Abstract
The number of functional hormone receptors expressed by a cell in large part determines its responsiveness to the hormonal signal. The regulation of hormone receptor gene expression is therefore a central component of hormone action. Vertebrate steroid and thyroid hormones act by binding to nuclear receptors (NR) that function as ligand-activated transcription factors. Nuclear receptor genes are regulated by diverse and interacting intracellular signaling pathways. Nuclear receptor ligands can regulate the expression of the gene for the NR that mediates the hormone's action (autoregulation), thus influencing how a cell responds to the hormone. Autoregulation can be either positive or negative, the hormone increasing or decreasing, respectively, the expression of its own NR. Positive autoregulation (autoinduction) is often observed during postembryoninc development, and during the ovarian cycle, where it enhances cellular sensitivity to the hormonal signal to drive the developmental process. By contrast, negative autoregulation (autorepression) may become important in the juvenile and adult for homeostatic negative feedback responses. In addition to autoregulation, a NR can influence the expression other types of NRs (cross-regulation), thus modifying how a cell responds to a different hormone. Cross-regulation by NRs is an important means to temporally coordinate cell responses to a subsequent (different) hormonal signal, or to allow for crosstalk between hormone signaling pathways.
I. Introduction
Steroid and thyroid hormones play central roles in animal development, physiology and behavior. The classical mode of signaling for these hormones is through binding to their cognate nuclear receptors (NRs), which are ligand-activated transcription factors that regulate the transcription of a subset of genes expressed by a cell. Nuclear receptors that mediate the actions of classical hormones can be divided into two groups based on their evolutionary relatedness, subcellular localization and mode of action (Germain et al., 2006). The type I NRs include the steroid hormone receptors: androgen – AR; estrogen – ER; glucocorticoid – GR; mineralocorticoid – MR; and progesterone receptor - PR. In the unliganded state, type I NRs are located in the cytoplasm associated with molecular chaperones (heat shock proteins) that maintain the receptors in a conformation that allows for ligand binding but not DNA binding. Upon hormone binding, type I NRs undergo a conformational change that results in the release of components of the chaperone complex, exposure of a nuclear localization sequence, and translocation to the nucleus where they associate with chromatin and regulate gene transcription. Both DNA binding-dependent (at hormone response elements - HREs) and DNA binding-independent actions of type I NRs have been described (Mangelsdorf et al., 1995; Reichardt et al., 1998).
The type II NRs include thyroid hormone (TR), Vitamin D (VDR), retinoic acid (RAR) and retinoid X (RXR) receptors. In contrast to the type I NRs, the type II NRs reside in the nucleus constitutively bound to DNA, and usually act as transcriptional repressors in the absence of hormone (e.g. TRs; Aranda and Pascual, 2001; Ribeiro et al., 1995). Both type I and type II NRs control gene transcription by recruiting proteins that cause chromatin remodeling, or posttranslational modification to histones that promote a relaxed or repressive chromatin structure (Robyr et al., 2000). Steroid and thyroid hormones also act via plasma membrane-associated receptors (Furuya et al., 2009; Kampa and Castanas, 2006; Levin, 2009; Tasker et al., 2006; Thomas, 2008; Zhu et al., 2008). The types of proteins responsible for transducing steroid and thyroid hormone (TH) actions at the plasma membrane include classical membrane-resident proteins such as G protein-coupled receptors and ligand-activated ion channels; but, there is also evidence for NRs associating with the plasma membrane and signaling via intracellular kinases. Furthermore, there may be crosstalk between plasma membrane (nongenomic) and nuclear (genomic) signaling pathways. Here we focus largely on the genomic signaling pathways initiated by binding of a hormone to its cognate NR in the cytosol or nucleus to influence NR gene expression.
Since their discovery there has been great interest in delineating the molecular mechanisms by which NRs regulate target gene expression owing to their central roles in development, metabolism and behavior. Comparatively less attention has been paid to the physiological and molecular mechanisms by which the expression of NR genes is regulated. The number of functional receptors expressed by a cell determines its hormone responsiveness, and the expression of NRs may change with stage of development, physiological or pathological state. For these reasons, knowledge of the mechanisms by which NR gene expression is controlled is critical for understanding hormone action. This review focuses on two aspects of the regulation of NR gene expression. First, we discuss the molecular mechanisms and physiological significance of NR autoregulation, the regulation of the expression of a NR by its ligand. We then discuss NR cross-regulation, the regulation of the expression of one NR gene by a different type of NR. In each case, the amount of functional NR in a cell is regulated, but the mechanisms by which this occurs may be transcriptional or posttranscriptional, or both. Where data are available we attempt to describe these regulatory mechanisms, and we also discuss the physiological significance of changes in NR expression. The focus of this review is on vertebrate NRs; however, similar phenomena have been described in invertebrates. For example, the ecdysone receptor is autoinduced during insect metamorphosis (Riddiford et al., 2001; Thummel, 1995). Auto- and cross-regulation of NRs is an ancient and evolutionarily conserved regulatory mechanism for controlling cell responses to hormones.
II. Nuclear Receptor Autoregulation
The regulation of the expression of a NR gene by its hormone-bound protein product is an important means for modulating hormone action in developing animals, within the reproductive system of adult animals, and for maintaining homeostasis. Autoregulation of NR genes can be positive (autoinduction; i.e., homologous upregulation) or negative (autorepression; homologous down-regulation). Autoinduction leads to the biosynthesis of more NRs by the cell, thus enhancing cellular responsiveness to the hormone. Autorepression is a homeostatic mechanism to modulate hormone action by downregulating the receptor for the hormone.
There are several mechanisms by which NR expression may be autoregulated that can be broadly described as transcriptional or posttranscriptional (Fig. 1). At the transcriptional level, regulation can be either direct, where the ligand-bound NR associates with its own gene to positively or negatively regulate transcription, or indirect, where the NR induces expression of a transcription factor(s) that regulates transcription of the NR gene (Fig. 1A,B). Both direct and indirect regulation may also occur, where the NR binds to and regulates expression of its own gene, but full transcriptional regulatory activity depends on the induction of a transcription factor(s) that regulates transcription of the NR gene (cooperative regulation; Fig. 1C). At the posttranscriptional level, the NR may induce expression of genes that influence mRNA stability or protein stability (Fig 1D,E,F; or perhaps also protein translation or protein bioactivity through posttranslational modifications).
Figure 1.
Molecular mechanisms for regulation of nuclear receptor (NR) expression. The level of functional NRs in a cell can be regulated at transcriptional or posttranscriptional levels, or some combination of the two. A. Direct transcriptional regulation involves the hormone (filled oval)-NR complex (NRA; shown as a homodimer, but type II NRs also function as heterodimers) directly binding to a hormone response element located within the nra gene that activates or represses its expression (for cross-regulation the NRA binds to and regulates a different NR gene). Some NRs may influence target genes through hormone response elements that are far upstream or downstream of the regulated locus. Also, some NRs regulate target genes through protein-protein interactions rather than by direct DNA binding. B. Indirect transcriptional regulation involves the hormone-NRA complex inducing the expression of a gene that codes for a transcription factor (TF) that then positively or negatively regulates nra gene transcription (for cross-regulation the NRA regulates a TF that binds to and regulate a different NR gene). C. Cooperative transcriptional regulation involves both direct and indirect transcriptional mechanisms shown in parts A and B. D. The NRA may regulate the expression of a gene that increases or decreases stability of the nra mRNA (for cross-regulation, the mRNA for a different NR gene). E. Hormone binding to the NRA can stabilize it, thus increasing its half-life (t ½). F. The NRA may induce expression of a ubiquitin ligase that ubiquitinates (Ub) NRs in the cell (either the NRA or a different NR) and targets it to the proteasome for degradation. NR protein stability and bioactivity may also be influenced through posttranslational modifications such as (e.g., phosphorylation).
Under physiological conditions, NR autoinduction is often observed when positive feedback within the neuroendocrine system increases hormone production, leading to a progressive rise in plasma hormone concentrations. Within hormone target tissues, NR autoinduction generates a feed-forward response that ends when the positive feedback on hormone production is terminated. For example, during the mammalian ovarian cycle gonadotropins secreted by the pituitary gland stimulate estrogen production by the ovarian follicle, and rising plasma estrogen concentration causes positive feedback on the hypothalamo-pituitary axis. During the mid- to late follicular phase, when plasma estrogen titers increase, estrogen stimulates proliferation of uterine endometrial cells; estrogen action may be facilitated by the autoinduction of the ER in these cells, a feed-forward response. These actions are terminated, and plasma estrogen concentration drops upon ovulation induced by the surge in plasma luteinizing hormone caused by the positive feedback signal of estrogen. Nuclear receptor autoinduction is also seen during animal development where it serves as a feed-forward mechanism to drive the developmental process. The most studied example of this phenomenon is amphibian metamorphosis, where rising plasma TH titers cause autoinduction of TRs in target tissues (Tata, 2000).
Nuclear receptor autorepression may function as a component of negative autoregulatory feedback loops to maintain homeostasis. For example, the GR is expressed in the brain in limbic structures involved with fear and anxiety (amygdala, bed nucleus of the stria terminalis), and learning and memory (hippocampus), and in neurosecretory neurons that regulate the hypothalamo-pituitary-adrenal (HPA) axis. The GR plays a central role in mediating negative feedback on the HPA axis, but also may facilitate stress-related behaviors through feed-forward actions on limbic structures. A physiological means to limit negative feedback of glucocorticoids on the HPA axis once the system has returned to baseline, and to ‘reign-in’ the facilitating effects of glucocorticoids on fear and anxiety is to downregulate GR expression in the hypothalamus and limbic structures, respectively.
A. Type I Nuclear Receptors
Sex steroids (estrogens, androgens and progestins) control the development of the male and female urogenital tracts and secondary sex characteristics, sexual behavior and gametogenesis. Corticosteroids (glucocorticoids and mineralocorticoids) control developmental, physiological and behavioral responses to stress and regulate hydromineral balance and blood pressure. Vitamin D regulates plasma calcium and phosphorous homeostasis, and skeletal mineralization and remodeling.
1. Estrogen Receptor (ER)
All jawed vertebrates that have been studied to date have two ER subtypes, designated ERα and ERβ (Escriva et al., 2004; Germain et al., 2006); some bony fishes have additional paralogs. Estrogen receptor expression is regulated by estrogens, most commonly through autoinduction. The molecular mechanisms that have been identified involve direct transcriptional regulation of ER genes by the ER and modulation of ER mRNA stability. ER autoinduction appears to be important for estrogen action on the female reproductive tract.
Autoinduction
One of the earliest reports of NR autoinduction was the demonstration that estrogen injection increased ER in liver cell nuclei of male frogs (Xenopus laevis; Westley and Knowland, 1979). Vitellogenin (Vtg) is a yolk protein synthesized by the female liver in nonmammalian species, and Vtg synthesis and deposition in eggs occurs over weeks to months, a process called vitellogenesis. The synthesis of Vtg is regulated by estrogen, and although males normally do not synthesize Vtg (and have very low ER levels in liver cells), it can be induced by estrogen injection. If male frogs are given a second injection of estrogen several weeks after the synthesis of Vtg caused by the initial injection has waned, the kinetics of Vtg production is significantly faster than that of the initial injection (Clemens, 1974). This led Westley and Knowland (1979) to hypothesize, and to confirm, that the primary estrogen injection caused the synthesis of ERs that then sensitized cells to a second exposure to the hormone. Subsequent studies confirmed and extended these findings in the frog (Barton and Shapiro, 1988; Shapiro et al., 1989; Varriale and Tata, 1990). Indeed, a single injection of estrogen into male X. laevis caused a robust (18 fold) and prolonged elevation of liver ER mRNA that persisted at the same level for the 125 day duration of the experiment (Barton and Shapiro, 1988). Autoinduction of ER, and a parallel increase in vitellogenin mRNA was also reported to occur in liver of several fish species (Bowman et al., 2002; Marlatt et al., 2008; Pakdel et al., 1991; Yadetie et al., 1999).
In addition to the liver, ER autoinduction was reported in frog oviduct cells (Varriale and Tata, 1990), mouse uterus (Bergman et al., 1992; Kamiya et al., 1996; Nephew et al., 2000; Yamashita et al., 1990) and rat pituitary gland (Friend et al., 1995). Estrogen also causes ER autoinduction in testis and prostate of male rodents (Prins, 1992; Prins and Birch, 1997; Sato et al., 1994; Tena-Sempere et al., 2000) where neonatal exposure to high doses of estrogen caused atrophy of the testes, suppressed prostate growth and reduced responsiveness to androgen (Brown-Grant et al., 1975; Naslund and Coffey, 1986; Prins and Korach, 2008; Rajfer and Coffey, 1978).
Autorepression
In the mammary gland the ER is negatively regulated by estrogen. Treatment with estrogen decreased ER mRNA levels in the estrogen-sensitive human breast cancer cell line MCF-7 (Berkenstam et al., 1989; Read et al., 1989). Injections of estrogen into lactating mice decreased ER mRNA in the mammary gland; the reduction in ER expression may be important for the normal transition from lactation to the postlactational phase (Hatsumi and Yamamuro, 2006).
Molecular mechanisms of ER autoregulation
Transcriptional activation of ER genes by estrogen via identified estrogen response elements (EREs) has been shown in fish, frog and mammal cells. Estrogen activated ER gene transcription in the breast cancer cell line MCF7 as measured by nuclear run-on assay (Saceda et al., 1988). Treilleux et al. (1997) identified three half estrogen response elements (EREs) upstream of the transcription start site of the human ER gene that supported estrogen induction in MCF7 cells. In the frog ER gene a functional ERE was identified about 480 bp upstream of the transcription start site within the protein coding region (Lee et al., 1995). The rainbow trout ER gene is regulated by an ERE located in the proximal promoter region of the gene (Le Drean et al., 1995; Petit et al., 1999).
Estrogen has also been shown to stabilize ER mRNAs in fish liver at the onset of vitellogenesis (Flouriot et al., 1996), and in mammalian (sheep) uterus at the time of the preovulatory estrogen surge (Ing and O'Malley, 1995; Ing, 2005; Ing et al., 2008; Ing and Ott, 1999; Mitchell and Ing, 2003). These actions of estrogen were likely mediated by the ER, since they could be blocked by estrogen receptor antagonists.
The molecular mechanisms by which the ER is negatively regulated by its ligand are poorly understood. Hatsumi and Yamamuro (2006) showed that in mouse mammary gland estrogen injection decreased phosphorylation of the signal transducer and activator of transcription 5, a known transcriptional regulator of the ER gene (Hatsumi and Yamamuro, 2006). However, the doses of estrogen used in this study were supraphysiological, and it is therefore uncertain whether the findings are of physiological relevance.
Physiological significance of ER autoinduction
Estrogen receptor autoinduction may be necessary for cells to achieve maximal response to the hormonal signal. Studies in frog hepatocytes and oviductal cells showed a close association between ER number in cell nuclei and the rate of de novo transcription of ER target genes (vitellogenin and FOSP-1 genes (Perlman et al., 1984; Varriale and Tata, 1990). Inhibition of protein synthesis that blocked the estrogen-induced increase in ER protein, or co-treatment with ER antagonists reduced estrogen-dependent accumulation of vitellogenin and FOSP-1 mRNAs, suggesting that ER target gene activation depends on the level of ER in the cell. Vitellogenin synthesis becomes responsive to estrogen at metamorphic climax in X. laevis tadpoles (Huber et al., 1979; Knowland, 1978; May and Knowland, 1980), which corresponds to the developmental stage when the ER can be autoinduced (May and Knowland, 1981; Rabelo et al., 1994). This suggests that the increase in Vtg biosynthesis is causally linked to the increase in ER. In mammals, estrogen is known to increase proliferation of epithelial and stromal cells of the uterine endometrium, coincident with ER autoinduction (Kamiya et al., 1996; Nephew et al., 2000; Yamashita et al., 1990).
2. Androgen Receptor (AR)
Autoregulation of the AR has been reported in virtually every cell type that expresses the gene. This regulation is predominantly negative, although there are some examples where androgens cause autoinduction of the AR. The regulation of AR mRNA and protein is complex, occuring at both transcriptional and posttranscriptional levels.
Autoinduction
The AR is autoinduced in specific regions of the prostate gland (Takeda et al., 1991; although, the AR is negatively autoregulated in other regions of the gland – discussed below), other organs of the male urogenital tract and in tissues displaying male secondary sex characteristics. For example, testosterone induced AR mRNA in rat penile smooth muscle cells (Gonzalez-Cadavid et al., 1993) and in hamster harderian gland, a tubulo-alveolar gland found in the orbital activity (Dominguez et al., 1994; Varriale, 1996). Evidence for positive regulation of AR mRNA by androgens has also been shown for lizard and bird testis (Cardone et al., 1998; Nastiuk and Clayton, 1994). In the frog Rana esculenta, testosterone treatment increased AR mRNA in the harderian gland and thumb pad (Varriale and Serino, 1994).
