Abstract
Background:
Viscoelastic diagnostics that monitor the hemostatic function of whole blood (WB), such as thromboelastography, have been developed with demonstrated clinical utility. By measuring the cumulative effects of all components of hemostasis, viscoelastic diagnostics have circumvented many of the challenges associated with more common tests of blood coagulation.
Methods:
We describe a new technology, called sonorheometry, that adaptively applies acoustic radiation force to assess coagulation function in WB. The repeatability (precision) of coagulation parameters was assessed using citrated WB samples. A reference range of coagulation parameters, along with corresponding measurements from prothrombin time (PT) and partial thromboplastin time (PTT), were obtained from WB samples of 20 healthy volunteers. In another study, sonorheometry monitored anticoagulation with heparin (0 – 5 IU/ml) and reversal from varied dosages of protamine (0 – 10 IU/ml) in heparinized WB (2 IU/ml).
Results:
Sonorheometry exhibited low CVs for parameters: clot initiation time (TC1), < 7%; clot stabilization time (TC2), < 6.5%; and clotting angle (θ), < 3.5%. Good correlation was observed between clotting times, TC1 and TC2, and PTT (r = 0.65 and 0.74 respectively; n=18). Linearity to heparin dosage was observed with average linearity r > 0.98 for all coagulation parameters. We observed maximum reversal of heparin anticoagulation at protamine to heparin ratios of 1.4:1 from TC1 (P=0.6) and 1.2:1 from θ (P=0.55).
Conclusions:
Sonorheometry is a non-contact method for precise assessment of WB coagulation.
Keywords: acoustic radiation force, hemostasis, point-of-care, reference range, repeatability, sonorheometry
Introduction
Hemostasis, the physiological control of bleeding, is a complex process incorporating the vasculature, platelets, coagulation factors (FI-FXIII), fibrinolytic proteins, and coagulation inhibitors [1]. Disruption of hemostasis plays a central role in the onset of myocardial infarction, stroke, pulmonary embolism, and deep vein thrombosis [2,3]. Consequently, in vitro diagnostics (IVD) are critically needed to quantify hemostatic dysfunction and direct appropriate treatment. This need is particularly acute during cardiac surgeries requiring cardiopulmonary bypass (CPB), where post-surgical bleeding is a common complication requiring transfusion of blood products.
Existing IVDs include endpoint biochemical assays, platelet aggregation assays, and clot viscoelastic measurement systems. Endpoint biochemical assays such as the prothrombin time (PT) and the partial thromboplastin time (PTT) are widely used to assess coagulation. However, these tests measure only a part of the hemostatic process and operate under non-physiological conditions incorporating only the function of plasma. As a result of these limitations, complications such as postoperative bleeding often occur despite normal perioperative PT and PTT measurements [4].
Activated clotting time (ACT) is an endpoint assay that is most often applied in support of CPB. This assay applies strong initiation of the surface activation (intrinsic) pathway to quantify heparinization [1,5-7]. Limitations of the ACT include its disregard for platelet function, lysis, and coagulation kinetics along with the use of large aliquots of WB (generally 2 ml) and moving mechanical parts, which do not mimic physiologic conditions. For these reasons, the ACT is used for rapid assessment of heparinization and associated protamine reversal with limited utility for additional applications.
Platelets play a crucial role in the progression of pathological clotting of artificial surface devices and quelling arterial bleeding [8-10]. Furthermore, the modern cell-based theory of hemostasis [1] recognizes that platelets play a modulating role in coagulation. Platelet function is monitored clinically via both central lab assays [11] and point of care (POC) tests [12], which use anticoagulated WB. Both approaches are limited in that they use platelet aggregation as a proxy for overall platelet function. Furthermore, disabling coagulation, these methods neglect the interaction between platelets and the coagulation cascade.
Techniques that monitor the viscoelastic properties of whole blood (WB), such as thromboelastography (TEG), circumvent many of the limitations of endpoint biochemical assays and platelet aggregation assays by measuring the combined effects of all components of hemostasis [13]. TEG has been shown to diagnose hyperfibrinolysis in bleeding patients [14-15], indicate transfusion requirements better than standard biochemical assays [16-18], and reduce transfusion requirements during CPB when used with transfusion algorithms [19-24]. While these tests offer valuable clinical information, the devices are typically complex to operate and difficult to interpret. Moreover, the TEG applies relatively large shear strains, which transgress the non-linear viscoelastic regime, thereby disrupting clot formation [25-26]. For these reasons, the TEG sees very limited utility as a POC test [27].
Our group previously described sonorheometry as an ultrasound-based technique for measuring the viscoelastic properties of WB during coagulation [28]. In this paper we extend sonorheometry by incorporating an adaptive force technique. This method increases the dynamic range of stiffness measurements to nearly five orders of magnitude while maintaining low shear strains. In this paper, we show that sonorheometry can precisely quantify coagulation kinetics in WB. We quantify the coefficients of variability (CVs) for key sonorheometry coagulation parameters, establish a reference range of these parameters from 20 healthy volunteers, assess the linearity of sonorheometry response to heparin dose, and demonstrate that sonorheometry can be used to monitor the protamine reversal of heparin. While sonorheometry is capable of monitoring clot dissolution during lysis as well as platelet contributions to clotting strength [29], in this manuscript we focus on clot formation and kinetics.
