Abstract
A major reason for the devastating and permanent disabilities after spinal cord and other types of CNS injury is the failure of injured axons to regenerate and to re-build the functional circuits. Thus, a long-standing goal has been to develop strategies that could promote axon regeneration and restore functions. Recent studies revealed that simply removing extracellular inhibitory activities is insufficient for successful axon regeneration in the adult CNS. On the other side, evidence from different species and different models is accumulating to support the notion that diminished intrinsic regenerative ability of mature neurons is a major contributor to regeneration failure. This review will summarize the molecular mechanisms regulating intrinsic axon growth capacity in the adult CNS and discuss potential implications for therapeutic strategies.
Introduction
Understanding why injured axons cannot regenerate after injury in the adult mammals has been a major challenge for both basic and clinical neuroscientists. Previous elegant studies by Aguayo and his colleagues showing that some injured CNS axons were able to grow into a permissive graft transplanted to the lesion site suggested that inhibitory activities in the lesion sites might be primarily responsible for preventing axon regeneration [1]. Thus, extensive studies in the past decades have been aimed at characterizing the molecular identities and functional mechanisms of these inhibitors. As a result, multiple molecules highly inhibitory to axon growth have been identified. They are associated with either myelin debris (eg, MAG, Nogo-A, and Omgp), or with glial scar formation (eg. CSPG and tenasin) [2–6]. Signaling pathways for these inhibitors have also been discovered. For example, a recent study suggests that a receptor tyrosine phosphatase acts as a functional receptor for CSPGs [7*]. However, removing these molecules by genetic deletions or pharmacological inhibitions only allows limited sprouting, but is not sufficient for long-distance axon regeneration [5,8]. These observations demand re-consideration of the intrinsic regenerative ability of mature neurons.
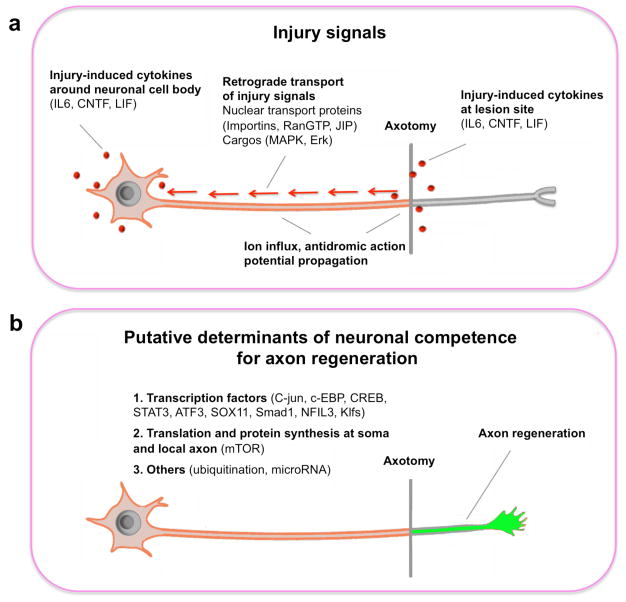
It is known that axon re-growth involves expressions of regeneration-associated genes (RAGs) such as GAP-43, Cap23, Arg1, and Sprr1a. In order to initiate the transcription program for axon regeneration, an injury signal is first generated by the lesioned axon and relayed to the neuronal soma (Figure 1a). However, not all neurons respond to injury signals in the same way. Whether successful axon regeneration could occur depends on the intrinsic competence of injured neurons in launching a growth program (Figure 1b). Recent studies from c. elegans, zebrafish and rodents revealed the possible molecular identities of injury signals and competence determinants.
Figure 1.
Two aspects of neuronal intrinsic mechanisms for axon regeneration. (a) Schematic of axotomy-triggered retrograde signals. In addition to acute axotomy-induced changes such as ion influx and antidromic action potential propagation, cytokines such as IL-6, CNTF and LIF could be up-regulated at the lesion site and/or around the cell body. Activated signaling components in the axon or at the cell body could be transported to the nucleus by nuclear transport proteins such as importins, RanGTP and JIP. (b) Putative determinants of neuronal competence for regenerative responses. These include the steps required for synthesis and assembly of materials for axon extension: transcription, translation and other post-translational modifications.
Injury signals
Upon axotomy, different changes could occur at the injured axonal terminal, along the axon shaft, as well as in the neuronal soma. For example, the lesion site may have rapid ion influxes. In cultured neurons, axotomy leads to a significant increase in local calcium concentrations that rapidly trigger various responses in the soma [9]. At least in Aplysia, such calcium increase is important for initiating axonal regrowth program [10, 11]. In addition to these acute changes, extensive evidence also suggest a set of slower propagating injury-induced signals propagating slower than calcium waves which comprise a decrease in the trafficking of trophic factors to the soma and an increase in the transport of new injury-induced molecular signals from the lesion site to the soma [12–14].
Nuclear transport proteins: Importins, RanGTP and JIP
Early studies in Aplysia and recent findings in the PNS neurons of rodents suggested that injuries triggered certain signaling molecules with nuclear localization signal (NLS) to be transported to the nucleus and initiate transcriptional program for axon regeneration. At least three classes of nuclear transport systems have been implicated and these include importins [15], RanGTP [16] and JNK-interacting proteins (JIP) [17]. Some components of importins and RanGTP are constitutively expressed in the axons of intact PNS neurons, but are insufficient to be functional. Axotomy triggers the local synthesis of other critical components such as importin-b [15], and Ran-binding protein RanBP1 [16], which allow the activation of these nuclear import systems. Similarly, injury also results in the activation of JNK3 in the axon, which will be relayed to the nucleus in activating the expression of c-Jun and other molecules [18, 19].
