Abstract
OBJECTIVE
Concerns about the safety and efficacy of over-the-counter cold medications have led to a recent US Food and Drug Administration public health advisory against their use in children <2 years of age. Our goal was to examine caregiver understanding of the age indication of over-the-counter cold medication labels and identify factors, associated with caregiver understanding.
METHODS
Caregivers of infant children (≤1 year old) were recruited from clinics at 3 institutions. Questions were administered regarding the use of 4 previously common “infant” over-the-counter cold and cough medicines labeled to consult a physician if used in children <2 years of age. Literacy and numeracy skills were assessed with validated instruments.
RESULTS
A total of 182 caregivers were recruited; 87% were the infants’ mothers. Mean education level was 12.5 years, and 99% had adequate literacy skills, but only 17% had >9th-grade numeracy skills. When examining the front of the product label, 86% of the time parents thought these products were appropriate for use in children <2 years of age. More than 50% of the time, parents stated they would give these over-the-counter products to a 13-month-old child with cold symptoms. Common factors that influenced parental decisions included label saying “infant,” graphics (eg, infants, teddy bears, droppers), and dosing directions. Caregivers were influenced by the dosing directions only 47% of the time. Caregivers with lower numeracy skills were more likely to provide inappropriate reasons for giving an over-the-counter medication.
CONCLUSIONS
Misunderstanding of over-the-counter cold products is common and could result in harm if medications are given inappropriately. Label language and graphics seem to influence inappropriate interpretation of over-the-counter product age indications. Poorer parental numeracy skills may increase the misinterpretation of these products. Opportunities exist for the Food and Drug Administration and manufacturers to revise existing labels to improve parental comprehension and enhance child safety.
Keywords: nonprescription drugs, literacy, safety
Over-the-counter (OTC) cough and cold products for infants have come under increased scrutiny in the past year by the US Food and Drug Administration (FDA), the Centers for Disease Control and Prevention (CDC), and consumer health care groups.1–5 Use of OTC cold and cough products containing antihistamines and decongestants have been implicated in the deaths of >100 infants nationwide in the past 40 years and have been associated with many adverse events and emergency department visits from overdose or misuse.5–7 Although these products are FDA-approved for use in adults, they have not been adequately tested for safety or efficacy in young children,8–15 and several studies have not found significant clinical efficacy.8,10–15 Recently, an FDA advisory committee recommended against the use of OTC cough and cold medications in children under 6 years of age, and a subsequent FDA public health advisory was issued recommending against the use of these medications in children <2 years of age.9,16,17 Although most manufacturers voluntarily removed products aimed toward children <2 years of age from the market, and have revised labels to warn against use in children under 4, some major manufacturers have disagreed with the recommendations against the use of these products for children ages 4 and above.18,19 Various pediatric cold and cough products, including many products aimed at children age ≥4, remain on the market, with no specific FDA ban.9,17 The FDA expects a long process before any new regulations governing these medications are enacted, and studies of how parents use and interpret child OTC cold products may inform help this process.9,20,21
Many parents may have difficulty understanding the indications and appropriate dosing of OTC medications. In 2003, the National Assessment of Adult Literacy found that ~90 million Americans have basic or below basic literacy skills, and >110 million people have basic or poor quantitative skills.22 Low-literacy skills are associated with worse understanding of medication labels, worse knowledge of one’s disease, and worse clinical outcomes.23–33 The role of quantitative skills, or numeracy, has been less studied,34,35 but one recent study found that low numeracy was associated with poorer understanding of food labels.36 Little is known about caregivers’ ability to choose and dose medications appropriately for their infants and young children.37
The objectives of this study were to (1) examine caregiver understanding of the age indication of OTC cold and cough medication labels of products that were commonly marketed for infants or young children <2 years of age, (2) assess the relationship between caregiver literacy and numeracy skills and understanding of OTC labels, and (3) determine how language and graphics on OTC products influences caregivers’ decisions. Although the OTC products used in this study were subsequently voluntarily removed from the market by manufacturers, these products could potentially return to the market, and the study has important implications relevant to caregiver understanding and safety of all OTC medications marketed for use with infants and children.
METHODS
Setting and Participants
Participants were recruited from general pediatric clinics at 3 academic medical centers from September 1, 2006 through October 16, 2007. Potential participants were approached if they were English-speaking and the caregivers for infants (≤14 months) who actively received care at the clinics involved. Exclusion criteria included: vision worse than 20/50 using a Rosenbaum pocket vision screener, or self-report of significant dementia, mental illness, or blindness. Over 96% of the study participants were the parents of the infant child. Appropriate institutional review board approval or exemption was obtained from all 3 institutions. Exemption was obtained under 45CFR46.101(b)(2) for survey data without identifying information. All subjects gave verbal or written consent to participate.
Study Design
Participants were asked to complete a 30 to 45 minute series of surveys administered by trained research assistants in a clinic waiting area or examination room. In-room child care, when available, was provided to minimize distraction. Participants were given $10 in cash or a gift certificate after completing the study. Literacy was assessed by using the previously validated Shortened Test of Functional Health Literacy in Adults (STOFHLA).38 If a participant achieved a score of ≤15 on the STOFHLA (inadequate literacy), the remainder of the surveys were read aloud to the participant to mitigate the impact of inadequate reading skills. Numeracy skills were assessed with the previously validated Wide Range Achievement Test 3 (WRAT-3).39
Assessment of OTC-Label Understanding
A 5-item survey was created to gauge how caregivers understood and characterized the labeling on OTC cold and cough medication products for young children. Common OTC products were chosen to reflect a variety of manufacturers, graphics, colors, and styles of labeling. The survey was administered verbally by a trained research assistant. Participants were asked a set of 5 questions about 4 different infant cough and cold products (Table 1). Questions 2 and 3 (Q2 and Q3) were asked with the parent able to view only the front panel of the OTC product as they would view it on a store shelf: “Looking only at the front of this product, what age group is this medicine for?” and “Why did you make that choice?” Q4 and Q5 were asked after the parent was able to view the entire product, including the “Drug Facts” label: “Would you give this product to a 13-month-old child with cold symptoms?” and “Why did you make that choice?”
