Abstract
The binding mode of telomestatin to G-quadruplex DNA has been investigated using electrospray mass spectrometry, by detecting the intact complexes formed in ammonium acetate. The mass measurements show the incorporation of one extra ammonium ion in the telomestatin complexes. Experiments on telomestatin alone also show that the telomestatin alone is able to coordinate cations in a similar way as a crown ether. Finally, density functional theory calculations suggest that in the G-quadruplex-telomestatin complex, potassium or ammonium cations are located between the telomestatin and a G-quartet. This study underlines that monovalent cation coordination capabilities should be integrated in the rational design of G-quadruplex binding ligands.
1. Introduction
The formation of G-quadruplex folds by telomeric DNA is thought to play a role in telomere regulation. It has been shown that G-quadruplex ligands binding specifically to the telomeric G-quadruplex structure effectively alter telomere capping and cause the senescence or apoptosis of cancer cells [1–5]. A variety of ligands have now been described as G-quadruplex binders, but a key issue in ligand design is often the specificity for G-quadruplexes over duplex sequences [4, 6–8]. Identifying binding modes that make a ligand a specific and highly active G-quadruplex binder is crucial for the rational design of novel molecules.
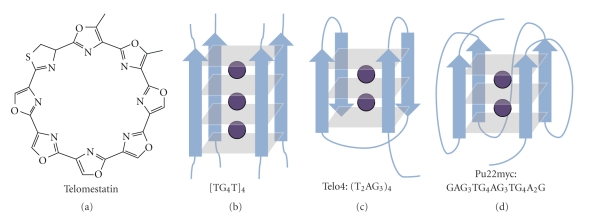
Telomestatin (Figure 1) is one of the most emblematic G-quadruplex ligands. The molecule was first extracted from Streptomyces anulatus 3533-SV4 [9]. It is highly specific for G-quadruplexes, with no significant binding to duplexes [10–12]. Telomestatin was found to effectively inhibit the DNA binding of telomere-associated proteins such as telomerase [13], POT1 and TRF2 [14], and even Topo III in ALT cell lines [15]. It therefore induces telomere shortening and apoptosis [16–19] not only via telomerase inhibition but also via telomere uncapping, and therefore has potential activity against many cancer cell types.
Figure 1.
Chemical structure of telomestatin and folding pattern of the three G-quadruplexes studied here. [TG4T]4 adopts a parallel fold and can incorporate three ammonium ions between its four G-quartets [21], the 4-repeat telomeric sequence (T2AG3)4 adopts an intramolecular antiparallel fold in ammonium acetate and incorporates up to two ammonium ions [22], and the Pu22myc promoter sequence GAG3TG4AG3TG4A2G adopts a predominantly parallel fold in ammonium acetate and incorporates two ammonium ions [23].
Telomestatin binds to G-quadruplexes, among which is the human telomeric G-quadruplex, by external stacking [12]. One G-quadruplex unit can therefore accommodate two telomestatin ligands, one on each end. A recent modeling study showed that telomestatin has a tendency to capture a potassium ion, either from the G-quadruplex itself or from the solution [20]. Here we show that mass spectrometry provides experimental evidence for the accommodation of one extra cation when a telomestatin molecule is bound to a G-quadruplex. This will be illustrated for three typical G-quadruplexes: the tetramolecular [TG4T]4 quadruplex, the 4-repeat telomeric sequence (T2AG3)4, and the Pu22myc promoter sequence GAG3TG4AG3TG4A2G. The typical folds adopted by each of these three G-quadruplexes in ammonium acetate were studied previously [21–23] and are summarized in Figure 1.