Materials and Methods
Sonorheometry
Sonorheometry applies acoustic radiation force to induce small, localized displacements within the blood sample. These displacements are quantified using ultrasound motion tracking with measured displacements analyzed to determine viscoelastic properties [30]. Since sonorheometry applies a body force generated by propagating ultrasound [31,32], our technique does not require direct contact between the transducer and the blood sample.
In prior work, we applied a fixed force similar to the TEG, which limited the range of stiffness measurements we could detect while operating at low strain levels [25-26,28]. To overcome this challenge, our lab has developed an adaptive force technique, which can be briefly described as follows: maximum and minimum displacement thresholds are preset in our custom designed software. If the maximum displacement threshold is breached, then the pulse interval (ΔT) (from Fig. 1a) is increased for the next acquisition to reduce strain. Similarly, if displacement is below the minimum threshold, then ΔT is decreased for the next acquisition. Using this method, sonorheometry can operate at low strains with a dynamic range of stiffness measurements of approximately five orders of magnitude.
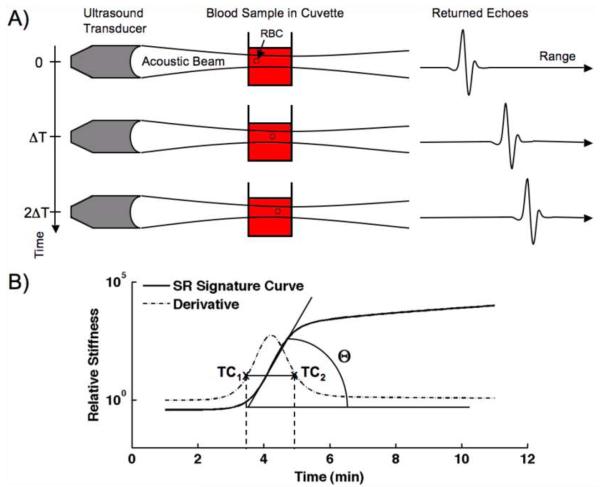
Fig. 1.
(a) Depiction of the sonorheometry mechanism. An ultrasound transducer generates acoustic radiation force, which is incident upon a 1 ml sample of whole blood. Resulting displacements are evident as shifts in the returning echoes. (b) Diagram of the sonorheometry signature curve with coagulation parameters clot initiation time (TC1), clot stabilization time (TC2), and clotting angle (θ).
As illustrated in Fig. 1a, sonorheometry applies multiple acoustic pulses to displace the blood sample. This technique is used to quantify relative stiffness by computing the applied force, which is adaptively set by specifying ΔT, and the estimated displacement. The relative stiffness, S, of the blood sample is then defined:
| (1) |
where d is displacement, PRF is pulse repetition frequency (or 1/ΔT), and Φ is a constant that incorporates sample geometry and the contributions of absorption, speed of sound, and temporal averaged intensity to the applied radiation force. Sonorheometry measurements are repeated once every 6 seconds to render information pertaining to the formation of a blood clot and these measurements are collected to form a characteristic sonorheometry curve (Fig. 1b).
The 3 coagulation parameters investigated in this paper are illustrated in Figure 1B. The parameter TC1 is termed the “clot initiation time” parameter and indicates an initial generation of fibrin. This is similar to the TEG R time. Likewise, TC2 is termed the “clot stabilization time” and is related to the sum of TEG R time and K time. Both sonorheometry parameters are determined from the derivative of the characteristic curve; the parameters are the times corresponding to 20% of the maximum of the derivative. The clotting angle, θ, is indicated by the maximum angle of the characteristic curve during coagulation and can be determined from the peak of the derivative. This parameter is related to the TEG α parameter. Other parameters indicating fibrin polymerization rate, such as clotting slope, can be extracted from the data. For comparative purposes, we focused on clotting angle in this paper.
In this work, the relative stiffness curves represent the fit of the raw sonorheometry data to a modified sigmoidal function, which facilitated parameter extraction. In general, the fit between sonorheometry data and the sigmoidal fit had a correlation greater than 0.995. The modified sigmoidal function is described in detail in Appendix I.
Sonorheometry experiments were performed using a single element piston transducer with a 1 cm aperture, a 4 cm fixed focus, and a center frequency of 10 MHz with roughly 50% fractional bandwidth (Olympus NDT Inc., Waltham, MA). Acoustic radiation force was applied using 16 cycle acoustic pulses with PRFs between 25 Hz and 12.8 kHz. For each experiment, a 1 ml blood sample was placed in a polystyrene cuvette. The cuvette was placed in a water bath that maintained a temperature of 37°C. This bath held the sample at body temperature and provided a propagation medium for the ultrasound. Temperature equilibrium of the blood sample within 3°C occurred within approximately 2 min. An external laptop computer controlled custom circuitry via a USB 2.0 connection. Received echoes were amplified, filtered, digitized at 65 MHz, and then processed in MATLAB (MathWorks Inc., Natick, MA) to display the sonorheometry characteristic curve in real-time.
Study Design
Blood samples were collected by venipuncture into 1.8 ml citrated Vacutainers™ (Becton Dickinson, Franklin Lakes, NJ) containing 3.2% (0.105M) sodium citrate. Plasma was obtained by centrifuging blood samples at 2000 × g for 10 min at 4°C and stored at −80°C until PTT and PT analysis. The first tube of citrated WB was always discarded. Remaining tubes were placed on a rocker and analyzed via sonorheometry starting 30 min after collection. These studies were approved by the Investigational Review Board at the University of Virginia.