TABLE 1.
OTC-Label Survey Items
|
Responses to Q2 (perceived age indication based on the front of the product) were dichotomized as including an age indication of <24 months or not. Responses to Q4 were dichotomized on the basis of whether the caregiver would give the product to a 13-month-old child with cold symptoms after viewing the entire package. Responses to Q4 were further coded as “appropriate” or “inappropriate” on the basis of the reason provided for the parent’s response. A response was considered inappropriate if the caregiver stated they would give the product to a 13-month-old child, or if they stated they would not give the product but for an inappropriate reason. An example of an inappropriate reason is if the caregiver stated she would not give the product to a 13-month-old, because she thought the child was too old for the product was only for young babies, or that the product was not strong enough. However, if the caregiver stated he or she would not give the product without first consulting a physician, this was deemed “appropriate.”
Open-ended responses to Q3 (reasons for perceived age indication) were coded into 1 of 7 categories: (1) the word “infant” on the front of the product; (2) a picture of an infant or other infantile graphic; (3) other language on the label besides the word “infant”; (4) dosing device provided (eg, dropper); (5) previous experience; (6) flavor; or (7) other. Open-ended responses to Q5 (influence of product labeling) were coded into 1 of 7 categories: (1) directions for dosing of the product; (2) a picture of an infant or other infantile graphic; (3) other language on the label besides the directions; (4) dosing device provided; (5) previous experience; (6) flavoring of the product; or (7) other.
Analysis
All analyses were performed by using Stata 9.2 (Stata Corp, College Station, TX). Caregivers’ characteristics and responses were described by using means with SDs for continuous variables and percentages for categorical variables. Open-ended questions were coded on the basis of the schema described above by 2 independent reviewers; discrepancies were resolved by a third reviewer.
Analyses using generalized estimating equations (GEE) to adjust for clustering at the caregiver level were performed to examine the relationship between caregiver characteristics and each of the following: (1) caregiver response that the product was appropriate for children <24 months of age when looking at the front of the package (Q2); (2) caregiver response that they would give the product to a 13-month-old with cold symptoms (Q4); and (3) whether the response to Q4 was “inappropriate.” A similar set of analyses were performed comparing caregiver responses about the OTC-label characteristics to the 3 outcomes described above.
To adjust for potential confounding factors, we performed 3 separate adjusted GEE analyses to examine the association between caregiver characteristics and the 3 binary outcomes listed above. In initial unadjusted analysis we found the relationship between numeracy and the 3 main outcomes to be nonlinear. To address this, we developed a linear spline model with a knot at 9th grade to model a nonlinear trend of the association. For ease of interpretation, numeracy was included in the model as the approximate grade equivalent, allowing different slopes before and after 9th-grade equivalent. Along with these 2 splines, for each GEE model, the following variables were chosen a priori: age, gender, race, and educational attainment (years). Similarly, we also performed 3 separate adjusted GEE analyses examining the association between label characteristics and the 3 main outcomes listed above. For each of these GEE models, the influence of each label characteristic was adjusted for age, gender, race, educational attainment, and numeracy level.
RESULTS
From September 1, 2006, to October 16, 2007, 182 caregivers participated. Caregiver characteristics are outlined in Table 2. The majority of caregivers interviewed were mothers, and the youngest child in the family was an average of 4.5 months of age (range: 0–13 months). Mean educational attainment was 12.8 years. Although 99% of caregivers had adequate literacy skills, only 17% of caregivers had >9th-grade numeracy skills, and 36% had <6th-grade numeracy skills. More than 50% of caregivers reported having used OTC medications to treat a child’s fever, and 29% used OTC medications to treat a child’s cold.
TABLE 2.
Parent/Caregiver Characteristics
| Variable, n | Mean (SD) or Percentage |
|---|---|
| Mean parent or guardian age (SD), y (179) | 25.5 (6.1) |
| Female (182) | 89 |
| Relationship to child (181) | |
| Mother | 87 |
| Father | 11 |
| Other | 2 |
| Race (181) | |
| White | 36 |
| Black | 51 |
| Other | 13 |
| Hispanic/Latino | 12 |
| Born in the United States (182) | 88 |
| Insurance status (182) | |
| Medicaid/Tenncare | 77 |
| Private | 17 |
| Other | 6 |
| On WIC program (182) | 78 |
| Income (182) | |
| <$20 000 | 42 |
| $20 000–$39 000 | 34 |
| ≥$40 000 | 10 |
| Don’t know or refused | 13 |
| Mean (SD) level of education, y (181) | 12.8 (3.7) |
| Education ≤ high school (181) | 58 |
| Adequate literacy (STOFHLA) (182) | 99 |
| Numeracy level (WRAT-3) (182) | |
| <6th grade | 36 |
| 6th–8th grade | 47 |
| <8th grade | 17 |
| Numeracy (WRAT-3) standard score (SD) (182) | 82.6 (12.0) |
| Mean No. (SD) of children in family (182) | 2.3 (1.5) |
| Age of youngest child (SD), mo (182) | 4.5 (3.7) |
| Has received written information from doctor or nurse about caring for infant (182) | 89 |
| Read any books or magazines about caring for infant (182) | 96 |
| Uses OTC medications to treat child’s fever (180) | 52 |
| Uses OTC meds to treat a cold (182) | 29 |
WIC indicates the Supplemental Nutrition Program for Women, Infants, and Children.