2. Experimental Section
2.1. Materials
All oligonucleotides were purchased from Eurogentec (Seraing, Belgium), solubilized in water doubly distilled in house, and the 400 μM stock solutions were stored at −20°C. The solvents used include methanol (absolute, HPLC grade, Biosolve, Valkenswaard, The Netherlands), bi-distilled water, and aqueous ammonium acetate (5 M stock solution from Fluka, diluted with bi-distilled water). KCl for the evaluation of the complexation of telomestatin alone was puriss, p.a., ≥99.5% (T) (Fluka). G-quadruplexes were formed by annealing (heating the oligonucleotides for 5 minutes at 85°C, followed by slow cooling to room temperature) in 150 mM ammonium acetate. The G-quadruplex-forming oligonucleotides were dTG4T (annealed at 200 μM single strand to form 50 μM tetramolecular G-quadruplex); the telomeric sequence (T2AG3)4 and the Pu22myc promoter sequence GAG3TG4AG3TG4A2G (annealed at 50 μM single strand to form intramolecular G-quadruplexes). Telomestatin was isolated and purified as described elsewhere [9, 24] to obtain a 1 mM stock solution in DMSO. For the electrospray mass spectrometry analysis of the complexes, the final injected solutions were 5 μM in G-quadruplex and 5 to 10 μM in telomestatin (only 10 μM results are shown), in 80/20 (v/v) aqueous ammonium acetate (150 mM)/methanol.
2.2. Mass Spectrometry
Electrospray mass spectrometry experiments were performed on a Q-TOF Ultima Global (Waters, Manchester, UK). The spectra of the intact G-quadruplexes and their noncovalent complexes with telomestatin were recorded in the negative ion mode (capillary voltage = −2.2 V, source and desolvation temperatures = 70°C, cone = 100 V, RF Lens1 Energy = 45 V, source pirani pressure = 3.94 mbar, collision energy = 10 V), smoothed (mean function, 3 × 20 channels) and subtracted (polynomial order 99, 0.1% below curve). The spectra of telomestatin in the absence of G-quadruplex were recorded in the positive ion mode (capillary voltage = +2.8 V, source and desolvation temperatures = 80 and 100°C, resp., cone = 100 V, RF Lens1 Energy = 50 V, source pirani pressure = 3.33 mbar and collision energy = 7 V) and were not subjected to smoothing or background subtraction.
2.3. Calculations
For the [telomestatin + cation] binary complexes, the ammonium, potassium, and sodium cations were manually docked within the telomestatin ring, and the resulting structures were optimized using density functional theory (DFT) with the hybrid functional B3LYP and the 6-31G(d,p) basis set. For the ternary complexes between [telomestatin + cation + one G-quartet], the telomestatin was manually docked on top of an optimized structure of a G-quartet coordinated with the cation (ammonium, potassium, sodium). The ternary complexes were then optimized using DFT B3LYP at the 6-31G(d,p) level. All electronic structure calculations were performed using the Gaussian 03 rev. D.02 software suite [25]. Comparison with a larger basis set (6-311 + G(d,p)) was performed for one of the complexes (G-tetrad + K + telomestatin), and the results were similar in terms of both energies (0.8 kcal/mol) and geometries (RMSD 0.16 Ǻ). 6-31G(d,p) basis set was therefore used for all calculations. Other hybrid functional BHandHLYP and new meta-GGA hybrid MPWB1K [26] have also been tested for comparison with B3LYP.
3. Results and Discussion
When operated in soft source conditions, electrospray mass spectrometry allows detecting intact noncovalent complexes [27–30]. In the analysis of quadruplex-ligand complexes, it therefore allows determining the number of strands, the number of ligands, and the number of cations in each complex. Electrospray mass spectrometry of nucleic acid noncovalent complexes is typically performed in ammonium acetate solution in order to obtain clean spectra [31]. Ammonium ions present in the counter-ion shell around phosphates are lost during the final stages of desolvation in the electrospray source, even in soft conditions (low acceleration voltages). In contrast, ammonium ions bound sufficiently tightly to the G-quadruplex, such as the ammonium ions trapped between the G-quartets, will persist at higher acceleration voltages in the source than the nonspecifically bound ones [21].
