Abstract
Place conditioning is widely used to study the conditioned rewarding effects of drugs. In the standard version, one reward (cocaine) is compared to no reward (saline). A modified variant of this task, “reference-conditioning” procedure, compares two potentially rewarding stimuli (high versus low cocaine dose). There has been little research on the utility of this procedure. Experiment 1 used the standard protocol with saline administered before confinement to the reference compartment of a place-conditioning chamber. On alternating days, saline, 2.5, 5, 7.5, 10, or 20 mg/kg cocaine was administered before confinement to the opposite compartment. In Experiments 2 and 3, reference-compartment saline was replaced with 5 and 7.5 mg/kg cocaine, respectively. Relative to saline, 7.5–20 mg/kg cocaine had comparable conditioned rewarding effects (i.e., similar increase in time in paired compartment). When cocaine replaced saline, there was competition at doses lower than 7.5 mg/kg. Rats that received 7.5 versus 2.5 mg/kg spent similar time in each compartment, indicating competition. Competition was not seen with 5 versus 20 mg/kg; preference was for the 20 mg/kg compartment. Experiment 4 showed that the competition at 2.5 mg/kg was not due to reward sensitization—. The reference-conditioning procedure has increased sensitivity for measuring associatively-motivated choice behavior.
Keywords: conditioned place preference, Pavlovian drug conditioning, learning, stimulant abuse, rat
Introduction
Drug addicts report that exposure to drug paraphernalia, videos of purchasing drugs, and imagery of drug use evoke feelings of drug craving (Ehrman et al., 1992; Kilts et al., 2001; Robbins et al., 1997; Sell et al., 2000). These stimuli likely acquire this effect through Pavlovian conditioning processes (Bevins and Palmatier 2004; Di Chiara 1999; Koob and LeMoal 2001; Robinson and Berridge 2001). The place conditioning procedure is commonly used to study these Pavlovian conditioned associations in rodents (Bardo and Bevins 2000; Bevins and Cunningham 2006; Tzschentke 1998, 2007). In this procedure, the drug of interest is administered before exposure to one environment but not a second distinct environment. In a subsequent test, the animal is given a choice between the two environments without the drug. The paired environment (CS or conditional stimulus) presumably acquires conditioned appetitive value by virtue of being repeatedly experienced with the rewarding effect of the drug US (unconditioned stimulus). Such acquired appetitive properties will evoke approach and other appetitive behaviors (Bardo and Bevins 2000; Bolles 1975; Panksepp et al., 2004), producing an increase in time spent in the paired environment relative to a control value on the choice test.
Although the place conditioning task is widely used to study the processes mediating the conditioned rewarding effects of a drug, it is rarely used to study the impact of learning history on choice behavior (cf. Bardo and Bevins 2000). In the typical procedure just described, the test day reflects a choice between a place where reward had occurred (drug-paired environment) versus a place where no explicit reward had occurred (unpaired ‘saline’ environment). Setting up such a choice tends to lead to an “all-or-none” preference for the paired environment. That is, once the dose of drug has crossed some threshold for reward, higher doses do not typically generate greater preferences for the paired environment (Bardo et al., 1995; Groblewski et al., 2008; Mueller and Stewart 2000; O’Dell et al., 1996). In a demonstration of this all-or-none effect, Bevins (2005) gave rats repeated pairings of i.v. cocaine (0.1, 0.3, 0.45, 0.6, 0.9, or 1.2 mg/kg) with one distinct environment; the other environment remained unpaired (i.e., no drug). The 0.45 mg/kg dose was the lowest dose of i.v. cocaine that conditioned a preference. Notably, as the cocaine dose increased, the preference for the paired compartment remained stable. This insensitivity to drug dose has been discussed in numerous reviews as a key limitation of the place conditioning task (e.g., Carr et al., 1989; Bardo and Bevins 2000).
This limitation has been overcome in a modified variant of the place conditioning task referred to as a “reference-conditioning” procedure. Rather than setting up a choice between reward and no reward, a reference-conditioning procedure compares a known rewarding stimulus (e.g., cocaine) to some other value of the same stimulus (e.g., another cocaine dose) or to a different stimulus [e.g., novelty, pups, etc. (see Barr et al., 1985; Bevins 2005; Mattson et al., 2001; Reichel and Bevins 2008, 2010)]. This change appears to produce a method that has increased sensitivity for detecting conditioned reward over the standard place conditioning procedure (Barr et al., 1985; Bevins 2005; Groblewski et al., 2008).
As an example, recall the earlier description where place preference with i.v. cocaine was similar whether 0.45 or 1.2 mg/kg was used in the standard protocol (Bevins 2005). In this same study, the reference-conditioning procedure yielded a more graded dose-effect curve. That is, 0.45 mg/kg i.v. cocaine, the reference dose, was paired with one environment and the comparison dose of cocaine (vehicle, 0.6, or 1.2 mg/kg) was paired with the other environment. Vehicle did not compete with the conditioning rewarding effects of 0.45 mg/kg cocaine. However, 0.6 mg/kg competed with this reference dose such that rats equally preferred the two environments. Notably, rats preferred the 1.2 mg/kg paired environment over the 0.45 mg/kg environment, indicating that 1.2 mg/kg cocaine had more conditioned rewarding value than the reference 0.45 mg/kg dose. This latter finding, and hence the conclusions, was in contrast to the standard protocol, where the preference conditioned by 0.45 and 1.2 mg/kg were comparable.
Groblewski et al. (2008) noted two key limitations with previously published reference-conditioning research using drugs (i.e., Barr et al., 1985; Bevins 2005). First, neither study used a dose of drug below the reference dose. Second, these studies also used only a single reference dose. In the studies by Groblewski et al. (2008) designed to remediated these limitations, a dose of ethanol (0.5 g/kg) that did not condition a place preference in DBA/2J male mice, when compared to saline (standard protocol), was able to compete for conditioned approach when known rewarding doses of ethanol (i.e., 1.5 or 2 g/kg) were used as the comparison dose.
