Abstract
A 55-year-old woman with primary Immunoglobulin light chain (AL) systemic amyloidosis died due to spontaneous rupture of her liver following treatment with high-dose melphalan and autologous stem cell transplant (HDM/SCT). She was first diagnosed after developing nephrotic-range proteinuria. Spontaneous rupture of her liver occurred 10 days after treatment with HDM/SCT and was complicated by septic shock. She was not eligible for surgical intervention and died shortly after. Amyloid fibrils were extracted from the autopsied liver sample (05-135L) and the biochemical nature of the fibrils was analyzed using electrophoretic and immunohistochemical techniques. Our testing showed that the fibrils were composed of immunoglobulin lambda light chains that were not glycosylated.
While the liver is often involved in AL amyloidosis, this is the first documented case of a spontaneous hepatic rupture in a patient during treatment with HDM/SCT. A literature review of spontaneous liver rupture in patients with amyloidosis is presented.
Keywords: AL amyloidosis, liver rupture, stem cell transplantation
Introduction
AL (immunoglobulin light chain) amyloidosis is a plasma cell dyscrasia in which clonal immunoglobulin light chains misfold, forming amyloid fibrils that are deposited in tissues and vital organs, leading to organ dysfunction and failure [1]. The most common sites of fibril deposition are the kidney and heart, although all parts of the body except the central nervous system are possible targets [2]. The liver is affected in 9% of all cases of amyloidosis [3]. Spontaneous hepatic rupture associated with AL amyloidosis is a rare but often fatal complication. Several cases of hepatic rupture have been reported in the literature; this is the first report of hepatic rupture in a patient with AL amyloidosis undergoing treatment with high-dose melphalan and autologous stem cell transplantation.
Materials and methods
Case report
A 55-year-old woman first presented to the emergency department in July 2005 complaining of severe right-sided abdominal pain. Exploratory laparoscopy following several studies resulted in the discovery of a subcapsular hepatic contusion. Marked reduction in pain occurred with supportive management. However, the patient continued to experience hypertension despite appropriate medications. She was referred to a nephrologist to explore alternate causes of hypertension associated with proteinuria and nephrotic syndrome. A renal biopsy was performed in September 2005 showing amyloid deposits that stained positive with Congo red and immunohisto-chemistry showed staining with antibody to lambda light chains. She was subsequently diagnosed with light-chain (AL) systemic amyloidosis with renal involvement and referred to the Amyloidosis Treatment & Research Program at Boston University Medical Center.
Upon evaluation, the patient complained of dyspnea upon exertion, fatigue and weight loss of 45 pounds over the previous 6 months. The physical examination revealed mild hepatomegaly extending 2 cm below the right costal margin and peripheral edema of lower extremities. There was no ascites. An abdominal fat pad aspiration was positive for amyloid deposits. DNA from her bone marrow plasma cells was cloned and the monoclonal light chain was identified as a λ6. Serum and urine immunofixation electrophoresis (IFE) revealed a monoclonal IgA lambda gammopathy. The serum free light chain lambda level was slightly elevated at 34.1 mg/l (5.7–26.3 mg/l), with a normal free kappa level of 12.1 mg/l (3.3–19.4 mg/l) and kappa to lambda ratio of 0.36 (0.26–1.65). Early cardiac involvement was suspected due to a 12 mm interventricular septal thickness, a decreased voltage on ECG in the limb leads, and BNP of 202 pg/ml, although the ejection fraction was normal at 70%. Hepatic involvement was suspected due to hepatomegaly and history of hepatic contusion. Renal involvement was histologically confirmed. Laboratory findings are included in Table I. Therefore, this patient had AL amyloidosis with renal, liver and early cardiac involvement with an underlying plasma cell dyscrasia.
Table I.
