Summary
Adult height is a risk factor in numerous human cancers that involve aberrant receptor tyrosine kinase (RTK) signaling. However, its importance is debated due to conflicting epidemiological studies and the lack of useful in vivo models. In Xiphophorus fishes (Platyfishes/Swordtails), a functional RTK, Xiphophorus melanoma receptor kinase (Xmrk), serves as the dominant oncogene and has been maintained for several million years despite being deleterious and in an extremely unstable genomic region. Here we show that the Xmrk genotype is positively correlated with standard length in male and female wild caught Xiphophorus cortezi sampled throughout their phylogeographic distribution. Histopathology confirms the occurrence of malignant melanomas in both sexes; however, melanoma incidence was extremely male biased. Furthermore, males collected with malignant melanomas in the field were significantly larger than both Xmrk males collected without melanomas and wildtype (Xmrk deficient) males. These results not only provide a novel selective mechanism for the persistence of the germline Xmrk oncogene but also create an innovative avenue of melanoma research within the Xiphophorus fishes. Wildlife cancer in natural systems is a growing concern, therefore, future research investigating life history characteristics associated with certain phenotypes and genotypes that predispose an individual to cancer will be fundamental to increasing our understanding of the evolutionary biology of cancer in nature as well as in humans.
Keywords: Fish, Molecular Evolution, Cancer, Evolutionary Biology, Natural Selection, Sexual Selection
Introduction
The documentation of cancer in natural populations is limited as many wild animals live and die in anonymity. Identifying cancer and its prevalence in a population requires detailed knowledge of the organism under investigation (e.g. demography, distribution, migration, etc) and extensive monitoring and sampling which often precludes such studies. However, this relative dearth of cancer research in natural settings should not be mistaken for its lack of importance in determining an organism’s survival and in shaping ecological dynamics. A long term study of an isolated population of Beluga whales found cancer was the second leading cause of mortality, accounting for 18% of the observed mortalities (Martineau et al. 2002) and is comparable to observed cancer death rate for humans in the United States (22.9%; Centers for Disease Control and Prevention 2004). Significant observations of cancer have been reported in several species including a few endangered species such as the Tasmanian devil, western barred bandicoot and Attwater’s prairie chicken (McAloose & Newton 2009). As a result there is an immediate and growing concern for cancer in wildlife. Human encroachment and subsequent habitat degradation introduce and/or increase exposure of organisms to many known carcinogens (e.g. UV light, carcinogenic contaminants, xenohormones). Because many of these carcinogens are novel, exposed organisms have not evolved effective defense mechanisms against them thereby making wildlife particularly vulnerable to carcinogenesis.
Although many cancers occur from somatic mutation or environmental risk factors, some genes capable of producing tumors are transmitted through the germ line. Therefore, despite their deleterious nature, such oncogenes can be maintained over evolutionary time periods across many generations (Meierjohann & Schartl 2006). Central to our current understanding of the evolutionary biology of cancer is genetic pleiotropy and genomic conflict (Graham 1992; Greaves 2000; Leroi et al. 2003; Crespi & Summers 2006). For example, the X-linked androgen receptor (AR) gene binds testosterone (Ross et al. 1998), a hormone that not only increases reproductive fitness in early adulthood but also the risk of developing prostate cancer later in life (Summers & Crespi 2008). Seemingly, the potential for increased reproductive benefits in young males due to the effects of testosterone outweighs its deleterious effects later in life when reproductive fitness is decreased (Williams 1957). This highlights an important concept regarding the continued evolutionary maintenance of oncogenes. The relative costs associated with cancer depend on several factors including when the disease manifests itself, rate of progression, and the affected area (Graham 1992).
In the late 1920s it was realized that certain Xiphophorus fishes carry a dominant oncogene (Xiphophorus melanoma receptor kinase, Xmrk) that could result in the formation malignant melanomas after hybridization (Gordon 1927; Kosswig 1928). Xmrk is not only sufficient to induce melanomas within Xiphophorus, but melanomas do not occur if this gene is disrupted (Schartl et al. 1999). In hybrid and non-hybrid melanomagenesis, the latency to tumor formation and the degree of malignancy is quite variable and depends on the individual species or species used in generating hybrid species (Kallman 1971; Borowsky 1973; Weis & Schartl 1998; Fernandez & Bowser 2008). Xiphophorus cortezi, one of the species known to form melanomas of non-hybrid origin in the laboratory, is particularly interesting because melanomas form within the first year of life (Kallman 1971; Schartl et al. 1995). Moreover, males are more susceptible to melanomas than females and malignancies appear most frequently in sexually active males of ‘high social rank’ (Schartl et al. 1995). Because X. cortezi forms melanomas during the prime reproductive period, when costs associated with malignancies are increased, this is a model species for investigating the possible benefits the Xmrk oncogene confers to the individual carrier.
Very little is known about melanomagenesis in natural populations of Xiphophorus. Thus, the primary goals of this study were to sample several populations of X. cortezi to determine the frequency of Xmrk oncogene and its associated melanin pattern and to document the occurrence of melanomas in these wild populations. X. cortezi is polymorphic for Xmrk and the associated Macromelanophore (M) pattern Spotted caudal (Sc; Kallman 1971; Schartl et al. 1995), which is an extremely asymmetrical melanin based pattern in the caudal fin. Non-malignant expression of Sc consists of one or more irregular elongations that commence at the base of the caudal fin and extend roughly one-third of the length of the caudal fin (Kallman, 1971). Pedigree crossing experiments suggest that Sc is under autosomal determination (Kallman 1971), although it has not been characterized at the genomic level. Xmrk is an essential component for the phenotypic expression of the Sc M pattern (Schartl et al. 1995; Fernandez 2010). Xmrk can be located on the X and/or Y chromosomes (Froschauer et al. 2002) and underlies the melanomas that originate from Sc M pattern in the laboratory (Kallman 1971; Schartl et al. 1995). However, because the Sc phenotype has incomplete penetrance (Kallman 1971), individuals that lack phenotypic expression of Sc can have Xmrk (Fernandez 2010). Recently, positive selection for the Sc pattern and Xmrk genotype was demonstrated using behavioral studies (Fernandez and Morris 2008; Fernandez 2010). However, no studies have addressed any physical characteristics that might be correlated with this potent oncogene that could mitigate the risk of developing cancer. Because height is a risk factor in numerous human cancers (reviewed by Gunnell et al. 2001), we were also interested in determining if there was a relationship between body size and the Xmrk genotype within individuals.
