Abstract
Dichoroacetate (DCA) and trichloroacetate (TCA) were found to be hepatotoxic and hepatocarcinogenic in rodents. To investigate the role of oxidative stress in the long term hepatotoxicity of the compounds, groups of mice were administered 7.7, 77, 154 and 410 mg/kg/day, of either DCA or TCA, by gavage, for 4 weeks (4-W) and 13 weeks (13-W), and superoxide anion (SA), lipid peroxidation (LP) and DNA-single strand breaks (SSBs) were determined in the hepatic tissues. Significant increases in all of the biomarkers were observed in response to the tested doses of both compounds in the two tested periods, with significantly greater increases observed in the 13-W, as compared with the 4-W period. Hepatomegaly was only observed with a DCA dose of 410 mg/kg/day in the 13-W treatment period, and that was associated with significant declines in the biomarkers, when compared with the immediately lower dose. With the exception of LP production in the 13-W treatment period that was similarly induced by the two compounds, the DCA-induced increases in all of the biomarkers were significantly greater than those of TCA. Since those biomarkers were significantly induced by the compounds' doses that were shown to be carcinogenic but at earlier periods than those demonstrating hepatotoxicity/ haptocarcinogencity, they can be considered as initial events that may lead to later production of those long term effects. The results also suggest LP to be a more significant contributing mechanism than SA and DNA damage to the long term hepatotoxicity of TCA.
Keywords: Dichloroacetate, Trichloroacetate, Superoxide anion, Lipid Peroxidation, DNA damage, Liver
INTRODUCTION
Municipal water supplies contain significant amounts of halogenated organic compounds that are formed from the reaction of chlorine with the organic material present in those supplies. Dicloroacetate (DCA) and trichloroacetate (TCA) are among the compounds present in those supplies (Coleman et al., 1976; Krasner et al., 1989; Uden and Miller, 1983), and were found to be toxic in laboratory animals and humans. Risk potential of the compounds arises from possible long term exposure through different sources. TCA and DCA are found in the municipal water supplies at concentrations ranging between 30-160 μg/ml (Jolly, 1985; Udden and Miller, 1983), and drinking from those supplies was found to be associated with bladder cancer (McGeen et., 1993; Vena et al., 1993). The widely used trichloroethylene solvent is found as a contaminant in the surface water in some industrial areas, and in vivo metabolism of this solvent was found to generate DCA and TCA in humans and experimental animals (Decant et al., 1984; Green and Prout, 1985; Hathway, 1980). DCA is also used for therapeutic purposes, especially for the treatment of lactic acidosis in patients with mitochondrial dysfunction (Kimura et al., 1997; Mori et al., 2004; Oishi et al., 2003; Spruijt et al., 2001), but long term use of a certain dose by patients was found to be associated with adverse effects that included mild liver dysfunction, hypoglycemia and changes in the central and peripheral nervous system. (Mori et al., 2004; Oishi etal., 2003; Spruijt et al, 2001). Other sources of DCA and TCA include agricultural uses as herbicides and fungicides and industrial uses as laboratory reagents.
Hepatotoxicity and hepatocarcinogenicity have been identified as the most prominent effects of DCA and TCA that are produced in rodents in general, and in B6C3F1 male mice in particular, after long term exposure (Bull et al., 1990; Daniel et al, 1992; DeAngelo et al., 1991; Herren-Freund et al., 1987). However, hepatic tumor induction by the two compounds in the B6C3F1 male mice was suggested to involve two different mechanisms (Bull et al., 1990). While long term treatment with DCA resulted in the development of hepatomegaly that was associated with massive accumulations of glycogen in the hepatocytes with areas of focal necrosis, TCA treatment resulted in small increases in cell size and much more modest accumulation of glycogen with no focal necrotic damage, but of greater and marked lipofuscin accumulation, compared to the DCA treatment (Bull et al., 1990). Studies of Herren-Freund et al. (1987) have also demonstrated the hepatocarcinogenic effects of DCA and TCA in B6C3F1 mice maintained on drinking water containing high concentrations of the compounds for 61 weeks and suggested the compounds to act as complete hepatocarcinogens.
Production of reactive oxygen species (ROS), such as superoxide anion (SA), together with their associated effects on different cellular components were found to be associated with the exposure to some xenobiotics, as well as with the pathogenesis of several long term diseases (Davies, 1995). Production of SA, Lipid preoxidation (LP) and DNA-single strand breaks (SSBs) were found to be significantly induced in the hepatic tissues of mice exposed to single high doses of DCA and TCA and have been suggested as possible mechanisms for the compounds'-induced hepatotoxicity (Austin et al, 1996; Hassoun and Dey, 2008; Larson and Bull, 1992; Nelson and Bull, 1988; Nelson et al., 1989; Parrish et al., 1996). However, several of those studies have demonstrated DCA to be more potent than TCA in the induction of those biomarkers.
