Abstract
Aims
To evaluate whether there is a correlation between the subcellular localization of maspin and the histological, molecular and biological features of pulmonary adenocarcinoma, particularly addressing the hypothesis that the tumour inhibitor properties of maspin may be linked to a nuclear, compared with a combined nuclear and cytoplasmic expression pattern.
Methods and results
The subcellular expression of maspin was determined in 80 resected pulmonary adenocarcinomas (Stage I, 46; Stage II, 10; Stage III, 20; Stage IV, 4) and correlated with histological grade, proliferative rate, p53 expression, vascular endothelial growth factor (VEGF)-A levels, and prognosis (mean follow-up of 41.5 months). Cases with nuclear (N) maspin (n = 47), compared with the [N + cytoplasmic (C)] group (n = 28), showed lower (P ≤ 0.05): histological grade, proliferative rate, p53 expression and VEGF-A levels. Cox multivariate analysis revealed in stage I adenocarcinomas (N) maspin as the only predictor of improved survival.
Conclusions
(N) maspin selects lung adenocarcinomas with distinct molecular and clinical features, supporting the hypothesis that its tumour inhibitor properties may be linked to its nuclear localization.
Keywords: lung adenocarcinoma, maspin, p53, prognosis, Stage I, subcellular localization, VEGF-A
Introduction
Maspin is a mammary homologue serine protease inhibitor (serpin) gene, located in the serpin cluster on chromosome 18q 21.3–q23, that exerts its tumour suppressor activity by modulating cancer cell growth and invasiveness, including apoptosis, angiogenesis, cell adhesion and motility,1–3 although the exact biochemical pathways leading to these biological endpoints are incompletely characterized. Paradoxically, in spite of its tumour suppressor activity, both a decrease4–7 and an increase of maspin levels8–11 have been described to parallel tumour progression. Nuclear (N) maspin segregates with molecular markers associated with favourable outcome,12 low histological grade13 and increased overall survival/disease-free interval in breast12 and ovarian13,14 cancer. In vitro, chemical transformation of bronchial epithelial cells15–18 is linked to selective down-regulation of nuclear maspin, with a consequent reduction of its nuclear/cytoplasmic ratio. Nuclear expression is associated with lower histological grade, proliferative rate and p53 immunopositivity in lung adenocarcinoma,19 and, in non-small cell lung cancer (NSCLC), improved survival.20 These data support the hypothesis that maspin’s subcellular localization may influence its biology and, particularly, that its tumour inhibitor activity may be dependent on its nuclear localization. However, studies on the relation of maspin expression and its cellular localization with both prognosis and molecular features in NSCLC have reported conflicting findings,19–25 so while these differences may be, at least in part, secondary to technical differences, the issue remains unsettled.
In the current study we tested the hypothesis that the higher nuclear/cytoplasmic levels may portend more favourable biology in NSCLC. Since, in our experience,19 confirmed by others,21 a combined nuclear–cytoplasmic (N + C) expression pattern is much more common in squamous than in adenocarcinoma, we focused on this latter histotype to study whether the maspin subcellular expression pattern is linked to differences in morphological, molecular and clinical features of pulmonary adenocarcinoma.
Materials and methods
Representative tumour samples from 80 formalin-fixed paraffin-embedded resected lung adenocarcinomas were selected to build a tissue array, using 2.5-mm cores. These cases were unselected, consecutive resected adenocarcinomas, identified retrospectively. Fourteen of these cases were included in the previous study.19 The project was approved by the hospital ethics committee, the Institutional Review Board, and adhered to its guidelines. Histological sections were deparaffinized and subject to immunohistochemistry, using standard streptavidin-biotin-peroxidase techniques, with diaminobenzidine (DAB) as the chromogen. In brief, 4–5 μm thick sections were antigen retrieved by steam treatment in a citrate buffer, quenched for 5 min with 3% H2O2, saturated in 0.05% bovine serum albumin (BSA) and preincubated with normal serum at 1:20 in 2% BSA/phosphate-buffered saline (PBS) for 15 min at room temperature. After incubation with the primary antibodies, slides were rinsed with PBS for 30 s and the secondary antibody was applied at 1:500 in 1% BSA/PBS for 60 min at room temperature. Following rinses with PBS for 30 s, slides were incubated with streptavidin–peroxidase at 1:500 in 1% BSA/PBS for 45 min at room temperature, then rinsed with PBS for 30 s, incubated for 15 min in 0.06% DAB (Zymed, Cambridge, MA, USA) and counterstained with Harris modified haematoxylin (Fisher, Middletown, VA, USA). Positive controls, consisting of cases with known reactivity for each antibody, and negative controls, obtained omitting the primary antibody, were included with each run.
