Abstract
We present the first demonstration of oral transmission of Trypanosoma cruzi to raccoons (Procyon lotor), a natural reservoir host in the United States, by ingestion of trypomastigotes and infected bugs, but not infected tissue. To investigate an alternative, non-vector–based transmission method, we tested the hypothesis that raccoons scavenging on infected hosts results in patent infection. Macerated tissue from selected organs infected with amastigote stages of T. cruzi was orally administered to experimental groups of raccoons (n = 2/group) at 2, 12, or 24 hr after collection of the tissue samples. Additionally, raccoons (n = 1) in control groups were inoculated intravenously or per os with trypomastigotes. To further elucidate transmission routes of T. cruzi to raccoons, infected Rhodnius prolixus were fed to raccoons (n = 2). Raccoons did not become infected after ingestion of amastigote-infected tissues as evidenced by negative polymerase chain reaction results from blood and tissue, lack of seroconversion, and negative parasitemias. However, per os transmission can occur by ingestion of the infective trypomastigote stage or infected reduviid bugs. We conclude from these findings that oral transmission of T. cruzi may be a route of infection for wildlife in sylvatic cycles, but the scavenging behavior of animals is not likely a significant transmission route.
Trypanosoma cruzi, a hemoflagellate protozoan parasite, has a complex life cycle that includes both domiciliary and sylvatic cycles. Reservoir hosts for the etiologic agent of Chagas’ disease include a range of wild mammals in endemic regions of the Americas. In the United States, there have been few documented human cases, but the prevalence of T. cruzi in U.S. wildlife based on serology, culture isolation, and/or polymerase chain reaction (PCR) can be equally as high as in South America (Barr et al., 1991). Studies conducted in the southeastern United States indicate that raccoons (Procyon lotor) and Virginia opossums (Didelphis virginiana) have the highest prevalence of T. cruzi compared with other mammals.
Classic transmission of infective trypomastigotes from the vector (triatomine bugs) to mammals requires invasion of the oral, nasal, or ocular mucosa, or invasion through an abrasion or cut in the dermis near the bug defecation site. Many have proposed alternative modes of transmission of T. cruzi in wildlife species because of inconsistent use of dens by animals and the apparent low density of vectors in dens (Jansen et al., 1994). Experimental per os infection trials in Virginia opossums (Yeager, 1971) and striped skunks (Mephitis mephitis) (Davis et al., 1980) have implied direct oral transmission as the presumptive natural route with the ingestion of infected triatomid bugs or oral lavage with infected intestinal contents, respectively. Conversely, microcosm experiments have demonstrated that opossums rarely, if ever, prey on infected bugs in simulated dens, but nonetheless acquire T. cruzi (Schweigmann et al., 1995).
In addition to the ingestion of vectors, numerous claims have been made regarding the importance of carnivory in maintenance of the sylvatic cycle (see Miles, 2004; Coura, 2006; Dias, 2006). However, no experimental data in natural reservoirs have been produced to support or refute such assertions. The objective of the current study was to elucidate the natural transmission for wildlife reservoir hosts and to determine the role of carnivory in T. cruzi transmission by simulating natural eating habits of raccoons. We hypothesized that the ingestion of amastigote-infected tissues would produce a detectable T. cruzi infection, which would be identified by seroconversion, development of a patent parasitemia, histologic detection of amastigotes, and amplification of T. cruzi DNA from blood and tissues. Additionally, raccoons were expected to develop patent infections after ingestion of infected triatomine bugs and food contaminated with culture-derived trypomastigotes.