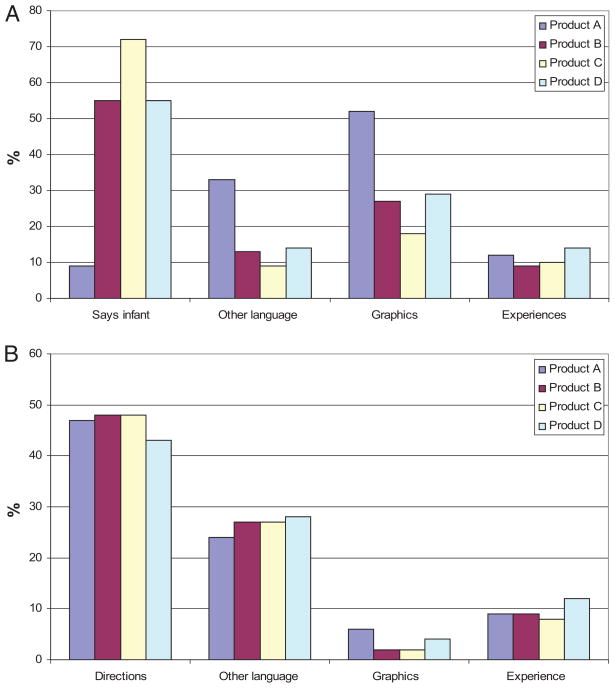
Responses to the OTC-label survey are shown in Table 3 and Figure. Up to one third of caregivers reported having previously used at least one of the OTC cold or cough products. When allowed to view only the front of the product packaging, 86% of the time caregivers thought a product was appropriate for children <24 months of age; results were similar across the different products. Nearly all (98%) of the caregivers thought that at least 1 of the 4 products they examined was appropriate for a child <24 months of age. The 3 most common features of product packaging that influenced caregiver perception of product age indication were: the word “infant” on the package, infant-related graphics (eg, depictions of infants, teddy bears, droppers), or other language on the package (eg, symptoms the product said it treated, the words “pediatrician recommended”) (Figure).
TABLE 3.
OTC-Label Survey Results
| Product | Has Used Product Before, % | Thinks Product Is Appropriate for Children <24 Mo of Age, %a | Age Range Influenced by These Main Factors, %a | Would Give Product to 13-mo-Old With Coldb | Inappropriate Responseb | Answer Influenced by These Main Factors, % |
|---|---|---|---|---|---|---|
| (A) decongestant nose drops | 19 | 89 | Says “infant”: 9 Other language: 33 Graphics: 52 Experience: 12 |
52 | 58 | Directions: 47 Other language: 24 Graphics: 6 Experience: 9 |
| (B) infant drops, long-acting cough | 14 | 80 | Says “infant”: 55 Other language: 13 Graphics: 27 Experience: 9 |
43 | 57 | Directions: 48 Other language: 27 Graphics: 2 Experience: 9 |
| (C) infant cough medicine | 15 | 91 | Says “infant”: 72 Other language: 9 Graphics: 18 Experience:10 |
50 | 57 | Directions: 48 Other language: 27 Graphics: 2 Experience: 8 |
| (D) infant drops for fever plus cold symptoms | 33 | 86 | Says “infant”: 55 Other language: 14 Graphics: 29 Experience: 14 |
58 | 60 | Directions: 43 Other language: 28 Graphics: 4 Experience: 12 |
Response based on examining the front of the package only.
Examined the entire package. Column is counted as inappropriate if the caregiver said he or she would give the product to a 13-month-old or that he or she would not give the product but did not have an appropriate explanation for why not.
FIGURE.
Factors that influence parental perception of OTC products. A, factors that influence perceived age range (viewing the front of the package only). B, factors that influence if the product is perceived as appropriate or not for 13 month-old with cold symptoms (responses when viewing the entire package).
Despite the fact that each product package specifically recommends consulting a physician before administering the medication to a child <24 months of age, after being allowed to examine the entire product package, 51% of the time caregivers stated that they would give a product to a 13-month-old with cold symptoms. And 72% of caregivers stated that they would administer at least 1 of the 4 products to a 13-month-old child with cold symptoms. Among all responses, 58% of responses were deemed inappropriate for a variety of reasons including: stating it was okay to give the product to the child without consulting a physician, stating a 13-month-old child is too old for the medicine, stating the medicine is “not strong enough,” or other responses not specifically related to the age indication for the product.
Table 4 shows the relationship between caregiver characteristics and caregiver perception that OTC products are appropriate for children <24 months of age. In unadjusted and adjusted analyses, nonwhite race was associated with increased odds of thinking the products were appropriate for children <24 months of age (adjusted odds ratio [aOR]: 1.95 [95% confidence interval (CI): 1.09–3.47]). Among caregivers with <9th-grade numeracy skills (83% of subjects), higher numeracy skills trended toward lower odds of thinking the products were appropriate for children <24 months of age (aOR: 0.80 [95% CI: 0.63–1.01]). Specific features of product packaging significantly influenced caregiver perception that OTC products were appropriate for children <24 months of age, including the word “infant” on the product label and product flavor (Table 4). In adjusted analysis, caregivers who were influenced by seeing the word infant on the product label were more likely to think the product was suitable for younger children (aOR: 3.08 [95% CI: 1.74–5.46]).