Working conditions, including pretreatment, antibody source, dilutions and incubation times were as follows: monoclonal antibody against maspin (G167-7; Becton Dickinson-Pharmingen, Franklin Lake, NJ, USA), 1:100, 2 h at 4°C; Ki67 monoclonal antibody (clone K2; Ventana, Tucson, AZ, USA), heat pretreatment in ethylenediamine tetraaceticacid (EDTA) buffer, prediluted, 16 min at 37°C; monoclonal anti-p53 (clone D07; Ventana), heat pretreatment in EDTA buffer, prediluted, 32 min at 37°C; monoclonal antivascular endothelial growth factor (VEGF)-A (Clone C1; Santa Cruz, Santa Cruz, CA, USA), pretreatment with protease for 10 min, 1:50, 32 min at 37°C.
p53 was considered positive when ≥1/3 intensity nuclear immunoreactivity occurred in ≥10% of tumour cells. All maspin-positive cases showed reactivity in ≥50% of cells; maspin expression was stratified as either negative or positive; positive cases were subdivided into N and N + C. The result of the Ki67 stain was expressed as a continuum, as the average number of positive tumour cell nuclei per 100 tumour cells, and was assumed to reflect the overall proliferative rate of the tumour. All cases showed some immunoreactivity, which ranged from 5% (generally displayed by bronchioloalveolar carcinoma) to ≥90%. The intensity of reactivity and the percentage of tumour cells reactive for VEGF-A were recorded separately, each on a scale of 1–3 (intensity of positivity = 1, lowest to 3, highest; extent of positivity: 1 = 0–10%; 2 = 11–49%; 3 = 51–100%), then a final score was obtained by multiplying the two.
Cases with a score of 1–3, i.e. that either showed no reactivity, or focal (≤10%) but intense reactivity were considered to have low VEGF-A levels, tumours with scores of 4–9, high VEGF-A levels.
Since standard definitions of grade for lung cancer are not available, the histological grade was defined by architectural criteria, using the percentage of solid growth displayed by the tumour. Similar scoring criteria are used to grade the growth patterns in breast cancer, (Nottingham modified Bloom–Richardson grading system), and endometrioid adenocarcinoma of uterus (FIGO grading system). Thus, cases where a solid versus patent acinar/tubular growth pattern was present in ≥80% of the tumour were classified as poorly differentiated; in 21–79%, moderately differentiated; in ≤20%, well differentiated.
After obtaining approval from the institutional review board, a retrospective analysis of the medical records of adenocarcinoma lung cancer patients who underwent surgical resection between August 1996 and March 2005 was performed. Demographic, physiological and clinical data including age, gender, race and smoking history were collected. Lung cancer was staged according to the tumour node metastasis (TNM) international staging system (adapted from the American Joint Committee on Cancer TNM Cancer Staging Manual), utilizing all the clinical, radiological and pathological data available. Further data including positron emission tomography scan, progression of disease, treatment modalities and response to treatment were obtained from the patients’ medical records.
Patients’ survival status and cause of death were determined from the medical records, and the Metropolitan Detroit Cancer Surveillance System, through the epidemiology and population studies section at the Barbara Ann Karmanos Cancer Center (Detroit, MI, USA).
The chi-squared Fisher’s exact test was applied for statistical analysis. Kaplan–Meier analysis was performed for survival. Cox regression was applied to identify the significance of maspin expression in relation to other variables in predicting improved survival in Stage I adenocarcinoma.
Results
RELATION OF MASPIN SUBCELLULAR EXPRESSION TO CLINICAL FEATURES
The overall clinical features of the series, including age, gender, stage distribution and follow-up, are listed in Table 1. Immunohistochemistry for maspin revealed immunoreactivity in 75/80 cases, 47 of which showed (N) (Figure 1), 28 combined (N + C) (Figure 2) stain. Table 2 lists clinical parameters according to maspin expression. Since the negative cases were only five, the following correlative analyses were limited to the two main groups of (N) versus (N + C) expression. No statistical correlation was present between maspin expression pattern and age or gender. Table 3 shows that the mean survival, for Stage I cases only, was higher for the N compared with (N + C) group. Kaplan–Meier analysis showed the statistically significant survival advantage for cases with N only expression, in Stage I (Figure 3), but not in advanced stage (data not shown).
Table 1.
Overall clinical features of cases studied
| Gender (n = 80) | Male (33), female (47) |
|---|---|
| Age (years) | Range (33–87); mean 67 |
| Stage | Stage I (46), Stage II (10) Stage III (20), Stage IV (4) |
| Follow-up (months) | Range (0.5–102); mean 41.5; median 37.5 |
Figure 1.
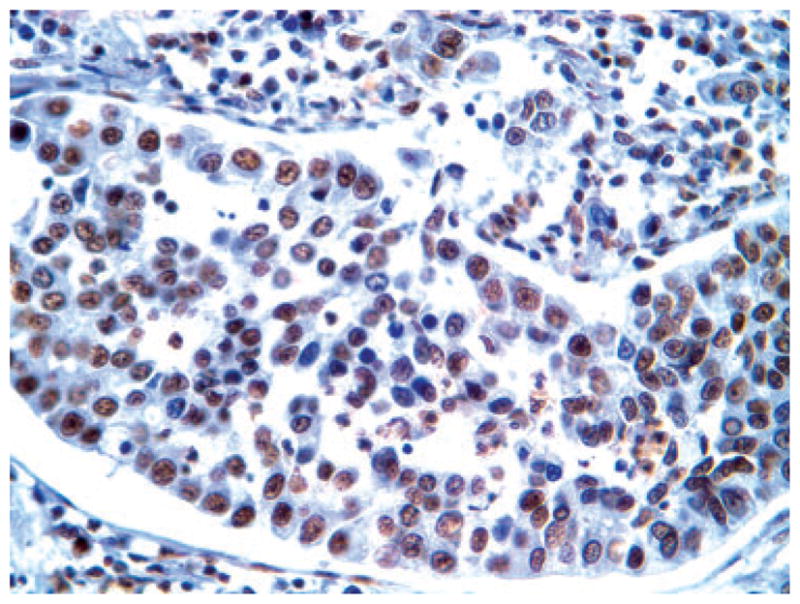
Representative immunohistochemical picture of nuclear expression of maspin in adenocarcinoma.