Materials and methods
Fish localities
Samples were collected from four natural populations that represent all three drainages within the natural distribution of X. cortezi (Rauchenberger et al. 1990): Arroyo Tanute (Tampaón drainage) 21°39′123″N, 99°02′127″W; Arroyo Conchita (Moctezuma drainage) 21°33′500″N, 98°59′320″W; Arroyo Chalpuhuacanita (Tempoal drainage) 21°12′364″N, 98°40′153″W; and Río San Martín (Tempoal drainage) 21°22′173″N, 98°39′543″W (Hidalgo and San Luis Potosí provinces; Mexico). Conchita and Tanute populations were sampled in December 2005 whereas Chalpuhuacanita and San Martín were sampled in April 2006. The following Xiphophorus species were sympatric with X. cortezi at the four collection sites: X. variatus (Arroyo Tanute, Arroyo Chalpuhuacanita, Río and San Martín), X. multilineatus (Arroyo Tanute), X. birchmanni (Arroyo Chalpuhuacanita and Río San Martín). X. cortezi was the only Xiphophorus species observed at Arroyo Conchita, therefore, the observed melanomas in this population were not the result of hybridization. Collection sites were selected to maximize sampling within the known distribution of X. cortezi (Rauchenberger et al. 1990) and were based upon the phylogenetic reconstruction of X. cortezi haplotypes (Gutierrez-Rodriguez et al. 2007). Adult fish (males and females) were randomly sampled from each of these four field sites using three different collection techniques: electroshock, seine, and bait traps. Upon capture, fish were anesthetized with tricane methanesulphonate (MS-222) in order to accurately measure standard length (SL; defined as the distance from the tip of the snout to the base of the caudal peduncle) and digitally photograph each individual. Before releasing fish, a small piece of the caudal fin was removed and preserved in 95% ethanol for subsequent DNA extraction and genotyping. After the collection trips, digital photographs of individual fish were scored for the presence/absence of the Sc phenotype and this information was used to estimate the frequencies of the Sc phenotype within populations (Table 1).
Table 1.
Frequencies of Sc and Xmrk in surveyed populations.
| Population | Sex | N | Sc % | Xmrk % | Penetrance of Sc % |
|---|---|---|---|---|---|
| Tanute | Male | 51 | 33.3 (17) | 60.8 (31) | 54.8 |
| Female | 41 | 2.4 (1) | 14.6 (6) | 16.7 | |
| Total | 92 | 17.2 (16) | 40.2 (37) | 43.2 | |
| Conchita | Male | 44 | 43.2 (19) | 81.8 (36) | 52.8 |
| Female | 28 | 32.1 (9) | 57.1 (16) | 56.3 | |
| Total | 72 | 38.9 (28) | 72.2 (52) | 53.8 | |
| Chalpu. | Male | 45 | 33.3 (15) | 37.8 (17) | 88.2 |
| Female | 45 | 26.7 (12) | 35.6 (16) | 75.0 | |
| Total | 90 | 29.7 (27) | 36.7 (33) | 81.8 | |
| San Martín | Male | 62 | 1.6 (1) | 4.8 (3) | 33.3 |
| Female | 53 | 1.9 (1) | 3.8 (2) | 50.0 | |
| Total | 115 | 1.7 (2) | 4.3 (5) | 40.0 | |
Parentheses indicate the actual number of individuals. The penetrance of Sc phenotype was calculated as the number of Sc individuals divided by the number of Xmrk individuals in that particular row.
DNA isolation, PCR, and Xmrk genotyping
DNA was extracted from the preserved fin clips collected in the field using DNeasy® tissue kit (Qiagen Inc., Valencia, CA, USA) following the manufacturer’s instructions. Total elution volume was 100 μl. The presence of Xmrk was determined by cross-referencing the polymerase chain reaction (PCR) products of two newly developed primers sets. These primers were designed from published Xiphophorus montezumae sequences in GenBank (Accession #s AY298857, AY298858). The published sequences in Genbank are derived from Xmrk specific clones (Volff et al. 2003), however there are regions of these sequences that are shared by both the Xmrk oncogene and the protooncogene (egfr-b). The following primer set was used to screen for the presence of the Xmrk genotype: “Montoncoup” sense primer 5’-GGGTCATAAATCACTCATCCATC - 3’ located in the promoter region at nt 21-43 (nt numbering according to AY298858; Volff et al. 2003) and “Dwnmont2” antisense primer 5’- ACAAGTTTGTGGAAATAAACCTGAACTC - 3’ located in Intron 1 at nt 688-715 (nt numbering according to AY298858; Volff et al. 2003). Because the Montoncoup primer corresponds to a region that is specific to the Xmrk oncogene, this primer set amplifies a single ~ 700 bp fragment if the individual male has the Xmrk oncogene (Xmrk deficient = no band). For the amplification of oncogene and protooncogene products, the following primers were developed: “Montoncoup5” sense primer 5’- GATGTTACTTTAGTTCTGGAGTC - 3’ located at nt 2956-2978 (nt numbering according to AY298857; Volff et al. 2003) and “Montoncodwn1” the antisense primer 5’- TCAGTTTGTTGGATCAGAGATG - 3’ located at nt 266-287 (nt numbering according to AY298858; Volff et al. 2003). The Montoncoup5 primer corresponds to a sequence found in both the oncogene and protooncogene, therefore the second primer set (Montoncoup5/Montoncodwn1) produces bands for both the protooncogene and the oncogene. The use of this second primer set enabled (1) the validation of the findings of the first PCR screening (i.e. Montoncoup/Dwnmont2) and (2) verification of the presence of amplifiable DNA because the protooncogene is ubiquitous in Xiphophorus. The final concentration of the primers was 100 nM.
The total reaction volume of all PCR amplifications was 10 μl. 1 μl of DNA template was used per reaction. PCR amplification was done under different conditions for each primer set used. For the Montoncoup/Dwnmont2 primer set, initial denaturation was at 94 °C for 3 min, then 29 cycles of denaturation at 95 °C for 30 s, annealing at 59 °C for 30 s, and extension at 72 °C for 45 s, followed by a final extension at 72 °C for 5 min. For the Montoncoup5/Montoncodwn1 primer set, initial denaturation was at 94 °C for 3 min, then 29 cycles of denaturation at 95 °C for 30 s, annealing at 62 °C for 30 s, and extension at 72 °C for 75 s, followed by a final extension at 72 °C for 5 min. 5 μl aliquots of the amplification products were fractionated by electrophoresis on an 1.0% agarose gel in 1X TAE (48 mM Tris-acetate, 1 mM EDTA) buffer and visualized after staining with ethidium bromide (0.5μg/ml TAE) and UV transillumination. The molecular marker used was Promega 1 KB (Madison, WI). The gel image was taken with a Gel Logic100 system (Kodak, Rochester, NY, USA).