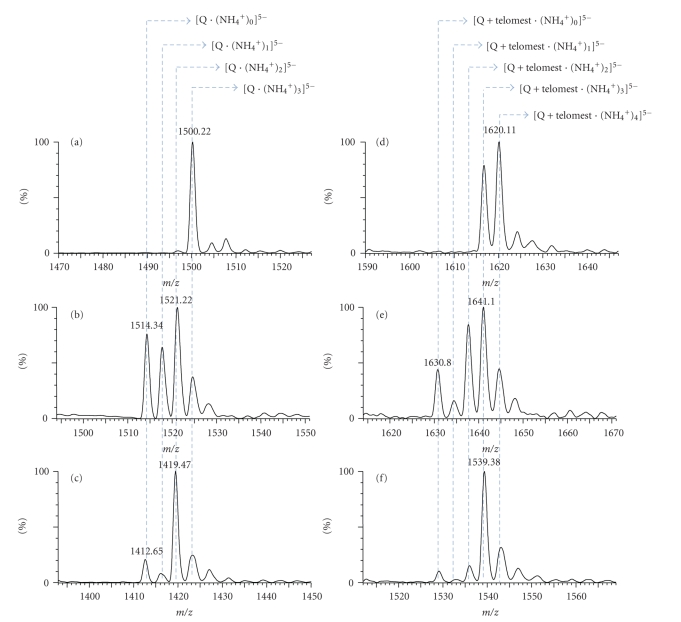
Figure 2 shows the electrospray mass spectra of three typical G-quadruplexes: (a) [TG4T]4, (b) the telomeric sequence (T2AG3)4, and (c) the Pu22myc promoter sequence GAG3TG4AG3TG4A2G and on their 1 : 1 complexes with telomestatin (d–f, resp.). The injected mixtures are 5 μM in each G-quadruplex 10 μM in telomestatin, in 80/20 aqueous ammonium acetate (150 mM)/methanol, and the spectra were recorded using soft source conditions to preserve the specifically bound ammonium ions. The free [TG4T]4 G-quadruplex (Figure 2(a)) contains three ammonium ions: the 5− charge state is found at m/z = 1500.22, corresponding to [Q·(NH4 +)3]5−. The major peaks of the telomeric (Figure 2(b)) and Pu22myc (Figure 2(c)) G-quadruplexes at charge state 5− are corresponding to the intramolecular G-quadruplex with two ammonium ions, at (m/z) = 1521.22 and 1419.47, respectively. For the charge state z = 5, with the average mass of telomestatin being m = 582.5 Da, the Δ(m/z) between the G-quadruplex and its complex with one telomestatin, with no change in the amount of ammonium ions incorporated, is expected to be equal to 582.5/5 = 116.5. In contrast, the observed Δ(m/z) between the major peak of the quadruplex and the major peak of its complex with one ligand is equal to 119.9 (compare Figure 2(a) with Figure 2(d), and Figure 2(b) with Figure 2(e), Figure 2(c) with Figure 2(f)). This corresponds to the addition of one telomestatin molecule, one extra ammonium ion, and the subtraction of one proton for the charge balance (119.9 = (585.5 + 18−1)/5). The complex with one telomestatin ligand therefore systematically contains one more ammonium ion than the corresponding unbound G-quadruplex. This extra ammonium ion is lost when the acceleration voltage is increased.
Figure 2.
Negative ion mode electrospray mass spectra of mixtures of 10 μM telomestatin and 5 μM of G-quadruplexes. Zooms on the peaks of free G-quadruplex (a) [TG4T]4, (b) telomeric sequence (T2AG3)4, and (c) Pu22myc promoter sequence GAG3TG4AG3TG4A2G, and on the complexes between (d) one telomestatin and [TG4T]4, (e) one telomestatin and (T2AG3)4, and (f) one telomestatin and GAG3TG4AG3TG4A2G. The spectra were recorded from and 80/20 aqueous ammonium acetate (150 mM)/methanol solution, using soft source conditions to preserve the specifically bound ammonium ions.