TABLE 4.
Odds of Saying That Product Is Suitable for Children < 24 Months of Age
| OR (95% CI) | aOR (95% CI)a | |
|---|---|---|
| Model: patient characteristics | ||
| Age | 1.03 (0.98–1.09) | 1.03 (0.97–1.08) |
| Female gender | 1.61 (0.61–4.26) | 1.70 (0.67–4.34) |
| Nonwhite race | 2.08 (1.14–3.79) | 1.95 (1.09–3.47) |
| Born in the United States | 0.74 (0.30–1.78) | — |
| On WIC program | 0.81 (0.37–1.81) | — |
| Education, y | 1.05 (0.92–1.20) | 1.04 (0.89–1.20) |
| Literacy (STOFHLA) | 0.94 (0.86–1.04) | — |
| Numeracy 2nd–8th grade (WRAT-3), per grade levelb | 0.80 (0.63–1.00) | 0.80 (0.63–1.01) |
| Numeracy 9th–16th grade (WRAT-3), per grade levelb | 1.14 (0.74–1.76) | 1.28 (0.79–2.06) |
| Model: label characteristicsc | ||
| Says “infant” | 2.99 (1.66–5.39) | 3.08 (1.74–5.46) |
| Graphics | 1.34 (0.69–2.60) | 1.54 (0.79–2.99) |
| Other language | 0.54 (0.28–1.03) | 0.56 (0.30–1.07) |
| Other experience | 0.30 (0.17–0.54) | 0.26 (0.15–0.47) |
| Flavor | d | d |
| Other factors | 0.50 (0.13–1.95) | 0.48 (0.13–1.87) |
WIC indicates the Supplemental Nutrition Program for Women, Infants, and Children.
Adjusted for age, gender, race, education level, and numeracy level.
OR for numeracy for each additional grade level of numeracy skill.
Each label characteristic was adjusted for age, gender, race, education level, and numeracy level.
Unable to calculate because all caregivers (n = 12) who were influenced by the flavor thought that the product was suitable for children <24 months of age.
Table 5 shows the relationship between caregiver characteristics and whether they would give the products to a 13-month-old child with cold symptoms. In adjusted analysis, among caregivers with <9th-grade numeracy skills, each additional grade level of numeracy skills was associated with lower odds of endorsing OTC product use for a 13-month-old with cold symptoms (aOR: 0.84 [95% CI: 0.71–0.99]) or answering inappropriately about the age indication (aOR: 0.78 [95% CI: 0.64–0.94]). Paradoxically, among caregivers with higher numeracy skills (≥9th-grade level), higher numeracy skills were associated with higher odds of endorsing the product for use in a 13-month-old (aOR: 1.78 [95% CI: 1.07–2.96]). Several OTC-packaging features significantly influenced whether the product would be given to a 13-month-old child with cold symptoms or caregivers responding “inappropriately.” Infant-specific graphics, or specific language (ie, infant or pediatrician recommended), were significantly associated with increased odds of giving the OTC products to a 13-month-old or answering inappropriately about the age indication. Caregivers who examined the dosing instructions were significantly less likely to state they would use the product in a 13-month-old (aOR: 0.19 [95% CI: 0.13–0.29]) or to answer inappropriately (aOR: 0.02 [95% CI: 0.01–0.03]). However, caregivers reported being influenced by the dosing directions on the packages only 47% of the time.
TABLE 5.
Odds of Saying They Would Use Cold Product for a 13-Month-Old With Cold Symptoms
| Odds of Using Product |
Odds of Answering Question Inappropriatelya |
|||
|---|---|---|---|---|
| OR (95% CI) | aOR (95% CI)b | OR (95% CI) | aOR (95% CI)b | |
| Model: patient characteristics | ||||
| Age | 0.98 (0.94–1.01) | 0.99 (0.95–1.03) | 1.00 (0.97–1.04) | 1.02 (0.98–1.07) |
| Female gender | 2.21 (1.02–4.78) | 1.95 (0.87–4.34) | 1.80 (0.81–4.00) | 1.74 (0.74–4.09) |
| Nonwhite race | 1.17 (0.72–1.89) | 1.24 (0.75–2.05) | 1.35 (0.79–2.30) | 1.38 (0.78–2.44) |
| Born in the United States | 1.42 (0.73–2.76) | — | 1.00 (0.45–2.22) | — |
| On WIC program | 1.26 (0.72–2.22) | — | 1.33 (0.74–2.39) | — |
| Education | 0.97 (0.86–1.08) | 0.98 (0.86–1.11) | 0.89 (0.78–1.02) | 0.87 (0.74–1.02) |
| Literacy (STOFHLA) | 1.00 (0.92–1.08) | — | 0.97 (0.87–1.08) | — |
| Numeracy 2nd–8th grade (WRAT-3), per grade levelc | 0.82 (0.70–0.96) | 0.84 (0.71–0.99) | 0.75 (0.63–0.90) | 0.78 (0.64–0.94) |
| Numeracy 9th–16th grade (WRAT-3), per grade levelc | 1.70 (1.06–2.73) | 1.78 (1.07–2.96) | 1.71 (1.08–2.70) | 1.95 (1.21–3.14) |
| Model: label characteristicsd | ||||
| Dosing directions | 0.20 (0.13–0.30) | 0.19 (0.13–0.29) | 0.03 (0.01–0.05) | 0.02 (0.01–0.03) |
| Graphics | 4.62 (1.95–11.00) | 5.93 (2.56–13.74) | 27.89 (3.05–254.89) | 70.9 (5.06–994.3) |
| Other language | 3.89 (2.61–5.78) | 3.53 (2.35–5.30) | 5.27 (3.53–7.86) | 5.98 (3.84–9.33) |
| Other experience | 1.35 (0.75–2.45) | 1.46 (0.80–2.68) | 3.19 (1.71–5.94) | 3.13 (1.69–5.81) |
| Flavor | 4.10 (0.97–17.24) | d | 1.16 (0.41–3.29) | d |
| Dosing tool | 1.42 (0.31–6.57) | 1.31 (0.24–7.08) | 3.84 (1.74–8.49) | d |
| Other factors | 1.21 (0.70–2.11) | 1.23 (0.68–2.22) | 6.23 (3.03–12.81) | 7.77 (3.11–19.39) |
Counted as inappropriate if the caregiver said they would give the product to a 13-month-old, or they said they would not give the product but did not have a satisfactory explanation for why not.