In an attempt to investigate the role of early induction of SA, LP and DNA-SSBs to the long term hepatoxic/hepatocarcinogenic effects of the compounds, this study was designed to determine those biomarkers in the hepatic tissues of B6C3F1 mice exposed to doses of DCA and TCA that were previously shown to be in the range of non hepatotoxic/hepatocarcinogenic to those producing maximal hapatocarcinogenesis in B6C3F1 male mice (Bull et al., 1990; DeAngelo et al, 1991), but at periods earlier than those demonstrating any of those effect. The results of the study have been presented in part, in the Society of Toxicology (SOT) meeting (Hassoun et al., 2009).
MATERIALS AND METHODS
Chemicals
All of the chemicals and reagents used for this study, including sodium dichloroacetate (DCA) and sodium trichloroacetate (TCA), were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO), and were at the highest grade available.
Animals and treatments
B6C3F1 male mice were found to be sensitive to the acute and long term effects of DCA and TCA (Austin et al, 1996; Bull et al., 1990; Hassoun and Dey, 2008; Herren-Freund et al., 1987; Daniel et al., 1992; DeAngelo et al., 1991; Larson and Bull, 1992; Nelson and Bull, 1988; Nelson et al., 1989; Parrish et al., 1996), and they were therefore used for this study. The animals were about 6 weeks of age and weighing approximately 20 g when received. They were allowed to acclimate for 3 days prior to the experimental use, caged at 21° C with a 12 hr light/dark cycle, maintained on a standard laboratory chow from Harlan Teklad (Madison, Wisconsin), and allowed a free access to food and water. DCA and TCA solutions were prepared in distilled water, and pHs of the solutions were adjusted to 7.0 by sodium hydroxide solution. Groups of mice (7 animals/ group) were treated post orally (by gavage), with daily doses of 7.7, 77, 154, and 410 mg/kg body weight of DCA or TCA, for 4 weeks (4-W) and 13 weeks (13-W). These doses were based on previous studies that investigated the abilities of DCA and TCA to induce hepatotoxic and hepatocarcinogenic effects in B6C3F1 mice when administered at concentrations ranging from 1-5 g/l in the drinking water for 52-75 weeks (Bull et al., 1990; deAngelo et al, 1991; Herren-Freund et al., 1987). DeAngelo et al. (1991) have calculated the time-weighted mean daily doses that correspond to 0.05, 0.5, 3.5 and 5 g/l of DCA in the drinking water and found them to be equivalent to 7.6, 77, 410 and 486 mg /kg/day, respectively. The studies have also shown that compounds concentrations equivalent to 7.6, 77, and 410 mg DCA/kg/day are the doses that correspond to the non carcinogenic dose, the threshold carcinogenic dose and the dose that results in 100% tumor prevalence, respectively. Also, Bull et al. (1990) have found that 1 and 2 g/l of DCA or DCA received by the B6C3F1 mice in the drinking water for 52 weeks were hepatocarcinogenic. They also calculated the total doses of either compound that correspond to 1-2 g/l drinking water concentrations administered for 52 weeks and found them to be respectively equivalent to 55-110 g/kg of DCA, and 60-120 g/kg TCA. Accordingly, the daily doses of DCA that correspond to 1-2 g/l for 52 weeks are 150-300 and those of TCA given for the same period of time are165-330 mg/kg/day. Control animals received distilled water at a rate of 5.0 ml/kg body weight/day, after adjusting its pH to 7.0 with sodium hydroxide solution. Animals were euthanized at the end of the treatment periods, using carbon dioxide anesthesia followed by cervical dislocation.
Homogenization of the hepatic tissues
After animals were euthanized, the livers were removed and weighed. A portion of each liver was homogenized in Tris-KCl buffer (0.05 M Tris and 1.15% KCl, pH 7.4) to produce 10% homogenate for the determination of LP and SA production and another portion was homogenized in the homogenization buffer of White et al. (1981), to produce 20% homogenate for the determination of DNA-SSBs.
Determination of superoxide anion (SA)
SA production was determined in the hepatic tissues, using the cytochrome c reduction assay (Babior et al., 1973) with modifications. The specificity of this method for SA production in the hepatic tissues of B6C3F1 male mice has been previously tested and confirmed (Hassoun and Dey, 2008). In brief 25 μl of the hepatic tissue homogenate was added to 2 ml of 0.05 mM cytochrome c in phosphate buffered saline (PBS), pH 7.2. Reaction tubes were incubated for 15 minutes at 37 ° C, and the tubes where then placed on ice to terminate the reactions. The reaction tubes were then centrifuged at 3000 rpm for 15 minutes and absorbances of the supernatant fractions were measured at 550 nm, using Spectronic 20 spectrophotometer (Rochester, NY). Absorbance values were converted to nmoles of cytochrome c reduced/min, using the extinction coefficient 2.1 × 104M−1 cm−1.
