Abstract
AIMS
To investigate if mosapride, a prokinetic agent, was an effective adjunct to acid suppression in improving the symptoms of reflux oesophagitis.
METHODS
Patients (n= 96) with reflux oesophagitis were randomly assigned to either mosapride (5 mg three times daily) or placebo for 4 weeks. Symptom severity was assessed by a validated questionnaire at enrolment, 4 and 8 weeks after medication. The primary outcome for the first 4 weeks was decrease in symptom scores. After a 3 day washout period, patients initially allocated to mosapride crossed over to placebo and vice versa for the next 4 weeks. The outcome of the second phase was maintenance of symptom control. All patients received lansoprazole (30 mg once daily) throughout study.
RESULTS
The decreased symptom score after 4 weeks of treatment with lansoprazole and mosapride (n= 50) was 13.42 ± 1.16 (mean ± SEM), similar to that of lansoprazole plus placebo (10.85 ± 1.03, n= 46), with an insignificant difference of 2.57 (95% CI −0.53, 5.67, P= 0.103). However, a sub-group analysis for patients with pre-treatment scores of >18 points (n= 48) revealed that lansoprazole plus mosapride achieved a greater reduction of symptom score than lansoprazole plus placebo (18.22 ± 1.91 vs. 12.88 ± 1.65; mean difference of 5.34, 95% CI 0.28, 10.40, P= 0.039). In the second phase, there was no difference between lansoprazole with mosapride or placebo in maintaining symptom control (39/44 or 86.64% vs. 41/50 or 82%, P= 0.401). Subgroup analysis for those with substantial residual symptoms revealed similar results.
CONCLUSION
Compared with placebo, mosapride generally does not provide additional benefit to a standard dose of lansoprazole in patients with reflux oesophagitis, except possibly in the subgroup of severely symptomatic patients.
Keywords: gastro-oesophageal reflux disease, lansoprazole, mosapride, reflux oesophagitis, therapeutic effectiveness
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Reflux oesophagitis is a common clinical disorder associated with significant morbidity. Proton pump inhibitors are the current pharmacotherapy of choice, but not all treated patients achieve symptom relief. Little is known about the efficacy of mosapride, a prokinetic agent which decreases episodes of gastro-oesophageal reflux, as an adjunct to proton pump inhibitors in improving the symptoms of reflux oesophagitis.
WHAT THIS STUDY ADDS
Mosapride was generally not more effective than placebo as an adjunct therapy to a standard dose of lansoprazole in decreasing the symptom burden of patients with reflux oesophagitis. However, in a subgroup with more severe symptoms, combination therapy with lansoprazole and mosapride was possibly superior to monotherapy with lansoprazole.
Introduction
Gastroesophageal reflux disease (GERD) is a common clinical disorder with an estimated prevalence of 10–28% in Europe and North America, and 2.5–6.7% in Asia [1–3]. Quality of life is significantly impaired in GERD patients with troublesome symptoms [4], and the associated socio-economic impact is enormous [2]. Furthermore, GERD is associated with serious, albeit infrequent, complications such as haemorrhage, peptic stricture, specialized intestinal metaplasia of the oesophageal epithelium (Barrett's oesophagus) and oesophageal adenocarcinoma [5, 6].
Proton pump inhibitors (PPI), with proven therapeutic effectiveness [7, 8], are the current recommended pharmacotherapy for GERD [9–11]. However, not all treated patients achieve symptom relief [12, 13]. It has been noted that the symptoms of 40–50% of patients with non-erosive reflux disease (NERD) are not relieved with PPI [14, 15]. Although a greater therapeutic response to PPI is observed in patients with reflux oesophagitis (RE) when compared with NERD, some10–15% of RE patients experience PPI failure [15].
Prokinetic medication like mosapride, which effectively decreases episodes of gastro-oesophageal reflux, may theoretically be beneficial to GERD patients [16–18]. Although a prokinetic agent cannot be regarded as ideal monotherapy [8–10], its use as an adjunct to acid suppression has been suggested yet sparsely studied [9, 19–21]. Moreover, a recent non-randomized trial reported that patients with more severe pre-treatment symptoms benefited from the addition of mosapride to the standard dose of PPI [21].
This study aimed to investigate whether mosapride was more effective than placebo in achieving or maintaining symptom relief in RE patients who were already treated with a standard PPI regimen, i.e. lansoprazole. The subgroup of symptomatically severe patients was specifically examined.
Methods
Setting and patients
This double-blind randomized crossover trial was conducted in a regional general hospital (Lotung Poh-Ai Hospital) in Taiwan. Adult outpatients presenting to the gastroenterology service were enrolled if they were aged between 18 and 90 years old, and consulting for acid regurgitation, heartburn or belching. All of the patients underwent upper gastrointestinal endoscopy to demonstrate the presence of erosive oesophagitis.