Laboratory values.
| Laboratory studies | Patient baseline | Patient day 10 | Normal |
|---|---|---|---|
| Hemoglobin | 12.7 g/dl | 7.0 g/dl | 12–16 g/dl |
| Hematocrit | 38.7% | 19.9% | 38–47% |
| Platelets | 448 × 103/mcl | 15 × 103/mcl | 150–450 × 103/mcl |
| WBC | 11.6 × 103/mcl | 0.3 × 103/mcl | 4.0–11 × 103/mcl |
| INR | 0.88 | 1.15 | 0.8–1.4 |
| PTT | 32 s | 35 s | 27–3 8 s |
| Factor X | 128% | * | 75–150% |
| D-dimer | > 1.0 mcg/ml | * | <0.25 mcg/ml |
| Albumin | 3.7 g/dl | 2.4 g/dl | 3.5–5.0 g/dl |
| Alkaline phosphatase | 147 U/l | 76 U/l | 25–125 U/l |
| ALT | 36 U/l | 170 U/l | 1–45 U/l |
| AST | 35 U/l | 163 U/l | 7–42 U/l |
| Total bilirubin | 0.3 mg/dl | 3.0 mg/dl | 0.3–1.0 mg/dl |
| Creatinine | 1.2 mg/dl | 3.2 mg/dl | 0.8–1.2 mg/dl |
| Urine total protein | 8955 mg/24 h | * | < 150 mg/24 h |
WBC = white blood count; INR = international normalized ratio; PTT = partial thromboplastin time; ALT = alanine transaminase; AST = aspartate transaminase
Results unavailable.
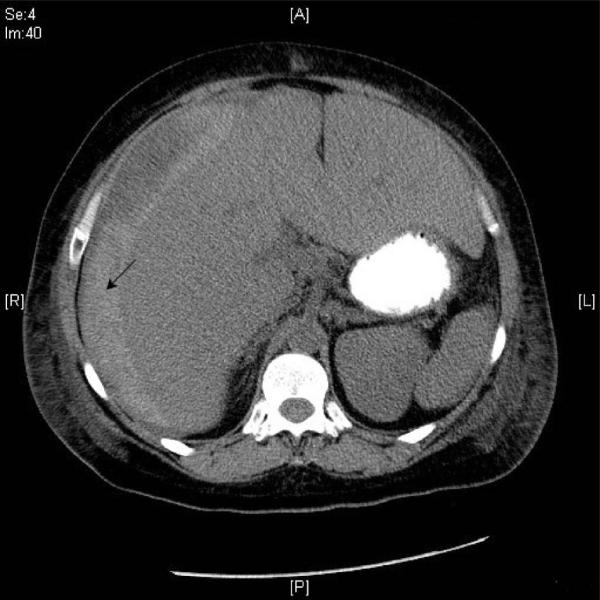
The patient underwent treatment with high-dose intravenous melphalan and autologous stem cell transplant (HDM/SCT). Peripheral blood stem cells were collected following GCSF mobilization and a total of 9.3 × 106 CD 34 + cells/kg were obtained. She received IV melphalan at 200 mg/m2 in two divided doses, the following week. She then received an autologous stem cell infusion of 5.58 × 106 CD34 + cells/kg 48 h later (Day 0). The following day, she was started on prophylactic medications to prevent nausea, vomiting, stomatitis, bacterial, fungal, and viral infections. She was hospitalized on D + 8 with a neutropenic fever and a red non-raised rash on her right thigh and posterior knee. Her status was complicated the next day when she developed septic shock from Escherichia coli bacteremia, acute renal failure, pancytopenia including a platelet count of 15,000/mm3 and diarrhea. The following day (Day + 10), she complained of severe abdominal pain and developed hematochezia. A CT scan was obtained which showed blood and fluid around the liver consistent with hepatic rupture (Figure 1). The surgical service concluded that she was not a candidate for surgery due to her neutropenia and sepsis. The patient's family expressed wishes for comfort measures only. The patient subsequently died three days later (Day + 13).
Figure 1.
Hyperattenuated area (arrow) indicates acute subcapsular hemorrhage of the liver.