Cargos of the injury-activated nuclear transport systems are also being revealed. Perlson et al suggested that vimentin might be also a candidate of cargo for injury-induced retrograde transport [20]. Zou et al showed that peripheral axon injury could activate the nuclear import of Smad1, a critical signaling mediator of BMPs, which promote axon growth in adult sensory neurons [21]. Recent interesting genetic studies in C. elegans identified the DLK (dual leucine zipper-bearing kinase 1) MAP kinase pathway as a positive regulator of growth cone formation and axon regeneration [22**, 23**]. DLK-1 is a component of a conserved MAPK cascade that includes the MAP kinase kinase MKK-4 and the p38 MAP kinase PMK-3. Loss-of-function mutations of the dlk-1, mkk-4 or pmk-3 gene result in axon regeneration defects, suggesting that this entire signaling pathway is required for axon regeneration. Furthermore, activated PMK-3 is likely to be transported to the nucleus for its biological function [22**]. It is less clear though, whether these pathways are important for neuronal survival after injury, or directly involved in regulating axon re-growth, or both in the adult CNS. For example, Erk pathway was shown to be critical for BDNF-induced corticospinal regeneration after a subcortical injury model [24]. Similarly, Mammalian sterile 20-like kinase-3b (Mst3b, encoded by Stk24), an Erk downstream signaling molecule, has also been implicated in promoting axon growth and regrowth [25]. However, over-expression of Erk1/2 promoted neuronal survival, but not axon regeneration, after optic nerve injury model [26].
Injury-triggered expression of cytokines
Several studies suggested that axotomy triggers the expression of cytokines such as interleukin-6 (IL-6), cilliary neurotrophic factor (CNTF) in the lesion sites after peripheral nerve injury [27–29]. These cytokines are known to act through their receptor complexes with a shared protein gp130 [30]. Downstream signaling mediators of this pathway are JAK-STAT cascade. Injury at the peripheral, but not central, axon branch of dorsal root ganglion (DRG) sensory neurons results in accumulation of phospho-STAT3 in the nucleus [31, 32], which correlates with the activation of axon regeneration program [33, 34]. In vitro, while inhibitors of ERK or PI3K could block neurite growth in embryonic DRG neurons, a JAK2 inhibitor could efficiently abolish outgrowth from adult DRG neurons with a peripheral lesion [35]. These findings suggest a critical role of this pathway in mediating the effects of a peripheral lesion on enhancing the regenerative ability. Furthermore, cytokines such as IL-6 could mimic the growth-promoting effects of a conditioning lesion and cAMP [36, 37]. However, IL6 knockout mice showed normal axon regeneration with or without a conditioning lesion [37].
Several reports have shown that cytokines such as CNTF and LIF are up-regulated in the retina, likely in astrocytes, after optic nerve injury [38, 39], although how axotomy leads to their up-regulation remains unknown. The importance of injury-induced cytokines in axon regeneration was shown in studies of lens injury-triggered enhancement of axon regeneration of adult retinal ganglion cells (RGCs) [40*]. Lens injury or intravitreal application of zymosan could switch RGCs into an active regenerative state, enabling these neurons to survive axotomy and to regenerate axons into the injured optic nerve [41, 42]. Molecules such as oncomodulin were previously shown to be involved in lens injury enhanced axon regeneration [43–45]. More recently, Leibinger et al showed that the effect of lens injury is reduced in CNTF−/− mice and completely blocked in CNTF−/− LIF−/− double mutant mice [40*].
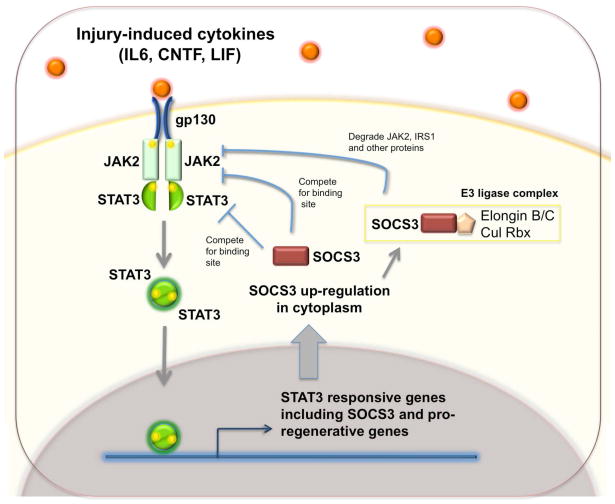
Surprisingly, exogenously delivered cytokines have limited effects on promoting survival and regeneration following either optic nerve [46, 47] or spinal cord injury [48]. These findings have puzzled the field until a recent study provided a plausible explanation. Park et al developed a highly efficient method to conditionally delete genes in adult retinal ganglion neurons (RGCs) by injecting Cre-expressing AAV into the vitreous body of floxed mice [49**]. This allows one to assess the effects of RGC-specific gene knockout on axon regeneration after optic nerve injury in adult mice. Among different floxed alleles tested, Smith et al found extensive axon regeneration upon conditional deletion of SOCS3, a negative regulator of the JAK-STAT pathway [50**]. By contrast, no RGC axon regeneration was observed when SOCS3 and gp130 were both deleted, indicating that the regeneration of SOCS3 mutant axons is dependent on cytokine ligands of gp130. Consistently, exogenous application of CNTF to SOCS3 deleted mice could dramatically increase the extent of axon regeneration [50**]. Another interesting observation is that cAMP-induced outgrowth-promoting effects may be partially mediated by down-regulation of SOCS3 [38]. Since SOCS3 is a well-known transcription target of the JAK-STAT pathway [51], in wildtype neurons, despite injury-induced induction of cytokines, the presence and induced expression of SOCS3 put a strong negative brake to prevent axon regeneration (Figure 2). In addition to confirming the role of the JAK/STAT pathway in promoting axon regeneration, these new observations highlight the dominant role of negative regulators of signaling pathways in restricting axon regeneration in adult neurons. Thus, it will be interesting to find out what other negative regulators of JAK-STAT and other growth factor signaling pathways (for example BDNF as show in [52]) also act as intrinsic barriers of axon regeneration.