The goal of the present research was to extend previous research from our laboratory, using the reference-conditioning procedure with cocaine (Bevins 2005), in several important ways. First, we assessed i.p. rather than i.v. administration of cocaine. Such an extension is important. That is, most place conditioning research uses i.p. or s.c.) as the route of drug administration. In fact, one advantage of place conditioning over self-administration as an assessment of reward is that surgery is not required (Bardo and Bevins 2000; Carr et al., 1989). Thus, to encourage more wide-spread use of the reference-conditioning procedure, it should show enhanced sensitivity when cocaine is administered i.p.. Second, we included in the reference procedure at least one dose of cocaine below the reference dose, to determine whether sensitivity of the reference procedure extends to the lower end of the dose-effect curve (cf. Groblewski et al., 2008). Third, we used multiple reference doses in order to gain a more complete understanding of the conditioned rewarding effects of cocaine in the standard versus reference-conditioning protocol (cf. Groblewski et al., 2008).
Methods
Subjects
Two-hundred and nineteen adult male Sprague-Dawley rats (275–300 g) from Harlan (Indianapolis, IN) were housed separately in polycarbonate cages lined with wood shavings, in a temperature- and humidity-controlled colony. Rat chow and water were continuously available in the home cage. All sessions were conducted during the light portion of a 12:12 h light/dark cycle. Experimental protocols were approved by the University of Nebraska-Lincoln IACUC and followed the “Guide for the Care and Use of Laboratory Animals” (National Research Council, 1996).
Apparatus
Place conditioning was assessed in one of two chambers with Plexiglas ceiling, front and back walls; the side walls were aluminum. Each chamber had two distinct end compartments [42 × 16 × 20 cm (l×w×h)] separated by a smaller center placement area [11.5 × 15.5 × 18.5 cm (l×w×h)]. Interchangeable floors were used to create the distinct environments. One floor had approximately 340 holes (1.3-cm diameter) drilled into a 16-gauge aluminum sheet. The other floor was made of 1-cm stainless steel rods. Two rods were mounted side-by-side on an acrylic base with the following adjacent rod pair separated from the next pair by 1 cm
Experiment 1: Cocaine dose-effect with saline as reference (standard protocol)
Habituation
The day before the first conditioning session, rats were injected with saline immediately before placement in the center area and allowed to freely explore the apparatus for 10 min.
Conditioning
Rats were assigned to conditioning dose (0, 2.5, 5, 7.5, 10, or 20 mg/kg cocaine; n=8 for 0 and 2.5; n=11 for 5, 7.5, 10, and 20) in an unbiased fashion (cf. Bevins and Cunningham, 2006). Conditioning occurred across 8 consecutive days with one session per day. On odd days (1, 3, 5, 7) rats were injected with their assigned dose of cocaine or saline immediately before confinement to one end compartment for 30 min. On even days rats were injected i.p. with the opposite solution immediately before confinement in the other end compartment for 30 min. Placement was counterbalanced according to rods/holes, spatial orientation, and whether cocaine was administered on odd or even days. To the extent allowed by the sample size. To compare this standard place conditioning protocol with that of the following experiments in this report, common terms for the end compartments are used. Thus, for all experiments we will refer to the compartment in which a common history is received by all rats as the “reference compartment”. In Experiment 1, the reference compartment is the one in which saline was administered before placement. The opposite end compartment where the learning history is varied by group will be referred to as the “comparison compartment”.
Testing
Approximately 24 h after the last conditioning session, a 10-min drug-free choice test occurred. This test was identical to habituation.
Experiment 2: Cocaine dose-effect with 5 mg/kg cocaine as reference
Habituation and testing were identical to Experiment 1. Rats were randomly assigned to a dose of cocaine to be paired with the comparison compartment (0, 2.5, 5, 7.5, 10, or 20 mg/kg; n=10 per comparison dose). For the conditioning phase the procedural details (number of trials, duration of confinement, counterbalancing, etc.) were similar to Experiment 1 except that 5 mg/kg of cocaine rather than saline was paired with the reference compartment.
Experiment 3: Cocaine dose-effect with 7.5 mg/kg cocaine as reference
For Experiment 3, the dose of cocaine paired with the reference compartment was increased to 7.5 mg/kg. All other procedural details for habituation, conditioning (0, 2.5, 5, 7.5, 10, or 20 mg/kg cocaine; n=10 per comparison dose), and testing were the same as Experiment 2.
Experiment 4: Test of reward sensitization
One noteworthy difference between the standard procedure and the reference procedure is increased cocaine exposure in the reference procedure. This increased exposure might enhance the rewarding value of the lowest cocaine dose (i.e., sensitization). Experiment 4 was designed to examine this possibility. Habituation was identical to Experiment 1. For conditioning, rats were assigned to one of the following groups: 0/0, 2.5/0, 5/0, or 2.5/7.5 (n=9 for 5/0 and n=10 for all other groups). The first number of the group name denotes the dose of cocaine (mg/kg) administered before placement in the comparison compartment. The second number denotes the dose of cocaine administered in the home cage at least 4 h after conditioning in the reference compartment. Regardless of group assignment, rats were injected with saline before placement in the reference compartment. All remaining details were similar to the previous experiments.
Dependent Measures
All test sessions were videotaped for later observations. Time spent in each end compartment was the primary measure during habituation and test sessions. A rat was considered to be in an end compartment when forelegs, head, and shoulders were positioned inside the compartment. Observers naïve to experimental conditions conducted inter-observer reliability checks on this behavior. The Pearson-product moment correlation was high for the 64 common observations (r=0.98, p<0.001). Data were converted to a ratio with respect to the comparison compartment using the following formula: (time in comparison compartment)÷(time in comparison compartment + time in reference compartment). By using time in the comparison compartment as the numerator one can readily determine whether the comparison dose of cocaine was more, less, or similar in its rewarding effects to the reference dose. For example, if the comparison dose is more rewarding than the reference dose, then rats will spend more time in the comparison compartment, resulting in a ratio value above 0.5. A ratio of 0.5 indicates that the conditioned rewarding effects of the comparison dose were similar to that of the reference dose. Conversely, a value less than 0.5 indicates that the reference dose was more rewarding than the comparison dose of cocaine. For rats in the control group of Experiments 1 and 4 that received saline in both end compartments, we calculated a ratio by randomly assigning a “comparison compartment”, with the restriction that half of the rats had the holes and half had the rods.