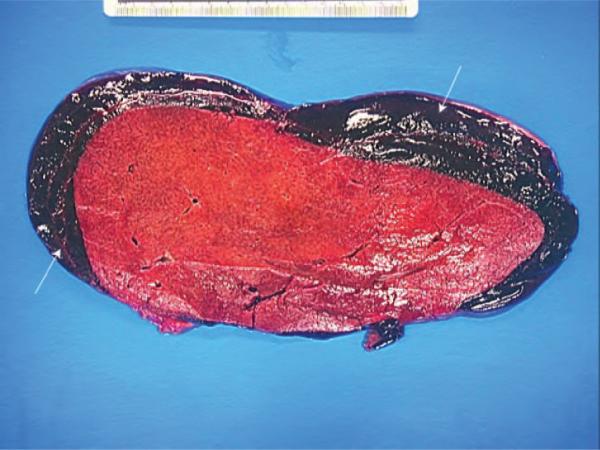
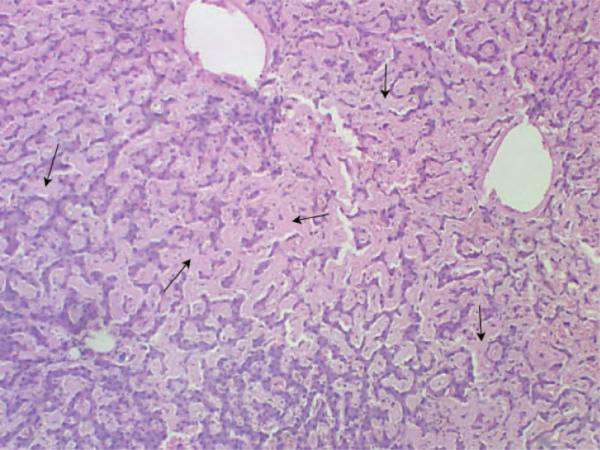
At autopsy, 3 l of bloody fluid were evacuated from the peritoneal cavity. The liver appeared grossly enlarged and weighed 5600 g (Figure 2). A massive subcapsular hematoma with capsular perforations in the right lobe was also noted. The liver showed widespread parenchymal and vascular amyloid infiltration (Figure 3). The heart was enlarged to 440 g. The left ventricular wall was noticeably thickened to 1.9 cm. The left and right kidneys were also enlarged and weighed 350 and 310 g, respectively. The cortical surface of both kidneys exhibited a granular appearance. Finally, the spleen also appears to have been enlarged, weighing 320 g.
Figure 2.
Liver at autopsy revealing hepatomegaly and subcapsular hematoma.
Figure 3.
Microscopic examination of the liver showing diffuse amyloid deposition on H&E stain.
Protein extraction and electrophoretic analysis
Amyloid fibrils were isolated from 20 g of liver tissue (05-135L) obtained at autopsy, using the classical water extraction method detailed previously [4]. This method involves repeated homogenization of the tissue in 0.9% NaCl to remove proteins noncovalently associated with the fibrils followed by four homogenizations with deionized H2O.
A portion of the lyophilized water-washed liver sample was analyzed by SDS-PAGE and immuno-blotting to assess size and light chain subtype (kappa or lambda). The approximate molecular weight of proteins was analyzed by horizontal SDS-PAGE on gradient 10–15% polyacrylamide gels using the automated Phast system (Amersham Biosciences) [5,6]. Protein bands were visualized with Coomassie blue stain. The identification of light chain subtype (lambda vs. kappa) was demonstrated by immunoblotting. Proteins were initially separated by SDS-PAGE, electrophoretically transferred to nitrocellulose, and then visualized by antibody-linked staining [7].