Androgen receptor may also be autoinduced in non-reproductive organs. Treatment of human osteoblast cell lines with androgen increased AR mRNA in a time and dose-dependent manner that was blocked by cotreatment with the antiandrogen flutamide (Takeuchi et al., 1994; Wiren et al., 1997). Indirect evidence for AR autoinduction in rat hippocampus was suggested by the findings that castration, or injection of AR antagonist in gonad-intact males caused a reduction in AR mRNA levels (Kerr et al., 1995).
Autorepression
By contrast to AR autoinduction, the AR appears to be autorepressed in most tissues in which the gene is expressed. For example, castration of male rats increased, while testosterone replacement decreased AR mRNA levels in the anterior prostate gland, epididymis, kidney and brain (Prins and Woodham, 1995; Quarmby et al., 1990). Negative AR mRNA autoregulation was also observed in the human prostate cancer line LNCaP (Blok et al., 1992; Wolf et al., 1993) and in breast cancer cell lines T47D and MFM-223 (Hackenberg et al., 1992; Wolf et al., 1993).
Molecular mechanisms of AR autoregulation
Direct transcriptional regulation of the human AR gene by AR protein is supported by the discovery of several putative androgen response elements (AREs) located within a 350 bp stretch of the AR coding region (Dai and Burnstein, 1996; Grad et al., 1999). This DNA sequence conferred androgen-dependent activation to a minimal promoter-reporter construct in transfection assays of prostatic PC3 cells, and electrophoretic mobility shift assays and DNAse I footprinting showed that the AR can bind to this region (Dai and Burnstein, 1996; Grad et al., 1999). In addition, Wiren et al. (1997) found that a 2.9 kb fragment of the 5’ flanking region of the human AR gene (-2330 to +572) conferred positive androgen regulation on a chloramphenicol acetyltransferase reporter construct transfected into osteoblast cells. These investigators did not define the specific sequences within this region of the human AR gene responsible for androgen regulation. Promoter analysis of the AR gene in hamsters identified an androgen/glucocorticoid response element located 473 bp upstream of the transcription start site, and this region was shown to associate with AR in gel shift assays (Varriale and Esposito, 2005). Whether a homologous DNA element is present in the human AR gene has not been reported. Taken together, the findings suggest that there may be multiple AREs present in mammalian AR genes that mediate autoinduction. The molecular basis for autorepression of the AR gene is not well understood, but thought to occur independent of the exonic AREs that are proposed to function in AR autoinduction (Burnstein, 2005).
Physiological significance of AR autoinduction
The development of secondary sex characteristics in males depends on circulating androgens. There are distinct sex differences in AR expression that may depend on androgen action, and the level of AR expressed in a tissue likely determines the physiological and morphological effects that androgens have on that tissue. For example, in hamsters, sexual dimorphism of the harderian gland is attributed to the different circulating levels of androgen between sexes (Payne, 1994). Both male and female hamster harderian glands express AR, but receptor levels are thirty times higher in male than in female, suggesting that the expression level of AR is in part responsible for the sex-associated phenotypic differences in the gland (Dominguez et al., 1994). A similar sex difference in AR expression was observed in hamster facial motor neurons where females express 50% less AR mRNA compared to males (Drengler et al., 1996). In this study, they showed that gonadectomy decreased AR mRNA in males but not in females, and replacement of testosterone increased AR mRNA in males. The sex-dependent difference in AR autoinduction may be a mechanism for testosterone to augment androgen responsiveness of facial nerve regeneration in males compared with females (Jones, 1993).
At the cellular level, studies in cell lines derived from rat prostate tumor or hamster ductus deferens smooth muscle showed that androgen treatment promoted cell proliferation, and the proliferative response correlated with an increase in the number of functional ARs in the cells (Syms et al., 1985; Syms et al., 1983a; Syms et al., 1983b). The upregulation of AR mRNA in osteoblast and prostate cancer cells has been associated with sensitization to androgens (Burnstein, 2005).
3. Progesterone Receptor (PR)
Unlike the ER and AR that show both autoinduction and autorepression, the PR appears to be exclusively negatively regulated by its ligand. Autorepression of PR has been shown in guinea pig and rabbit uterus (Isomaa et al., 1979; Milgrom et al., 1973), rat and guinea pig brain (Blaustein and Turcotte, 1990; Parsons et al., 1981), chicken oviduct (Mester and Baulieu, 1977; Syvala et al., 1998) and in human breast cancer cell lines (Alexander et al., 1989; Mullick and Katzenellenbogen, 1986; Nardulli and Katzenellenbogen, 1988; Read et al., 1988).
The progesterone-mediated decrease in PR expression may occur at both transcriptional and posttranscriptional levels, where progesterone decreases PR mRNA (Alexander et al., 1989; Read et al., 1988; Savouret et al., 1991; Savouret et al., 1994a) and promotes PR turnover (Blaustein and Turcotte, 1990; Isomaa et al., 1979; Mester and Baulieu, 1977; Mullick and Katzenellenbogen, 1986; Read et al., 1988). The reduction in PR mRNA level is accompanied by a parallel decrease in receptor binding activity (Blaustein and Turcotte, 1990; Parsons et al., 1981). DNase I footprinting assay identified several binding sites for the PR located in the 5’ flanking region, and binding sites for the ER in the 5’ flanking region and near the translation initiation site of the rabbit PR gene. The PR gene is positively regulated by the ER (discussed in greater detail below), and both transactivation by the ER, and transrepression by the PR are mediated by intragenic sequences surrounding the translation initiation site that correspond to the identified ERE, but not by the putative hormone response elements in the 5’ flanking region of the gene (Savouret et al., 1991). Although repression by progestins was mediated by the region containing the ERE, the PR did not bind to this element (Savouret et al., 1994b). The mechanism for PR autorepression may involve antagonism of estrogen-dependent induction of the PR gene through protein-protein interactions with the ER (Savouret et al., 1994b).
In addition to negative regulation of PR gene transcription, progestins can also reduce the level of PR protein in the cell through alterations in PR turnover. For example, in chicken oviduct, progesterone causes the PR to become ubiquitinated, which targets the receptor to the proteasomal pathway for degradation (Syvala et al., 1998).
4. Corticosteroid Receptors (GR and MR)
Vertebrates have two corticosteroid receptors, the MR (type I) and the GR (type II). Both autoinduction and autorepression have been shown for both receptors, although autorepression appears to be more common. Of the two receptors the GR has been better studied.
Autoinduction
Autoinduction of GR was demonstrated in human leukemic T cells (CEM C7 and 6TG1.1) that exhibit glucocorticoid-dependent cell lysis (Denton et al., 1993; Eisen et al., 1988; Ramdas et al., 1999; Thompson et al., 1992). In these cells, the glucocorticoid-induced increase in GR mRNA preceded apoptosis, and was therefore not a consequence of cell lysis (Eisen et al., 1988). GR autoinduction was resistant to protein synthesis inhibition, and was completely blocked by the GR antagonist RU486, suggesting that GR autoinduction in these cells is directly mediated by the GR (Denton et al., 1993). Autoinduction of GR was also reported in tadpole intestine (Krain and Denver, 2004).
Autorepression
Downregulation of GR expression by glucocorticoids has been described in mammals and in frogs, and may be a more common form of autoregulation of the GR than autoinduction. The GR plays a central role in mediating negative feedback by glucocorticoids on the activity of the hypothalamic-pituitary-adrenal (HPA) axis (reviewed by De Kloet et al., 1998). It is expressed in the hypothalamus and limbic structures (e.g., amygdala, bed nucleus of the stria terminalis, hippocampus) of the brain, and in the anterior pituitary gland where it participates in negative feedback by glucocorticoids on corticotropin-releasing factor (CRF) and adrenocorticotropic hormone (ACTH; reviewed by Denver, 2009b; Yao et al., 2008). Glucocorticoid receptor expressed in these tissues is predominantly negatively regulated by glucocorticoids. For example, administration of glucocorticoids decreased GR protein and/or mRNA throughout the brain of rats and frogs (Chao et al., 1998; Ghosh et al., 2000; Han et al., 2007; Holmes et al., 1995; Hugin-Flores et al., 2004; Reul et al., 1989b; Sapolsky et al., 1984; Spencer et al., 2000; Yao et al., 2008). In rats, adrenalectomy increased GR protein in the hippocampus, hypothalamus, and cortex (Kalman and Spencer, 2002; O'donnell et al., 1995; Spencer et al., 2000) and GR mRNA in the hippocampus; the latter could be reversed by supplementation with glucocorticoids (Chao et al., 1998; Han et al., 2007; Holmes et al., 1995; Hugin-Flores et al., 2004; Okret et al., 1991; Reul et al., 1989b; Tornello et al., 1982).
Autorepression of the GR by circulating glucocorticoids may be an important mechanism for regulating the responsiveness of the HPA axis during chronic stress. For example, rats exposed to repeated or chronic stressors had reduced GR mRNA expression in hypothalamic and limbic structures involved in regulating the HPA axis (Gomez et al., 1996; Herman and Watson, 1995; Makino et al., 2002; Makino et al., 1995; Nishimura et al., 2004; Sapolsky et al., 1984). The GR also appears to be negatively regulated by corticosteroids in other cell types; e.g., lymphocytes, liver, lung and pancreatic acinar cells (Dong et al., 1988; Okret et al., 1991; Okret et al., 1986; Rosewicz et al., 1988).
In contrast to the GR, which shows both forms of autoregulation, only autorepression has been reported for the MR. In the rat kidney, adrenalectomy increased MR mRNA (Kalinyak et al., 1992) and receptor binding (Claire et al., 1981; Stephenson and Funder, 1987). In rodent brain, the GR is widely expressed compared to the MR, whose expression is largely restricted to the hippocampus (Chao et al., 1989). Similar to kidney, adrenalectomy elevated MR mRNA (Chao et al., 1998; Herman et al., 1989; Kwak et al., 1993; Patchev et al., 1994; Reul et al., 1989a) and protein levels (Chao et al., 1989; Kalman and Spencer, 2002; Stephenson and Funder, 1987) in the hippocampus. Both acute and chronic stress caused downregulation of MR mRNA in the hippocampus (Fujikawa et al., 2000; Herman and Watson, 1995; Lopez et al., 1998). Use of specific agonists for the GR or MR showed that MR downregulation was mediated by the MR (Chao et al., 1998).
Molecular mechanisms of GR and MR autoregulation
Several molecular and cellular mechanisms have been proposed to explain the downregulation of GR expression by glucocorticoids. These include 1) the inhibition of GR gene transcription (Dong et al., 1988; Okret et al., 1991; Rosewicz et al., 1988), 2) posttranscriptional regulation (e.g., decreased mRNA stability or translatability; Burnstein et al., 1991; Meyer and Schmidt, 1995; Okret et al., 1986; Vedeckis et al., 1989), and 3) posttranslational regulation (e.g., decreased protein stability and/or increased protein degradation; Dong et al., 1988; Okret et al., 1991; Webster et al., 1997).
Glucocorticoid administration decreased the rate of transcription of the GR gene, as measured by nuclear run on assay, and this was independent of protein synthesis, suggesting that the GR gene is directly regulated by the GR (Dong et al., 1988; Okret et al., 1991; Okret et al., 1986; Rosewicz et al., 1988). In further support of this hypothesis, GR was found to associate with the coding and 3’ untranslated regions of the GR gene by immunoprecipitation and DNase I footprinting assays (Burnstein et al., 1990; Okret et al., 1986). Deletion of a 1 kb fragment within the human GR coding region abolished negative GR autoregulation, suggesting that there are intragenic sequences responsible for autorepression (Burnstein et al., 1990). The decrease in GR mRNA was paralleled by a decrease in GR protein levels, which could be due to decreased transcription and increased GR protein turnover or both (Dong et al., 1988; Yao et al., 2008). Upon hormone binding the GR translocates to the nucleus where it binds DNA to regulate gene transcription. It is subsequently degraded through a proteasome-mediated mechanism (Conway-Campbell et al., 2007; Deroo et al., 2002). Although the molecular mechanism for MR autorepression is not known, there is evidence that it can occur at the level of gene transcription (Herman and Watson, 1995).
Physiological significance of GR autoinduction
Support for the biological significance of GR autoinduction in glucocorticoid-induced cell death was provided by Ramdas et al. (1999) where they were able to rescue the apoptotic effects of glucocorticoids in a cell line expressing mutant GR by expressing varying levels of wild type GR. They transfected a wild type human GR expression plasmid into ICR27TK.3 cells, a cell line derived from the human leukemic cell line 6TG1.1 that expresses an activation deficient mutant human GR. They found that basal levels of GR expression in human leukemic T cells had minimal effect on cell growth and cell death, while overexpression of GR to levels similar to those seen after GR autoinduction resulted in growth arrest and apoptosis. These findings suggest that basal levels of GR are not sufficient to elicit an apoptotic response, and that GR autoinduction may provide a means to amplify the hormone signal needed for cell death in response to steroid treatment (Ramdas et al., 1999).
B. Type II Nuclear Receptors
1. Retinoic Acid Receptor (RAR)
Retinoic acid receptors play important roles in mediating the actions of retinoic acid on cell differentiation and proliferation during vertebrate development. There are three RAR receptor subtypes (RAR α, β, γ) encoded by three separate genes (Benbrook et al., 1988; Brand et al., 1988; Giguere et al., 1987; Krust et al., 1989; Petkovich et al., 1987; Zelent et al., 1989). Of the three subtypes, both human and rodent RARβ have been found to be positively regulated by retinoic acid in several tissues (liver, liver, testes; de The et al., 1989; de The et al., 1990; Haq et al., 1991). Autoinduction of RARβ occurred at the level transcription as evidenced by nuclear run on assays and treatment with inhibitors of RNA and protein synthesis (de The et al., 1989). Deletion mapping of the human RARβ promoter identified a region immediately upstream of the transcription start site that contained a functional retinoic acid responsive element (RARE; de The et al., 1990); an identical, functional RARE was identified in the homologous region of the mouse gene (Sucov et al., 1990). The mouse RARα (the RARα2 isoform) was also found to be autoinduced in a mouse embryonic carcinoma cell line although not as strongly as RARβ (Leroy et al., 1991b). The autoregulation of RARα2 was attributed to the presence of a functional RARE located in the RARα proximal promoter that closely resembles the RARE in the RARβ promoter (Leroy et al., 1991a).
2. Vitamin D Receptor (VDR)
The vitamin D receptor mediates the actions of 1,25-dihydroxyvitamin D3 (vitamin D3) on calcium homeostasis. Autoregulation of the VDR plays an important role in the actions of vitamin D3, and so far, only positive regulation has been reported. For example, autoinduction of the VDR has been reported in several cell lines (Mahonen and Maenpaa, 1994; McDonnell et al., 1987; Pan et al., 1991). In animals, autoregulation of the VDR is tissue-specific; for example, vitamin D3 increases VDR mRNA levels in the kidney (Healy et al., 2005; Healy et al., 2003), parathyroid gland (Naveh-Many et al., 1990) and skin (Zineb et al., 1998), but not in the intestine (Wiese et al., 1992; Zineb et al., 1998; but see Strom et al., 1989). Autoinduction of VDR in the kidney depends on a normal serum calcium level (Healy et al., 2005; Healy et al., 2003). Vitamin D3 causes upregulation of VDR mRNA in cultured osteoblastic cells, which suggests that the gene can be autoinduced in bone (Mahonen and Maenpaa, 1994; Zella et al., 2006; Zella et al., 2010).
Vitamin D receptor autoinduction involves both transcriptional and posttranscriptional mechanisms. Earlier studies found that vitamin D3 stabilizes the VDR protein, thus increasing its half-life (Arbour et al., 1993; Davoodi et al., 1995; Wiese et al., 1992). Recent work in mouse osteoblastic cells identified functional vitamin D3 response elements located within intronic regions of the VDR gene, supporting a direct transcriptional mechanism for VDR autoinduction (Zella et al., 2006). Recently, Zella et al. (2010) used chromatin immunoprecipitation scanning to identify two other potential vitamin D3 response elements, one located 6 kb upstream of the transcription start site of the mouse VDR gene (Zella et al., 2010); however, whether these DNA sequences mediate VDR actions in vivo is uncertain.
3. Thyroid Hormone Receptor (TR)
Thyroid hormone receptors exhibit autoinduction and autorepression, although the former has been best documented and studied. The most detailed studies of TR autoregulation have been conducted on tadpoles of the South African clawed frog Xenopus laevis. Tadpole metamorphosis is dependent on TH, and TR autoinduction is hypothesized to be essential to the metamorphic process. Similar phenomena may occur in developing mammalian brain, although this has received less attention.