Figure 2.
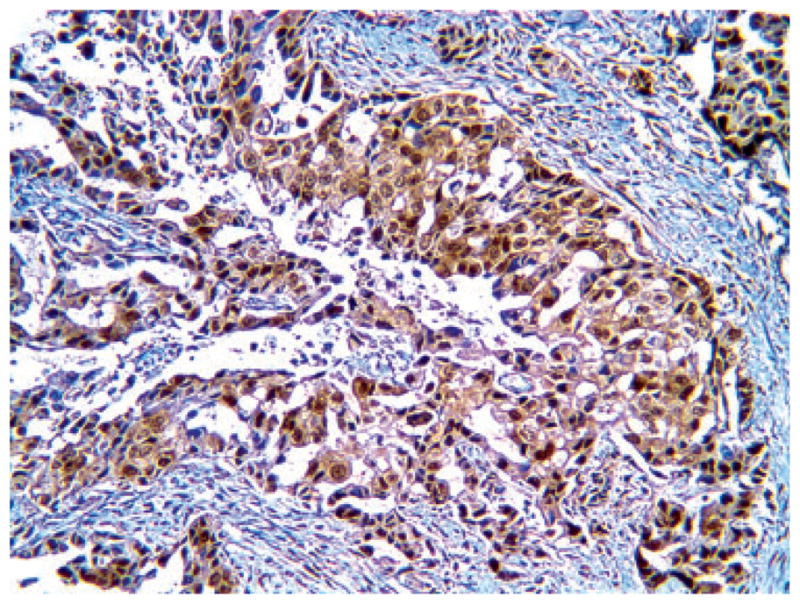
Representative immunohistochemical picture of combined nuclear–cytoplasmic expression of maspin in adenocarcinoma.
Table 2.
Clinical features of series divided by maspin expression
| N (47) | N + C (28) | Negative (5) | |
|---|---|---|---|
| Age (years) | Mean 66.9, range (33–87) | Mean 67.2, range (47–87) | Mean 67.8, range (57–80) |
| Gender | M 20, F 27 | M 12, F 16 | M 2, F 3 |
| Follow-up period (months) | Mean 43, range (0.5–102) | Mean 38.8, range (8–88) | Mean 42.6, range (1–88) |
| Stage Stage I |
25 |
16 |
5 |
| Stage II | 6 | 5 | 0 |
| Stage III | 15 | 4 | 0 |
| Stage IV | 1 | 3 | 0 |
N, maspin nuclear stain; N + C, maspin nuclear + cytoplasmic stain.
Table 3.
Survival by maspin status in local versus advanced stage
| Maspin status | Mean survival (months) ± standard error | |
|---|---|---|
| Local (I) | Advanced (II–IV) | |
| N | 87.7 ± 6.9 | 48.6 ± 8.6 |
| (N + C) | 52.7 ± 6.5 | 52.7 ± 10.3 |
N, maspin nuclear stain; N + C, maspin nuclear + cytoplasmic stain
Figure 3.
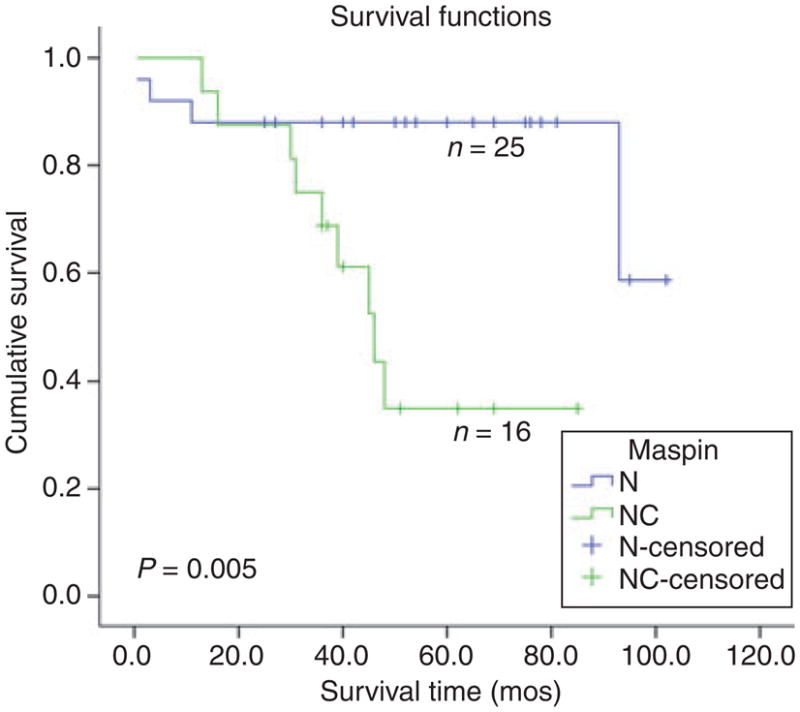
Kaplan–Meier survival curve in Stage I adenocarcinoma, shows a statistically significant advantage in survival for cases with nuclear (N) compared with combined nuclear–cytoplasmic (N + C) maspin expression.