Determination of lipid peroxidation (LP) in the hepatic tissues
The thiobarbituric acid reactive substances (TBARS) formation was used as an index of LP in the whole hepatic tissue homogenate, and was determined using the assay of Uchiyama and Mihara (1978), with modifications. In brief, 0.5 ml of tissue homogenates were added to tubes containing 3 ml of 1% H3PO4 and 1 ml of 0.6% thiobarbituric acid solution. Butylated hydroxy toluene (0.25 mg/ml) was also added to each tube to prevent formation of other oxidants during the heating process. The mixtures were heated in a water bath at a boiling temperature for 45 minutes. After cooling to room temperature, 1 ml of n-butanol was added to each tube, and the tubes were vortexed and centrifuged at 3000g for 10 min. Absorbances of the n-butanol layers were determined at 535 nm, using Spectronic 20 spectrophotometer (Rochester, NY), and absorbance values were converted into nmoles, using an extinction coefficient of 1.56 × 105 M−1Cm−1.
Determination of DNA-SSBs
DNA damage was measured in the nuclear fraction of the hepatic tissue homogenates, as single strand breaks (SSBs), using the alkaline elution technique (Wahba et al., 1988; White et al., 1981). The hepatic tissue homogenates were centrifuged at 3000 rpm for 15 minutes, and nuclear fractions were separated and re-suspended in half of the original volume of the homogenizing buffer. The hepatic nuclear homogenates (0.1 ml) were layered onto 5.0 μm SMWP Millipore filters (Millipore Corporation, Bedford, MA). Using a monostat cassette pump, the nuclear fractions were lysed for 20 minutes at a rate of 200 μl/min with a lysing solution containing 2% (w/v) sodium dodecyl sulfate (SDS) and 25 mM EDTA, pH 10.3, adjusted with NaOH and were then eluted at a rate of 100 μl/min with an elution solution containing 0.1% w/v SDS and 20 mM EDTA, pH 12.3, adjusted with disodium salt of tetraethyl ammonium hydroxide. Seven fractions, 3 ml each were collected using an Isco fraction collector (Isco, Lincoln, NE) and DNA was precipitated by adding 0.1 ml bovine serum albumin solution (BSA) (2.5 mg/ml) and 1.0 ml of 40% trichloroacetic acid to each fraction. The fractions were kept in a refrigerator for 2-3 h and were then centrifuged at 3000 rpm for 10 min. The precipitates were washed once with 3 ml ethanol: HCL (36:1 v/v) solution and were then allowed to dry overnight, in a fume hood. After complete dryness, 0.1 ml of 27% 3,5-diamino benzoic acid dihydrochloride solution was added to each tube, and tubes were incubated in a water bath for 45 minutes at 60° C. DNA contents were measured microflurometrically (excitation 436 nm and emission 521 nm) in a Shimadzu RF 5000U Spectroflurorometer. The elution rate constant (k) was determined by plotting the log 10 of the %DNA remaining on the filter after each fraction collection, against the cumulative volume of the eluate, where k = −2.3 × slope of this plot.
Determination of protein
The protein amounts of the hepatic tissue homogenates were determined according to the method of Lowry et al. (1951), using BSA as the standard.
Statistical methods
Data were analyzed using Microsoft Excel data analysis tool package. Data are expressed as means of 7 samples (animals) ± S.D. A two-factor Analysis of Variance (ANOVA) with replication was used to determine the statistical differences between the effects of different doses and time points on each biomarker. A two-factor ANOVA without replication was used to compare the dose-response curves of DCA and TCA for each of the tested biomarkers at each time point. Student's t-test was used to compare the effects of various doses on the liver weight (LW), and the % liver/body (L/B) weight in each period of treatment, with the corresponding control in that period. Scheffe's S method was used as a post hoc test and a significance level of p< 0.05 was employed.
RESULTS
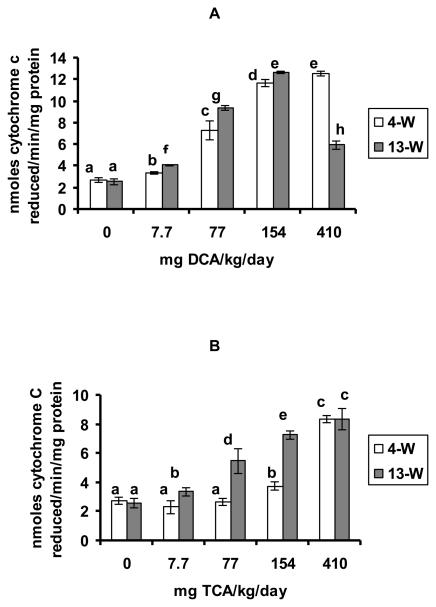
The effects of DCA and TCA on SA production in the hepatic tissues are demonstrated in figures 1 A and B, respectively. Significant SA production was observed in response to all of the tested doses of DCA at the 4-W and the 13-W treatment periods, as compared with the corresponding controls. Except for 410 mg of DCA/kg/day in the 13-W treatment period that displayed a decline in the effect when compared with the immediately lower dose at that period, the increases in the two periods were dose-dependent (figure 1A). While SA production was significantly induced in response to TCA doses of 154 and 410 mg/kg/day after 4-W of treatment, it was significantly induced by all of the tested doses in the 13-W period, as compared with the corresponding controls (figure 1B). Except for a TCA dose of 410 mg/kg/day where no significant difference in SA production was seen when comparing the two periods, all other doses displayed significantly greater production of SA in the 13-W treatment period, as compared with the 4-W period.
