Abstract
Introduction
To assess clinical features of bronchioloalveolar carcinoma (BAC) based upon the 1999 WHO Classification (“pure BAC”), compare pure BAC patients with patients previously diagnosed as BAC not meeting the 1999 definition, and compare survival changes of pure BAC based on the old and new (2009) staging systems.
Methods
A pulmonary pathologist reviewed each BAC tumor diagnosed between January 1, 1997 and December 31, 2007 identifying cases meeting the new criteria. Cases were restaged according to the seventh edition of the TNM classification introduced in 2009. Pure BAC patients were analyzed under both staging systems for changes in overall survival estimation.
Results
Of 338 total patients who were diagnosed with BAC, 117 were classified as pure and 221 were non pure BAC. Seventy-eight of the 117 and 178 of the 221 had no other primary lung cancer. One-year and five-year survival for the 78 pure BAC patients were 94.8% and 83.5%, and for the 178 patients were 92.6% and 46.4%, respectively. Restaging for pure BAC cases resulted in 9 of the 78 cases (12%) changing stage. Compared to the old staging, patients with advanced stage under the new stage had a worse 5-year survival (53% vs. 45%) but no change was observed for stage IA.
Conclusions
For patients with pure BAC, the new pathologic system favorably affects survival and the new staging system may more accurately reflect prognosis in advanced stage cancer. Our results have important implications for researchers, clinicians, and patients.
Keywords: Bronchioloalveolar carcinoma, lung cancer, epidemiology
Introduction
The description of disease consistent with bronchioloalveolar carcinoma (BAC) dates back to the 1800's. The reported incidence of BAC in the literature has ranged from 4% to 29% of non-small cell lung cancer (NSCLC) cases.1, 2 Lack of precision in prevalence and survival estimates relates to wide variations in criteria distinguishing BAC from adenocarcinoma.1–3
In 1999, the World Health Organization (WHO) Classification of Lung Tumors established strict criteria for BAC diagnosis restricting it to tumors demonstrating pure bronchioloalveolar growth pattern with no stromal, lymphatic, or vascular invasion.4 Our current understanding of BAC is based on data reported before publication of the 1999 WHO Classification or more recent studies, which did not include central pathologic review of all cases using the WHO Classification. In addition, the International Association for the Study of Lung Cancer (IASLC) has revised the lung cancer staging system.5 The impact of the definition and staging changes on the epidemiology of BAC has not been clearly defined.
The goals of the current study were to: (1) assess the clinical features of BAC based upon the 1999 WHO Classification; (2) compare these patients with patients previously diagnosed as BAC but not currently meeting the 1999 WHO Classification; (3) compare the survival change of BAC based on the old and new staging system. Cases received diagnosis and/or treatment at Mayo Clinic in Rochester, Minnesota, during the 11-year period between January 1, 1997 and December 31, 2007.
Materials and Methods
Study Cohort
Only patients who provided informed consent as approved by the Mayo Clinic Institutional Review Board (IRB) were included in this study. Patients were ascertained daily from a computerized pathology reporting system that identified all lung cancer cases who received care in our institution.
Clinical data was abstracted from medical records for each patient during a baseline visit at the time of diagnosis and included demographics, history of tobacco exposure, alcohol use, family cancer history, comorbid medical conditions, and lung cancer histology, staging, and treatment. Clinical staging was assigned by results from available chest radiography, computerized tomography (CT), bone scans, positron emission tomography (PET) scans, and magnetic resonance imaging. If a patient received any type of diagnostic procedure or therapy elsewhere, authorization for the release of medical information and copies of relevant medical records were requested. Tobacco use history included age of regular smoking initiation, average cigarettes smoked per day, total years smoked, and other types of tobacco products used. Never smokers were defined by self-report as having smoked less than 100 cigarettes during their lifetime. Former smokers were defined as reporting at least six months of smoking abstinence at the time of diagnosis. Current smokers were daily cigarette smokers or those who smoked within 6 months of the time of diagnosis.
The vital status of each patient was verified through the Mayo Clinic registration database, next-of-kin reports, death certificates and obituary documents, the Mayo Clinic Tumor Registry, and the Social Security Death Index internet site. Vital status information for all patients in the lung cancer cohort is updated at the beginning of each calendar year. Clinical information was obtained from the most recent clinical note or the last mailed study questionnaire. The mailed study questionnaire obtained information on new diseases and treatments that occurred after the initial diagnosis. For deceased patients, a follow-up packet was sent to the next-of-kin to obtain proxy information.