Autoinduction
One of the better understood examples of NR autoinduction and its biological significance is the autoinduction of the beta isoform of the TH receptor (TR) that occurs during amphibian metamorphosis. The transformation of a tadpole into a frog is dependent on TH, and TH biosynthesis and secretion increases during metamorphosis reaching a peak at metamorphic climax (reviewed by Denver, 2009a). The frog X. laevis, like all jawed vertebrates studied to date, has two TH receptor subtypes, TRα and TRβ encoded by four genes (TRαA and B, TRβA and B) owing to its pseudotetraploidy (Yaoita et al., 1990). The mRNAs for TR subtypes are detected at the time of hatching, and expression of TRα increases shortly thereafter to reach peak levels in the tadpole that are sustained through metamorphosis (Shi, 2000). By contrast, after hatching TRβ mRNA expression is very low, but then rises in parallel with the elevation in plasma TH that occurs during metamorphosis, reaching a peak at metamorphic climax that coincides with maximal production of TH and a period of rapid tissue transformation (Shi, 2000). Autoinduction of TRβ is also seen in X. laevis tissue culture cells (i.e XTC-2 and XL177), which has allowed for detailed investigations of response kinetics and molecular mechanisms of gene regulation (Kanamori and Brown, 1992; Machuca and Tata, 1992).
The patterns of TRα and TRβ expression in rat brain parallel those seen in the tadpole during metamorphosis. For example, TRα expression is high and relatively constant throughout late fetal and neonatal periods; whereas, TRβ expression is low at birth and shows a dramatic increase during the first 2 weeks postnatally that is coincident with rising plasma TH concentrations (Strait et al., 1990). The human TRβ promoter has two functional TREs that mediate transactivation in transient transfection assays (discussed below), but to our knowledge, the occurrence and significance of TRβ autoinduction during mammalian development has not been investigated.
Autorepression
Examples of autorepression of TR genes are isoform specific and have been seen mostly in pituitary cells. Thyroid hormone treatment of a rat pituitary tumor cell line, GH1, decreased TR receptor number as measured by nuclear 3,5,3’-triiodothyronine (T3) binding capacity (Samuels et al., 1977). In rats, TRβ2 (a TRβ isoform generated by alternative splicing) is elevated in the hypothyroid state, which becomes normalized or decreased below basal in the transition to the euthyroid or hypothyroid state, respectively (Childs et al., 1991; Ercan-Fang et al., 1996; Hodin et al., 1990.) However, there were no changes in nuclear TR binding capacity when TRβ 2 mRNA was decreased, thus questioning the physiological relevance of the downregulation of the TRβ2 gene (Ercan-Fang et al., 1996; Hodin et al., 1990).
Molecular mechanisms of TR autoregulation
Autoinduction of TRβ in the tadpole can be explained by the presence of TH response elements (TREs) located in the proximal promoter region of the genes (Ranjan et al., 1994); Machuca et al., 1995). The proximal TRβA promoter of X. laevis contains three putative TREs, two of which are near optimal direct repeat +4 (DR+4) TREs and are located just upstream or proximal to the transcription start site (Machuca et al., 1995; Ranjan et al., 1994). Transfection assays and mutational analysis have shown that both DR+4 TREs support TH-dependent transactivation, and also transrepression in the absence of TH. TR-RXR heterodimers, but not TR or RXR homodimers bound the DR+4 regions in a ligand-dependent manner (Machuca et al., 1995; Ranjan et al., 1994; Ulisse et al., 1996). The human TRβ gene has two functional TREs located in the 5’ flanking region that mediate autoinduction in transient transfection assays using the rat pituitary tumor cell line GH3 (Sakurai et al., 1992; Suzuki et al., 1994).
Further support for the functionality of the proximal TRE in the X. laevis TRβA promoter was shown by studies using a promoter-reporter construct transfected into living tadpole tail muscle in vivo (Ulisse et al., 1996). Treatment with TH activated transcription of a TRβA promoter-reporter construct containing the proximal DR+4 TRE cotransfected with TRβ expression plasmids. This activity was abolished by cotransfection of a mutant TRβ capable of binding DNA but not hormone (Ulisse et al., 1996). Taken together, both in vitro and in vivo experiments support that the frog TRβA gene is a direct TR target. However, TH induction of TRβ was found to be partially sensitive to protein synthesis inhibition (Kanamori and Brown, 1992; Machuca and Tata, 1992), suggesting that the synthesis of proteins other than preexisting TRs may be required for full TRβ autoinduction.
Autoinduction of TRβ was originally thought to be the earliest TH response in premetamorphic tadpole tissues (Yaoita and Brown, 1990), but it was subsequently discovered, through the use of gene expression screens, that other genes respond to TH more rapidly than TRβ (Shi, 2000). One of the most rapidly responding TH-induced genes in tadpole tissues is the transcription factor Krüppel-like factor 9 (KLF9; also known as basic transcription element binding protein 1; BTEB1). In X. laevis tadpole tissues (brain, tail, intestine), KLF9 is the most rapidly responding TH-induced gene, and its expression parallels the increase in plasma TH that occurs during tadpole metamorphosis (Denver, 1997; Furlow and Kanamori, 2002; Gomez et al., 1996; Hoopfer et al., 2002; Shi, 2000; Wang and Brown, 1993). The TH responsiveness of the frog gene is attributed to a near perfect DR+4 TRE located ~6.5 kb upstream of the transcription start site (Furlow and Kanamori, 2002); TREs have also been described in mammalian KLF9 genes (Denver and Williamson, 2009); P. Bagamasbad and R.J. Denver, unpublished data).
As mentioned above, although TREs were identified in the frog TRβA gene, studies with the protein synthesis inhibitor cycloheximide suggested that the synthesis of other factors was necessary for maximal TRβ autoinduction. The discovery that KLF9 is a rapidly responding TH-induced gene, and that the TRβA promoter has several potential binding sites for KLF9 (Ranjan et al., 1994) led to the hypothesis that TRβA is a target gene for KLF9 (Bagamasbad et al., 2008). Analysis of the kinetics of expression of TRβA and KLF9 mRNAs showed that KLF9 is upregulated earlier than TRβA in cultured Xenopus fibroblast cells (XTC-2) and in the brain of premetamorphic tadpoles treated with TH (Bagamasbad et al., 2008). Electrophoretic mobility shift assays showed that KLF9 can bind to GC rich regions of the proximal TRβA promoter, and chromatin-immunoprecipitation (ChIP) assays found KLF9 associates with this region in vivo in a TH and developmental stage-dependent manner. Moreover, forced expression of KLF9 in XTC-2 cells or tadpole brain in vivo accelerated and enhanced TRβA autoinduction (Bagamasbad et al., 2008; F. Hu, P. Bagamasbad and R.J. Denver, unpublished). These findings support the hypothesis that the immediate early gene KLF9 functions as an accessory transcription factor necessary for TRβ autoinduction.
Physiological significance of TR autoinduction
The discovery that TRβ was autoinduced in tadpole tissues led to the hypothesis that the increase in TRβ is necessary for driving metamorphosis, whereas, the early expression of TRα was necessary to establish tissue competence to respond to the hormone (Tata, 2000). There are at least two potential mechanisms by which TRβ autoinduction could function to promote metamorphosis: 1) Receptor autoinduction could cause a general increase in tissue sensitivity to TH, thereby amplifying gene expression responses to TH, or 2) TRβ could regulate a set of genes/processes that are distinct from those regulated by TRα. There are several lines of evidence that support a role for TRα in establishing tissue competence to respond to TH, and in the proliferative actions of the hormone, which are the earliest responses to TH that occur in target tissues. Studies of the temporal and spatial expression of the different TR subtypes, and the use of TR subtype-specific TH analogs support a role for TRα in cell proliferation in the hindlimb and brain. By contrast to TRα, the expression pattern of TRβ is dependent on the rise in plasma TH during metamorphosis, and findings using the TRβ specific TH analogs GC1 and GC24 support the hypothesis that TRβ functions in cell differentiation and apoptosis (reviewed by Furlow and Neff, 2006; Denver et al., 2009)
Other lines of evidence support a role for TRβ autoinduction during metamorphosis. For example, treatment with prolactin, which blocked TH-induced tadpole limb bud growth and tail regression, decreased TRα and completely blocked TRβ autoinduction (Baker and Tata, 1992; Tata et al., 1991). These correlative data suggest that one mechanism by which prolactin blocks metamorphosis is through inhibition of TRβ autoinduction, and therefore that TRβ autoinduction is necessary for tissue transformations.
Obligate paedomorphic salamanders (they attain reproductive maturity while retaining larval characteristics) do not respond to TH by initiating metamorphosis. Safi et al. (1997) failed to find evidence for expression of TRβ mRNA in the mudpuppy Necturus maculosus using reverse transcriptase polymerase chain reaction, and suggested that this may be the reason that the animals do not respond to TH by metamorphosing. However, this species was found to have genes for both TR subtypes that are expressed in some tissues and code for functional proteins as evidenced by DNA binding, hormone binding, and transactivation of target genes in in vitro transfection assays (Safi et al., 2006). Contrary to the initial report, Safi et al. (2006) have since found that both TRs are expressed in different tissues at low levels in N. maculosus, but TRβ shows no, or only modest responses to high doses of TH in gill and tail fin, two structures that undergo apoptosis in metamorphosing salamanders but not in N. maculosus (Safi et al., 2006). However, TRβ may be induced in the brain of the mudpuppy where TH responses may have been retained to support neurological development (M. Miller and R.J. Denver, unpublished). Safi and colleagues suggested that loss of metamorphosis in the lineage of salamanders that includes N. maculosus may have depended on the loss of TH-dependent control of key genes required for tissue transformation. However, it is also possible that the failure to upregulate TRβ in organs that normally undergo metamorphic transformation in metamorphosing species (e.g., the gill, tail, etc.) is responsible for the paedomorphic life history.
III. Nuclear Receptor Cross-regulation
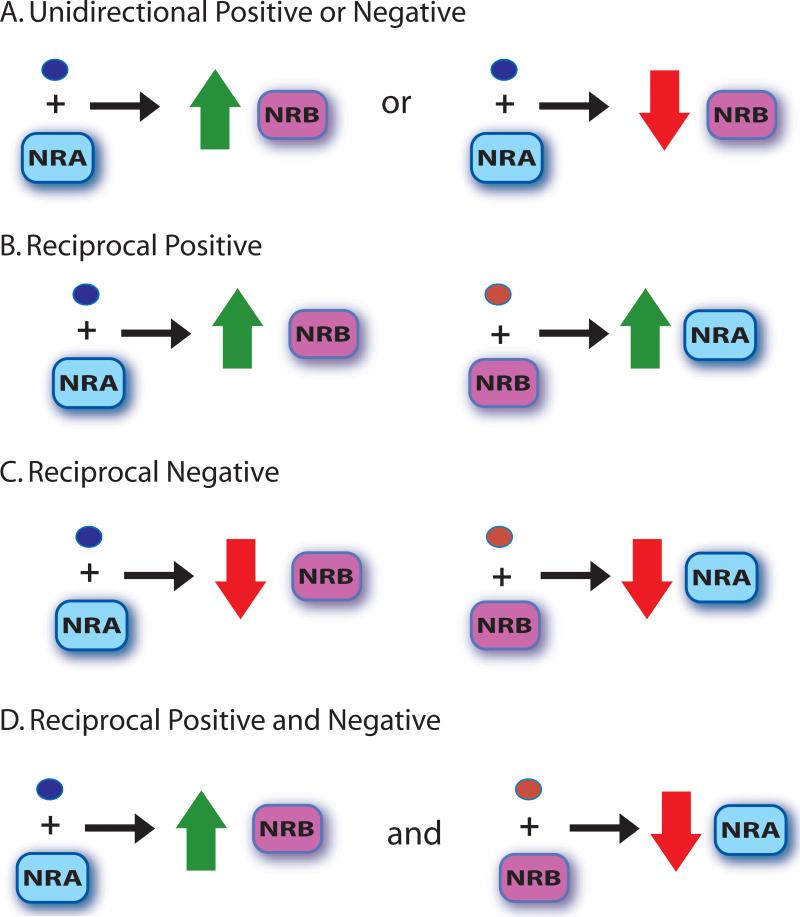
In addition to hormones autoregulating expression of their receptor, the regulation of expression of other NRs (cross-regulation) is an important means for modulating cellular responsiveness to different hormonal signals. Nuclear receptor cross-regulation is complex and can take different forms; e.g., the regulation can be undirectional positive or negative (Fig. 2A), reciprocal positive (Fig. 2B), reciprocal negative (Fig. 2C), or reciprocal positive and negative; Fig. 2D). Also, cross-regulation can operate at both transcriptional and posttranscriptional levels (see Fig. 1).
Figure 2.
Forms of nuclear receptor (NR) cross-regulation. Hormone-bound NRs can regulate the expression of other types of NRs, and NR cross-regulation can take several forms. A. Unidirectional cross regulation involves a NR (NRA) regulating the expression of a different NR (NRB) while NRB does not affect the expression of NRA. B. In reciprocal positive cross-regulation, the hormone-bound NR (NRA) positively regulates the expression of another type of NR (NRB) and at the same time, hormone-bound NRB upregulates the expression of NRA. C. Reciprocal negative cross-regulation occurs when a hormone-bound NR (NRA) downregulates expression of another NR (NRB), and hormone-bound NRB negatively regulates expression of NRA. D. In another form of cross-regulation, a hormone-bound NR (NRA) positive regulates the expression of another NR (NRB), while NRB downregulates expression of NRA.
Nuclear receptor cross-regulation is a means to coordinate, in a temporal manner, the responsiveness of a cell to a subsequent (different) hormonal signal, or to allow for crosstalk between hormonal signaling pathways. For example, cross-regulation between ER and PR is important for the morphological changes in the uterus, and the expression of female sexual behavior that occurs during the mammalian ovarian cycle. During amphibian metamorphosis cross-regulation between GR and TRs sensitizes tissues to the TH signal, thereby accelerating metamorphosis. These and other examples of NR cross-regulation are discussed below. This survey is not comprehensive, and new examples of NR cross-regulation continue to be discovered. Our goal is to provide a few examples of NR cross-regulation covering the different forms that it can take that are described above and depicted in Fig. 2.
A. Cross-regulation Between Estrogen and Progestin Receptors
One of the better studied forms of NR cross-regulation is the reciprocal positive and negative regulation between the ER and PR that occurs during mammalian ovarian cycles; liganded ER upregulates PR, but liganded PR downregulates ER expression. During the rodent estrous cycle circulating estrogen concentrations rise at the beginning of proestrus and reach peak levels during mid-proestrus prior to the LH surge (reviewed by McCarthy and Becker, 2002). Progesterone increases at late proestrus and peaks during the transition from proestrus to estrus. These cyclical hormonal changes cause increased cell proliferation and differentiation of uterine cells and initiate female sexual behavior.
Rising plasma estrogen concentration promotes proliferation of uterine epithelial cells (the proliferative phase; Quarmby and Korach, 1984). After ovulation, the uterine epithelium shifts from proliferation to secretion, mediated by the actions of progesterone. Progesterone blocks estrogen-dependent epithelial cell proliferation, promotes proliferation and differentiation of uterine stromal cells (decidualization) and if pregnancy occurs, it suppresses myometrial contractions (reviewed by Graham and Clarke, 1997). These opposing and compartment-specific effects of estrogen and progesterone can be explained by cross-regulation of their NRs.
Estrogen acts on the uterus causing ER autoinduction (discussed earlier) and increased PR mRNA and protein (Evans and Leavitt, 1980; Kamiya et al., 1996; Kraus and Katzenellenbogen, 1993; Tibbetts et al., 1998; Yamashita et al., 1990). The molecular basis for the estrogen-dependent upregulation of PR is attributed to functional estrogen responsive elements (ERE) in the promoter region of the PR gene (Kraus et al., 1993; Kraus et al., 1994). Support for positive cross-regulation of PR by ER in vivo comes from studies of ERα knockout mice where PR was expressed in the uterus, but could not be induced by estrogen (Couse et al., 1995).
The upregulation of PR by liganded ER is important for sensitizing uterine cells to the actions of progesterone, the plasma concentration of which peaks shortly after the estrogen surge during the ovarian cycle. On the other hand, progesterone blocks the estrogen-mediated increase in ER and PR (Evans and Leavitt, 1980; Kraus and Katzenellenbogen, 1993), and the progesterone-mediated block of ER autoinduction is accompanied by inhibition of epithelial cell proliferation at the beginning of the secretory phase. The elevated plasma concentration of estrogen increased PR expression, and uterine stromal cells are thus sensitized to the rising plasma concentration of progesterone that promotes decidualization. Thus, cross-regulation of ER and PR expression is central to the morphological and physiological changes that occur in uterine cells during the mammalian ovarian cycle (Graham and Clarke, 1997).