Figures 1A and B.
SA production, determined as cytochrome c reduced/min/mg protein in the hepatic tissues of the DCA- treated (A) and TCA-treated mice (B), 4 and 13 weeks after treatment. Each value is the mean of 7 samples (7 animals) ± S.D. The effects of different doses, including the control, were compared against each other in the same treatment period, as well as in the other period of treatment. Columns with non identical superscripts are significantly different (p< 0.05)
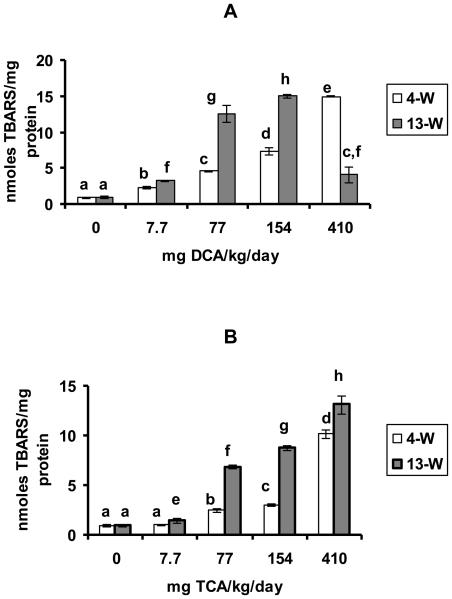
DCA and TCA effects on the production of LP are demonstrated in figures 2 A and B, respectively. LP production was significantly increased in response to all of the tested doses of DCA in the 4-W and 13-W treatment periods when compared with the corresponding controls (figure 2A). Those increases were dose-dependent in response to all of the tested doses in the 4-W treatment period and also in response to doses ranging from 7.7-154 mg/kg/day, in the 13-W treatment period (figure 2 A). With the exception of 410 mg DCA/kg/day dose where a significant decline in the response was observed in the 13-W period when compared with the immediately lower dose at that period, the increases in response to the rest of the tested doses were significantly greater in the 13-w treatment period as compared with the 4-W period. TCA treatment on the other hand, resulted in dose-dependent increases in LP production at doses ranging between 77-410mg/kg/day and 7.7-410 mg/kg/day in the 4-W and 13-W treatment periods, respectively (figure 2B). The figure also indicates that TCA-induced LP production in response to the different doses of TCA in the 13-W treatment period were significantly greater than those produced by the corresponding doses in the 4-W period (figure 2B).
Figures 2A and B.
LP production, determined as nmole TBARS produced/mg protein in the hepatic tissues of the DCA- treated (A) and TCA-treated (B) mice, 4 and 13 weeks after treatment. Each value is the mean of 7 samples (7 animals) ± S.D. The effects of different doses, including the control, were compared against each other in the same treatment period, as well as in the other period of treatment. Columns that do not share an identical superscript are significantly different (p< 0.05)
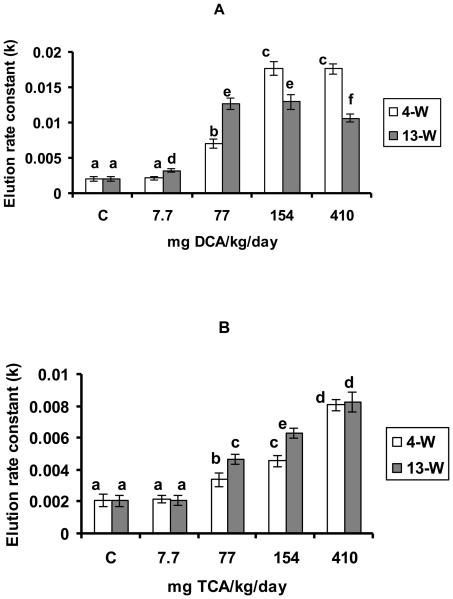
DCA administration at doses ranging between 77-410 mg/kg/day and 7.7-410 mg/kg/day resulted in significant increases in DNA-SSBs in the 4-W and 13-W periods, respectively, as compared with the corresponding controls (figure 3A). However, the differences between the observed increases in response to 154-410 and 77-154 mg TCA/kg/day in the 4-W and 13-W periods, respectively were non significant (figure 3 A). Figure 3 A also shows a significant decline in the level of DNA damage occurring in response to 410 mg DCA/kg/day in the 13-W treatment period, as compared with the immediately lower dose in that period. TCA on the other hand resulted in dose-dependent increases in DNA-SSBs when administered at doses ranging between 77-410 mg/kg/day in the two tested treatment periods, with significantly greater increases observed in the 13-W treatment period as compared with the 4-W period (figure 3B). The figure also demonstrates similar increases in the level of DNA damage in response to 410 mg TCA/kg/day in the two tested treatment periods.