Results
Amyloid liver fibril protein characterization
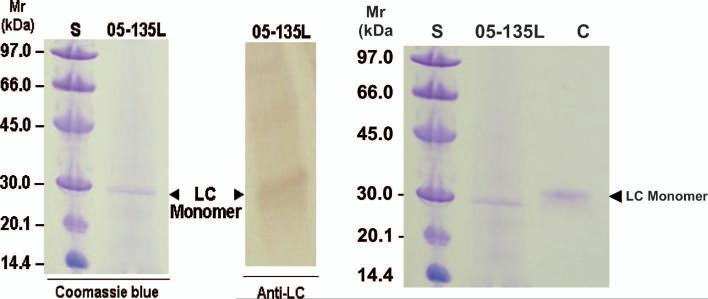
SDS-PAGE analysis of 05-135L liver-extracted fibrils demonstrated a major protein with an approximate molecular weight slightly less that 30 kDa (Figure 4). The corresponding immunoblot showed reactivity of the 30 kDa band to anti-human immunoglobulin lambda light chain antibodies. Periodic acid-Schiff (PAS) staining of a separate SDS-PAGE gel containing the 05-135L liver-extracted fibrils showed no staining of the 30 kDa band. This suggested that the fibril-deposited lambda light chain was not glycosylated.
Figure 4.
Electrophoretic analysis of a water-washed liver extraction sample (05-135L) performed under reducing conditions. Monomeric light chain (LC) of the lambda subclass is demonstrated by treatment with Commasie blue (left) and immunoblotting with polyclonal anti-human lambda LC antibodies (right). A low Mr standard (S) included phosphorylase b, Mr = 97,000, albumin, Mr = 66,000, ovalbumin, Mr = 45,000, carbonic anhydrase, Mr = 30,000, trypsin inhibitor, Mr = 20,100, and alpha-lactalbumin, Mr = 14,400.
Discussion
Liver rupture is often a fatal complication that is associated with various conditions such as trauma, pregnancy [8], anticoagulant therapy [9], connective tissue disorders [10], liver infiltrative diseases [11], graft-versus-host disease [12], and hepatocellular carcinomas [13]. There have only been a handful of reported cases where systemic AL amyloidosis is implicated as the cause for spontaneous liver rupture [14–22]. It has been fatal in all but three cases. Hepatic parenchymal and capsular infiltration with amyloid have been implicated as contributing factors to liver ruptures. Intrahepatically, amyloid has been found in the parenchyma in 100% of cases and in the vasculature in 68% of cases [23], possibly leading to structural weakening of the liver. This can lead to intrahepatic hemorrhaging with eventual capsular rupture. The degree of amyloid infiltration of the liver shows no distinct correlation with liver dysfunction. As such, liver involvement cannot be reliably detected with liver panel tests. In addition, hepatomegaly is also an unreliable indicator of liver involvement [24]. Liver biopsy still remains the definitive method in detecting liver involvement. However, care should be taken as up to 8% of patients may suffer from procedure related hemorrhaging [25].
In this patient, spontaneous liver contusion led to her diagnosis. Although she had a λ6 monoclonal protein, which is most frequently associated with renal amyloidosis [26], we and others have found that patients with λ6 can have other organ involvement. With few effective therapies for AL amyloidosis available at that time and improvement in liver dysfunction reported in 60% of patients after HDM/SCT, she was offered treatment with HDM/SCT. In addition to the underlying hepatic involvement with amyloid, other factors that may have contributed to her subsequent hepatic rupture and death may have included sepsis and thrombocytopenia. Severe sepsis, defined as sepsis with accompanying organ system failure, poses a difficult problem to manage and has a mortality rate of 28.6% and was the cause of death in 3% of patients completing HDM/SCT at our center prior to 2004 [27]. Currently, the preferred method of treatment of hepatic rupture is surgical intervention [28]. Conservative treatment was reported to be successful in some instances [29], but in one case, the patient suffered from a hepatic hematoma without rupture of the capsule [18]. Nonetheless, presenting feature of liver contusion should be considered as a relative contraindication for subsequent HDM/SCT.
Conclusion
Liver rupture has a poor prognosis and is an extremely rare complication of systemic AL amyloidosis. Causes for hepatic rupture are multi-factorial. It should be suspected in patients with AL amyloidosis and severe right upper quandrant pain, abdominal distention, guarding, and/or acute anemia or hypotension. Diagnosis is established by ultrasound or CT scanning. The mainstay of treatment is surgical, but mortality remains high.
Acknowledgements
This research was supported by grants from the National Institutes of Health (P01 HL68705), the Gerry Foundation, and the Amyloid Research Fund at Boston University. We thank Dr. Akira Murakami for providing the images of radiological studies and Dr. Carl O'Hara for autopsy images.
