Abstract
Hypoxia and epithelial stretch that are commonly observed in patients with acute lung injury have been shown to promote the release of serotonin (5-hydroxytryptamine, 5-HT) in vitro. However, whether 5-HT contributes to the decrease of alveolar epithelial fluid transport, which is a hallmark of lung injury, is unknown. Thus, we investigated the effect of 5-HT on ion and fluid transport across the alveolar epithelium. 5-HT caused a dose-dependent inhibition of the amiloride-sensitive current across primary rat and human alveolar epithelial type II cell monolayers, but did not affect Na+/K+ ATPase function. Furthermore, we found that the 5-HT induced inhibition of ion transport across the lung epithelium was receptor independent, as it was not prevented by the blockade of 5-HT2R (5-HT receptor 2), 5-HT3R (5-HT receptor 3), or by pretreatment with an intracellular calcium-chelating agent, BAPTA-AM (1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetra(acetoxymethyl) ester). In addition, the stimulation of 5-HT1R (5-HT receptor 1), 5-HT2R (5-HT receptor 2), 5-HT4R (5-HT receptor 4), and 5-HT7R (5-HT receptor 7) failed to reproduce the 5-HT effect on amiloride-sensitive sodium transport. We ascertained that 5-HT directly inhibited the function of rat αβγ epithelial sodium channel (ENaC), as determined by heterologous expression of rat ENaC in Xenopus oocytes that do not express endogenous ENaC nor 5-HT receptors (5-HTR). Exposure of mice to hypoxia for 1 hour induced a 30% increase of 5-HT secretion into the distal airways of mice. Finally, the intratracheal instillation of 5-HT inhibited the amiloride-sensitive fraction of alveolar fluid clearance in mice. Together, these results indicate that 5-HT inhibits the amiloride-sensitive fraction of the alveolar epithelial fluid transport via a direct interaction with ENaC, and thus can be an endogenous inhibitor of this ion channel.
Keywords: alveolar, epithelial, ion transport, serotonin
CLINICAL RELEVANCE.
The results of this study demonstrate that serotonin (5-HT) significantly inhibits amiloride-sensitive sodium transport across rat and human lung alveolar epithelial cell monolayers via a receptor-independent inhibition of epithelial sodium channel (ENaC) activity. As 5-HT also significantly inhibits the amiloride-sensitive fraction of the alveolar fluid clearance in mice, 5-HT can be considered an endogenous inhibitor of ENaC.
Active vectorial Na+ transport through the distal lung epithelium is essential to maintain a physiological lung fluid balance. This vectorial fluid and ion transport is altered under pathological conditions, such as acute lung injury (ALI). Indeed, the majority of patients with ALI have impaired alveolar epithelial fluid transport, a finding that is associated with more prolonged acute respiratory failure and higher mortality (1). The epithelial sodium channel (ENaC), which participates in the vectorial movement of sodium through the apical membrane of the distal lung epithelium, has been shown to be critical for the control of fluid transport across this epithelium (2, 3). Several mediators, such as hypoxia, adenosine, and free radicals, have been reported to affect the sodium transport in distal airways (4–6). However, the mechanisms of inhibition of the fluid transport across the distal lung epithelium under pathological conditions such as ALI are not completely understood.
Serotonin (5-HT), a widely occurring biogenic amine derived from tryptophan, is involved in a variety of processes, such as neurotransmission, vasoconstriction, bronchoconstriction, and intestinal motility. Its role in the pathophysiology of asthma and pulmonary hypertension is well established (7–9). The lung neuroendocrine system, represented by pulmonary neuroepithelial cells in upper airway and neuroepithelial bodies in distal airway (including alveoli), is able to secrete 5-HT into the airspace under various stimuli, such as hypoxia and epithelial stretch (10, 11), two conditions commonly observed in patients with ALI. Therefore, under these conditions, locally secreted 5-HT could modulate the function of epithelial ion channels, including ENaC, and affect its function. In fact, 5-HT has been shown to inhibit sodium conductance in mammalian cells, including tracheal cells (12–15). However, even though functional 5-HT receptors have been identified in alveolar cells (16, 17), very little is known about the effect of 5-HT on the ion transport properties of the distal lung epithelium.
In this study, we investigated the mechanisms by which 5-HT affects fluid and ion transport across the distal lung epithelium. The results indicate that 5-HT causes a significant decrease of alveolar epithelial fluid transport via a receptor-independent inhibition of ENaC.
MATERIALS AND METHODS
Reagents and Chemicals
Serotonin hydrochloride, amiloride hydrochloride, dexamethasone, ketanserin, 5-carboxamidotryptamine, Y-25130 hydrochloride,1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride (±)-2,5-dimethoxy-4-iodoamphetamine hydrochloride, and ouabain were purchased from Sigma-Aldrich (Brussels, Belgium). Fura-2 acetoxymethyl ester (FURA2-AM) was purchased from Molecular Probe (Brussels, Belgium).
Cell Culture
A549 cells were grown in culture flasks in RPMI-1640 media (Invitrogen, Merelbeke, Belgium) adjusted to contain 4.5 g/L glucose, and supplemented with 1% penicillin/streptomycin, 5% FBS (Bio-Greneir, Brussels, Belgium), and 10 nM dexamethasone. Upon reaching confluence, cells were removed from the flask with trypsin-EDTA and subcultured at a 1:5 ratio. For patch-clamp experiments, cells were plated onto glass coverslips (15 mm; VWR, Leuven, Belgium) at 50% confluence. The media was changed every other day. Cells were used between Days 1 and 3 after plating.
Isolation and Culture of Rat Alveolar Epithelial Type II Cells
Rat alveolar epithelial type II (ATII) cells were isolated as described previously (18, 19) after obtaining approval from the University of California San Francisco (UCSF) Committee on Animal Research. Briefly, cells were isolated by elastase digestion, followed by negative selection with four monoclonal antibodies against cell-surface molecules expressed on rat macrophages (CD4, CD32, CD45, and rat macrophage activator) purchased from Pharmingen (Erembodegem, Belgium). These monoclonal antibodies were preincubated with Dynabeads M-450 (magnetic beads with sheep anti-mouse IgG; Dynal Biotech ASA, Oslo, Norway) in 0.1% BSA in PBS. After removing unbound monoclonal antibodies, rat ATII cells were mixed with the bead suspension and rocked gently for 30 minutes at 4°C. Unbound cells were isolated and plated on polycarbonate Transwell dishes (0.4 μm pore size; Corning Costar, Cambridge, MA). Cells were seeded at a concentration of 1.5 × 106 cells/cm2 in Dulbecco's modified Eagles medium (DMEM)/H21 medium containing 10% low-endotoxin FBS and 1% penicillin/streptomycin, and kept at 37°C in a humidified 95% air and 5% CO2 environment. After 24 hours, nonadherent epithelial cells were removed by washing with PBS, and fresh medium was added to the lower compartments of the Transwell dishes, thus maintaining the ATII cell monolayers with an air–liquid interface on their apical side. After 72–96 hours, cells that formed confluent monolayers reaching a transepithelial electrical resistance (TER) greater than 1,500 Ω/cm2 were used for experimentation.
