Abstract
Purpose
This study examined the effect of a yearlong exercise intervention on F2-isoprostane, a specific marker of lipid peroxidation and a general marker of oxidative stress.
Methods
In a randomized, controlled trial, 173 overweight or obese, postmenopausal, sedentary women were randomized to an aerobic exercise intervention (60–75% observed maximal heart rate) for ≥ 45 min·d−1, 5 d·wk−1 (n = 87), or to a stretching control group (n = 86), on an intent-to-treat basis. Baseline and 12-month measures included: urinary F2-isoprostane, maximal O2 uptake, body weight, body fat percentage, waist circumference, and intra-abdominal fat surface area. Urine samples were available from 172 and 168 women at baseline and 12-months, respectively.
Results
Over the 12-month study, controls minimally changed maximal O2 uptake (+0.2%) and body weight (+0.1 kg), whereas exercisers increased maximal O2 uptake (+13.6%; p-value < 0.0001 versus controls) and decreased body weight (−1.3 kg; p-value = 0.007 versus controls). F2-isoprostane increased slightly among controls (+3.3%) and decreased in exercisers (−6.2%), although the effect was not statistically significant (p-value = 0.26). In planned subgroup analyses, F2-isoprostane decreased linearly with gain in maximal O2 uptake (ptrend = 0.005) relative to controls; exercisers who increased maximal O2 uptake >15% decreased F2-isoprostane 14.1% (p-value = 0.005 versus controls). A borderline statistically significant trend was observed between decreased waist circumference and F2-isoprostane (p-value = 0.06). Similar subgroup analyses by 12-month changes in body fat percentage, weight, and intra-abdominal fat were not statistically significant.
Conclusions
These findings suggest that aerobic exercise, when accompanied by relatively marked gains in aerobic fitness, decreases oxidative stress among previously sedentary older women, and that these effects occur with minimal change in mass or body composition.
Keywords: intervention, women, aerobic, maximal oxygen uptake, F2-isoprostane, overweight/obese
Introduction
Oxidative stress occurs when the production of reactive species, derived largely from oxygen and nitrogen, exceeds degradation by the antioxidant defense system. The ensuing damage to DNA, protein, and lipid has been implicated in cardiovascular and pulmonary diseases, diabetes, neurodegenerative disorders, and some cancers (3,19). Thus, intervention strategies to reduce oxidative stress, especially among overweight/obese persons who generally have high oxidative stress levels (19), have widespread appeal. Such efforts with human study subjects have been hindered by difficulties in the measurement of oxidative damage. Recently, however, sensitive and stable methods have become available to measure F2-isoprostanes (7). F2-isoprostanes are a family of isomeric F2-prostaglandin-like compounds, derived from free radical-catalyzed peroxidation of arachidonic acid, independent of the cyclooxygenase enzyme. A recent multi-institutional study concluded that F2-isoprostane was the most accurate method to assess oxidative stress in vivo from urine or plasma samples (9).
Reduced oxidative stress, probably achieved through improved antioxidant defenses and/or reduced reactive species formation, may be one mechanism that links physical activity to reduced risk of chronic disease (3,19). Non-controlled exercise intervention studies among premenopausal women have noted 25% (6) and 34% (18) decreased F2-isoprostane concentrations after 12–15 weeks of exercise training; however, no intervention effects were observed in an 8-week controlled exercise trial among older type 2 diabetics (15). Given the lack of data on this topic, we investigated the effect of a yearlong aerobic exercise intervention compared to a stretching control program on F2-isoprostane concentrations in postmenopausal women. We hypothesized that the 12-month exercise intervention would decrease F2-isoprostane, and that this effect would be mediated by changes in maximal O2 uptake and by measures of body size/ fat distribution.
This work was conducted with ancillary funding, using previously collected urine specimens and data from the Physical Activity for Total Health study (ClinicalTrials.gov Identifier: NCT00668174). The main objective of the parent trial was to examine the effects of exercise on steroid hormones among 173 postmenopausal, previously sedentary, overweight/obese women, as described previously (11–13). A subgroup of participants (n = 115) from the parent trial, who met additional inclusion criteria, were included in separately funded studies of the effects of exercise on immune function (5) and inflammation (4).