Histopathology Re-Review
A review of histologic slides of surgically-resected specimens was required in order to make a diagnosis of BAC. A pulmonary pathologist on our investigative team (MCA) reviewed tumor slides for each tumor diagnosed as BAC and identified cases that met criteria for diagnosis of BAC according to the 1999 and 2004 WHO Classification.6 A tumor was adjudicated as BAC if it was an adenocarcinoma that demonstrated a pure bronchioloalveolar growth pattern with no lymphatic, vascular or pleural invasion, and no distortion of alveolar architecture. Tumors initially classified as BAC no longer meeting the new WHO classification were labeled as “non pure” BAC to contrast with the “pure” BAC tumors. Tumors previously diagnosed as BAC on the basis of cytology or needle biopsy specimens were excluded from this study. Recorded histologic descriptors of each tumor included histologic subtype (mucinous or non-mucinous), and presence of scar.
Lung Cancer Staging and Restaging
All patients were staged at the time of enrollment by the sixth edition of the TNM classification for Malignant Tumors introduced in 2002. The patients were then restaged by the seventh edition of the TNM classification of Malignant Tumors introduced in 2009. The 2009 TNM classification includes additional cutoffs for tumor size subgrouping T1 into T1a (≤2cm) and T1b (>2cm but ≤3cm), T2 into T2a (>3cm but ≤5cm) and T2b (>5cm but ≤7cm) and reclassifying tumors >7cm to T3.7 Moreover, the 2009 staging system reclassifies satellite lesions in the same lobe from T4 to T3 and reclassifies additional nodules in another ipsilateral lobe from M1 to T4.7 Pleural effusion is reclassified from T into M1 category.7
Statistical Analysis
We compared clinical characteristics and outcomes of two groups of patients identified after review of histopathology of all patients who received a diagnosis of BAC: (1) patients who met the 1999 WHO criteria for BAC (“pure BAC”); and (2) patients who did not meet the criteria were termed (“non pure BAC.”) Descriptive analyses were conducted on patient characteristics, clinical features of disease, and clinical outcomes. All statistical tests used were two-sided with an alpha = 0.05 significance threshold. Comparative analyses to identify differences in patient and disease characteristics at baseline were performed using Chi-square tests or Fisher's Exact tests (for sparse tables) on categorical variables and Kruskal-Wallis tests on continuous variables. Similar tests were also conducted to identify differences based on histologic subtype.
Survival was defined as the time from BAC diagnosis to death or date the patient was last reported to be alive. Patients known to be alive at the last contact were censored. The Kaplan-Meier method was used to create survival curves and to estimate survival at yearly increments. Overall survival differences in gender, age at diagnosis, smoking status, histologic subtype, stage of disease, history of other cancer (excluding non-melanoma skin cancer), and presence of symptoms were assessed for statistical significance by the log-rank test. Differences in survival at one year were calculated by censoring all deaths that occurred after one year. Similarly, differences in survival at five years and ten years were calculated by censoring all deaths that occurred after five years and ten years, respectively. Multivariable Cox proportional hazards models were constructed to estimate the relative risk of mortality using the predetermined predictors of age at diagnosis, gender, smoking status, and presence of symptoms at presentation.
Results
Between January 1, 1997 and December 31, 2007, 338 patients were diagnosed with BAC. Of these, 117 were classified as pure BAC by the 1999/2004 WHO definition and 221 were classified as non pure BAC. Of the 117 pure BAC patients, 78 had no other non BAC lung cancer.
The mean age at diagnosis of the 78 pure BAC patients was 66 years, 68% were women and 28% were never smokers (Table 1). Seventeen percent had a family history of lung cancer among first degree relatives and 65% had a family history of other cancers among first degree relatives. Five percent had another BAC primary (Table 2). Eighteen percent were mucinous type (Table 3). No significant differences were observed between males and females with respect to treatments received (Table 3). The one-year survival rate was 94.8% (95% CI: 90.0% to 99.9%), and the five-year survival rate was 83.5% (95% CI: 74.9% to 93.1%) (Table 4).
Table 1.