Isolation and Culture of Human ATII Cells
Human alveolar ATII cells were isolated by a modification of methods previously described (18–20) after obtaining approval from the UCSF Committee on Human Research. Briefly, alveolar type II cells were isolated from human lungs that were not used by the Northern California Transplant Donor Network. Our studies indicated that these lungs were in good condition, both physiologically and pathologically (21). Cells were isolated after the lungs had been preserved for 4–8 hours at 4°C. A lobe of human lung that had no evidence of injury on the preharvest chest radiograph, that could be normally inflated, and that had no area of consolidation or hemorrhage was selected. The pulmonary artery for this segment was perfused with 37°C PBS solution, and the distal airspaces of a segmental bronchus were lavaged 10 times with 37°C Ca2+/Mg2+–free PBS solution containing 0.5 mM each of EGTA and EDTA; 60–90 ml of pancreatic porcine elastase (8 U/ml) diluted in Ca2+/Mg2+–free Hank's balanced saline solution was instilled into the airspaces of 50 g of the chosen segment of lung tissue. The lung was incubated in a water bath for 30 minutes at 37°C and minced finely in the presence of FBS and DNase I (500 μg/ml). The cell-rich fraction was filtered sequentially through one- and two-layer gauze and 150 and 30 μm nylon mesh. The cell suspension was then layered onto a discontinuous Percoll density gradient (1.04–1.09 g/ml) and centrifuged at 400 × g for 20 minutes to remove red blood cells. The cells that accumulated at the interface of the solution and the Percoll were a mixture of type II pneumocytes and alveolar macrophages. These cells were recovered by centrifugation at 200 × g for 10 minutes at 4°C. The pellet was resuspended in DMEM containing 10% FCS. The cells were incubated in DMEM containing magnetic beads coated with anti-CD14 antibody (Dynabeads M-450 CD14; Dynal Biotech ASA) at 4°C for 40 minutes under constant mixing to eliminate macrophages. Cell viability was assessed by Trypan blue exclusion. The purity of isolated human alveolar type II cells was checked by Papanicolaou staining or by staining with anti-human type II cell antibody (obtained from Leland Dobbs, UCSF), and the purity was consistently greater than 90% (data not shown). Human alveolar type II cells were seeded on collagen I–coated Transwell dishes at a density of 1 × 106 cells/cm2. At 5 days after the cells were seeded, the monolayer developed a TER greater than 1,500 ohms/cm2, as reported for rat ATII cell monolayers.
Isolation and Culture of Human Tracheal Cells
Human tracheal primary cultures were isolated and cultured as previously described (22, 23). Briefly, strips of epithelium were removed from the underlying tissues and treated with protease overnight. Cells were plated (106 cells/cm2) on permeable filter supports (0.4-μm pore size, 1-cm2 area, Transwell; Corning Costar, Cambridge, MA) precoated with human placental collagen (15 μg/cm2). Human tracheal cells were grown in a 1:1 mixture of DMEM and Ham's F-12 nutrient medium (DMEM/F-12) supplemented with 2% Ultroser G (Pall Corporation, Port Washington, NY). All media used in cell isolation and primary culture contained the following antibiotics: 100 U/ml penicillin, 100 μg/ml streptomycin, 2.5 mg/ml fungizone, and 100 μg/ml gentamicin. Tracheal sheets were grown to confluence in an air–liquid interface in a tissue culture incubator gassed with 5% CO2 and air. Transepithelial potential and TER were monitored routinely under sterile condition with an EVOM voltmeter (World Precision Instruments, Sarasota, FL). Cell sheets were used for transepithelial experiments when transepithelial potential was larger than −30 mV.
Short Circuit Measurements
Freshly isolated ATII cells were seeded on polycarbonate Snapwell membranes (pore size, 0.4 μm; surface area, 1.13 cm2). The culture medium was changed daily. Hydrocortisone (10−7 M) and insulin–transferrin–Sel-G were added to the culture medium for human cells. The cells were grown in an air–liquid interface 72 hours after seeding. At 120–144 hours, the Snapwell inserts were mounted in an Ussing chamber system (Physiologic Instruments Inc., San Diego, CA). Experiments performed in symmetrical conditions used a bathing solution containing (in): 140 mM NaCl, 4 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM Hepes, and 15 mM glucose (pH = 7.3, adjusted with NaOH; final [Na] = 144 mM). Experiments with basolateral membrane permeabilization were performed with a low-Na+ solution: 10 mM NaCl, 130 mM N-methyl-D-glucamine, 4 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM Hepes, and 15 mM glucose (pH = 7.3, adjusted with HCl) in the basolateral compartment. Permeabilization was achieved by adding nystatin (400 μg/ml) in the basolateral chamber. Permeabilized cell monolayers were also pretrated with ouabain (100 μM) before exposure to 5-HT (4 mM). The voltage was clamped at 0 volts for all the short-circuit current (Isc) measurements. Both hemichambers were bubbled with 5% CO2, and measurements were performed at 37°C. The hemichambers were connected to a VCC MC6 voltage clamp (Physiologic Instruments Inc., San Diego, CA) via Ag/AgCl electrodes and 3 M KCl agar bridges. Isc data were acquired by PowerLab (ADInstruments Inc, Colorado Springs, CO) and recorded in Chart software (ADInstruments Inc., Colorado Springs, CO).
Functional Expression of Rat ENaC in Xenopus Oocytes and Related Ion Current Measurements
Expression of ENaC in Xenopus oocytes.
In vitro–transcribed cRNA for the α, β, and γ subunits of rat ENaC (rENaC) were injected into stage V–VI Xenopus oocytes (2 ng of cRNA of each subunit in a total volume of 50 nl), as described previously (24). cDNA of rENaC encoding the αβγ subunits were generously provided by B. Rossier and J.-D. Horisberger (Department of Pharmacology, Lausanne University, Lausanne, Switzerland). Injected oocytes were incubated at 18.5°C in a low-Na+ modified Barth's solution (10 mM NaCl, 80 mMN-methyl-D-glucamine [NMDG]-Cl, 2.0 mM KCl, 0.7 mM CaCl2, 0.8 mM MgCl2, 0.33 mM Ca(NO3)2, 10.0 mM NMDG-Hepes [pH 7.4]) supplemented with penicillin (100 U/ml) and streptomycin (100 mg/ml). The low Na+ concentration was chosen to prevent excessive Na+ loading during the time needed for ENaC expression. Electrophysiological experiments were performed 1 or 2 days after cRNA injection.
Ion current measurements in whole oocytes.
The amiloride-sensitive Na+ current was measured as previously described (25) with the two-electrode voltage-clamp technique by means of a Dagan TEV voltage-clamp apparatus (Dagan, Minneapolis, MN), at room temperature (22–24°C) and at a holding potential of −60 mV in a solution containing (in): 100 mM Na gluconate, 0.4 mM CaCl2, 10 mM Na-Hepes (pH 7.4), 5 mM BaCl2, and 10 mM tetraethyl-ammonium chloride. Low Cl− concentration and K+ channel blockers (Ba2+, tetraethyl-ammonium) were used to reduce the background membrane conductance. Extracellular solutions flowed under gravity at flow rates of 6–8 ml/min. The current signal was filtered at 20 Hz with the internal filter of the Dagan apparatus (Dagan), and continuously recorded and sampled at 1,000 Hz with Pclamp software (Axon Laboratory, Sunnyvale, CA).
Patch clamp experiments.
Dexamethasone-treated (10 nM) A549 cells were plated on uncoated plastic coverslips and cultured for 48 hours. The cells were patched at 50% confluence. Nystatin-perforated whole-cell patch-clamp experiments were conducted as described previously (26). Briefly, normal bathing media contained 140 mM NaCl, 4 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM Hepes, and 15 mM glucose (pH = 7.3, adjusted with NaOH; final [Na] = 144 mM). For whole-cell measurements, the pipette solution contained 10 mM NaCl, 10 mM KCl, 130 mM K-gluconate, 10 mM Hepes, 15 mM glucose (pH = 7.2, adjusted with KOH; final [K] = 144 mM). In all experiments, 70% compensation of series resistance (<30 MΩ) was used. The mean membrane capacitances of the cells used in the study were 20 (±0.9) pF.
Intracellular calcium measurements.
Rat ATII cells were isolated and cultured on coverslips for 15 hours. The ATTII cells were plated at low density to allow observation of individual cells or cell clusters. The cells were then rinsed with a Krebs solution (140 mM NaCl, 4 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM Hepes, and 15 mM glucose (pH = 7.3) before loading with FURA2-AM. The cells were loaded with 5 μM FURA2-AM and incubated for 35 minutes at 37°C. Once placed in the perfusion chamber of the Zeiss inverted microscope (Zaventem, Belgium), cells were alternatively illuminated (128 Hz) with two excitation wavelengths (340 ± 10 and 380 ± 10 nm) obtained from a xenon high-pressure lamp (75 W) coupled to two monochromators. The emitted light from the ATII cells was collected by a photomultiplier through a 500 (±12) nm filter. Data acquisition was performed with a modified version of the KS400 system from Zeiss. The results are expressed as the ratio between the signals emitted at 340 and 380 nm.