Patients were excluded if they were pregnant or lactating, allergic to lansoprazole or mosapride, co-morbid with serious illness (e.g. end-stage renal disease, cirrhosis, major psychiatric disease), unwilling to participate, or had received a PPI in the previous 1 month. The Institutional Review Board of Lotung Poh-Ai hospital approved the study protocol (ClinicalTrials.gov number, NCT00729339) and all participants provided written informed consent.
Endoscopic procedures and FSSG assessment
Upper gastrointestinal endoscopy was performed with a standard video-endoscope (GIF-XQ240 or GIF-XQ260, Olympus, Tokyo, Japan) by an experienced endoscopist who had previously carried out a minimum of 2000 upper endoscopies (YCH, WLH, ZHY, HTW, MFC, YZC, HJL). RE was diagnosed and classified according to the Los Angeles Classification [22]. Representative endoscopic pictures were taken and stored as digital files. One investigator (HJL) reviewed all endoscopic pictures of eligible patients before enrolment in order to control inter-observer variability in interpreting erosive oesophagitis. Those with endoscopically suspected oesophageal metaplasia (whether or not specialized intestinal metaplasia was histologically confirmed) without discernible mucosal breaks were not eligible.
Patients with endoscopically confirmed RE were then evaluated using the frequency scale for symptoms of gastro-oesophageal reflux disease (FSSG) questionnaire for severity of symptoms. The FSSG questionnaire is a validated graphic scale consisting of 12 questions developed specifically to evaluate GERD symptoms and has been widely used in Japan and other countries [21, 23, 24]. Each question was rated with a five-point Likert scale from zero (frequency of the symptom was never) to four points (frequency of the symptom was always). Every response to each question of the FSSG questionnaire was visually spaced with equal distance. Since we aimed to investigate whether the therapeutic response was different in subgroups divided by pre-treatment FSSG scores, we chose to use seven points as the cut-off value in order to include patients with wider ranges of baseline symptom burden [23].
Randomization and blinding process
Enrolled patients were randomized with a 1:1 proportion to receive either one tablet of mosapride 5 mg (Mopride®, TTY Biopharm Company, Taoyuan, Taiwan) or comparable placebo three times daily for 4 weeks. Placebo receivers in the first 4 weeks crossed over to receive mosapride for another 4 weeks in the second phase of study, and vice versa for those who initially received mosapride. Allocation of treatment sequence was determined by a computer-generated randomization code prepared prior to enrolment. Both study medications were identical in appearance and treatment allocation could only be identified by the randomization code, which was concealed from both the patients and treating physicians throughout the study. All patients received lansoprazole 30 mg (Takepron®, Takeda Pharmaceutical Company, Osaka, Japan) once daily for 2 months regardless of treatment assignment. There was a 3 day washout period between the first and second phases, during which all patients took only lansoprazole. With the half-life of mosapride being around 2 h, the pharmacological effect of the first phase would not be carried over to the second phase. This study did not allow any other medication for symptom rescue. Patients who were not satisfied with their study medication and requested change of treatment during the trial period were considered as protocol violators and were removed from the study.
Outcome measurements and study endpoints
The enrolled patients were evaluated using the FSSG questionnaire three times in this study. In addition to the first assessment upon enrolment, the second and third evaluations were performed after completing 4 weeks (first phase) and 8 weeks (second phase) of therapy. The total score was regarded as the indicator of symptom severity. Primary endpoint for the first phase was defined as change between the first and second FSSG scores.
Patients with severe pre-treatment symptoms were considered as a specific subgroup and were defined as those who had initial FSSG scores of more than 18 points [21]. The endpoint for the second phase was maintenance of symptom response and was defined as the proportion of patients whose FSSG scores at week 8 were no more than their FSSG scores at week 4. Since this study defined patients with FSSG score of 7 points or more as having significant symptoms, which was required upon entry, patients whose FSSG scores remained 7 points or more after 4 weeks of therapy were regarded as having substantial residual symptoms. A subgroup analysis of the second phase was performed for those with substantial residual symptoms despite the first-phase therapy.
Compliance was assessed by counting pills remaining in the medication bottles returned at the follow-up interview. Poor compliance was defined as more than eight (20%) pills found in the bottle.
Statistical analysis
Sample size estimation was based on an expected difference of at least 2 points between changes of FSSG scores in each treatment arm (10 vs. 8) after the first phase of therapy [21, 23]. A sample size of 48 patients in each group was needed to detect the difference to a power of 0.9 at an alpha level of 0.05. Because the FSSG questionnaire is considered as an interval scale, the sum of FSSG scores was expressed as mean ± standard error of mean (SEM) and Student's t-test was used to compare differences between the two groups. Pearson's chi-square test and Fisher's exact test were used (if the expected frequency in any of the cells was <10) to compare categorical variables.
Results were interpreted principally with intention-to-treat (ITT) analysis, but the per-protocol (PP) approach was also performed. In ITT analysis, patients who did not complete the study protocol were considered as not responsive to the study medication and their symptom scores did not improve at all. All statistical examinations were two tailed with a P value <0.05 defined as statistically significant.