Estrogen and progesterone produced during the ovarian cycle act on the brain to promote female sexual behavior (lordosis; McCarthy and Becker, 2002). Progesterone acting through the PR is necessary for the expression of lordosis. Exposure to estrogen (priming) appears necessary for the subsequent action of progesterone to ‘release’ the behavior. Studies in rats and guinea pigs showed that estrogen induces PR expression in the hypothalamus and preoptic area (reviewed by McCarthy and Becker, 2002). In ovariectomized guinea pigs, only animals primed with estrogen responded to a subsequent injection of progesterone by exhibiting lordosis behavior, supporting an important role for the estrogen-dependent increase in PR for inducing sexual receptivity during the estrous cycle (Blaustein and Feder, 1979; Blaustein and Turcotte, 1989; DonCarlos et al., 1989; Parsons et al., 1981).
B. Cross-regulation Between Sex Steroid and Glucocorticoid Receptors
Reciprocal regulation occurs between hormones of the reproductive and stress axes. For example, glucocorticoids act at several levels of the hypothalamo-pituitary-gonadal (HPG) axis to inhibit sex steroid biosynthesis and secretion. Sex steroids are also known to influence HPA axis function, and there are sex differences in the regulation of the HPA axis; e.g., testosterone inhibits, while estrogen enhances HPA activity (Handa et al., 1994; McEwen, 1994; Viau, 2002). However, despite the large amount of information on the interaction between the stress and gonad axes, few studies have investigated cross-regulation among NRs that mediate the actions of sex steroids and corticosteroids.
Evidence for cross-regulation between sex steroid receptors (AR and ER) and the GR shows that the regulation is mostly unidirectional and negative, with the sex steroids downregulating GR expression. For example, injections of testosterone or estrogen in rats decreased GR mRNA levels in the hippocampus (Burgess and Handa, 1993; Kerr et al., 1996). A similar phenomenon was observed in the rat lung where antenatal testosterone treatment decreased GR mRNA and protein levels, consistent with the inhibitory effect of androgens on fetal lung development (fetal lung development is dependent on glucocorticoids; Sweezey et al., 1998).
Glucocorticoids and estrogens have been shown to have opposing actions in some tissues commonly targeted by both hormones. Estrogen, together with progesterone, is critical for normal mammary gland development where it promotes cell proliferation (reviewed by Sutherland et al., 1998). In breast cancer cell lines, glucocorticoids prevent estrogen-mediated cell proliferation and the increase in PR expression (Zhou et al., 1989). Antagonistic actions between glucocorticoids and estrogen are also observed in bone, where estrogens reduce bone loss while glucocorticoids promote it (Gallagher et al., 2001; Rackoff and Rosen, 1998). The opposing effects of these two hormones may be explained by the cross-regulation of their NRs. For example, in a breast cancer cell line, estrogen repressed GR-mediated transcription from the synthetic MMTV promoter that contains a glucocorticoid response element, and from GR target genes. This repression was due, at least in part, to estrogen inducing the expression of Mdm2, an E3 ubiquitin ligase, that targets GR for proteasomal degradation (Kinyamu and Archer, 2003).
By contrast to the negative cross-regulation between ER and GR in mammary cells, glucorticoids potentiated ER autoinduction, and the expression of the ER target gene vitellogenin in cultured X. laevis hepatocytes (Ulisse and Tata, 1994) suggesting that the interplay between the ER and GR may be tissue specific (or perhaps species specific). More study is needed to understand interactions between the stress and reproductive axes occurring through cross-regulation among the NRs that mediate sex steroid and corticosteroid actions.
C. Cross-regulation Between Estrogen and Thyroid Hormone Receptors
Thyroid hormone regulates diverse developmental and physiological processes such as brain development, growth, energy metabolism and reproduction. Estrogen plays a critical role in reproductive development, physiology and sexual behavior. These two endocrine systems interact to influence aspects of reproduction and sexual behavior. For example, TH and estrogen cooperate to regulate the synthesis of yolk proteins by the liver in frogs. Thyroid hormone potentiated ER autoinduction in primary cultures of frog (X. laevis) hepatocytes, and in the liver in vivo (Rabelo and Tata, 1993; Ulisse and Tata, 1994). Thyroid hormone treatment also accelerated the upregulation of the estrogen target gene vitellogenin. This ability of TH to enhance ER autoinduction in the liver is developmental stage-dependent, appearing at metamorphic climax (May and Knowland, 1980; Rabelo et al., 1994). Thyroid hormone induction of ER gene expression has also been reported in rat liver, although whether it potentiates ER autoinduction was not investigated (Freyschuss et al., 1994).
Cross-regulation between TH and ER has also been observed in the tadpole brain (Rana pipiens (Hogan et al., 2007), and TH increased ERα mRNA in GH3 cells, thereby potentiating the actions of estrogen (Fujimoto et al., 2004; Fujimoto et al., 1997). To our knowledge, TREs have not yet been demonstrated in ER genes of any species, and the molecular basis for the cross-regulation of ER expression by TR is not understood.
Thyroid hormone and estrogen play central roles in the control of sexual behavior. Interplay between TH and estrogen actions on the brain may provide a mechanism for assessing metabolic state, thus affecting reproductive physiology and behavior. For example, in birds and mammals TH promotes the transition to anestrus. In some mammals, exposure to cold temperatures increases plasma TH levels, suggesting that the cross-regulation between TH and estrogen is involved in mediating environmental effects on reproductive behavior (reviewed by Vasudevan et al., 2002). In rodents, TH decreases estrogen–dependent lordosis behavior. For example, in ovariectomized (OVX) rats and mice, estrogen replacement plus TH treatment decreased lordosis behavior compared to estrogen replacement alone (Dellovade et al., 1996; Morgan et al., 2000). Similarly, thyroid-intact, OVX female rats that received estrogen replacement showed delayed onset of lordosis behavior compared to animals that were thyroidectomized, OVX+estrogen (Dellovade et al., 1996). In addition, TRβ knockout mice that were OVX+estrogen showed increased lordosis behavior as compared to wild type OVX+estrogen mice; whereas, the same experimental paradigm comparing TRα knockout mice showed decreased sexual receptivity compared to wild type (Dellovade et al., 2000), suggesting that the actions of TH on sexual behavior are complex and TR subtype-specific.
The findings described above suggested the hypothesis that TH inhibits lordosis behavior by decreasing plasma estrogen levels, or by decreasing ER expression in the hypothalamus. However, TH administration did not affect plasma estrogen levels (Dellovade et al., 1996; Morgan et al., 2000), and instead increased ER immunoreactivity in the hypothalamus (Dellovade et al., 1996), which suggested that TH effects on lordosis may not be mediated through effects on ER signaling. Subsequent studies investigated possible effects of TH on the expression of estrogen target genes in the brain that are known to be involved in sexual behavior (Dellovade et al., 2000; Scott et al., 1997; Vasudevan et al., 2001a; Vasudevan et al., 2001c; Zhu et al., 2001; Zhu et al., 1996). For example, expression of the oxytocin gene, which is a known ER target, was downregulated by TH, and this action was TR subtype-specific (Dellovade et al., 2000; Vasudevan et al., 2001b). Estrogen-dependent induction of the preproenkephalin (PPE) gene was also attenuated by TH (Vasudevan et al., 2001c; Zhu et al., 2001; Zhu et al., 1996). Molecular analysis of the cross-regulation between TH and estrogen in the regulation of the PPE gene found that TR and ER both associate with the same segment of DNA located in the PPE promoter (a predicted ERE; Vasudevan et al., 2001c; Zhu et al., 2001). Since both ER and TR have identical consensus hormone response element half sites (Glass et al., 1988; Truss and Beato, 1993) and TR can bind to the EREs of ER target genes, TR may decrease lordosis behavior by inhibiting ER transactivation through competition with ER for DNA binding, or perhaps through protein-protein interactions (Vasudevan et al., 2001a; Zhu et al., 2001). Another proposed mechanism is competition between TR and ER for coactivators such as SRC-1 (Vasudevan et al., 2001a; Vasudevan et al., 2001b).
D. Crossregulation Between Thyroid Hormone and Glucocorticoid Receptors
The thyroid and stress endocrine axes interact at multiple levels to influence development, physiology and behavior. Here we focus on the cross-regulation of NRs for TH and corticosteroids. In amphibians and other nonmammalian species, interactions between the thyroid and stress endocrine axes have been demonstrated at both central and peripheral levels. Hypothalamic CRF regulates both the stress and thyroid axes by stimulating secretion of ACTH and TSH (De Groef et al., 2006). In amphibians, this dual role for CRF allows tadpoles to modify their rate of metamorphosis in response to a changing environment (Denver, 1997; Denver, 2009b). At the periphery, corticosteroids synergize with TH to accelerate metamorphosis, and it is at this level that NR cross-regulation has been observed (Denver, 2009b).
In tadpole tail, corticosteroid treatment accelerated TH-induced tail shrinkage, a measure of metamorphic progression (reviewed by Denver, 2009b). Treatment of tadpole tail explants with corticosteroids increased nuclear binding capacity for TH (Kikuyama et al., 1983; Suzuki and Kikuyama, 1983) and TR mRNAs; similar findings on corticosteroid actions on TR mRNA expression were observed in tadpole tail, intestine and brain in vivo (Krain and Denver, 2004; R. Bonett, E.D. Hoopfer and R.J. Denver, unpublished data). These findings suggest that corticosteroids can enhance sensitivity of target tissues to TH by increasing the expression of TRs, thus accelerating metamorphosis. Corticosteroids have also been shown to increase expression of type 2 deiodinase in tadpole tissues, which catalyzes the conversion of thyroxine (T4) to the more active form T3 (Galton, 1990; R. Bonett, E.D. Hoopfer and R.J. Denver, unpublished data). This would increase bioavailability of the ligand for TR, and thus facilitate TH action on target tissues.
There is also evidence that TH can regulate GR mRNA in some tadpole tissues. Treatment of tadpoles with T3 downregulated GR mRNA in brain and intestine, but upregulated it in tail (Krain and Denver, 2004). The synergistic actions of TH and corticosteroids on tadpole tail shrinkage may be explained, in part, by the reciprocal positive cross-regulation of TR and GR, which would sensitize the tissue to further hormone action.
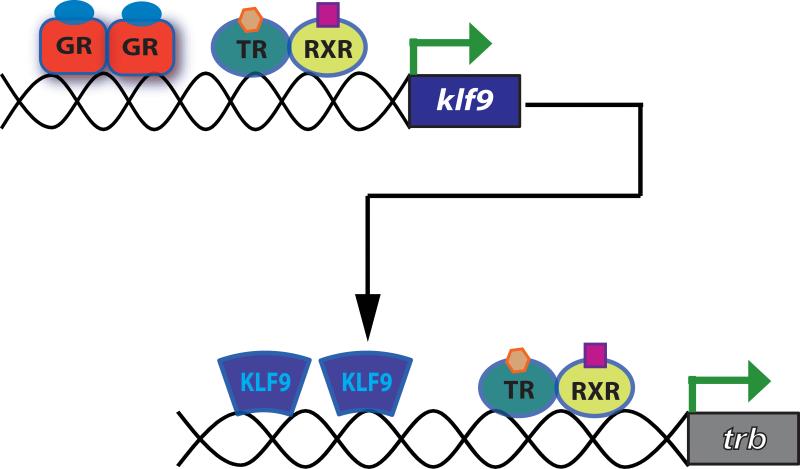
The molecular basis for GR (or MR) regulation of TR gene expression has not been investigated. Therefore, it is not known whether this regulation is direct on TR gene transcription, or indirect through induction of other transcription factors, or through effects on mRNA transcript or protein stabilization. We recently found that KLF9, which we earlier showed could associate with the frog TRβA promoter and enhance autoinduction, is a direct GR target gene in frog and mouse brain (Bonett et al., 2009; P. Bagamasbad. T. Ziera, S.A. Borden and R.J. Denver, unpublished data). Thus, one mechanism by which corticosteroids may increase TRβ gene expression is through induction of KLF9 (see Fig. 3). In this regard, it is noteworthy that combined treatment with TH and corticosterone synergistically activated KLF9 transcription in frog and mouse brain (R. Bonett, P. Bagamasbad and R.J. Denver, unpublished).
Figure 3.
Role of the thyroid hormone (TH)-induced immediate early transcription factor Krüppel-like factor 9 (KLF9) in TH receptor (TR) autoinduction. Ligand-bound TR-retinoid X receptor (RXR) heterodimers bind to and induce expression of klf9 and trb genes. The upregulation of KLF9 by TH precedes TRβ. KLF9 associates with the promoter region of TRβ and enhances TRβ autoinduction (Bagamadbad et al., 2008). Aside from being a TH direct target gene, klf9 is also a direct glucocorticoid receptor (GR) target gene that is induced by stress (Bonett et al., 2009; P. Bagamasbad, T. Ziera, S.A. Borden and R.J. Denver, unpublished data). Thyroid hormone and glucocorticoids synergistically activate KLF9 expression, thereby further enhancing TRβ autoinduction.
Conclusions
Nuclear receptor auto- and cross-regulation are important mechanisms for amplifying hormone signals, regulating hormone activity through negative feedback, and coordinating hormone action in a temporal and tissue-specific manner. In this review we have highlighted some of the better known examples of auto- and cross-regulation of classical nuclear hormone receptors, but there are other examples not discussed here, and perhaps many more to be discovered. Also, other classes of NR genes may be similarly auto- or cross-regulated.
Autoinduction of NR genes appears to have arisen very early in animal evolution and may have been maintained by natural selection because of a pivotal role in controlling development. This feed-forward mechanism may be essential for developmental progression from larva or fetus to the juvenile adult animal. While this conclusion is supported by correlational data and some functional studies, direct tests are still needed to understand the importance of NR autoinduction to animal development and physiology.