METHODS and PROCEDURES
Setting and Participants
Participants were 173 women who resided in the Seattle, WA area. Eligibility criteria included: age 50–75 years; body mass index (BMI) between 25 and 40 kg·m−2 (or 24.0–24.9 kg·m−2 if body fat percentage > 33%); postmenopausal, not taking hormones in the previous 6 months; non-smoker; sedentary at baseline (moderate-or vigorous-activity < 60 min·wk−1 and maximal O2 uptake < 25 mL·kg−1·min−1); alcohol consumption of fewer than 2 drinks per day; fasting glucose < 140 mg·dL−1; and no history of cancer, diabetes, or cardiovascular disease. All women provided written, informed consent and all procedures were approved by the Fred Hutchinson Cancer Research Center Institutional Review Board.
Randomization and Exercise Intervention
Randomization was stratified by BMI (< 27.5 or ≥ 27.5 kg·m−2) to ensure similar numbers of heavier and lighter women in each group. The exercise intervention progressed to ≥ 45 min·d−1 of moderate-intensity aerobic exercise (60–75% of observed maximal heart rate), 5 d·wk−1, by the 8th week of the trial, where it was maintained to the end of study. For months 1–3, the intervention participants attended 3 mandatory exercise sessions at a study facility (University of Washington or a commercial gym) and exercised twice per week at home. For months 4–12, the intervention group attended at least 1 session per week at a study facility and conducted the remaining sessions at home or at a study facility.
Exercisers wore Polar Heart Rate monitors during all exercise sessions and maintained exercise logs that included information on the duration, mode, relative perceived exertion, and peak heart rate of all sport and recreational activities estimated at ≥ 3 METS (1). Exercise logs were reviewed weekly by study staff to monitor adherence with the study protocol and to intervene when needed. Women in the control group attended once-weekly 45 minute stretching and relaxation sessions and were asked to not otherwise change exercise habits for the duration of the trial.
All women were asked to not change their dietary habits for the duration of the trial. At baseline (immediately prior to randomization), 3-months, and 12-months, participants completed self-reported questionnaires on diet (120-item food frequency questionnaire), alcohol consumption, medications, and dietary supplement usage, as described previously (17).
Outcomes
At baseline and 12-months, all women had a clinic visit for spot urine collection after a 12h fast. All samples were processed within one hour of collection, aliquoted into 1.8-ml tubes, and stored at −70°C. Date and time of collection and time since last meal were recorded, as well as medications used, vigorous activities in the past eight hours, and consumption of alcoholic beverages in the previous 48 hours. Participants were instructed to refrain from alcohol (48 hours) and vigorous exercise (8–12 hours) prior to their clinic appointments. Participants who did not comply had their clinic appointments rescheduled. All participants were contacted 1 week after their clinic visit to track recent illness; participants who reported being ill during this interval returned for a second clinic visit when symptom free.
F2-isoprostane was measured by its major urinary metabolite, 15-F2t-isoprostane, 2,3-dinor-8-iso-prostaglandin F2α via gas chromatography-mass spectrometry–based methodology (14). The assay was done on a Hewlett-Packard 5971 MSD quadrupole instrument, using selective ion monitoring, after solid-phase chromatography, thin-layer separations, and derivatization.
Baseline and 12-month samples from each participant were assayed in the same batch. Each batch contained an approximately equal number of exerciser and control samples. Pooled quality control (QC) samples were included in each batch. Lab personnel were blinded to intervention/QC status. Intra-assay and inter-assay coefficients of variation were 5.8% and 9.8%, respectively. Assay results were measured as ng·mg−1 of creatinine, to account for hydration status. F2-isoprostane values were non-normally distributed and were log-transformed and presented as geometric means with 95% confidence intervals.
Maximal O2 uptake (aerobic fitness) was assessed at baseline and at 12-months in all participants by a maximal-graded treadmill test using a Medgraphics automated metabolic cart (Medgraphics, St. Paul, MN), using a previously described protocol (5). Also measured at baseline and 12-months were: body weight to the nearest 0.1 kg and height to the nearest 0.1 cm, both in duplicate, to compute BMI; waist circumference to the nearest 0.1 cm; total body fat and body fat percentage by dual-energy X-ray absorptiometry (DXA, QDR 1500; Hologic, Waltham, MA); and intra-abdominal and subcutaneous fat images by a 1-slice computed tomography scan at L4-5 (CT; model CT 9800 scanner; General Electric, Waukesha, WI).