Results
Demographics and clinical characteristics at baseline
Between June 2008 and April 2009, 114 RE patients were eligible, but 18 were excluded because of unwillingness to participate (n= 11), serious co-morbidity (n= 4), history of allergy (n= 2) and pregnancy (n= 1). The remaining 96 patients were randomized to mosapride (n= 50) or placebo (n= 46) in the first phase of treatment (Figure 1). Demographic and baseline characteristics of the two arms were similar (Table 1). Two patients initially randomized to the placebo group did not complete their first-phase treatment because of unsatisfactory therapeutic response. Therefore, 94 patients entered the second phase of study. There were seven additional dropouts in the second phase (n= 4 in the mosapride group and n= 3 in the placebo group). All adverse effects, which included headache (n= 2), diarrhoea (n= 2), abdominal fullness (n= 1) and palpitation (n= 1), were mild and did not differ between treatment groups. There was no serious adverse reaction in any study subject.
Figure 1.
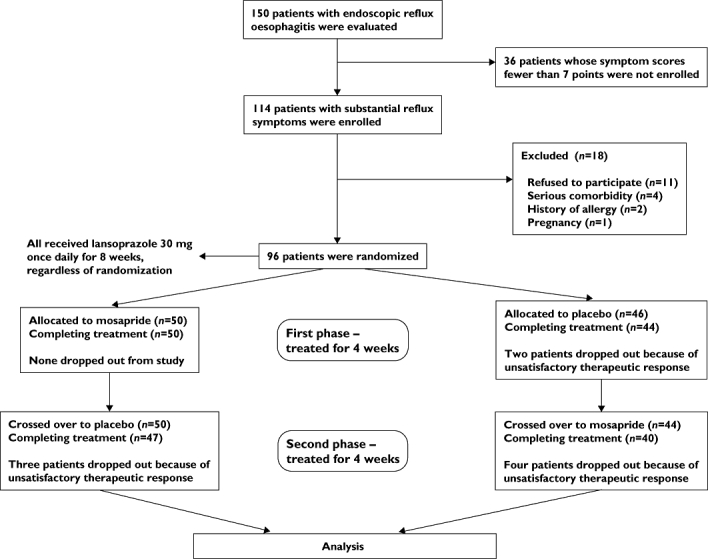
CONSORT flow diagram of the study
Table 1.
Clinical variables of the study patients on entry
| Mosapride (n= 50) in the first 4 weeks | Placebo (n= 46) in the first 4 weeks | P | |
|---|---|---|---|
| Age (years) | 47 ± 2.1 | 47 ± 2.2 | 0.82 |
| Male sex (%) | 23 (46%) | 25 (54.35%) | 0.41 |
| BMI (kg m−2) | 23.66 ± 0.51 | 23.89 ± 0.68 | 0.79 |
| Waist circumference (cm) | 80.67 ± 1.60 | 80.78 ± 1.53 | 0.96 |
| Smoking (%) | 14 (28%) | 14 (30.43%) | 0.83 |
| Alcohol (%) | 14 (28%) | 18 (39.13%) | 0.25 |
| LA classification | 0.79 | ||
| Grade A | 36 (72%) | 31 (67.39%) | |
| Grade B | 13 (26%) | 13 (28.26%) | |
| Grade C | 1 (2%) | 2 (4.35%) | |
| Initial symptom scores | 19.72 ± 1.31 | 20.15 ± 1.29 | 0.81 |
BMI, body mass index. Continuous data were expressed as mean ± SEM.
Symptom improvement in the first-phase treatment
Both treatment arms achieved similar symptomatic improvement after 4 weeks of therapy (Figure 2). The ITT analysis revealed that the mean decrease of FSSG scores in patients initially managed with mosapride and lansoprazole (mean ± SEM, 13.42 ± 1.16) was similar to that (10.85 ± 1.03) in those treated with placebo plus lansoprazole, with an insignificant mean difference of 2.57 (95% CI −0.53, 5.67, P= 0.103). The corresponding figures in the PP analysis were 13.42 ± 1.16 vs. 11.34 ± 1.02, with the mean difference being also insignificant (2.08; 95% CI −1.02, 5.18, P= 0.186). However, in the subgroup of severely symptomatic patients with pre-treatment FSSG scores >18 (n= 48), mosapride plus lansoprazole (n= 23) achieved a greater reduction of FSSG scores (18.22 ± 1.91) than placebo plus lansoprazole (n= 25) did (12.88 ± 1.65). The difference of 5.34 was marginally significant (95% CI 0.28, 10.40, P= 0.039). The PP analysis also demonstrated similar findings (18.22 ± 1.91 vs. 13.42 ± 1.63, with difference of 4.80, 95% CI −0.24, 9.84, P= 0.062).
Figure 2.