Five volunteers with no history of hemostatic disorder participated in our repeatability experiments; three subjects were male (ages 23, 23, and 30 y) and 2 subjects were female (22 and 24 y). For each subject, WB samples were collected into 11, 3.2% sodium citrate tubes. The samples were analyzed by first placing a 1 ml aliquot of citrated WB into a 4 ml polysytrene cuvette. Next, 62 μL of 0.2 M CaCl2 with 100 μL of 0.5% (w/v) kaolin (Mallinckrodt Backer Inc., Phillipsburg, NJ) in sterile sodium chloride solution (Becton Dickinson, Franklin Lakes, NJ) was added to reverse sodium citrate anticoagulation and to stimulate coagulation through activation of the surface activated pathway. Sonorheometry was initiated 1 min later, with measurements performed every 6 sec for a total observation time of 11 min. During the 1 min period before sonorheometry was initiated, the sample was inverted 5 times and placed into a water bath held at 37°C. The sample was situated with its center at the transducer focus. This procedure was repeated 10 times for each subject. The sonorheometry parameters TC1, TC2, and θ were analyzed to assess the repeatability of our technique.
Further experiments were performed to assess a reference range for sonorheometry coagulation parameters in healthy subjects and to test the hypothesis that sonorheometry clotting times (TC1 and TC2) were correlated with PTT. Twenty healthy volunteers [10 male (aged 26.2 ± 5.5 y), 10 female (24.0 ± 2.7 y)] participated in the study. WB samples were collected into 5, 3.2% sodium citrate tubes. The first tube was discarded while 2 others were centrifuged to collect plasma for PTT and PT analysis on a Beckman-Coulter ACL TOP® coagulation analyzer (Beckman-Coulter, Inc., Fullterton, CA). PTT and PT analysis was performed in the Core Laboratory of the University of Virginia Health System following clinical guidelines. An identical procedure was followed for sonorheometry measurements in these experiments as outlined above for repeatability assessment.
Sonorheometry was performed on citrated WB samples from five healthy subjects [3 male (ages 24, 24, 31 y), 2 females (18 and 24 y)] to assess the linearity of sonorheometry to heparin dose. WB samples were collected into 6, 3.2% sodium citrate tubes. Control experiments were performed by adding 62 μl of 0.2 mol/l CaCl2 with 100 μl of 7.0% (w/v) kaolin in sterile sodium chloride solution to a 1 ml aliquot of citrated WB. The additional blood samples were dosed with 1-5 IU heparin/ml (American Pharmaceutical Partners, Inc., Schaumburg, IL).
Experiments were also performed to test the hypothesis that sonorheometry can detect anticoagulation reversal from incremental dosages of protamine. Five healthy subjects volunteered for these experiments [3 male (ages 23, 30, and 40), 2 female (ages 20 and 24)]. The same procedure for sonorheometry analysis was executed as outlined above. The control sample contained no heparin or protamine. The additional blood samples were analyzed using the following procedure: 1 ml of heparinized blood was pipetted into a 4 ml polystyrene cuvette, titrated quantities of protamine (Sigma Chemical Co., St. Louis, MO) was pipetted into the sample at dosages 0, 1.2, 1.6, 2, 2.4, 2.8, 5, and 10 IU. After 10 min of incubation, 62 μl of 0.2 mol/l CaCl2 with 100 μl of 7.0% (w/v) kaolin was added to the sample. Sonorheometry was initiated 1 min later. During the 1 min prior to sonorheometry initiation, samples were inverted 5 times and placed into the sample water bath held at 37°C. Sodium chloride solution was added to maintain constant sample volume across experiments.
Data Analysis
Signal processing of returned echoes, parameter estimation, and statistical comparison were performed in MATLAB. Descriptive statistics are reported as mean ± standard deviation (SD) for parametric and median ± interquartile range (IQR) for non-parametric data. An unpaired, 2-tailed t-test was used to assess the significance of differences between observed variables when data was parametric. When the data was non-parametric, a Mann-Whitney U-test was used. In all instances a P< 0.05 was considered significant. The Kolmogorov-Smirnov test was used to assess normality. Reference ranges for coagulation parameters are determined according to standard guidelines by calculating the 2.5 and 97.5% percentiles [27]. Least squares linear regression analysis was used to estimate a linear relationship between coagulation parameters with PTT results and heparin dosage. Subjects with PTT values outside the standard normal range (PTT 25-39 sec) were excluded from data used to establish a reference range of sonorheometry parameters.
Results
The effects of system variability on sonorheometry coagulation parameters
The CVs for each parameter (TC1, clot initiation time; TC2, clot stabilization time; and θ, clotting angle) were determined over 10 sonorheometry measurements from each individual subject when clotting was initiated via the surface activated (intrinsic) pathway. The results were: TC1 5.7% (range 3.1-6.8%), TC2 5.5% (3.90-6.1) and θ 1.8 (1.6-3.2) indicating clotting kinetics.