MATERIALS AND METHODS
Inoculation material
Trypanosoma cruzi isolated from a raccoon trapped in Torreya State Park, Florida, in 2005 (FL RAC 9) was used as the inoculation source in the experiments. This isolate was previously shown to be a type IIa, which is the group most often associated with raccoon infections (Roellig et al., 2008). The original isolate was stored in liquid nitrogen before culture in liver-infusion tryptose (LIT) medium at the commencement of this study. Epimastigotes were passaged from LIT medium into DH82 canine macrophage monolayers at 1:5 dilutions to yield the culture-derived-trypomastigotes. Trypomastigotes were pelleted from culture by centrifugation at 1,620 g for 15 min and resuspended in minimum essential medium (MEM). Amastigote-containing tissue was obtained from parasitemic, juvenile raccoons (n = 2) 18 days after intravenous (i.v.) inoculation with 1 × 106 culture-derived trypomastigotes. Heart, spleen, quadriceps muscle, diaphragm, urinary bladder, and liver were collected at necropsy, pooled, and coarsely ground using a tissue grinder. Trypanosoma cruzi infection of tissues was confirmed by PCR of individual and ground tissue and observation of pseudocysts in hematoxylin- and eosin-stained sections of heart tissue (data not shown). Laboratory-reared Rhodnius prolixus nymphs (fourth and fifth instars) (n = 6) were fed until repletion on T. cruzi-infected raccoons (n = 2) with detectable parasitemias and allowed to digest the blood meal for 19 days.
Animals and experimental design
Eleven juvenile raccoons obtained from Ruby Fur Farm, Inc. (New Sharon, Iowa) were housed individually or in pairs in climate-controlled animal housing at the College of Veterinary Medicine, University of Georgia (Athens, Georgia); they were given food and water ad libitum, except food was withheld for 24 hr pre-exposure. All animals used in this study were maintained in accordance with the guidelines of the Institutional Animal Care and use Committee and under animal use protocol approved by the Institutional Animal Care and Use Committee at the University of Georgia. Before use, all raccoons were determined to be negative for antibodies reactive with T. cruzi (as described below).
Animals were separated into 6 experimental groups. Group 1 (n = 1) served as a positive control for parasite infectivity and was inoculated i.v. with 1 × 106 culture-derived trypomastigotes. Individuals in group 2, 3, or 4 (n = 2) ingested approximately 33.3 g of pooled amastigote-infected tissue that was held at room temperature for 2, 12, or 24 hr post-mortem, respectively. Group 5 raccoons (n = 2) each ingested 3 R. prolixus nymphs shedding metacyclic trypomastigotes in feces as determined by light microscopy. Group 6 (n = 1) was inoculated per os (p.o.) by feeding 1 × 106 culture-derived trypomastigotes mixed with commercial canned cat food. Two negative control raccoons were inoculated i.v. with equivalent volumes of media as group 1.
All animals were anesthetized with an intramuscular injection of a mixture of 20 mg/kg ketamine (Fort Dodge Laboratories, Inc., Fort Dodge, Iowa) and 4 mg/kg xylazine (Mobay Corporation, Shawnee, Kansas) for handling and blood collection. Blood samples were aseptically collected from the jugular vein into ethylenediaminetetraacetic acid (EDTA) tubes every 7 days post-inoculation (DPI) until being killed. The raccoon in group 1 was killed at 28 DPI, group 5 at 35 DPI, and all others at 42 DPI. Animals were humanely killed under anesthesia by exsanguination and intracardiac injection of sodium pentobarbital (1 mg/kg; Butler Company, Columbus, Ohio).
Direct and molecular detection of T. cruzi
Parasitemias were determined by examining 5 μl of whole blood under an 18-mm cover glass at ×400 magnification with a compound microscope. The entire volume of blood was scanned and the number of counted parasites converted to parasites per milliliter.
DNA was extracted from 100 μl of whole blood using the DNeasy blood and tissue kit (QIAGEN, Valencia, California) following the manufacturer’s protocol. Extracted DNA was used as template in PCR amplification of D7 divergent domain of the 24Sα rDNA gene using a modified nested reaction with primers D75 and D76 (Briones et al., 1999) in the primary reaction and primers D71 and D72 in a secondary reaction (Souto et al., 1996). Total volume of each reaction mixture was 25 μl and contained 5× buffer, 2 μM of each dNTP, 1 μM of each primer, 2.5 mM MgCl2, and 1.25 U of GoTaq Taq polymerase (Promega, Madison, Wisconsin). The temperature and cycling profile were described previously (Souto et al., 1996). Stringent protocols and controls were used in all PCR assays to prevent and to detect contamination. DNA extraction, amplification, and product analysis were performed in separate dedicated laboratory areas. A negative water control was included in each set of extractions and PCR reactions as contamination controls. The 120-bp amplicons were visualized on an ethidium bromide-stained 1.5% agarose gel by transillumination.