Auto- and cross-regulation can occur through mechanisms that involve direct DNA binding of NRs to the homologous NR gene, or through indirect transcriptional or posttranscriptional mechanisms. While some of these mechanisms have been elucidated for some NRs, much remains to be learned about the physiological and molecular mechanisms that regulate NR gene expression. Elucidating these mechanisms is essential to understand the diverse roles that NRs play in animal development, and in adult physiology and behavior.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Alexander IE, Clarke CL, Shine J, Sutherland RL. Progestin inhibition of progesterone receptor gene expression in human breast cancer cells. Mol. Endocrinol. 1989;3:1377–1386. doi: 10.1210/mend-3-9-1377. [DOI] [PubMed] [Google Scholar]
- Aranda A, Pascual A. Nuclear hormone receptors and gene expression. Physiol. Rev. 2001;81:1269–1304. doi: 10.1152/physrev.2001.81.3.1269. [DOI] [PubMed] [Google Scholar]
- Arbour NC, Prahl JM, Deluca HF. Stabilization of the vitamin-D receptor in rat osteosarcoma cells through the action of 1,25-dihydroxyvitamin-D(3). Mol. Endocrinol. 1993;7:1307–1312. doi: 10.1210/mend.7.10.8264662. [DOI] [PubMed] [Google Scholar]
- Bagamasbad P, Howdeshell KL, Sachs LM, Demeneix BA, Denver RJ. A role for basic transcription element-binding protein 1 (BTEB1) in the autoinduction of thyroid hormone receptor beta. J. Biol. Chem. 2008;283:2275–2285. doi: 10.1074/jbc.M709306200. [DOI] [PubMed] [Google Scholar]
- Baker BS, Tata JR. Prolactin prevents the autoinduction of thyroid hormone receptor mRNAs during amphibian metamorphosis. Dev. Biol. 1992;149:463–467. doi: 10.1016/0012-1606(92)90301-v. [DOI] [PubMed] [Google Scholar]
- Barton MC, Shapiro DJ. Transient administration of estradiol-17 beta establishes an autoregulatory loop permanently inducing estrogen receptor messenger RNA. Proc. Natl. Acad. Sci. USA. 1988;85:7119–7123. doi: 10.1073/pnas.85.19.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrook D, Lernhardt E, Pfahl M. A new retinoic acid receptor identified from a hepatocellular carcinoma. Nature. 1988;333:669–672. doi: 10.1038/333669a0. [DOI] [PubMed] [Google Scholar]
- Bergman MD, Schachter BS, Karelus K, Combatsiaris EP, Garcia T, Nelson JF. Up-regulation of the uterine estrogen receptor and its messenger ribonucleic acid during the mouse estrous cycle - the role of estradiol. Endocrinology. 1992;130:1923–1930. doi: 10.1210/endo.130.4.1547720. [DOI] [PubMed] [Google Scholar]
- Berkenstam A, Glaumann H, Martin M, Gustafsson JA, Norstedt G. Hormonal regulation of estrogen receptor messenger ribonucleic acid in T47Dco and MCF-7 breast cancer cells. Mol. Endocrinol. 1989;3:22–28. doi: 10.1210/mend-3-1-22. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Feder HH. Cytoplasmic progestin-receptors in guinea pig brain: characteristics and relationship to the induction of sexual behavior. Brain Res. 1979;169:481–497. doi: 10.1016/0006-8993(79)90398-6. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Turcotte JC. Estradiol-induced progestin receptor immunoreactivity is found only in estrogen receptor-immunoreactive cells in guinea pig brain. Neuroendocrinology. 1989;49:454–461. doi: 10.1159/000125152. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Turcotte JC. Down-regulation of progestin receptors in guinea pig brain: new findings using an immunocytochemical technique. J. Neurobiol. 1990;21:675–685. doi: 10.1002/neu.480210502. [DOI] [PubMed] [Google Scholar]
- Blok LJ, Themmen AP, Peters AH, Trapman J, Baarends WM, Hoogerbrugge JW, Grootegoed JA. Transcriptional regulation of androgen receptor gene expression in Sertoli cells and other cell types. Mol. Cell. Endocrinol. 1992;88:153–164. doi: 10.1016/0303-7207(92)90020-7. [DOI] [PubMed] [Google Scholar]
- Bonett RM, Hu F, Bagamasbad P, Denver RJ. Stressor and glucocorticoid-dependent induction of the immediate early gene Krüppel-like factor 9: implications for neural development and plasticity. Endocrinology. 2009;150:1757–1765. doi: 10.1210/en.2008-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman CJ, Kroll KJ, Gross TG, Denslow ND. Estradiol-induced gene expression in largemouth bass (Micropterus salmoides). Mol. Cell. Endocrinol. 2002;196:67–77. doi: 10.1016/s0303-7207(02)00224-1. [DOI] [PubMed] [Google Scholar]
- Brand N, Petkovich M, Krust A, Chambon P, de The H, Marchio A, Tiollais P, Dejean A. Identification of a second human retinoic acid receptor. Nature. 1988;332:850–853. doi: 10.1038/332850a0. [DOI] [PubMed] [Google Scholar]
- Brown-Grant K, Fink G, Greig F, Murray MA. Altered sexual development in male rats after oestrogen administration during the neonatal period. J. Reprod. Fertil. 1975;44:25–42. doi: 10.1530/jrf.0.0440025. [DOI] [PubMed] [Google Scholar]
- Burgess LH, Handa RJ. Estrogen-induced alterations in the regulation of mineralocorticoid and glucocorticoid receptor messenger RNA expression in the female rat anterior pituitary gland and brain. Mol. Cell. Neurosci. 1993;4:191–198. doi: 10.1006/mcne.1993.1023. [DOI] [PubMed] [Google Scholar]
- Burnstein KL. Regulation of androgen receptor levels: Implications for prostate cancer progression and therapy. J. Cell. Biochem. 2005;95:657–669. doi: 10.1002/jcb.20460. [DOI] [PubMed] [Google Scholar]
- Burnstein KL, Bellingham DL, Jewell CM, Powelloliver FE, Cidlowski JA. Autoregulation of glucocorticoid receptor gene expression. Steroids. 1991;56:52–58. doi: 10.1016/0039-128x(91)90124-e. [DOI] [PubMed] [Google Scholar]
- Burnstein KL, Jewell CM, Cidlowski JA. Human glucocorticoid receptor cDNA contains sequences sufficient for receptor down-regulation. J. Biol. Chem. 1990;265:7284–7291. [PubMed] [Google Scholar]
- Cardone A, Angelini F, Varriale B. Autoregulation of estrogen and androgen receptor mRNAs and downregulation of androgen receptor mRNA by estrogen in primary cultures of lizard testis cells. Gen. Comp. Endocrinol. 1998;110:227–236. doi: 10.1006/gcen.1998.7063. [DOI] [PubMed] [Google Scholar]
- Chao HM, Choo PH, McEwen BS. Glucocorticoid and mineralocorticoid receptor mRNA expression in rat brain. Neuroendocrinology. 1989;50:365–371. doi: 10.1159/000125250. [DOI] [PubMed] [Google Scholar]
- Chao HM, Ma LY, McEwen BS, Sakai RR. Regulation of glucocorticoid receptor and mineralocorticoid receptor messenger ribonucleic acids by selective agonists in the rat hippocampus. Endocrinology. 1998;139:1810–1814. doi: 10.1210/endo.139.4.5896. [DOI] [PubMed] [Google Scholar]
- Childs GV, Taub K, Jones KE, Chin WW. Triiodothyronine receptor beta-2 messenger ribonucleic acid expression by somatotropes and thyrotropes: effect of propylthiouracil-induced hypothyroidism in rats. Endocrinology. 1991;129:2767–2773. doi: 10.1210/endo-129-5-2767. [DOI] [PubMed] [Google Scholar]
- Claire M, Oblin ME, Steimer JL, Nakane H, Misumi J, Michaud A, Corvol P. Effect of adrenalectomy and aldosterone on the modulation of mineralocorticoid receptors in rat kidney. J. Biol. Chem. 1981;256:142–147. [PubMed] [Google Scholar]
- Clemens M. The regulation of egg yolk synthesis by steroid hormones. Progress in Biophys. Mol. Biol. 1974;28:69–74. doi: 10.1016/0079-6107(74)90017-0. [DOI] [PubMed] [Google Scholar]
- Conway-Campbell BL, McKenna MA, Wiles CC, Atkinson HC, de Kloet ER, Lightman SL. Proteasome-dependent down-regulation of activated nuclear hippocampal glucocorticoid receptors determines dynamic responses to corticosterone. Endocrinology. 2007;148:5470–5477. doi: 10.1210/en.2007-0585. [DOI] [PubMed] [Google Scholar]
- Couse JF, Curtis SW, Washburn TF, Lindzey J, Golding TS, Lubahn DB, Smithies O, Korach KS. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol. Endocrinol. 1995;9:1441–1454. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- Dai JL, Burnstein KL. Two androgen response elements in the androgen receptor coding region are required for cell-specific up-regulation of receptor messenger RNA. Mol. Endocrinol. 1996;10:1582–1594. doi: 10.1210/mend.10.12.8961268. [DOI] [PubMed] [Google Scholar]
- Davoodi F, Brenner RV, Evans SRT, Schumaker LM, Shabahang M, Nauta RJ, Buras RR. Modulation of vitamin D receptor and estrogen receptor by 1,25(OH2)-vitamin D3 in T47D human breast cancer cells. J. Steroid Biochem. Mol. Biol. 1995;54:147–153. doi: 10.1016/0960-0760(95)00128-m. [DOI] [PubMed] [Google Scholar]
- De Groef B, Van der Geyten S, Darras VM, Kuhn ER. Role of corticotropin-releasing hormone as a thyrotropin-releasing factor in non-mammalian vertebrates. Gen. Comp. Endocrinol. 2006;146:62–68. doi: 10.1016/j.ygcen.2005.10.014. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- de The H, Marchio A, Tiollais P, Dejean A. Differential expression and ligand regulation of the retinoic acid receptor alpha and beta genes. EMBO J. 1989;8:429–433. doi: 10.1002/j.1460-2075.1989.tb03394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de The H, Vivanco-Ruiz MM, Tiollais P, Stunnenberg H, Dejean A. Identification of a retinoic acid responsive element in the retinoic acid receptor beta gene. Nature. 1990;343:177–180. doi: 10.1038/343177a0. [DOI] [PubMed] [Google Scholar]
- Dellovade TL, Chan J, Vennstrom B, Forrest D, Pfaff DW. The two thyroid hormone receptor genes have opposite effects on estrogen-stimulated sex behaviors. Nat. Neurosci. 2000;3:472–475. doi: 10.1038/74846. [DOI] [PubMed] [Google Scholar]
- Dellovade TL, Zhu YS, Krey L, Pfaff DW. Thyroid hormone and estrogen interact to regulate behavior. Proc. Natl. Acad. Sci. U S A. 1996;93:12581–12586. doi: 10.1073/pnas.93.22.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton RR, Eisen LP, Elsasser MS, Harmon JM. Differential autoregulation of glucocorticoid receptor expression in human T- and B-cell lines. Endocrinology. 1993;133:248–256. doi: 10.1210/endo.133.1.8319574. [DOI] [PubMed] [Google Scholar]
- Denver RJ. Environmental stress as a developmental cue: corticotropin-releasing hormone is a proximate mediator of adaptive phenotypic plasticity in amphibian metamorphosis. Horm. Behav. 1997;31:169–179. doi: 10.1006/hbeh.1997.1383. [DOI] [PubMed] [Google Scholar]
- Denver RJ. Endocrinology of complex life cycles: Amphibians. In: Pfaff DW, et al., editors. Hormones, Brain and Behavior. Elsevier; San Diego: 2009a. [Google Scholar]
- Denver RJ. Stress hormones mediate environment-genotype interactions during amphibian development. Gen. Comp. Endocrinol. 2009b;164:20–31. doi: 10.1016/j.ygcen.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Denver RJ, Hu F, Scanlan TS, Furlow JD. Thyroid hormone receptor subtype specificity for hormone-dependent neurogenesis in Xenopus laevis. Dev. Biol. 2009;326:155–168. doi: 10.1016/j.ydbio.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Denver RJ, Williamson KE. Identification of a thyroid hormone response element in the mouse Krüppel-like factor 9 gene to explain its postnatal expression in the brain. Endocrinology. 2009;150:3935–3943. doi: 10.1210/en.2009-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroo BJ, Rentsch C, Sampath S, Young J, DeFranco DB, Archer TK. Proteasomal inhibition enhances glucocorticoid receptor transactivation and alters its subnuclear trafficking. Mol. Cell. Biol. 2002;22:4113–4123. doi: 10.1128/MCB.22.12.4113-4123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez P, Antolin I, Boga JA, Uria H, Menendez-Pelaez A. Androgen regulation of gene expression in the Syrian hamster Harderian gland. Mol. Cell. Endocrinol. 1994;106:81–89. doi: 10.1016/0303-7207(94)90189-9. [DOI] [PubMed] [Google Scholar]
- DonCarlos LL, Greene GL, Morrell JI. Estrogen plus progesterone increases progestin receptor immunoreactivity in the brain of ovariectomized guinea pigs. Neuroendocrinology. 1989;50:613–623. doi: 10.1159/000125290. [DOI] [PubMed] [Google Scholar]
- Dong Y, Poellinger L, Gustafsson JA, Okret S. Regulation of glucocorticoid receptor expression: evidence for transcriptional and posttranslational mechanisms. Mol. Endocrinol. 1988;2:1256–1264. doi: 10.1210/mend-2-12-1256. [DOI] [PubMed] [Google Scholar]
- Drengler SM, Handa RJ, Jones KJ. Regulation of androgen receptor mRNA expression in hamster facial motoneurons: differential effects of non-aromatizable and aromatizable androgens. Brain Res. Mol. Brain Res. 1996;41:8–15. doi: 10.1016/0169-328x(96)00060-5. [DOI] [PubMed] [Google Scholar]
- Eisen LP, Elsasser MS, Harmon JM. Positive regulation of the glucocorticoid receptor in human T-cells sensitive to the cytolytic effects of glucocorticoids. J. Biol. Chem. 1988;263:12044–12048. [PubMed] [Google Scholar]
- Ercan-Fang S, Schwartz HL, Oppenheimer JH. Isoform-specific 3,5,3'-triiodothyronine receptor binding capacity and messenger ribonucleic acid content in rat adenohypophysis: effect of thyroidal state and comparison with extrapituitary tissues. Endocrinology. 1996;137:3228–3233. doi: 10.1210/endo.137.8.8754744. [DOI] [PubMed] [Google Scholar]
- Escriva H, Bertrand S, Laudet V. The evolution of the nuclear receptor superfamily. Essays in Biochemistry: Nuclear Receptor Superfamily. 2004;40:11–26. doi: 10.1042/bse0400011. [DOI] [PubMed] [Google Scholar]
- Evans RW, Leavitt WW. Progesterone inhibition of uterine nuclear estrogen receptor: dependence on RNA and protein synthesis. Proc. Natl. Acad. Sci. U S A. 1980;77:5856–5860. doi: 10.1073/pnas.77.10.5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flouriot G, Pakdel F, Valotaire Y. Transcriptional and post-transcriptional regulation of rainbow trout estrogen receptor and vitellogenin gene expression. Mol. Cell. Endocrinol. 1996;124:173–183. doi: 10.1016/s0303-7207(96)03960-3. [DOI] [PubMed] [Google Scholar]
- Freyschuss B, Sahlin L, Masironi B, Eriksson H. The hormonal regulation of the oestrogen receptor in rat liver: an interplay involving growth hormone, thyroid hormones and glucocorticoids. J. Endocrinol. 1994;142:285–298. doi: 10.1677/joe.0.1420285. [DOI] [PubMed] [Google Scholar]
- Friend KE, Ang LW, Shupnik MA. Estrogen regulates the expression of several different estrogen receptor messenger RNA isoforms in rat pituitary. Proc. Natl. Acad. Sci. U S A. 1995;92:4367–4371. doi: 10.1073/pnas.92.10.4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa T, Soya H, Fukuoka H, Alam KS, Yoshizato H, McEwen BS, Nakashima K. A biphasic regulation of receptor mRNA expressions for growth hormone, glucocorticoid and mineralocorticoid in the rat dentate gyrus during acute stress. Brain Res. 2000;874:186–193. doi: 10.1016/s0006-8993(00)02576-2. [DOI] [PubMed] [Google Scholar]
- Fujimoto N, Jinno N, Kitamura S. Activation of estrogen response element dependent transcription by thyroid hormone with increase in estrogen receptor levels in a rat pituitary cell line, GH3. J. Endocrinol. 2004;181:77–83. doi: 10.1677/joe.0.1810077. [DOI] [PubMed] [Google Scholar]
- Fujimoto N, Watanabe H, Ito A. Up-regulation of the estrogen receptor by triiodothyronine in rat pituitary cell lines. J. Steroid Biochem. Mol. Biol. 1997;61:79–85. doi: 10.1016/s0960-0760(97)00009-5. [DOI] [PubMed] [Google Scholar]
- Furlow JD, Kanamori A. The transcription factor basic transcription element-binding protein 1 is a direct thyroid hormone response gene in the frog Xenopus laevis. Endocrinology. 2002;143:3295–3305. doi: 10.1210/en.2002-220126. [DOI] [PubMed] [Google Scholar]