Figures 3A and B.
DNA-SSBs production, indicated by the elution rate constant (k), in the hepatic tissues of the DCA- treated (A) and TCA-treated (B) mice, 4 and 13 weeks after treatment. Each value is the mean of 7 samples (7 animals) ± S.D. The effects of different doses, including the control, were compared against each other in the same treatment period, as well as in the other period of treatment. Columns with non identical superscripts are significantly different (p< 0.05)
Table 1 shows the effects of DCA and TCA treatment on LW and the % L/B weight at the end of the treatment periods. Except for the 410 mg of DCA/kg/day administered for 13-W that produced significant increases in the LW and the % L/B weight when compared with the corresponding control at that period of treatment, all other doses of either compound at either period of treatment did not result in significant changes in those parameters.
Table 1.
DCA and TCA effects on the liver weight (LW) and the % liver/body (L/B) weight at the end of the treatment periods. Each value is the mean of 7 samples (animals) ± S.D
| LW (g) | % L/B weight | |
|---|---|---|
| Control | ||
| 4-W | 1.57 ± 0.16 | 5.5 ± 0.5 |
| 13-W | 1.99 ± 0.26 | 5.7 ± 0.5 |
|
DCA (mg/kg/day) 4-W |
||
| 7.7 | 1.63 ± 0.11 | 5.5 ± 0.1 |
| 77 | 1.57 ± 0.19 | 5.4 ± 0.5 |
| 154 | 1.72 ± 0.12 | 5.7 ± 0.1 |
| 410 | 1.80 ± 0.26 | 6.2 ± 0.6 |
|
DCA (mg/kg/day) 13-W |
||
| 7.7 | 2.00 ± 0.12 | 5.7 ± 0.2 |
| 77 | 1.93 ± 0.15 | 5.6 ± 0.2 |
| 154 | 2.20 ± 0.27 | 6.3 ± 0.4 |
| 410 | 2.60 ± 0.15* | 7.5 ± 0.3* |
|
TCA (mg/kg/day) 4-W |
||
| 7.7 | 1.57 ± 0.16 | 5.5 ± 0.4 |
| 77 | 1.63 ± 0.16 | 5.5 ± 0.3 |
| 154 | 1.67 ± 0.16 | 5.4 ± 0.3 |
| 410 | 1.67 ± 0.23 | 5.6 ± 0.4 |
|
TCA (mg/kg/day) 13-W |
||
| 7.7 | 1.99 ± 0.26 | 5.7 ± 0.5 |
| 77 | 2.00 ± 0.16 | 5.7 ± 0.3 |
| 154 | 1.96 ± 0.15 | 5.3 ± 0.2 |
| 410 | 1.99 ± 0.20 | 5.5 ± 0.4 |
Significantly different from the corresponding control, p< 0.05, using t-test.
Table 2 shows the results of comparisons between the effects of DCA and TCA on the production of SA, LP and DNA-SSBs by the tested doses at each period of treatment. Data of the DCA dose-response curve for each biomarker (7 data points/dose), in each of the tested time points were pooled and compared with the corresponding pooled data of TCA, using two-factor ANOVA with out replication. Except for LP production after 13-W of treatment where no significant difference between the effects of DCA and TCA was reported (p> 0.05), all other differences between the effects of the two compounds were significantly different (p< 0.05), with DCA producing greater effects than TCA.
Table 2.
p-Values for comparisons between the effects of DCA and TCA on the production of SA, LP and DNA-SSBs at each period of treatment, using two-factor ANOVA with out replication. p> 0.05 is non significant.
| SA | LP | DNA | |
|---|---|---|---|
| (4-W) | 1.3 × 10−8 | 4.1 × 10−10 | 3.2 × 10−6 |
| (13-W) | 0.0069 | 0.117 | 31.8 × 10−7 |
DISCUSSION
Previous studies have reported a relative increase of 136% in L/B weight, in B6C3F1 mice treated with a DCA dose of 0.5g/l for 60 weeks (equivalent to 77 mg DCA/kg/day), and have suggested that dose to be the threshold dose for cancer production (DeAngelo et al, 1991). The results of the present study demonstrate an approximate increase in the L/B weight of about 132% over the control, in response to 410 mg DCA/kg/day administration for 13-W. The observed increase in this study is equivalent to that produced by 77 mg DCA /kg/day in the 60-75 weeks studies, and accordingly, the 410 mg DCA/kg/day may be considered as a threshold dose in the 13-W period. The tumorgenic response to high doses of DCA has been shown to be greatly influenced by the compound's-induced hepatomegaly associated with focal necrotic lesions (Bull et al, 1990) and that, DCA-induced hepatomegaly and focal lesions result in a significantly high and sustained level of cell proliferation (Sanchez and Bull, 1989). Inline with that, a DCA dose of 410 mg/kg/day given for 13 weeks is expected to be associated with those cellular changes. While Bull et al. (1990) have demonstrated a significant increase in L/B weight by 1g TCA/l (equivalent to 150 mg/kg/day), after 52 weeks of exposure, no significant increase in the L/B was observed with any of the TCA tested doses at either period of treatment in this study. Therefore, a higher dose than 410 mg TCA/kg/day, and /or a longer treatment period than 13-W may be required to observe any effect.