After death, raccoons were necropsied and representative samples of major organs (retropharyngeal lymph nodes, skeletal muscle [diaphragm and quadriceps], heart, lungs, liver, spleen, gastrointestinal tract, pancreas, kidney, adrenal glands, reproductive organs, urinary bladder, quadriceps muscle, and brain) were collected. One portion of each sample was preserved in 10% neutral buffered formalin for histologic examination, and the remaining portion was stored at −20 C until PCR analysis. Frozen tissues were thawed, and 1, 25-mg section of each was aseptically excised. DNA was isolated from tissue using the DNeasy blood and tissue kit (QIAGEN) following the manufacturer’s protocol with a 24-hr tissue lysation step.
Serology
Indirect immunofluorescent antibody assay was performed as described previously (Yabsley et al., 2004) with plasma at a 1:40 dilution. Briefly, epimastigotes were fixed to serology slides (Fisher Scientific, Rome, Georgia) by air drying and fixation in an acetone wash for 2 min. Diluted serum samples and positive and negative controls were added to respective wells and incubated for approximately 25 min. Two, 5-min washes with 1× phosphate-buffered saline (PBS) and a 5-min distilled water wash were performed, and the slides were dried. Diluted fluorescein isothiocyanate-labeled goat anti-raccoon antibodies (Kirkegaard and Perry Laboratories, Gaithersburg, Maryland; 1:50) were added to slides and incubated for approximately 25 min. Two, 5 min PBS washes were performed and were counterstained using a final wash of 1.65% Eriochrome black in distilled water.
Hemoculture
At the time of death, hemoculture in DH82 macrophages (Yabsley et al., 2004; Hall et al., 2007) was carried out with 1 ml of EDTA-anticoagulated whole blood and checked daily for the presence of trypomastigotes. Briefly, in a 50-ml tube, approximately 35 ml of ACE lysing buffer was added to blood, gently inverted for 5 min, and centrifuged at 1,620 g for 10 min. The supernatant was discarded, and the procedure was repeated. The buffy coat pellet was resuspended in 5 ml of MEM and added to a confluent monolayer of DH82 cells.
Histopathology
Formalin-fixed tissues were embedded in paraffin, sectioned, and stained with hematoxylin and eosin by standard methods. Inflammation was blindly scored based on the number of foci detected per fields viewed and compared with negative control tissues. These scores were then evaluated and assigned to 1 of 4 categories, i.e., very mild, mild, moderate, and severe inflammation. Presence of other histologic lesions and pseudocysts was also noted.
RESULTS
Patent infections were only detected in i.v.-inoculated (group 1), bug-fed (group 5), and p.o.-inoculated (group 6) raccoons. Parasitemias were first detected in i.v.-inoculated raccoons, followed by bug-fed and then p.o.-inoculated raccoons (Fig. 1). None of the negative control raccoons or raccoons that ingested amastigote-infected tissue developed parasitemias.
Figure 1.
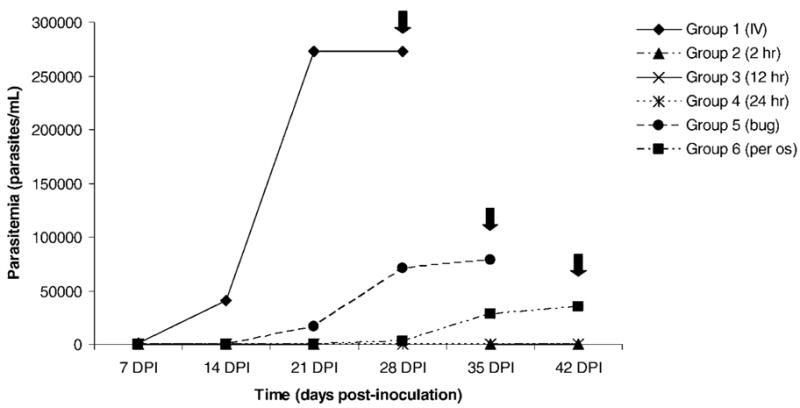
Parasitemias of raccoons experimentally infected with Trypanosoma cruzi via different inoculation methods. Inoculation methods were as follows: group 1 intravenously with 1 × 106 culture-derived trypomastigotes; groups 2, 3, and 4 per os with 33.3 g of amastigote-infected tissue at 2, 12, and 24 hr postmortem, respectively; group 5 per os with 3 infected R. prolixis fourth or fifth instar nymphs; and group 6 per os with 1 × 106 culture-derived trypomastigotes. Black arrows indicate day on which animals were euthanized.