The effects of intrasubject variability on sonorheometry coagulation parameters and the relationship to conventional biochemical assays, the PTT and PT
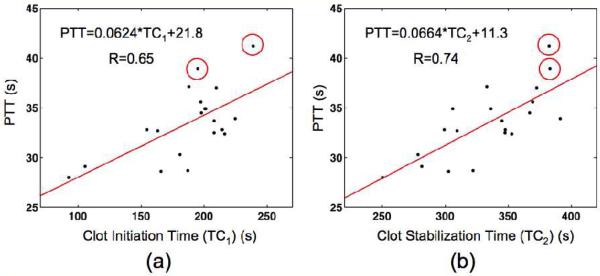
Sonorheometry measurements were preformed on WB from 20 healthy subjects with coagulation activated via the surface activated pathway. The mean and median values with SD and IQR intervals are reported in Table 1 for coagulation parameters from 18 of 20 healthy subjects. The Core Laboratory flagged the two subjects not included in the reference range as outside the normal range. Also listed in Table 1 are the mean with SD intervals and range of PT and PTT values from these same 18 subjects. There was no significant difference between male and female subjects. The data exhibits good correlation between sonorheometry clotting times, TC1 and TC2, and measures from PTT with correlation values of 0.65 and 0.74 respectively. Scatter plots of the data with linear regression fits are illustrated in Figure 2. There was no correlation (R<0.5) between clotting angle and PTT or any sonorheometry coagulation parameter and the PT test. Circles are shown around data points for two subjects who exhibited PTT results above the normal range as determined by the Core Laboratory.
Table 1.
Observed coagulation parameters from sonorheometry (TC1, TC2 and Θ), PT, and PTT (n=18)
| Parameter | Mean ± SD | Reference Range |
|---|---|---|
| TC1 (min) | 3.07 ± 0.61 | 1.54 - 3.75 |
| TC2 (min) | 5.47 ± 0.63 | 4.17 - 6.52 |
| PT (s) | 15.1 ± 1.9 | 13.1 - 21.6 |
| PTT (s) | 32.8 ± 2.8 | 28.0 - 37.1 |
|
| ||
| Parameter | Median ± IQR | Reference Range |
|
| ||
| Θ (degrees) | 69.28 ± 3.26 | 65.27 - 75.29 |
Fig. 2.
PTT test results and sonorheometry measurements were obtained from citrated WB samples of 20 healthy subjects. Correlation values and linear regression analysis are shown for PTT versus sonorheometry parameters (a) clot initiation time, TC1, and (b) clot stabilization time, TC2. Lines indicate the least squares best-fit line through the data. Circles mark two subjects who were flagged by the Core Laboratory at the University of Virginia as having high PTT test results.
The effects of heparin on sonorheometry coagulation parameters
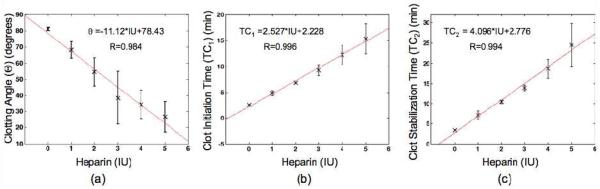
Linearity between heparin dosages of 0-5 IU was observed for θ, TC1, and TC2. Plots of sonorheometry parameter values versus heparin dose are illustrated in Fig. 3. Error bars indicate IQR from the median (Fig. 3a; clotting angle θ) or one standard deviation from the mean (Fig. 3b-c; clotting times TC1 and TC2). Correlations between heparin dose and median θ or average TC1 and TC2 over 5 subjects were 0.984, 0.996, and 0.994 respectively.
Fig. 3.
Correlation values and linear regression analysis (n=5) are shown for (a) clotting angle ϴ (median ± IQR), (b) clot initiation time TC1 (mean ± SD), (c) and clot stabilization time TC2 (mean ± SD) versus heparin dosage.
The effects of protamine on heparinized WB as measured by sonorheometry coagulation parameters
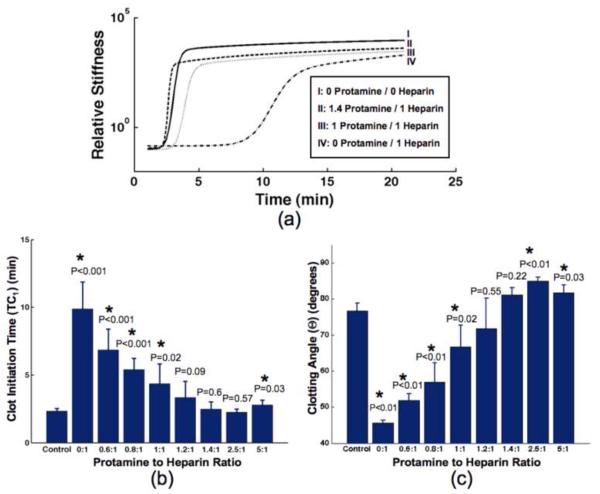
Protamine was added to heparinized blood from healthy subjects to give final protamine to heparin ratios between 0 and 5. The coagulation parameters from sonorheometry were compared against a control where no heparin or protamine was added to 1 ml of WB. Sample sonorheometry characteristic curves, which were fit to a modified sigmoidal function with > 0.995 correlation, are shown in Figure 4a. Heparin anticoagulation reversal was seen at protamine to heparin ratios of 1.2:1, 1.4:1, and 2.5:1 according to the sonorheometry TC1 parameter with maximum reversal at 1.4:1 (Fig. 4b). According to sonorheometry θ parameter, heparin anticoagulation was reversed at protamine to heparin ratios of 1.2:1 and 1.4:1 with maximum reversal at 1.2:1 (Fig. 4c). At all other ratios, clot initiation time and clotting angle were statistically different from the control with P<0.05.
Fig. 4.