Figure 2.
gp130-dependent cytokines promote axon regeneration and SOCS3 act as a critical negative regulator of the signaling pathway. Injury-induced cytokines act on their receptor complexes with a shared component gp130 and activate the JAK-STAT cascade in both PNS and CNS neurons. Phosphorylated STAT-3 is translocated to the nucleus and initiate gene expression for axon regeneration. However, in the adult CNS, the activation of this pathway leads to the up-regulation of SOCS3 which will inhibit JAK2 and STAT3 and in turn inhibit this pathway. Thus, in SOCS3 deleted RGCs, both endogenous and exogenous cytokines such as CNTF promote significant axon regeneration [ 50**].
Neuronal competence of axon regeneration
Obviously, CNS neurons differ in their responses to injuries and injury-induced signals. While some axotomized neurons undergo cell death, those that survive the injuries differ in their abilities to initiate axon regeneration. Thus, an important question is what determines the intrinsic competence of neurons to regenerate injured axons.
Regeneration-associated transcription factors
In order to identify possible master control gene(s) for axon regeneration, microarray based experiments have been performed to compare gene expression differences in neurons with or without enhanced axon regeneration. Several models have been used and these include DRG neurons with or without pre-conditioning lesions [53–56], adult RGCs with or without lens injuries [57], and adult RGCs from regeneration-competent species such as zebrafish [58*]. In addition, Goldberg et al found a development-dependent decline of axon growth ability of rodent RGCs [59] and thus RGCs of different developmental stages were also used in searching for genes critical for axon regeneration [60].
Studies from DRG neurons with a conditioning lesion identified several transcription factors such as c-Jun [61, 62], c-EBP [63], CREB [64], STAT3 [32, 65], ATF3 [19, 62, 66, 67], SOX11 [68, 56], and Smad1 [21], as positively associated with axon regeneration. Indeed, constitutive expression of ATF3 in neurons of Thy-1.2 ATF3 transgenic mice enhanced PNS regeneration [69]. C/EBPβ is also up-regulated and phosphorylated after peripheral injury in rodent, which is needed for injury-induced up-regulation of α-tubulin and GAP-43 [63]. On the flip side, transcription factor such as NFIL3 was implicated as an injury-induced transcription suppressor of axon regeneration, likely by antagonizing the positive effects of CREB family members [70].
Recent studies from several groups pointed to a critical role of Kruppel-like factors (KLFs), which are zinc-finger transcription factors, in axon growth control. These genes were initially implicated in regulating cell cycle exit and terminal differentiation in non-neuronal cells [71]. KLF4 is one of the four transcription factors sufficient to transform fibroblasts into pluripotent stem cells [72]. In zebrafish, KLF6 and KLF7 were identified among the group of up-regulated genes in regenerating RGCs [58*]. Importantly, knockdown of these molecules reduces axon growth [58*, 73]. In an independent study, KLF4 was found to be a potent inhibitory molecule for axon growth in embryonic hippocampal neurons and RGCs [60**]. Interestingly, these different KLFs differ in their expression levels over the course of development: while KLF6/7 are down-regulated, KLF4/9 are up-regulated in adult RGCs [60**]. Consistently, over-expression of different KLFs results in opposite effects on neurite growth in cortical neurons [60**]. These studies provide an example of how complicated transcriptional factor networks regulate the process of axon growth and regeneration. Thus, differences in the ability to express growth-promoting versus growth-inhibitory transcription factors may result in different intrinsic regenerative competence in adult neurons.
mTOR and protein translation in axon regeneration
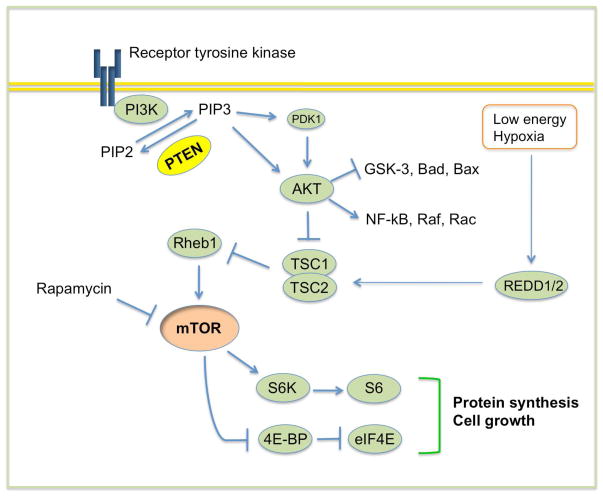
In an effort to analyze the effects of knockout of individual genes involved in cellular growth control on axon regeneration in an optic nerve injury model, Park et al discovered robust long-distance axon regenerations in adult mice with targeted deletion of the phosphatase and tensin homolog (PTEN) gene [49**]. PTEN is a well-established dual phosphatase which could convert phosphatidylinositol (3,4,5) trisphosphate (PIP3) to phosphatidylinositol (4,5) bisphosphate (PIP2), in antagonizing the activity of phosphoinositide 3-kinases (PI3K). PTEN deletion leads to the accumulation of PIP3, which in turn recruits and activates phosphatidylinositol-dependent kinase 1/2 (PDK1/2), resulting in the activation of Akt [74, 75] (Figure 3). Among the multiple down-stream targets of this pathway, mTOR controls cap-dependent protein translation [76] and GSK-3 can regulate cytoskeleton assemble and axonal transport [77]. Rapamycin administration abolishes the regeneration effect of PTEN deletion, suggesting a requirement of mTOR in axon regeneration. In addition, targeted deletion of tuberous sclerosis protein 1 (TSC1), a specific negative regulator of mTOR (Figure 3), also promotes axon regeneration. However, the extent of axon regeneration after TSC1 is less than that after PTEN deletion. These results suggest a model in which mTOR activity may control the protein synthesis for axon growth, while mTOR-independent pathways such as GSK-3 may promotes axonal cytoskeleton assembly and axonal transport [78].
Figure 3.