In our previous report on the MS detection of telomestatin binding to telomeric DNA [11], we have missed this “extra ammonium” for two reasons. Firstly, for the 3.5-repeat telomeric sequence studied previously it is difficult to preserve two inner ammoniums even in soft conditions. Secondly, soft conditions could not be used because a long duplex had to be detected simultaneously with the G-strand, and the electrospray source conditions were chosen as a compromise.
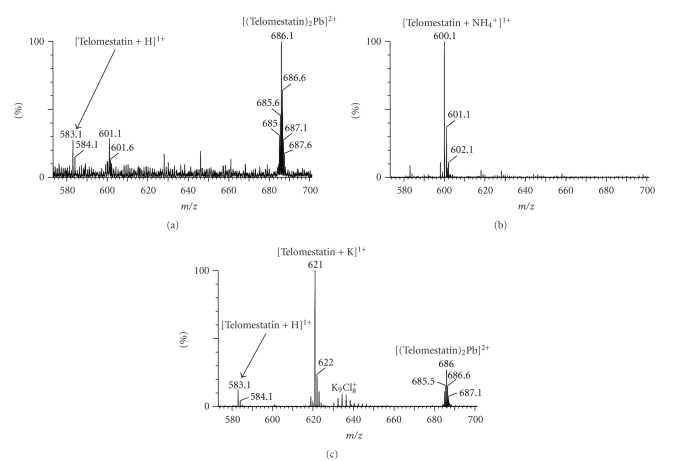
Electrospray mass spectrometry is also powerful to analyze caged supramolecular complexes such as crown ethers bound to cations [32–35]. To probe whether telomestatin is able to coordinate a cation already in the absence of G-quadruplex, we used ESI-MS in the positive ion mode on telomestatin solutions. Figure 3(a) shows the spectrum obtained with telomestatin dilution in bi-distilled water. The signal-to-noise ratio of all peaks is weak, indicating low protonation and cationization efficiencies. Surprisingly, we found that the major peak was a doubly charged ion at m/z = 686.1 (base peak), whose isotopic distribution matches with that of a complex between two telomestatin ligands and one lead ion adduct. The fragment ion spectrum shows the loss of one telomestatin, and the isotopic distribution of the resulting [Telomestatin + Pb] complex confirms the presence of lead. Traces of lead come from the purification of telomestatin from Streptomyces anulatus 3533-SV4 [24]. Another weak doubly charged peak is tentatively assigned to a complex between two telomestatin and two H3O+ ions, and the fragment ion spectrum shows only the singly protonated telomestatin.
Figure 3.
Positive ion mode electrospray mass spectra of 15 μM telomestatin (a) in 80/20 water/methanol, (b) in 80/20 water methanol and 5 μM ammonium acetate, and (c) in 80/20 water methanol and 5 μM potassium chloride.
We then doped the solution with either ammonium acetate or with potassium chloride, in substoichiometric amounts, to probe if telomestatin was able to coordinate monovalent cations such as those typically coordinated to G-quartets. In the presence of ammonium ions, the lead complex totally disappears and the complex [telomestatin + NH4 +] is detected at m/z = 600. In the presence of potassium ions, the complex [telomestatin + K+] is detected at m/z = 621, but the lead complex has not disappeared completely.