Statistical Analyses
Spearman correlation coefficients were calculated for the associations between baseline F2-isoprostane values and baseline values for maximal O2 uptake, percentage body fat, body weight, waist circumference, and intra-abdominal body fat, and between 12-month changes in F2-isoprostane values and 12-month changes in maximal O2 uptake, percentage body fat, body weight, waist circumference, and intra-abdominal body fat. Correlation coefficients were computed among all women, with no consideration of intervention group assignment. Age (continuous) was included in the correlation models.
The main intervention effect was assessed based on a comparison between exercisers and controls as defined at randomization, regardless of exercise adherence (i.e. intent-to-treat). The difference between groups was assessed with a generalized estimating equation (GEE), which accounts for repeated observations on the same subjects over time (20). In the primary trial analysis, the GEE model was specified with follow-up (i.e. 12-month) F2-isoprostane values as the outcome variable, and group assignment (exercise or control) as the main predictor variable. Baseline F2-isoprostane values and age (continuous) were included as covariates in the primary GEE model.
In planned secondary analyses we examined subgroup effects with controls (referent group) compared to exercisers stratified by exercise adherence and changes in maximal O2 uptake and body composition/distribution. For exercisers, these subgroups were meant to create approximately equal yet easily interpretable tertiles. Because the control group was expected to have minimal changes in any of these parameters, we decided a priori to not further stratify the control group. The subgroups for exercisers were defined as: average exercise adherence over 12-months of < 136 min·wk−1, 136–195 min·wk−1, or > 195 min·wk−1; 12-month increases in maximal O2 uptake of < 5%, 5–15% or > 15%; body fat percentage decrease of < 0.1%, decrease of 0.1–2%, or decrease of > 2%; body weight decrease of < 0.5 kg, decrease of 0.5–3 kg, or decrease of > 3 kg; waist circumference decrease of < 0.5 cm, decrease of 0.5 to 3 cm, or decrease of > 3 cm; and intra-abdominal fat (IAF) surface area decrease of < 1 cm−2, decrease of 1–8 cm−2, or decrease of > 8 cm−2).
In the subgroup analyses, each GEE model included follow-up F2-isoprostane values as the outcome variable, with group assignment as the main predictors. The group assignment variable had four mutually exclusive values: controls (denoted by a ‘0’ indicator variable) and 3 indicator values to denote the exercise group (‘1’, ‘2’, ‘3’), depending on their exercise adherence or category of 12-month change in maximal O2 uptake or body composition/distribution, as defined above. Tests for linear trends were obtained by including the 4-level group assignment variable (i.e., 0, 1, 2, 3) as a continuous value in the GEE regression model. Baseline F2-isoprostane values and age (continuous) were again included as covariates in these models. Missing outcome data were omitted from GEE analyses. All statistical tests were 2-sided.