- Furlow JD, Neff ES. A developmental switch induced by thyroid hormone: Xenopus laevis metamorphosis. Trends Endocrinol. Metab. 2006;17:38–45. doi: 10.1016/j.tem.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Furuya F, Lu CX, Guigon CJ, Cheng SY. Nongenomic activation of phosphatidylinositol 3-kinase signaling by thyroid hormone receptors. Steroids. 2009;74:628–634. doi: 10.1016/j.steroids.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher JC, Fowler SE, Detter JR, Sherman SS. Combination treatment with estrogen and calcitriol in the prevention of age-related bone loss. J. Clin. Endocrinol Metab. 2001;86:3618–3628. doi: 10.1210/jcem.86.8.7703. [DOI] [PubMed] [Google Scholar]
- Galton VA. Mechanisms underlying the acceleration of thyroid hormone-induced tadpole metamorphosis by corticosterone. Endocrinology. 1990;127:2997–3002. doi: 10.1210/endo-127-6-2997. [DOI] [PubMed] [Google Scholar]
- Germain P, Staels B, Dacquet C, Spedding M, Laudet V. Overview of nomenclature of nuclear receptors. Pharmacol. Rev. 2006;58:685–704. doi: 10.1124/pr.58.4.2. [DOI] [PubMed] [Google Scholar]
- Ghosh B, Wood CR, Held GA, Abbott BD, Lau C. Glucocorticoid receptor regulation in the rat embryo: a potential site for developmental toxicity? Toxicol. Appl. Pharmacol. 2000;164:221–229. doi: 10.1006/taap.2000.8904. [DOI] [PubMed] [Google Scholar]
- Giguere V, Ong ES, Segui P, Evans RM. Identification of a receptor for the morphogen retinoic acid. Nature. 1987;330:624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- Glass CK, Holloway JM, Devary OV, Rosenfeld MG. The thyroid hormone receptor binds with opposite transcriptional effects to a common sequence motif in thyroid hormone and estrogen response elements. Cell. 1988;54:313–323. doi: 10.1016/0092-8674(88)90194-8. [DOI] [PubMed] [Google Scholar]
- Gomez F, Lahmame A, deKloet ER, Armario A. Hypothalamic-pituitary-adrenal response to chronic stress in five inbred rat strains: Differential responses are mainly located at the adrenocortical level. Neuroendocrinology. 1996;63:327–337. doi: 10.1159/000126973. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Cadavid N, Vernet D, Fuentes Navarro A, Rodriguez JA, Swerdloff RS, Rajfer J. Up-regulation of the levels of androgen receptor and its mRNA by androgens in smooth-muscle cells from rat penis. Mol. Cell. Endocrinol. 1993;90:219–229. doi: 10.1016/0303-7207(93)90155-d. [DOI] [PubMed] [Google Scholar]
- Grad JM, Dai JL, Wu S, Burnstein KL. Multiple androgen response elements and a Myc consensus site in the androgen receptor (AR) coding region are involved in androgen-mediated up-regulation of AR messenger RNA. Mol. Endocrinol. 1999;13:1896–1911. doi: 10.1210/mend.13.11.0369. [DOI] [PubMed] [Google Scholar]
- Graham JD, Clarke CL. Physiological action of progesterone in target tissues. Endocr. Rev. 1997;18:502–519. doi: 10.1210/edrv.18.4.0308. [DOI] [PubMed] [Google Scholar]
- Hackenberg R, Hawighorst T, Filmer A, Slater EP, Bock K, Beato M, Schulz KD. Regulation of androgen receptor messenger RNA and protein level by steroid hormones in human mammary cancer cells. J. Steroid Biochem. Mol. Biol. 1992;43:599–607. doi: 10.1016/0960-0760(92)90284-p. [DOI] [PubMed] [Google Scholar]
- Han F, Ozawa H, Matsuda KI, Lu H, De Kloet ER, Kawata M. Changes in the expression of corticotrophin-releasing hormone, mineralocorticoid receptor and glucocorticoid receptor mRNAs in the hypothalamic paraventricular nucleus induced by fornix transection and adrenalectomy. J. Neuroendocrinol. 2007;19:229–238. doi: 10.1111/j.1365-2826.2006.01519.x. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Haq R, Pfahl M, Chytil F. Retinoic acid affects the expression of nuclear retinoic acid receptors in tissues of retinol-deficient rats. Proc. Natl. Acad. Sci. U S A. 1991;88:8272–8276. doi: 10.1073/pnas.88.18.8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsumi T, Yamamuro Y. Downregulation of estrogen receptor gene expression by exogenous 17 beta-estradiol in the mammary glands of lactating mice. Exp. Biol. Med. 2006;231:311–316. doi: 10.1177/153537020623100311. [DOI] [PubMed] [Google Scholar]
- Healy KD, Frahm MA, DeLuca HF. 1,25-Dihydroxyvitamin D-3 up-regulates the renal vitamin D receptor through indirect gene activation and receptor stabilization. Arch. Biochem. Biophys. 2005;433:466–473. doi: 10.1016/j.abb.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Healy KD, Zella JB, Prahl JM, DeLuca HF. Regulation of the murine renal vitamin D receptor by 1,25-dihydroxyvitamin D-3 and calcium. Proc. Natl. Acad. Sci. U S A. 2003;100:9733–9737. doi: 10.1073/pnas.1633774100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Patel PD, Akil H, Watson SJ. Localization and regulation of glucocorticoid and mineralocorticoid receptor messenger RNAs in the hippocampal formation of the rat. Mol. Endocrinol. 1989;3:1886–1894. doi: 10.1210/mend-3-11-1886. [DOI] [PubMed] [Google Scholar]
- Herman JP, Watson SJ. Stress regulation of mineralocorticoid receptor heteronuclear RNA in rat hippocampus. Brain Res. 1995;677:243–249. doi: 10.1016/0006-8993(95)00152-g. [DOI] [PubMed] [Google Scholar]
- Hodin RA, Lazar MA, Chin WW. Differential and tissue-specific regulation of the multiple rat c-erbA messenger RNA species by thyroid hormone. J. Clin. Invest. 1990;85:101–105. doi: 10.1172/JCI114398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan NS, Crump KL, Duarte P, Lean DR, Trudeau VL. Hormone cross-regulation in the tadpole brain: developmental expression profiles and effect of T3 exposure on thyroid hormone- and estrogen-responsive genes in Rana pipiens. Gen. Comp. Endocrinol. 2007;154:5–15. doi: 10.1016/j.ygcen.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Holmes MC, Yau JL, French KL, Seckl JR. The effect of adrenalectomy on 5-hydroxytryptamine and corticosteroid receptor subtype messenger RNA expression in rat hippocampus. Neuroscience. 1995;64:327–337. doi: 10.1016/0306-4522(94)00407-v. [DOI] [PubMed] [Google Scholar]
- Hoopfer ED, Huang LY, Denver RJ. Basic transcription element binding protein is a thyroid hormone-regulated transcription factor expressed during metamorphosis in Xenopus laevis. Devel. Growth & Differentiation. 2002;44:365–381. doi: 10.1046/j.1440-169x.2002.00650.x. [DOI] [PubMed] [Google Scholar]
- Huber S, Ryffel GU, Weber R. Thyroid hormone induces competence for estrogen dependent vitellogenin synthesis in developing Xenopus laevis liver. Nature. 1979;278:65–67. doi: 10.1038/278065a0. [DOI] [PubMed] [Google Scholar]
- Hugin-Flores ME, Steimer T, Aubert ML, Schulz P. Mineralo- and glucocorticoid receptor mrnas are differently regulated by corticosterone in the rat hippocampus and anterior pituitary. Neuroendocrinology. 2004;79:174–184. doi: 10.1159/000078099. [DOI] [PubMed] [Google Scholar]
- Ing H, O'Malley BW. The steroid hormone receptor superfamily: Molecular mechanisms of action. Raven Press, Ltd.; New York: 1995. [Google Scholar]
- Ing NH. Steroid hormones regulate gene expression posttranscriptionally by altering the stabilities of messenger RNAs. Biol. Repro. 2005;72:1290–1296. doi: 10.1095/biolreprod.105.040014. [DOI] [PubMed] [Google Scholar]
- Ing NH, Massuto DA, Jaeger LA. Estradiol up-regulates AUF1p45 binding to stabilizing regions within the 3 '-untranslated region of estrogen receptor alpha mRNA. J. Biol. Chem. 2008;283:1764–1772. doi: 10.1074/jbc.M704745200. [DOI] [PubMed] [Google Scholar]
- Ing NH, Ott TL. Estradiol up-regulates estrogen receptor-alpha messenger ribonucleic acid in sheep endometrium by increasing its stability. Biol. Repro. 1999;60:134–139. doi: 10.1095/biolreprod60.1.134. [DOI] [PubMed] [Google Scholar]
- Isomaa V, Isotalo H, Orava M, Janne O. Regulation of cytosol and nuclear progesterone receptors in rabbit uterus by estrogen, antiestrogen and progesterone administration. Biochim. Biophys. Acta. 1979;585:24–33. doi: 10.1016/0304-4165(79)90321-0. [DOI] [PubMed] [Google Scholar]
- Jones KJ. Recovery from facial paralysis following crush injury of the facial nerve in hamsters: differential effects of gender and androgen exposure. Exp. Neurol. 1993;121:133–138. doi: 10.1006/exnr.1993.1079. [DOI] [PubMed] [Google Scholar]
- Kalinyak JE, Bradshaw JG, Perlman AJ. The role of development and adrenal steroids in the regulation of the mineralocorticoid receptor messenger RNA. Horm. Metab. Res. 1992;24:106–109. doi: 10.1055/s-2007-1003269. [DOI] [PubMed] [Google Scholar]
- Kalman BA, Spencer RL. Rapid corticosteroid-dependent regulation of mineralocorticoid receptor protein expression in rat brain. Endocrinology. 2002;143:4184–4195. doi: 10.1210/en.2002-220375. [DOI] [PubMed] [Google Scholar]
- Kamiya K, Sato T, Nishimura N, Goto Y, Kano K, Iguchi T. Expression of estrogen receptor and proto-oncogene messenger ribonucleic acids in reproductive tracts of neonatally diethylstilbestrol-exposed female mice with or without post-puberal estrogen administration. Exp. Clin. Endocrinol. Diabetes. 1996;104:111–122. doi: 10.1055/s-0029-1211432. [DOI] [PubMed] [Google Scholar]
- Kampa M, Castanas E. Membrane steroid receptor signaling in normal and neoplastic cells. Mol. Cell. Endocrinol. 2006;246:76–82. doi: 10.1016/j.mce.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Kanamori A, Brown DD. The regulation of thyroid hormone receptor beta genes by thyroid hormone in Xenopus laevis. J. Biol. Chem. 1992;267:739–745. [PubMed] [Google Scholar]
- Kerr JE, Allore RJ, Beck SG, Handa RJ. Distribution and hormonal regulation of androgen receptor (AR) and AR messenger ribonucleic acid in the rat hippocampus. Endocrinology. 1995;136:3213–3221. doi: 10.1210/endo.136.8.7628354. [DOI] [PubMed] [Google Scholar]
- Kerr JE, Beck SG, Handa RJ. Androgens modulate glucocorticoid receptor mRNA, but not mineralocorticoid receptor mRNA levels, in the rat hippocampus. J. Neuroendocrinol. 1996;8:439–447. doi: 10.1046/j.1365-2826.1996.04735.x. [DOI] [PubMed] [Google Scholar]
- Kikuyama S, Niki K, Mayumi M, Shibayama R, Nishikawa M, Shintake N. Studies on corticoid action on the toad tadpole tail in vitro. Gen Comp Endocrinol. 1983;52:395–399. doi: 10.1016/0016-6480(83)90178-8. [DOI] [PubMed] [Google Scholar]
- Kinyamu HK, Archer TK. Estrogen receptor-dependent proteasomal degradation of the glucocorticoid receptor is coupled to an increase in mdm2 protein expression. Mol. Cell. Biol. 2003;23:5867–5881. doi: 10.1128/MCB.23.16.5867-5881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowland J. Induction of vitellogenin synthesis in Xenopus laevis tadpoles. Differentiation. 1978;12:47–51. doi: 10.1111/j.1432-0436.1979.tb00989.x. [DOI] [PubMed] [Google Scholar]
- Krain LP, Denver RJ. Developmental expression and hormonal regulation of glucocorticoid and thyroid hormone receptors during metamorphosis in Xenopus laevis. J. Endocrinol. 2004;181:91–104. doi: 10.1677/joe.0.1810091. [DOI] [PubMed] [Google Scholar]
- Kraus WL, Katzenellenbogen BS. Regulation of progesterone receptor gene expression and growth in the rat uterus: modulation of estrogen actions by progesterone and sex steroid hormone antagonists. Endocrinology. 1993;132:2371–2379. doi: 10.1210/endo.132.6.8504742. [DOI] [PubMed] [Google Scholar]
- Kraus WL, Montano MM, Katzenellenbogen BS. Cloning of the rat progesterone receptor gene 5'-region and identification of two functionally distinct promoters. Mol. Endocrinol. 1993;7:1603–1616. doi: 10.1210/mend.7.12.8145766. [DOI] [PubMed] [Google Scholar]
- Kraus WL, Montano MM, Katzenellenbogen BS. Identification of multiple, widely spaced estrogen-responsive regions in the rat progesterone receptor gene. Mol. Endocrinol. 1994;8:952–969. doi: 10.1210/mend.8.8.7997237. [DOI] [PubMed] [Google Scholar]
- Krust A, Kastner P, Petkovich M, Zelent A, Chambon P. A third human retinoic acid receptor, hRAR-gamma. Proc. Natl. Acad. Sci. U S A. 1989;86:5310–5314. doi: 10.1073/pnas.86.14.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak SP, Patel PD, Thompson RC, Akil H, Watson SJ. 5'-Heterogeneity of the mineralocorticoid receptor messenger ribonucleic acid: differential expression and regulation of splice variants within the rat hippocampus. Endocrinology. 1993;133:2344–2350. doi: 10.1210/endo.133.5.8404687. [DOI] [PubMed] [Google Scholar]
- Le Drean Y, Lazennec G, Kern L, Saligaut D, Pakdel F, Valotaire Y. Characterization of an estrogen-responsive element implicated in regulation of the rainbow trout estrogen receptor gene. J. Mol. Endocrinol. 1995;15:37–47. doi: 10.1677/jme.0.0150037. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kim J, Shapiro DJ. Regulation of Xenopus laevis estrogen receptor gene expression is mediated by an estrogen response element in the protein coding region. DNA Cell Biol. 1995;14:419–430. doi: 10.1089/dna.1995.14.419. [DOI] [PubMed] [Google Scholar]
- Leroy P, Krust A, Zelent A, Mendelsohn C, Garnier JM, Kastner P, Dierich A, Chambon P. Multiple isoforms of the mouse retinoic acid receptor alpha are generated by alternative splicing and differential induction by retinoic acid. EMBO J. 1991a;10:59–69. doi: 10.1002/j.1460-2075.1991.tb07921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy P, Nakshatri H, Chambon P. Mouse retinoic acid receptor alpha 2 isoform is transcribed from a promoter that contains a retinoic acid response element. Proc. Natl. Acad. Sci. U S A. 1991b;88:10138–10142. doi: 10.1073/pnas.88.22.10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER. Membrane oestrogen receptor alpha signalling to cell functions. J. Physiol. London. 2009;587:5019–5023. doi: 10.1113/jphysiol.2009.177097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez JF, Chalmers DT, Little KY, Watson SJ. A.E. Bennett Research Award. Regulation of serotonin1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. Biol. Psychiatry. 1998;43:547–573. doi: 10.1016/s0006-3223(97)00484-8. [DOI] [PubMed] [Google Scholar]
- Machuca I, Esslemont G, Fairclough L, Tata JR. Analysis of structure and expression of the Xenopus thyroid hormone receptor-beta gene to explain its autoinduction. Mol. Endocrinol. 1995;9:96–107. doi: 10.1210/mend.9.1.7760854. [DOI] [PubMed] [Google Scholar]
- Machuca I, Tata JR. Autoinduction of thyroid hormone receptor during metamorphosis is reproduced in Xenopus XTC-2 cells. Mol. Cell. Endocrinol. 1992;87:105–113. doi: 10.1016/0303-7207(92)90238-2. [DOI] [PubMed] [Google Scholar]
- Mahonen A, Maenpaa PH. Steroid hormone modulation of vitamin D receptor levels in human Mg-63 osteosarcoma cells. Biochem. Biophys. Res. Comm. 1994;205:1179–1186. doi: 10.1006/bbrc.1994.2790. [DOI] [PubMed] [Google Scholar]
- Makino S, Hashimoto K, Gold PW. Multiple feedback mechanisms activating corticotropin-releasing hormone system in the brain during stress. Pharmacol. Biochem. Behav. 2002;73:147–158. doi: 10.1016/s0091-3057(02)00791-8. [DOI] [PubMed] [Google Scholar]