PTEN-regulated signaling pathway. In response to receptor tyrosine kinase activation, PI3K phosphorylates and converts the lipid second messenger phosphatidylinositol (4,5) bisphosphate (PIP2) into phosphatidylinositol (3,4,5) trisphosphate (PIP3), which recruits and activates phosphatidylinositol-dependent kinase 1/2 (PDK1/2). PDK1/2, in turn, phosphorylates and activates Akt. PTEN catalyzes the conversion from PIP3 to PIP2. Thus, inactivation of PTEN results in the accumulation of PIP3 and the activation of Akt. Akt controls a host of signaling molecules, including GSK-3 and TSC1/2. Inactivation of the TSC1/2 complex leads to activation of mTOR, which integrates various cellular signals including nutrient availability to control protein translation, cell growth, and other processes. The ribosomal protein S6 kinase (RP-S6) and the eukaryotic initiation factor 4E (eIF-4E) binding protein 1 (4E-BP1) are the mTOR effector molecules executing these functions. Cellular stresses such as hypoxia and low energy induce expression of Redd1/2, which augments TSC1/2 activity and in turn suppress the mTOR activity.
In further support for the loss of mTOR activity as a major intrinsic barrier for axon regeneration, Park et al found that mTOR signaling is down-regulated in the CNS neurons over the course of development [49**]. The residual mTOR activity in adult RGCs are further diminished by axotomy-triggered stress response [49**]. By this two-step mTOR suppression, injured RGCs retain extremely low level of mTOR activity, and hence very limited ability to initiate new protein synthesis required for axon regeneration. It remains to be determined whether local protein synthesis in axons [79–81] or translation in the soma is crucial for axon regeneration.
The mechanisms that mediate the changes of mTOR activity during development and after injury are largely unknown. The localization and function of PTEN may be controlled by myosin V [82]. In addition, Nedd4, an E3 ligase, was shown to promote axonal branching by down-regulating PTEN [83]. Many other molecules and pathways such as REDD1 [84–86], sestrin [87], could down-regulate mTOR activity in the cells under stress conditions. At present, whether any of these above-mentioned pathways mediate axotomy-triggered mTOR suppression is unknown.
Other mechanisms
In addition to transcription and translation regulations, evidence also suggested the involvement of other protein post-translational modifications in the process of axon regeneration. The anaphase-promoting complex (APC), an E3 ligase complex that was extensively studied in cell cycle, is expressed in postmitotic neurons and may degrade molecules required for axon growth [88, 89]. It will be interesting to test the axon regeneration effects of these molecular mechanisms on axon regeneration in vivo. Recently, an elegant study demonstrated a critical role of microRNA-206 in promoting regeneration of neuromuscular synapses in mice [90]. The roles of microRNA in axon growth and regeneration await to be explored. In addition, it will be interesting to find out how other established pathways such as cAMP/Arg1 [91, 92] cross-talk with the mechanisms discussed above.
Taken together, these recent studies identified several critical intrinsic barriers preventing axon regeneration in adult CNS neurons. Thus, removing these negative brakes may allow mature neurons to regain regenerative ability after injury. As recently demonstrated [93–96], combinatorial strategies to deal with extrinsic and intrinsic mechanisms may represent a promising avenue to promoting axon regeneration and functional recovery after CNS injury.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- 2.Schwab ME, Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev. 1996;76:319–370. doi: 10.1152/physrev.1996.76.2.319. [DOI] [PubMed] [Google Scholar]
- 3.Filbin MT. Recapitulate development to promote axonal regeneration: good or bad approach? Philos Trans R Soc Lond B Biol Sci. 2006;361:1565–1574. doi: 10.1098/rstb.2006.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitch MT, Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol. 2008;209:294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harel NY, Strittmatter SM. Can regenerating axons recapitulate developmental guidance during recovery from spinal cord injury? Nat Rev Neurosci. 2006;7:603–616. doi: 10.1038/nrn1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7*.Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, He Z, Silver J, Flanagan JG. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326:592–596. doi: 10.1126/science.1178310. This study provides evidence for a transmembrane protein tyrosine phosphatase, PTPsigma as a receptor of CSPGs. In culture, PTPsigma(−/−) neurons show reduced inhibition by CSPG. After spinal cord injury, PTPsigma gene disruption enhanced the ability of axons to penetrate regions containing CSPG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JK, Chan AF, Luu SM, Zhu Y, Ho C, Tessier-Lavigne M, Zheng B. Reassessment of corticospinal tract regeneration in Nogo-deficient mice. J Neurosci. 2009;29:8649–8654. doi: 10.1523/JNEUROSCI.1864-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandolesi G, Madeddu F, Bozzi Y, Maffei L, Ratto GM. Acute physiological response of mammalian central neurons to axotomy: ionic regulation and electrical activity. FASEB J. 2004;18:1934–1936. doi: 10.1096/fj.04-1805fje. [DOI] [PubMed] [Google Scholar]
- 10.Spira ME, Oren R, Dormann A, Gitler D. Critical calpain-dependent ultrastructural alterations underlie the transformation of an axonal segment into a growth cone after axotomy of cultured Aplysia neurons. J Comp Neurol. 2003;457:293–312. doi: 10.1002/cne.10569. [DOI] [PubMed] [Google Scholar]