The results indicate production of dose- and time- dependent increases in SA by DCA and TCA. Free radicals that are transiently generated from certain xenobiotics are known to be re-oxidized by oxygen, generating SA (Mason, 1982, Kappus, 1981), and DCA and TCA metabolism that involves generation of free radicals through reductive dechlorination pathways (Lasrson and Bull, 1992) may have contributed to SA overproduction.
Changes in the levels of LP and DNA damage in response to the tested doses of each of DCA and TCA in the two periods of treatment have similar profiles to that of SA production by the same doses of each compound. This may suggest the contribution of SA production to the observed production of LP and DNA damage by the compounds.
While LP production by DCA in the 4-W treatment period was significantly greater than that of TCA in the same period, it was similarly induced by the two compounds in the 13-W period. These findings clearly demonstrate the slower induction of LP by TCA. Previous studies have demonstrated a more extensive metabolism and rapid rate of elimination of DCA, relative to TCA in B6C3F1 mice and rats, after oral administration of the compounds (Larson and Bull, 1992; Shultz et al, 1999). Also Larson and Bull (1992) have indicated a TCA pathway of metabolism that involves a one-electron reduction and hemolytic cleavage catalyzed by cyp-450, forming dichloroacetyl radical that may abstract a hydrogen atom from lipids yielding DCA. Therefore, the slow metabolism of TCA to DCA, together with the abstraction of a hydrogen atom from the lipids may have contributed to the more significant effects of TCA on LP production in the later period, as compared with the earlier one. The fact that SA production by TCA in the 13-W treatment period was significantly less than that of DCA in the same period suggests the contribution of additional mechanisms, besides SA, to the TCA-induced LP production. For example, H2O2 is another ROS that can be formed through SA dismutation by superoxide dismutase (SOD), and like the other ROS, it can induce LP production (Davies, 1995). Hence, modulation of SOD activity by DCA and TCA may lead to changes in SA/H2O2 levels in the cell that may contribute differently to LP production by either compound. Preliminary studies in our lab have indicated significant inhibition and induction of SOD by DCA and TCA, respectively. However, further studies on this and other antioxidant enzymes are required to confirm this suggestion. Although DNA-SSBs production is suggested to be related to SA production by the compounds, the contribution of the fore mentioned mechanisms and /or ROS to that damage is not excluded.
Based on the observation that maximal increases in SA and DNA damage production were reached in response to 410 mg TCA/kg/day in the two periods of treatment, no further increases in those biomarkers are expected to be seen in response to that dose with possible extension of the treatment period. This may also indicate that the TCA-induced increases in SA and DNA damage production, although significant, but were not sufficient to produce changes in the liver, such as hepatomegaly. On the contrary, LP production by TCA did never plateau with any of the treatments, and is therefore suggested to play a more significant role than SA and DNA damage in the TCA induced long-term hepatotoxicity/ hepatocarcinogenicity. DCA on the other hand resulted in maximal increases in SA, LP and DNA damage production when administered at doses of 410 and 154 for 4-W and 13-W, respectively, with declines in those biomarkers associated with hepatomegaly occurring at a dose of 410 mg/kg/day, after 13-W exposure. This may indicate that those maximal levels are sufficient to initiate the primary hepatic damage by DCA, and that the damage is associated with cellular changes that make them more resistant to further induction of those biomarkers by the compound. Studies in our lab are underway to validate this suggestion.
Except for LP production by DCA and TCA that was found to be similarly induced by the two compounds in the 13-W treatment period, significantly greater induction of all other biomarkers, including LP in the 4-W period, was observed with DCA, as compared with TCA. These may be contributed to differences in the kinetics of the compounds. Larson and Bull (1992) have demonstrated the excretion of more than 50% of any single dose of TCA as unchanged compound in the urine of B6C3F1 mice, compared to less than 2% of any dose of DCA. Since the compounds'-metabolism has been suggested as a contributing mechanism to the generation of free radicals and ROS by the compounds, it is therefore expected to observe less effects with the 50% of the TCA dose that undergoes metabolism, compared with the 98% of the DCA dose.
In summary, exposure of mice to doses of DCA and TCA that were previously found to range between non hepatocarcinogenic to those producing maximal hepatocarcinogenic response, result in dose- and time-dependent increases in SA, LP and DNA-SSBs production at periods much earlier than those required to produce hepatic cancer. The results suggest that significant induction of those biomarkers to a certain level by DCA and TCA can be the early events that may lead to the primary initiation of those long term effects. Also, while the three tested biomarkers are suggested to contribute to DCA long term effects, LP production appears to contribute more than SA and DNA damage to the TCA-induced effects.