Adjusted for age, gender, race, education level, and numeracy level.
OR for numeracy for each additional grade level of numeracy skill.
Each label characteristic is adjusted for age, gender, race, education level, and numeracy level.
Unable to calculate because almost all caregivers who were influenced by this characteristic would use the product or answered inappropriately.
DISCUSSION
Many OTC-product labels contain a high volume of textual information, which may be confusing to many parents, particularly those with lower literacy and numeracy levels. The drug facts component of the label, which is the FDA-regulated section of the label that addresses usage and warning information, is complex and can be challenging for parents to navigate and understand. In addition, OTC products marketed for infants and children often include pictures of small children or childlike graphics and other marketing claims that can be misleading. In the current study, we found that graphics and other language on the front of the label adversely influenced caregivers’ perceptions of the appropriateness of OTC cough and cold medications for young children. Overall, >4 of 5 caregivers of infants were already using or would use OTC cold and cough medications in children <2 years of age, despite the fact that the dosage instructions on the labels comply with FDA labeling standards, recommending to first consult a physician before use. Although caregivers who identified the dosing instruction on the products were less likely to use these products without first consulting a physician, less than half of the time did caregivers report being influenced by the products’ dosing directions.
To our knowledge, this is the first study to comprehensively evaluate how caregivers interpret OTC pediatric cold and cough medicine labels and to assess the role of caregiver literacy and numeracy skills on this interpretation. Previous research has demonstrated that many adults, particularly those with lower literacy skills, struggle to understand prescription drug labels, dosing instructions, and warning labels. Adult patients can be confused by difficult language, graphics, numerical concepts, and distracting marketing information on prescription drug labels.28–30,32,40–43 Among caregivers of young children, errors in dosing of OTC cold and cough medications could potentially lead to significant threats to the safety of infants or young children.44–46 Recent reports suggest that inappropriate usage of OTC medications may lead to dangerous overdoses, particularly in infants with parents of lower socioeconomic status or limited English proficiency.3–5,7,9,17,46
Similar to several other studies,35,36,47 in this study we found many caregivers with adequate literacy skills (on the STOFHLA) but poor math skills (by the WRAT-3). We found that the majority of caregivers had lower numeracy skills (<9th-grade level) and that among these caregivers, the lower one’s numeracy level the more likely they were to endorse inappropriate use of the OTC products in young children. However, paradoxically, among caregivers with higher numeracy skills (>9th-grade skills), higher numeracy was also associated with being more likely to endorse inappropriate usage. One explanation may be that caregivers with higher numeracy skills (>9th-grade level) rely more heavily on their own judgment about medication use than they do on the label recommendations. However, caregivers with lower numeracy skills, may truly be having difficulty in understanding the age indication of the OTC products. Of concern, some caregivers believed these products to be appropriate only for infants <13 months of age, and others indicated that the medication would not be strong enough for children >12 months of age. Several caregivers did not understand how to interpret the age guidelines in the dosing directions, and thought that the language “for children under 2 years of age,” despite being followed by the language “consult a physician,” actually endorsed use of the product for children in that age group.
This study has several limitations. We recruited a convenience sample of caregivers that were predominantly supported by public insurance and sought care at academic pediatric residency clinics. Our study also only focused on English-speaking subjects. Our results, therefore, might not be generalizable to all populations. In general, the participants in our study had high levels of education and literacy. Including caregivers with lower literacy or limited English proficiency probably would have resulted in even higher rates of OTC-label misinterpretation. Our measure of literacy, the STOFHLA, had a ceiling effect, limiting our ability to assess the value of literacy. We found a modest relationship between numeracy skills and caregiver responses, but our results were limited by our relatively small sample size. The OTC products examined in this study have since been voluntarily removed from the market by the manufacturers, although there are no specific regulations preventing the products from being made available at a later date, and products aimed at children 4 years of age and older currently remain on the market. Finally, our study concentrated on caregiver understanding of the age indications of OTC products but did not examine caregiver ability to administer medication according to directions.