- 11.Spira ME, Oren R, Dormann A, Ilouz N, Lev S. Calcium, protease activation, and cytoskeleton remodeling underlie growth cone formation and neuronal regeneration. Cell Mol Neurobiol. 2001;21:591–604. doi: 10.1023/A:1015135617557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ambron RT, Walters ET. Priming events and retrograde injury signals. A new perspective on the cellular and molecular biology of nerve regeneration. Mol Neurobiol. 1996;13:61–79. doi: 10.1007/BF02740752. [DOI] [PubMed] [Google Scholar]
- 13.Rishal I, Michaelevski I, Rozenbaum M, Shinder V, Medzihradszky KF, Burlingame AL, Fainzilber M. Axoplasm isolation from peripheral nerve. Dev Neurobiol. 2010;70:126–133. doi: 10.1002/dneu.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newbern JM, Shoemaker SE, Snider WD. Taking off the SOCS: cytokine signaling spurs regeneration. Neuron. 2009;64:591–592. doi: 10.1016/j.neuron.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 15.Hanz S, Perlson E, Willis D, Zheng JQ, Massarwa R, Huerta JJ, Koltzenburg M, Kohler M, van-Minnen J, Twiss JL, et al. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron. 2003;40:1095–1104. doi: 10.1016/s0896-6273(03)00770-0. [DOI] [PubMed] [Google Scholar]
- 16.Yudin D, Hanz S, Yoo S, Iavnilovitch E, Willis D, Gradus T, Vuppalanchi D, Segal-Ruder Y, Ben-Yaakov K, Hieda M, et al. Localized regulation of axonal RanGTPase controls retrograde injury signaling in peripheral nerve. Neuron. 2008;59:241–252. doi: 10.1016/j.neuron.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abe N, Cavalli V. Nerve injury signaling. Curr Opin Neurobiol. 2008;18:276–283. doi: 10.1016/j.conb.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cavalli V, Kujala P, Klumperman J, Goldstein LS. Sunday Driver links axonal transport to damage signaling. J Cell Biol. 2005;168:775–787. doi: 10.1083/jcb.200410136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindwall C, Kanje M. Retrograde axonal transport of JNK signaling molecules influence injury induced nuclear changes in p-c-Jun and ATF3 in adult rat sensory neurons. Mol Cell Neurosci. 2005;29:269–282. doi: 10.1016/j.mcn.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Perlson E, Hanz S, Ben-Yaakov K, Segal-Ruder Y, Seger R, Fainzilber M. Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron. 2005;45:715–726. doi: 10.1016/j.neuron.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 21.Zou H, Ho C, Wong K, Tessier-Lavigne M. Axotomy-induced Smad1 activation promotes axonal growth in adult sensory neurons. J Neurosci. 2009;29:7116–7123. doi: 10.1523/JNEUROSCI.5397-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22**.Hammarlund M, Nix P, Hauth L, Jorgensen EM, Bastiani M. Axon regeneration requires a conserved MAP kinase pathway. Science. 2009;323:802–806. doi: 10.1126/science.1165527. In a forward genetic screen, this study demonstrates that the DLK-1 MAP kinase pathway is essential for regeneration in c. elegans motor neurons. Loss of this pathway eliminates regeneration, whereas activating it improves regeneration. Further, these proteins also regulate the later step of growth cone migration. This is a nice demonstration of an injury-induced signaling pathway required for initiation of axon regeneration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23**.Yan D, Wu Z, Chisholm AD, Jin Y. The DLK-1 kinase promotes mRNA stability and local translation in C. elegans synapses and axon regeneration. Cell. 2009;138:1005–1018. doi: 10.1016/j.cell.2009.06.023. This study together with [22**] shows an essential role of DLK-1 kinase in axon regeneration in c. elegans. In addition to regulate synapse formation and axon morphology, the authors demonstrate that DLK-1 affects axon regeneration by influencing the stability of the mRNA encoding CEBP-1, a bZip protein related to CCAAT/enhancer-binding proteins, via its 3′UTR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollis ER, 2nd, Jamshidi P, Low K, Blesch A, Tuszynski MH. Induction of corticospinal regeneration by lentiviral trkB-induced Erk activation. Proc Natl Acad Sci U S A. 2009;106:7215–7220. doi: 10.1073/pnas.0810624106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lorber B, Howe ML, Benowitz LI, Irwin N. Mst3b, an Ste20-like kinase, regulates axon regeneration in mature CNS and PNS pathways. Nat Neurosci. 2009;12:1407–1414. doi: 10.1038/nn.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pernet V, Hauswirth WW, Polo AD. Extracellular signal-regulated kinase 1/2 mediates survival, but not axon regeneration, of adult injured central nervous system neurons in vivo. Journal of Neurochemistry. 2005;93:72–83. doi: 10.1111/j.1471-4159.2005.03002.x. [DOI] [PubMed] [Google Scholar]
- 27.Banner LR, Patterson PH. Major changes in the expression of the mRNAs for cholinergic differentiation factor/leukemia inhibitory factor and its receptor after injury to adult peripheral nerves and ganglia. Proc Natl Acad Sci U S A. 1994;91:7109–7113. doi: 10.1073/pnas.91.15.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curtis R, Scherer SS, Somogyi R, Adryan KM, Ip NY, Zhu Y, Lindsay RM, DiStefano PS. Retrograde axonal transport of LIF is increased by peripheral nerve injury: correlation with increased LIF expression in distal nerve. Neuron. 1994;12:191–204. doi: 10.1016/0896-6273(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 29.Sun Y, Zigmond RE. Leukaemia inhibitory factor induced in the sciatic nerve after axotomy is involved in the induction of galanin in sensory neurons. Eur J Neurosci. 1996;8:2213–2220. doi: 10.1111/j.1460-9568.1996.tb00744.x. [DOI] [PubMed] [Google Scholar]
- 30.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 31.Schweizer U, Gunnersen J, Karch C, Wiese S, Holtmann B, Takeda K, Akira S, Sendtner M. Conditional gene ablation of Stat3 reveals differential signaling requirements for survival of motoneurons during development and after nerve injury in the adult. J Cell Biol. 2002;156:287–297. doi: 10.1083/jcb.200107009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu J, Cafferty WB, McMahon SB, Thompson SW. Conditioning injury-induced spinal axon regeneration requires signal transducer and activator of transcription 3 activation. J Neurosci. 2005;25:1645–1653. doi: 10.1523/JNEUROSCI.3269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson PM, Issa VM. Peripheral injury enhances central regeneration of primary sensory neurones. Nature. 1984;309:791–793. doi: 10.1038/309791a0. [DOI] [PubMed] [Google Scholar]