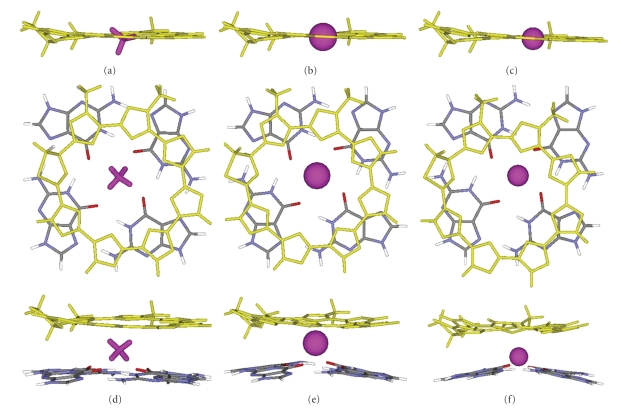
All experimental results suggest that telomestatin has significant affinity for monovalent cations like the ammonium ion, and this could influence its binding mode to the G-quadruplex DNA. We performed DFT calculations in order to ascertain the possible coordination geometries of the monovalent cations to telomestatin. The geometries of the [telomestatin + cation] binary complexes are shown in Figures 4(a)–4(c), and the geometries of the ternary complexes between [telomestatin + cation + one G-quartet] are shown in Figures 4(d)–4(f). In the isolated [telomestatin + cation] complexes, all cations are coordinated in the plane of the telomestatin. In the ternary complexes with the G-quartet, the cation clearly moves towards the G-quartet. The structures of the complexes with K+ and NH4 + are similar, with the cation coordinated midway between the telomestatin and the G-quartet. Sodium, on the other hand, sits closer to the G-quartet than to the telomestatin.
Figure 4.
Optimized geometries (DFT B3LYP, 6-31G(d,p)) of the telomestatin—cation complexes (side view) for (a) NH4 +, (b) K+, and (c) Na+, and optimized geometries of the telomestatin—cation—G-quartet complexes (top view and side view) for (d) NH4 +, (e) K+, and (f) Na+. Telomestatin is shown in yellow, the cation is shown in purple, and the G-quartet is colored by elements.
The mode of cation coordination to the telomestatin—G-quartet system is similar to the coordination mode already described for successive G-quartets [36]. In terms of coordination geometries, potassium tends to sit between G-quartets while sodium tends to fit in the middle of one G-quartet because it is smaller. K+ and NH4 + have similar ionic radii [37, 38] and therefore behave similarly, and the same trend is observed for our telomestatin-G-quartet complex. The optimized geometries are similar for all functionals tested (B3LYP, BHandHLYP, MPBW1K) (see Figure S1 in Supplementary Material available online at doi: 10.4061/2010/121259), with the cation in the plane of telomestatin in the absence of G-quartet, and between telomestatin and the G-quartet in the ternary complex. However, the functionals have a greater influence on the computed interaction energies. The root mean square distances (RMSDs) for two-by-two comparisons of hybrid functional, and the interaction energies of NH4 +, K+ and Na+ with telomestatin alone, telomestatin + one G-quartet are given in supplementary Tables S1 and S2, respectively.
4. Conclusion
We have therefore shown that telomestatin readily coordinates monovalent cations such as K+ and NH4 +, and that telomestatin retains this cation while binding to G-quadruplexes. The observed stoichiometry and the calculations are consistent with a cation trapped midway between the telomestatin and the G-quartet. Telomestatin therefore acts like an analog of a G-quartet. This study underlines that monovalent cation coordination capabilities should be integrated in the rational design of G-quadruplex binding ligands.
Supplementary Material
Comparison between three density functionals: overlay of structures (Figure S1), RMSD (Table S1) and interaction energies (Table S2).
Acknowledgments
The authors wish it to be known that, in their opinion, the first two authors should be regarded as joint first authors. This work was supported by the Fonds de la Recherche Scientifique (FNRS) (FRFC 2.4.623.05 to Edwin De Pauw, CC.1.5.286.09.F to Valérie Gabelica, research associate position to Valérie Gabelica, postdoctoral fellowship to Frédéric Rosu, and logistician fellowship to Nicolas Smargiasso), and by the University of Liége (starting Grant FRSD-08/10 to V.G.).