We also evaluated horizontal activity in each end compartment. Horizontal activity was defined as the number of times the rat’s front paws crossed a center line that bisected the end compartment. Activity (number of line crosses÷seconds spent in compartment) was compared for both sides on the habituation and the test day. Inter-observer reliability on this behavior was high for the 64 common observations (r=0.86, p<0.001).
Drug
(−)-Cocaine hydrochloride, a gift from NIDA or purchased from Sigma (St Louis, MO), was dissolved in 0.9% saline and injected i.p. at 1 ml/kg. All doses are reported as salt.
Data Analyses
During habituation, time in seconds and the activity in each of the two end compartments (i.e., comparison between rods and holes) were analyzed by a two-way (Comparison dose × Floor type) mixed analysis of variance (ANOVA), to assess the unbiased construction of our apparatus. Ratios on the test day were analyzed by a one-way (Comparison dose) between-subjects ANOVA. An a priori analysis was conducted with one-sample t-tests to compare each ratio to a hypothetical value of 0.5 (i.e., the value indicating similar conditioned rewarding effects of the reference and comparison dose) on the test day. Activity on the test day was analyzed with two-way (Comparison dose × Side) mixed subjects ANOVA. Follow-up comparisons used Fisher’s Protected Least Significant Difference (LSD) tests. Statistical significance was declared at p<0.05 for all tests.
Results
Experiment 1: Cocaine dose-effect with saline as reference (standard protocol)
Table 1 shows time spent in each end compartment, as well as activity on the habituation day. Before conditioning, rats did not exhibit a bias toward one particular stimulus (holes vs. rods). More so, group assignment did not interact with compartment choice in habituation. These observations were supported by non-significant effects of Floor type, Comparison dose, and Comparison dose × Floor type interaction (Fs≤1.84, NS). Further, activity scores did not differ between Floor type or Comparison dose, nor did Comparison dose interact with Floor type (Fs≤1.3, NS).
Table 1.
Time spent (sec) and activity scores (line crosses/time spent) in each compartment on the habituation day.
| Time in Compartment | Activity Scores | |||
|---|---|---|---|---|
| Rod Flooring (Mean ± S.E.M.) | Hole Flooring (Mean ± S.E.M.) | Rod Flooring (Mean ± S.E.M.) | Hole Flooring (Mean ± S.E.M.) | |
| Experiment 1 | ||||
| Cocaine (mg/kg) | ||||
| Saline | 221.9 ± 13.63 | 258.7 ± 9.84 | 0.148 ± 0.016 | 0.134 ± 0.012 |
| 2.5 | 232.4 ± 10.75 | 267.2 ± 6.55 | 0.120 ± 0.009 | 0.137 ± 0.015 |
| 5 | 248.9 ± 8.69 | 254.4 ± 10.02 | 0.127 ± 0.007 | 0.127 ± 0.009 |
| 7.5 | 254.3 ± 8.44 | 252.9 ± 6.30 | 0.139 ± 0.007 | 0.134 ± 0.008 |
| 10 | 253.3 ± 10.91 | 249.1 ± 7.19 | 0.136 ± 0.009 | 0.116 ± 0.007 |
| 20 | 240.6 ± 8.75 | 240.6 ± 10.13 | 0.125 ± 0.007 | 0.133 ± 0.010 |
| Experiment 2 | ||||
| Cocaine (mg/kg) | ||||
| Saline | 269.5 ± 7.33 | 244.6 ± 6.12 | 0.094 ± 0.012 | 0.099 ± 0.010 |
| 2.5 | 261.1 ± 6.06 | 241.5 ± 8.30 | 0.090 ± 0.011 | 0.094 ± 0.012 |
| 5 | 263.1 ± 6.73 | 245.8 ± 10.50 | 0.089 ± 0.010 | 0.093 ± 0.012 |
| 7.5 | 250.2 ± 8.49 | 255.6 ± 8.13 | 0.093 ± 0.010 | 0.091 ± 0.010 |
| 10 | 274.7 ± 7.72 | 237.0 ± 7.99 | 0.087 ± 0.009 | 0.090 ± 0.008 |
| 20 | 248.2 ± 28.29 | 279.2 ± 36.65 | 0.077 ± 0.012 | 0.085 ± 0.011 |
| Experiment 3 | ||||
| Cocaine (mg/kg) | ||||
| Saline | 240.3 ± 29.4 | 285.0 ± 36.4 | 0.108 ± 0.015 | 0.108 ± 0.014 |
| 2.5 | 252.2 ± 4.15 | 249.5 ± 4.3 | 0.119 ± 0.006 | 0.119 ± 0.090 |
| 5 | 264.0 ± 6.76 | 244.5 ± 6.93 | 0.108 ± 0.008 | 0.124 ± 0.014 |
| 7.5 | 254.8 ± 7.8 | 250.8 ± 8.9 | 0.118 ± 0.006 | 0.123 ± 0.011 |