Cytotoxicity test.
The toxicity test consisted of a live/dead staining experiment with the cell-mediated cytotoxicity kit from Invitrogen. This kit is based on the differential uptake of Acrydine orange and propidium iodide. After 48 hours in culture on glass coverslips, the rat ATII cells were rinsed and incubated with 0.1 μM of both Acrydine orange (for 10 min) and propidium iodide (for 5 min). The cells were again washed, and they were then observed under a fluorescence microscope with green and red filters. Dead cells fluoresce red, whereas living cells fluoresce green.
Hypoxia studies.
C57BL6 mice were exposed to 1 hour of hypoxia (fraction of inspired oxygen [FiO2], 11–13%) or normoxia (n = 10 in each group). Exposure to hypoxia was achieved by using a large, sealed plastic cage (allowing ventilation) constantly flushed with nitrogen. The FiO2 was monitored constantly and adjused with a PROOX-110 (Biospherix Ltd., Redfield, NY). After exposure to hypoxia, mice were quickly anesthetized (10 mg/kg nembutal) for bronchoalveolar lavage (BAL) sampling. BAL was performed with 1 ml of a PBS solution containing 3% EDTA. 5-HT concentration in BAL fluids was then measured with a 5-HT ELISA kit (IBL-International GMBH, Hamburg, Germany).
Alveolar fluid clearance measurement.
All alveolar fluid clearance (AFC) measurements were performed on C57BL6 mice (20 and 25 g) after obtaining approval from the UCSF Committee on Animal Research. The mice were anesthetized by an intraperitoneal injection of nembutal (10 mg/kg). Following a previous protocol (27), the mice were then quickly tracheotomized and bled before fluid instillation. Each mouse was instilled with 0.4 ml of 5% BSA/physiological saline solution containing 5 μCi 125I. After instilation into the airways, a first aliquot was taken as the reference sample. The tracheotomy cannula was then immediately connected to continuous positive-pressure circuit delivering air with a positive pressure of 8 cm H2O. The mice were subject to the continuous positive pressure for 30 minutes, after which duplicate aliquots were sampled from the distal airspace. All the samples were weighed and radioactivity was measured using a γ counter. The distal airways fluid clearance is expressed as the percentage of alveolar fluid volume cleared during 30 minutes by the following equation: distal airways fluid clearance = 1 − (PInstilled/PFinal) × 100, where PInstilled is the initial 125I-albumin concentration, and PFinal is the final 125I-albumin concentration. Animal experiments were performed in accordance with the Declaration of Helsinki.
Statistics
All the data are summarized as means (±SD), unless otherwise specified. One-way ANOVA and Fisher's exact t test were used to compare three or more experimental groups. The nonpaired t test was used to compare two experimental groups. A P value of less than 0.05 was considered statistically significant.
RESULTS
5-HT Decreases Transepithelial Current across Rat and Human Distal Lung Epithelial Cell Monolayers, as well as Human Tracheal Cell Monolayers
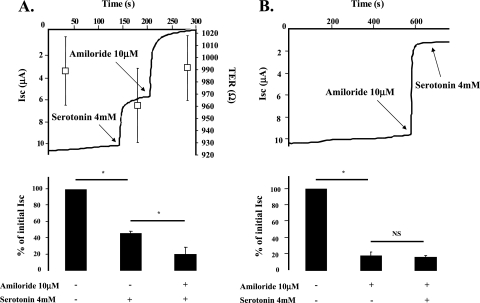
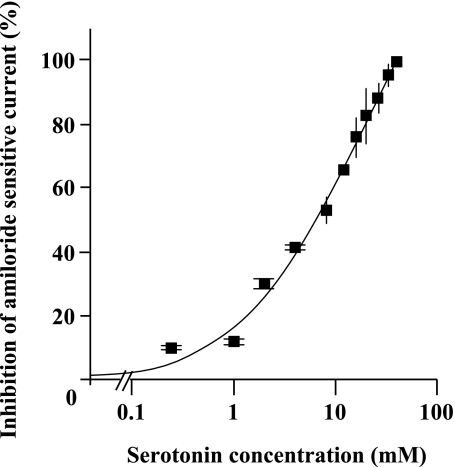
In the first series of experiments, we investigated the effect of 5-HT on ion transport across rat ATII cell monolayers with Ussing chambers. These experiments showed that 5-HT (4 mM) induced an immediate inhibition of the Isc across rat ATII cell monolayers in Ussing chambers (Figure 1A). In addition, amiloride (10 μM) completely prevented the 5-HT–mediated inhibition of Isc (Figure 1B), indicating that 5-HT inhibits the amiloride-sensitive fraction of the alveolar epithelial sodium transport. The effect of 5-HT on these cells was completely reversible (data not shown), and 5-HT did not significantly modify the transepithelial resistance (Figure 1A). The 5-HT–induced inhibition of amiloride-sensitive current across ATII monolayers was dose dependent, with an IC50 (half maximal inhibitory concentration) of 7.6 mM and a Hill coefficient of 0.96 (estimation based on a logistic Hill regression) (Figure 2).
Figure 1.
Serotonin (5-HT) decreases the amiloride-sensitive fraction of the short-circuit current (Isc) across rat distal lung epithelial cell monolayers. (A) 5-HT induces a rapid decrease in the Isc across primary cultures of polarized rat alveolar epithelial type II (ATII) cell monolayers. Rat ATII cell monolayers cultured at an air–liquid interface for 4 days were placed in Ussing chambers and exposed to 5-HT (4 mM), then to amiloride (10 μM). Exposure to amiloride further decreased the Isc across rat ATII cell monolayers. One representative of four experiments is shown. Open squares represent averaged tranepithelial resistances (±SD) before and after 5-HT treatment. The transepithelial resistance scale is displayed on the left. (B) Pretreatment with amiloride abolishes the 5-HT–induced decrease in Isc across primary cultures of polarized rat ATII cell monolayers. Rat ATII cell monolayers cultured at an air–liquid interface for 4 days were placed in Ussing chambers and exposed to amiloride (10 μM), then 5-HT (4 mM). Exposure to 5-HT (4 mM) did not further inhibit the Isc. In all experiments, the effect of 5-HT was reversible. All the experiments were performed at 37°C under symmetrical conditions with saline solution (140 mM NaCl, 4 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM Hepes, and 15 mM glucose [pH = 7.3, adjusted with NaOH]). The EasyMount system, from Physiological Instruments, Inc. (San Diego, CA) was used for all experiments. The voltage was clamped at 0 mV during the whole experiments. One representative experiment is shown. For all experiments, histogram data are the means (±SD) of four experiments done in triplicate; *P < 0.05. NS, not significant; TER, transepithelial electrical resistance.
Figure 2.
5-HT induces a dose-dependent decrease in TEC across primary cultures of polarized rat ATII cell monolayers. Rat ATII cell monolayers cultured at an air–liquid interface for 4 days were exposed to 5-HT (250 μM−38 mM). All experiments were performed under symmetric conditions with saline solution (140 mM NaCl, 4 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM Hepes, and 15 mM glucose [pH = 7.3, adjusted with NaOH]). The EasyMount system from was used for all experiments. The voltage was clamped at 0 mV during the whole experiments. The polynomial regression provided an IC50 (half maximal inhibitory concentration) of 7.6 mM and a Hill coefficient of 0.96 (Microcal Origin Software 5.0; Origin Laboratories, Northampton, MA). Curve fitting was performed with a logistic Hill regression. Each point represents the mean of at least three experiments (±SD).