- 34.Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23:83–91. doi: 10.1016/s0896-6273(00)80755-2. [DOI] [PubMed] [Google Scholar]
- 35.Liu RY, Snider WD. Different signaling pathways mediate regenerative versus developmental sensory axon growth. J Neurosci. 2001;21:RC164. doi: 10.1523/JNEUROSCI.21-17-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cafferty WB, Gardiner NJ, Gavazzi I, Powell J, McMahon SB, Heath JK, Munson J, Cohen J, Thompson SW. Leukemia inhibitory factor determines the growth status of injured adult sensory neurons. J Neurosci. 2001;21:7161–7170. doi: 10.1523/JNEUROSCI.21-18-07161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao Z, Gao Y, Bryson JB, Hou J, Chaudhry N, Siddiq M, Martinez J, Spencer T, Carmel J, Hart RB, et al. The cytokine interleukin-6 is sufficient but not necessary to mimic the peripheral conditioning lesion effect on axonal growth. J Neurosci. 2006;26:5565–5573. doi: 10.1523/JNEUROSCI.0815-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park KK, Hu Y, Muhling J, Pollett MA, Dallimore EJ, Turnley AM, Cui Q, Harvey AR. Cytokine-induced SOCS expression is inhibited by cAMP analogue: impact on regeneration in injured retina. Mol Cell Neurosci. 2009;41:313–324. doi: 10.1016/j.mcn.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Muller A, Hauk TG, Fischer D. Astrocyte-derived CNTF switches mature RGCs to a regenerative state following inflammatory stimulation. Brain. 2007;130:3308–3320. doi: 10.1093/brain/awm257. [DOI] [PubMed] [Google Scholar]
- 40*.Leibinger M, Muller A, Andreadaki A, Hauk TG, Kirsch M, Fischer D. Neuroprotective and axon growth-promoting effects following inflammatory stimulation on mature retinal ganglion cells in mice depend on ciliary neurotrophic factor and leukemia inhibitory factor. J Neurosci. 2009;29:14334–14341. doi: 10.1523/JNEUROSCI.2770-09.2009. This study provide definitive evidence for an essential role of CNTF and LIF in neuroprotection and axon regeneration-promoting effects of inflammatory stimulation on adult RGCs. These effects are partially reduced in CNTF knockout mice, completely abolished in CNTF/LIF double knockout mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fischer D, Pavlidis M, Thanos S. Cataractogenic lens injury prevents traumatic ganglion cell death and promotes axonal regeneration both in vivo and in culture. Invest Ophthalmol Vis Sci. 2000;41:3943–3954. [PubMed] [Google Scholar]
- 42.Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI. Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci. 2000;20:4615–4626. doi: 10.1523/JNEUROSCI.20-12-04615.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin Y, Cui Q, Gilbert HY, Yang Y, Yang Z, Berlinicke C, Li Z, Zaverucha-do-Valle C, He H, Petkova V, et al. Oncomodulin links inflammation to optic nerve regeneration. Proc Natl Acad Sci U S A. 2009;106:19587–19592. doi: 10.1073/pnas.0907085106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fischer D. What are the principal mediators of optic nerve regeneration after inflammatory stimulation in the eye? Proc Natl Acad Sci U S A. 2010;107:E8. doi: 10.1073/pnas.0912942107. author reply E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benowitz LI, Carmichael ST. Promoting axonal rewiring to improve outcome after stroke. Neurobiol Dis. 2010;37:259–266. doi: 10.1016/j.nbd.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cui Q, Lu Q, So KF, Yip HK. CNTF, not other trophic factors, promotes axonal regeneration of axotomized retinal ganglion cells in adult hamsters. Invest Ophthalmol Vis Sci. 1999;40:760–766. [PubMed] [Google Scholar]
- 47.Watanabe M, Tokita Y, Kato M, Fukuda Y. Intravitreal injections of neurotrophic factors and forskolin enhance survival and axonal regeneration of axotomized beta ganglion cells in cat retina. Neuroscience. 2003;116:733–742. doi: 10.1016/s0306-4522(02)00562-6. [DOI] [PubMed] [Google Scholar]
- 48.Lacroix S, Chang L, Rose-John S, Tuszynski MH. Delivery of hyper-interleukin-6 to the injured spinal cord increases neutrophil and macrophage infiltration and inhibits axonal growth. J Comp Neurol. 2002;454:213–228. doi: 10.1002/cne.10407. [DOI] [PubMed] [Google Scholar]
- 49**.Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. This study demonstrates robust axon regeneration after optic nerve injury in the mice with targeted deletion of PTEN or TSC1 in adult RGCs. They also show that mTOR activity is down-regulated over the course of development and further reduced by axotomy-triggered stress responses. Because of the critical role of mTOR in controlling protein translation, this two-step mTOR down-regulation may be a major mechanism of regeneration failure in adult RGCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50**.Smith PD, Sun F, Park KK, Cai B, Wang C, Kuwako K, Martinez-Carrasco I, Connolly L, He Z. SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron. 2009;64:617–623. doi: 10.1016/j.neuron.2009.11.021. By the method used in [49**], this study shows that targeted deletion of SOCS3 in RGCs leads to significant axon regeneration after optic nerve injury. This effect is dependent on gp130 ligands generated either constitutively or induced after injury. Application of CNTF to SOCS3 deleted mice shows further enhanced axon regeneration. This study provides definitive evidence for a regeneration-promoting effect of gp130-dependent JAK/STAT pathway and also reveals the critical role of negative regulators such as SOCS3 in limiting neuronal regenerative responses after injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Croker BA, Kiu H, Nicholson SE. SOCS regulation of the JAK/STAT signalling pathway. Semin Cell Dev Biol. 2008;19:414–422. doi: 10.1016/j.semcdb.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen H, Chung JM, Chung K. Expression of neurotrophin mRNAs in the dorsal root ganglion after spinal nerve injury. Brain Res Mol Brain Res. 1999;64:186–192. doi: 10.1016/s0169-328x(98)00314-3. [DOI] [PubMed] [Google Scholar]