| 10 | 244.2 ± 11.6 | 271.0 ± 8.4 | 0.116 ± 0.009 | 0.097 ± 0.005 |
| 20 | 245.1 ± 9.8 | 262.1 ± 13.5 | 0.134 ± 0.008 | 0.118 ± 0.007 |
| Experiment 4 | ||||
| Cocaine (mg/kg) | ||||
| Saline | 243.44 ± 13.14 | 225.44 ± 14.08 | 0.129 ± 0.014 | 0.130 ± 0.012 |
| 2.5/0 | 255.89 ± 13.14 | 216.28 ± 14.07 | 0.129 ± 0.014 | 0.135 ± 0.012 |
| 5/0 | 238.50 ± 13.85 | 228.92 ± 14.83 | 0.133 ± 0.014 | 0.131 ± 0.013 |
| 2.5/7.5 | 260.20 ± 13.12 | 210.77 ± 14.07 | 0.137 ± 0.014 | 0.153 ± 0.012 |
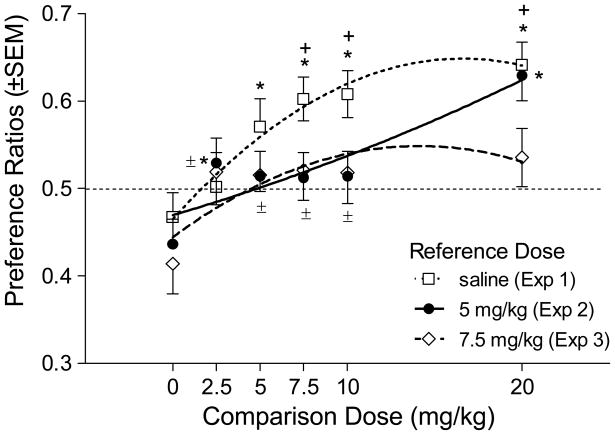
Figure 1 (open squares, dotted line) shows the test-day preference ratios for the groups in Experiment 1. When compared to a hypothetical value of 0.5, rats conditioned with saline in both compartments or 2.5 mg/kg cocaine did not differ in compartment choice [ts≤1.18, NS]. The 5 mg/kg cocaine dose was on the cusp of having consistent conditioning rewarding effects [t(10)=2.23, p=.0502]. The remaining higher comparison doses (7.5, 10, and 20 mg/kg) were significantly greater than the hypothetical mean of 0.5, indicating that the conditioned rewarding effects of these doses competed against the saline reference [ts(10)≥3.98, ps≤0.001]. The ANOVA revealed a significant effect of Comparison dose [F(5,54)=4.75, p<0.001]. Specifically, groups conditioned with 5 through 20 mg/kg cocaine as the comparison dose had higher preference ratios than the saline control (Fisher’s LSD=0.08). Further, rats conditioned with 7.5, 10, and 20 mg/kg cocaine had higher preference ratios than the 2.5 mg/kg group. Table 2 shows the mean time spent in each end compartment on the test day.
Figure 1.
Mean preference ratios (time spent in cocaine-paired compartment/time in both end compartments) on the conditioning tests for Experiments 1 to 3. Symbols represent the mean ratio value at a particular comparison dose of cocaine. The line represents the second-order polynomial of best fit of the values for each experiment. * Significant difference from saline (p<0.05); + Significant difference from 2.5 mg/kg cocaine (p<0.05); ± Significant difference from 20 mg/kg cocaine (p<0.05).
Table 2.
Time spent (sec) in the Comparison and Reference compartments on the test day.
| Comparison side (Mean ± S.E.M.) | Reference side (Mean ± S.E.M.) | |
|---|---|---|
| Experiment 1 | ||
| Cocaine (mg/kg) | ||
| Saline | 208.6 ± 14.0 | 236.7 ± 13.3 |
| 2.5 | 222.5 ± 21.7 | 215.6 ± 13.9 |
| 5 | 250.8 ± 13.3 | 192.3 ± 17.6 |
| 7.5 | 262.7 ± 11.6 | 174.4 ± 13.1 |
| 10 | 281.7 ± 15.9 | 180.2 ± 11.7 |
| 20 | 299.6 ± 15.3 | 165.9 ± 11.5 |
| Experiment 2 | ||
| Cocaine (mg/kg) | ||
| Saline | 182.0 ± 13.5 | 234.9 ± 13.5 |
| 2.5 | 222.3 ± 16.2 | 195.2 ± 11.2 |
| 5 | 223.7 ± 11.6 | 212.7 ± 13.9 |
| 7.5 | 218.2 ± 6.6 | 207.9 ± 7.2 |
| 10 | 219.3 ± 17.4 | 204.9 ± 13.5 |
| 20 | 285.2 ± 15.5 | 168.1 ± 13.4 |
| Experiment 3 | ||
| Cocaine (mg/kg) | ||
| Saline | 172.4 ± 15.6 | 243.3 ± 14.9 |
| 2.5 | 219.6 ± 16.4 | 203.8 ± 16.2 |
| 5 | 228.9 ± 11.2 | 214.2 ± 7.8 |
| 7.5 | 219.4 ± 14.0 | 203.9 ± 17.1 |
| 10 | 210.7 ± 14.7 | 196.9 ± 15.7 |
| 20 | 231.5 ± 18.4 | 199.1 ± 14.7 |
| Experiment 4 | ||
| Cocaine (mg/kg) | ||
| Saline | 205.6 ± 14.3 | 211.6 ± 14.8 |
| 2.5/0 | 237.0 ± 21.6 | 204.1 ± 19.5 |
| 5/0 | 237.6 ± 17.0 | 191.7 ± 12.4 |
| 2.5/7.5 | 222.8 ± 13.9 | 202.6 ± 7.1 |
As displayed in Table 3, activity on the test day was generally higher in the reference (i.e., saline) compartment [main effect of Side, F(1,54)=12.26, p<0.001]. This effect was more apparent for rats conditioned with the higher doses of cocaine [Comparison dose × Side interaction, F(5,54)=2.48, p<0.05]. More specifically, rats conditioned with 7.5, 10, and 20 mg/kg cocaine were significantly more active in the reference than the comparison compartment (Fisher’s LSD=0.063).
Table 3.