A) Symptom resolution was similar between mosapride and placebo after 4 weeks of treatment in all enrolled patients (n= 96). B) Sub-group analysis for those with pre-treatment scores >18 (n= 48) revealed mosapride was more effective than placebo in symptomatically severe patients. Data are expressed as mean ± SEM. Abbreviations: ITT, intention to treat; PP, per protocol. Mosapride ( ); Placebo (□)
); Placebo (□)
Maintaining symptom control in the second-phase treatment
With regard to the second phase of treatment, symptomatic response was maintained or further improved in most patients, regardless of treatment allocation (Figure 3). FSSG scores after the second month of study remained the same or further decreased in 39 out of 44 patients who received mosapride plus lansoprazole, and in 41 out of 50 patients with placebo plus PPI (86.64% vs. 82%, P= 0.401). The results did not change if dropouts were removed from the analysis (39/40 or 97.50% in the mosapride group vs. 41/47 or 87.23% in the placebo group, P= 0.118). In a subgroup analysis for those who remained substantially symptomatic after the first phase of therapy (n= 38), similar proportions of patients achieved symptom control whether mosapride or placebo was added to lansoprazole (15/20 or 75% vs. 16/18 or 88.89%, P= 0.410 by ITT analysis; 15/16 or 93.75% vs. 16/17 or 94.12%, P= 1.0 by PP analysis).
Figure 3.
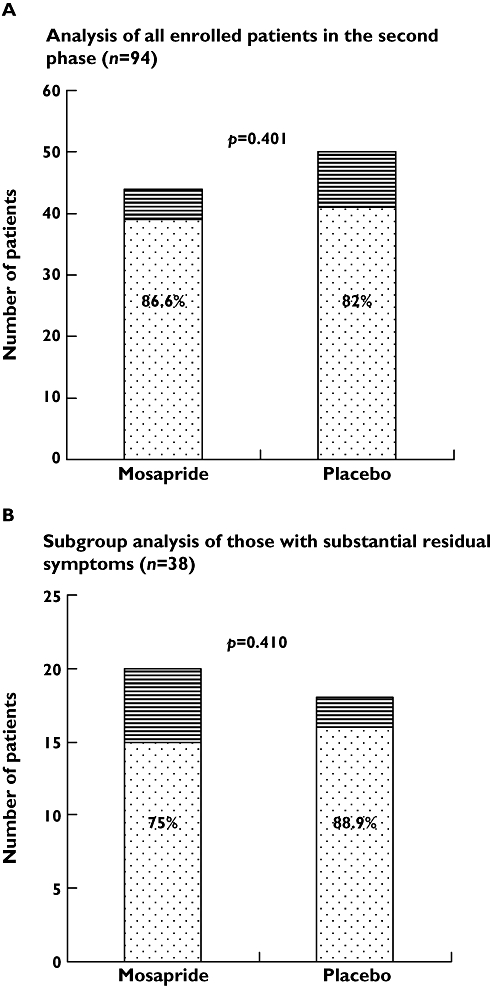
A) Proportions of enrolled patients (n= 94) with symptom control in the second phase were not different between the two treatment groups. B) Results were similar in the sub-group analysis for those whose residual symptoms remained substantial (scores ≥7) after the first phase of therapy (n= 38). All dropouts were regarded as ‘no symptom control’ based on the ITT principle. symptoms uncontrolled ( ); symptom controlled (
); symptom controlled ( )
)
Symptom scores from baseline to week 4 and week 8 are shown in Table 2 in five groups divided by baseline scores. Patients with higher pre-treatment scores had a greater symptom burden than those with lower initial scores after pharmacotherapy for 4 weeks (P < 0.0001) and 8 weeks (P= 0.001). Figure 4 illustrates the evolution of symptom scores over the 8-week trial period between the two treatment arms in subgroups with FSSG scores > and ≤18 points at baseline. In the first 4 weeks, mosapride was superior to placebo only in the subgroup with baseline scores >18 points (P= 0.039).
Table 2.
Symptom scores of participants from initiation to week 4 and week 8, in different groups according to baseline FSSG scores
| Groups of different baseline scores | Baselines scores | Week 4 | Week 8 |
|---|---|---|---|
| 7–12 (n= 22) | 9.59 ± 2.02 | 2.36 ± 1.89 | 1.23 ± 1.19 |
| 13–18 (n= 26) | 15.62 ± 1.90 | 5.23 ± 4.40 | 3.65 ± 4.78 |
| 19–24 (n= 23) | 21.52 ± 1.78 | 6.78 ± 4.67 | 3.65 ± 3.76 |
| 25–30 (n= 12) | 27.33 ± 2.15 | 16.25 ± 10.15 | 9.42 ± 10.60 |
| >31 (n= 13) | 36.38 ± 4.5 | 15.69 ± 12.84 | 8.46 ± 10.63 |
Difference of symptom scores among distinct groups of patients according to baseline FSSG scores was examined by anova.
Figure 4.