Histology
Nine Xiphophorus cortezi (8 males, 1 female) collected from the Arroyo Conchita appeared to have abnormal enhancement of the Sc phenotype and, therefore, were examined microscopically. Whole fish were fixed in 10 % neutral buffered formalin and decalcified with sodium EDTA. They were then trimmed in the transverse plane to produce sections representative of the head as well as 4 sections of the body. The trimmed transverse body sections were then embedded in paraffin, sectioned and stained with hematoxylin and eosin stains for histological evaluation (Luna 1968). All observed microscopic lesions were documented and melanomas were classified according to the system proposed by Gimenez-Conti et al. (2001). A primary criterion for differentiation of melanoma from normal melanization is that in the latter the melanocytes are limited to the dermis and basal layer of the skin. In melanoma the melanocytes invade through the basal layer of the skin and may destroy the muscle bundles to varying degrees.
Data Analysis
Because many Xiphophorus species are sexually dimorphic with respect to SL and the interpretation of any observed differences in SL would be sex dependent, males and females were analyzed separately. We used unpaired t-tests to compare the SL of fish with and without the Xmrk genotype (Table 2). However, because fish with the Xmrk genotype can either express the associated Sc phenotype or not (Fernandez 2010), we examined this subset (i.e. Xmrk individuals only) of the total dataset further. With Xmrk individuals, we used unpaired t-tests to ask if there was a difference in those individuals with the Xmrk genotype and the Sc phenotype and those individuals with the Xmrk genotype who lacked phenotypic expression of Sc (Table 3). Males and females were again analyzed separately. The eight wild caught males with melanomas from the La Conchita population, who also possessed Sc and Xmrk, were not included in any of the above analyses. However, the SL of these males was compared against the SL of all Xmrk bearing males and all wild type (Xmrk deficient) males in a one-way ANOVA (Figure 2). All statistical assumptions of this analysis were met. All statistical analyses were conducted using SPSS 16.0.1.
Table 2.
Relationship between the Xmrk genotype and standard length (SL, mm) in males and females.
| Sex | Xmrk genotype | Mean SL ± SEM | N | t statistic, df | Probability |
|---|---|---|---|---|---|
| Male | |||||
| No Xmrk | 37.4 ± 0.38 | 115 | 3.59, 192 | P = 0.0004 | |
| Xmrk | 39.6 ± 0.49 | 79 | |||
| Female | |||||
| No Xmrk | 37.8 ± 0.38 | 127 | 3.61, 164 | P = 0.0004 | |
| Xmrk | 40.8 ± 0.81 | 39 | |||
Table 3.
Relationship between the Sc phenotype and standard length (SL, mm) in males and females with the Xmrk genotype.
| Sex | Xmrk/Sc trait | Mean SL ± SEM | N | t statistic, df | Probability |
|---|---|---|---|---|---|
| Male | |||||
| Xmrk/No Sc | 38.8 ± 0.60 | 35 | 1.619, 77 | P = 0.101 | |
| Xmrk/Sc | 40.3 ± 0.72 | 44 | |||
| Female | |||||
| Xmrk/No Sc | 38.9 ± 1.35 | 17 | 2.083, 37 | P = 0.044 | |
| Xmrk/Sc | 42.2 ± 0.89 | 22 | |||
Figure 2.
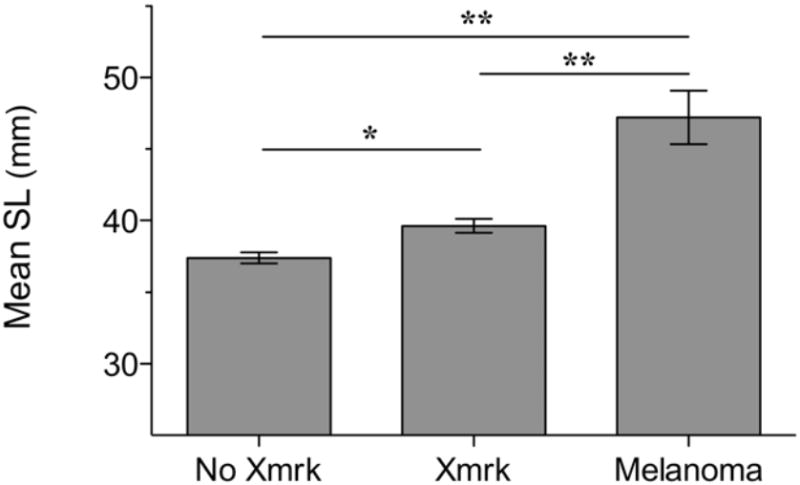
The standard lengths of adult males without the Xmrk genotype (N = 115), males bearing Xmrk (N = 79), and wild caught males bearing Xmrk with melanomas (N = 8) were significantly different from one another. Lines above columns provide significance levels according to Tukey and Bonferroni post hoc tests. * P = 0.001 and ** P < 0.0001.
Results
All surveyed populations of X. cortezi were found to be polymorphic for not only the Xmrk oncogene but also the Sc phenotype (Table 1) from which Xmrk induced melanomas arise. In both males and females, we found that individuals bearing the Xmrk oncogene, regardless of whether they expressed Sc, were significantly larger than wildtype individuals (Table 2). In many taxa, including teleost fishes, individuals with larger body size experience numerous competitive advantages (e.g. competitive ability, fecundity, female preference, predator avoidance). Within Xiphophorus, it has been demonstrated that females prefer larger males to smaller males (Ryan et al. 1990; Basolo 1998b; Zimmerman & Kallman 1989), and that larger males tend to be dominant to (Earley & Dugatkin 2006) and win fights against smaller males (Ribowski & Franck 1993). In addition to sexual selection, Xiphophorus with larger body sizes experience decreased predation risk in both natural (Basolo & Wagner 2004) and laboratory populations (Basolo 2008) compared to smaller individuals. Therefore, this result establishes selection for the Xmrk oncogene in the context of both sexual selection and natural selection. Sexually selected traits often burden their carriers in terms of natural selection (Zuk & Kolluru 1998), however, in Xiphophorus because both types of selection favor large body size this synergism likely contributes to the continued evolutionary maintenance of the deleterious Xmrk oncogene.