Activity scores (line crosses/time spent) in the Comparison and Reference compartments on the test of reference conditioning.
| Comparison side (Mean ± S.E.M.) | Reference side (Mean ± S.E.M.) | |
|---|---|---|
| Experiment 1 | ||
| Cocaine (mg/kg) | ||
| Saline | 0.201 ± 0.021 | 0.180 ± 0.024 |
| 2.5 | 0.193 ± 0.023 | 0.175 ± 0.018 |
| 5 | 0.148 ± 0.016 | 0.202 ± 0.025 |
| 7.5* | 0.140 ± 0.010 | 0.211 ± 0.029 |
| 10* | 0.119 ± 0.012 | 0.208 ± 0.016 |
| 20* | 0.136 ± 0.013 | 0.241 ± 0.036 |
| Experiment 2 | ||
| Cocaine (mg/kg) | ||
| Saline | 0.136 ± 0.027 | 0.158 ± 0.019 |
| 2.5 | 0.152 ± 0.017 | 0.175 ± 0.026 |
| 5 | 0.182 ± 0.027 | 0.201 ± 0.021 |
| 7.5 | 0.171 ± 0.016 | 0.185 ± 0.014 |
| 10 | 0.160 ± 0.019 | 0.179 ± 0.021 |
| 20 | 0.201 ± 0.020 | 0.248 ± 0.017 |
| Experiment 3 | ||
| Cocaine (mg/kg) | ||
| Saline | 0.160 ± 0.019 | 0.173 ± 0.022 |
| 2.5 | 0.168 ± 0.022 | 0.208 ± 0.042 |
| 5 | 0.163 ± 0.017 | 0.156 ± 0.016 |
| 7.5 | 0.155 ± 0.018 | 0.171 ± 0.020 |
| 10 | 0.155 ± 0.017 | 0.165 ± 0.019 |
| 20 | 0.159 ± 0.018 | 0.202 ± 0.026 |
| Experiment 4 | ||
| Cocaine (mg/kg) | ||
| Saline | 0.168 ± 0.017 | 0.154 ± 0.013 |
| 2.5/0 | 0.143 ± 0.016 | 0.157 ± 0.009 |
| 5/0 | 0.140 ± 0.019 | 0.175 ± 0.020 |
| 2.5/7.5 | 0.179 ± 0.015 | 0.179 ± 0.015 |
Significantly different from time spent on the Reference side (p<0.05).
Experiment 2: Cocaine dose-effect with 5 mg/kg cocaine as reference
Rats did not exhibit a bias toward one particular stimulus (holes vs. rods) over the other before the conditioning phase (see Table 1), and group assignment did not interact with compartment choice on the habituation day [main effects of Floor type, Comparison dose, and Comparison dose × Floor type interaction (Fs≤1)]. Rats were more active on the hole side [main effect of Floor type, F(1,54)=4.01, p<0.05]. However, there was no significant effect of Comparison dose, or Comparison dose × Floor type interaction (Fs≤1).
The filled circles and solid line of Figure 1 shows preference ratios for the groups in Experiment 2 (see Table 2 for time spent in each end compartment). All preference ratios were compared to a hypothetical value of 0.5. As in Experiment 1, the 5 mg/kg reference dose was not able to condition a significant preference relative to saline [t(9)=2.12, p=0.064]. The 2.5 through 10 mg/kg doses of cocaine were comparable to the 5 mg/kg cocaine [ts<1]. In contrast, the preference ratio for the 20 mg/kg cocaine dose was significantly above the hypothetical 0.5 value [t(10)=4.4, p<0.002], indicating that the conditioned rewarding effects of 20 mg/kg cocaine were greater than those of the 5 mg/kg dose. The overall ANOVA on preference ratio revealed a significant effect of Comparison dose [F(5,54)=4.65, p<0.001], with rats conditioned with comparison doses of 2.5 and 20 mg/kg differing from saline (Fisher’s LSD=0.08). Further, rats conditioned with 2.5 through 10 mg/kg had lower preference ratios than the 20 mg/kg group.
As displayed in Table 3, activity in general was higher in the reference compartment [main effect of Side, F(1,54)=6.09, p<0.02]. Further, rats conditioned with 20 mg/kg cocaine had higher overall activity than the other groups [main effect of Comparison dose, F(5,54)=2.50, p<0.05, and Fisher’s LSD=0.049]. Comparison dose did not interact with Side (F<1).
Experiment 3: Cocaine dose-effect with 7.5 mg/kg cocaine as reference
Consistent with the first two experiments, rats did not exhibit a bias toward one particular stimulus (holes vs. rods) over the other (see Table 1). More so, Comparison dose did not interact with compartment choice on the habituation day (Fs≤1). Further, activity scores did not differ between Floor types or Comparison dose, nor did Comparison dose interact with Floor type (Fs≤1.55, NS).
The open diamond symbol and dashed line of Figure 1 show the data for rats conditioned with saline, 2.5, 5, 7.5, 10, or 20 mg/kg cocaine in comparison to 7.5 mg/kg cocaine (see Table 2 for time spent in each end compartment). Comparisons between preference ratios and a hypothetical value of 0.5 show that a reference dose of 7.5 mg/kg cocaine was more rewarding than saline [t(9)=2.50, p<0.05]. The comparison doses of 2.5 through 20 mg/kg cocaine were comparable to 7.5 mg/kg cocaine, as time was split relatively evenly between end compartments [ts≤1.06, NS]. On the test of place conditioning there was no significant main effect of Comparison dose [F=1.83, NS]. Activity scores (Table 3) did not differ [main effect of Side, Comparison dose, and Comparison dose × Side interaction, Fs≤1.99, NS].