Comparison of Patients with Pure and Non-Pure Bronchioloalveolar Carcinoma with No Other Non-BAC Lung Cancer
| Pure BAC (N=78) | Non-pure BAC (N=178) | Total (N=256) | p-valuea | |
|---|---|---|---|---|
| Age at diagnosis | 0.0540b | |||
| Mean (SD) | 65.9 (9.61) | 67.9 (12.27) | 67.3 (11.54) | |
| Median | 67.0 | 69.0 | 69.0 | |
| Q1, Q3 | 61.0, 73.0 | 62.0, 76.0 | 61.5, 75.0 | |
| Age at diagnosis | 0.0438 | |||
| ≤55 | 12 (15.4%) | 26 (14.6%) | 38 (14.8%) | |
| 56–74 | 53 (67.9%) | 96 (53.9%) | 149 (58.2%) | |
| ≥75 | 13 (16.7%) | 56 (31.5%) | 69 (27%) | |
| Gender | 0.0099 | |||
| Female | 53 (67.9%) | 90 (50.6%) | 143 (55.9%) | |
| Male | 25 (32.1%) | 88 (49.4%) | 113 (44.1%) | |
| Race | 0.1510 | |||
| Missing | 0 (-) | 8 (-) | 8 (-) | |
| Non-Caucasianc | 8 (10.3%) | 9 (5.3%) | 17 (6.9%) | |
| Caucasian | 70 (89.7%) | 161 (94.7%) | 231 (93.1%) | |
| Smoking status at diagnosis | 0.8704 | |||
| Never smoker | 22 (28.2%) | 56 (31.5%) | 78 (30.5%) | |
| Former smoker | 41 (52.6%) | 90 (50.6%) | 131 (51.2%) | |
| Current smoker | 15 (19.2%) | 32 (18%) | 47 (18.4%) | |
| Pack-years (ever smokers) | 0.2376 | |||
| Missing | 0 (-) | 17 (-) | 17 (-) | |
| 0–20 | 17 (30.4%) | 30 (28.6%) | 47 (29.2%) | |
| 21–40 | 22 (39.3%) | 30 (28.6%) | 52 (32.3%) | |
| 41–60 | 11 (19.6%) | 21 (20%) | 32 (19.9%) | |
| >60 | 6 (10.7%) | 24 (22.9%) | 30 (18.6%) | |
| SHSd exposure (never smokers) | 0.9900 | |||
| Missing | 5 (-) | 18 (-) | 23 (-) | 1.0000e |
| Not exposed | 4 (23.5%) | 9 (23.7%) | 13 (23.6%) | |
| Exposed | 13 (76.5%) | 29 (76.3%) | 42 (76.4%) | |
| Family history of lung cancer (1st degree) | 0.7612 | |||
| No | 65 (83.3%) | 151 (84.8%) | 216 (84.4%) | |
| Yes | 13 (16.7%) | 27 (15.2%) | 40 (15.6%) | |
| Family history of other cancer (1st degree) | 0.0044 | |||
| No | 27 (34.6%) | 96 (53.9%) | 123 (48%) | |
| Yes | 51 (65.4%) | 82 (46.1%) | 133 (52%) |
All p-values are from chi-square tests unless otherwise noted.
Kruskall-Wallis test
Non-Caucasian includes Alaskan/Native American, black, Asian/Pacific Islander, and other unspecified races.
SHS - Secondhand smoke
Fisher's exact test
Table 2.
Comparison of Disease Characteristics between Patients with Pure and Non-Pure Bronchioloalveolar Carcinoma with No Other Non-BAC Lung Cancer
| Pure BAC (N=78) | Non-pure BAC (N=178) | Total (N=256) | p-valuea | |
|---|---|---|---|---|
| Grade | 0.0006 | |||
| Well differentiated | 78 (100%) | 148 (83.1%) | 226 (88.3%) | <0.0001b |
| Moderately differentiated | 0 (0%) | 14 (7.9%) | 14 (5.5%) | |
| Nongradable & missing | 0 (0%) | 16 (9.0%) | 16 (6.3%) | |
| Stage (old) | <0.0001 | |||
| Missing | 0 (-) | 7 (-) | 7 (-) | |
| IA | 61 (78.2%) | 70 (40.9%) | 131 (52.6%) | |
| IB | 7 (9%) | 36 (21.1%) | 43 (17.3%) | |
| II/IIIA | 0 (0%) | 14 (8.2%) | 14 (5.6%) | |
| IIIB/IV | 10 (12.8%) | 51 (29.8%) | 61 (24.5%) | |
| Tumor (old) | <0.0001 | |||
| Missing | 0 (-) | 9 (-) | 9 (-) | |
| T1 | 63 (80.8%) | 76 (45.0%) | 139 (56.3%) | |
| T2 | 10 (12.8%) | 52 (30.8%) | 62 (25.1%) | |
| T3–4c | 5 (6.4%) | 21 (12.4%) | 26 (10.5%) | |
| Not assessable | 0 (0%) | 20 (11.8%) | 20 (8.1%) | |
| Multiple BACd primaries | 0.8252 | |||
| No | 74 (94.9%) | 170 (95.5%) | 244 (95.3%) | 0.7595b |
| Yes | 4 (5.1%) | 8 (4.5%) | 12 (4.7%) | |
| Recurrence/progression of BACd | 0.3003 | |||
| No | 72 (92.3%) | 170 (95.5%) | 242 (94.5%) | 0.3706b |
| Yes | 6 (7.7%) | 8 (4.5%) | 14 (5.5%) |
All p-values are from chi-square tests unless otherwise noted.
