Hypersensitivity syndrome, also known as drug rash with eosinophilia and systemic symptoms (DRESS), is a serious idiosyncratic drug reaction. It is associated with fever, skin eruption, eosinophilia and multiple organ involvement (lymph node enlargement, hepatitis, pneumonitis, renal dysfunction, pancreatitis, myositis, myocarditis, central nervous system manifestations and hyper- and/or hypothyroidism) [1].
Anticonvulsant drugs are known to be responsible for this reaction. Among them, oxides producing aromatic anticonvulsants such as carbamazepine (CBZ) are the particular drugs most frequently implicated in the DRESS syndrome. The latter has been attributed to a disorder in the metabolic patterns of the aromatic anticonvulsants leading to an excess of toxic metabolite [2].
Allopurinol (ALP) is also frequently associated with the onset of DRESS syndrome. The physiopathology of ALP-induced DRESS is not yet well elucidated. The induction of a significant lymphocyte proliferation by ALP itself or its metabolite (the oxypurinol), suggested an immunological cell-mediated mechanism [3].
Cross-reactivity explained by a chemical or antigenic similarity has been well described between anticonvulsant drugs [4, 5]. However, limited data have reported the development of drug hypersensitivity after a history of the DRESS syndrome induced by other chemically unrelated drugs [6, 7].
We report two cases of amoxicillin (AMX) hypersensitivity after DRESS syndrome due to carbamazepine in the first case, and to allopurinol in the second one.
In case 1, a 34-year-old male with a 20-year history of epilepsy was treated with valproic acid (500 mg three times daily) and phenobarbital (200 mg once a day). As he had frequent convulsive fits, CBZ was added. Thirty-four days later, the patient developed hyperthermia and cervical lymphadenopathy. Initially, he was diagnosed with lymphadenitis and, therefore, amoxicillin-clavulanic acid (1 g twice a day) and acetaminophen (500 mg three times a day), were started. Two days later, a generalized cutaneous eruption (exfoliated and confluent maculae and papulae associated with facial angioedema) was also observed. The laboratory findings showed an abnormal white cell count (16.1 × 103 µl−1, 17% eosinophils), liver dysfunction with an aspartate aminotransferase (AST) concentration of 50 IU l−1, an alanine aminotransferase concentration (ALT) of 116 IU l−1 (normal 4–40 IU l−1), and a lactate dehydrogenase concentration of 3197 (normal 190–430 IU l−1). The platelet count, INR, serum concentrations of immunoglobulins and renal function were conversely normal and no atypical lymphocytes were found. Thoracic imaging did not show any abnormalities. The serologic test for Human Herpes Virus 6 (HHV6) was positive (IgM anti-HHV6 detected). Tests for other viral infections including cytomegalovirus (CMV), Epstein–Barr virus (EBV), hepatitis B and C and human immunodeficiency virus (HIV) were all negative. CBZ, amoxicillin-clavulanic acid and acetaminophen, but not phenobarbital and valproic acid, were then discontinued and cetirizine (10 mg once a day) was administered. About 1 month later, the skin eruption, fever, lymphadenopathy, liver dysfunction and eosinophilia progressively disappeared. Six weeks after complete recovery, a 2 day-patch testing occlusion to CBZ (Carbatol®, Dar Aldawa) was performed on the back of the patient at a concentration of 5% in petrolatum (using Finn Chambers). Two healthy controls underwent skin testing to CBZ according to the same procedure. The patch test was strongly positive (PR0 CR3 according to the International Contact Dermatitis Research Group [8]) at the 48 h reading in our patient, and negative in healthy controls (Figure 1).
Figure 1.
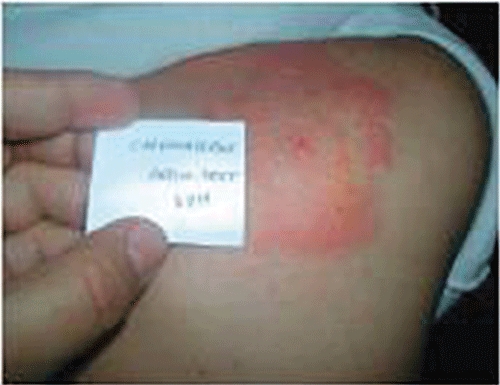
Positive patch test to carbamazepine at the 48 h reading
About 2 years later, the patient was treated with AMX (2 g daily) for a dental abutment. On day 2 of this treatment, he presented with a generalized maculo-papular eruption with neither fever nor lymph node swelling. The laboratory findings showed eosinophilia (white cell count: 8.1 × 103 µl−1, 7% eosinophils) with normal hepatic and renal function. AMX was discontinued and the symtoms had subsided 1 week later. Two months later, an intradermal test to AMX (20 mg ml−1) was performed, which was positive (according to the ENDA/EAACI group) at the 48 h reading in our patient (Figure 2). This test was negative in two healthy controls undergoing the test according to the same protocol. An intradermal test to penicillin G (10 000 IU ml−1), cefazolin (2 mg ml−1) and cefotaxime (2 mg ml−1) was conversely negative (Figure 2).