Experiment 4: Test of reward sensitization
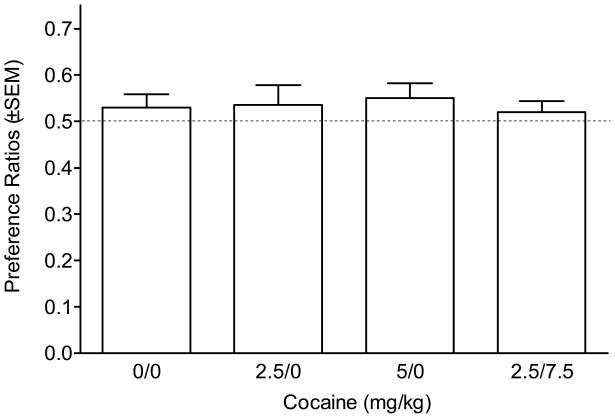
Table 1 shows time spent on the end compartments, as well as activity counts on the habituation day. Rats appeared to prefer the floor with the rods as there was a significant main effect of Floor type [F(1,35)=7.97, p<0.01]. However, this preference did not impact the groups differently, as Comparison dose did not interact with Floor type on habituation [main effects and Comparison dose × Floor type interaction: Fs≤1]. Further, activity scores did not differ between Floor types or Cocaine dose, nor did Cocaine dose interact with Floor type (Fs≤1.12, NS). Figure 2 shows the preference ratios on the post-conditioning test day; none of the groups differed from a hypothetical value of 0.5 [ts≤1.60, NS]. Further, the one-way ANOVA was not significant (F<1). Activity scores (Table 3) were similar across groups [main effect of Side and Comparison dose, and Comparison dose × Side interaction, Fs≤1.96, NS].
Figure 2.
Mean preference ratios (time spent in comparison compartment/time in both end compartments) for the follow up test of sensitization.
Discussion
The place conditioning task is widely used to study the neuropharmacological and neurobiological processes underlying the conditioned rewarding effects of abused drugs. Given the popularity of the task, along with the interest of addiction researchers in choice behavior, it is surprising that the place conditioning task has not been more widely used to measure choice among different rewards. This omission is especially notable given that the few systematic uses of the reference procedure to study associatively-motivated choice have shown the procedure to be a more sensitive index of the effects of drugs than the standard procedure. For instance, in the first use of the reference procedure, Barr et al., (1985) found a graded morphine-dose effect curve with 1 mg/kg morphine as the reference dose. Bevins (2005) demonstrated a similar graded dose-effect with i.v. cocaine. Groblewski et al., (2008) extended these observations to ethanol. The present research adds i.p.-administered cocaine to this list. This addition is important considering that place conditioning with cocaine is done much more frequently with i.p. than i.v. administration. A Medline search of “cocaine place conditioning” as a keyword returns 365 articles indexed between 1999 and 2009; of these, only 23 also include “intravenous” as a search term; only 9 of these 23 articles examined place conditioning with i.v. cocaine. Of course, there are drawbacks that may limit adoption of the reference-conditioning procedure. Namely, it require extensive preliminary research to identify the reference doses and the designs are between-subject requiring a large number of animals and experimenter time (see Bevins, 2005)
We measured activity (line crosses) in all of the experiments. The only notable effect was in Experiment 1. Using the standard training protocol we found that rats were less active in the paired than the unpaired compartment on the test day. This outcome is consistent with previous findings from our laboratory (Reichel and Bevins 2008, 2010) and others (e.g., Martin-Iverson and Reimer 1996; Parker, 1992). Thus, conditioned locomotor sensitization in the cocaine-paired compartment is not necessary for the expression of a cocaine-conditioned place preference. At present, however, it is unclear as to the nature of the conditioned response evoked by the paired environment that produces a decrease in line crosses (e.g., greater pause and contact with elements of the environment).
In Experiment 2, we chose the 5 mg/kg dose of cocaine as the reference dose, given that it appeared “marginally” rewarding in the standard protocol. That is, in Experiment 1 the 5 mg/kg cocaine group differed from the saline control with the post-hoc LSD, but not from the hypothetical mean of 0.5 (i.e., p=0.0502). As additional studies were conducted with this dose, we would now conclude that in the standard conditioning protocol, 5 mg/kg cocaine does not have reliable rewarding effects in our hands. This conclusion is consistent with other laboratories showing that male rats do not typically express compartment preferences with 5 mg/kg cocaine (e.g., Shippenberg and Heidbreder 1995; Russo et al., 2003). A total of 31 rats had 5 mg/kg cocaine paired in one compartment and saline in the other [Experiment 1: n=11; Experiment 2: n=11; Experiment 4: n=9]. Across these studies, 18 of the 31 rats spent more time in the 5 mg/kg cocaine-paired compartment (binominal distribution, p=0.096). Compare this outcome to the 7.5 mg/kg cocaine dose which conditioned a preference in 18 of 21 rats that had this dose in competition with saline [Experiment 1: n=11; Experiment 3: n=10; binomial distribution, p<0.002]. Regardless, the use of this dose was important for observing some of the increased sensitivity in the reference conditioning procedure. That is, although both the 5 and the 7.5 mg/kg dose revealed effects of 2.5 mg/kg cocaine not seen in the standard protocol, only the experiment with the 5 mg/kg dose displayed a pattern in which a higher dose (i.e., 20 mg/kg) out-competed the conditioned association engendered by the lower dose.
In the standard protocol, the group that received 2.5 mg/kg cocaine was statistically indistinguishable from the saline control. This outcome suggests a lack of conditioned reward at this dose. However, the reference-conditioning procedure revealed that 2.5 mg/kg of cocaine competed with 7.5 mg/kg for conditioned approach. One potential account of this observation is based on past research indicating that pre-exposure to drug before place conditioning can enhance (sensitize) its rewarding effect in a place conditioning task (e.g., Shippenberg and Heidbreder 1995). Take as an example the protocol from Experiment 3. Rats assigned to the 2.5 mg/kg comparison dose also receive four administrations of 7.5 mg/kg before the test. This may be the equivalent of cocaine pre-exposure, with the net effect being sensitization of the rewarding effects of the 2.5 mg/kg dose. We tested this sensitization account in Experiment 4. The key group (2.5/7.5) in that study received 2.5 mg/kg of cocaine paired with one end compartment (saline in the other), but received 7.5 mg/kg in the home cage in a temporal pattern similar to Experiment 3. We found no evidence that this procedure enhanced the conditioned rewarding effects of cocaine; the 2.5/7.5 group did not differ from a saline/saline control (group 0/0), or the group that received the standard conditioning protocol with 2.5 mg/kg. This conclusion is consistent with the studies by Groblewski et al. (2008) using the reference-conditioning procedure with ethanol in mice. In two of their three experiments total ethanol exposure was equated by providing the remaining ethanol in the home cage after each saline conditioning day.