Fisher's exact test.
All 5 pure BAC patients and 19 non-pure BAC cases were T4. The remaining two non-pure BAC patients were T3, one due to lung atelectasis and one due to satellite nodules in the same lobe.
BAC defined as any current BAC diagnosis for the pure BAC group, defined as an ever diagnosis of BAC for the non-pure BAC group.
Table 3.
Comparison of Disease Characteristics, Comorbidities, Symptoms and Performance Status, and Treatment between Male and Female Patients with Pure Bronchioloalveolar Carcinoma and No Other Non-BAC Lung Cancer
| Female (N=53) | Male (N=25) | Total (N=78) | p-valuea | |
|---|---|---|---|---|
| Histologic subtype | 0.7458 | |||
| Non-mucinous | 44 (83%) | 20 (80%) | 64 (82.1%) | 0.7591b |
| Mucinous | 9 (17%) | 5 (20%) | 14 (17.9%) | |
| Stage (new) | 0.3900 | |||
| IA | 41 (77.4%) | 20 (80%) | 61 (78.2%) | 0.3542b |
| IB | 2 (3.8%) | 2 (8%) | 4 (5.1%) | |
| II/IIIA | 8 (15.1%) | 1 (4%) | 9 (11.5%) | |
| IIIB/IV | 2 (3.8%) | 2 (8%) | 4 (5.1%) | |
| Tumor (new) | 0.8638 | |||
| T1 | 42 (79.3%) | 20 (80%) | 62 (79.5%) | 1.0000b |
| T2 | 6 (11.3%) | 2 (8%) | 8 (10.3%) | |
| T3–4 | 5 (9.4%) | 3 (12%) | 8 (10.3%) | |
| Comorbid non-lung cancerc | 0.6626 | |||
| Missing | 12 (-) | 3 (-) | 15 (-) | |
| No | 21 (51.2%) | 10 (45.5%) | 31 (49.2%) | |
| Yes | 20 (48.8%) | 12 (54.5%) | 32 (50.8%) | |
| Comorbid non-LCc (not non-melanoma skin) | 0.5951 | |||
| Missing | 12 (-) | 3 (-) | 15 (-) | |
| No | 27 (65.9%) | 13 (59.1%) | 40 (63.5%) | |
| Yes | 14 (34.1%) | 9 (40.9%) | 23 (36.5%) | |
| Comorbid lung diseased | 0.0671 | |||
| Missing | 12 (-) | 3 (-) | 15 (-) | |
| No | 21 (51.2%) | 6 (27.3%) | 27 (42.9%) | |
| Yes | 20 (48.8%) | 16 (72.7%) | 36 (57.1%) | |
| Comorbid non-lung diseasee | 0.2192 | |||
| Missing | 12 (-) | 3 (-) | 15 (-) | |
| No | 9 (22%) | 8 (36.4%) | 17 (27%) | |
| Yes | 32 (78%) | 14 (63.6%) | 46 (73%) | |
| Any symptoms present | 0.3898 | |||
| No | 33 (62.3%) | 13 (52%) | 46 (59%) | |
| Yes | 20 (37.7%) | 12 (48%) | 32 (41%) | |
| Weight loss | 0.2496 | |||
| Missing | 1 (-) | 2 (-) | 3 (-) | 0.2960b |
| No | 46 (88.5%) | 18 (78.3%) | 64 (85.3%) | |
| Yes | 6 (11.5%) | 5 (21.7%) | 11 (14.7%) | |
| Performance status | 0.2145 | |||
| 0 | 41 (77.4%) | 16 (64%) | 57 (73.1%) | |
| ≥1 | 12 (22.6%) | 9 (36%) | 21 (26.9%) | |
| Type of surgery | 0.5629 | |||
| Major resectionf | 2 (3.8%) | 0 (0%) | 2 (2.6%) | 0.8002b |
| Lobectomy | 39 (73.6%) | 18 (72%) | 57 (73.1%) | |
| Other resectiong | 12 (22.6%) | 7 (28%) | 19 (24.4%) | |
| Any chemotherapy | 0.5208 | |||
| No | 49 (92.5%) | 22 (88%) | 71 (91%) | 0.6741b |
| Yes | 4 (7.5%) | 3 (12%) | 7 (9%) | |
| Chemotherapy response | 0.3679 | |||
| Missing | 2 (-) | 1 (-) | 3 (-) | 1.0000b |
| Complete | 1 (50%) | 0 (0%) | 1 (25%) | |
| Progression | 0 (0%) | 1 (50%) | 1 (25%) | |
| Incomplete | 1 (50%) | 1 (50%) | 2 (50%) | |
| Any radiation | 0.5816 | |||
| No | 52 (98.1%) | 24 (96%) | 76 (97.4%) | 0.5411b |
| Yes | 1 (1.9%) | 1 (4%) | 2 (2.6%) |
All p-values are from chi-square tests unless otherwise noted
Fisher's exact test
Comorbid cancer includes bladder, breast, head and neck, kidney, liver, lymphoma, melanoma, non-melanoma skin, prostate, thyroid, uterine, and other unspecified cancers.