(a) Sonorheometry characteristic curves from a single volunteer are displayed for varied dosages of heparin and protamine: (I) Control; no protamine, no heparin (II) 1.4:1; 2.8 IU protamine, 2 IU heparin/ml blood (III) 1:1; 2 IU protamine, 2 IU heparin/ml blood (IV) 0:1; 0 IU protamine, 2 IU heparin/ml blood. (b) Clot initiation times, TC1, and (c) clotting angle, θ, are illustrated versus protamine to heparin dosage ratios (n=5). At all protamine to heparin ratios, concentration of heparin is 2 IU/ml blood. Results are expressed as mean ± SD (TC1; 4b) or median ± IQR (θ; 4c). Significance values (P values) were calculated for each protamine to heparin ratio in comparison to the control. *P<0.05 versus control using unpaired, 2-tailed t-test (TC1; 4b) or Mann-Whitney U-test (θ; 4c).
Discussion
Monitoring of blood coagulation prior to and during surgeries, such as CPB, is a critical step to better understand the causes of hemorrhage, guide hemostatic treatment, and predict risk associated with bleeding [27]. Moreover, the Society of Thoracic Surgeons and Society of Cardiovascular Anesthesiologists have expressed the need for transfusion of blood products to be guided by point-of-care devices that assess hemostatic function accurately and in a timely manner [4]. Devices that monitor the viscoelastic properties of WB during hemostasis, such as TEG, have demonstrated clinical utility in monitoring blood disorders and directing transfusion requirements. However, the uncontrolled and large strains applied along with the complexities of these devices make them difficult to use and interpret [25-26]. As a result, these devices have been moved into the central laboratory in some hospitals [27]. In contrast, adaptive force sonorheometry has advantages that include non-contact measurements with low strain, no moving mechanical parts, and high precision over a large dynamic range.
All sonorheometry experiments reported in this paper were performed on recalcified, citrated whole blood. As reported by several studies, citrate affects the coagulability of whole blood with respect to native samples [33,34]. It has been demonstrated, for instance, that citrated samples do not prevent significant thrombin generation from occurring, which results in shortened TEG clotting times and increased TEG clotting angle. However, it has also been demonstrated that samples are relatively stable if recalcified in the time period between approximately 30 minutes and 8 hours. This was the time frame over which experiments were performed for this study. While we do not expect that our results match those expected for native blood samples, we do expect that they are consistent for citrated samples incubated at room temperature for between 30 minutes and 8 hours before experimentation.
In the repeatability study, we examined the precision of sonorheometry in controlled tests. The range of CVs for these parameters showed good precision for clot initiation time, TC1, and clot stabilization time, TC2, parameters with CVs < 7%. Excellent precision was observed for the clotting angle parameter, θ, with CVs < 3.5%. The range of CVs for sonorheometry coagulation parameters was on the lower end of typical CVs seen for comparable TEG parameters. For instance, the CV range in sonorheometry for TC1 was 3.06-6.78% compared with CVs of 3-12% for the R, or clotting time, parameter in TEG when the intrinsic pathway was initiated [35]. The precision of sonorheometry also compares favorably to other techniques that monitor coagulation kinetics, such as the PT, PTT, and the (ACT), which typically exhibit CVs between 5-10% [35-38].
A reference range of sonorheometry coagulation parameters was established using WB from 20 healthy volunteers. Two subjects, despite reporting themselves as healthy individuals who had not taken any medication that could interfere with clotting, had test result values that fell outside the standard normal range for PTT and were excluded from the reported reference range (Table 1). The SD or IQR along with the reference range of values observed from sonorheometry was comparable to previous studies performed using TEG [35,39]. Differences in parameter values and ranges may be accounted for by variations in kaolin concentration used in clotting assays and dissimilar techniques used to measure the mechanical properties of the forming clot.
We observed a good correlation between sonorheometry clotting times, TC1 and TC2, and the PTT test with correlation values of 0.65 and 0.74 respectively. This correlation was expected because sonorheometry was performed when the intrinsic pathway was initiated and PTT examines this same coagulation pathway. It is likely that correlation values were not closer to unity because of the within run variability in both instruments with CVs < 7% for sonorheometry and CVs 5-10% for the PTT. Further, the PTT uses only plasma and excludes non-plasma components in WB, which influence sonorheometry clotting times.
Sonorheometry was used to determine the linearity between coagulation parameters and heparin. As illustrated in Figure 3, not only were the clotting times TC1 and TC2 highly linear with heparin, but the clotting angle was also highly linear. A linear model was chosen since it is the least complex model with an acceptably high correlation coefficient (> 0.98) between the data and the model. However, the relationship between sonorheometry clotting parameters and heparin concentration is no longer linear above clinically relevant heparin concentrations, which we have observed from our experiments and others have observed from studies with the ACT [7]. To model the behavior of sonorheometry coagulation parameters over a large range of heparin dosages would require a higher order model. The measurements taken for heparin concentrations between 0 and 5 IU indicate that increased heparin dosages delay the time to the beginning of fibrin formation and also decrease the rate at which the crosslinked fibrin clot is formed. The high correlations between sonorheometry coagulation parameters and heparin dosage are comparable with literature values seen from the TEG and ACT [5,7,40].