References
- 1.Rezler EM, Bearss DJ, Hurley LH. Telomere inhibition and telomere disruption as processes for drug targeting. Annual Review of Pharmacology and Toxicology. 2003;43:359–379. doi: 10.1146/annurev.pharmtox.43.100901.135733. [DOI] [PubMed] [Google Scholar]
- 2.Bearss DJ, Hurley LH, Von Hoff DD. Telomere maintenance mechanisms as a target for drug development. Oncogene. 2000;19(56):6632–6641. doi: 10.1038/sj.onc.1204092. [DOI] [PubMed] [Google Scholar]
- 3.Bailey SM, Murnane JP. Telomeres, chromosome instability and cancer. Nucleic Acids Research. 2006;34(8):2408–2417. doi: 10.1093/nar/gkl303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neidle S. The structures of quadruplex nucleic acids and their drug complexes. Current Opinion in Structural Biology. 2009;19(3):239–250. doi: 10.1016/j.sbi.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Alberti P, Lacroix L, Guittat L, Hélène C, Mergny JL. Nucleic acids as targets for antitelomerase agents. Mini Reviews in Medicinal Chemistry. 2003;3(1):23–36. doi: 10.2174/1389557033405485. [DOI] [PubMed] [Google Scholar]
- 6.Monchaud D, Teulade-Fichou M-P. A hitchhiker’s guide to G-quadruplex ligands. Organic and Biomolecular Chemistry. 2008;6(4):627–636. doi: 10.1039/b714772b. [DOI] [PubMed] [Google Scholar]
- 7.Riou J-F, Gomez D, Morjani H, Trentesaux C. Quadruplex ligand recognition: biological aspects. In: Neidle S, Balasubramanian S, editors. Quadruplex Nucleic Acids. Cambridge, UK: The Royal Soceity of Chemistry; 2006. pp. 154–179. [Google Scholar]
- 8.Searle MS, Balkwill GD. DNA quadruplex-ligand recognition: structure and dynamics. In: Neidle S, Balasubramanian S, editors. Quadruplex Nucleic Acids. Cambridge, UK: The Royal Soceity of Chemistry; 2006. pp. 131–153. [Google Scholar]
- 9.Shin-Ya K, Wierzba K, Matsuo K, Ohtani T, Yamada Y, Furihata K, Hayakawa Y, Seto H. Telomestatin, a novel telomerase inhibitor from Streptomyces anulatus. Journal of the American Chemical Society. 2001;123(6):1262–1263. doi: 10.1021/ja005780q. [DOI] [PubMed] [Google Scholar]
- 10.De Cian A, Guittat L, Shin-Ya K, Riou JF, Mergny JL. Affinity and selectivity of G4 ligands measured by FRET. Nucleic Acids Symposium Series. 2005;(49):235–236. doi: 10.1093/nass/49.1.235. [DOI] [PubMed] [Google Scholar]
- 11.Rosu F, Gabelica V, Shin-Ya K, De Pauw E. Telomestatin-induced stabilization of the human telomeric DNA quadruplex monitored by electrospray mass spectrometry. Chemical Communications. 2003;9(21):2702–2703. doi: 10.1039/b309394h. [DOI] [PubMed] [Google Scholar]
- 12.Kim M-Y, Vankayalapati H, Shin-Ya K, Wierzba K, Hurley LH. Telomestatin, a potent telomerase inhibitor that interacts quite specifically with the human telomeric intramolecular G-quadruplex. Journal of the American Chemical Society. 2002;124(10):2098–2099. doi: 10.1021/ja017308q. [DOI] [PubMed] [Google Scholar]
- 13.Tauchi T, Shin-Ya K, Sashida G, Sumi M, Nakajima A, Shimamoto T, Ohyashiki JH, Ohyashiki K. Activity of a novel G-quadruplex-interactive telomerase inhibitor, telomestatin (SOT-095), against human leukemia cells: involvement of ATM-dependent DNA damage response pathways. Oncogene. 2003;22(34):5338–5347. doi: 10.1038/sj.onc.1206833. [DOI] [PubMed] [Google Scholar]