ACKNOWLEDGEMENT
The project described was supported by Grant Number R15ES013706-01A from the National Institute of Environmental Health Sciences (NIEHS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS.
REFERNCES
- Austin EW, Parrish JM, Kinder DH, Bull RJ. Lipid peroxidation and formation of 8-hydroxyguanosine from acute doses of halogenated acetic acids. Fundam. Appl. Toxicol. 1996;31:77–82. doi: 10.1006/faat.1996.0078. [DOI] [PubMed] [Google Scholar]
- Babior BM, Kipnes RS, Curnutte JT. Biological defense mechanism. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Invest. 1973;52:741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull RJ, Sanchez IM, Nelson MA, Larson JL, Lansing AJ. Liver tumor induction in B6C3F1 mice by dichloroacetate and dichloroacetate. Toxicology. 1990;63:341–359. doi: 10.1016/0300-483x(90)90195-m. [DOI] [PubMed] [Google Scholar]
- Coleman WE, Lingg RD, Melton RG, Kopfler FC. The occurrence of volatile organics in five drinking water supplies using gas chromatography/mass spectroscopy. In: Keith LH, editor. Identification and Analysis of Organic Pollutants of Water. Ann Arbor Science Pub; Michigan: 1976. pp. 305–327. [Google Scholar]
- Daniel FB, DeAngelo AB, Stober JA, Olson GR, Page NP. Hepatocarcinogenicity of chloral hydrate, 2-chloroaldehyde and dichloroacetic acid in the male B6C3F1 mouse. Fundam. Appl. Toxicol. 1992;19:159–168. doi: 10.1016/0272-0590(92)90147-a. [DOI] [PubMed] [Google Scholar]
- Davies KJA. Oxidative stress: the paradox of aerobic life. In: Rice-Evans C, Halliwell B, Lunt GG, editors. Free radical and oxidative stress: Environment, Drugs and Food Additives. Portland Press Ltd; London: 1995. pp. 3–31. [Google Scholar]
- DeAngelo AB, Daniel FB, Stober JA, Olson GR. The carcinogenicity of dichloroacetic acid in the male B6C3F1 mouse. Fundam. Appl. Toxicol. 1991;16:337–347. doi: 10.1016/0272-0590(91)90118-n. [DOI] [PubMed] [Google Scholar]
- Decant W, Metzler M, Henschler D. Novel metabolites of trichloroethylene through dechlorination reactions in mice and humans. Biochem. Pharmacol. 1984;33:2021–2027. doi: 10.1016/0006-2952(84)90568-9. [DOI] [PubMed] [Google Scholar]
- Green T, Prout MS. Species differences in response to trichloroethylene. II Biotransformation in rats and mice. Toxicol. Appl. Pharmacol. 1985;79:401–411. doi: 10.1016/0041-008x(85)90138-3. [DOI] [PubMed] [Google Scholar]
- Hassoun E, Cearfoss J, Spildener J. The roles of oxidative stress an phagocytic activation in the subacute toxicity of the water chlorination by products, di- and tri-chloroacetate. The Toxicologist. Supplement to Toxicological Sciences. 2009;108:66. [Google Scholar]
- Hassoun EA, Dey S. Dichloroacetate- and trichloroacetate-induced phagocytic activation and production of oxidative stress in the hepatic tissues of mice after acute exposure. J. Biochem. Mol. Toxicol. 2008;22:27–34. doi: 10.1002/jbt.20210. [DOI] [PubMed] [Google Scholar]
- Hathway DE. Consideration of evidence of mechanism of 1,1,2 trichloroethylenemetabolism, including new identification of its dichloroacetic acid and trichloroacetic acid metabolites in mice. Cancer Lett. 1980;8:263–269. doi: 10.1016/0304-3835(80)90012-9. [DOI] [PubMed] [Google Scholar]
- Herren-Freund SL, Pereira MA, Khoury MD, Olson G. The carcinogenicity of trichloroethylene and its metabolites, trichloroacetic acid and dichloroacetic acid, in mouse liver. Toxicol. Appl. Pharmacol. 1987;90:183–189. doi: 10.1016/0041-008x(87)90325-5. [DOI] [PubMed] [Google Scholar]
- Jolly RL. Basic issues in water chlorination: A chemical perspective. In: Jolly RL, Bull RJ, Davies WP, Katz S, Roberts MH Jr., Jacobs VA, editors. Water chlorination. Vol. 5. Lewis Pub., Inc.; Michigan: 1985. pp. 19–38. [Google Scholar]
- Kappus H, Sies H. Toxic drug effects associated with oxygen metabolism: Redox cycling and lipid peroxidation. Experientia. 1981;37:1233–1241. doi: 10.1007/BF01948335. [DOI] [PubMed] [Google Scholar]