We repeated Ussing chamber experiments with primary human alveolar type II and human tracheal cell monolayers. 5-HT (4 mM) inhibited the Isc across human ATII cell monolayers. The currents before and after treatment with 5-HT– (4 mM) were, respectively, 6.2 (±1.9) μA and 0.72 (±0.5) μA. In human tracheal cells, 5-HT also caused a dose-dependent inhibition of the amiloride-sensitive fraction of the Isc. The currents before and after treatment with 5-HT (4 mM) were, respectively, 118 (±11) μA and 67 (±9) μA. 5-HT did not further decrease the Isc in primary human tracheal cell monolayers pretreated with amiloride (10 μM), confirming that 5-HT inhibits the amiloride-sensitive fraction of the sodium transport.
5-HT Directly Inhibits ENaC Function
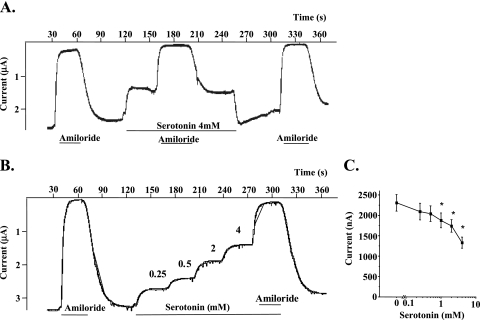
Because 5-HT inhibits the amiloride-sensitive fraction of the Isc across rat and human ATII cell monolayers, we tested whether 5-HT specifically and directly inhibits ENaC. To do so, we measured the effet of 5-HT on amiloride-sensitive current at −60 mV with the two-electrode voltage-clamp tchnique in rENaC-expressing oocytes. By convention, downward current corresponds to a cation flux from the extracellular space to the intracellular side of cell membrane. In the presence of amiloride (10 μM), the current level is close to zero, confirming that the measured current is completely amiloride sensitive. 5-HT reversibly inhibited ENaC function (Figure 3A) in a dose-dependent manner (Figures 3B and 3C).
Figure 3.
5-HT reversibly inhibits the amiloride-sensitive current in Xenopus oocytes expressing rat αβγ epithelial sodium channel (ENaC). (A) 5-HT induces a rapid decrease of inward current in Xenopus oocytes expressing rat αβγENaC. The sodium current sensitive to 10 μM amiloride was measured at −60 mV holding potential before and during exposure to 4 mM 5-HT. This representative experiment also shows the reversibility of 5-HT–induced current inhibition. No effect of 4 mM 5-HT could be detected in noninjected oocytes (n = 5). (B) 5-HT induces a dose-dependent decrease of inward current in Xenopus oocytes expressing rat αβγENaC. This representative experiment was performed with increasing 5-HT doses (0.25, 0.5, 1, 2, and 4 mM). (C) 5-HT induces a dose-dependent decrease of inward current in Xenopus oocytes expressing rat αβγENaC. Mean values of amiloride-sensitive sodium current measured before and after perfusion with 0.25, 0.5, 1, 2, or 4 mM 5-HT are shown (n = 26, 15, 25, 20, 14, and 26, respectively). For all experiments, histogram data are the means (±SD); *P < 0.05 from control animals.
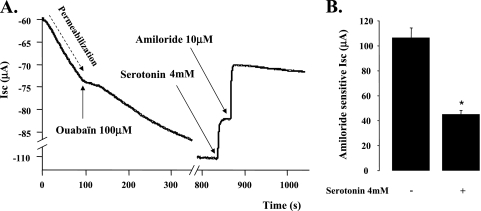
To exclude any short-term effect of 5-HT on the Na+/K+ ATPase function in rat ATII cell monolayers, we used Ussing chambers to perform Isc measurements on basolateraly permeabilized rat ATII monolayers. This series of experiments showed that blocking the Na+/K+ ATPase does affect the inhibitory effect of 5-HT on the Isc across these cell monolayers (Figures 4A and 4B).
Figure 4.
5-HT–induced inhibition of the amiloride senitive Na+ transport across primary cultures of polarized rat ATII cell monolayers is not mediated through the Na+/K+ ATPase. (A) Neither the kinetic of the 5-HT–mediated inhibition nor the magnitude of 5-HT effect was affected by the Na+/K+ ATPase blockade and basolateral membrane permeabilization. Permeabilization was achieved with nystatin (400 μg/ml) in the basolateral chamber. All experiments were performed under asymmetric conditions with different saline solutions for apical and basolateral chambers (apical: 140 mM NaCl, 4 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM Hepes, and 15 mM glucose [pH = 7.3, adjusted with NaOH]; basolateral: 10 mM NaCl, 130 mM N-methyl-D-glucamine, 4 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM Hepes, and 15 mM glucose [pH = 7.3, adjusted with HCl). The EasyMount system was used for all experiments. The voltage was clamped at 0 mV during the whole experiments. (B) This histogram summarizes the effect of 5-HT after Na+/K+ ATPase inhibition and permeabilization of the basolateral membrane of ATII cell monolayers. The results are means (±SD) of four independent experiments; *P < 0.05 from controls.
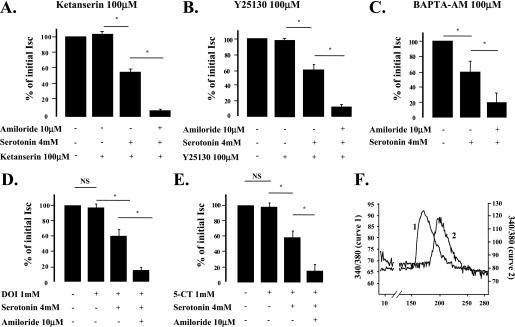
5-HT–Induced ENaC Inhibition in the Distal Lung Epithelium Is Not Receptor Mediated
Although we have previously shown that 5-HT is able to directly inhibit ENaC, these experiments did not exclude an involvement of 5-HT receptors to explain the effect of 5-HT on ion transport across the lung epithelium. The 5-HT receptors, 5-HTR(1A), 5-HTR(1B), 5-HTR(1E), 5-HTR(1F), 5-HTR(2A), 5-HTR(4), 5-HTR(6), 5-HTR(7), and 5-HTR(3), have been detected in alveolar type II cells (16, 17). We thus performed experiments with antagonists or agonists of several of these receptors, as well as with BAPTA-AM, a calcium chelator that inhibits the intracellular signaling induced by the activation of some of these receptors. Pretreatment with the 5-HTR(2) receptor antagonist, ketanserin (100 μM), and the 5-HTR(3) receptor antagonist, Y-25130 (100 μM), did not alter the response of rat ATII cells to 5-HT (Figures 5A and 5B), indicating that the effect of 5-HT was neither mediated through the 5-HTR(2), nor through the 5-HTR(3) receptors. Preincubation of rat ATII cells with BAPTA-AM (100 μM) for 30 minutes did not prevent the effect of 5-HT on amiloride-sensitive current (Figure 5C), indicating that the observed 5-HT-induced ENaC inhibition is not calcium mediated. Finally, through another approach, we tested whether 5-HT receptor stimulation would mimic the effects of 5-HT on amiloride-sensitive current. Exposure of rat ATII cells to the 5-HTR(2) agonist, (±)-2,5-dimethoxy-4-iodoamphetamine (1 mM), and the nonspecific 5-HTR(1), (4), and (7) agonist, 5-carboxamidotryptamine (1 mM) did not reproduce the effect of 5-HT–induced inhibition of amiloride-sensitive current across rat ATII cell monolayers (Figures 5D and 5E). Intracellular calcium measurements performed on rat ATII cells showed that these agonists are effective at stimulating an intracellular calcium increase by using a concentration of 100 μM (Figure 5F). The specific role of 5-HTR(6) receptors was not investigated, because they have been shown to activate adenylate cyclase, and therefore could not mediate ENaC inhibition (28). Taken together, these results indicate that the inhibitory effect of 5-HT on ENaC function in the distal lung epithelium is not receptor mediated.
Figure 5.