Heparin anticoagulation reversal was quantified using the clotting time parameter TC1 and clotting angle parameter θ. For brevity, TC2 was neglected although similar trends were observed. Sample characteristic sonorheometry curves from one of the five volunteers for this study are illustrated in Figure 4a. Two primary conclusions are illustrated from these curves. First, the time of the initial uptrend in the heparin curves (II, III, and IV), as measured by TC1, do not match the time seen in the control curve (I) until a protamine to heparin ratio of 1.4:1, which corresponds to curve II. Second, the maximum slope of the control curve, quantified by θ, appears to match most closely with the maximum slope of curve III, which has a protamine to heparin ratio of 1.2:1. It is clear that the maximum slope of curve II is greater than the slope of the control curve. These trends that are visually apparent in the sample curves are summarized in Figs. 4b and 4c. According to the clotting time parameter, heparin anticoagulation was maximally reversed at a protamine to heparin ratio of 1.4:1 (Fig. 4b) while the clotting angle indicates maximum reversal at a ratio of 1.2:1 (Fig. 4c). It is likely that the actual ratio of maximum anticoagulation reversal is in between the ratios of 1.2:1 and 1.4:1, which is in good agreement with past literature that indicated maximum reversal at a ratio of 1.3:1 using the ACT with a kaolin assay [40-41].
The data in Figure 4 strongly suggest the potential for sonorheometry to become an appropriate method for monitoring protamine reversal of heparin anticoagulation. Both clotting time and clotting angle show a statistically significant lack of anticoagulation reversal at ratios <1.2:1. Only the clotting angle parameter indicated excess protamine at a ratio of 2.5:1, which is not indicated in the time to clot data. Since excess protamine has been shown to weaken clot structure and decrease platelet function, it is important to differentiate excess protamine from residual heparin anticoagulation [41]. As with the ACT, decreased clotting times may be misinterpreted as residual heparin anticoagulation in clinical applications such as monitoring bleeding after cardiopulmonary bypass (CPB). However, our data show that the sonorheometry clotting angle increases with excess protamine. These findings indicate that sonorheometry will differentiate inadequately high dosages of protamine, which is currently not detected with current methods such as the ACT.
While this paper has addressed several essential questions regarding the utility of sonorheometry and its correlation to standard assays, important clinical and technical questions remain. In previous work we addressed the effects of platelet adhesive function on sonorheometry parameters [29]. However, the effects of particulate concentrations of platelets, red blood cells, and white blood cells have yet to be established. Other clinical questions include the possible correlation between coagulation parameters and release of prothrombin activation fragment (F1.2), as well as the correlation between the area under the curve from TC1 to TC2 with total thrombin generation. Key technical developments for sonorheometry instrumentation include a means of estimating acoustic attenuation. This feature will allow for estimation of absolute mechanical properties, such as Elastic Modulus, and will ensure that sonorheometry can account for differences in hematocrit between subjects.
In conclusion, we have demonstrated that adaptive force sonorheometry is a non-contact method for rapid quantification of coagulation kinetics in WB. The CVs for sonorheometry coagulation parameters are low (< 7%) and compare favorably to existing technologies such as the ACT, PT, PTT, and TEG. A reference range for sonorheometry coagulation parameters was provided from a sample of 18 healthy volunteers, which may serve as a guideline for future studies aimed at detecting abnormal coagulation. Furthermore, we have demonstrated that sonorheometry coagulation parameters respond with high linearity to doses of heparin between 0-5 IU. Our data suggests that sonorheometry may be an appropriate technique for monitoring heparin concentration along with heparin anticoagulation reversal from dosages of protamine.
Acknowledgements
We gratefully acknowledge funding from the National Institutes of Health (NIH) / National Institute of Biomedical Imaging and Bioengineering (NIBIB) grant R01 EB005433-01 and the Wallace H. Coulter Translational Partnership Award at the University of Virginia.
List of Abbreviations
- WB
whole blood
- PT
prothrombin time
- PTT
partial thromboplastin time
- IVD
in vitro diagnostics
- CPB
cardiopulmonary bypass
- ACT
activated clotting time
- POC
point of care
- TEG
thromboelastography
- CVs
coefficient of variability
- ΔT
pulse interval
- IRB
Investigational Review Board
- SD
standard deviation
- IQR
interquartile range
Appendix I
A modified sigmoidal function was fit to raw sonorheometry characteristic curve data. Coagulation parameters were then extracted from the fitted model and the derivative of the fitted model. The modified sigmoidal function is given by:
| (2) |
where t is experimental time and α, β, γ, δ, and ε are constants. Constants were found using an unconstrained nonlinear optimization fit to raw data in MATLAB called fminsearch. The derivative of eqn (2) with respect to t is:
| (3) |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: We would like to disclose that Dr. Viola, Dr. Walker, and Dr. Lawrence, all of whom are co-authors of this manuscript, are also co-founders of HemoSonics, LLC, a start-up company formed to bring to market diagnostic instruments based on sonorheometry.