RESULTS
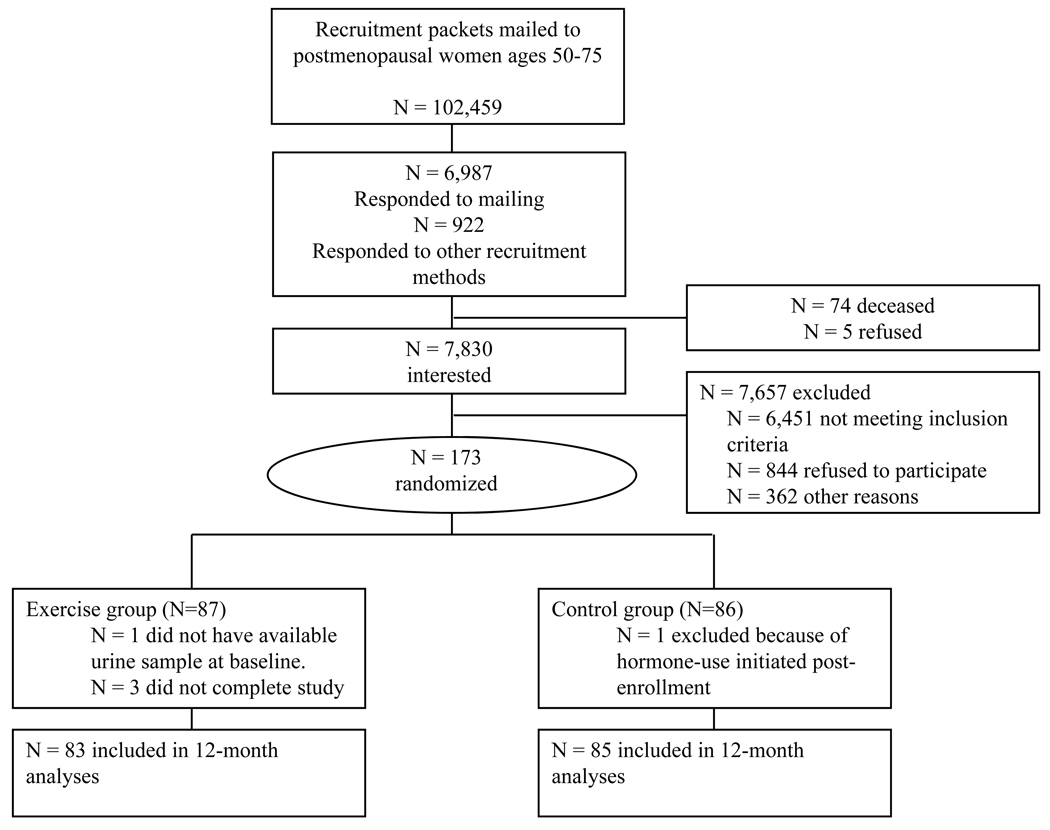
Eighty-seven women were randomized to the 12-month exercise intervention, 86 to the stretching control group. One exerciser did not have a baseline urine sample; one control was excluded from the study for hormone-use initiated after randomization and three exercisers did not complete the study (>97% retention) (Figure 1). Women were, on average, 61 years of age (range 50–75 years). At baseline, participants had a mean BMI of 30 kg·m−2, 47% body fat, and relatively low maximal O2 uptake (mean: 20 ml·kg−1·min−1). There were no statistically significant differences between exercise and control groups at baseline for any of the descriptive demographic, smoking, dietary, phenotypic, or physiologic parameters (Table 1). Over 12-months, exercisers averaged 171.3 min·wk−1 (SD: 87.9; median: 170 min·wk−1) of moderate-to-vigorous activity. Exercisers increased maximal O2 uptake 13.6% whereas controls increased maximal O2 uptake 0.2% (p-value <0.0001). Exercisers decreased body weight and body fat percentage compared to controls (exercisers: −1.3 kg and −1.4%; controls: +0.1 kg and −0.1%; p-values = 0.007 and 0.001, respectively).
Figure 1.
Flow of participants through the trial.
Table 1.
Baseline characteristics of exercisers and controls.
| Exercisers (n=87) | Controls (n=86) | ||
|---|---|---|---|
| Mean (standard deviation) or N | p-value* | ||
| Age, years | 60.7 (6.7) | 60.6 (6.8) | 0.89 |
| Self-described race/ethnicity | |||
| American Indian | 0 | 2 | |
| African American | 4 | 3 | |
| Asian/Pacific Islander | 6 | 3 | |
| Hispanic/Latino | 0 | 2 | |
| Non-Hispanic White | 74 | 75 | |
| Other/ no response | 3 | 1 | 0.29 |
| Body Mass Index, kg·m−2 | 30.4 (4.1) | 30.5 (3.8) | 0.87 |
| n < 30 kg·m−2 | 45 | 45 | |
| n ≥ 30 kg·m−2 | 42 | 41 | 0.94 |
| Body fat percentage (DEXA) | 47.5 (4.8) | 47.4 (4.6) | 0.86 |
| Maximal O2 uptake, ml·kg−1·min−1 | 20.1 (3.5) | 20.5 (3.0) | 0.38 |
| Education | |||
| High school or less | 10 | 9 | |
| Some college or college degree | 41 | 45 | |
| Graduate or professional degree | 36 | 32 | 0.79 |
| Income | |||
| <$35,000 | 21 | 33 | |
| $35,000–$75,000 | 41 | 29 | |
| >$75,000 | 24 | 19 | 0.08 |
| Smoking status | |||
| Never smoker | 46 | 46 | |
| Former smoker | 41 | 40 | 0.94 |
| Average daily caloric intake, kcal | 1635 (792) | 1722 (671) | 0.43 |
| Average daily alcohol intake, g | 4 (8.4) | 4.6 (7.2) | 0.62 |
| Average daily servings of fruit, number | 1.8 (1.4) | 1.8 (1.4) | 0.79 |
| Average daily servings of vegetables, number |
2.1 (1.4) | 2.3 (1.6) | 0.40 |
| Total physical activity per week, minutes | 143 (127) | 127 (214) | 0.29 |
Values are means (standard deviation) or frequency counts. N, number of subjects.