- 53.Bonilla IE, Tanabe K, Strittmatter SM. Small proline-rich repeat protein 1A is expressed by axotomized neurons and promotes axonal outgrowth. J Neurosci. 2002;22:1303–1315. doi: 10.1523/JNEUROSCI.22-04-01303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Costigan M, Befort K, Karchewski L, Griffin RS, D’Urso D, Allchorne A, Sitarski J, Mannion JW, Pratt RE, Woolf CJ. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 2002;3:16. doi: 10.1186/1471-2202-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nilsson A, Moller K, Dahlin L, Lundborg G, Kanje M. Early changes in gene expression in the dorsal root ganglia after transection of the sciatic nerve; effects of amphiregulin and PAI-1 on regeneration. Brain Res Mol Brain Res. 2005;136:65–74. doi: 10.1016/j.molbrainres.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 56.Tanabe K, Bonilla I, Winkles JA, Strittmatter SM. Fibroblast growth factor-inducible-14 is induced in axotomized neurons and promotes neurite outgrowth. J Neurosci. 2003;23:9675–9686. doi: 10.1523/JNEUROSCI.23-29-09675.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fischer D, Petkova V, Thanos S, Benowitz LI. Switching mature retinal ganglion cells to a robust growth state in vivo: gene expression and synergy with RhoA inactivation. J Neurosci. 2004;24:8726–8740. doi: 10.1523/JNEUROSCI.2774-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58*.Veldman MB, Bemben MA, Thompson RC, Goldman D. Gene expression analysis of zebrafish retinal ganglion cells during optic nerve regeneration identifies KLF6a and KLF7a as important regulators of axon regeneration. Dev Biol. 2007;312:596–612. doi: 10.1016/j.ydbio.2007.09.019. To identify genes involved in successful nerve regeneration in RGCs in zebrafish, this study used micro-array analysis, quantitative RT-PCR and in situ hybridization and identified several genes associated with axon regeneration. Knockdown of six highly induced regeneration-associated genes identified two, KLF6a and KLF7a, that together were necessary for robust RGC axon re-growth. [DOI] [PubMed] [Google Scholar]
- 59.Goldberg JL, Klassen MP, Hua Y, Barres BA. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science. 2002;296:1860–1864. doi: 10.1126/science.1068428. [DOI] [PubMed] [Google Scholar]
- 60**.Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, Goldberg JL. KLF family members regulate intrinsic axon regeneration ability. Science. 2009;326:298–301. doi: 10.1126/science.1175737. By screening genes developmentally regulated in RGCs, this study shows KLF4 as a transcriptional repressor of axon growth in RGCs and other CNS neurons. RGCs lacking KLF4 showed increased axon growth both in vitro and after optic nerve injury in vivo. Related KLF family members suppressed or enhanced axon growth to differing extents, and several growth-suppressive KLFs were up-regulated postnatally, whereas growth-enhancing KLFs were down-regulated. Together with [58*], this study suggest that coordinated activities of different KLFs regulate the regenerative capacity of CNS neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Broude E, McAtee M, Kelley MS, Bregman BS. c-Jun expression in adult rat dorsal root ganglion neurons: differential response after central or peripheral axotomy. Exp Neurol. 1997;148:367–377. doi: 10.1006/exnr.1997.6665. [DOI] [PubMed] [Google Scholar]
- 62.Raivich G, Bohatschek M, Da Costa C, Iwata O, Galiano M, Hristova M, Nateri AS, Makwana M, Riera-Sans L, Wolfer DP, et al. The AP-1 transcription factor c-Jun is required for efficient axonal regeneration. Neuron. 2004;43:57–67. doi: 10.1016/j.neuron.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 63.Nadeau S, Hein P, Fernandes KJ, Peterson AC, Miller FD. A transcriptional role for C/EBP beta in the neuronal response to axonal injury. Mol Cell Neurosci. 2005;29:525–535. doi: 10.1016/j.mcn.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 64.Gao Y, Deng K, Hou J, Bryson JB, Barco A, Nikulina E, Spencer T, Mellado W, Kandel ER, Filbin MT. Activated CREB is sufficient to overcome inhibitors in myelin and promote spinal axon regeneration in vivo. Neuron. 2004;44:609–621. doi: 10.1016/j.neuron.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 65.Miao T, Wu D, Zhang Y, Bo X, Subang MC, Wang P, Richardson PM. Suppressor of cytokine signaling-3 suppresses the ability of activated signal transducer and activator of transcription-3 to stimulate neurite growth in rat primary sensory neurons. J Neurosci. 2006;26:9512–9519. doi: 10.1523/JNEUROSCI.2160-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsujino H, Kondo E, Fukuoka T, Dai Y, Tokunaga A, Miki K, Yonenobu K, Ochi T, Noguchi K. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: A novel neuronal marker of nerve injury. Mol Cell Neurosci. 2000;15:170–182. doi: 10.1006/mcne.1999.0814. [DOI] [PubMed] [Google Scholar]