Of note, some caregivers in our study indicated that they would give the OTC products because of previous product endorsement from their physician. Two previous health care provider surveys have suggested that as many as one half of the physicians surveyed have recommended using OTC cold products in children <12 months of age.48,49 Although this high rate of physician endorsement for child OTC products may drop in light of recent recommendations by the CDC, the FDA, and the American Academy of Pediatrics, our study highlights the opportunity for additional work with physicians to discourage inappropriate use of these medications.
Our results have important and timely implications for federal agencies and manufacturers considering revised labeling and availability of OTC products for children.20 Recently, the CDC and an FDA public health advisory recommended against providing OTC cold medications to children <2 years of age because of lack of efficacy as well as safety concerns.5,9,17 Although an FDA advisory panel suggested banning these medications for children <6 of age, the FDA has not endorsed this recommendation. In addition, the FDA has stated that it could take several years before any specific regulations against current OTC cold medication products are enforced.9,20,21 The FDA has also suggested that any regulations would likely involve placing new warnings on current products as opposed to an outright ban.20 On the basis of our study, we suspect that a single warning statement or revision of dosing guidelines will not adequately guard against inappropriate medication use by parents of young children. Our study also raises possible concerns about how parents interpret current OTC medications that are marketed towards children age 4 and older, and whether parents may be inappropriately using these products for younger children. On the basis of our findings, an FDA endorsement to improve the accuracy of language and ban misleading graphics on all OTC-product packaging would provide greater caregiver understanding of OTC dosing recommendations and help prevent significant harm to children. Although such actions would help all children, they could be particularly valuable to children of parents with lower literacy or numeracy.
CONCLUSIONS
This study demonstrates that the age indications of OTC pediatric cold and cough products are difficult for caregivers to understand. Caregivers were often influenced by infant-like graphics and the term infant on the product. Although dosing directions were modestly helpful in promoting appropriate use, these directions were not always examined or properly understood. These misinterpretations may pose significant hazards to child safety. Although this study focused on OTC products aimed at children <2 years of age, it raises important concerns about parental understanding of all OTC products and potential threats to the safety of children of all ages. We applaud the initial steps taken by the FDA and drug manufacturers to improve current pediatric medication labeling. However, if OTC pediatric cold and cough products are to remain on, or be reintroduced to, the market, significant changes are necessary to make product packaging and labeling more clearly understood by all caregivers. These changes should include labels written at lower literacy and numeracy levels, using uniform language, and avoiding misleading graphics to help proper product selection and safe use.
What’s Known on This Subject
The misuse or overdosage of OTC cold and cough medications has been implicated in many adverse events and the deaths of >100 infants. Caregivers may have difficulties understanding OTC-label directions, but this has not been well studied.
What This Study Adds
OTC labels can be misleading and difficult for many caregivers to understand. Label graphics and claims can lead to misappropriate understanding of the age indication of products. Poorer understanding may be more common among caregivers with lower numeracy skills.
Acknowledgments
Dr Rothman is currently supported by National Institute of Diabetes and Digestive and Kidney Diseases career development award NIDDK-5K23DK065294, and Dr Perrin is currently supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development career development award NICHD-3K23-HD051817. Dr Sanders’ work was supported in part by the Robert Wood Johnson Foundation Generalist Physician Scholars Program. This research was also supported with funding from the Vanderbilt Program on Effective Health Communication.
We acknowledge Leslie Newsome, Steven Pattishall, and Matthew Oettinger for their aid in recruitment, and Ayumi Shintani, PhD, for aid in statistical support.
Abbreviations
- OTC
over-the-counter
- CDC
Centers for Disease Control and Prevention
- FDA
Food and Drug Administration
- STOFHLA
Shortened Test of Functional Health Literacy in Adults
- WRAT-3
Wide Range Achievement Test 3
- GEE
generalized estimating equations
- aOR
adjusted odds ratio
- CI
confidence interval
- Qn
question n
Footnotes
Financial Disclosure: Dr Sanders previously received research funding from the Pfizer Clear Health Communication Initiative and serves on the Pfizer Health Literacy Fellowship Advisory Board; and Dr Rothman previously received funding from the Pfizer Clear Health Communication Initiative and serves on the Pfizer Health Literacy Fellowship and Visiting Professor Advisory Boards. The other authors have no financial relationships relevant to this article to disclose.
References
- 1.Dlugosz CK, Chater RW, Engle JP. Appropriate use of nonprescription analgesics in pediatric patients. J Pediatr Health Care. 2006;20(5):316–325. doi: 10.1016/j.pedhc.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Kuehn BM. FDA: cold medications risky for young children. JAMA. 2007;298(10):1151. doi: 10.1001/jama.298.10.1151. [DOI] [PubMed] [Google Scholar]
- 3.US Food and Drug Administration, Center for Drug Evaluation and Research Public Health Advisory. Nonprescription Cough and Cold Medicine Use in Children. Washington, DC: US Food and Drug Administration; 2007. [Google Scholar]
- 4.Harris G. U.S. reviewing safety of children’s cough drugs. [Accessed March 10, 2007];New York Times. 2007 March 2; Available at: www.nytimes.com/2007/03/02/health/02cough.html.
- 5.Centers for Disease Control and Prevention. Infant deaths associated with cough and cold medications: two states, 2005. MMWR Morb Mortal Wkly Rep. 2007;56(1):1–4. [PubMed] [Google Scholar]
- 6.Pifer J. Child deaths lead to FDA hearing on cough, cold meds. [Accessed October 25, 2007];CNN. 2007 October 17; Available at: www.cnn.com/2007/HEALTH/10/17/cough.syrup.deaths/index.html.