- 14.Gomez D, Wenner T, Brassart B, Douarre C, O’Donohue M-F, El Khoury V, Shin-Ya K, Morjani H, Trentesaux C, Riou J-F. Telomestatin-induced telomere uncapping is modulated by POT1 through G-overhang extension in HT1080 human tumor cells. Journal of Biological Chemistry. 2006;281(50):38721–38729. doi: 10.1074/jbc.M605828200. [DOI] [PubMed] [Google Scholar]
- 15.Temime-Smaali N, Guittat L, Sidibe A, Shin-Ya K, Trentesaux C, Riou J-F. The G-quadruplex ligand telomestatin impairs binding of topoisomerase IIIα to G-quadruplex-forming oligonucleotides and uncaps telomeres in ALT cells. PLoS ONE. 2009;4(9, article e6919) doi: 10.1371/journal.pone.0006919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tauchi T, Shin-Ya K, Sashida G, Sumi M, Okabe S, Ohyashiki JH, Ohyashiki K. Telomerase inhibition with a novel G-quadruplex-interactive agent, telomestatin: in vitro and in vivo studies in acute leukemia. Oncogene. 2006;25(42):5719–5725. doi: 10.1038/sj.onc.1209577. [DOI] [PubMed] [Google Scholar]
- 17.Binz N, Shalaby T, Rivera P, Shin-Ya K, Grotzer MA. Telomerase inhibition, telomere shortening, cell growth suppression and induction of apoptosis by telomestatin in childhood neuroblastoma cells. European Journal of Cancer. 2005;41(18):2873–2881. doi: 10.1016/j.ejca.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 18.Sumi M, Tauchi T, Sashida G, Nakajima A, Gotoh A, Shin-Ya K, Ohyashiki JH, Ohyashiki K. A G-quadruplex-interactive agent, telomestatin (SOT-095), induces telomere shortening with apoptosis and enhances chemosensitivity in acute myeloid leukemia. International Journal of Oncology. 2004;24(6):1481–1487. [PubMed] [Google Scholar]
- 19.Shammas MA, Shmookler Reis RJ, Li C, Koley H, Hurley LH, Anderson KC, Munshi NC. Telomerase inhibition and cell growth arrest after telomestatin treatment in multiple myeloma. Clinical Cancer Research. 2004;10(2):770–776. doi: 10.1158/1078-0432.ccr-0793-03. [DOI] [PubMed] [Google Scholar]
- 20.Agrawal S, Ojha RP, Maiti S. Energetics of the human Tel-22 quadruplex—telomestatin interaction: a molecular dynamics study. Journal of Physical Chemistry B. 2008;112(22):6828–6836. doi: 10.1021/jp7102676. [DOI] [PubMed] [Google Scholar]
- 21.Rosu F, Gabelica V, Houssier C, Colson P, De Pauw E. Triplex and quadruplex DNA structures studied by electrospray mass spectrometry. Rapid Communications in Mass Spectrometry. 2002;16(18):1729–1736. doi: 10.1002/rcm.778. [DOI] [PubMed] [Google Scholar]
- 22.Baker ES, Bernstein SL, Gabelica V, De Pauw E, Bowers MT. G-quadruplexes in telomeric repeats are conserved in a solvent-free environment. International Journal of Mass Spectrometry. 2006;253(3):225–237. [Google Scholar]
- 23.Gabelica V, Baker ES, Teulade-Fichou M-P, De Pauw E, Bowers MT. Stabilization and structure of telomeric and c-myc region intramolecular G-quadruplexes: the role of central cations and small planar ligands. Journal of the American Chemical Society. 2007;129(4):895–904. doi: 10.1021/ja065989p. [DOI] [PubMed] [Google Scholar]
- 24.Seto H, Shin-Ya K, Wierzba K. Substance GM-95, process for producing the same and utilization thereof. WO 00/24747, October 1999.