MASPIN SUBCELLULAR LOCALIZATION AND ITS RELATION TO GRADE AND MOLECULAR PARAMETERS
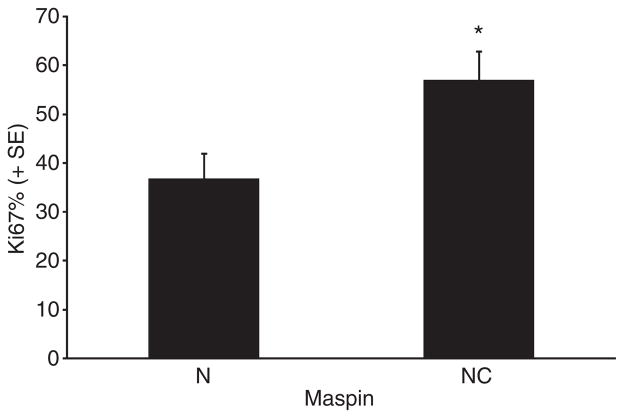
Maspin subcellular expression was then correlated with histological grade and select molecular markers (Tables 4–7, Figure 4). A statistically significant association of (N) maspin was present with low histological grade (i.e. the combined group of bronchioloalveolar and well-differentiated adenocarcinoma) (Table 4), negative p53 expression (Table 5) and lower proliferative rate (Figure 4). The mean proliferative rate of cases with (N) maspin was 36.6, that of (N + C) cases 56.89%. A highly significant association between (N) maspin expression and low VEGF-A expression levels was also present (see Materials and methods for definition; Figures 5 and 6) (Table 6).
Table 4.
Maspin versus histological grade
| Maspin N | Maspin N + C | Negative | |
|---|---|---|---|
| BAC and WD | 27 | 8 | 2 |
| MD and PD | 20 | 20 | 3 |
N, maspin nuclear stain; N + C, maspin nuclear + cytoplasmic stain; BAC, bronchioloalveolar carcinoma; WD, MD, PD, well, moderately, poorly differentiated.
Maspin N versus N + C, P = 0.0183.
Table 7.
Multivariate Cox regression analysis of maspin, grade, molecular variables, and survival, in stage I adenocarcinoma
| P-value | |
|---|---|
| Maspin subcellular expression | 0.050 |
| Histological grade | 0.321 |
| p53 | 0.183 |
| Ki67 | 0.676 |
| VEGF-A | 0.618 |
| Size | 0.387 |
Figure 4.
Bar graph showing the Ki67 score, in nuclear versus nuclear–cytoplasmic maspin, with standard error bars. The means of the two groups were, respectively 36.64 ± 35.08 and 56.89 ± 30.50; the 95% confidence intervals 25.97, 47.30 and 44.82, 68.96. The differences were statistically significant.
Table 5.
Maspin versus p53
| Maspin N | Maspin N + C | Maspin negative | |
|---|---|---|---|
| p53− | 33 | 13 | 4 |
| p53+ | 12 | 15 | 1 |
N, maspin nuclear stain; N + C, maspin nuclear + cytoplasmic stain.
Maspin N versus N + C, P = 0.0264.
Figure 5.
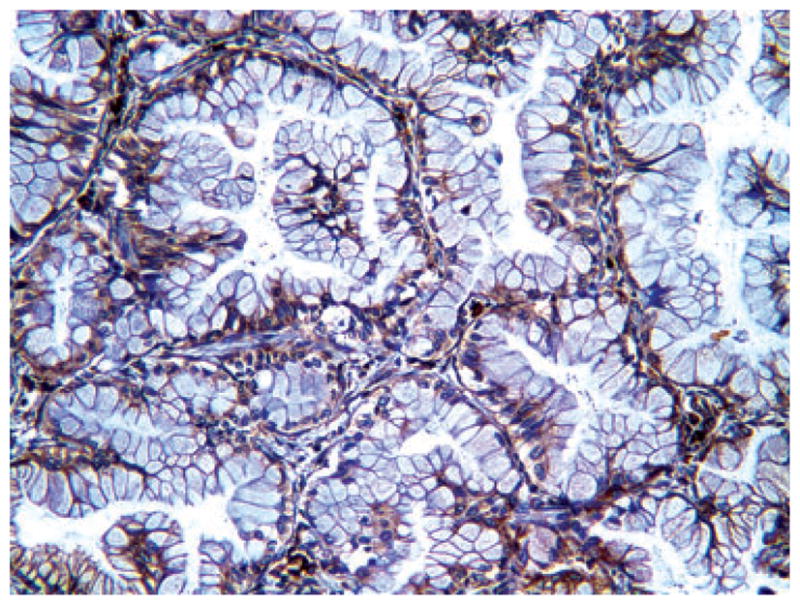
Representative immunohistochemical picture of low expression level of vascular endothelial growth factor-A (score of 3 = 1 intensity × 3 extent of positivity) in adenocarcinoma.
Figure 6.