- Kimura S, Ohtuki N, Nezu A, Tanaka M, Takeshita S. Clinical and radiologic improvement in mitochondrial encephalopathy following sodium dichloroacetate therapy. Brain & development. 1997;19:535–540. doi: 10.1016/s0387-7604(97)00074-0. [DOI] [PubMed] [Google Scholar]
- Krasner SW, McGuire MJ, Jacagelo JG, Patania NL, Reagan KM, Aieta EM. The occurrence of disinfection by-products in U.S. drinking water. J. Amer. Water Works Assoc. 1989;81:41–53. [Google Scholar]
- Larson JL, Bull RJ. Metabolism and lipoperoxidative activity of trichloroacetate and dichloroacetate in rats and mice. Toxicol. Appl. Pharmacol. 1992;115:268–277. doi: 10.1016/0041-008x(92)90332-m. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr WL, Randall RJ. Protein measurements with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mason RP. In: Free radical intermediates in the metabolism of toxic chemicals, in: free Radicals in Biology. Pryor WA, editor. Vol. 5. Academic Press; New York: 1982. pp. 161–222. [Google Scholar]
- McGeen MA, Reif JS, Becher JC, Mangione EJ. Case-control study of bladder cancer and water disinfection methods in Colorado. Am. J. Epidemiol. 1993;138:492–501. doi: 10.1093/oxfordjournals.aje.a116883. [DOI] [PubMed] [Google Scholar]
- Mori M, Yamagata T, Goto T, Saito S, Momoi MY. Dichloroacetate treatment for mitochondrial cytopathy: Long-term effects in MELAS. Brain & Development. 2004;26:453–458. doi: 10.1016/j.braindev.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Nelson MA, Bull RJ. Induction of strand breaks in DNA by trichloroethylene and metabolites in rat liver in vivo. Toxicol. Appl. Pharmacol. 1988;94:45–54. doi: 10.1016/0041-008x(88)90335-3. [DOI] [PubMed] [Google Scholar]
- Nelson MA, Lansing AJ, Sanchez IM, Bull RJ, Springer DL. Dichloroacetic acid- and Trichloroacetic acid-induced DNA single strand breaks are independent of peroxisome proliferation. Toxicology. 1989;58:239–248. doi: 10.1016/0300-483x(89)90139-x. [DOI] [PubMed] [Google Scholar]
- Oishi K, Yoshioka M, Ozawa R, Yamamoto T, Oya Y, Ogawa M, Kawai M. Dichloroacetate treatment for adult patients with mitochondrial disease. Clinical Neurology. 2003;43:154–161. [PubMed] [Google Scholar]
- Parrish JM, Austin EW, Stevens DK, Kinder DH, Bull RJ. Haloacetate-induced oxidative damage to DNA in the liver of male B6C3F1 mice. Toxicology. 1996;110:103–111. doi: 10.1016/0300-483x(96)03342-2. [DOI] [PubMed] [Google Scholar]
- Sanchez IM, Bull RJ. Hepatotoxic effects of dichloroacetate (DCA) and trichloroacetate (TCA) in B6C3F1 mice. Toxicologist. 1989;9:845. [Google Scholar]
- Shultz IR, Merdink JL, Gonzalez-Leon A, Bull RJ. Comaprative toxicokinetics of chlorinated and brominated haloacetates in F344 rats. Toxicol. Appl. Pharmacol. 1999;158:103–114. doi: 10.1006/taap.1999.8698. [DOI] [PubMed] [Google Scholar]
- Spruijt L, Naviaux RK, McGowan KA, Nyhan WL, Sheehan G, Haas RH, Barsho BA. nerve conduction changes in patients with mitochondrial diseases treated with dichloroacetate. Muscle & Nerve. 2001;24:916–924. doi: 10.1002/mus.1089. [DOI] [PubMed] [Google Scholar]
- Uchiyama M, Mihara M. Determination of malondialdehyde precursor in tissues by thiobarbituric acid test. Analyt. Biochem. 1973;86:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- Uden PC, Miller JW. Chlorinated acids and chloral in drinking water. J. Am. Water Works Assoc. 1983;75:524–526. [Google Scholar]
- Vena JE, Graham S, Freundenheim J, Marshall J, Zeilzny M, Swanson M, Sufrin G. Drinking water, fluid intake and bladder cancer in Western New York. Arch. Environ. Health. 1993;48:191–198. doi: 10.1080/00039896.1993.9940820. [DOI] [PubMed] [Google Scholar]
- Wahba ZZ, Lawson TA, Stohs SJ. Induction of hepatic DNA-single strand breaks in rats by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Cancer Lett. 1988;29:281–286. doi: 10.1016/0304-3835(88)90071-7. [DOI] [PubMed] [Google Scholar]
- White RD, Sipes IG, Gandolfi AJ, Bowden GT. Characterization of the hepatic DNA damage caused by 1,2-dibromoethane using the alkaline elution techniques. Carcinogenesis. 1981;2:839–843. doi: 10.1093/carcin/2.9.839. [DOI] [PubMed] [Google Scholar]