- 7.Makers pull cold medicines sold for infants. [Accessed October 25, 2007];CNN. 2007 October 11; Available at: http://transcripts.cnn.com/TRANSCRIPTS/0710/11/htm.03.html.
- 8.Clemens CJ, Taylor JA, Almquist JR, Quinn HC, Mehta A, Naylor GS. Is an antihistamine-decongestant combination effective in temporarily relieving symptoms of the common cold in preschool children? J Pediatr. 1997;130(3):463–466. doi: 10.1016/s0022-3476(97)70211-7. [DOI] [PubMed] [Google Scholar]
- 9.Harris G. FDA panel urges ban on medicine for child colds. [Accessed October 20, 2007];New York Times. 2007 October 19; Available at: www.nytimes.com/2007/10/20/washington/20fda.html?adxnnl=1&adxnnlx=1193328357-ozlzGtQ0iACjnxOlung0Jg.
- 10.Hutton N, Wilson MH, Mellits ED, et al. Effectiveness of an antihistamine-decongestant combination for young children with the common cold: a randomized, controlled clinical trial. J Pediatr. 1991;118(1):125–130. doi: 10.1016/s0022-3476(05)81865-7. [DOI] [PubMed] [Google Scholar]
- 11.Schroeder K, Fahey T. Should we advise parents to administer over the counter cough medicines for acute cough? Systematic review of randomised controlled trials. Arch Dis Child. 2002;86(3):170–175. doi: 10.1136/adc.86.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith MB, Feldman W. Over-the-counter cold medications. A critical review of clinical trials between 1950 and 1991. JAMA. 1993;269(17):2258–2263. doi: 10.1001/jama.269.17.2258. [DOI] [PubMed] [Google Scholar]
- 13.Sutter AI, Lemiengre M, Campbell H, Mackinnon HF. Antihistamines for the common cold. Cochrane Database Syst Rev. 2003;(3):CD001267. doi: 10.1002/14651858.CD001267. [DOI] [PubMed] [Google Scholar]
- 14.Taylor JA, Novack AH, Almquist JR, Rogers JE. Efficacy of cough suppressants in children. J Pediatr. 1993;122(5 pt 1):799–802. doi: 10.1016/s0022-3476(06)80031-4. [DOI] [PubMed] [Google Scholar]
- 15.Paul IM, Yoder KE, Crowell KR, et al. Effect of dextromethorphan, diphenhydramine, and placebo on nocturnal cough and sleep quality for coughing children and their parents. Pediatrics. 2004;114(1) doi: 10.1542/peds.114.1.e85. Available at: www.pediatrics.org/cgi/content/full/114/1/e85. [DOI] [PubMed]
- 16.Food and Drug Administration. [Accessed February 19, 2008];FDA releases recommendations regarding use of over-the-counter cough and cold products. 2008 January 17; Available at: www.fda.gov/bbs/topics/NEWS/2008/NEW01778.html.
- 17.FDA panel: No cold medicines to children under 6. [Accessed October 25, 2007];CNN. 2007 October 19; Available at: www.cnn.com/2007/HEALTH/10/19/coldmed.fda/index.html.
- 18.Sharfstein JM, North M, Serwint JR. Over the counter but no longer under the radar: pediatric cough and cold medications. N Engl J Med. 2007;357(23):2321–2324. doi: 10.1056/NEJMp0707400. [DOI] [PubMed] [Google Scholar]
- 19.Harris G. Child warning added to cold remedies. [Accessed March 30, 2009];New York Times. 2008 October 7; Available at: www.nytimes.com/2008/10/08/us/08cough.html?EXTKEY=172RSSB.
- 20.FDA press conference following OTC medication hearing. CNN Live Feed. 2007 October 19; [Google Scholar]
- 21.Food and Drug Administration. Over the counter cough and cold medications for pediatric use; notice of public hearing (docket No. FDA-2008-N-0466) Fed Regist. 2008;73(165):50033–50036. [Google Scholar]
- 22.Kutner M, Greenberg E, Jin Y, Boyle B, Hsu Y, Dunleavy E. NCES 2007480. Washington, DC: US Department of Education; Mar 3, 2007. Literacy in Everyday Life: Results From the 2003 National Assessment of Adult Literacy. [Google Scholar]
- 23.Doak LG, Doak CC. Lowering the silent barriers to compliance for patients with low literacy skills. Promot Health. 1987;8(4):6–8. [PubMed] [Google Scholar]
- 24.Schillinger D, Grumbach K, Piette J, et al. Association of health literacy with diabetes outcomes. JAMA. 2002;288(4):475–482. doi: 10.1001/jama.288.4.475. [DOI] [PubMed] [Google Scholar]
- 25.Institute of Medicine Committee on Health Literacy. Health Literacy: A Prescription to End Confusion. Washington, DC: National Academies Press; 2004. [PubMed] [Google Scholar]
- 26.Rothman RL, Dewalt DA, Malone R, et al. Influence of patient literacy on the effectiveness of a primary care-based diabetes disease management program. JAMA. 2004;292(14):1711–1716. doi: 10.1001/jama.292.14.1711. [DOI] [PubMed] [Google Scholar]
- 27.Dewalt DA, Berkman ND, Sheridan S, Lohr KN, Pignone MP. Literacy and health outcomes. A systematic review of the literature. J Gen Intern Med. 2004;19(12):1228–1239. doi: 10.1111/j.1525-1497.2004.40153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolf MS, Davis TC, Tilson HH, Bass PF, III, Parker RM. Misunderstanding of prescription drug warning labels among patients with low literacy. Am J Health Syst Pharm. 2006;63(11):1048–1055. doi: 10.2146/ajhp050469. [DOI] [PubMed] [Google Scholar]