Comorbid lung diseases include asthma, asthmatic bronchitis, acute bronchitis, chronic bronchitis, unspecified bronchitis, COPD, emphysema, pneumonia, and other unspecified pulmonary diseases.
Comorbid non-lung diseases include arthritis, diabetes, GI disease, heart disease, hypertension, kidney disease, liver disease, peripheral vascular disease, rheumatoid arthritis, stroke, and other unspecified non-pulmonary diseases.
Major resections include pneumonectomy and bilobectomy.
Other resections include segmentectomy, wedge resection, other lung surgeries, and mets resection.
Table 4.
Comparison of Survival between Patients with Pure and Non-Pure Bronchioloalveolar Carcinoma with No Other Non-BAC Lung Cancer
| Overall (n=256) | Pure BAC (N=78, 30.5%) | Non-Pure BAC (N=178, 69.5%) | p-value | |
|---|---|---|---|---|
| Number of events (deaths) | 109 (42.6%) | 15 (19.2%) | 94 (52.8%) | |
|
| ||||
| Median survival (years) | 7.59 | 10.41 | 4.30 | |
|
| ||||
| Overall post-diagnosis survival | ||||
| 6 months | 93.7% (90.7, 96.7) | 96.1% (91.9, 100) | 92.6% (88.8, 96.6) | <0.0001 |
| 1 year | 89.2% (85.4, 93.1) | 94.8% (90.0, 99.9) | 86.7% (81.8, 91.9) | <0.0001a |
| 2 years | 77.5% (72.4, 82.9) | 93.5% (88.1, 99.2) | 70.4% (63.8, 77.6) | <0.0001b |
| 3 years | 71.8% (66.3, 77.7) | 92.1% (86.2, 98.4) | 62.6% (55.6, 70.5) | |
| 4 years | 63.8% (57.8, 70.4) | 85.4% (77.3, 94.3) | 54.0% (46.7, 62.4) | |
| 5 years | 57.9% (51.5, 64.9) | 83.5% (74.9, 93.1) | 46.4% (38.9, 55.3) | |
| 6 years | 54.4% (47.8, 61.8) | 81.3% (72.1, 91.7) | 42.2% (34.6, 51.5) | |
| 7 years | 53.3% (46.7, 60.9) | 81.3% (72.1, 91.7) | 40.8% (33.1, 50.3) | |
| 8 years | 48.0% (40.5, 56.8) | 77.0% (65.6, 90.4) | 35.5% (27.2, 46.2) | |
| 9 years | 42.2% (33.8, 52.8) | 69.3% (53.4, 90.1) | 31.1% (22.6, 42.9) | |
| 10 years | 38.7% (29.2, 51.3) | 69.3% (53.4, 90.1) | 26.7% (17.2, 41.4) | |
Follow-up time was capped at 10 years and all deaths after 10 years were censored (n=1).
Follow-up time was capped at 5 years and all deaths after 5 years were censored (n=23).
Of the 221 non pure BAC patients, 178 had no other non BAC lung cancer and would have been classified as BAC before the use of 1999 WHO definition. Compared to the 78 pure BAC, a greater percentage of the 178 non pure BAC patients were diagnosed ≥ 75 years of age, were more likely to be male, had no family history of other cancers in first degree relatives (Table 1), and had higher grade and stage tumors (Table 2). Both the one-year survival rate of 86.7% (95% CI: 81.8% to 91.9%) and the five-year survival rate of 46.4% (95% CI: 38.9% to 55.3%) were remarkably lower (p = 0.0618 for 1 year and p < 0.0001 for 5 year) than pure BAC patients (Table 4).