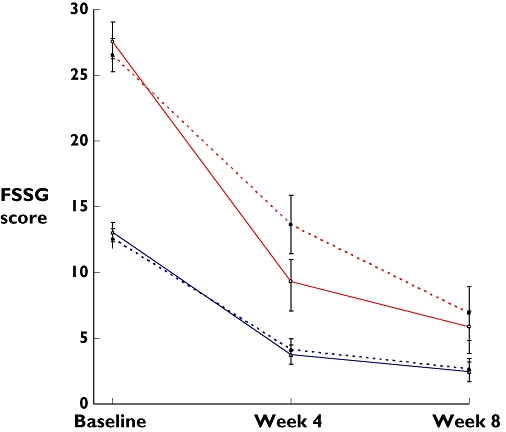
FSSG scores over the trial period between the two treatment arms in subgroups of patients according to baseline scores. The upper two lines (red) indicate patients with baseline FSSG scores >18 points and the lower two lines (blue) refer to those with 18 points or fewer. Changes of symptom scores between the two medications were different only in the subgroup with baseline FSSG scores >18 at week 4. Data are expressed as mean ± SEM. FSSG, frequency scale for symptoms of gastro-oesophageal reflux disease. Placebo-mosapride ( ) (
) ( ); Mosapride-placebo (
); Mosapride-placebo ( ) (
) ( )
)
Discussion
This study demonstrated that mosapride generally was not more effective than placebo as an adjunct to a standard dose of lansoprazole in ameliorating or controlling reflux symptoms in unselected RE patients. However, in those with severe reflux symptoms, combination therapy with lansoprazole and mosapride as compared with lansoprazole plus placebo resulted in greater symptom improvement after 4 weeks of treatment. FSSG symptom scores of >18 points may be a useful predictor for those who may benefit from combination therapy.
There is little evidence to support or refute the use of a prokinetic agent as an adjunct to adequate acid suppression in managing GERD patients [9–11]. As monotherapy, PPI is unequivocally superior to other medications either in relieving symptoms or in healing mucosal erosions in RE [7, 8], but whether combination therapy with a prokinetic drug is more effective has remained undetermined. In a randomized placebo-controlled trial comprising 61 GERD patients [20], Madan et al. reported that pantoprazole plus mosapride was more effective than pantoprazole plus placebo in resolving reflux symptoms in patients with RE (n= 32), but not in those with NERD (n= 29). In their trial, 18 out of 19 (94.7%) RE patients with combination therapy reported symptom resolution, a proportion significantly higher than those in the placebo group (6/13, 46.2%; P= 003). Nonetheless, it is worth noting that their participants were probably more severe or difficult to treat, inasmuch as the response rate after treatment with pantoprazole for 8 weeks was only 46.2%, remarkably lower than that observed in most previous studies [7].
Our finding that patients with higher pre-treatment symptom scores would maintain a greater symptom burden throughout study period (Table 2) was consistent with previous GERD studies, which have found that pretreatment symptom severity was a predictor for poor response to PPI. How to improve outcomes in this group of patients remains sparsely investigated. In a non-randomized trial evaluating efficacy of mosapride in GERD patients who remained dissatisfied after treatment for 12 weeks with PPI, Miyamoto and colleagues reported that patients with high pretreatment FSSG scores (17.4 ± 1.4) were more likely to respond poorly to monotherapy of PPI and to benefit from the addition of mosapride. However, effectiveness of mosapride over placebo could not be ascertained in their trial, which had no control group for comparison. In our double-blind placebo-controlled trial, mosapride plus PPI was more effective than placebo plus PPI in reducing symptom scores with a statistically significant mean difference of 5.34 points. Nonetheless, the magnitude of the 95% CI (0.28, 10.40) warned against an undisputable conclusion. Furthermore, although symptom scores have been frequently applied in gastrointestinal research that measures subjective symptomatic improvement, there remains controversy over the clinical relevance of symptom scores. Hence, results of this pilot study should be validated before they can be recommended in daily practice. Further statistically empowered randomized trials that include patients' global assessment of symptom relief as a study endpoint are now warranted.
Mosapride, a selective 5-hydroxytryptamine (5-HT) 4-receptor agonist, may alleviate GERD symptoms through its effect on oesophageal motility. Ruth et al. demonstrated mosapride to be more effective than placebo in decreasing reflux episodes and total acid exposure time in GERD patients [16, 17]. Cho et al. reported that mosapride effectively decreased bolus transit time and increased rates of total bolus transit in asymptomatic volunteers [18], a finding that implied the efficacy of mosapride in oesophageal acid clearance. In fact, before the era of PPI, a number of earlier studies had evaluated the role of cisapride, another 5-HT4-receptor agonist, in the management of GERD [25–27]. As a combination therapy, cisapride plus ranitidine has been shown to be more effective than ranitidine alone in maintaining symptom remission (66% vs. 49%, P= 0.05) in a randomized trial conducted by Vigneri and colleagues [19]. Since cisapride has been withdrawn from clinical use because of its cardiovascular side effects, mosapride has become the 5-HT4-receptor agonist of choice and consequently was adopted in the current trial.