- 29.Davis TC, Wolf MS, Bass PF, III, et al. Literacy and misunderstanding prescription drug labels. Ann Intern Med. 2006;145(12):887–894. doi: 10.7326/0003-4819-145-12-200612190-00144. [DOI] [PubMed] [Google Scholar]
- 30.Davis TC, Wolf MS, Bass PF, III, et al. Low literacy impairs comprehension of prescription drug warning labels. J Gen Intern Med. 2006;21(8):847–851. doi: 10.1111/j.1525-1497.2006.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Committee on Health Literacy. Health literacy: report of the Council on Scientific Affairs. Ad Hoc Committee on Health Literacy for the Council on Scientific Affairs, American Medical Association. JAMA. 1999;281(6):552–557. [PubMed] [Google Scholar]
- 32.Holt GA, Hollon JD, Hughes SE, Coyle R. OTC labels: can consumers read and understand them? Am Pharm. 1990;NS30(11):51–54. doi: 10.1016/s0160-3450(16)33630-3. [DOI] [PubMed] [Google Scholar]
- 33.Estrada CA, Martin-Hryniewicz M, Peek BT, Collins C, Byrd JC. Literacy and numeracy skills and anticoagulation control. Am J Med Sci. 2004;328(2):88–93. doi: 10.1097/00000441-200408000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Golbeck AL, Ahlers-Schmidt CR, Paschal AM, Dismuke SE. A definition and operational framework for health numeracy. Am J Prev Med. 2005;29(4):375–376. doi: 10.1016/j.amepre.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 35.Montori VM, Rothman RL. Weakness in numbers: the challenge of numeracy in health care. J Gen Intern Med. 2005;20(11):1071–1072. doi: 10.1111/j.1525-1497.2005.051498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rothman RL, Housam R, Weiss H, et al. Patient understanding of food labels: the role of literacy and numeracy. Am J Prev Med. 2006;31(5):391–398. doi: 10.1016/j.amepre.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 37.Patel V, Eisenmon T, Arocha J. Comprehending instructions for using pharmaceutical products in rural Kenya. Instr Sci. 1990;19(1):71–84. [Google Scholar]
- 38.Parker RM, Baker DW, Williams MV, Nurss JR. The test of functional health literacy in adults: a new instrument for measuring patients’ literacy skills. J Gen Intern Med. 1995;10(10):537–541. doi: 10.1007/BF02640361. [DOI] [PubMed] [Google Scholar]
- 39.Wilkinson GS. Wide Range Achievement Test: Administration Manual. Wilmington, DE: Wide Range, Inc; 1993. [Google Scholar]
- 40.Hwang SW, Tram CQ, Knarr N. The effect of illustrations on patient comprehension of medication instruction labels. BMC Fam Pract. 2005;6(1):26. doi: 10.1186/1471-2296-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katz MG, Kripalani S, Weiss BD. Use of pictorial aids in medication instructions: a review of the literature. Am J Health Syst Pharm. 2006;63(23):2391–2397. doi: 10.2146/ajhp060162. [DOI] [PubMed] [Google Scholar]
- 42.Wolf MS, Davis TC, Shrank WH, Neuberger M, Parker RM. A critical review of FDA-approved medication guides. Patient Educ Couns. 2006;62(3):316–322. doi: 10.1016/j.pec.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 43.Wolf MS, Davis TC, Shrank W, et al. To err is human: patient misinterpretations of prescription drug label instructions. Patient Educ Couns. 2007;67(3):293–300. doi: 10.1016/j.pec.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 44.Kaushal R, Bates DW, Landrigan C, et al. Medication errors and adverse drug events in pediatric inpatients. JAMA. 2001;285(16):2114–2120. doi: 10.1001/jama.285.16.2114. [DOI] [PubMed] [Google Scholar]
- 45.Gunn VL, Taha SH, Liebelt EL, Serwint JR. Toxicity of over-the-counter cough and cold medications. Pediatrics. 2001;108(3) doi: 10.1542/peds.108.3.e52. Available at: www.pediatrics.org/cgi/content/full/108/3/e52. [DOI] [PubMed]
- 46.Rimsza ME, Newberry S. Unexpected infant deaths associated with use of cough and cold medications. Pediatrics. 2008;122(2) doi: 10.1542/peds.2007-3813. Available at: www.pediatrics.org/cgi/content/full/122/2/e318. [DOI] [PubMed]
- 47.Cavanaugh K, Huizinga MM, Wallston KA, et al. Association of numeracy and diabetes control. Ann Intern Med. 2008;148(10):737–746. doi: 10.7326/0003-4819-148-10-200805200-00006. [DOI] [PubMed] [Google Scholar]
- 48.Cohen-Kerem R, Ratnapalan S, Djulus J, Duan X, Chandra RV, Ito S. The attitude of physicians toward cold remedies for upper respiratory infection in infants and children: a questionnaire survey. Clin Pediatr (Phila) 2006;45(9):828–834. doi: 10.1177/0009922806295281. [DOI] [PubMed] [Google Scholar]
- 49.Gadomski AM, Rubin JD. Cough and cold medicine use in young children: a survey of Maryland pediatricians. Md Med J. 1993;42(7):647–650. [PubMed] [Google Scholar]