References
- 1.Hoffman M, Monroe DM. A cell-based model of hemostasis. Thromb Haemost. 2001;85:958–65. [PubMed] [Google Scholar]
- 2.Hoyert DL, Kung HC, Smith BL. Deaths: preliminary data for 2003. National Vital Statistics Reports. 2005;53:1–48. [PubMed] [Google Scholar]
- 3.Hambleton J, Leung LL, Levi M. Coagulation: consultative hemostasis. Hematology Am Soc Hematol Educ Program. 2002;1:335–52. doi: 10.1182/asheducation-2002.1.335. [DOI] [PubMed] [Google Scholar]
- 4.Ferraris VA, Ferraris SP, Saha SP, Hessel EA, Haan CK, Royston BD, Bridges CR, Higgins RSD, Despotis G, Brown JR, Spiess BD, Shore-Lesserson L, Stafford-Smith M, Mazer CD, Bennett-Guerrero E, Hill SE, Body S. Perioperative blood transfusion and blood conservation in cardiac surgery: the society of thoracic surgeons and the society of cardiovascular anesthesiologists clinical practice guideline. Ann Thorac Surg. 2007;83:S27–86. doi: 10.1016/j.athoracsur.2007.02.099. [DOI] [PubMed] [Google Scholar]
- 5.Cohen JA. Activated coagulation time method for control of heparin is reliable during cardiopulmonary bypass. Anesthesiology. 1984;60:121–4. doi: 10.1097/00000542-198402000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Slight RD, Buell R, Nzewi OC, McClelland DBL, Mankad PS. A comparison of activated coagultion time-based techniques for anticoagulation during cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2008;22:47–52. doi: 10.1053/j.jvca.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Despotis GJ, Alsoufiev AL, Spitznagel E, Goodnough LT, Lappas DG. Response of kaolin act to heparin: evaluation with an automated assay and higher heparin doses. Ann Thorac Surg. 1996;61:795–9. doi: 10.1016/0003-4975(95)00821-7. [DOI] [PubMed] [Google Scholar]
- 8.Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2008;357:2482–94. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 9.Sheppeck R, Bentz M, Dickson C, Hribar S, White J, Janosky J, Berceli S, Borovetz H, Johnson P. Examination of the roles of glycoprotein Ib and glycoprotein IIb/IIIa in platelet deposition on an artificial surface using clinical antiplatelet agents and monoclonal antibody blockade. Blood. 1991;78:673–80. [PubMed] [Google Scholar]
- 10.Rodvien R, Mielke C. Role of platelets in hemostasis and thrombosis. West J Med. 1976;125:181–6. [PMC free article] [PubMed] [Google Scholar]
- 11.Cox D. Methods for monitoring platelet function. Am Heart J. 1998;135:S160–9. doi: 10.1016/s0002-8703(98)70244-3. [DOI] [PubMed] [Google Scholar]
- 12.Harrison P, Segal H, Blasbery K, Furtado C, Silver L, Rothwell PM. Screening for aspirin responsiveness after transient ischemic attack and stroke: comparison of 2 point-of-care platelet function tests with optical aggregometry. Stroke. 2005;36:1001–5. doi: 10.1161/01.STR.0000162719.11058.bd. [DOI] [PubMed] [Google Scholar]
- 13.Hartert H. Blutgerinnungstudien mit der Thrombelastographie, einem neuen Untersuchungsverfahren. Klin Wochenschrift. 1948;26:557–83. doi: 10.1007/BF01697545. [DOI] [PubMed] [Google Scholar]
- 14.Luddington RJ. Thrombelastography/thromboelastometry. Clin Lab Haematol. 2005;27:81–90. doi: 10.1111/j.1365-2257.2005.00681.x. [DOI] [PubMed] [Google Scholar]
- 15.Spiel AO, Mayr FB, Firbas C, Quehenberger P, Jima B. Validation of rotation thrombelastography in a model of systemic activation of fibrinolysis and coagulation in humans. J Thromb Haemost. 2006;4:411–6. doi: 10.1111/j.1538-7836.2006.01715.x. [DOI] [PubMed] [Google Scholar]
- 16.Plotkin AJ, Wade CE, Jenkins DH, Smith KA, Noe JC, Park MS, Perkins JG, Holcomb JB. A reduction in clot formation rate and strength assessed by thrombelastography is indicative of transfusion requirements in patients with penetrating injuries. J Trauma. 2008;64:S64–8. doi: 10.1097/TA.0b013e318160772d. [DOI] [PubMed] [Google Scholar]
- 17.Schreiber MA, Differding J, Thorborg P, Mayberry JC, Mullins RJ. Hypercoagulability is most prevalent early after injury and in female patients. J Trauma. 2005;58:475–81. doi: 10.1097/01.ta.0000153938.77777.26. [DOI] [PubMed] [Google Scholar]
- 18.Essell JH, Martin TJ, Salinas J, Thompson JM, Smith VC. Comparison of thromboelastography to bleeding time and standard coagulation tests in patients after cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 1993;7:410–5. doi: 10.1016/1053-0770(93)90161-d. [DOI] [PubMed] [Google Scholar]
- 19.Shore-Lesserson L, Manspeizer HE, DePerio M, Francis S, Vela-Cantos F, Ergin MA. Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth Analg. 1999;88:312–319. doi: 10.1097/00000539-199902000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Anderson L, Quasim I, Soutar R, Steven M, Macfie A, Korte W. An audit of red cell and blood product use after the instiution of thromboelastometry in a cardiac intensive care unit. Transfus Med. 2006;16:31–39. doi: 10.1111/j.1365-3148.2006.00645.x. [DOI] [PubMed] [Google Scholar]