- Makino S, Smith MA, Gold PW. Increased expression of corticotropin-releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: association with reduction in glucocorticoid receptor mRNA levels. Endocrinology. 1995;136:3299–3309. doi: 10.1210/endo.136.8.7628364. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlick P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily. The second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt VL, Martyniuk CJ, Zhang D, Xiong H, Watt J, Xia X, Moon T, Trudeau VL. Auto-regulation of estrogen receptor subtypes and gene expression profiling of 17beta-estradiol action in the neuroendocrine axis of male goldfish. Mol. Cell. Endocrinol. 2008;283:38–48. doi: 10.1016/j.mce.2007.10.013. [DOI] [PubMed] [Google Scholar]
- May FEB, Knowland J. The Role of Thyroxine in the transition of vitellogenin synthesis from non-inducibility to inducibility during metamorphosis in Xenopus laevis. Dev. Biol. 1980;77:419–430. doi: 10.1016/0012-1606(80)90485-6. [DOI] [PubMed] [Google Scholar]
- May FEB, Knowland J. Estrogen receptor levels and vitellogenin synthesis during development of Xenopus laevis. Nature. 1981;292:853–855. doi: 10.1038/292853a0. [DOI] [PubMed] [Google Scholar]
- McCarthy M, Becker J. Neuroendocrinology of sexual behavior in the female. In: Becker J, et al., editors. Behavioral Endocrinology. MIT Press; Cambridge, MA: 2002. pp. 117–152. [Google Scholar]
- McDonnell DP, Mangelsdorf DJ, Pike JW, Haussler MR, Omalley BW. Molecular cloning of complementary DNA encoding the avian receptor for vitamin D. Science. 1987;235:1214–1217. doi: 10.1126/science.3029866. [DOI] [PubMed] [Google Scholar]
- McEwen BS. How do sex and stress hormones affect nerve cells? Ann. N.Y. Acad. Sci. 1994;743:1–16. doi: 10.1111/j.1749-6632.1994.tb55784.x. discussion 17-18. [DOI] [PubMed] [Google Scholar]
- Mester J, Baulieu EE. Progesterone receptors in the chick oviduct. Determination of the total concentration of binding sites in the cytosol and nuclear fraction and effect of progesterone on their distribution. Eur. J. Biochem. 1977;72:405–414. doi: 10.1111/j.1432-1033.1977.tb11265.x. [DOI] [PubMed] [Google Scholar]
- Meyer AS, Schmidt TJ. Potential mechanisms underlying autoregulation of glucocorticoid receptor mRNA levels in the DHD/K12/PROb rat colonic adenocarcinoma cell line. J. Steroid Biochem. Mol. Biol. 1995;55:219–228. doi: 10.1016/0960-0760(95)00168-y. [DOI] [PubMed] [Google Scholar]
- Milgrom E, Thi L, Atger M, Baulieu EE. Mechanisms regulating the concentration and the conformation of progesterone receptor(s) in the uterus. J. Biol. Chem. 1973;248:6366–6374. [PubMed] [Google Scholar]
- Mitchell DC, Ing NH. Estradiol stabilizes estrogen receptor messenger ribonucleic acid in sheep endometrium via discrete sequence elements in its 3 '-untranslated region. Mol. Endocrinol. 2003;17:562–574. doi: 10.1210/me.2002-0313. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Dellovade TL, Pfaff DW. Effect of thyroid hormone administration on estrogen-induced sex behavior in female mice. Horm. Behav. 2000;37:15–22. doi: 10.1006/hbeh.1999.1553. [DOI] [PubMed] [Google Scholar]
- Mullick A, Katzenellenbogen BS. Progesterone receptor synthesis and degradation in MCF-7 human breast cancer cells as studied by dense amino acid incorporation. Evidence for a non-hormone binding receptor precursor. J. Biol. Chem. 1986;261:13236–13246. [PubMed] [Google Scholar]
- Nardulli AM, Katzenellenbogen BS. Progesterone receptor regulation in T47D human breast cancer cells: analysis by density labeling of progesterone receptor synthesis and degradation and their modulation by progestin. Endocrinology. 1988;122:1532–1540. doi: 10.1210/endo-122-4-1532. [DOI] [PubMed] [Google Scholar]
- Naslund MJ, Coffey DS. The differential effects of neonatal androgen, estrogen and progesterone on adult rat prostate growth. J. Urol. 1986;136:1136–1140. doi: 10.1016/s0022-5347(17)45239-6. [DOI] [PubMed] [Google Scholar]
- Nastiuk KL, Clayton DF. Seasonal and tissue specific regulation of canary androgen receptor messenger ribonucleic acid. Endocrinology. 1994;134:640–649. doi: 10.1210/endo.134.2.8299561. [DOI] [PubMed] [Google Scholar]
- Naveh-Many T, Marx R, Keshet E, Pike JW, Silver J. Regulation of 1,25-dihydroxyvitamin D3 receptor gene expression by 1,25-dihydroxyvitamin-D3 in the parathyroid in vivo. J. Clin. Invest. 1990;86:1968–1975. doi: 10.1172/JCI114931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew KP, Long XH, Osborne E, Burke KA, Ahluwalia A, Bigsby RM. Effect of estradiol on estrogen receptor expression in rat uterine cell types. Biol. Repro. 2000;62:168–177. doi: 10.1095/biolreprod62.1.168. [DOI] [PubMed] [Google Scholar]
- Nishimura K, Makino S, Tanaka Y, Kaneda T, Hashimoto K. Altered expression of p53 mRNA in the brain and pituitary during repeated immobilization stress: Negative correlation with glucocorticoid receptor mRNA levels. J. Neuroendocrinol. 2004;16:84–91. doi: 10.1111/j.1365-2826.2004.01131.x. [DOI] [PubMed] [Google Scholar]
- O'donnell D, Francis D, Weaver S, Meaney MJ. Effects of adrenalectomy and corticosterone replacement on glucocorticoid receptor levels in rat brain tissue: a comparison between Western blotting and receptor binding assays. Brain Res. 1995;687:133–142. doi: 10.1016/0006-8993(95)00479-a. [DOI] [PubMed] [Google Scholar]
- Okret S, Dong Y, Bronnegard M, Gustafsson JA. Regulation of glucocorticoid receptor expression. Biochimie. 1991;73:51–59. doi: 10.1016/0300-9084(91)90074-b. [DOI] [PubMed] [Google Scholar]
- Okret S, Poellinger L, Dong Y, Gustafsson JA. Down-regulation of glucocorticoid receptor mRNA by glucocorticoid hormones and recognition by the receptor of a specific binding sequence within a receptor cDNA clone. Proc. Natl. Acad. Sci. U S A. 1986;83:5899–5903. doi: 10.1073/pnas.83.16.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakdel F, Feon S, Le Gac F, Le Menn F, Valotaire Y. In vivo estrogen induction of hepatic estrogen receptor mRNA and correlation with vitellogenin mRNA in rainbow trout. Mol. Cell. Endocrinol. 1991;75:205–212. doi: 10.1016/0303-7207(91)90162-l. [DOI] [PubMed] [Google Scholar]
- Pan P, Reddy K, Lee S, Studzinski GP. Differentiation related regulation of 1,25-dihydroxyvitamin D3 receptor messenger RNA in human leukemia cells Hl-60. Cell Proliferation. 1991;24:159–170. doi: 10.1111/j.1365-2184.1991.tb01146.x. [DOI] [PubMed] [Google Scholar]
- Parsons B, McGinnis MY, McEwen BS. Sequential inhibition of progesterone: effects on sexual receptivity and associated changes in brain cytosol progestin binding in the female rat. Brain Res. 1981;221:149–160. doi: 10.1016/0006-8993(81)91069-6. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Brady LS, Karl M, Chrousos GP. Regulation of HSP90 and corticosteroid receptor mRNA by corticosterone levels in vivo. Mol. Cell. Endocrinol. 1994;103:57–64. doi: 10.1016/0303-7207(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Payne AP. The harderian gland: a tercentennial review. J Anat. 1994;185:1–49. [PMC free article] [PubMed] [Google Scholar]
- Perlman AJ, Wolffe AP, Champion J, Tata JR. Regulation by estrogen receptor of vitellogenin gene transcription in Xenopus hepatocyte cultures. Mol. Cell. Endocrinol. 1984;38:151–161. doi: 10.1016/0303-7207(84)90113-8. [DOI] [PubMed] [Google Scholar]
- Petit FG, Metivier R, Valotaire Y, Pakdel F. Synergism between a half-site and an imperfect estrogen-responsive element, and cooperation with COUP-TFI are required for estrogen receptor (ER) to achieve a maximal estrogen-stimulation of rainbow trout ER gene. Euro. J. Biochem. 1999;259:385–395. doi: 10.1046/j.1432-1327.1999.00072.x. [DOI] [PubMed] [Google Scholar]
- Petkovich M, Brand NJ, Krust A, Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987;330:444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Prins GS. Neonatal estrogen exposure induces lobe-specific alterations in adult rat prostate androgen receptor expression. Endocrinology. 1992;130:3703–3714. doi: 10.1210/endo.130.6.1597166. [DOI] [PubMed] [Google Scholar]
- Prins GS, Birch L. Neonatal estrogen exposure up-regulates estrogen receptor expression in the developing and adult rat prostate lobes. Endocrinology. 1997;138:1801–1809. doi: 10.1210/endo.138.5.5106. [DOI] [PubMed] [Google Scholar]
- Prins GS, Korach KS. The role of estrogens and estrogen receptors in normal prostate growth and disease. Steroids. 2008;73:233–244. doi: 10.1016/j.steroids.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins GS, Woodham C. Autologous regulation of androgen receptor messenger ribonucleic acid in the separate lobes of the rat prostate gland. Biol. Repro. 1995;53:609–619. doi: 10.1095/biolreprod53.3.609. [DOI] [PubMed] [Google Scholar]
- Quarmby VE, Korach KS. The influence of 17 beta-estradiol on patterns of cell division in the uterus. Endocrinology. 1984;114:694–702. doi: 10.1210/endo-114-3-694. [DOI] [PubMed] [Google Scholar]
- Quarmby VE, Yarbrough WG, Lubahn DB, French FS, Wilson EM. Autologous down-regulation of androgen receptor messenger ribonucleic acid. Mol. Endocrinol. 1990;4:22–28. doi: 10.1210/mend-4-1-22. [DOI] [PubMed] [Google Scholar]
- Rabelo EM, Baker BS, Tata JR. Interplay between thyroid hormone and estrogen in modulating expression of their receptor and vitellogenin genes during Xenopus metamorphosis. Mech. Dev. 1994;45:49–57. doi: 10.1016/0925-4773(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Rabelo EM, Tata JR. Thyroid hormone potentiates estrogen activation of vitellogenin genes and autoinduction of estrogen receptor in adult Xenopus hepatocytes. Mol. Cell. Endocrinol. 1993;96:37–44. doi: 10.1016/0303-7207(93)90092-x. [DOI] [PubMed] [Google Scholar]
- Rackoff PJ, Rosen CJ. Pathogenesis and treatment of glucocorticoid-induced osteoporosis. Drugs Aging. 1998;12:477–484. doi: 10.2165/00002512-199812060-00005. [DOI] [PubMed] [Google Scholar]
- Rajfer J, Coffey DS. Sex steroid imprinting of the immature prostate. Long-term effects. Invest. Urol. 1978;16:186–190. [PubMed] [Google Scholar]
- Ramdas J, Liu W, Harmon JM. Glucocorticoid-induced cell death requires autoinduction of glucocorticoid receptor expression in human leukemic T cells. Cancer Res. 1999;59:1378–1385. [PubMed] [Google Scholar]
- Ranjan M, Wong J, Shi YB. Transcriptional repression of Xenopus TR beta gene is mediated by a thyroid hormone response element located near the start site. J. Biol. Chem. 1994;269:24699–24705. [PubMed] [Google Scholar]
- Read LD, Greene GL, Katzenellenbogen BS. Regulation of estrogen receptor messenger ribonucleic acid and protein levels in human breast cancer cell lines by sex steroid hormones, their antagonists, and growth factors. Mol. Endocrinol. 1989;3:295–304. doi: 10.1210/mend-3-2-295. [DOI] [PubMed] [Google Scholar]
- Read LD, Snider CE, Miller JS, Greene GL, Katzenellenbogen BS. Ligand-modulated regulation of progesterone receptor messenger ribonucleic acid and protein in human breast cancer cell lines. Mol. Endocrinol. 1988;2:263–271. doi: 10.1210/mend-2-3-263. [DOI] [PubMed] [Google Scholar]
- Reichardt HM, Kaestner KH, Tuckermann J, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Angel P, Schutz G. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93:531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- Reul J, Pearce PT, Funder JW, Krozowski ZS. Type I and type II corticosteroid receptor gene expression in the rat: Effect of adrenalectomy and dexamethasone administration. Mol. Endocrinol. 1989a;3:1674–1680. doi: 10.1210/mend-3-10-1674. [DOI] [PubMed] [Google Scholar]
- Reul JM, Pearce PT, Funder JW, Krozowski ZS. Type I and type II corticosteroid receptor gene expression in the rat: effect of adrenalectomy and dexamethasone administration. Mol. Endocrinol. 1989b;3:1674–1680. doi: 10.1210/mend-3-10-1674. [DOI] [PubMed] [Google Scholar]
- Ribeiro RC, Kushner PJ, Baxter JD. The nuclear hormone receptor gene superfamily. Annual review of medicine. 1995;46:443–453. doi: 10.1146/annurev.med.46.1.443. [DOI] [PubMed] [Google Scholar]
- Riddiford LM, Cherbas P, Truman JW. Ecdysone receptors and their biological actions. Vitamins and Hormones - Advances in Research and Applications. 2001;60:1–73. doi: 10.1016/s0083-6729(00)60016-x. [DOI] [PubMed] [Google Scholar]
- Robyr D, Wolffe AP, Wahli W. Nuclear hormone receptor coregulators in action: diversity for shared tasks. Mol. Endocrinol. 2000;14:329–347. doi: 10.1210/mend.14.3.0411. [DOI] [PubMed] [Google Scholar]
- Rosewicz S, McDonald AR, Maddux BA, Goldfine ID, Miesfeld RL, Logsdon CD. Mechanism of glucocorticoid receptor down-regulation by glucocorticoids. J. Biol. Chem. 1988;263:2581–2584. [PubMed] [Google Scholar]
- Saceda M, Lippman ME, Chambon P, Lindsey RL, Ponglikitmongkol M, Puente M, Martin MB. Regulation of the estrogen receptor in MCF-7 cells by estradiol. Mol. Endocrinol. 1988;2:1157–1162. doi: 10.1210/mend-2-12-1157. [DOI] [PubMed] [Google Scholar]
- Safi R, Begue A, Hanni C, Stehelin D, Tata JR, Laudet V. Thyroid hormone receptor genes of neotenic amphibians. J. Mol. Evol. 1997;44:595–604. doi: 10.1007/pl00006182. [DOI] [PubMed] [Google Scholar]
- Safi R, Vlaeminck-Guillem V, Duffraisse M, Seugnet I, Plateroti M, Margotat A, Duterque-Coquillaud M, Crespi EJ, Denver RJ, Demeneix B, Laudet V. Pedomorphosis revisited: thyroid hormone receptors are functional in Necturus maculosus. Evol. Dev. 2006;8:284–292. doi: 10.1111/j.1525-142X.2006.00099.x. [DOI] [PubMed] [Google Scholar]
- Sakurai A, Miyamoto T, Degroot LJ. Cloning and characterization of the human thyroid hormone receptor beta-1 gene promoter. Biochem. Biophys. Res. Comm. 1992;185:78–84. doi: 10.1016/s0006-291x(05)80957-x. [DOI] [PubMed] [Google Scholar]
- Samuels HH, Stanley F, Shapiro LE. Modulation of thyroid hormone nuclear receptor levels by 3,5,3'-triiodo-L-thyronine in GH1 cells. Evidence for two functional components of nuclear-bound receptor and relationship to the induction of growth hormone synthesis. J. Biol. Chem. 1977;252:6052–6060. [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Stress down-regulates corticosterone receptors in a site-specific manner in the brain. Endocrinology. 1984;114:287–292. doi: 10.1210/endo-114-1-287. [DOI] [PubMed] [Google Scholar]
- Sato T, Chiba A, Hayashi S, Okamura H, Ohta Y, Takasugi N, Iguchi T. Induction of estrogen receptor and cell division in genital tracts of male mice by neonatal exposure to diethylstilbestrol. Reprod. Toxicol. 1994;8:145–153. doi: 10.1016/0890-6238(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Savouret JF, Bailly A, Misrahi M, Rauch C, Redeuilh G, Chauchereau A, Milgrom E. Characterization of the hormone responsive element involved in the regulation of the progesterone receptor gene. EMBO J. 1991;10:1875–1883. doi: 10.1002/j.1460-2075.1991.tb07713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savouret JF, Chauchereau A, Misrahi M, Lescop P, Mantel A, Bailly A, Milgrom E. The progesterone receptor. Biological effects of progestins and antiprogestins. Hum. Reprod. 1994a;9(Suppl 1):7–11. doi: 10.1093/humrep/9.suppl_1.7. [DOI] [PubMed] [Google Scholar]
- Savouret JF, Rauch M, Redeuilh G, Sar S, Chauchereau A, Woodruff K, Parker MG, Milgrom E. Interplay between estrogens, progestins, retinoic acid and AP-1 on a single regulatory site in the progesterone receptor gene. J. Biol. Chem. 1994b;269:28955–28962. [PubMed] [Google Scholar]