P-values from independent sample t-tests or chi-square goodness of fit tests. DEXA, dual energy X-ray absorptiometry
There were moderate correlations between baseline F2-isoprostane values and baseline values for maximal O2 uptake (r = −0.37; p-value < 0.0001), percentage body fat (r = 0.45; p-value < 0.0001), body weight (r = 0.30; p-value = 0.0002), waist circumference (r = 0.40; p-value < 0.0001), and intra-abdominal body fat (r = 0.31; p-value < 0.0001). There was a moderate association between 12-month changes in F2-isoprostanes and 12-month changes in maximal O2 uptake (r = −0.27; p-value = 0.02); while correlations between 12-month changes in F2-isoprostanes and 12-month changes in body fat (r = −0.08; p-value = 0.52), body weight (r = 0.12; p-value = 0.32), waist circumference (r = 0.20; p-value = 0.10), and intra-abdominal body fat (r = −0.14; p-value = 0.24) were weaker and not statistically significant.
There were no statistically significant between-group differences for controls versus exercisers, including analyses where exercisers were stratified by exercise adherence, from baseline to 12-months for dietary intake of total energy, fat, protein, carbohydrate, cholesterol, fiber, sucrose, calcium and alcohol or for the number of servings per week of vegetables and fruits, as described in detail previously (17). There were also no statistically significant between-group differences for use of non-steroidal anti-inflammatory drugs, any dietary supplements, and multivitamins over the yearlong trial (17). Additionally, there were no statistically significant between-group differences for any of the above dietary/lifestyle/pharmacologic factors when compared across subgroups of gain in maximal O2 uptake, with the singular exception of a dietary fat intake decrease (p-value = 0.05) among exercisers who gained > 15% maximal O2 uptake relative to controls. Inclusion of change in dietary fat intake into the GEE model for gain in maximal O2 uptake had no material effect on the study results (data not shown)
From baseline to 12-months, F2-isoprostane increased slightly among controls (+3.3%) and decreased modestly in exercisers (−6.2%), although the exercise intervention effect was not statistically significant (p-value = 0.26), as shown in Table 2. In planned subgroup analyses, however, with controls again as the referent group, F2-isoprostane decreased linearly among exercisers with gain in maximal O2 uptake (ptrend = 0.005); exercisers who increased aerobic fitness > 15% decreased F2-isoprostane 14.1% (p-value = 0.005 versus controls). Similar linear trends, although not statistically significant, were observed for exercise adherence (ptrend = 0.11) and waist circumference (ptrend = 0.06). When 12-month changes in maximal O2 uptake and waist circumference were included in the same GEE model, the p-value for waist circumference was attenuated further toward the null (ptrend = 0.25), while the p-value for gain in maximal O2 uptake was only marginal attenuated by inclusion of change in waist circumference (ptrend = 0.008).
Table 2.
Effect of a yearlong exercise intervention relative to a stretching control program: overall and stratified by exercise adherence, and 12 month changes in aerobic fitness, adiposity, and adipose tissue distribution.