- 25.Gaussian 03, Revision D.02. Wallingford, Conn, USA: Gaussian; 2004. [Google Scholar]
- 26.Zhao Y, Truhlar DG. How well can new-generation density functional methods describe stacking interactions in biological systems? Physical Chemistry Chemical Physics. 2005;7(14):2701–2705. doi: 10.1039/b507036h. [DOI] [PubMed] [Google Scholar]
- 27.Breuker K. New mass spectrometric methods for the quantification of protein-ligand binding in solution. Angewandte Chemie - International Edition. 2003;43(1):22–25. doi: 10.1002/anie.200301695. [DOI] [PubMed] [Google Scholar]
- 28.Siegel MM. Early discovery drug screening using mass spectrometry. Current Topics in Medicinal Chemistry. 2002;2(1):13–33. doi: 10.2174/1568026023394551. [DOI] [PubMed] [Google Scholar]
- 29.Pramanik BN, Bartner PL, Mirza UA, Liu Y-H, Ganguly AK. Electrospray ionization mass spectrometry for the study of non-covalent complexes: an emerging technology. Journal of Mass Spectrometry. 1998;33(10):911–920. doi: 10.1002/(SICI)1096-9888(1998100)33:10<911::AID-JMS737>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 30.Loo JA. Studying noncovalent protein complexes by electrospray ionization mass spectrometry. Mass Spectrometry Reviews. 1997;16(1):1–23. doi: 10.1002/(SICI)1098-2787(1997)16:1<1::AID-MAS1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 31.Rosu F, De Pauw E, Gabelica V. Electrospray mass spectrometry to study drug-nucleic acids interactions. Biochimie. 2008;90(7):1074–1087. doi: 10.1016/j.biochi.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Schalley CA. Molecular recognition and supramolecular chemistry in the gas phase. Mass Spectrometry Reviews. 2001;20(5):253–309. doi: 10.1002/mas.10009. [DOI] [PubMed] [Google Scholar]
- 33.Leize E, Jaffrezic A, Van Dorsselaer A. Correlation between solvation energies and electrospray mass spectrometric response factors. Study by electrospray mass spectrometry of supramolecular complexes in thermodynamic equilibrium in solution. Journal of Mass Spectrometry. 1996;31(5):537–544. [Google Scholar]
- 34.Blair SM, Brodbelt JS, Marchand AP, Kumar KA, Chong H-S. Evaluation of binding selectivities of caged crown ligands toward heavy metals by electrospray ionization/quadrupole ion trap mass spectrometry. Analytical Chemistry. 2000;72(11):2433–2445. doi: 10.1021/ac991125t. [DOI] [PubMed] [Google Scholar]
- 35.Kempen EC, Brodbelt JS, Bartsch RA, Jang Y, Kim JS. Investigation of alkali metal cation selectivities of lariat ethers by electrospray ionization mass spectrometry. Analytical Chemistry. 1999;71(24):5493–5500. [Google Scholar]
- 36.Gu J, Leszczynski J. Origin of Na+/K+ selectivity of the guanine tetraplexes in water: the theoretical rationale. Journal of Physical Chemistry A. 2002;106(3):529–532. [Google Scholar]
- 37.Hud NV, Schultze P, Sklenář V, Feigon J. Binding sites and dynamics of ammonium ions in a telomere repeat DNA quadruplex. Journal of Molecular Biology. 1999;285(1):233–243. doi: 10.1006/jmbi.1998.2327. [DOI] [PubMed] [Google Scholar]
- 38.Schultze P, Hud NV, Smith FW, Feigon J. The effect of sodium, potassium and ammonium ions on the conformation of the dimeric quadruplex formed by the Oxytricha nova telomere repeat oligonucleotide d(G4T4G4) Nucleic Acids Research. 1999;27(15):3018–3028. doi: 10.1093/nar/27.15.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison between three density functionals: overlay of structures (Figure S1), RMSD (Table S1) and interaction energies (Table S2).