The lack of evidence for sensitization of conditioned reward prompts an important question: how can 2.5 mg/kg compete for conditioned approach with 7.5 mg/kg cocaine (a dose that reliably conditions a preference) despite the lack of evidence for any rewarding effects with this low dose? If we follow the lead of Groblewski et al., (2008), then we would conclude that this low dose of cocaine had rewarding effects that were not revealed until it was placed in a choice situation with competing reward learning. With this conclusion, these authors provide a theory partly based on the Rescorla-Wagner model of conditioning (Rescorla and Wagner 1972) and includes a hypothesized “response floor”, “response ceiling”, and a “performance range” [for more detail see the Discussion and Figure 2 of Groblewski et al., (2008)].
We would like to suggest an alternative account to explain why 0.5 g/kg of ethanol in Groblewski et al. (2008), or 2.5 mg/kg cocaine in present research, does not condition a preference in the standard conditioning protocol, but competes with the rewarding effects of higher doses in the reference procedure. This alternative possibility suggests that 2.5 mg/kg cocaine has perceptible interoceptive stimulus effects and that these stimulus effects enter into an association with the environment (flooring CS). Further, the stimulus effects of 2.5 mg/kg overlap to some extent with those of higher doses (e.g., 7.5 and 10 mg/kg). This overlap in the stimulus properties evokes approach behavior to the 2.5 mg/kg compartment on the test day (i.e., generalization). The drug discrimination literature supports this stimulus generalization account. Rats trained to discriminate 10 mg/kg cocaine from saline show partial substitution of drug-appropriate responding to 3 mg/kg cocaine and have an ED50 of 2.49 mg/kg (Costanza et al., 2001). Thus, low doses of cocaine have perceptible stimulus effects that overlap with a higher and demonstrably rewarding dose of cocaine. Presumably, overlap in the stimulus effects that permits for generalization of schedule-maintained responding in an operant conditioning task would also allow for generalization of conditioned approach behavior in the place conditioning task.
From this line of reasoning, the results of the present research provide insight into the nature of the conditioned association in the place conditioning task. The environmental CS appears not only to be associated with the appetitive or rewarding effects of the drug, but also with other stimulus effects of the drug that are not necessarily reward related. For example, cocaine-induced activation of peripheral systems serves as an interoceptive cue that has formed an association with the environmental CS (cf. Wise et al., 2008). Experiment 2, using a reference dose of 5 mg/kg cocaine, provides the best exemplar of this point. In that experiment, 5 mg/kg cocaine competed with 2.5, 5, 7.5 and 10 mg/kg in a manner that produced an equivalent preference for each end compartment. The 2.5 and 5 mg/kg doses did not condition a significant preference in the standard protocol. We take this result to reflect the balanced design of our apparatus despite the acquisition of a flooring cue–cocaine stimulus effect association in each environment. However, the 7.5 and 10 mg/kg doses conditioned a preference in the standard protocol. Thus, we take the ability of 5 mg/kg to compete with these doses to indicate that the comparison flooring–cocaine reward+stimulus effects association generalized with the reference flooring–stimulus effects association.
Importantly, there are limits to this generalization. Rats that had a comparison dose of 20 mg/kg spent more time in that compartment than in the 5 mg/kg reference compartment. The generalization account suggests at least two potential explanations. One, the conditioned rewarding effects of 20 mg/kg dose are sufficiently high as to out-compete any generalized conditioned approach evoked by the overlap in stimulus effects in the reference compartment. Two, the stimulus effects of 20 mg/kg cocaine are sufficiently different from those of 5 mg/kg as to diminish generalization between compartments. Admittedly, these accounts may not be mutually exclusive as appetitive effects of cocaine may be one aspect of the cocaine stimulus. Regardless, the conditioned association in place conditioning includes more than just the affective properties of the drug US; there is clearly information regarding the stimulus feature of the US (cf. Corbit & Balleine 2005; Delamater & Holland 2008; Konorski 1967). If these richer associations that include stimulus features of the drug do develop in place conditioning, the import and their potential influence on the outcome and interpretation of place conditioning studies is not clear at this point. However, it will be necessary to understand their influences given the widespread use of the place conditioning task to study mechanisms underlying conditioned reward.
Acknowledgments
We thank Marcela Bravo, Drea Wilson, and Erin Zwart for their assistance scoring the video tapes. This research was supported by NIH grant DA017086. R.A. Bevins was supported, in part, by grant DA018114 while preparing this report. CM Reichel was supported by DA023283 while preparing this manuscript for publication.