| F2-isoprostane (ng·mg−1 of creatinine) | |||||||
|---|---|---|---|---|---|---|---|
| n | Baseline Geometric Mean (95% CI) |
n | 12 month Geometric Mean (95% CI) |
% change |
p- valuea |
ptrendb | |
| Controls (referent group) | 86 | 0.60 (0.54, 0.66) | 85 | 0.62 (0.56, 0.68) | 3.3 | ||
| Exercisers - Overall | 86 | 0.65 (0.60, 0.71) | 83 | 0.61 (0.55, 0.68) | −6.2 | 0.26 | |
| Exercisers - Subgroups | |||||||
| Exercise Adherence | |||||||
| Exercised < 136 min·wk−1 | 28 | 0.70 (0.60, 0.83) | 27 | 0.67 (0.56, 0.81) | −4.3 | 0.87 | |
| Exercised 136–195 min·wk−1 | 31 | 0.66 (0.59, 0.74) | 29 | 0.63 (0.54, 0.74) | −4.5 | 0.57 | |
| Exercised > 195 min·wk−1 | 27 | 0.59 (0.49, 0.72) | 27 | 0.54 (0.45, 0.65) | −8.5 | 0.10 | 0.11 |
| Change in Aerobic Fitness | |||||||
| Gained < 5% VO2max | 35 | 0.68 (0.59, 0.80) | 32 | 0.73 (0.61, 0.86) | 7.4 | 0.17 | |
| Gained 5–15% VO2max | 23 | 0.62 (0.54, 0.71) | 23 | 0.56 (0.45, 0.68) | −9.7 | 0.15 | |
| Gained > 15% VO2max | 28 | 0.64 (0.54, 0.75) | 28 | 0.55 (0.47, 0.63) | −14.1 | 0.005 | 0.005 |
| Change in body fat percentage | |||||||
| No change or gained fat | 26 | 0.79 (0.67, 0.93) | 23 | 0.72 (0.57, 0.91) | −8.9 | 0.64 | |
| Body fat decreased ≤ 2% | 34 | 0.55 (0.48, 0.63) | 34 | 0.53 (0.46, 0.60) | −3.6 | 0.17 | |
| Body fat decreased > 2% | 26 | 0.67 (0.59, 0.77) | 26 | 0.64 (0.54, 0.77) | −4.5 | 0.68 | 0.36 |
| Change in weight (kg) | |||||||
| No change or gained body mass | 32 | 0.75 (0.65, 0.86) | 29 | 0.73 (0.62, 0.86) | −2.7 | 0.74 | |
| Body mass decreased ≤ 3 kg | 34 | 0.55 (0.48, 0.63) | 34 | 0.53 (0.46, 0.61) | −3.6 | 0.16 | |
| Body mass decreased > 3 kg | 20 | 0.69 (0.58, 0.82) | 20 | 0.60 (0.47, 0.77) | −13.0 | 0.34 | 0.16 |
| Change in waist circumference (cm) | |||||||
| No change or increased waist circumference | 34 | 0.65 (0.55, 0.77) | 31 | 0.65 (0.54, 0.78) | 0 | 0.95 | |
| Waist circumference decreased ≤ 3 cm | 30 | 0.64 (0.55, 0.74) | 30 | 0.61 (0.53, 0.71) | −4.7 | 0.61 | |
| Waist circumference decreased > 3 cm | 22 | 0.67 (0.58, 0.78) | 22 | 0.56 (0.45, 0.70) | −16.4 | 0.04 | 0.06 |
| Change in intra-abdominal fat (cm2) | |||||||
| No change or increased intra-abdominal fat | 30 | 0.68 (0.58, 0.79) | 27 | 0.62 (0.53, 0.74) | −8.8 | 0.36 | |
| Intra-abdominal fat decreased ≤ 8 cm2 | 22 | 0.60 (0.49, 0.74) | 22 | 0.58 (0.46, 0.74) | −3.3 | 0.42 | |
| Intra-abdominal fat decreased > 8 cm2 | 34 | 0.66 (0.58, 0.75) | 34 | 0.62 (0.53, 0.73) | −6.1 | 0.50 | 0.40 |
P-value from generalized estimating equation (GEE) regression model with F2-isoprostane values at twelve months as the outcome, and group assignment as a categorical predictor variable. Control group is the referent for all comparisons.
P-value for test of trend in GEE models with F2-isoprostane values at twelve months as the outcome, and group assignment entered as a linear predictor variable (e.g. controls = ‘0’; exercised < 136 min·wk−1 = ‘1’; exercised 136–195 min·wk−1 = ‘2’; exercised > 195 min·wk−1 = ‘3’).
Baseline F2-isoprostane and age (continuous) were included as co-variates in all models.
Discussion
We examined the effect of a yearlong aerobic exercise intervention compared to a stretching control program on urinary F2-isoprostane, a marker of systemic oxidative stress, among previously sedentary, overweight/obese, postmenopausal women. Overall, exercisers decreased F2-isoprostane concentrations, as predicted by our primary hypothesis, but the effect was not statistically significant, except in planned analyses by strata of gain in aerobic fitness.