Trypanosoma cruzi DNA was amplified from raccoons in groups 1 (i.v.), 5 (bug), and 6 (p.o.); no T. cruzi DNA was detected by PCR in tissue-fed raccoons. The i.v.-inoculated raccoon was PCR-positive on day 7 post-inoculation (PI) and every bleed date thereafter. Interestingly, the first detection of T. cruzi DNA in the p.o.-inoculated and bug-fed individuals was 1 wk later, at 14 days PI; animals remained PCR-positive through the completion of the study. The amplified product in all cases was 120 bp, consistent with the lineage typing of the inoculation strain (FL RAC 9) (Roellig et al., 2008). On the last day of the experiment, for those animals that were parasitemic (groups 1, 5, and 6), T. cruzi DNA was amplified from all tissues collected. Trypanosoma cruzi DNA was not amplified from the blood or tissues of any raccoon that ingested T. cruzi-infected tissues.
Similar to the parasitemia results, seroconversion to T. cruzi only occurred in i.v.-inoculated, p.o.-inoculated, and bug-fed groups. The intravenously inoculated raccoon seroconverted sooner (7 days PI) than those that ingested infected bugs (21 days PI) or trypomastigote contaminated food (28 days PI).
After 8 wk, cultures from tissue-fed animals (groups 2–4) were considered negative for T. cruzi. Hemoculture was not performed for bug-fed animals, which were parasitemic at the time of their death, but i.v.- and p.o.-inoculated groups were positive by hemoculture, confirming patent infections detected by other methods.
No major histologic lesions were noted other than varying levels of inflammation and pseudocysts in individuals that were successfully infected with T. cruzi (Table I). The i.v.- and p.o.-inoculated raccoons had greater levels of inflammation than the bug-fed raccoons, and tissue-fed raccoons had no appreciable inflammation.
TABLE I.
Inflammatory lesions of tissues in raccoons experimentally infected with Trypanosoma cruzi via different routes. Inoculation methods were as follows: group 1 intravenously (i.v.) with 1 × 106 culture-derived trypomastigotes; groups 2, 3, and 4 per os (p.o.) with 33.3 g of amastigote-infected tissue at 2, 12, and 24 hr post-mortem, respectively; group 5 p.o. with 3 infected R. prolixis fourth or fifth instar nymphs; and group 6 p.o. with 1 × 106 culture-derived trypomastigotes.
| Animal | Tissue |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LN* | Skeletal muscle | Heart | Lung | Liver | Spleen | GI* | Pancreas | Adrenal gland | Kidney | Bladder | Sex organ | Brain | |
| Group 1 (i.v.) | |||||||||||||
| RAC A | Vm* | S*† | S† | S | Mo* | Vm | Vm | M*† | M† | Vm | M | M | M |
| Group 2 (2 hr) | |||||||||||||
| RAC B | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm |
| RAC C | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm |
| Group 3 (12 hr) | |||||||||||||
| RAC D | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm |
| RAC E | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm |
| Group 4 (24 hr) | |||||||||||||
| RAC F | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm |
| RAC G | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm |
| Group 5 (bug) | |||||||||||||
| RAC H | Vm | M | Mo | Vm | S | Vm | Vm | Vm | Vm | Vm | Mo | Vm | Vm |
| RAC I | Vm | M | Mo† | Vm | S | Vm | Vm | Vm | Vm | Vm | Vm | Vm | Vm |
| Group 6 (p.o. control) | |||||||||||||
| RAC J | Vm | Mo | M | Vm | Mo | Vm† | Vm | Vm | S | M | Vm | M | Vm |
LN, lymph node; GI, gastrointestinal tract; Vm, very mild; M, mild; Mo, moderate; S, severe.