Impact of the New Staging System
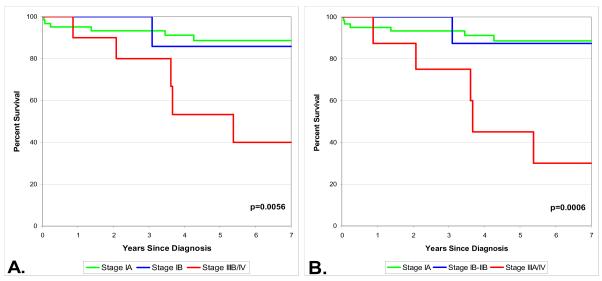
When the 78 pure BAC cases were restaged in accordance with the seventh edition of the TNM classification of Malignant Tumors, the assigned stage changed for 9 cases (12%), a change occurred from IB to IIA for 3 cases, IIIB to IIB for 2 cases and IV to IIIA for 4 cases.
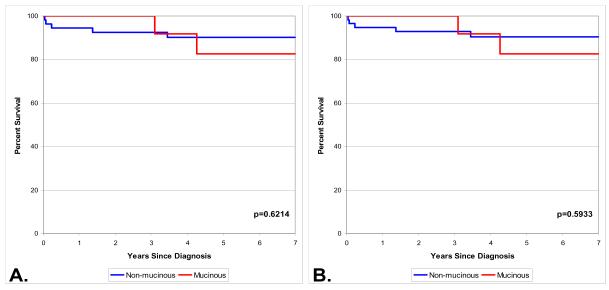
Among the 78 pure BAC cases, based upon the old staging system, patients with stage IIIB/IV had significantly worse survival than those with stage IA (Figure 1, Panel A). Based upon the 2009 staging system, patients with stage IIIA/IV had significantly worse survival than patients with stage IA (Figure 1, Panel B). No differences were observed in survival between non mucinous and mucinous histologies under either staging system (Figure 2).
Figure 1.
Survival by old (1999) stage (A) and new (2009) stage (B) for 78 patients with pure bronchioloalveolar carcinoma and no other non-BAC lung cancer.
Figure 2.
Survival by histologic subtype in early stage patients with pure bronchioloalveolar carcinoma and no other non-BAC lung cancer. Old stage (A, n=68, stages IA and IB), new stage (B, n=70, stages IA–IIB).
Discussion
Important findings from the current study are: (1) use of the 1999 WHO definition to re-review 338 BACs diagnosed at Mayo Clinic, Rochester, between 1997 and 2007 resulted in only 117 (35%) meeting the new criteria; (2) patients with pure BAC by the 1999 definition had a greater 5-year survival (83.5%) compared to the patients with BAC under the old definition (46.4%); and (3) the 2009 staging system better distinguished between early versus late stage BAC with 5-year survival at 88.5% vs 45.0%, respectively.
We observed a mean age of 65.9 ± 9.6 years among patients with pure BAC at the time of diagnosis, and the mean age of patients with BAC in the SEER database has been reported to be 67.1 years.8 In a study of the association between age and NSCLC among 293,427 incident cases of NSCLC in the SEER database from 1973 to 2002, the mean age of 13,859 patients with BAC was 66.99 ± 10.66 years.9 A multivariable analysis of the SEER data showed that patients with BAC were more likely to be female (adjusted OR 2.06, 95% C.I. 1.99 to 2.14) and we observed that almost two-thirds of pure BAC cases were women. Although the SEER data is limited by the inability to confirm the diagnosis of BAC by independent pathologists, our findings relating to gender and age in patients with BAC are similar.
We observed higher 1- and 5-year survival rates (94.8% and 83.5%, respectively) among the pure BAC patients identified in our cohort than has been reported previously. In a study analyzing data from the population-based Cancer Surveillance Programs of three Southern California counties from 1995 to 2003, survival for BAC patients was 69.6% at one year and 41.4% at 5 years.10 The investigators concluded that the observed survival benefit likely reflects changes in the revised 1999 WHO classification. However, the study did not involve central pathologic review of all cases and included BAC cases from different institutions in the three Southern California counties. Further, it is unlikely that the 1999 classification was uniformly applied by clinical pathologists immediately after publication of the new WHO classification and that the cases of BAC diagnosed after 1999 were indeed pure BAC as established by the 1999 WHO classification.
Our observation that 59% of pure BAC patients had no symptoms present at the time of diagnosis is also consistent with previous investigations. In a study of 274 patients with NSCLC undergoing surgical resection, patients with incidentally detected lung cancer were three times as likely to have BAC histology.11 Among patients who received a baseline CT scan in the Early Lung Cancer Action Project (ELCAP), more than two-thirds had BAC histology.12 Our study supports the prevailing notion in the literature that most BAC lung cancer is discovered incidentally.
We observed that 28.2% of pure BAC patients were never smokers. Smoking has not always been thought to be a risk factor for BAC. However, case-control studies have demonstrated an association between BAC and intensity of cigarette smoking.13, 14 Between 24–33% of BAC patients, 15% of adenocarcinoma patients, and 5% of squamous cell carcinoma patients are never smokers.3, 13–15 16 However, the exact importance of tobacco smoke exposure, active and passive, on BAC risk is not known. Possible other risk factors for BAC that have been considered include pulmonary parenchymal damage and scarring from pulmonary diseases, occupational exposures and viral infection.