Because individuals with the Xmrk oncogene can either express the Sc phenotype or not, we wanted to distinguish the contributions of these two traits in the observed size correlation. We performed the same type of analysis on a subset of the total data set; that is, only those fish with the Xmrk genotype. Within Xmrk individuals, we found there was no correlation between male size and Sc expression; however, Xmrk/Sc females were larger than those females who had Xmrk but lacked Sc expression (Table 3). Therefore, at least in the case of males, the observed correlation between an individual’s size and Xmrk genotype can’t be attributed to any developmental factors (genetic or environmental) that underlie the expression of Sc pattern. Rather these data suggest that the Xmrk oncogene, or possibly genetically linked factors associated with Xmrk, is responsible for an individual’s larger size. Although female size was positively correlated with expression of the Sc pattern, this relationship was close to being non-significant (P = 0.044). Furthermore, we are cautious of this result because the sample size of females was much smaller than of males (Xmrk females = 39; Xmrk males = 79) due to the decreased frequency of Xmrk in females as compared to males (Table 1). With that said, it should be noted that Sc is a visual signal in X. cortezi and males respond with less aggression when observing an individual with Sc to that same individual without the Sc phenotype (Fernandez 2010). Thus, it is possible that Xmrk/Sc females might receive less aggression and/or harassment from males than Xmrk/no Sc females which might afford them more time for activities that would factor into their larger size (e.g. feeding).
Histopathology confirmed our suspicion that X. cortezi collected in natural populations possessed malignant melanomas. Melanomas were found in all nine fish examined (Table 4) and classified according to the established nomenclature for the Xiphophorus melanoma model (Gimenez-Conti et al. 2001). In this fish, the invasion of melanocytes into the underlying musculature was a consistent finding and considered the determining feature for the identification of melanoma. The melanomas observed in these specimens were not the result of interspecies hybridization as X. cortezi is the only Xiphophorus species at the Arroyo Conchita site. The most harmful lesions within this animal model, melanophorus-macromelanophorus polymorphic melanoma, were observed in the region of the caudal peduncle and originated from the Sc pigment pattern (Figure 1). In addition, melanosis was found in a variety of locations in all fish (Table 4), however, these cells did not appear to show any invasive behavior and typically consisted of a single layer in thickness with only occasional foci of several cells (20-30) being found. Although determining the age of these fish was not possible, all eight males and the single female appeared to be otherwise healthy and in breeding condition. Thus, it is likely that melanoma formation influences the reproductive fitness of the tumor-bearing fish as has been previously suggested (Fernandez & Morris 2008).
Table 4.
Summary of histopathology for wild caught X. cortezi individuals.
| Fish | SL | Benign Melanosis | Malignant Melanomas |
|---|---|---|---|
| Male 1 | 44.1 | MHP of the skin | MM in the skin and musculature of CP and CF; MMM in the skin and musculature of CP and CF |
| Male 2 | 55.1 | MHP of the skin; Melanosis circumscribing globe of both eyes |
MM in the skin and musculature of CP and CF; MMM in the skin and musculature of CP and CF |
| Male 3 | 43.8 | MHP of the skin | MM in the skin and musculature of CP and CF; MMM in the skin and musculature of CP and CF |
| Male 4 | 43.3 | MHP of the skin | MM in the musculature of CP |
| Male 5 | 49.8 | MHP of the skin; Melanosis circumscribing globe of both eyes |
MM in the musculature of CP |
| Male 6 | 51.7 | MHP of the skin | MM in the skin and musculature of CP and CF; MMM in the skin and musculature of CP and CF |
| Male 7 | 42.7 | MHP of the skin | MMM in the skin and musculature of CP and CF |
| Male 8 | 38.8 | MHP of the skin | MM in the skin and musculature of CP and CF; Foci of MM dorsal to caudal vein and artery in CP; MMM in the skin and musculature of CP and CF |
| Female 1 | 38.5 | MHP of the skin | MM in the musculature of CP |
MHP = Melanotic Hyperpigmentation; MM = Melanocytic melanoma; MMM = Melanophorus-Macromelanophorus Polymorphic Melanoma; CP = Caudal Peduncle; CF = Caudal fin
Figure 1.
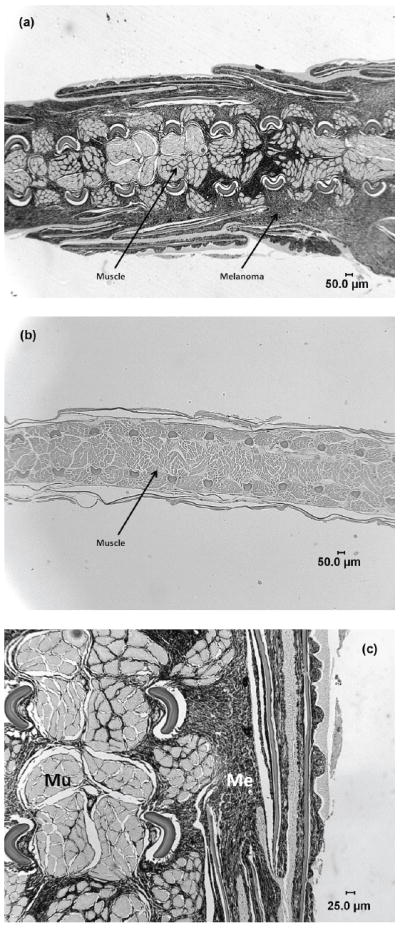
(a) Photomicrograph of Xiphophorus cortezi in the region of the caudal peduncle and caudal fin with melanophorus-macromelanophorus polymorphic melanoma. The melanocytes can be seen invading into the muscle. (b) Photomicrograph of Xiphophorus cortezi in the region of the caudal peduncle and caudal fin with no melanoma. (c) Higher magnification of Xiphophorus cortezi in the region of the caudal peduncle and caudal fin with melanophorus-macromelanophorus polymorphic melanoma. Invasion of melanocytes into the muscle are more clearly visible.
Lastly, we wanted to determine if melanomagenesis was correlated with an individual’s size in the wild caught tumor-bearing males. We found males with melanoma were significantly larger than Xmrk males without melanomas as well as wildtype males that lacked the Xmrk oncogene (SL ± SEM: melanoma males: 46.2 ± 1.92, Xmrk males: 39.6 ± 0.49, wildtype males: 37.4 ± 0.38; F2,199 = 19.4, P < 0.0001; Figure 2). Interestingly, this result supports the human melanoma literature in which studies have shown that taller men (Shors et al. 2001) and women (Olsen et al. 2008) have an elevated melanoma risk. Mammals (including humans) and Xiphophorus share many of the identified downstream signaling pathways underlying melanoma formation and progression (Meierjohann & Schartl 2006); therefore, the Xiphophorus melanoma model is an ideal system to elucidate the precise mechanisms underlying the correlation between size and melanoma susceptibility in humans.