Pseudocyst(s) found within tissue sample.
DISCUSSION
Although different transmission routes for T. cruzi have been identified experimentally, the mechanism by which wildlife reservoirs predominately become infected with T. cruzi in the United States is unknown. Classic stercorarian vector transmission is unlikely because the 2 main reservoirs, raccoons and opossums, rarely use permanent dens, and competent vectors are rarely found in, or near, temporary dens (Walton et al., 1958); some species of native vectors, such as Triatoma sanguisuga, have delayed defecation times after the acquisition of a bloodmeal (Zeledón, 1974). Vertical, or transplacental, transmission has been demonstrated in rodent models (Andrade, 1982; Moreno et al., 2003) and naturally in humans (Hoff et al., 1978; Muños et al., 2007), but similar experiments have not been performed with raccoons. Infective trypomastigote stages in breast milk have also been reported in rodent models (Miles, 1972), but experimental infection studies in rodents (Mazza et al., 1936) and opossums (Jansen et al., 1994) disprove this as a route of transmission. Oral, or intragastric, transmission has been responsible for numerous outbreaks in humans, particularly when associated with ingesting vector-contaminated juices (Ianni and Mady, 2005). Additionally, researchers have hypothesized this as a mode of transmission in wildlife reservoirs (Yaeger, 1971; Miles et al., 2004; Coura, 2006; Dias, 2006), including via the ingestion of infected bugs and other animals.
In previous intragastric inoculation studies with mice, researchers found that T. cruzi trypomastigotes are able to penetrate the gastric mucosa and establish infection; this process was dependent on the expression of surface proteins gp90 and gp82 (Cortez et al., 2006; Covarrubias et al., 2007). When ingested, metacyclic trypomastigotes found in the feces of triatomes were able to produce detectable parasitemias prior to day 20 PI (Covarrubias et al., 2007) and were more infective than blood-form trypomastigotes (Calvo Méndez et al., 1992). Because a patent infection occurred after oral ingestion of our culture-derived trypomastigotes, we believe the parasites were more similar to bloodstream forms, and our results may simulate the indirect consequences of mesomammal carnivory by ingesting highly parasitemic, infected blood.
The oral transmission of T. cruzi via ingestion of tissues has only been presented in the literature once, but with a nonreservoir species, in which Phyllostomus sp. (bats) became infected after ingesting T. cruzi-infected mice (Thomas et al., 2007). Our findings, however, suggest that raccoons, a major wildlife reservoir in the United States, do not become readily infected after consuming infected tissues. Discrepancies between our findings and those of Thomas et al. (2007) may be explained by the ingestion of bloodstream trypomastigotes. In the previous study, the bats may have become infected because the mice were highly parasitemic; in the present study, raccoons were also fed tissue from parasitemic animals. However, less infective forms may have been present in this “inoculum” because the killed animals were exsanguinated at the time of death and remaining trypomastigotes may have died after the animals’ death and clotting of remaining blood. Feeding of tissues from these animals more readily mimics natural exposure, i.e., scavenging of carcasses by wildlife, where the blood of deceased animals has clotted. Based on our results, carnivory does not seem to be a major contributor to the high prevalence of infections seen in wildlife.
As has been demonstrated with opossums and striped skunks (Yaeger, 1971; Davis et al., 1980), raccoons develop patent infections upon ingestion of infected bugs. The metacyclic trypomastigotes are able to withstand the acidic gastric environment and penetrate the gut mucosa to establish an infection. These findings parallel the human cases of T. cruzi resultant from ingesting vector parts in food or drink (Shikanai-Yasuda et al., 1991; Ianni and Mady, 2005), because in all cases the infective stage, metacyclics, are ingested. Together, these data suggest that consumption of bugs by raccoons and opossums (both omnivorous), is the major transmission route for T. cruzi in the United States because alternative transmission routes such as stercorarian vector transmission and ingestion of infected tissues seem to be insignificant.
Acknowledgments
We thank Kate McMillan, Mason Savage, Jessica Murdock, and Emily Brown (SCWDS) for laboratory assistance. This study was supported by the National Institutes of Health National Institute of Allergy and Infectious Disease grant R15 AI067304.