Abbreviations
- HDM/SCT
high-dose melphalan and autologous stem cell transplant
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden. The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
References
- 1.Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. N Engl J Med. 1997;337:898–909. doi: 10.1056/NEJM199709253371306. [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Gertz MA. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin Hematol. 1995;32:45–59. [PubMed] [Google Scholar]
- 3.Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349:583–596. doi: 10.1056/NEJMra023144. [DOI] [PubMed] [Google Scholar]
- 4.Skinner M, Shirahama T, Cohen AS, Deal CL. The association of amyloid P-component (AP) with the amyloid fibril: an updated method for amyloid fibril protein isolation. Prep Biochem. 1982;12:461–476. doi: 10.1080/10826068208070597. [DOI] [PubMed] [Google Scholar]
- 5.Weber K, Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969;244:4406–4412. [PubMed] [Google Scholar]
- 6.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 7.Jagersten C, Edstrom A, Olsson B, Jacobson G. Blotting from PhastGel media after horizontal sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Electrophoresis. 1988;9:662–665. doi: 10.1002/elps.1150091007. [DOI] [PubMed] [Google Scholar]
- 8.Nelson DB, Dearmon V, Nelson MD. Spontaneous rupture of the liver during pregnancy: a case report. J Obstet Gynecol Neonatal Nurs. 1989;18:106–113. doi: 10.1111/j.1552-6909.1989.tb00473.x. [DOI] [PubMed] [Google Scholar]
- 9.Dizadji H, Hammer R, Strzyz B, Weisenberger J. Spontaneous rupture of the liver. A complication of oral anticoagulant therapy. Arch Surg. 1979;114:734–735. doi: 10.1001/archsurg.1979.01370300088017. [DOI] [PubMed] [Google Scholar]
- 10.Ng SC, Muiesan P. Spontaneous liver rupture in Ehlers-Danlos syndrome type IV. J R Soc Med. 2005;98:320–322. doi: 10.1258/jrsm.98.7.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Croitoru AG, Hytiroglou P, Schwartz ME, Saxena R. Liver transplantation for liver rupture due to light chain deposition disease: a case report. Semin Liver Dis. 2006;26:298–303. doi: 10.1055/s-2006-947301. [DOI] [PubMed] [Google Scholar]
- 12.Barnett SJ, Weisdorf-Schindle S, Baker KS, Saltzman DA. Spontaneous liver rupture in a child with graft-versus-host disease. J Pediatr Surg. 2004;39:e1–e3. doi: 10.1016/j.jpedsurg.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 13.Lai EC, Lau WY. Spontaneous rupture of hepatocellular carcinoma: a systematic review. Arch Surg. 2006;141:191–198. doi: 10.1001/archsurg.141.2.191. [DOI] [PubMed] [Google Scholar]
- 14.Ooi LL, Lynch SV, Graham DA, Strong RW. Spontaneous liver rupture in amyloidosis. Surgery. 1996;120:117–119. doi: 10.1016/s0039-6060(96)80251-0. [DOI] [PubMed] [Google Scholar]
- 15.Bujanda L, Beguiristain A, Alberdi F, Cosme A, Ruíz de la Hermosa J, Gutiérrez-Stampa, Arenas JI. Spontaneous rupture of the liver in amyloidosis. Am J Gastroenterol. 1997;92:1385–1386. [PubMed] [Google Scholar]
- 16.Satue JA, Ortuño T, Carabias E, Cisneros C, Araque A, Fernandez Zatarain G, Morales JM. Fatal spontaneous liver rupture in a renal transplant patient with amyloidosis. Nephron. 1996;73:355–356. doi: 10.1159/000189084. [DOI] [PubMed] [Google Scholar]