Discussion
This study found that a potent oncogene is positively correlated with larger body size in X. cortezi males and females. Furthermore, this larger adult body size could not be attributed to the Xmrk associated Sc pattern that is involved in sexual selection. This suggests that, unlike previous explanations for the evolutionary maintenance of this deleterious gene (genetic hitch-hiking: Meierjohann & Schartl 2006; sexual selection: Fernandez & Morris 2008; Fernandez 2010), the preservation of the Xmrk oncogene within the germline of X. cortezi does not hinge entirely on its association with the Sc M pigment pattern. This study also demonstrated that non-hybrid melanomas do occur in natural populations and, therefore, are not a laboratory artifact as previously suggested (Kallman 1971). Melanomas were decisively male-biased which has also been documented in laboratory populations of X. cortezi (Kallman 1971; Schartl et al. 1995) and in platyfish-swordtail hybrids (Siciliano et al. 1971). Finally, our data revealed that these tumor-bearing males are significantly larger than either males with Xmrk and no melanomas or wildtype males without Xmrk. Because melanoma risk in humans is also correlated with height (Shors et al. 2001; Olsen et al. 2008), this finding opens up a novel avenue of melanoma research and places renewed importance on the well-studied Xiphophorus melanoma model.
Given that Xmrk confers advantages in male-male competition (Fernandez 2010), female mate choice (Fernandez & Morris 2008), and natural selection it remains to be determined why the Xmrk oncogene is not fixed in all populations of X. cortezi. In the four populations surveyed there was considerable variation in the frequency of the Xmrk oncogene and the Sc phenotype (Table 1). Furthermore, every Xiphophorus species that has retained a functional Xmrk is polymorphic at this locus (Schartl 2008). A likely explanation is that site-specific and species-specific factors are involved in maintaining a balanced polymorphism within this system. For example, a recent study on three divergent populations of X. cortezi found that female preferences for Sc depend on the frequency of Sc in males and females across populations (i.e. frequency dependent selection; Fernandez & Morris 2008). This variation in female preferences for Sc appears to result from the variable cost associated with choosing Xmrk males as mates due to homozygous Xmrk offspring being less viable than heterozygous Xmrk siblings because of genetic incompatibility (Kallman 1971; Schartl et al. 1998). Similarly, because Sc is a visual signal under sexual selection and Xmrk is positively correlated with aggression, the frequency of these traits could influence developmental aspects such as juvenile growth rates and age at sexual maturity, which in Xiphophorus are influenced by the degree of male ornamentation in the population (Borowsky 1978; Walling et al. 2007). In addition to population structuring, stochastic biotic (predation intensity) and abiotic (annual rainfall, UV exposure) ecological factors would also contribute to fluctuations in the costs associated with an individual bearing the Sc phenotype and Xmrk oncogene (Setlow et al. 1993; Franck et al. 2001). Future research that focuses on these more ecological and developmental aspects are necessary to fully determine why the Xmrk oncogene remains polymorphic in all species in which it is found.
We can think of several possible non-mutually exclusive mechanisms by which individual melanoma susceptibility is positively correlated with body size. First, the experimental supplementation of androgens in juvenile and adult Xiphophorus induces melanoma formation (Schartl et al. 1981; Schartl et al. 1982; Schartl et al. 1995). Juvenile aggression is common in Poeciliids (Gorlick 1976, A. A. Fernandez personal observation) and aggression is positively correlated with testosterone levels in Xiphophorus (Hannes 1986). The gonads of fry are capable of producing sex steroids (Schreibman et al. 1982). Thus, it is possible that during development the adult tumor bearing fish had relatively higher levels of circulating testosterone than their wildtype counterparts that induced melanoma formation but also allowed them greater access to food resources and ultimately their larger body size. Second, although X. cortezi does not have genetically determined size classes like some of its closely related taxa (P alleles; Kallman et al. 1973), it is possible for genetic factors on the X and/or Y chromosome that predispose large body size to become linked with the Xmrk oncogene. This would explain why Xmrk individuals are larger than wildtype individuals; however, an additional stimulus would be needed to initiate melanoma formation in these larger Xmrk individuals. Third, numerous copies of melanocortin receptors (Mcrs), which have various physiological functions including pigmentation, regulation of appetite and energy balance (Metz et al. 2006), are found in the immediate genomic vicinity of Xmrk (Froschauer et al. 2002) and are likely genetically linked. Furthermore, both Mc1r and Mc4r are overexpressed in Xiphophorus melanoma tissue (Selz et al. 2006) and, therefore, could be intimately involved in the observed correlation between body size and melanoma susceptibility. Lastly, because Xmrk is an oncogenic version of epidermal growth factor receptor (EGFR), a logical trigger for melanoma formation could be the binding of native and/or non-native growth factor ligands that could concomitantly increase body size. In fact, in mammalian cell lines it has been demonstrated that both growth hormone (GH) and insulin-like growth factor -1 (IGF-1) cross-react with and activate EGF receptors (Huang et al. 2003; Saxena et al. 2008). The GH/IGF-1 axis not only plays a major role increasing growth during development (Juul et al. 1995; Butler & Le Roith 2001) but also has a pivotal role in the growth and progression of a number of cancers (reviewed by Holly et al. 1999; Renehan et al. 2004). In fish, circulating levels of IGF-1 are positively correlated with SL and aggression (Mommsen 1998; Vera Cruz & Brown 2007). Because SL (this study) and aggression (Fernandez 2010) are positively correlated with the presence of Xmrk oncogene, we believe IGF-1 and/or GH are involved in the observed relationship between larger males and melanoma susceptibility.