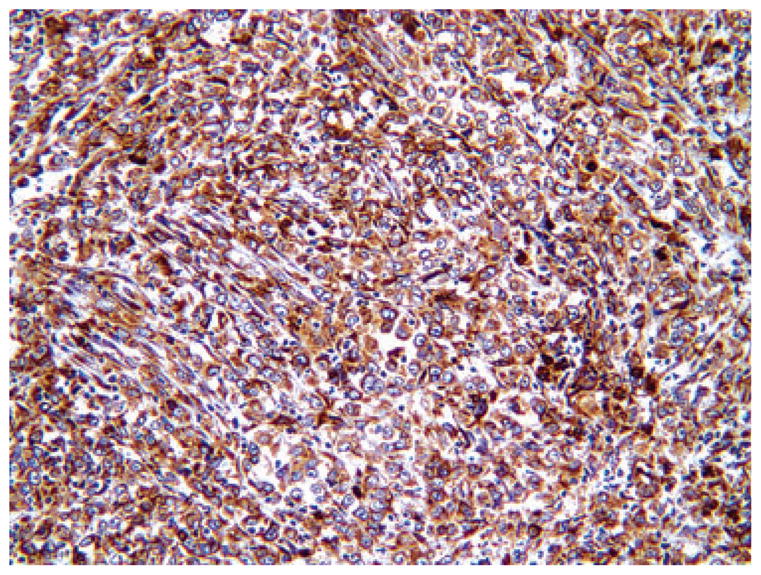
Representative immunohistochemistry of high expression level of vascular endothelial growth factor-A (score of 9 = 3 intensity × 3 extent of positivity) in adenocarcinoma.
Table 6.
Maspin versus vascular endothelial growth factor (VEGF)-A
| Maspin N | Maspin N + C | Maspin negative | |
|---|---|---|---|
| Low VEGF-A (score 1–3) | 41 | 17 | 3 |
| High VEGF-A (score 4–9) | 2 | 11 | 2 |
N, maspin nuclear stain; N + C, maspin nuclear + cytoplasmic stain.
Maspin N versus N + C, P = 0.0004.
Cox regression analysis of maspin subcellular localization, size, VEGF level (low versus high score), p53 expression (negative versus positive), histological grade (low versus high) and Ki67 (50% or less versus more than 50%), showed that maspin (N) versus (N + C) expression was the only independent predictor of improved survival in Stage I cases (Table 7).
Discussion
Our data show an association of nuclear maspin with lower histological grade, lower proliferative rate and absent p53 immunopositivity, confirming previous findings,19 and further establish a novel correlation between nuclear maspin and low VEGF-A levels. No standard criteria exist for the histological grading of lung cancer. In the current study we adopted a grading system based on architectural patterns. Although we recognize the somewhat arbitrary nature of this classification, yet we have to underscore its at least internal consistency and reproducibility and, most importantly, its correlation with the cellular localization of maspin. Indeed, the current analysis included many more cases of the previous study. Yet the authors, blinded to the results of the molecular evaluation until the end of the study, were able to reproduce the correlation of maspin cellular localization and histological grade described previously.19 We were not able to find any correlation between maspin cellular immunoreactivity pattern and the histological subtypes of lung cancer, as described in the World Health Organization 2004 classification of lung tumours, i.e. acinar versus, papillary versus, solid. However, we found that virtually all (10/11) the bronchioloalveolar carcinomas studied displayed a nuclear pattern of maspin reactivity. These data had been previously reported19 and corroborate the association we describe between nuclear maspin and favourable histological features in lung adenocarcinoma. They further justify studies of tumours showing combined non-invasive/invasive growth patterns to address the role of maspin cellular localization in tumour progression. These data also raise the question of whether a relation exists between nuclear maspin and epidermal growth factor receptor mutations, which, although beyond the scope of the current study, deserves further investigation. Since all the bronchioloalveolar carcinomas in our series were non-mucinous, it also remains unclear what the pattern of maspin immunoreactivity is in the mucinous variant.
No attempts were made in the current study to include nuclear features as a component of the grade, and thus its correlation to prognosis and the other molecules studied awaits further characterization.
The proliferative rate, an important feature in other grading systems, was here evaluated by the Ki67 stain and studied as a separate parameter. A significant statistical correlation of nuclear maspin with lower proliferative rates was found (Figure 4).
VEGF-A has been shown in NSCLC to constitute a marker of angiogenesis, correlating with vessel density, 26–28 predict a worse prognosis26–29 and constitute an effective target of molecular therapy.29 Studies of maspin and VEGF-A have been published for other tumour types,14,30,31 and a link between nuclear maspin and VEGF-A described in ovarian cancer, but ours is the first study to find this link in NSCLC.14 Overall, our data support the hypothesis that maspin subcellular localization selects adenocarcinoma cases with distinct molecular and morphological features.