5-HT–induced inhibition of ENaC is receptor independent in rat ATII cell monolayers. (A) Pretreatment with ketanserin, a 5-HTR(2) receptor antagonist, did not affect the magnitude of 5-HT–induced decrease in Isc across primary cultures of polarized rat ATII cell monolayers. Rat ATII cell monolayers cultured at an air–liquid interface for 4 days were placed in Ussing chambers and exposed to 5-HT (4 mM). In some experiments, ketanserin (100 μM) was added 10 minutes before 5-HT, and amiloride (10 μM) was added 3 minutes after 5-HT treatment. (B) Pretreatment with Y-25130, an 5-HTR(3) (5-HT receptor 3) receptor antagonist, did not affect the magnitude of 5-HT–induced decrease of Isc across primary cultures of polarized rat ATII cell monolayers. Rat ATII cell monolayers cultured at an air–liquid interface for 4 days were placed in Ussing chambers and exposed to 5-HT (4 mM). In some experiments, Y-25130 (100 μM) was added 10 minutes before 5-HT, and amiloride (10 μM) was added 3 minutes after 5-HT treatment. (C) Pretreatment with BAPTA-AM (1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetra(acetoxymethyl) ester), a calcium chelator, did not affect the magnitude of 5-HT–induced decrease of Isc across primary cultures of polarized rat ATII cell monolayers. Rat ATII cell monolayers, cultured at an air–liquid interface for 4 days, were placed in Ussing chambers and exposed to 5-HT (4 mM). BAPTA-AM (100 μM) was added 35 minutes before 5-HT, and amiloride (10 μM) was added 3 minutes after 5-HT treatment. (D) 5-HT2R (5-HT receptor 2) stimulation by (±)-2,5-dimethoxy-4-iodoamphetamine (DOI) hydrochloride did not reproduce the effect of 5-HT on Isc. Rat ATII cell monolayers, cultured at an air–liquid interface for 4 days, were placed in Ussing chambers and successively exposed to DOI (1 mM), 5-HT (4 mM), and amiloride (10 μM). (E) Nonspecific stimulation of 5-HT1R (5-HT receptor 1), 5-HT4R (5-HT receptor 4), and 5-HT7R (5-HT receptor 7) by 5-carboxamidotryptamine (5-CT) did not reproduce the effect of 5-HT on Isc. Rat ATII cell monolayers, cultured at an air–liquid interface for 4 days, were placed in Ussing chambers and successively exposed to 5-CT (1 mM), 5-HT (4 mM), and amiloride (10 μM). All these experiments were performed at 37°C under symmetrical conditions with saline solution (140 mM NaCl, 4 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM Hepes, and 15 mM glucose [pH = 7.3, adjusted with NaOH]). The EasyMount system was used for all experiments; the voltage was clamped at 0 mV during the whole experiments. (F) Both DOI and 5-CT cause an increase in intracellular calcium in rat ATII cells. Rat ATII cells were cultured on glass coverslips for 15 hours before experiments. The cells were loaded with 5 μM FURA2-AM. DOI and 5-CT (curves 1 and 2, respectively) elicited a significant increase in intracellular calcium. One representative of four experiments is shown. For all experiments, the histogram results are expressed as means (±SD) of four experiments repeated in triplicate; *P < 0.05. NS, not significant.
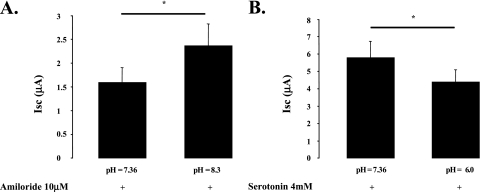
5-HT and Amiloride Present Comparable Inhibition Properties of ENaC
Because 5-HT inhibited the amiloride-sensitive fraction of the Isc across rat and human ATII cell monolayers, we hypothesized that both molecules could inhibit ENaC by comparable mechanisms. The inhibition of ENaC by amiloride has been attributed to its strong positive charge at physiological pH. Indeed, amiloride-induced inhibition of ENaC current has been shown to be voltage dependent (29). Therefore, we tested whether 5-HT–induced inhibition of amiloride-sensitive current was charge dependent. We performed these inhibition experiments with rat ATII cell monolayers in Ussing chambers with amiloride (10 μM) and 5-HT at 2 mM, a dose that still allows a significant current inhibition by amiloride, under different pH conditions (Figures 6A and 6B). The results indicate that the pH of the extracellular bath influenced the magnitude of amiloride as well as 5-HT–dependent current inhibition. Indeed, increasing the pH closer to amiloride's isoelectric point (from 7.36 to 8.3) resulted in a 48% decrease in the amiloride-induced current inhibition. Conversely, lowering the pH from 5-HT isoelectric point (from 7.36 to 6.0) resulted in a 24% increase in the 5-HT–induced current inhibition. These values of pH were chosen because they bracket the pH of alveolar lining fluid, which is estimated at 6.8 (30).
Figure 6.
Amiloride- and 5-HT–mediated inhibition of ENaC are charge dependent. (A) Amiloride-induced inhibition of Isc across primary cultures of polarized rat ATII cell monolayers is pH dependent. Rat ATII cell monolayers, cultured at an air–liquid interface for 4 days, were placed in Ussing chambers and exposed to amiloride (10 μM). The two sets of experiments were performed in symmetrical conditions at two different pH levels. The average amiloride-induced inhibition of TEC (transepithelial current) was 48% lower at pH 8.3 than at physiological pH (7.36). (B) 5-HT–induced inhibition of Isc across primary cultures of polarized rat ATII cell monolayers is pH dependent. Rat ATII cell monolayers, cultured at an air–liquid interface for 4 days, were placed in Ussing chambers and exposed to 5-HT (2 mM). The two sets of experiments were performed in symmetrical conditions at two different pH levels. The average 5-HT–induced inhibition of TEC was 24% greater at pH 6.0 than at physiological pH (7.36). All the experiments were performed at 37°C under symmetrical conditions with saline solution (140 mM NaCl, 4 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM Hepes, and 15 mM glucose). The EasyMount system was used for all experiments. The voltage was clamped at 0 mV during the whole experiments. Results are the means (±SD) of at least three experiments done in triplicate; *P < 0.05 from cell monolayers exposed to a pH of 7.36.
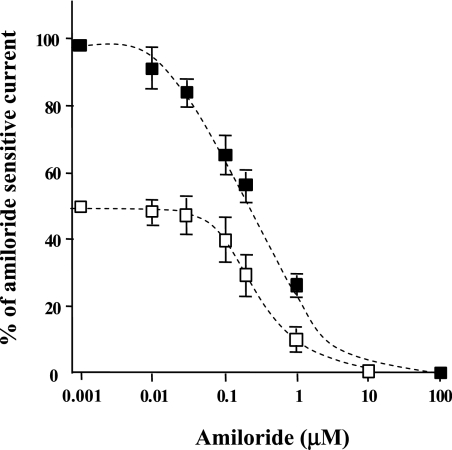
We next examined whether 5-HT and amiloride would be competitive inhibitors of ENaC activity on alveolar epithelial cells. Whole-cell patch-clamp experiments that were performed on A549 cells with increasing concentrations of amiloride were compared in the absence or presence of 2 mM 5-HT, a dose that still allows a significant current inhibition by amiloride. Any competition or interaction with the same binding site would modify the dissociation constant (Kd) for amiloride. The results indicate that the presence of 2 mM 5-HT did not change the Kd for amiloride (Figure 7). Indeed, curve fitting with the Boltzman sigmoidal regression yields values of 292 (±34) nM and 288 (±18) nM for the inflection point in the two series of experiments. Therefore, this result indicates that, although amiloride and 5-HT have a structural analogy and comparable kinetics of ENaC inhibition, the site/region of their interaction with ENaC is different. Whole-cell patch-clamp experiments on A549 confirmed that ENaC is the specific target of 5-HT, as likewise observed for rat ATII cells. Indeed, pretreatment of the cells with amiloride (10 μM) prevented the effect of 5-HT, and the reversal potential of the serotonin sensitive current was +55 mV, which corresponds to a highly selective Na+ channel.
Figure 7.
5-HT and amiloride do not compete to mediate ENaC inhibition in A549 cells. A549 cells were used 24 hours after plating on glass coverslips. A549 cells were cultured in the presence of 10 nM dexamethasone. All these experiments were performed in whole-cell configuration. Closed squares represent the dose–response curve for amiloride alone. Open squares represent the dose–response curve for amiloride in the presence of 2 mM 5-HT. The two curves were obtained after fitting experimental data with the Boltzman equation. Exposure to serotonin did not modify amiloride's Kd in A549 cells. We observed that the inhibition of the amiloride-sensitive current by 5-HT was completely reversible (data not shown). Results are the means (±SD) of at least four experiments done in triplicate.