Decrease of symptom scores and the proportion of patients who maintain symptom control were chosen as primary outcomes for the first and second phase of the study, respectively, in order to reflect the characteristics of symptomatic responses to PPI in RE patients. Because treatment with lansoprazole for 4 weeks generally leads to significant symptom relief in responsive patients, it may be less appropriate to consider magnitude of symptom improvement as the study outcome in the second phase [28, 29]. Moreover, after receiving different study medications in the first phase, patients could not be regarded as comparable at entry to the second phase. A crossover design was deliberately chosen because if there had been therapeutic differences in the first phase, the results of the second phase would have been biased should the study medications have remained the same (expectancy effect) [30]. An alternative way was to re-randomize the patients at entry to the second phase, thus generating four treatment arms. Regrettably, this study was not statistically empowered for a factorial design with four arms.
This study has the following strengths. First, the first phase of study was in essence a double-blind randomized trial, which eliminated probable bias and confounders. Second, inclusion of only symptomatic RE patients identified a clearly defined study population. NERD patients were not enrolled because they represent a more heterogeneous group of patients [31–33]. In addition, a subgroup analysis of severely symptomatic patients explored the interaction between treatment and baseline symptom severity. Because such patients who may benefit from combination therapy are not uncommon in general practice, our study has important clinical implications. Lastly, application of the validated FSSG symptom score as a means to distinguish clinically relevant subgroups is simple and objective.
Several limitations warrant discussion. First, the second phase was not a randomized trial in itself, because a crossover design was adopted to reduce the effect of ‘subject expectancy’. However, with a high response rate in either the mosapride or placebo group (86.64% vs. 82%, P= 0.401), any difference would have been clinically irrelevant. In any case, results of the first phase were not influenced despite this limitation. Second, whether or not adding mosapride to lansoprazole would hasten healing of erosive oesophagitis was not investigated. Because the rate of mucosal healing generally is higher and more predictable than that of symptom resolution in RE patients, this limitation may be of less clinical importance. Third, the negative result in the whole study sample with a size of 96 patients raises the concern of a type II error. Nonetheless, changes of symptoms scores between the two treatment arms were nearly identical in patients with baseline FSSG scores of 18 points or fewer (Figure 4). Therefore, the conclusion that mosapride was not more effective than placebo as an adjunct to a standard dose of PPI in RE patients is convincing. Finally, this study did not address the issue of maintenance therapy in the long term. It would be interesting to explore further whether mosapride has any role, combined with a low or standard dose of PPI, with a strategy of on-demand or continuous administration, in the long-term treatment of GERD.
In conclusion, our results do not support addition of mosapride to a standard dose of PPI in every RE patient, but indicates that mosapride as an adjunct to PPI may be beneficial in a subgroup of patients with severe symptoms.
Acknowledgments
The authors wish to thank Miss Betty Tzu-en Lin, Miss Tze Yu Tung, and Mr Alex Jen-hao Lin for their assistance. This study was supported by a research grant from the Tomorrow Medical Foundation.
Competing interests
Yao-Chun Hsu reports receiving grant support from Takeda Pharmaceutical Company for another study. There are no other competing interests to declare.
REFERENCES
- 1.Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastro-esophageal reflux disease: a systematic review. Gut. 2005;54:710–17. doi: 10.1136/gut.2004.051821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camilleri M, Dubois D, Coulie B, Jones M, Kahrilas PJ, Rentz AM, Sonnenberg A, Stanghellini V, Stewart WF, Tack J, Talley NJ, Whitehead W, Revicki DA. Prevalence and socio-economic impact of upper gastro-intestinal disorders in the United States: results of the US Upper Gastrointestinal Study. Clin Gastroenterol Hepatol. 2005;3:543–52. doi: 10.1016/s1542-3565(05)00153-9. [DOI] [PubMed] [Google Scholar]