References
- Anagnostaras S, Robinson T. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behav Neurosci. 1996;110:1397–1414. doi: 10.1037//0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- Bardo M, Bevins R. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bardo M, Rowlett J, Harris M. Conditioned place preference using opiate and stimulant drugs: A meta-analysis. Neurosci Biobehav Rev. 1995;19:39–51. doi: 10.1016/0149-7634(94)00021-r. [DOI] [PubMed] [Google Scholar]
- Barr G, Paredes W, Bridger W. Place conditioning with morphine and phencyclidine: Dose dependent effects. Life Sciences. 1985;36:363–68. doi: 10.1016/0024-3205(85)90122-5. [DOI] [PubMed] [Google Scholar]
- Bevins R. The reference-dose place conditioning procedure yields a graded dose-effect function. Int J Comp Psychol. 2005;18:101–11. [Google Scholar]
- Bevins R, Cunningham C. Place conditioning: A methodological analysis. In: Anderson M, editor. Tasks and Techniques: A Sampling of Methodologies for the Investigation of Animal Learning, Behavior, and Cognition. Nova Science Publisher; Hauppauge New York: 2006. pp. 99–110. [Google Scholar]
- Bevins R, Palmatier M. Extending the role of associative learning processes in nicotine addiction. Behav Cogn Neurosci Rev. 2004;3:143–58. doi: 10.1177/1534582304272005. [DOI] [PubMed] [Google Scholar]
- Bolles R. Theory of Motivation. 2. Harper & Row Publishers; New York NY: 1975. [Google Scholar]
- Carr G, Phillips A, Fibiger H. Independence of amphetamine reward from locomotor stimulation demonstrated by conditioned place preference. Psychopharmacology. 1988;94:221–6. doi: 10.1007/BF00176849. [DOI] [PubMed] [Google Scholar]
- Corbit L, Balleine B. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of Pavlovian-instrumental transfer. J Neurosci. 2005;25:962–70. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanza R, Barber D, Terry P. Antagonism of the discriminative stimulus effects of cocaine at two training doses by dopamine D2-like receptor antagonists. Psychopharmacology. 2001;158:146–53. doi: 10.1007/s002130100872. [DOI] [PubMed] [Google Scholar]
- Delamater A, Holland P. The influence of CS-US interval on several different indices of learning in appetitive conditioning. J Exp Psychol: Anim Behav Process. 2008;34:202–22. doi: 10.1037/0097-7403.34.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. Drug addiction as dopamine-dependent associative learning disorder. Eur J Pharmacol. 1999;375:13–30. doi: 10.1016/s0014-2999(99)00372-6. [DOI] [PubMed] [Google Scholar]
- Ehrman R, Robbins S, Childress A, O’Brien C. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology. 1992;107:523–9. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Groblewski P, Bax L, Cunningham C. Reference-dose place conditioning with ethanol in mice: Empirical and theoretical analysis. Psychopharmacology. 2008;201:97–106. doi: 10.1007/s00213-008-1251-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts C, Schweitzer J, Quinn C, Gross R, Faber T, Muhammad F, et al. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–41. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Koob G, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Konorski J. Integrative activity of the brain. Chicago: University of Chicago Press; 1967. [Google Scholar]
- Martin-Iverson MT, Reimer AR. Classically conditioned motor effects do not occur with cocaine in an unbiased conditioned place preferences procedure. Behav Pharmacol. 1996;7:303–14. doi: 10.1097/00008877-199608000-00001. [DOI] [PubMed] [Google Scholar]
- Mattson B, Williams S, Rosenblatt J, Morrell J. Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav Neurosci. 2001;115:683–94. doi: 10.1037//0735-7044.115.3.683. [DOI] [PubMed] [Google Scholar]
- Mueller D, Stewart J. Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behav Brain Res. 2000;115:39–47. doi: 10.1016/s0166-4328(00)00239-4. [DOI] [PubMed] [Google Scholar]
- O’Dell L, Khroyan T, Neisewander J. Dose-dependent characterization of the rewarding and stimulant properties of cocaine following intraperitoneal and intravenous administration in rats. Psychopharmacology. 1996;123:144–53. doi: 10.1007/BF02246171. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Nocjar C, Burgdorf J, Panksepp J, Huber R. The role of emotional systems in addiction: a neuroethological perspective. In: Bevins RA, Bardo MT, editors. Motivational Factors in the Etiology of Drug Abuse: Volume 50 of the Nebraska Symposium on Motivation. Nebraska Press; Lincoln NE: 2004. pp. 85–126. [PubMed] [Google Scholar]
- Parker LA. Place conditioning in a three- or four-choice apparatus: role of stimulus novelty in drug-induced place conditioning. Behav Neurosci. 1992;106:294–306. doi: 10.1037//0735-7044.106.2.294. [DOI] [PubMed] [Google Scholar]
- Reichel C, Bevins R. Competition between the conditioned rewarding effects of cocaine and novelty. Behav Neurosci. 2008;122:140–50. doi: 10.1037/0735-7044.122.1.140. [DOI] [PubMed] [Google Scholar]
- Reichel C, Bevins R. Competition between novelty and cocaine conditioned reward is sensitive to drug dose and retention interval. Behavioral Neurosci. 2010;124:141–51. doi: 10.1037/a0018226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla R, Wagner A. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasky WF, editors. Classical Conditioning II. Appleton-Century-Crofts; New York: 1972. pp. 64–99. [Google Scholar]
- Robbins S, Ehrman R, Childress A, O’Brien C. Relationships among physiological and self-report responses produced by cocaine-related cues. Addictive Behaviors. 1997;22:157–67. doi: 10.1016/s0306-4603(96)00007-x. [DOI] [PubMed] [Google Scholar]
- Robinson T, Berridge K. Incentive-sensitization and addiction. Addiction. 2001;96:103–14. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Russo S, Jenab S, Fabian S, Festa E, Kemen L, Quinones-Jenab V. Sex differences in the conditioned rewarding effects of cocaine. Brain Research. 2003;970:214–20. doi: 10.1016/s0006-8993(03)02346-1. [DOI] [PubMed] [Google Scholar]
- Sell L, Morris J, Bearn J, Frackowiak R, Friston K, Dolan R. Neural responses associated with cue evoked emotional states and heroin in opiate addicts. Drug Alcohol Depend. 2000;60:207–16. doi: 10.1016/s0376-8716(99)00158-1. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shippenberg T, Heidbreder C. Sensitization to the conditioned rewarding effects of cocaine: pharmacological and temporal characteristics. J Pharmacol Exp Ther. 1995;273:808–15. [PubMed] [Google Scholar]
- Thiel K, Okum A, Neisewander J. Social reward-conditioned place preference: a model revealing an interaction between cocaine and social context rewards in rats. Drug Alcohol Depend. 2008;96:202–12. doi: 10.1016/j.drugalcdep.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke T. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–72. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Tzschentke T. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Wise R, Wang B, You Z. Cocaine serves as a peripheral interoceptive conditioned stimulus for central glutamate and dopamine release. PLoS One. 2008;3(e2846):1–9. doi: 10.1371/journal.pone.0002846. [DOI] [PMC free article] [PubMed] [Google Scholar]