5-HT Does Not Have Cytotoxic Effects
We investigated the toxicity of 5-HT on rat ATII cells by double cell labeling with propidium iodide and Acrydine orange. We did not observe cytotoxic effects of 5-HT at 4 mM (data not shown), indicating that the observed inhibition of amiloride-sensitive current by 5-HT was not due to cell death.
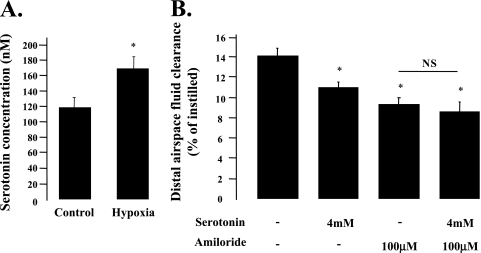
5-HT Is Present in Distal Airways and Inhibits the AFC in Mice
The last series of experiments were conducted to determine the in vivo effect of 5-HT on AFC in mice. First, we tested whether 5-HT is present in distal airways, and whether its concentration can be increased by a physiological stimulus. To do so, we exposed mice to 1 hour of hypoxia (FiO2, 11–13%) and performed BAL to estimate 5-HT concentration in the BAL fluid. The results show that there was a significant increase in BAL fluid 5-HT concentration in mice exposed to hypoxia compared with control animals (Figure 8A). The values under normoxia and hypoxia were, respectively, 118.8 (±11.5) nM and 167.9 (±15.3) nM. After correction for the dilution factor, the 5-HT concentration in the distal airway lining fluid under normoxia and hypoxia are, respectively, 12 μM and 17 μM.
Figure 8.
5-HT inhibits the amiloride-sensitive fraction of the alveolar epithelial fluid transport in mice. (A) 5-HT is present in distal airways of mice, and its concentration is increased after exposure to hypoxia. C57BL6 mice were either exposed to 11–13% fraction of inspired oxygen for 1 hour or to air. The results are the means (±SD) for 10 mice in each group; *P < 0.03 from mice exposed to air. (B) Intratracheal instillation of 5-HT decreases the amiloride-sensitive fraction of the alveolar epithelial fluid transport in mice. Alveolar fluid clearance (AFC) was measured as described in Materials and Methods. Results are the means (±SD) of 5–7 mice per experimental group. The addition of 5-HT (4 mM) to the alveolar instillate did not cause an additional significant decrease in AFC of rats instilled with amiloride (100 μM). *P < 0.05 from control animals instilled with albumin solution alone. NS, not significant.
Intratracheal instillation of 5-HT (4 mM) induced a significant decrease in AFC (Figure 8B). There was no further decrease in AFC when 5-HT was added to amiloride, indicating that 5-HT inhibits the amiloride-sensitive fraction of the AFC in mice.
DISCUSSION
The results of this study shows for the first time that: (1) in a dose-dependent manner, 5-HT significantly inhibits the amiloride-sensitive fraction of sodium transport across rat and human alveolar epithelial cell monolayers; (2) 5-HT directly inhibits the function of the apical cell membrane sodium channel, ENaC, interacting with a site or a region independent of the amiloride binding site; (3) the inhibition of the sodium channel, ENaC, is receptor independent, despite the presence of functional 5-HT receptors in these cells; (4) 5-HT, which is present in the distal airways, significantly inhibits the amiloride-sensitive fraction of the AFC in vivo in mice.
Previous studies have shown that 5-HT inhibits sodium transport across the tracheal epithelium, although the mechanisms for this inhibition were not defined (12, 14, 15). Our results show that 5-HT inhibits the amiloride-sensitive fraction of sodium transport across the distal lung epithelium (Figure 1B). The rapid and reversible inhibition of ENaC by 5-HT was comparable to the amiloride-induced inhibition of ENaC (Figure 1A). We therefore examined whether 5-HT could directly affect the ENaC channel. We found that 5-HT directly inhibits ENaC expressed in Xenopus oocytes in a dose-dependent manner (Figures 3A–3C). Moreover, we also found that the 5-HT–induced inhibition of the amiloride-sensitive transepithelial current was not mediated through the Na+/K+ ATPase (Figures 4A and 4B). Finally, the hypothesis of a direct inhibition of ENaC by 5-HT was supported by our data on 5-HT receptors. Indeed, because functional 5-HT receptors have been identified and characterized in alveolar epithelial cells (16, 17), the fact that 5-HT directly inhibits ENaC in Xenopus oocytes did not exclude that its effect could also be receptor mediated. Thus, we next tested whether the effect of 5-HT on transepithelial sodium transport was in part dependent on the activation of its receptors expressed on alveolar epithelial cells. Among the characterized receptors, 5-HTR(1), 5-HTR(2), and 5HTR(3) were candidates for mediating ENaC inhibition, because they are expressed on ATII cells (17), and signal via second messengers that could down-regulate Na+ transport in these cells. Furthermore, all three receptors have been shown to elicit intracellular calcium increase in alveolar cells (17). The 5-HTR(1) receptors are coupled to Gi/o (pertussis toxin–sensitive Giα2, Giα3, and Go), which is known to mediate a decrease in adenylate cyclase activity, and have been shown to elicit intracellular calcium increase (17). The 5-HTR(2) receptors are known to be coupled to Gαq that, in turn, stimulates phospholipase C and finally promote intracellular calcium increase via inositol 1,4,5-trisphosphate (IP3) and protein kinase C (PKC) stimulation (31). PKC is a known inhibitor of ENaC activity and expression in alveolar epithelial cells (31). It has been shown that PKC activation by phorbol 12-myristate 13-acetate induces a decrease in total and amiloride-sensitive current across alveolar epithelial cell monolayers (32, 33). The 5-HTR(3) receptors are ligand-gated, nonselective, cationic channels that allow movement of sodium, potassium, and calcium across the cell membrane. In neurons and airway cells, opening of these channels induces a membrane depolarization due to calcium entry. Therefore, as 5-HTR(3) receptors may be responsible for an important calcium influx, they could also be involved in the inhibitory effect of 5-HT on ENaC function. Despite the potential involvement of 5-HTR(1), 5-HTR(2), and 5-HTR(3) receptors through the Ca2+–PKC pathway, we ruled out any receptor involvement in the effect of 5-HT on ENaC by three approaches. First, we treated rat ATII cell monolayers with the 5-HTR(2) antagonist, ketanserin, and the 5-HTR(3) antagonist, Y-25130 (Figures 5A and 5B). We observed that 5-HTR(2) and 5-HTR(3) blockade did not prevent the effect of 5-HT on amiloride-sensitive current, indicating that this effect was not mediated by these receptors. Second, we treated rat ATII cell monolayers with 5-HTR(1), 5-HTR(2), 5-HTR(4), and 5-HTR(7) agonist (Figures 5D and 5E). We found that none of these agonists reproduced the effect of 5-HT on the amiloride-sensitive current. Moreover, these agonists did not modify the response to 5-HT. Third, we tested whether the 5-HT effect on amiloride-sensitive current was calcium mediated. We observed that the intracellular calcium-chelating agent, BAPTA-AM, did not prevent the effect of 5-HT (Figure 5C), ruling out the involvement of calcium in this process. The 5-HT inhibition of ENaC through a G protein–coupled receptor family, trace amine-associated receptors, is also excluded by our results. These receptors use calcium as a second messenger, as reported for the 5-HT receptors (34), and BAPTA had no effect on the 5-HT inhibition of ENaC.