- 21.Spalding GJ, Hartrumpf M, Sierig T, Oesberg N, Kirschke CG, Albes JM. Cost reduction of perioperative coagulation management in cardiac surgery: value of ‘bedside’ thrombelastography (ROTEM) Eur J Cardiothorac Surg. 2007;31:1052–1057. doi: 10.1016/j.ejcts.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 22.Avidan MS, Alcock EL, Da FJ, Ponte J, Desai JB, Despotis GJ, Hung BJ. Comparison of structured use of routine laboratory tests or near-patient assessment with clinical judgement in the management of bleeding after cardiac surgery. Br J Anaesth. 2004;92:178–186. doi: 10.1093/bja/aeh037. [DOI] [PubMed] [Google Scholar]
- 23.Miller BE, Guzzetta NA, Tosone SR, Levy JH. Rapid evaluation of coagulopathies after cardiopulmonary bypass in children using modified thromboelastography. Anesth Analg. 2000;90:1324–1330. doi: 10.1097/00000539-200006000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Williams GD, Bratton SL, Riley EC, Ramamoorthy C. Coagulation tests during cardiopulmonary bypass correlate with blood loss in children undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 1999;13:398–404. doi: 10.1016/s1053-0770(99)90210-0. [DOI] [PubMed] [Google Scholar]
- 25.Evans PA, Hawkins K, Williams PR, Williams RL. Rheometrical detection of incipient blood clot formation by fourier transform mechanical spectroscopy. J Non-Newtonian Fluid Mech. 2008;148:122–126. [Google Scholar]
- 26.Burghardt WR, Goldstick TK, Leneschmidt J, Kempka K. Nonlinear viscoelasticity and thromboelastography. 1. Studies on bovine plasma clots. Biorheology. 1995;32:621–630. doi: 10.1016/0006-355X(95)00041-7. [DOI] [PubMed] [Google Scholar]
- 27.Ganter MT, Hofer CK. Coagulation monitoring: current techniques and clinical use of viscoelastic point-of-care coagulation devices. Anesth Analg. 2007;106:1366–1375. doi: 10.1213/ane.0b013e318168b367. [DOI] [PubMed] [Google Scholar]
- 28.Viola F, Kramer MD, Lawrence MB, Oberhauser JP, Walker WF. Sonorheometry: a non-contact method for dynamic assessment of thrombosis. Annals of Biomedical Engineering. 2004;32:696–705. doi: 10.1023/b:abme.0000030235.72255.df. [DOI] [PubMed] [Google Scholar]
- 29.Viola F, Mauldin FW, Lin-Schmidt X, Haverstick D, Lawrence M, Walker WF. A novel ultrasound-based method to evaluate hemostatic function of whole blood. Clinica Chimica Acta. 2010;411:106–113. doi: 10.1016/j.cca.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mauldin FW, Viola F, Walker WF. Reduction of echo decorrelation via complex principal component filtering. Ultrasound Med Biol. 2009;35:1325–1343. doi: 10.1016/j.ultrasmedbio.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torr GR. The acoustic radiation force. Am J Phys. 1984;52:402–408. [Google Scholar]
- 32.Nyborg W. Acoustic streaming. In: Mason W, editor. Physical Acoustics. IIB. Academic; New York: 1965. pp. 265–331. [Google Scholar]
- 33.Camenzind V, Bombeli T, Seifert B, Jamnicki M, Popovic D, Pasch T, Spahn DR. Citrate storage affects thrombelastograph® analysis. Anesthesiology. 2000;92:1242–1249. doi: 10.1097/00000542-200005000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Roche AM, James MFM, Grocott MPW, Mythen MC. Citrated blood does not reliably reflect fresh whole blood coagulability in trials of in vitro hemodilution. Anesth Analg. 2003;96:58–61. doi: 10.1097/00000539-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Lang T, Bauters A, Braun S, Potzsch B, von Pape K, Kolde H, Lakner M. Multi-centre investigation on reference range for ROTEM thromboelastometry. Blood coaglation and fibrinolysis. 2005;16:301–310. doi: 10.1097/01.mbc.0000169225.31173.19. [DOI] [PubMed] [Google Scholar]
- 36.Prisco D, Paniccia R. Point-of-care testing of hemostasis in cardiac surgery. Thromb J. 2003;1:1. doi: 10.1186/1477-9560-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan MY, Rusconi CP, Alexander JH, Tonkens RM, Harrington RA, Becker RC. A randomized, repeat-dose, pharmacodynamic and safety study of an antidote-controlled factor IXa inhibitor. J Thromb Haemost. 2008;6:789–796. doi: 10.1111/j.1538-7836.2008.02932.x. [DOI] [PubMed] [Google Scholar]
- 38.Pi DW, Raboud JM, Filby C, Carter CJ. Effect of thromboplastin and coagulometer interaction on the precision of the international normalized ratio. J Clin Pathol. 1995;48:13–17. doi: 10.1136/jcp.48.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edwards RM, Naik-Mathuria BJ, Gay AN, Olutoye OO, Teruya J. Parameters of thromboelastography in healthy newborns. Am J Clin Pathol. 2008;130:99–102. doi: 10.1309/LABNMY41RUD099J2. [DOI] [PubMed] [Google Scholar]
- 40.Chavez J, Foley D, Snider C, Howell J, Cohen E, Muenchen R, Carroll R. A Novel thromboelastograph® tissue factor/kaolin assay of a ctivated clotting times for monitoring heparin anticoagulation during cardiopulmonary bypass. Anesth Analg. 2004;99:1290–1294. doi: 10.1213/01.ANE.0000133909.66768.C8. [DOI] [PubMed] [Google Scholar]
- 41.Mochizuki T, Olson P, Szlam F, Ramsay J, Levy J. Protamine reversal of heparin affects platelet aggregation and activated clotting time after cardiopulmonary bypass. Anesth Analg. 1998;87:781–785. doi: 10.1097/00000539-199810000-00008. [DOI] [PubMed] [Google Scholar]