- 67.Seijffers R, Allchorne AJ, Woolf CJ. The transcription factor ATF-3 promotes neurite outgrowth. Mol Cell Neurosci. 2006;32:143–154. doi: 10.1016/j.mcn.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 68.Jankowski MP, Cornuet PK, McIlwrath S, Koerber HR, Albers KM. SRY-box containing gene 11 (Sox11) transcription factor is required for neuron survival and neurite growth. Neuroscience. 2006;143:501–514. doi: 10.1016/j.neuroscience.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seijffers R, Mills CD, Woolf CJ. ATF3 increases the intrinsic growth state of DRG neurons to enhance peripheral nerve regeneration. J Neurosci. 2007;27:7911–7920. doi: 10.1523/JNEUROSCI.5313-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.MacGillavry HD, Stam FJ, Sassen MM, Kegel L, Hendriks WT, Verhaagen J, Smit AB, van Kesteren RE. NFIL3 and cAMP response element-binding protein form a transcriptional feedforward loop that controls neuronal regeneration-associated gene expression. J Neurosci. 2009;29:15542–15550. doi: 10.1523/JNEUROSCI.3938-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Laub F, Lei L, Sumiyoshi H, Kajimura D, Dragomir C, Smaldone S, Puche AC, Petros TJ, Mason C, Parada LF, et al. Transcription Factor KLF7 Is Important for Neuronal Morphogenesis in Selected Regions of the Nervous System. Mol Cell Biol. 2005;25:5699–5711. doi: 10.1128/MCB.25.13.5699-5711.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 73.Veldman MB, Bemben MA, Goldman D. Tuba1a gene expression is regulated by KLF6/7 and is necessary for CNS development and regeneration in zebrafish. Mol Cell Neurosci. 2010 doi: 10.1016/j.mcn.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maehama T, Taylor GS, Dixon JE. PTEN and myotubularin: novel phosphoinositide phosphatases. Annu Rev Biochem. 2001;70:247–279. doi: 10.1146/annurev.biochem.70.1.247. [DOI] [PubMed] [Google Scholar]
- 75.Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: of feedbacks and crosstalks. Oncogene. 2008;27:5527–5541. doi: 10.1038/onc.2008.247. [DOI] [PubMed] [Google Scholar]
- 76.Ma XM, Yoon SO, Richardson CJ, Julich K, Blenis J. SKAR links pre-mRNA splicing to mTOR/S6K1-mediated enhanced translation efficiency of spliced mRNAs. Cell. 2008;133:303–313. doi: 10.1016/j.cell.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 77.Zhou FQ, Snider WD. Cell biology. GSK-3beta and microtubule assembly in axons. Science. 2005;308:211–214. doi: 10.1126/science.1110301. [DOI] [PubMed] [Google Scholar]
- 78.Park KK, Liu K, Hu Y, Kanter JL, He Z. PTEN/mTOR and axon regeneration. Exp Neurol. 2010 doi: 10.1016/j.expneurol.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 79.Willis DE, Twiss JL. The evolving roles of axonally synthesized proteins in regeneration. Curr Opin Neurobiol. 2006;16:111–118. doi: 10.1016/j.conb.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 80.Lin AC, Holt CE. Function and regulation of local axonal translation. Curr Opin Neurobiol. 2008;18:60–68. doi: 10.1016/j.conb.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gumy LF, Tan CL, Fawcett JW. The role of local protein synthesis and degradation in axon regeneration. Exp Neurol. 2009 doi: 10.1016/j.expneurol.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Diepen MT, Parsons M, Downes CP, Leslie NR, Hindges R, Eickholt BJ. MyosinV controls PTEN function and neuronal cell size. Nat Cell Biol. 2009;11:1191–1196. doi: 10.1038/ncb1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Drinjakovic J, Jung H, Campbell DS, Strochlic L, Dwivedy A, Holt CE. E3 Ligase Nedd4 Promotes Axon Branching by Downregulating PTEN. Neuron. 2010;65:341–357. doi: 10.1016/j.neuron.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, Witters LA, Ellisen LW, Kaelin WG., Jr Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18:2893–2904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Corradetti MN, Inoki K, Guan KL. The stress-inducted proteins RTP801 and RTP801L are negative regulators of the mammalian target of rapamycin pathway. J Biol Chem. 2005;280:9769–9772. doi: 10.1074/jbc.C400557200. [DOI] [PubMed] [Google Scholar]
- 86.Reiling JH, Hafen E. The hypoxia-induced paralogs Scylla and Charybdis inhibit growth by down-regulating S6K activity upstream of TSC in Drosophila. Genes Dev. 2004;18:2879–2892. doi: 10.1101/gad.322704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Konishi Y, Stegmuller J, Matsuda T, Bonni S, Bonni A. Cdh1-APC controls axonal growth and patterning in the mammalian brain. Science. 2004;303:1026–1030. doi: 10.1126/science.1093712. [DOI] [PubMed] [Google Scholar]
- 89.Lasorella A, Stegmuller J, Guardavaccaro D, Liu G, Carro MS, Rothschild G, de la Torre-Ubieta L, Pagano M, Bonni A, Iavarone A. Degradation of Id2 by the anaphase-promoting complex couples cell cycle exit and axonal growth. Nature. 2006;442:471–474. doi: 10.1038/nature04895. [DOI] [PubMed] [Google Scholar]
- 90.Williams AH, Valdez G, Moresi V, Qi X, McAnally J, Elliott JL, Bassel-Duby R, Sanes JR, Olson EN. MicroRNA-206 delays ALS progression and promotes regeneration of neuromuscular synapses in mice. Science. 2009;326:1549–1554. doi: 10.1126/science.1181046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ma TC, Campana A, Lange PS, Lee HH, Banerjee K, Bryson JB, Mahishi L, Alam S, Giger RJ, Barnes S, et al. A large-scale chemical screen for regulators of the arginase 1 promoter identifies the soy isoflavone daidzeinas a clinically approved small molecule that can promote neuronal protection or regeneration via a cAMP-independent pathway. J Neurosci. 2010;30:739–748. doi: 10.1523/JNEUROSCI.5266-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Deng K, He H, Qiu J, Lorber B, Bryson JB, Filbin MT. Increased synthesis of spermidine as a result of upregulation of arginase I promotes axonal regeneration in culture and in vivo. J Neurosci. 2009;29:9545–9552. doi: 10.1523/JNEUROSCI.1175-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alto LT, Havton LA, Conner JM, Hollis Ii ER, Blesch A, Tuszynski MH. Chemotropic guidance facilitates axonal regeneration and synapse formation after spinal cord injury. Nat Neurosci. 2009;12:1106–1113. doi: 10.1038/nn.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci. 2009;12:1333–1342. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kadoya K, Tsukada S, Lu P, Coppola G, Geschwind D, Filbin MT, Blesch A, Tuszynski MH. Combined intrinsic and extrinsic neuronal mechanisms facilitate bridging axonal regeneration one year after spinal cord injury. Neuron. 2009;64:165–172. doi: 10.1016/j.neuron.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Garcia-Alias G, Barkhuysen S, Buckle M, Fawcett JW. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat Neurosci. 2009;12:1145–1151. doi: 10.1038/nn.2377. [DOI] [PubMed] [Google Scholar]