Few previous studies have examined the effects of exercise alone on F2-isoprostane concentrations (6,15,18), and no earlier studies, to our knowledge, were conducted exclusively among postmenopausal women. These studies suggest that exercise may reduce quite strongly F2-isoprostanes among relatively younger women (6,18), whereas this beneficial effect may be attenuated among older study subjects, as observed currently, and previously (15). The three earlier studies, and the current study, noted similarly decreased body mass and increased aerobic fitness after exercise intervention, despite the wide range of intervention periods and age groups studied. Thus, these data suggest a potential age effect for the capacity of exercise to reduce oxidative stress. The mechanisms to explain such an effect are discussed in detail elsewhere (2), and are largely based on experiments with lab animals: essentially, older animals have been observed to experience damage to cardiac and skeletal muscle after short-term exercise training if it is not initiated prior to a certain age, indicative of a physiologic age threshold. Alternatively, if exercise is initiated prior to this age, older animals experience the same health benefits as younger exercising animals. However, it is important to note that the older adults in the current study, and the participants in the previous study (15), marginally decreased oxidative stress levels, although the effects were not statistically significant, suggesting the lab animal data are not entirely generalizable to humans.
We observed an inverse linear association between aerobic fitness and oxidative stress, suggesting a hormetic effect of exercise training (10). This finding for aerobic fitness and oxidative stress compliments recent results from a subset of participants in this trial (n = 115) where we reported that the effect of exercise on inflammation (C-reactive protein and serum amyloid A) was restricted to participants who were obese (BMI ≥ 30 kg·m−2 or WC > 88 cm) at baseline (4). In contrast, baseline obesity status did not modify the influence of exercise on F2-isoprostane in the current study (data not shown). For our study participants in particular, these results suggest that gains in aerobic fitness improve oxidative stress levels, probably because of exercise-induced adaptations of the antioxidant-defense system (3,8,16), and this effect occurs independent of general- or abdominal-obesity status. While our subgroup analyses among exercisers by strata of weight/fat loss were largely not statistically significant, it is worth noting that this trial was not designed to elicit large amounts of weight loss, and our exercise participants only reduced body weight by about 1.3 kg, on average. Therefore, we could not assess the effects of large amounts of weight/fat loss on oxidative stress, a limitation that future studies should address.
Particular strengths of this study include: the selection of a sensitive and stable biomarker for oxidative stress (9); the long period of exercise intervention, with high participant retention; the good participant adherence to the exercise protocol; the bona fide effects of the aerobic exercise intervention, quantified by direct measures of maximal O2 uptake; and the gold-standard randomized, controlled trial design. Limitations of this study include the highly homogeneous study sample which limits comparisons to other groups, and that we studied only one biomarker of oxidative stress, F2-isoprostane; future studies should consider other measures of lipid peroxidation, such as TBARS, and biomarkers that additionally reflect oxidative damage to protein and DNA.
In conclusion, in a randomized, controlled trial with excellent retention and good adherence to the aerobic exercise intervention, conducted among 173 previously sedentary, overweight/obese, postmenopausal women, we observed a modest reduction in F2-isoprostane overall that was not statistically significant. When exercisers were stratified by gain in aerobic fitness, as measured by maximal O2 uptake, an inverse and linear association was observed between aerobic fitness and F2-isoprostane, suggesting a training-effect on oxidative stress.
Acknowledgments and Grant Support
The F2-isoprostane component of this study was supported by a research grant from the National Institutes of Health (CA RO3-130043). The parent trial, the Physical Activity for Total Health study, from which the urine samples and other data were acquired was supported by a research grant from the National Institutes of Health (CA RO1-69334). PTC was Research Fellow of the Canadian Cancer Society through an award from the National Cancer Institute of Canada and also supported by a NIH Transdisciplinary Research on Energetics and Cancer Postdoctoral Fellowship (NCI U54 CA116847). The sponsors had no role on: the design or conduct of the study, the collection, the management, the analyses, the interpretation of the data, or on preparing, reviewing, or approving the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: The authors have no conflicts of interest to disclose.
The results of this study do not constitute endorsement by the ACSM.