- 3.Wong BC, Kinoshita Y. Systematic review on epidemiology of gastro-esophageal reflux disease in Asia. Clin Gastroenterol Hepatol. 2006;4:398–407. doi: 10.1016/j.cgh.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Revicki DA, Wood M, Maton PN, Sorensen S. The impact of gastro-esophageal reflux disease on health-related quality of life. Am J Med. 1998;104:252–8. doi: 10.1016/s0002-9343(97)00354-9. [DOI] [PubMed] [Google Scholar]
- 5.Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastro-esophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–31. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 6.Avidan B, Sonnenberg A, Schnell TG, Chejfec G, Metz A, Sontag SJ. Hiatal hernia size, Barrett's length, and severity of acid reflux are all risk factors for esophageal adenocarcinoma. Am J Gastroenterol. 2002;97:1930–6. doi: 10.1111/j.1572-0241.2002.05902.x. [DOI] [PubMed] [Google Scholar]
- 7.Chiba N, De Gara CJ, Wilkinson JM, Hunt RH. Speed of healing and symptom relief in grade II to IV gastro-esophageal reflux disease: a meta-analysis. Gastroenterology. 1997;112:1798–810. doi: 10.1053/gast.1997.v112.pm9178669. [DOI] [PubMed] [Google Scholar]
- 8.Khan M, Santana J, Donnellan C, Preston C, Moayyedi P. Medical treatments in the short term management of reflux esophagitis. Cochrane Database Syst Rev. 2007;(2) doi: 10.1002/14651858.CD003244.pub2. CD003244. [DOI] [PubMed] [Google Scholar]
- 9.DeVault KR, Castell DO. Updated guidelines for the diagnosis and treatment of gastro-esophageal reflux disease. Am J Gastroenterol. 2005;100:190–200. doi: 10.1111/j.1572-0241.2005.41217.x. [DOI] [PubMed] [Google Scholar]
- 10.Kahrilas PJ, Shaheen NJ, Vaezi MF, Hiltz SW, Black E, Modlin IM, Johnson SP, Allen J, Brill JV. American Gastroenterological Association Medical Position Statement on the management of gastro-esophageal reflux disease. Gastroenterology. 2008;135:1383–91. doi: 10.1053/j.gastro.2008.08.045. 1391 e1–5. [DOI] [PubMed] [Google Scholar]
- 11.Fock KM, Talley NJ, Fass R, Goh KL, Katelaris P, Hunt R, Hongo M, Ang TL, Holtmann G, Nandurkar S, Lin SR, Wong BC, Chan FK, Rani AA, Bak YT, Sollano J, Ho KY, Manatsathit S. Asia-Pacific consensus on the management of gastro-esophageal reflux disease: update. J Gastroenterol Hepatol. 2008;23:8–22. doi: 10.1111/j.1440-1746.2007.05249.x. [DOI] [PubMed] [Google Scholar]
- 12.Carlsson R, Dent J, Watts R, Riley S, Sheikh R, Hatlebakk J, Haug K, de Groot G, van Oudvorst A, Dalvag A, Junghard O, Wiklund I. Gastro-esophageal reflux disease in primary care: an international study of different treatment strategies with omeprazole. International GORD Study Group. Eur J Gastroenterol Hepatol. 1998;10:119–24. [PubMed] [Google Scholar]
- 13.Inadomi JM, McIntyre L, Bernard L, Fendrick AM. Step-down from multiple- to single-dose proton pump inhibitors (PPIs): a prospective study of patients with heartburn or acid regurgitation completely relieved with PPIs. Am J Gastroenterol. 2003;98:1940–4. doi: 10.1111/j.1572-0241.2003.07665.x. [DOI] [PubMed] [Google Scholar]
- 14.Dean BB, Gano AD, Jr, Knight K, Ofman JJ, Fass R. Effectiveness of proton pump inhibitors in non-erosive reflux disease. Clin Gastroenterol Hepatol. 2004;2:656–64. doi: 10.1016/s1542-3565(04)00288-5. [DOI] [PubMed] [Google Scholar]
- 15.Fass R, Shapiro M, Dekel R, Sewell J. Systematic review: proton-pump inhibitor failure in gastro-esophageal reflux disease – where next? Aliment Pharmacol Ther. 2005;22:79–94. doi: 10.1111/j.1365-2036.2005.02531.x. [DOI] [PubMed] [Google Scholar]
- 16.Ruth M, Hamelin B, Rohss K, Lundell L. The effect of mosapride, a novel prokinetic, on acid reflux variables in patients with gastro-esophageal reflux disease. Aliment Pharmacol Ther. 1998;12:35–40. doi: 10.1046/j.1365-2036.1998.00268.x. [DOI] [PubMed] [Google Scholar]
- 17.Ruth M, Finizia C, Cange L, Lundell L. The effect of mosapride on esophageal motor function and acid reflux in patients with gastro-esophageal reflux disease. Eur J Gastroenterol Hepatol. 2003;15:1115–21. doi: 10.1097/00042737-200310000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Cho YK, Choi MG, Han HW, Park JM, Oh JH, Jeong JJ, Cho YS, Lee IS, Kim SW, Choi KY, Chung IS. The effect of mosapride on esophageal motility and bolus transit in asymptomatic volunteers. J Clin Gastroenterol. 2006;40:286–92. doi: 10.1097/01.mcg.0000210103.82241.97. [DOI] [PubMed] [Google Scholar]