LITERATURE CITED
- Andrade SG. The influence of the strain of Trypanosoma cruzi in placental infections in mice. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1982;76:123–128. doi: 10.1016/0035-9203(82)90036-0. [DOI] [PubMed] [Google Scholar]
- Barr SC, Brown CC, Dennis VA, Klei TR. The lesions and prevalence of Trypanosoma cruzi in opossums and armadillos from southern Louisiana. Journal of Parasitology. 1991;77:624–627. [PubMed] [Google Scholar]
- Briones MRS, Souto RP, Stolf BS, Zingales B. The evolution of two Trypanosoma cruzi subgroups inferred from rRNA genes can be correlated with the interchange of American mammalian faunas in the Cenozoic and has implications to pathogenicity and host specificity. Molecular and Biochemical Parasitology. 1999;104:219–232. doi: 10.1016/s0166-6851(99)00155-3. [DOI] [PubMed] [Google Scholar]
- Calvo Méndez ML, Nogueda Torres B, Alejandre Aguilar R. The oral route: An access port for Trypanosoma cruzi. Revista Latinoamericana de Microbiología. 1992;34:39–42. [PubMed] [Google Scholar]
- Cortez M, Silva MR, Neira I, Ferreira D, Sasso GR, Luquetti AO, Rassi A, Yoshida N. Trypanosoma cruzi surface molecule gp90 down regulates invasion of gastric mucosal epithelium in orally infected mice. Microbes and Infection. 2006;8:36–44. doi: 10.1016/j.micinf.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Coura RJ. Transmission of chagasic infection by oral route in the natural history of Chagas disease. Revista da Sociedade Brasileira de Medicina Tropical. 2006;39(Suppl 3):113–117. [PubMed] [Google Scholar]
- Covarrubias C, Cortez M, Ferreira D, Yoshida N. Interaction with host factors exacerbates Trypanosoma cruzi cell invasion capacity upon oral infection. International Journal for Parasitology. 2007;37:1609–1616. doi: 10.1016/j.ijpara.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Davis DS, Russell LH, Adams LG, Yaeger RG, Robinson RM. An experimental infection of Trypanosoma cruzi in striped skunks (Mephitis mephitis) Journal of Wildlife Diseases. 1980;16:403–406. doi: 10.7589/0090-3558-16.3.403. [DOI] [PubMed] [Google Scholar]
- Dias JCP. Notas sobre o Trypanosoma cruzi e suas características bio-ecológicas, como agente de enferemidades transmitidas por alimentos. Revista da Sociedade Brasileira de Medicina Tropical. 2006;39:370–375. doi: 10.1590/s0037-86822006000400010. [DOI] [PubMed] [Google Scholar]
- Hall CA, Polizzi C, Yabsley MJ, Norton TM. Trypanosoma cruzi prevalence and epidemiologic trends in lemurs on St. Catherines Island, Georgia. Journal of Parasitology. 2007;93:93–96. doi: 10.1645/GE-936R.1. [DOI] [PubMed] [Google Scholar]
- Hoff R, Mott KE, Milanesi ML, Bittencourt AL, Barbosa HS. Congenital Chagas’ disease in an urban population: Investigation of infected twins. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1978;72:247–250. doi: 10.1016/0035-9203(78)90203-1. [DOI] [PubMed] [Google Scholar]
- Ianni BM, Mady C. The sugarcane was delicious, but.…. Arquivos Brasileiros de Cardiologia. 2005;85:379–381. doi: 10.1590/s0066-782x2005001900001. [DOI] [PubMed] [Google Scholar]
- Jansen AM, Madeira FB, Deane MP. Trypanosoma cruzi infection in the opossum Didelphis marsupialis: Absence of neonatal transmission and protection by maternal antibodies in experimental infections. Memorias do Instituto Oswaldo Cruz. 1994;89:41–45. doi: 10.1590/s0074-02761994000100008. [DOI] [PubMed] [Google Scholar]