However, the biological meaning of maspin expression and subcellular localization remains controversial. Some data support the hypothesis that the tumour suppressor properties of maspin are linked to its nuclear localization. Thus, Smith et al.20 found nuclear localization to correlate with overall better prognosis, in 48 resectable NSCLCs with median follow-up of 16 months, not subgrouped by histology and stage. Hirai and Kuzumi found a cytoplasmic localization to be independently associated with higher stages and reduced survival in NSCLC.23 Others have described an association of increased maspin levels with improved survival, compatible with the view that maspin is a tumour suppressor gene, but they did not report this to be linked to its nuclear localization. Thus, high levels of maspin, determined by real-time polymerase chain reaction (PCR), were found to represent an independent predictor of improved survival in 55 NSCLCs,24 and the same positive correlation with prognosis was found, but limited to SqCCa when maspin levels were determined by immunohistochemistry.25 Likewise, Nakashima et al. have shown in adenocarcinoma (78 cases) a positive association between maspin expression and prognosis.22 However, others have found no association between maspin expression and survival.21 The reasons for these variations are probably manifold. A review of the literature discloses wide differences in techniques of detection. The differences in results obtained by immunohistochemistry versus real-time PCR may be explained by the wide divergences that exist between maspin mRNA and protein levels.32
Among immunohistochemical studies, a major source of variation may reside in the failure to identify the cellular localization of maspin in positive cases.25 Furthermore, wide differences exist in the immunohistochemical detection methods used,20 including antibody concentrations, incubation times and temperatures.19–23,25 In our experience, such methodological differences may affect drastically the immunohistochemical results, and we suspect that they may play a large role in such variations. Fixation time is another parameter, known to affect immunoreactivity, that may play a role. Ultimately, however these discrepancies may not be resolved until groups with diverging results accept cross-validation of their series, or multicentric studies are conducted with standardized methods. Data on the relation of maspin with p53 in NSCLC are contrasting, some authors finding p53 immunopositivity to correlate with maspin nuclear reactivity,21 others with high immunohistochemical maspin levels,25 yet others finding no association.23 However, our finding that a combined (N + C) expression of maspin is more frequent in squamous cell carcinoma than in adenocarcinoma was confirmed by others (NSCLC n = 487),21 supporting the hypothesis that different expression patterns may co-segregate with different histological types of NSCLC.
Our novel finding, that in Stage I adenocarcinoma N maspin predicts a more favourable outcome, supports the hypothesis that the site of maspin subcellular localization is an aspect of its biology with clinical relevance. Both Stage 1A and 1B were included in this series, implying that tumours ≤30 mm and ≥30 mm were grouped. The small number of cases precluded separate analysis of these two subgroups (only 10 cases were stage 1A) and thus the question of whether significant differences exists among them awaits further, larger studies. However, Cox regression analysis revealed that size, expressed as a continuum, was not a significant parameter in predicting survival in Stage 1 tumours (Table 7). These data are consistent with our previous finding that in vitro, chemical transformation of immortalized bronchial cells induces selective reduction of nuclear maspin, with a consequent reduction in its nuclear–cytoplasmic ratio,19 and support the hypothesis that the tumour inhibitor activity of maspin may be linked to its nuclear localization. The pathways that link N maspin with low p53 expression, low proliferative rate and VEGF-A remain unknown. The biology of maspin is complex, since the protein is found in different cellular localizations, including nucleus, cytoplasm and membrane, and it may exert different functions in these different compartments. Data showing that differential molecular partners for maspin exist in these different subcellular compartments are starting to be unveiled,1–3, supporting this hypothesis. Known molecular partners of maspin include transcription factors and histone deacethylase (HDAc),1–3 and it is reasonable to hypothesize that through these, nuclear maspin may modulate the transcription of genes involved in cell cycle progression, and angiogenesis, such as VEGF-A. Indeed, known targets of HDAc include cell cycle kinase inhibitors p21 and p27.1–3 The reason for an association between nuclear maspin and wild-type p53 are at present not clear. However, wild-type p53 is known to transactivate maspin.1–3 On the basis of the demonstrated association between maspin and glutathione-S-reductase,1–3 one may further speculate that maspin has a role in preventing oxidative damage and may be selected out in earlier stage tumours, whereas tumour progression, mirrored by the appearance of p53 mutations, is paralleled by a shift of maspin to the cytoplasm. Indeed, although this dependence on subcellular localization may constitute a unique feature of maspin biology, many precedents exist for proteins whose activity is influenced by nucleus–cytoplasm or even cell membrane–nucleus shuffling, including tumour suppressors and adhesion molecules.33–35
In conclusion, we present data showing that nuclear versus combined nuclear–cytoplasmic expression of maspin is related not only to favourable histological and molecular features of lung adenocarcinoma but also, in early stage, improved prognosis. These data support the hypothesis that changes in the subcellular localization of maspin affect its biological function and warrant additional studies to test their biochemical basis. Standardization of methods of maspin detection and cross-validation among different groups, may, at the same time, help resolve controversy on the prognostic and biological role of the expression and subcellular localization of this protein in NSCLC.
Abbreviations
- BSA
bovine serum albumin
- C
cytoplasmic
- DAB
diaminobenzidine
- EDTA
ethylenediamine tetraaceticacid
- HDAc
histone deacethylase
- N
nuclear
- NSCLC
non-small cell lung cancer
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- TNM
tumour node metastasis
- VEGF
vascular endothelial growth factor
Contributor Information
Amy Frey, Departments of Pathology, Harper University Hospital, Wayne State University and Barbara Ann Karmanos Cancer Institute, Detroit, MI.
Ayman O Soubani, Division of Pulmonary, Allergy, Critical Care and Sleep Medicine, Internal Medicine, Harper University Hospital, Wayne State University and Barbara Ann Karmanos Cancer Institute, Detroit, MI.