- 19.Vigneri S, Termini R, Leandro G, Badalamenti S, Pantalena M, Savarino V, Di Mario F, Battaglia G, Mela GS, Pilotto A. A comparison of five maintenance therapies for reflux esophagitis. N Engl J Med. 1995;333:1106–10. doi: 10.1056/NEJM199510263331703. [DOI] [PubMed] [Google Scholar]
- 20.Madan K, Ahuja V, Kashyap PC, Sharma MP. Comparison of efficacy of pantoprazole alone versus pantoprazole plus mosapride in therapy of gastro-esophageal reflux disease: a randomized trial. Dis Esophagus. 2004;17:274–8. doi: 10.1111/j.1442-2050.2004.00424.x. [DOI] [PubMed] [Google Scholar]
- 21.Miyamoto M, Haruma K, Takeuchi K, Kuwabara M. Frequency scale for symptoms of gastroesophageal reflux disease predicts the need for addition of pro-kinetics to proton pump inhibitor therapy. J Gastroenterol Hepatol. 2008;23:746–51. doi: 10.1111/j.1440-1746.2007.05218.x. [DOI] [PubMed] [Google Scholar]
- 22.Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler SJ, Tytgat GN, Wallin L. Endoscopic assessment of esophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172–80. doi: 10.1136/gut.45.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kusano M, Shimoyama Y, Sugimoto S, Kawamura O, Maeda M, Minashi K, Kuribayashi S, Higuchi T, Zai H, Ino K, Horikoshi T, Sugiyama T, Toki M, Ohwada T, Mori M. Development and evaluation of FSSG: frequency scale for the symptoms of GERD. J Gastroenterol. 2004;39:888–91. doi: 10.1007/s00535-004-1417-7. [DOI] [PubMed] [Google Scholar]
- 24.Oridate N, Takeda H, Mesuda Y, Nishizawa N, Furuta Y, Asaka M, Fukuda S. Evaluation of upper abdominal symptoms using the Frequency Scale for the Symptoms of Gastroesophageal Reflux Disease in patients with laryngo-pharyngeal reflux symptoms. J Gastroenterol. 2008;43:519–23. doi: 10.1007/s00535-008-2189-2. [DOI] [PubMed] [Google Scholar]
- 25.Baldi F, Bianchi Porro G, Dobrilla G, Iascone C, Lobello R, Marzio L, Sabbatini F, Tittobello A, Verme G. Cisapride versus placebo in reflux esophagitis: a multi-center double-blind trial. J Clin Gastroenterol. 1988;10:614–8. doi: 10.1097/00004836-198812000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Richter JE. Efficacy of cisapride on symptoms and healing of gastro-esophageal reflux disease: a review. Scand J Gastroenterol Suppl. 1989;165:19–27. doi: 10.3109/00365528909091227. discussion 27–8. [DOI] [PubMed] [Google Scholar]
- 27.Ceccatelli P, Janssens J, Vantrappen G, Cucchiara S. Cisapride restores the decreased lower esophageal sphincter pressure in reflux patients. Gut. 1988;29:631–5. doi: 10.1136/gut.29.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong WM, Lai KC, Hui WM, Lam KF, Huang JQ, Hu WH, Wong NY, Lam CL, Xia HH, Chan AO, Lam SK, Wong BC. Double-blind, randomized controlled study to assess the effects of lansoprazole 30 mg and lansoprazole 15 mg on 24-h esophageal and intra-gastric pH in Chinese subjects with gastro-esophageal reflux disease. Aliment Pharmacol Ther. 2004;19:455–62. doi: 10.1046/j.1365-2036.2004.01846.x. [DOI] [PubMed] [Google Scholar]
- 29.Mulder CJ, Westerveld BD, Smit JM, Oudkerk Pool M, Otten MH, Tan TG, van Milligen de Wit AW, de Groot GH. A double-blind, randomized comparison of omeprazole Multiple Unit Pellet System (MUPS) 20 mg, lansoprazole 30 mg and pantoprazole 40 mg in symptomatic reflux oesophagitis followed by 3 months of omeprazole MUPS maintenance treatment: a Dutch multicentre trial. Eur J Gastroenterol Hepatol. 2002;14:649–56. doi: 10.1097/00042737-200206000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Jensen MP, Karoly P. Motivation and expectancy factors in symptom perception: a laboratory study of the placebo effect. Psychosom Med. 1991;53:144–52. doi: 10.1097/00006842-199103000-00004. [DOI] [PubMed] [Google Scholar]
- 31.DeVault KR. Review article: the role of acid suppression in patients with non-erosive reflux disease or functional heartburn. Aliment Pharmacol Ther. 2006;23(Suppl. 1):33–9. doi: 10.1111/j.1365-2036.2006.02798.x. [DOI] [PubMed] [Google Scholar]
- 32.Frazzoni M, De Micheli E, Zentilin P, Savarino V. Patho-physiological characteristics of patients with non-erosive reflux disease differ from those of patients with functional heartburn. Aliment Pharmacol Ther. 2004;20:81–8. doi: 10.1111/j.1365-2036.2004.01998.x. [DOI] [PubMed] [Google Scholar]
- 33.Savarino E, Pohl D, Zentilin P, Dulbecco P, Sammito G, Sconfienza L, Vigneri S, Camerini G, Tutuian R, Savarino V. Functional heartburn has more in common with functional dyspepsia than with non-erosive reflux disease. Gut. 2009;58:1185–91. doi: 10.1136/gut.2008.175810. [DOI] [PMC free article] [PubMed] [Google Scholar]