- Mazza S, Montaña A, Benitez C, Janzi EC. Transmisión de Schizotrypanum cruzi al niño por leche de la madre con enfermedad de Chagas. MEPRA (Mision de Estudios de Patolgia Regional Argentina, Publicación) 1936;28:41–46. [Google Scholar]
- Miles MA. Trypanosoma cruzi-Milk transmission of infection and immunity from mother to young. Parasitology. 1972;65:1–9. doi: 10.1017/s0031182000044188. [DOI] [PubMed] [Google Scholar]
- Miles M, Yeo AM, Gaunt MW. Epidemiology of American Trypanosomiasis. In: Maudlin I, Holmes IPH, Miles MA, editors. The trypanosomiases. CABI Publishing; Cambridge, Massachusetts: 2004. pp. 243–267. [Google Scholar]
- Moreno EA, I, Rivera M, Moreno SC, Alarcón ME, Lugo-Yarbuh A. Vertical transmission of Trypanosoma cruzi in Wistar rats during the acute phase of infection. Investigacion Clínica. 2003;44:241–254. [PubMed] [Google Scholar]
- Muños J, Portús M, Corachan M, Fumadó V, Gascon J. Congenital Trypanosoma cruzi infection in a non-endemic area. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2007;101:1161–1162. doi: 10.1016/j.trstmh.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Roellig DM, Brown EL, Barnabé C, Tibayrenc M, Steurer FJ, Yabsley MJ. Molecular typing of Trypanosoma cruzi isolates, United States. Emerging Infectious Diseases. 2008;14:1123–1125. doi: 10.3201/eid1407.080175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweigmann NJ, Pietrokovsky S, Bottazzi V, Conti O, Wisnivesky-Colli C. Interaction between Didelphis albiventris and Triatoma infestans in relation to Trypanosoma cruzi transmission. Memorias do Instituto Oswaldo Cruz. 1995;90:678–682. doi: 10.1590/s0074-02761995000600003. [DOI] [PubMed] [Google Scholar]
- Shikanai-Yasudo MA, Marcondes CB, Guedes LA, Siqueira GS, Barone AA, Dias JC, Amato Neto V, Tolezano JE, Peres BA, Arruda ER, et al. Possible oral transmission of acute Chagas’ disease in Brazil. Revista do Instituto de Medicina Tropical de São Paulo. 1991;33:351–357. doi: 10.1590/s0036-46651991000500003. [DOI] [PubMed] [Google Scholar]
- Souto RP, Fernandes O, Macedo AM, Campbell DA, Zingales B. DNA markers define two major phylogenetic lineages of Trypanosoma cruzi. Molecular and Biochemical Parasitology. 1996;83:141–152. doi: 10.1016/s0166-6851(96)02755-7. [DOI] [PubMed] [Google Scholar]
- Thomas ME, Rasweiler JJ, IV, D’Alessandro A. Experimental transmission of the parasitic flagellates Trypanosoma cruzi and Trypanosoma rangeli between triatomine bugs or mice and captive Neotropical bats. Memorias do Instituto Oswaldo Cruz. 2007;102:559–565. doi: 10.1590/s0074-02762007005000068. [DOI] [PubMed] [Google Scholar]
- Walton BC, Bauman PM, Diamond LS, Herman CM. The isolation and identification of Trypanosoma cruzi from raccoons in Maryland. American Journal of Tropical Medicine and Hygiene. 1958;7:603–610. doi: 10.4269/ajtmh.1958.7.603. [DOI] [PubMed] [Google Scholar]
- Yabsley MJ, Norton TM, Powell MR, Davidson WR. Molecular and serologic evidence of tick-borne Ehrlichiae in three species of lemurs from St. Catherines Island, Georgia, USA. Journal of Zoo and Wildlife Medicine. 2004;35:503–509. doi: 10.1638/03-116. [DOI] [PubMed] [Google Scholar]
- Yeager RG. Transmission of Trypanosoma cruzi infection to opossums via the oral route. Journal of Parasitology. 1971;57:1375–1376. [PubMed] [Google Scholar]
- Zeledón R. Trypanosomiasis and leishmaniasis with special reference to Chagas’ disease. Ciba Foundation Symposium 20. Associated Publishers; Amsterdam, Netherlands: 1974. Epidemiology, modes of transmission and reservoir hosts of Chagas’ disease; pp. 51–85. [Google Scholar]