Abdulgadir K Adam, Division of Pulmonary, Allergy, Critical Care and Sleep Medicine, Internal Medicine, Harper University Hospital, Wayne State University and Barbara Ann Karmanos Cancer Institute, Detroit, MI.
Shijie Sheng, Departments of Pathology, Harper University Hospital, Wayne State University and Barbara Ann Karmanos Cancer Institute, Detroit, MI.
Harvey I Pass, Divisions of Thoracic Surgery and Thoracic Oncology, Department of Cardiothoracic Surgery, NCI Comprehensive Cancer Center, New York University, New York, NY, USA.
Fulvio Lonardo, Departments of Pathology, Harper University Hospital, Wayne State University and Barbara Ann Karmanos Cancer Institute, Detroit, MI.
References
- 1.Bailey CM, Khalkhali-Elllis Z, Seftor EA, Hendrix MJ. Biological functions of maspin. J Cell Physiol. 2006;209:617–624. doi: 10.1002/jcp.20782. [DOI] [PubMed] [Google Scholar]
- 2.Sheng S. A role of novel serpin maspin in tumor progression: the divergence revealed through efforts to converge. J Cell Physiol. 2006;209:631–635. doi: 10.1002/jcp.20786. [DOI] [PubMed] [Google Scholar]
- 3.Khalkhali-Ellis Z. Maspin, the new frontier. Clin Cancer Res. 2006;12:7279–7283. doi: 10.1158/1078-0432.CCR-06-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czerwenka KF, Manavi M, Hosmann J, et al. Comparative analysis of two-dimensional protein patterns in malignant and normal human breast tissue. Cancer Detect Prev. 2001;25:268–279. [PubMed] [Google Scholar]
- 5.Maass N, Hojo T, Rosel F, Ikeda T, Jonat W, Nagasaki K. Down regulation of the tumor suppressor gene maspin in breast carcinoma is associated with a higher risk of distant metastasis. Clin Biochem. 2001;34:303–307. doi: 10.1016/s0009-9120(01)00220-x. [DOI] [PubMed] [Google Scholar]
- 6.Maass N, Teffner M, Rosei F, et al. Decline in the expression of the serine proteinase inhibitor maspin is associated with tumour progression in ductal carcinomas of the breast. J Pathol. 2001;195:321–326. doi: 10.1002/path.948. [DOI] [PubMed] [Google Scholar]
- 7.Zou Z, Anisowicz A, Hendrix MJ, et al. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994;263:526–529. doi: 10.1126/science.8290962. [DOI] [PubMed] [Google Scholar]
- 8.Bieche I, Girault I, Sabourin JC, et al. Prognostic value of maspin mRNA expression in ER alpha-positive postmenopausal breast carcinomas. Br J Cancer. 2003;88:863–870. doi: 10.1038/sj.bjc.6600812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stathopoulou A, Mavroudis D, Perraki M, et al. Molecular detection of cancer cells in the peripheral blood of patients with breast cancer: comparison of CK-19, CEA and maspin as detection markers. Anticancer Res. 2003;23:1883–1890. [PubMed] [Google Scholar]
- 10.Umekita Y, Ohi Y, Sagara Y, Yoshida H. Expression of maspin predicts poor prognosis in breast-cancer patients. Int J Cancer. 2002;100:452–455. doi: 10.1002/ijc.10500. [DOI] [PubMed] [Google Scholar]
- 11.Umekita Y, Yoshida H. Expression of maspin is up-regulated during the progression of mammary ductal carcinoma. Histopathology. 2003;42:541–545. doi: 10.1046/j.1365-2559.2003.01620.x. [DOI] [PubMed] [Google Scholar]
- 12.Mohsin SK, Zhang M, Clark GM, Craig Allred D. Maspin expression in invasive breast cancer: association with other prognostic factors. J Pathol. 2003;199:432–435. doi: 10.1002/path.1319. [DOI] [PubMed] [Google Scholar]
- 13.Sood AK, Fletcher MS, Gruman LM, et al. The paradoxical expression of maspin in ovarian carcinoma. Clin Cancer Res. 2002;8:2924–2932. [PubMed] [Google Scholar]
- 14.Solomon LA, Munkarah AR, Schimp VL, et al. Maspin expression and localization impact on angiogenesis and prognosis in ovarian cancer. Gynecol Oncol. 2006;101:385–389. doi: 10.1016/j.ygyno.2005.11.049. [DOI] [PubMed] [Google Scholar]
- 15.Langenfeld J, Lonardo F, Kiyokawa H, et al. Inhibited transformation of immortalized human bronchial epithelial cells by retinoic acid is linked to cyclin E down-regulation. Oncogene. 1996;13:1983–1990. [PubMed] [Google Scholar]
- 16.Lonardo F, Dragnev KH, Freemantle SJ, et al. Evidence for the epidermal growth factor receptor as a target for lung cancer prevention. Clin Cancer Res. 2002;8:54–60. [PubMed] [Google Scholar]
- 17.Lonardo F, Rusch V, Langenfeld J, Dmitrovsky E, Klimstra DS. Overexpression of cyclins D1 and E is frequent in bronchial preneoplasia and precedes squamous cell carcinoma development. Cancer Res. 1999;59:2470–2476. [PubMed] [Google Scholar]