A viral etiology has been suggested for BAC given its clinical and histologic similarities to sheep pulmonary adenomatosis (SPA) which is caused by the Jaagsiekte sheep retrovirus (JSRV).17 Indeed, this etiologic conclusion was based on the ability to produce SPA by inoculating sheep with cell-free lung secretions of infected animals, Dungal, 1946 #5355} and with full length JSRV proviral clones transfected in vivo into newborn lambs.18 JSRV has also been demonstrated in epithelial tumor cells by immunohistochemical staining,19 and the genomic sequence of JSRV has been derived from viral particle RNA purified from lung secretions of OPA-affected sheep.20, 21 The similarity of clinical course and histologic morphology between SPA and human BAC, particularly mucinous BAC, has raised interest on the possible role of JSRV in the development of human lung carcinoma. In 1994, researchers suggested that JSRV may be related to the human BAC.22 These findings were followed by an immunohistochemical study showing that 30% of BAC and 26% of typical adenocarcinomas stained positively with antiserum against the JSRV capsid protein.23 Although some researchers reported the presence of JSRV in tissue sections from human BAC depends on patients' geographical origin,24 molecular studies using PCR and RT-PCR techniques could not confirm this finding.25 SRV continues to be an interesting model for BAC although there is insufficient evidence to support a causal association.
Our study has several notable strengths. First, the patients in the current study are a subset of lung cancer patients who have prospectively enrolled at diagnosis; all patients with a diagnosis of lung cancer are invited to participate, which eliminates selection bias. Second, the diagnosis of BAC was confirmed by review of tumor slides by a pulmonary pathologist. This is particularly important given that many BAC epidemiology studies do not include central pathologic review, thus making it difficult for clinicians and researchers to interpret the results. Third, detailed information on each study patient was available for analysis including sites of other cancers for patients who had a cancer other than BAC.
One of the limitations of the current study relates to a potential referral bias. We have minimized the referral bias by including both patients referred to Mayo Clinic, Rochester and patients who live in the surrounding community. A second limitation is that most included patients were Caucasian. A third limitation relates to missing data for some clinical information. However, we do not believe that the missing data had any particular pattern that can be related to significant impact on the study results.
Our study provides new information on the clinical characteristics and excellent prognosis of patients with pure BAC. Because the new pathology and staging systems affect epidemiology, treatment, and life expectancy for BAC, they have important implications for researchers, clinicians, and patients.
Acknowledgements
We would like to thank Susan M. Ernst, M.A., for her technical assistance with the manuscript.
Funding Source This work was supported by Mayo Foundation Funds and grants CA80127 and CA 84354 (Yang) from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Conflicts of Interest None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Auerbach O, Garfinkel L. The changing pattern of lung carcinoma. Cancer. 1991;68:1973–1977. doi: 10.1002/1097-0142(19911101)68:9<1973::aid-cncr2820680921>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 2.Barsky SH, Cameron R, Osann KE, et al. Rising incidence of bronchioloalveolar lung carcinoma and its unique clinicopathologic features. Cancer. 1994;73:1163–1170. doi: 10.1002/1097-0142(19940215)73:4<1163::aid-cncr2820730407>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 3.Furak J, Trojan I, Szoke T, et al. Bronchioloalveolar lung cancer: occurrence, surgical treatment and survival. Eur J Cardiothorac Surg. 2003;23:818–823. doi: 10.1016/s1010-7940(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 4.Travis WD, Colby TV, Shimosato Y, et al. Histological Typing of Lung and Pleural Tumours. 3rd edition Springer-Verlag; New York: 1999. [Google Scholar]
- 5.Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136:260–271. doi: 10.1378/chest.08-0978. [DOI] [PubMed] [Google Scholar]