Other investigators have reported the effect of 5-HT on ion transport across the tracheal epithelium at lower concentrations than those used in our study (14, 15). However, the cell types, species, and experimental models were different from the ones used in our study. Indeed, Greczko and Tyrakowski (14) have shown that 5-HT (5 μM) can prevent the mechanically induced hyperpolarization in rabbit tracheal walls. Nam and Park (15) reported that apical 5-HT (50 μM) inhibits the amiloride-sensitive Na+ transport in rat tracheal cells. Their observed effect was maximal at 50 μM, and only induced a 14% inhibition of the sodium transport across these cell monolayers, whereas our results demonstrate a 50–60% inhibition at 4 mM. The differences observed between the previous studies and our results can be explained by the fact that the previous studies showed that the inhibitory effect of 5-HT on sodium transport across the tracheal epithelium was calcium and receptor dependent; receptor-mediated effects are known to require lower doses of ligands. As we did not observe receptor-dependent inhibition of ENaC by 5-HT, the observational differences between the previous studies and our studies further indicate that 5-HT inhibits ion transport in tracheal and alveolar epithelia by different mechanisms. Further studies dissecting the mechanism of 5-HT inhibition of sodium transport between different cell types and animal species remain to be conducted.
Because the 5-HT effect on transepithelial current was likely due to its direct interaction with the channel, we tested whether its mechanism of inhibition was comparable to the amiloride-induced inhibition of ENaC. Because amiloride is a pore blocker, acting through a charge-dependent complex binding to ENaC subunits (29, 35–37), we postulated that a comparable mechanism could be responsible for the 5-HT–induced ENaC inhibition. Therefore, we investigated the influence of 5-HT electrical charge on its inhibition of the transepithelial current. By modifying the extracellular pH, we have shown that 5-HT effect was, indeed, charge dependent (Figure 6B). Although it has been shown that extracellular protons can modulate the activity of human ENaCs (38), rENaCs have been shown to be unaffected by extracellular pH, indicating that the observations we reported in Figure 6 are only due to amiloride and 5-HT charge modification. Despite very similar mechanisms, there was no competitive inhibition of ENaC function between these compounds (Figure 7), suggesting different sites/regions of interaction with ENaC. Taken together, these results indicate that 5-HT released by neuroendocrine epithelial cells and mastocytes could act as an endogenous ENaC inhibitor in the lung.
The last important finding of our study is that 5-HT is released in the distal airspaces of the lung, and that its concentration is significantly increased after 1 hour of exposure to moderate hypoxia (FiO2, 11–13%) (Figure 8A). Furthermore, airspace instillation of 5-HT induces a significant decrease in the amiloride-sensitive fraction of AFC (Figure 8B). Although the concentrations of 5-HT measured in the distal airspaces of the lung did not reach millimolar levels that we used in the in vitro experiments, it indicates that 5-HT, an inhibitor of ENaC, is in direct contact with these channels on alveolar epithelial cells. Obviously, the 5-HT concentrations measured in BAL fluids constitute a rough estimation of what the real values could be in the microenvironment of the alveolar lining fluid. Interestingly, much higher concentrations of 5-HT (up to 300 μM) were measured in BAL fluids in an animal model of allergen-induced airway inflammation (39), suggesting that 5-HT concentrations close to the lower concentration used in the present study could be present in vivo in the distal airspaces of the lung.
The results of the present study may be relevant to pathological conditions involving massive 5-HT release in the airways, hypoxia, or epithelial stretch. The alveolar epithelium contains distal neuroendocrine cells (usually called neuroepithelial bodies) that secrete various mediators, including 5-HT. Alveolar type II cells also have a tryptophan hydroxylase activity that suggests their ability to produce 5-HT from the amino acid, tryptophan (40). Recent publications have shown that 5-HT can be released in the respiratory system by the neuroendocrine epithelial cells after exposure to hypoxia or epithelial stretch (10, 11), two common conditions found in patients with ALI. Hypoxia is known to cause an inhibition of ion transport across the distal lung epithelium (4). Indeed, hypoxia modifies transcriptional and post-transcriptional activity of ENaC, resulting in a decreased AFC (41). In this context, 5-HT could participate in the hypoxia-mediated decrease in AFC by its direct inhibition of ENaC. Finally, the inhibition of the amiloride-sensitive sodium transport by 5-HT in human tracheal monolayers may be relevant to the pathophysiology of acute asthma. The asthma-induced airway obstruction results from bronchoconstriction, mucosa edema, and glands hypersecretion. Among the mediators involved in this process, 5-HT (mostly from luminal mastocytes) is well known for its role in mediating bronchoconstriction, but it has also been shown to stimulate Cl− secretion and inhibit Na+ absorption in canine tracheal epithelial sheets (9). Therefore, the direct 5-HT–induced inhibition of ENaC described in this article may also be an important mechanism for airways fluid accumulation in acute severe asthma.
In summary, the results of this study demonstrate that 5-HT significantly inhibits amiloride-sensitive sodium transport across rat and human lung alveolar epithelial cell monolayers via a receptor-independent inhibition of ENaC activity. As 5-HT also significantly inhibits the amiloride-sensitive fraction of AFC in vivo in mice, 5-HT can be considered an endogenous inhibitor of ENaC.
Acknowledgments
The authors are grateful to Dr. Finkbeiner (University of California San Francisco, San Francisco, CA) for technical assistance during the human tracheal cells isolation. They are also grateful to Prof. Lebrun (Laboratoire de Pharmacodynamie, Université Libre de Bruxelles) for his help during intracellular calcium measurements.
This work was supported by funds from the Université Libre de Bruxelles, the Fonds DeFay and the Fonds National de la Recherche Scientifique (FNRS) (S.S.-S.), and by National Institutes of Health grant P50HL074005 (J.-F.P), National Heart, Lung, and Blood Institute grant HL51854 (M.A.M.), ALA (American Lung Association) Senior Research Training Fellowship and T32 GM008440 (J.R.), and by Canadian Institute of Health Research grant NRF 74706 (A.C.). A.C. is a Chercheur-boursier du Fonds de la Recherche en Santé du Québec. A.G. is a FRIA (Fonds pour la Recherche dans l'Industrie et l'Agronomie, FNRS) doctoral fellow at the Université Libre de Bruxelles. Vadim Shlyonsky is an European Respiratory Society fellow (fellowship no. 306) at Université Libre de Bruxelles.