- Scott RE, Wu-Peng XS, Yen PM, Chin WW, Pfaff DW. Interactions of estrogen- and thyroid hormone receptors on a progesterone receptor estrogen response element (ERE) sequence: a comparison with the vitellogenin A2 consensus ERE. Mol. Endocrinol. 1997;11:1581–1592. doi: 10.1210/mend.11.11.0003. [DOI] [PubMed] [Google Scholar]
- Shapiro DJ, Barton MC, McKearin DM, Chang TC, Lew D, Blume J, Nielsen DA, Gould L. Estrogen regulation of gene transcription and mRNA stability. Recent Prog. Horm. Res. 1989;45:29–58. doi: 10.1016/b978-0-12-571145-6.50006-6. discussion 58-64. [DOI] [PubMed] [Google Scholar]
- Shi YB. From Morphology to Molecular Biology. Wiley-Liss; New York: 2000. Amphibian Metamorphosis. [Google Scholar]
- Spencer RL, Kalman BA, Cotter CS, Deak T. Discrimination between changes in glucocorticoid receptor expression and activation in rat brain using western blot analysis. Brain Res. 2000;868:275–286. doi: 10.1016/s0006-8993(00)02341-6. [DOI] [PubMed] [Google Scholar]
- Stephenson G, Funder J. Hippocampal and renal type I receptors are differentially regulated. Am. J. Physiol. 1987;252:E525–529. doi: 10.1152/ajpendo.1987.252.4.E525. [DOI] [PubMed] [Google Scholar]
- Strait KA, Schwartz HL, Perez-Castillo A, Oppenheimer JH. Relationship of c-erbA mRNA content to tissue triiodothyronine nuclear binding capacity and function in developing and adult rats. J Biol Chem. 1990;265:10514–10521. [PubMed] [Google Scholar]
- Strom M, Sandgren ME, Brown TA, Deluca HF. 1,25-Dihydroxyvitamin-D3 up-Regulates the 1,25-Dihydroxyvitamin-D3 Receptor Invivo. Proc. Natl. Acad. Sci. U S A. 1989;86:9770–9773. doi: 10.1073/pnas.86.24.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucov HM, Murakami KK, Evans RM. Characterization of an autoregulated response element in the mouse retinoic acid receptor type beta gene. Proc. Natl. Acad. Sci U S A. 1990;87:5392–5396. doi: 10.1073/pnas.87.14.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RL, Prall OW, Watts CK, Musgrove EA. Estrogen and progestin regulation of cell cycle progression. J. Mammary Gland Biol. Neoplasia. 1998;3:63–72. doi: 10.1023/a:1018774302092. [DOI] [PubMed] [Google Scholar]
- Suzuki MR, Kikuyama S. Corticoids augment nuclear binding capacity for triiodothyronine in bullfrog tadpole tail fins. Gen. Comp. Endocrinol. 1983;52:272–278. doi: 10.1016/0016-6480(83)90122-3. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Miyamoto T, Opsahl A, Sakurai A, Degroot LJ. 2 Thyroid hormone response elements are present in the promoter of human thyroid hormone receptor beta-1. Mol. Endocrinol. 1994;8:305–314. doi: 10.1210/mend.8.3.8015548. [DOI] [PubMed] [Google Scholar]
- Sweezey NB, Ghibu F, Gagnon S, Schotman E, Hamid Q. Glucocorticoid receptor mRNA and protein in fetal rat lung in vivo: modulation by glucocorticoid and androgen. Am J Physiol. 1998;275:L103–109. doi: 10.1152/ajplung.1998.275.1.L103. [DOI] [PubMed] [Google Scholar]
- Syms AJ, Norris JS, Panko WB, Smith RG. Mechanism of androgen-receptor augmentation. Analysis of receptor synthesis and degradation by the density-shift technique. J Biol Chem. 1985;260:455–461. [PubMed] [Google Scholar]
- Syms AJ, Norris JS, Smith RG. Androgen stimulated elevation in androgen receptor levels is inhibited by the synthetic glucocorticoid triamcinolone acetonide. Biochem. Biophys. Res. Commun. 1983a;116:1020–1025. doi: 10.1016/s0006-291x(83)80244-7. [DOI] [PubMed] [Google Scholar]
- Syms AJ, Norris JS, Smith RG. Proliferation of a highly androgen-sensitive ductus deferens cell line (DDT1MF-2) is regulated by glucocorticoids and modulated by growth on collagen. In Vitro. 1983b;19:929–936. doi: 10.1007/BF02661714. [DOI] [PubMed] [Google Scholar]
- Syvala H, Vienonen A, Zhuang YH, Kivineva M, Ylikomi T, Tuohimaa P. Evidence for enhanced ubiquitin-mediated proteolysis of the chicken progesterone receptor by progesterone. Life Sci. 1998;63:1505–1512. doi: 10.1016/s0024-3205(98)00417-2. [DOI] [PubMed] [Google Scholar]
- Takeda H, Nakamoto T, Kokontis J, Chodak GW, Chang CS. Autoregulation of Androgen Receptor Expression in Rodent Prostate - Immunohistochemical and Insitu Hybridization Analysis. Biochem. Biophys. Res. Comm. 1991;177:488–496. doi: 10.1016/0006-291x(91)92010-h. [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Kakushi H, Tohkin M. Androgens directly stimulate mineralization and increase androgen receptors in human osteoblast-like osteosarcoma cells. Biochem. Biophys. Res. Comm. 1994;204:905–911. doi: 10.1006/bbrc.1994.2545. [DOI] [PubMed] [Google Scholar]
- Tasker JG, Di S, Malcher-Lopes R. Minireview: Rapid glucocorticoid signaling via membrane-associated receptors. Endocrinology. 2006;147:5549–5556. doi: 10.1210/en.2006-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata JR. Autoinduction of nuclear hormone receptors during metamorphosis and its significance. Insect Biochem. Mol. Biol. 2000;30:645–651. doi: 10.1016/s0965-1748(00)00035-7. [DOI] [PubMed] [Google Scholar]
- Tata JR, Kawahara A, Baker BS. Prolactin inhibits both thyroid hormone-induced morphogenesis and cell death in cultured amphibian larval tissues. Dev. Biol. 1991;146:72–80. doi: 10.1016/0012-1606(91)90447-b. [DOI] [PubMed] [Google Scholar]
- Tena-Sempere M, Navarro J, Pinilla L, Gonzalez LC, Huhtaniemi I, Aguilar E. Neonatal exposure to estrogen differentially alters estrogen receptor alpha and beta mRNA expression in rat testis during postnatal development. J. Endocrinol. 2000;165:345–357. doi: 10.1677/joe.0.1650345. [DOI] [PubMed] [Google Scholar]
- Thomas P. Characteristics of membrane progestin receptor alpha (mPR alpha) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions. Front. Neuroendocrinol. 2008;29:292–312. doi: 10.1016/j.yfrne.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson EB, Nazareth LV, Thulasi R, Ashraf J, Harbour D, Johnson BH. Glucocorticoids in malignant lymphoid cells: gene regulation and the minimum receptor fragment for lysis. J. Steroid Biochem. Mol. Biol. 1992;41:273–282. doi: 10.1016/0960-0760(92)90352-j. [DOI] [PubMed] [Google Scholar]
- Thummel CS. From embryogenesis to metamorphosis - the regulation and function of Drosophila nuclear receptor superfamily members. Cell. 1995;83:871–877. doi: 10.1016/0092-8674(95)90203-1. [DOI] [PubMed] [Google Scholar]
- Tibbetts TA, Mendoza-Meneses M, O'Malley BW, Conneely OM. Mutual and intercompartmental regulation of estrogen receptor and progesterone receptor expression in the mouse uterus. Biol. Reprod. 1998;59:1143–1152. doi: 10.1095/biolreprod59.5.1143. [DOI] [PubMed] [Google Scholar]
- Tornello S, Orti E, De Nicola AF, Rainbow TC, McEwen BS. Regulation of glucocorticoid receptors in brain by corticosterone treatment of adrenalectomized rats. Neuroendocrinology. 1982;35:411–417. doi: 10.1159/000123429. [DOI] [PubMed] [Google Scholar]
- Treilleux I, Peloux N, Brown M, Sergeant A. Human estrogen receptor (ER) gene promoter-P1: Estradiol-independent activity and estradiol inducibility in ER+ and ER-cells. Mol. Endocrinol. 1997;11:1319–1331. doi: 10.1210/mend.11.9.9973. [DOI] [PubMed] [Google Scholar]
- Truss M, Beato M. Steroid hormone receptors: interaction with deoxyribonucleic acid and transcription factors. Endocr. Rev. 1993;14:459–479. doi: 10.1210/edrv-14-4-459. [DOI] [PubMed] [Google Scholar]
- Ulisse S, Esslemont G, Baker BS, Krishna V, Chatterjee K, Tata JR. Dominant-negative mutant thyroid hormone receptors prevent transcription from Xenopus thyroid hormone receptor beta gene promoter in response to thyroid hormone in Xenopus tadpoles in vivo. Proc. Natl. Acad. Sci. U S A. 1996;93:1205–1209. doi: 10.1073/pnas.93.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulisse S, Tata JR. Thyroid hormone and glucocorticoid independently regulate the expression of estrogen receptor in male Xenopus liver cells. Mol. Cell. Endocrinol. 1994;105:45–53. doi: 10.1016/0303-7207(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Varriale B. Autoinduction of androgen receptor mRNA in primary cultures of hamster (Mesocricetus auratus) harderian gland cells. Gen. Comp. Endocrinol. 1996;102:386–393. doi: 10.1006/gcen.1996.0082. [DOI] [PubMed] [Google Scholar]
- Varriale B, Esposito T. The hamster androgen receptor promoter: a molecular analysis. J. Steroid Biochem. Mol. Biol. 2005;94:103–110. doi: 10.1016/j.jsbmb.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Varriale B, Serino I. The androgen receptor mRNA is up-regulated by testosterone in both the Harderian gland and thumb pad of the frog, Rana esculenta. J. Steroid Biochem. Mol. Biol. 1994;51:259–265. doi: 10.1016/0960-0760(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Varriale B, Tata JR. Autoinduction of estrogen receptor is associated with FOSP-1 mRNA induction by estrogen in primary cultures of Xenopus oviduct cells. Mol. Cell. Endocrinol. 1990;71:R25–31. doi: 10.1016/0303-7207(90)90035-7. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Davidkova G, Zhu YS, Koibuchi N, Chin WW, Pfaff D. Differential interaction of estrogen receptor and thyroid hormone receptor isoforms on the rat oxytocin receptor promoter leads to differences in transcriptional regulation. Neuroendocrinology. 2001a;74:309–324. doi: 10.1159/000054698. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Koibuchi N, Chin WW, Pfaff DW. Differential crosstalk between estrogen receptor (ER)alpha and ERbeta and the thyroid hormone receptor isoforms results in flexible regulation of the consensus ERE. Brain Res. Mol. Brain Res. 2001b;95:9–17. doi: 10.1016/s0169-328x(01)00165-6. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Ogawa S, Pfaff D. Estrogen and thyroid hormone receptor interactions: physiological flexibility by molecular specificity. Physiol. Rev. 2002;82:923–944. doi: 10.1152/physrev.00014.2002. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Zhu YS, Daniel S, Koibuchi N, Chin WW, Pfaff D. Crosstalk between oestrogen receptors and thyroid hormone receptor isoforms results in differential regulation of the preproenkephalin gene. J. Neuroendocrinol. 2001c;13:779–790. doi: 10.1046/j.1365-2826.2001.00693.x. [DOI] [PubMed] [Google Scholar]
- Vedeckis WV, Ali M, Allen HR. Regulation of glucocorticoid receptor protein and mRNA levels. Cancer Res. 1989;49:S2295–S2302. [PubMed] [Google Scholar]
- Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. J. Neuroendocrinol. 2002;14:506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Brown DD. Thyroid hormone-induced gene expression program for amphibian tail resorption. J. Biol. Chem. 1993;268:16270–16278. [PubMed] [Google Scholar]
- Webster JC, Jewell CM, Bodwell JE, Munck A, Sar M, Cidlowski JA. Mouse glucocorticoid receptor phosphorylation status influences multiple functions of the receptor protein. J. Biol. Chem. 1997;272:9287–9293. doi: 10.1074/jbc.272.14.9287. [DOI] [PubMed] [Google Scholar]
- Westley B, Knowland J. Estrogen causes a rapid, large and prolonged rise in the level of nuclear estrogen receptor in Xenopus laevis liver. Biochem. Biophys. Res. Commun. 1979;88:1167–1172. doi: 10.1016/0006-291x(79)91531-6. [DOI] [PubMed] [Google Scholar]
- Wiese RJ, Uhlandsmith A, Ross TK, Prahl JM, Deluca HF. Up regulation of the vitamin D receptor in response to 1,25-dihydroxyvitamin-D(3) results from ligand-induced stabilization. J. Biol. Chem. 1992;267:20082–20086. [PubMed] [Google Scholar]
- Wiren KM, Zhang X, Chang C, Keenan E, Orwoll ES. Transcriptional up-regulation of the human androgen receptor by androgen in bone cells. Endocrinology. 1997;138:2291–2300. doi: 10.1210/endo.138.6.5163. [DOI] [PubMed] [Google Scholar]
- Wolf DA, Herzinger T, Hermeking H, Blaschke D, Horz W. Transcriptional and posttranscriptional regulation of human androgen receptor expression by androgen. Mol. Endocrinol. 1993;7:924–936. doi: 10.1210/mend.7.7.8413317. [DOI] [PubMed] [Google Scholar]
- Yadetie F, Arukwe A, Goksoyr A, Male R. Induction of hepatic estrogen receptor in juvenile Atlantic salmon in vivo by the environmental estrogen, 4-nonylphenol. Sci. Total Environ. 1999;233:201–210. doi: 10.1016/s0048-9697(99)00226-0. [DOI] [PubMed] [Google Scholar]
- Yamashita S, Newbold RR, McLachlan JA, Korach KS. The role of the estrogen receptor in uterine epithelial proliferation and cytodifferentiation in neonatal mice. Endocrinology. 1990;127:2456–2463. doi: 10.1210/endo-127-5-2456. [DOI] [PubMed] [Google Scholar]
- Yao M, Hu F, Denver RJ. Distribution and corticosteroid regulation of glucocorticoid receptor in the brain of Xenopus laevis. J. Comp. Neurol. 2008;508:967–982. doi: 10.1002/cne.21716. [DOI] [PubMed] [Google Scholar]
- Yaoita Y, Brown DD. A correlation of thyroid hormone receptor gene expression with amphibian metamorphosis. Genes Dev. 1990;4:1917–1924. doi: 10.1101/gad.4.11.1917. [DOI] [PubMed] [Google Scholar]
- Yaoita Y, Shi Y, Brown D. Xenopus laevis alpha and beta thyroid hormone receptors. Proc. Natl. Acad. Sci. U S A. 1990;87:8684. doi: 10.1073/pnas.87.21.8685a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelent A, Krust A, Petkovich M, Kastner P, Chambon P. Cloning of murine alpha and beta retinoic acid receptors and a novel receptor gamma predominantly expressed in skin. Nature. 1989;339:714–717. doi: 10.1038/339714a0. [DOI] [PubMed] [Google Scholar]
- Zella LA, Kim S, Shevde NK, Pike JW. Enhancers located within two introns of the vitamin D receptor gene mediate transcriptional autoregulation by 1,25-dihydroxyvitamin D-3. Mol. Endocrinol. 2006;20:1231–1247. doi: 10.1210/me.2006-0015. [DOI] [PubMed] [Google Scholar]
- Zella LA, Meyer MB, Nerenz RD, Lee SM, Martowicz ML, Pike JW. Multifunctional Enhancers Regulate Mouse and Human Vitamin D Receptor Gene Transcription. Mol. Endocrinol. 2010;24:128–147. doi: 10.1210/me.2009-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Bouillard B, Pharaboz-Joly MO, Andre J. Non-classical antiestrogenic actions of dexamethasone in variant MCF-7 human breast cancer cells in culture. Mol. Cell. Endocrinol. 1989;66:189–197. doi: 10.1016/0303-7207(89)90031-2. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Hanna RN, Schaaf MJM, Spaink HP, Thomas P. Candidates for membrane progestin receptors-Past approaches and future challenges. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2008;148:381–389. doi: 10.1016/j.cbpc.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Zhu YS, Cai LQ, You X, Duan Y, Imperato-McGinley J, Chin WW, Pfaff DW. Molecular analysis of estrogen induction of preproenkephalin gene expression and its modulation by thyroid hormones. Brain Res. Mol. Brain Res. 2001;91:23–33. doi: 10.1016/s0169-328x(01)00109-7. [DOI] [PubMed] [Google Scholar]
- Zhu YS, Yen PM, Chin WW, Pfaff DW. Estrogen and thyroid hormone interaction on regulation of gene expression. Proc. Natl. Acad. Sci. U S A. 1996;93:12587–12592. doi: 10.1073/pnas.93.22.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zineb R, Zhor B, Odile W, Marthe RR. Distinct, tissue-specific regulation of vitamin D receptor in the intestine, kidney, and skin by dietary calcium and vitamin D. Endocrinology. 1998;139:1844–1852. doi: 10.1210/endo.139.4.5903. [DOI] [PubMed] [Google Scholar]