Figure 2.

Positive intradermal test to amoxicillin at the 48 h reading
In case 2, a 54-year-old patient had a history of congenital ankylosing spondylo-epiphyseal dysplasia treated with indometacin for many years, and a recent history of hypertension treated with methyldopa. As an asymptomatic hyperuricaemia was discovered, he was treated with ALP (100 mg day−1). Seventy-five days later, the patient was referred to the infectious diseases department for a cutaneous eruption and fever. The physical examination revealed a body temperature of 38°C, a maculo-papular rash with facial oedema, conjunctivitis, oral ulceration and inguinal and axillary lymphadenopathy. The laboratory tests showed an abnormal white cell count (14.7 × 103 µl−1 with 6% eosinophils), and liver dysfunction with AST and ALT concentrations of 644 IU l−1 and 716 IU l−1, respectively. Thoracic imaging did not show any abnormalities. The serological tests for viral infections including EBV, CMV, parvo-virus B19, HHV6 and HIV were negative. A skin biopsy revealed a vacuolar interface dermatitis with scattered eosinophils, compatible with the diagnosis of drug hypersensitivity. ALP was then discontinued and dexchlorpheniramine (6 mg once a day) with dexamethasone (8 mg day−1) were administered. The course of events was progressively favourable with a resolution of fever, the cutaneous lesions and the hepatic abnormalities within about 1 month. About 2 months after total recovery, a 2 day occlusion patching test to ALP (Purinol®, Ibn AL BAYTER) was performed on the back of the patient at a concentration of 10% in petrolatum, and was negative. A control patch test was conducted in two healthy controls according to the same procedure and was also negative. Two years later, this patient presented with a generalized cutaneous eruption 2 days after AMX intake. There was neither fever nor lymphadenopathy, and AST and ALT were at normal values. The laboratory findings showed an isolated eosinophilia (leucocytes 7.7 × 103 µl−1, 11% eosinophils). AMX was then withdrawn, with remission of the symptoms 1 week later. We noted that our patient was previously exposed to AMX without any history of hypersensitivity or to the other beta-lactam drugs. Two months after total recovery, an intradermal test to AMX (20 mg·ml−1), penicillin G (10 000 IU ml−1), and cefazolin (2 mg ml−1), was only positive for AMX (according to the ENDA/EAACI) (Figure 3), and negative in healthy controls at the 48 h reading.
Figure 3.
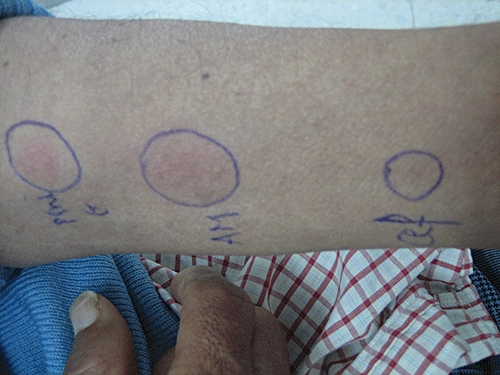
Intradermal test to penicillin G (pen G), amoxicillin, (AMX) and cefazolin (cef) (48 h reading)
We describe two cases of hypersensitivity to AMX after a DRESS induced by CBZ in the first patient and ALP in the second. We believe that DRESS would obviously be related to these drugs in view of the following arguments: (i) a clear temporal relationship between the administration of CBZ and ALP and the onset of symptoms (5 and 10 weeks, respectively, typically 1 to 12 weeks [9, 10]); (ii) remission of the symptomatological pattern after withdrawal of these drugs; (iii) the association of different symptoms evoking a clinical picture of DRESS syndrome; and (iv) a positive patch test to CBZ in the first case. Regarding the subsequent episodes of hypersensitivity in both patients, the responsibility of AMX was established according to the clear temporal relationship between AMX intake and the onset of the reaction, the remission of the symptoms after AMX withdrawal and the positive intradermal test to AMX.