- 17.Gastineau DA, Gertz MA, Daniels TM, Kyle RA, Bowie EJ. Inhibitor of the thrombin time in systemic amyloidosis: a common coagulation abnormality. Blood. 1991;77:2637–2640. [PubMed] [Google Scholar]
- 18.Levy-Lahad E, Steiner-Salz D, Berkman N, Chisin R, Levensart P, Leitersdorf E. Reversible functional asplenia and subcapsular liver hematoma—two distinctive manifestations of amyloidosis. Klin Wochenschr. 1987;65:1104–1107. doi: 10.1007/BF01736118. [DOI] [PubMed] [Google Scholar]
- 19.Okazaki K, Moriyasu F, Shiomura T, Yamamoto T, Suzaki T, Kanematsu Y, Akasaka S, Kobashi Y. Spontaneous rupture of the spleen and liver in amyloidosis—a case report and review of the literature. Gastroenterol Jpn. 1986;21:518–524. doi: 10.1007/BF02774637. [DOI] [PubMed] [Google Scholar]
- 20.Hurd WW, Katholi RE. Acquired functional asplenia. Association with spontaneous rupture of the spleen and fatal spontaneous rupture of the liver in amyloidosis. Arch Intern Med. 1980;140:844–845. doi: 10.1001/archinte.140.6.844. [DOI] [PubMed] [Google Scholar]
- 21.Kacem C, Helali K, Puisieux F. Recurrent spontaneous hepatic rupture in primary hepatic amyloidosis. Ann Intern Med. 1998;129:339. doi: 10.7326/0003-4819-129-4-199808150-00029. [DOI] [PubMed] [Google Scholar]
- 22.Mukhopadhya A, Raghuram L, Justus A, Joseph AJ, Eapen CE, Chandy GM. Transcatheter hepatic artery embolization for spontaneous rupture of amyloid liver. Indian J Gastroenterol. 2004;23:26–27. [PubMed] [Google Scholar]
- 23.Chopra S, Rubinow A, Koff RS, Cohen AS. Hepatic amyloidosis. A histopathologic analysis of primary (AL) and secondary (AA) forms. Am J Pathol. 1984;115:186–193. [PMC free article] [PubMed] [Google Scholar]
- 24.Park MA, Mueller PS, Kyle RA, Larson DR, Plevak MF, Gertz MA. Primary (AL) hepatic amyloidosis: clinical features and natural history in 98 patients. Medicine (Baltimore) 2003;82:291–298. doi: 10.1097/01.md.0000091183.93122.c7. [DOI] [PubMed] [Google Scholar]
- 25.Yood RA, Skinner M, Rubinow A, Talarico L, Cohen AS. Bleeding manifestations in 100 patients with amyloidosis. JAMA. 1983;249:1322–1324. [PubMed] [Google Scholar]
- 26.Comenzo RL, Zhang Y, Martinez C, Osman K, Herrera GA. The tropism of organ involvement in primary systemic amyloidosis: contributions of Ig V(L) germ line gene use and clonal plasma cell burden. Blood. 2001;98:714–720. doi: 10.1182/blood.v98.3.714. [DOI] [PubMed] [Google Scholar]
- 27.Skinner M, Sanchorawala V, Seldin DC, Dember LM, Falk RH, Berk JL, Anderson JJ, O'Hara C, Finn KT, Libbey CA, Wiesman J, Quillen K, Swan N, Wright DG. High-dose melphalan and autologous stem-cell transplantation in patients with AL amyloidosis: an 8-year study. Ann Intern Med. 2004;140:85–93. doi: 10.7326/0003-4819-140-2-200401200-00008. [DOI] [PubMed] [Google Scholar]
- 28.Chen ZY, Qi QH, Dong ZL. Etiology and management of hemmorrhage in spontaneous liver rupture: a report of 70 cases. World J Gastroenterol. 2002;8:1063–1066. doi: 10.3748/wjg.v8.i6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naito KS, Ichiyama T, Kawakami S, Kadoya M, Tabata T, Matsuda M, Ikeda S. AL amyloidosis with spontaneous hepatic rupture: successful treatment by transcatheter hepatic artery embolization. Amyloid J Protein Folding Dis. 2008;15:137–139. doi: 10.1080/13506120802006187. [DOI] [PubMed] [Google Scholar]