An alternative hypothesis to the direct selection of the Xmrk oncogene is the theory of genetic hitchhiking (Maynard Smith & Haigh 1974). Under this model, the frequency of a neutral or slightly deleterious gene (Xmrk) can be maintained due to its close genomic proximity to a second locus that is under positive selection (Maynard Smith & Haigh 1974). This mechanism has been proposed to explain the evolutionary maintenance of Xmrk via positive selection for species-specific M pigment patterns (Meierjohann & Schartl 2006). Many M patterns are encoded by the so called Macromelanophore determining locus (Mdl) that is believed to be in close proximity to Xmrk on the sex chromsomes (Froschauer et al. 2002). However, despite extensive efforts Mdl has not been characterized at the molecular level (Meierjohann & Schartl 2006). In addition to the Mdl locus, Xmrk is surrounded by a number of genes that are potentially important in sexual selection (e.g. red-yellow locus that determines body, fin and eye color; Kallman 1975; Froschauer et al. 2002). Thus, it easy to see how this mechanism might be relevant to the preservation of the deleterious Xmrk. However, there are several reasons why we believe there is direct selection for Xmrk. First, the Ka/Ks ratio of 0.21 for Xmrk and its protooncogene paralog (egfr-b) indicates that Xmrk is under purifying selection (Volff & Schartl 2003). This is compelling not only because Xmrk has avoided non-functionalization despite being a ‘dispensable’ duplicated gene, but also because the genomic region where it resides is very plastic and contains many mobile DNA elements (helitron transposons, retrotransposons) that disrupt genes (Froschauer et al. 2001; 2002; Zhou et al. 2006). Second, after duplication from the egfr-b protooncogene several millions ago (Weis & Schartl 1998), Xmrk has aquired two activating mutations that can make it constitutively active and able to signal without ligand binding (Gómez et al. 2001). Third, the expression of Xmrk is not limited to Macromelanophores within M patterns. Detectable levels of expression have been consistently observed in the brain, eye, gill, and non-pigmented fins (Woolcock et al. 1994; A. A. Fernandez unpublished data). This begs the question: Why would a dominant oncogene that is argued to not be under selection be expressed in so many vital tissues? Finally, in the case of X. cortezi, the Sc M pattern is autosomally determined (Kallman 1971) and therefore not in close proximity to the sex-linked Xmrk oncogene which makes it maintenance under the genetic hitchhiking model not applicable (Fernandez & Morris 2008).
This study is the first to demonstrate selection for the Xmrk oncogene that is completely independent of the closely associated M patterns. More importantly, it increases our understanding of how genes that predispose an organism to cancer persist under natural conditions. There is a tremendous need for life history studies that focus on known oncogenes, which will begin to elucidate how such ‘maladaptive’ genes persist over evolutionary time periods. For example, within Xiphophorus, does increased fecundity due to larger female size compensate for the deleterious effects associated with her carrying the Xmrk genotype? Such insights into the evolutionary biology of cancer will undoubtedly help refine future scientific investigations into the proximate mechanisms that underlie the initiation and progression of this disease.
Acknowledgments
I am grateful to numerous people in the laboratory of Molly R. Morris in the Department of Biomedical Sciences at Ohio University for their assistant in collecting specimens and their continual support and to Lorie Fernandez for insightful comments on this manuscript. We also thank Jeanine Peters-Kennedy, Section of Anatomic Pathology, Department of Biomedical Sciences, College of Veterinary Medicine, Cornell University, for helpful comments on our interpretation of the hisopathology reported in this manuscript. This research was funded by a NIH Institutional Research Training Grant (CA009480), a NIH NRSA predoctoral fellowship to A. A. Fernandez (GM077096-01), a NSF grant to Molly R. Morris (IBN-0316687), and an Ohio University Student Enhancement Award to A. A. Fernandez.
References
- Basolo AL. Shift in investment between sexually selected traits: tarnishing of the silver spoon. Animal Behaviour. 1998;55:665–671. doi: 10.1006/anbe.1997.0634. [DOI] [PubMed] [Google Scholar]
- Basolo AL. Evolution of pleiotropic alleles for maturation and size as a consequence of predation. Biology Letters. 2008;23:200–203. doi: 10.1098/rsbl.2007.0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basolo AL, Wagner WE., Jr Covariation between predation risk, body size and fin elaboration in the green swordtail. Biological Journal of the Linnean Society of London. 2004;83:87–100. [Google Scholar]
- Borowsky R. Melanomas in Xiphophorus variatus (Pisces: Poeciliidae) in the absence of hybridization. Experientia. 1973;29:1431–1433. doi: 10.1007/BF01922860. [DOI] [PubMed] [Google Scholar]
- Borowsky R. Social inhibition of maturation in natural populations of Xiphophorus variatus (Pisces: Poeciliidae) Science. 1978;201:933–935. doi: 10.1126/science.201.4359.933. [DOI] [PubMed] [Google Scholar]
- Butler A, Le Roith D. Control of growth by the somatropic axis: growth hormone and the insulin-like growth factors have related and independent roles. Annual Review of Physiology. 2001;63:141–164. doi: 10.1146/annurev.physiol.63.1.141. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. US Department of Health and Human Services; 2004. The burden of chronic diseases and their risk factors: national and state perspectives 2004. (online); http://www.cdc.gov/nccdphp/burdenbook2004. [Google Scholar]
- Crespi B, Summers K. Positive selection in the evolution of cancer. Biological Reviews. 2006;81:407–424. doi: 10.1017/S1464793106007056. [DOI] [PubMed] [Google Scholar]
- Earley RL, Dugakin LA. Merging social hierarchies: Effects on dominance rank in male green swordtail fish (Xiphophorus helleri) Behavioural Processes. 2006;73:290–298. doi: 10.1016/j.beproc.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Fernandez AA. A cancer-causing gene is positively correlated with male aggression in Xiphophorus cortezi. Journal of Evolutionary Biology. 2010;23:386–396. doi: 10.1111/j.1420-9101.2009.01914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez AA, Bowser PR. Two cases of nonhybrid melanoma formation in Xiphophorus nezahualcoyotl (Poecitiidae, Cyprinodontiformes) Journal of Fish Biology. 2008;72:292–300. [Google Scholar]