DRESS is a nosological entity characterized mainly by a potentially life-threatening drug-induced cutaneous eruption. Several drugs, including anticonvulsant agents, sulfonamides and allopurinol, have been associated with an increased risk of inducing such a syndrome.
According to the results of some studies [11, 12], it seems that several mechanisms must simultaneously occur to produce the massive specific and nonspecific T cell activity which is a characteristic feature of DRESS. Enough exposure time to a drug able to form chemically active toxic metabolites is required (or, alternatively, the native drug or its metabolites must be exposed on MHCII-matched antigen presenting cells to specific cytotoxic T cells). It has been suggested that a defect in the epoxide hydroxylases, which induces the accumulation of CBZ toxic metabolites, may lead to cell death or contribute to the formation of antigens triggering the immune reaction involved in DRESS [2]. However, in our patient since the patch test was positive to CBZ, it can be argued that the drug reaction may be caused by CBZ itself and not its reactive metabolite. In the case of ALP, the symptoms may be explained by a significant lymphocyte proliferation to ALP as well as its metabolite, oxypurinol, suggesting an immunological cell-mediated mechanism against these two products, mainly oxypurinol [3]. In our patient, it seems that the metabolite, rather than ALP itself, was responsible for eliciting the symptoms since the patch test to ALP was negative. It is reported that a reactivation of a latent HHV-6 infection during a period of transient immunosuppression would stimulate a massive expansion of HHV-6 specific and nonspecific bystander CD8+ and CD4+ T cells and cause full development of DRESS symptoms. However, it is not completely clear whether HHV6 reactivation is a consequence of a strong immune modulation during DRESS or a cofactor which favours the manifestation of this syndrome [13]. Anyway, we did not notice a reactivation of HHV6 in our patients since the serological test showed a primary infection in the first case and was negative in the second one.
As our patients exhibited a hypersensitivity reaction to AMX after a DRESS episode, it can be argued that a possible AMX-CBZ and AMX-ALP co-sensitization has occurred. AMX had been administered previously during an episode of DRESS only in the first patient. In both patients, we had a positive intradermal test to AMX at the 48 h reading, denoting a delayed hypersensitivity to this drug. The intradermal test to other betalactams performed on both patients was negative, revealing a lack of cross-reactivity between AMX and these drugs.
The hypersensitivity reaction that occurred in our patients was mild compared with the initial one. Indeed, we only noticed a skin rash and eosinophilia with no organ involvement.
Cross-reactivity between anticonvulsant drugs has been described widely in the literature when the initial reaction is DRESS. It is known to be as high as 80% among aromatic anticonvulsant drugs [5]. We have recently described a cross-reactivity between CBZ and lamotrigine, aromatic and non aromatic anticonvulsants, respectively [14]. To our knowledge, five clinical observations describing a sensitization to chemically different drugs taken during a previous episode of anticonvulsant-induced DRESS have been reported so far [6, 7]. Only two cases reported a possible AMX-CBZ co-sensitization. The first described a generalized rash and facial angioedema occurring 7 h after taking AMX in a patient who took this drug during a previous CBZ-related DRESS. The second refers to a 68-year-old woman with a history of CBZ-induced DRESS who had a positive patch test to AMX. However, to the best of our knowledge, and after performing an exhaustive search in Pubmed and Embase databases, hypersensitivity to AMX after ALP-related DRESS has never been reported previously.
In this respect, some authors have suggested that the supposed cross-reactions between such drugs, without any chemical or antigenic similarity, are due to the fact that the drug responsible for the second reaction was administered during the immunological depression occurring during a first DRESS episode [15]. Our first clinical observation appears to support this hypothesis. However, in the second case, AMX was not administered during a DRESS episode. In view of these considerations, it has been suggested that a DRESS episode may elicit a massive nonspecific activation of the immune system, which will provide the enhanced expression of co-stimulatory molecules and pro-inflammatory cytokines. The latter will allow a more efficient presentation of chemical antigens to antigen-presenting cells and consequently decrease the level of tolerance to drugs [6], in particular those known to be potentially immunogenic, such as AMX antibiotics whether the drug was taken or not during the initial phase of DRESS.
In conclusion, throughout these reports, we point out a possible co-sensitization to several chemically or antigenically unrelated drugs, CBZ and ALP, on the one hand, and AMX, on the other.
Thus, clinicians should be cautious when prescribing AMX to a patient with a previous history of DRESS syndrome. Skin tests to beta lactams should be performed in such a patient to identify whether he/she could or could not tolerate these drugs.
Acknowledgments
The authors are greatly indebted to Professor Adel Rdissi for his help with improving the language used in this article.