Originally Published in Press as DOI: 10.1165/rcmb.2008-0472OC on August 28, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Matthay MA, Robriquet L, Fang X. Alveolar epithelium: role in lung fluid balance and acute lung injury. Proc Am Thorac Soc 2005;2:206–213. [DOI] [PubMed] [Google Scholar]
- 2.Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev 2002;82:569–600. [DOI] [PubMed] [Google Scholar]
- 3.Hummler E, Baker P, Gatzy J, Beerman F, Verdumo C, Schmidt A, Boucher R, Rossier BC. Early death due to defective neonatal lung liquid clearance in alpha-ENaC–deficient mice. Nat Genet 1996;12:325–328. [DOI] [PubMed] [Google Scholar]
- 4.Planes C, Escoubet B, Blot-Chabaud M, Friedlander G, Farman N, Clerici C. Hypoxia downregulates expression and activity of epithelial sodium channels in rat alveolar epithelial cells. Am J Respir Cell Mol Biol 1997;17:508–518. [DOI] [PubMed] [Google Scholar]
- 5.Factor P, Mutlu GM, Chen L, Mohameed J, Akhmedov AT, Meng FJ, Jilling T, Lewis ER, Johnson MD, Xu A, et al. Adenosine regulation of alveolar fluid clearance. Proc Natl Acad Sci USA 2007;104:4083–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo Y, DuVall MD, Crow JP, Matalon S. Nitric oxide inhibits Na+ absorption across cultured alveolar type II monolayers. Am J Physiol 1998;274:369–377. [DOI] [PubMed] [Google Scholar]
- 7.Launay JM, Herve P, Peoc'h K, Tournois C, Callebert J, Nebigil CG, Etienne N, Drouet L, Humbert M, Simonneau G, et al. Function of the serotonin 5-hydroxytryptamine 2B receptor in pulmonary hypertension. Nat Med 2002;8:1129–1135. [DOI] [PubMed] [Google Scholar]
- 8.Marcos E, Fadel E, Sanchez O, Humbert M, Dartevelle P, Simonneau G, Hamon M, Adnot S, Eddahibi S. Serotonin transporter and receptors in various forms of human pulmonary hypertension. Chest 2005;128:552–553. [DOI] [PubMed] [Google Scholar]
- 9.Lechin F, van der Dijs B, Orozco B, Lechin M, Lechin AE. Increased levels of free serotonin in plasma of symptomatic asthmatic patients. Ann Allergy Asthma Immunol 1996;77:245–253. [DOI] [PubMed] [Google Scholar]
- 10.Fu XW, Nurse CA, Wong V, Cutz E. Hypoxia-induced secretion of serotonin from intact pulmonary neuroepithelial bodies in neonatal rabbit. J Physiol 2002;539:503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan J, Copland I, Post M, Yeger H, Cutz E. Mechanical stretch induced serotonin release from pulmonary neuroendocrine cells: implications for lung development. Am J Physiol Lung Cell Mol Physiol 2005;290:185–193. [DOI] [PubMed] [Google Scholar]
- 12.Tamaoki J, Chiyotani A, Takemura H, Konno K. 5-hydroxytryptamine inhibits Na absorption and stimulates Cl secretion across canine tracheal epithelial sheets. Clin Exp Allergy 1997;27:972–977. [PubMed] [Google Scholar]
- 13.Stepp LR, Novakoski MA. Effect of 5-hydroxytryptamine on sodium- and potassium-dependent adenosine triphosphatase and its reactivity toward ouabain. Arch Biochem Biophys 1997;337:43–53. [DOI] [PubMed] [Google Scholar]
- 14.Greczko I, Tyrakowski T. The effect of serotonin on airway transepithelial sodium ion pathways. Eur J Pharmacol 2001;412:113–119. [DOI] [PubMed] [Google Scholar]
- 15.Nam BD, Park HJ. Side specific effect of 5-hydroxytryptamine on NaCl transport in the apical and basolateral membrane of rat tracheal epithelia. Physiol Res 2006;55:397–403. [DOI] [PubMed] [Google Scholar]
- 16.Wang D, Post M, Cutz E. Expression of serotonin receptor 2c in rat type II pneumocytes. Am J Respir Cell Mol Biol 1994;20:1175–1180. [DOI] [PubMed] [Google Scholar]
- 17.Bayer H, Müller T, Myrtek D, Sorichter S, Ziegenhagen M, Norgauer J, Zissel G, Idzko M. Serotoninergic receptors on human airway epithelial cells. Am J Respir Cell Mol Biol 2007;36:85–93. [DOI] [PubMed] [Google Scholar]
- 18.Dobbs LG, Gonzalez R, Williams MC. An improved method for isolating type II cells in high yield and purity. Am Rev Respir Dis 1986;134:141–145. [DOI] [PubMed] [Google Scholar]
- 19.Dobbs LG. Isolation and culture of alveolar type II cells. J Physiol 1990;258:134–147. [DOI] [PubMed] [Google Scholar]
- 20.Fang X, Song Y, Hirsch J, Galietta LJV, Pedemonte N, Zemans RL, Dolganov G, Verkman AS, Matthay MA. Contribution of CFTR to apical–basolateral fluid transport in cultured human alveolar epithelial type II cells. Am J Physiol Lung Cell Mol Physiol 2006;290:242–249. [DOI] [PubMed] [Google Scholar]
- 21.Ware LB, Wang Y, Fang X, Warnock M, Sakuma T, Hall TS, Matthay MA. Assessment of lungs rejected for transplantation and implications for donor selection. Lancet 2002;360:619–620. [DOI] [PubMed] [Google Scholar]
- 22.Kondo M, Finkbeiner WE, Widdicombe JH. Cultures of bovine tracheal epithelium with differentiated ultrastructure and ion transport. In Vitro Cell Dev Biol 1992;29:19–24. [DOI] [PubMed] [Google Scholar]
- 23.Yamaya M, Finkbeiner WE, Chun SY, Widdicombe JH. Differentiated structure and function of cultures from human tracheal epithelium. Am J Physiol Lung Cell Mol Physiol 1992;262:L713–L724. [DOI] [PubMed] [Google Scholar]
- 24.Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 1994;367:463–467. [DOI] [PubMed] [Google Scholar]
- 25.Chrabi A, Horisberger JD. Stimulation of epithelial sodium channel activity by the sulfonylurea glibenclamide. J Pharmacol Exp Ther 1999;290:341–347. [PubMed] [Google Scholar]
- 26.Shlyonsky V, Goolaerts A, Van Beneden R, Sariban-Sohraby S. Differentiation of epithelial Na+ channel function: an in vitro model. J Biol Chem 2005;280:24181–24187. [DOI] [PubMed] [Google Scholar]
- 27.Frank J, Roux J, Kawakatsu H, Su G, Dagenais A, Berthiaume Y, Howard M, Canessa CM, Fang X, Sheppard D, et al. Transforming growth factor-beta1 decreases expression of the epithelial sodium channel alphaENaC and alveolar epithelial vectorial sodium and fluid transport via an ERK1/2-dependent mechanism. J Biol Chem 2003;278:43939–43950. [DOI] [PubMed] [Google Scholar]
- 28.Weinstein H, Osman R. On the structural and mechanistic basis of function, classification, and ligand design for 5-HT receptors. Neuropsychopharmacology 1990;3:397–409. [PubMed] [Google Scholar]
- 29.Hamilton KL, Eaton DC. Single-channel recordings from two types of amiloride-sensitive epithelial Na+ channels. Membr Biochem 1986;6:149–171. [DOI] [PubMed] [Google Scholar]
- 30.Song Y, Thiagarajah J, Verkman AS. Sodium and chloride concentrations, pH, and depth of airway surface liquid in distal airways. J Gen Physiol 2003;122:511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamagata T, Yamagata Y, Masse C, Tessier MC, Brochiero E, Dagenais A, Berthiaume Y. Modulation of Na+ transport and epithelial sodium channel expression by protein kinase C in rat alveolar epithelial cells. Can J Physiol Pharmacol 2005;83:977–987. [DOI] [PubMed] [Google Scholar]
- 32.Awayda MS. Specific and non-specific effects of protein kinase C on the epithelial Na+ channel. J Gen Physiol 2000;115:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stockand JD, Bao HF, Schenck J, Malik B, Middleton P, Schlanger LE, Eaton DC. Differential effects of protein kinace C on the levels of epithelial Na+ channel subunit proteins. J Biol Chem 2000;275:25760–25765. [DOI] [PubMed] [Google Scholar]
- 34.Lewin AH. Receptors of mammalian trace amines. AAPS J 2006;10:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmer LG, Andersen OS. Interactions of amiloride and small monovalent cations with the epithelial sodium channel: inferences about the nature of the channel pore. Biophys J 1989;55:779–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McNicholas CM, Canessa CM. Diversity of channels generated by different combinations of epithelial sodium channel subunits. J Gen Physiol 1997;109:681–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kashlan OB, Sheng S, Kleyman TR. On the interaction between amiloride and its putative alpha-subunit epithelial Na+ channel binding site. J Biol Chem 2005;280:26206–26215. [DOI] [PubMed] [Google Scholar]
- 38.Collier DM, Snyder PM. Extracellular protons regulate human ENaC by modulating Na+ self-inhibition. J Biol Chem 2009;284:792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee YL, Fu CL, Chiang BL. Administration of interleukin-12 exerts a therapeutic instead of a long-term preventive effect on mite Der p I allergen–induced animal model of airway inflammation. Immunology 1999;97:232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Lommel A. Pulmonary neuroendocrine cells (PNEC) and neuroepithelial bodies (NEB): chemoreceptors and regulators of lung development. Paediatr Respir Rev 2001;2:171–176. [DOI] [PubMed] [Google Scholar]
- 41.Hardiman KM, Matalon S. Modification of sodium transport and alveolar fluid clearance by hypoxia: mechanisms and physiological implications. Am J Respir Cell Mol Biol 2001;25:538–541. [DOI] [PubMed] [Google Scholar]