti MC, Messas E, Carrion C, Bissery A, Bruneval P, et al. Skeletal myoblast transplantation through a catheter-based coronary sinus approach: an effective means of improving function of infarcted myocardium. Eur Heart J. 2005;26:1551–6. doi: 10.1093/eurheartj/ehi151. [DOI] [PubMed] [Google Scholar]
- 11.Dimmeler S, Burchfield J, Zeiher AM. Cell-based therapy of myocardial infarction. Arterioscler Thromb Vasc Biol. 2008;28:208–16. doi: 10.1161/ATVBAHA.107.155317. [DOI] [PubMed] [Google Scholar]
- 12.Kang S, Yang YJ, Li CJ, Gao RL. Effects of intracoronary autologous bone marrow cells on left ventricular function in acute myocardial infarction: a systematic review and meta-analysis for randomized controlled trials. Coron Artery Dis. 2008;19:327–35. doi: 10.1097/MCA.0b013e328300dbd3. [DOI] [PubMed] [Google Scholar]
- 13.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–97. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 14.Yoon YS, Park JS, Tkebuchava T, Luedeman C, Losordo DW. Unexpected severe calcification after transplantation of bone marrow cells in acute myocardial infarction. Circulation. 2004;109:3154–57. doi: 10.1161/01.CIR.0000134696.08436.65. [DOI] [PubMed] [Google Scholar]
- 15.Breitbach M, Bostani T, Roell W, Xia Y, Dewald O, Nygren JM, et al. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood. 2007;110:1362–69. doi: 10.1182/blood-2006-12-063412. [DOI] [PubMed] [Google Scholar]
- 16.Lounev VY, Ramachandran R, Wosczyna MN, Yamamoto M, Maidment AD, Shore EM, et al. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J Bone Joint Surg Am. 2009;91:652–63. doi: 10.2106/JBJS.H.01177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kan L, Liu Y, McGuire TL, Palila Berger DM, Awatramani RB, Dymecki SM, et al. Dysregulation of Local Stem/Progenitor Cells as a Common Cellular Mechanism for Heterotopic Ossification. Stem Cells. 2008 doi: 10.1634/stemcells.2008-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Speer MY, Yang HY, Brabb T, Leaf E, Look A, Lin WL, et al. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 2009;104:733–41. doi: 10.1161/CIRCRESAHA.108.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Coppi P, Bartsch G, Jr, Siddiqui MM, Xu T, Santos CC, Perin L, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100–06. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 20.Takagawa J, Zhang Y, Wong ML, Sievers RE, Kapasi NK, Wang Y, et al. Myocardial infarct size measurement in the mouse chronic infarction model: comparison of area- and length-based approaches. J Appl Physiol. 2007;102:2104–11. doi: 10.1152/japplphysiol.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiavegato A, Bollini S, Pozzobon M, Callegari A, Gasparotto L, Taiani J, et al. Human amniotic fluid-derived stem cells are rejected after transplantation in the myocardium of normal, ischemic, immuno-suppressed or immuno-deficient rat. JMol Cell Cardiol. 2007;42:746–59. doi: 10.1016/j.yjmcc.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Anversa P, Leri A, Rota M, Hosoda T, Bearzi C, Urbanek K, et al. Concise review: stem cells, myocardial regeneration, and methodological artifacts. Stem Cells. 2007;25:589–601. doi: 10.1634/stemcells.2006-0623. [DOI] [PubMed] [Google Scholar]
- 23.Collett GD, Canfield AE. Angiogenesis and pericytes in the initiation of ectopic calcification. Circ Res. 2005;96:930–8. doi: 10.1161/01.RES.0000163634.51301.0d. [DOI] [PubMed] [Google Scholar]
- 24.Korff S, Riechert N, Schoensiegel F, Weichenhan D, Autschbach F, Katus HA, et al. Calcification of myocardial necrosis is common in mice. Virchows Arch. 2006;448:630–38. doi: 10.1007/s00428-005-0071-7. [DOI] [PubMed] [Google Scholar]
- 25.Meng H, Vera I, Che N, Wang X, Wang SS, Ingram-Drake L, et al. Identification of Abcc6 as the major causal gene for dystrophic cardiac calcification in mice through integrative genomics. ProcNatlAcadSciUSA. 2007;104:4530–35. doi: 10.1073/pnas.0607620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aherrahrou Z, Doehring LC, Kaczmarek PM, Liptau H, Ehlers EM, Pomarino A, et al. Ultrafine mapping of Dyscalc1 to an 80-kb chromosomal segment on chromosome 7 in mice susceptible for dystrophic calcification. Physiol Genomics. 2007;28:203–12. doi: 10.1152/physiolgenomics.00133.2006. [DOI] [PubMed] [Google Scholar]
- 27.Fitzpatrick LA, Turner RT, Ritman ER. Endochondral bone formation in the heart: a possible mechanism of coronary calcification. Endocrinology. 2003;144:2214–19. doi: 10.1210/en.2002-0170. [DOI] [PubMed] [Google Scholar]
- 28.Ribeiro KC, Mattos EC, Werneck-de-castro JP, Ribeiro VP, Costa-e-Sousa RH, Miranda A, et al. Ectopic ossification in the scar tissue of rats with myocardial infarction. Cell Transplant. 2006;15:389–97. [PubMed] [Google Scholar]