Competing interests
There are no competing interests to declare.
REFERENCES
- 1.Maria CC, Kimberly AY, Nancy BE, Beth AD. Drug-induced hypersensitivity syndrome in pediatric patients. Pediatrics. 2001;108:485–92. doi: 10.1542/peds.108.2.485. [DOI] [PubMed] [Google Scholar]
- 2.Shear NH, Spielberg SP. Anticonvulsant hypersensitivity syndrome: in vitro assessment of risk. Clin Investig. 1988;82:1826–32. doi: 10.1172/JCI113798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marrakchi C, Kanoun F, Kilani B, Tiouiri H, Goubontini A, Zouiten F, Ben Chaabane T. DRESS syndrome à l'allopurinol. Rev Med Interne. 2004;25:244–54. [Google Scholar]
- 4.Silje A, Stian L, Eylert B. Cross-reactivity pattern of rash from current aromatic antiepileptic drugs. Epilepsy Res. 2008;80:194–200. doi: 10.1016/j.eplepsyres.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch L, Arif H, Nahm EA, Buchsbaum R, Resor SR, Bazil CW. Cross-sensitivity of skin rashes with antiepileptic drug use. Neurology. 2008;7:1527–34. doi: 10.1212/01.wnl.0000334295.50403.4c. [DOI] [PubMed] [Google Scholar]
- 6.Gaig P, García-Ortega P, Baltasar M, Bartra J. Drug neosensitization during anticonvulsant hypersensitivity syndrome. J Investig Allergol Clin Immunol. 2006;16:321–6. [PubMed] [Google Scholar]
- 7.Aihara Y, Ito I, Aihara M, Yokota S. Two different adverse drug reactions in a pediatric patient separated by a 15-month interval: carbamazepine-induced hypersensitivity syndrome and cefaclor-induced cutaneous eruptions. Allergologie. 2004;27:163–5. [Google Scholar]
- 8.Lachapelle JM. A proposed relevance scoring system for positive allergic patch test reactions: practical implications and limitations. Contact Dermatitis. 1997;36:39–43. doi: 10.1111/j.1600-0536.1997.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 9.Gogtay NJ, Bavdeker SB, Kshirsagar NA. Anticonvulsant hypersensitivity syndrome: a review. Expert Opin Drug Saf. 2005;4:571–81. doi: 10.1517/14740338.4.3.571. [DOI] [PubMed] [Google Scholar]
- 10.Taillia H, Alla P, Fournier B, Bounolleau P, Ouelogem M, Ricard D, Sallansonnet-Froment M, de Greslan T, Renard JL. Anticonvulsant hypersensitivity syndrome and lamotrigine-associated anticonvulsant hypersensitivity syndrome. Rev Neurol. 2009;165:821–7. doi: 10.1016/j.neurol.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Kano Y, Inaoka M, Shiohara T. Association between anticonvulsant hypersensitivity syndrome and human herpesvirus 6 reactivation and hypogammaglobulinemia. Arch Dermatol. 2004;140:183–8. doi: 10.1001/archderm.140.2.183. [DOI] [PubMed] [Google Scholar]
- 12.Naisbitt DJ, Britschgi G. Hypersensitivity reactions to carbamazepine: characterization of the specificity, phenotype, and cytokine profile of drug-specific T cell clones. Mol Pharmacol. 2003;63:732–41. doi: 10.1124/mol.63.3.732. [DOI] [PubMed] [Google Scholar]
- 13.Aouam K, Belhadjali H, Youssef M, Chaabane A, Amri M, Boughattas NA, Zili J. Carbamazepine-induced DRESS and HHV6 primary infection: the importance of skin tests. Epilepsia. 2008;49:1630–3. doi: 10.1111/j.1528-1167.2008.01660.x. [DOI] [PubMed] [Google Scholar]
- 14.Aouam K, Ben Romdhane F, Loussaïf C, Salem R, Toumi A, Belhadjali H, Chaabane A, Boughattas NA, Chakroun M. Hypersensitivity syndrome induced by anticonvulsants: possible cross reactivity between carbamazepine and lamotrigine. J Clin Pharmacol. 2009;49:1488–91. doi: 10.1177/0091270009344985. [DOI] [PubMed] [Google Scholar]
- 15.Klassen BD. Induction of hypersensitivity to a previously tolerated antiepileptic drug by a second antiepileptic drug. Epilepsia. 2001;42:433–5. doi: 10.1046/j.1528-1157.2001.33400.x. [DOI] [PubMed] [Google Scholar]