References
- 1.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 2.Bar-Shai M, Carmeli E, Ljubuncic P, Reznick AZ. Exercise and immobilization in aging animals: the involvement of oxidative stress and NF-kappaB activation. Free Radic Biol Med. 2008;44(2):202–214. doi: 10.1016/j.freeradbiomed.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Bloomer RJ. Effect of exercise on oxidative stress biomarkers. Adv Clin Chem. 2008;46:1–50. doi: 10.1016/s0065-2423(08)00401-0. [DOI] [PubMed] [Google Scholar]
- 4.Campbell PT, Campbell KL, Wener MH, et al. A yearlong exercise intervention decreases CRP among obese postmenopausal women. Med Sci Sports Exerc. 2009;41(8):1533–1539. doi: 10.1249/MSS.0b013e31819c7feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell PT, Wener MH, Sorensen B, et al. Effect of exercise on in vitro immune function: a 12-month randomized, controlled trial among postmenopausal women. J Appl Physiol. 2008;104(6):1648–1655. doi: 10.1152/japplphysiol.01349.2007. [DOI] [PubMed] [Google Scholar]
- 6.Devries MC, Hamadeh MJ, Glover AW, Raha S, Samjoo IA, Tarnopolsky MA. Endurance training without weight loss lowers systemic, but not muscle, oxidative stress with no effect on inflammation in lean and obese women. Free Radic Biol Med. 2008;45(4):503–511. doi: 10.1016/j.freeradbiomed.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 7.Fam SS, Morrow JD. The isoprostanes: unique products of arachidonic acid oxidation-a review. Curr Med Chem. 2003;10(17):1723–1740. doi: 10.2174/0929867033457115. [DOI] [PubMed] [Google Scholar]
- 8.Ji LL, Gomez-Cabrera MC, Vina J. Role of free radicals and antioxidant signaling in skeletal muscle health and pathology. Infect Disord Drug Targets. 2009;9(4):428–444. doi: 10.2174/187152609788922573. [DOI] [PubMed] [Google Scholar]
- 9.Kadiiska MB, Gladen BC, Baird DD, et al. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic Biol Med. 2005;38(6):698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Mattson MP. Hormesis defined. Ageing Res Rev. 2008;7(1):1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McTiernan A, Tworoger SS, Rajan KB, et al. Effect of exercise on serum androgens in postmenopausal women: a 12-month randomized clinical trial. Cancer Epidemiol Biomarkers Prev. 2004;13(7):1099–1105. [PubMed] [Google Scholar]
- 12.McTiernan A, Tworoger SS, Ulrich CM, et al. Effect of exercise on serum estrogens in postmenopausal women: a 12-month randomized clinical trial. Cancer Res. 2004;64(8):2923–2928. doi: 10.1158/0008-5472.can-03-3393. [DOI] [PubMed] [Google Scholar]
- 13.McTiernan A, Ulrich CM, Yancey D, et al. The Physical Activity for Total Health (PATH) Study: rationale and design. Med Sci Sports Exerc. 1999;31(9):1307–1312. doi: 10.1097/00005768-199909000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Morales CR, Terry ES, Zackert WE, Montine TJ, Morrow JD. Improved assay for the quantification of the major urinary metabolite of the isoprostane 15-F(2t)-Isoprostane (8-iso-PGF(2alpha)) by a stable isotope dilution mass spectrometric assay. Clin Chim Acta. 2001;314(1–2):93–99. doi: 10.1016/s0009-8981(01)00637-4. [DOI] [PubMed] [Google Scholar]
- 15.Mori TA, Dunstan DW, Burke V, et al. Effect of dietary fish and exercise training on urinary F2-isoprostane excretion in non-insulin-dependent diabetic patients. Metabolism. 1999;48(11):1402–1408. doi: 10.1016/s0026-0495(99)90150-6. [DOI] [PubMed] [Google Scholar]
- 16.Radak Z, Chung HY, Goto S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic Biol Med. 2008;44(2):153–159. doi: 10.1016/j.freeradbiomed.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 17.Rhew I, Yasui Y, Sorensen B, et al. Effects of an exercise intervention on other health behaviors in overweight/obese post-menopausal women. Contemp Clin Trials. 2007;28(4):472–481. doi: 10.1016/j.cct.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Schmitz KH, Warren M, Rundle AG, Williams NI, Gross MD, Kurzer MS. Exercise effect on oxidative stress is independent of change in estrogen metabolism. Cancer Epidemiol Biomarkers Prev. 2008;17(1):220–223. doi: 10.1158/1055-9965.EPI-07-0058. [DOI] [PubMed] [Google Scholar]
- 19.Vincent HK, Taylor AG. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J Obes (Lond) 2006;30(3):400–418. doi: 10.1038/sj.ijo.0803177. [DOI] [PubMed] [Google Scholar]
- 20.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]