- Fernandez AA, Morris MR. Mate choice for more melanin as a mechanism to maintain a functional oncogene. Proceedings of the National Academy of Sciences USA. 2008;105:13503–13507. doi: 10.1073/pnas.0803851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck D, Dikomey M, Schartl M. Selection and the maintenance of a colour pattern polymorphism in the green swordtail (Xiphophorus helleri) Behaviour. 2001;138:467–486. [Google Scholar]
- Froschauer A, Körting C, Bernhardt W, Nanda I, Schmid M, Schartl M, Volff J-N. Genomic plasticity and melanoma formation in Xiphophorus. Marine Biotechnology. 2001;3:S72–80. doi: 10.1007/s10126-001-0049-7. [DOI] [PubMed] [Google Scholar]
- Froschauer A, Korting C, Katagiri T, Aoki T, Asakawa S, Shimizu N, Schartl M, Volff J-N. Construction and initial analysis of bacterial artificial chromosome (BAC) contigs from the sex-determining region of the platyfish Xiphophorus maculatus. Gene. 2002;295:247–254. doi: 10.1016/s0378-1119(02)00684-4. [DOI] [PubMed] [Google Scholar]
- Giminez-Conti I, Woodhead AD, Harshbarger JC, Kazianis S, Setlow RB, Nairn RS, Walter RB. A proposed classification scheme for Xiphophorus melanomas based on histopathologic analyses. Marine Biotechnology. 2001;3:S100–S106. doi: 10.1007/s10126001-0031-4. [DOI] [PubMed] [Google Scholar]
- Gómez A, Wellbrock C, Gutbrod H, Dimitrijevic N, Schartl M. Ligand-independent dimerization and activation of the oncogenic Xmrk receptor by two mutations in the extracellular domain. The Journal of Biological Chemistry. 2001;276:3333–3340. doi: 10.1074/jbc.M006574200. [DOI] [PubMed] [Google Scholar]
- Gordon M. The genetics of viviparous top-minnow Platypoecilus: the inheritance of two kinds of melanophores. Genetics. 1927;12:253–283. doi: 10.1093/genetics/12.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlick DL. Dominance hierarchies and factors influencing dominance in the guppy Poecilia reticulata (Peters) Animal Behaviour. 1976;24:336–346. [Google Scholar]
- Graham J. Cancer Selection: A New Theory of Evolution. Aculeus Press; Lexington, VA: 1992. [Google Scholar]
- Greaves M. Cancer: The Evolutionary Legacy. Oxford University Press; Oxford: 2000. [Google Scholar]
- Gunnell D, Okasha M, Davey Smith G, Oliver SE, Sandhu J, Holly JMP. Height, leg length, and cancer risk: A systematic review. Epidemiologic Reviews. 2001;23:313–342. doi: 10.1093/oxfordjournals.epirev.a000809. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Rodriguez C, Morris MR, Dubois NS, de Quieroz K. Genetic variation and phylogeography of the swordtail fish Xiphophorus cortezi (Cyprinodontiformes, Poeciliidae) Molecular Phylogenetics and Evolution. 2007;43:111–123. doi: 10.1016/j.ympev.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Hannes RP. Blood and whole-body androgen levels of male swordtails correlated with aggression measures in a standard-opponent test. Aggressive Behavior. 1986;12:249–254. [Google Scholar]
- Holly JMP, Gunnell DJ, Davey Smith G. Growth hormone, IGF-I and cancer. Less intervention to avoid cancer? More intervention to prevent cancer? Journal of Endocrinology. 1999;162:321–330. doi: 10.1677/joe.0.1620321. [DOI] [PubMed] [Google Scholar]
- Huang Y, Kim S-O, Jiang J, Frank SJ. Growth hormone-induced phosphorylation of epidermal growth factor (EGF) receptor in 3T3-F442A cells. The Journal of Biological Chemistry. 2003;278:18902–18913. doi: 10.1074/jbc.M300939200. [DOI] [PubMed] [Google Scholar]
- Juul A, Dalgaard P, Blum W. Serum levels of Insulin-like Growth Factor (IGF)-binding protein-3 (IGFBP-3) in healthy infants, children and adolescents: the relation to IGF-I, IGF-II, IGFBP-1, IGFBP-2, age, sex, body mass index and pubertal maturation. Journal of Clinical Endocrinology & Metabolism. 1995;80:2534–2542. doi: 10.1210/jcem.80.8.7543116. [DOI] [PubMed] [Google Scholar]
- Kallman KD. Inheritance of melanophore patterns and sex determination in the Montezuma swordtail, Xiphophorus montezumae cortezi (Rosen) Zoologica. 1971;56:77–94. [Google Scholar]
- Kallman KD. The platyfish, Xiphophorus maculatus. In: King RC, editor. Handbook of Genetics. Plenum Press; New York: 1975. pp. 81–132. [Google Scholar]
- Kallman KD, Schreibman MP, Borkoski V. Genetic control of gonadotrop differentiation in the platyfish, Xiphophorus maculatus (Poeciliidae) Science. 1973;181:678–680. doi: 10.1126/science.181.4100.678. [DOI] [PubMed] [Google Scholar]
- Kosswig C. Uber bastarde der Teleostier Platyopoecilus und Xiphophorus. Zeitschrift für induktive Abstammungs-und Vererbungslehre. 1928;47:150–158. [Google Scholar]
- Leroi AM, Koufopanou V, Burt A. Cancer Selection. Nature Reviews Cancer. 2003;3:226–231. doi: 10.1038/nrc1016. [DOI] [PubMed] [Google Scholar]
- Luna LG. Manual of histological staining of the Armed Forces Institute of Pathology. McGraw-Hill; New York: 1968. [Google Scholar]
- Martineau D, Lemberger K, Dallaire A, Labelle P, Lipscomb TP, Michel P, Mikaelian I. Cancer in wildlife, a case study: beluga from the St. Lawrence estuary, Québec, Canada. Environmental Health Perspectives. 2002;110:285–292. doi: 10.1289/ehp.02110285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard Smith J, Haigh J. The hitch-hiking effect of a favourable gene. Genetics Research. 1974;23:23–35. [PubMed] [Google Scholar]
- McAloose D, Newton AL. Wildlife cancer: a conservation perspective. Nature Reviews Cancer. 2009;9:517–526. doi: 10.1038/nrc2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz JR, Peters JJM, Flik G. Molecular biology and physiology of the melanocortin system in fish: A review. General and Comparative Endocrinology. 2006;148:150–162. doi: 10.1016/j.ygcen.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Meierjohann S, Schartl M. From Mendelian to molecular genetics: the Xiphophorus melanoma model. Trends in Genetics. 2006;22:654–661. doi: 10.1016/j.tig.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Mommsen TP. Growth and metabolism. In: Evans DH, editor. The Physiology of Fishes. CRC Press; Boca Raton: 1998. pp. 65–97. [Google Scholar]
- Olsen CM, Zens MS, Stukel TA, Sacerdote C, Chang Y-M, Armstrong BK, Bataille V, Berwick M, Elwood JM, Holly EA, Kirkpatrick C, Mack T, Newton Bishop J, Østerlind A, Swerdlow AJ, Zanetti R, Green AC, Karagas MR, Whiteman DC. Nevus density and melanoma risk in women: A pooled analysis to test the divergent pathway hypothesis. International Journal of Cancer. 2008;124:937–944. doi: 10.1002/ijc.24011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauchenberger M, Kallman KD, Morizot DC. Monophyly and geography of the Río Pánuco Basin swordtails (Genus Xiphophorus) with descriptions of four new species. American Museum Novitates. 1990;2975:1–41. [Google Scholar]
- Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like Growth Factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–53. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- Ribowski A, Franck D. Demonstration of strength and concealment of weakness in escalating fights of male swordfish (Xiphophorus helleri) Ethology. 1993;93:265–274. [Google Scholar]
- Ross RK, Pike MC, Coetzee GA, Reichardt JKV, Yu MC, Feigelson H, Stanczyk FZ, Kolonel LN, Henderson BE. Androgen metabolism and prostate cancer: establishing a model of genetic susceptibility. Cancer Research. 1998;58:4497–4504. [PubMed] [Google Scholar]
- Ryan MJ, Hews D, Wagner WE., Jr Sexual selection on alleles that determine body size in the swordtail Xiphophorus nigrensis. Behavioral Ecology and Sociobiology. 1990;26:231–237. [Google Scholar]
- Saxena NK, Taliaferro-Smith L, Knight BB, Merlin D, Anania FA, O’Regan RM, Sharma D. Bidirectional crosstalk between Leptin and Insulin-like Growth Factor-I signaling promotes invasion and migration of breast cancer cells via transactivation of epidermal growth factor receptor. Cancer Research. 2008;68:9712–9722. doi: 10.1158/0008-5472.CAN-08-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schartl M. Evolution of Xmrk: an oncogene, but also a speciation gene? BioEssays. 2008;30:822–832. doi: 10.1002/bies.20807. [DOI] [PubMed] [Google Scholar]
- Schartl A, Schartl M, Anders F. Promotion and regression of neoplasia by testosterone-promoted cell differentiation in Xiphophorus and Girardinus. In: Hecker E, Fusenig NE, Kunz W, Marks F, Thielmann HW, editors. Carcinogenesis, Volume 7. New Raven Press; New York: 1982. pp. 427–434. [PubMed] [Google Scholar]
- Schartl A, Malitschek B, Kazianis S, Borowsky R, Schartl M. Spontaneous melanoma formation in nonhybrid Xiphophorus. Cancer Research. 1995;55:159–165. [PubMed] [Google Scholar]
- Schartl M, Hornung U, Gutbrod H, Volff J-N, Wittbrodt J. Melanoma loss-of-function mutants in Xiphophorus caused by Xmrk-oncogene deletion and gene disruption by a transposable element. Genetics. 1999;153:1385–1394. doi: 10.1093/genetics/153.3.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schartl M, Schartl A, Anders F. Phenotypic conversion of malignant melanoma to benign melanoma and vice versa in Xiphophorus. In: Seji M, editor. Phenotypic expression in pigment cells. University of Tokyo Press; Tokyo: 1981. pp. 507–514. [Google Scholar]
- Schartl M, Wilde B, Hornung U. Triplet repeat variability in the signal peptide sequence of the Xmrk receptor tyrosine kinase gene in Xiphophorus fish. Gene. 1998;224:17–21. doi: 10.1016/s0378-1119(98)00520-4. [DOI] [PubMed] [Google Scholar]
- Schreibman MP, Berkowitz EJ, Van den Hurk R. Histology and histochemistry of the testis and ovary of the platyfish, Xiphophorus maculatus, from birth to sexual maturity. Cell and Tissue Research. 1982;224:81–87. doi: 10.1007/BF00217268. [DOI] [PubMed] [Google Scholar]
- Selz Y, Froschauer A, Hoffmann C, Schmidt C, Schultheis C, Zhou Q, Braasch I, Böhne A, Schartl M, Volff J-N. Two types of melanocortin receptors are overexpressed in melanoma in the fish Xiphophorus. Melanoma Research. 2006;16:S74–S75. [Google Scholar]
- Setlow RB, Grist E, Thompson K, Woodhead AD. Wavelengths effective in induction of malignant melanomas. Proceedings of the National Academy of Sciences USA. 2003;90:6666–6670. doi: 10.1073/pnas.90.14.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors AR, Solomon C, McTiernan A, White E. Melanoma risk in relation to height, weight, and exercise (United States) Cancer Causes & Control. 2001;12:599–606. doi: 10.1023/a:1011211615524. [DOI] [PubMed] [Google Scholar]
- Siciliano MJ, Perlmutter A, Clark E. Effect of sex on the development of melanoma in hybrid fish of the genus Xiphophorus. Cancer Research. 1971;31:725–729. [PubMed] [Google Scholar]
- Summers K, Crespi B. The androgen receptor and prostate cancer: a role for sexual selection and sexual conflict? Medical Hypotheses. 2008;70:435–443. doi: 10.1016/j.mehy.2007.04.044. [DOI] [PubMed] [Google Scholar]
- Vera Cruz EM, Brown CL. The influence of social status on the rate of growth, eye color pattern and Insulin-like Growth Factor-I gene expression in Nile tilapia, Oreochromis niloticus. Hormones and Behavior. 2007;51:611–619. doi: 10.1016/j.yhbeh.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Volff J-N, Koerting C, Froschauer A, Zhou Q, Wilde B, Schultheis C, Selz Y, Sweeney K, Duschl J, Wichert K, Altschmied J, Schartl M. The Xmrk oncogene can escape nonfunctionalization in a highly unstable subtelomeric region of the genome of the fish Xiphophorus. Genomics. 2003;82:470–479. doi: 10.1016/s0888-7543(03)00168-x. [DOI] [PubMed] [Google Scholar]
- Volff J-N, Schartl M. Evolution of signal transduction by gene and genome duplication in fish. Journal of Structural and Functional Genomics. 2003;3:139–150. [PubMed] [Google Scholar]
- Wallings CA, Royle NJ, Metcalfe NB, Lindström J. Green swordtails alter their age at maturation in response to the population level of male ornamentation. Biology Letters. 2007;3:144–146. doi: 10.1098/rsbl.2006.0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis S, Schartl M. The macromelanophore locus and the melanoma oncogene Xmrk are separate genetic entities in the genome of Xiphophorus. Genetics. 1998;149:1909–1920. doi: 10.1093/genetics/149.4.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- Woolcock BW, Schmidt BM, Kallman KD, Vielkind JR. Differences in transcription and promoters of Xmrk-1 and Xmrk-2 genes suggest a role for Xmrk-2 in pigment pattern development in the Platyfish, Xiphophorus maculatus. Cell Growth and Differentiation. 1994;5:575–583. [PubMed] [Google Scholar]
- Zhou Q, Froschauer A, Schultheis C, Schmidt C, Bienert GP, Wenning M, Dettai A, Volff J-N. Helitron transposons on the sex chromosomes of the platyfish Xiphophorus maculatus and their evolution in animal genomes. Zerbafish. 2006;3:39–52. doi: 10.1089/zeb.2006.3.39. [DOI] [PubMed] [Google Scholar]
- Zimmerer EJ, Kallman KD. Genetic basis for alternative reproductive tactics in the pygmy swordtail, Xiphophorus nigrensis. Evolution. 1989;43:1298–1307. doi: 10.1111/j.1558-5646.1989.tb02576.x. [DOI] [PubMed] [Google Scholar]
- Zuk M, Kolluru GR. Exploitation of sexual signals by predators and parasitoids. Quarterly Review of Biology. 1998;73:415–438. [Google Scholar]