- 18.Siddiq F, Sarker FH, Wali A, Pass HI, Lonardo F. Increased osteonectin expression is associated with malignant transformation and tumor associated fibrosis in the lung. Lung Cancer. 2004;45:197–205. doi: 10.1016/j.lungcan.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Lonardo F, Li X, Siddiq F, et al. Maspin nuclear localization is linked to favorable morphological features in pulmonary adenocarcinoma. Lung Cancer. 2006;51:31–39. doi: 10.1016/j.lungcan.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Smith SL, Watson SG, Ratschiller D, Gugger M, Betticher DC, Heighway J. Maspin—the most commonly-expressed gene of the 18q21.3 serpin cluster in lung cancer—is strongly expressed in preneoplastic bronchial lesions. Oncogene. 2003;22:8677–8687. doi: 10.1038/sj.onc.1207127. [DOI] [PubMed] [Google Scholar]
- 21.Woenckhaus M, Bubendorf L, Dalquen P, et al. Nuclear and cytoplasmic Maspin expression in primary non-small cell lung cancer. J Clin Pathol. 2007;60:483–486. doi: 10.1136/jcp.2005.033407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakashima M, Ohike N, Nagasaki K, Adachi M, Morohoshi T. Prognostic significance of the maspin tumor suppressor gene in pulmonary adenocarcinoma. J Cancer Res Clin Oncol. 2004;130:475–479. doi: 10.1007/s00432-004-0571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirai K, Koizumi K, Haraguchi S, et al. Prognostic significance of the tumor suppressor gene maspin in non-small cell lung cancer. Ann Thorac Surg. 2005;79:248–253. doi: 10.1016/j.athoracsur.2004.06.118. [DOI] [PubMed] [Google Scholar]
- 24.Katakura H, Takenaka K, Nakagawa M, et al. Maspin gene expression is a significant prognostic factor in resected non-small cell lung cancer (NSCLC). Maspin in NSCLC. Lung Cancer. 2006;51:323–328. doi: 10.1016/j.lungcan.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Nakagawa M, Katakura H, Adachi M, et al. Maspin expression and its clinical significance in non-small cell lung cancer. Ann Surg Oncol. 2006;13:1517–1523. doi: 10.1245/s10434-006-9030-z. [DOI] [PubMed] [Google Scholar]
- 26.Mineo TC, Ambrogi V, Baddi A, et al. Prognostic impact of VEGF, CD31, CD34, and CD105 expression and tumour vessel invasion after radical surgery for IB-IIA non-small cell lung cancer. J Clin Pathol. 2004;57:591–597. doi: 10.1136/jcp.2003.013508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han H, Siverman JF, Santucci TS, et al. Vascular endothelial growth factor expression in stage I non-small cell lung cancer correlates with neoangiogenesis and a poor prognosis. Ann Surg Oncol. 2001;8:72–79. doi: 10.1007/s10434-001-0072-y. [DOI] [PubMed] [Google Scholar]
- 28.Ohta Y, Tanaka Y, Watanabe G, Minato H. Predicting recurrence following curative surgery in stage I non-small cell lung cancer patients using an angiogenesis-associated factor. J Exp Clin Cancer Res. 2007;26:301–305. [PubMed] [Google Scholar]
- 29.Stinchcombe TE, Socinski MA. Bevacizumab in the treatment of non-small-cell lung cancer. Oncogene. 2007;26:3691–3698. doi: 10.1038/sj.onc.1210366. [DOI] [PubMed] [Google Scholar]
- 30.Cho JH, Kim HS, Park CS, et al. Maspin expression in early oral tongue cancer and its relation to expression of mutant-type p53 and vascular endothelial growth factor (VEGF) Oral Oncol. 2007;43:272–277. doi: 10.1016/j.oraloncology.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 31.Li JJ, Chen Y, Zhang SM, et al. Pathobiological significance of vascular endothelial growth factor and Maspin expressions in human gastric carcinoma. World J Gastroenterol. 2004;10:2624–2627. doi: 10.3748/wjg.v10.i18.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schedel J, Distler O, Woenckhaus M, et al. Discrepancy between mRNA and protein expression of tumour suppressor maspin in synovial tissue may contribute to synovial hyperplasia in rheumatoid arthritis. Ann Rheum Dis. 2004;63:1205–1211. doi: 10.1136/ard.2003.006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fabbro M, Henderson BR. Regulation of tumor suppressors by nuclear–cytoplasmic shuttling. Exp Cell Res. 2003;282:59–69. doi: 10.1016/s0014-4827(02)00019-8. [DOI] [PubMed] [Google Scholar]
- 34.Wang SC, Hung MC. Cytoplasmic/nuclear shuttling and tumor progression. Ann NY Acad Sci. 2005;1059:11–15. doi: 10.1196/annals.1339.002. [DOI] [PubMed] [Google Scholar]
- 35.Hervy M, Hoffman L, Beckerle MC, et al. From the membrane to the nucleus and back again: bifunctional focal adhesion proteins. Curr Opin Cell Biol. 2006;18:524–532. doi: 10.1016/j.ceb.2006.08.006. [DOI] [PubMed] [Google Scholar]