- 6.International Agency for Research on Cancer, International Academy of Pathology . World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. 1st ed. IARC Press, International Agency for Research on Cancer; Lyon, France: 2004. [Google Scholar]
- 7.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 8.Read WL, Page NC, Tierney RM, et al. The epidemiology of bronchioloalveolar carcinoma over the past two decades: analysis of the SEER database. Lung Cancer. 2004;45:137–142. doi: 10.1016/j.lungcan.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Raz DJ, Jablons DM. Bronchioloalveolar carcinoma is not associated with younger age at diagnosis: an analysis of the SEER database. J Thorac Oncol. 2006;1:339–343. [PubMed] [Google Scholar]
- 10.Zell JA, Ou SH, Ziogas A, et al. Epidemiology of bronchioloalveolar carcinoma: improvement in survival after release of the 1999 WHO classification of lung tumors. J Clin Oncol. 2005;23:8396–8405. doi: 10.1200/JCO.2005.03.0312. [DOI] [PubMed] [Google Scholar]
- 11.Raz DJ, Glidden DV, Odisho AY, et al. Clinical characteristics and survival of patients with surgically resected, incidentally detected lung cancer. J Thorac Oncol. 2007;2:125–130. doi: 10.1097/jto.0b013e31802f1cb1. [DOI] [PubMed] [Google Scholar]
- 12.Flieder DB, Vazquez M, Carter D, et al. Pathologic findings of lung tumors diagnosed on baseline CT screening. AM J Surg Pathol. 2006;30:606–613. doi: 10.1097/01.pas.0000202040.51967.d0. [DOI] [PubMed] [Google Scholar]
- 13.Falk RT, Pickle LW, Fontham ET, et al. Epidemiology of bronchioloalveolar carcinoma. Cancer Epidemiol Biomarkers Prev. 1992;1:339–344. [PubMed] [Google Scholar]
- 14.Morabia A, Wynder EL. Relation of bronchioloalveolar carcinoma to tobacco. BMJ. 1992;304:541–543. doi: 10.1136/bmj.304.6826.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barkley JE, Green MR. Bronchioloalveolar carcinoma. J Clin Oncol. 1996;14:2377–2386. doi: 10.1200/JCO.1996.14.8.2377. [DOI] [PubMed] [Google Scholar]
- 16.Thun MJ, Lally CA, Flannery JT, et al. Cigarette smoking and changes in the histopathology of lung cancer. J Natl Cancer Inst. 1997;89(21):1580–1586. doi: 10.1093/jnci/89.21.1580. [DOI] [PubMed] [Google Scholar]
- 17.Palmarini M, Fan H. Retrovirus-induced ovine pulmonary adenocarcinoma, an animal model for lung cancer. J Natl Cancer Inst. 2001;93:1603–1614. doi: 10.1093/jnci/93.21.1603. [DOI] [PubMed] [Google Scholar]
- 18.Palmarini M, Sharp JM, De las Heras M, et al. Jaagsiekte sheep retrovirus is necessary and sufficient to induce a contagious lung cancer in sheep. J Virol. 1999;73:6964–6972. doi: 10.1128/jvi.73.8.6964-6972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Platt JA, Kraipowich N, Villafane F, et al. Alveolar type II cells expressing jaagsiekte sheep retrovirus capsid protein and surfactant proteins are the predominant neoplastic cell type in ovine pulmonary adenocarcinoma. Vet Pathol. 2002;39:341–352. doi: 10.1354/vp.39-3-341. [DOI] [PubMed] [Google Scholar]
- 20.York DF, Vigne R, Verwoerd DW, et al. Isolation, identification, and partial cDNA cloning of genomic RNA of jaagsiekte retrovirus, the etiological agent of sheep pulmonary adenomatosis. J Virol. 1991;65:5061–5067. doi: 10.1128/jvi.65.9.5061-5067.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.York DF, Vigne R, Verwoerd DW, et al. Nucleotide sequence of the jaagsiekte retrovirus, an exogenous and endogenous type D and B retrovirus of sheep and goats. J Virol. 1992;66:4930–4939. doi: 10.1128/jvi.66.8.4930-4939.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Connell J, De las Heras M, Palmarini M. JSRV related sequence and capsid protein in human lung BAC/PAC suggests a retroviral connection. Mod Pathol. 1998;11:5A. Abstract. [Google Scholar]
- 23.De las Heras M, Barsky SH, Hasleton P, et al. Evidence for a protein related immunologically to the jaagsiekte sheep retrovirus in some human lung tumours. Eur Respir J. 2000;5:330–332. doi: 10.1034/j.1399-3003.2000.16b23.x. [DOI] [PubMed] [Google Scholar]
- 24.Rocca S, Sanna MP, Leoni A, et al. Presence of Jaagsiekte sheep retrovirus in tissue sections from human bronchioloalveolar carcinoma depends on patients' geographical origin. Hum Pathol. 2008;39:303–304. doi: 10.1016/j.humpath.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Yousem SA, Finkelstein SD, Swalsky PA, et al. Absence of jaagsiekte sheep retrovirus DNA and RNA in bronchioloalveolar and conventional human pulmonary adenocarcinoma by PCR and RT-PCR analysis. Hum Pathol. 2001;32:1039–1042. doi: 10.1053/hupa.2001.28249. [DOI] [PubMed] [Google Scholar]