Summary
Adaptive immunity has traditionally been considered a unique feature of vertebrate physiology. Unlike innate immune responses, which remain essentially unchanged upon exposure to a recurrent challenge with the same stimulus, adaptive immune cells possess the ability to learn and remember. Thus, secondary adaptive responses to a previously encountered challenge are qualitatively and/or quantitatively distinct from those elicited by a primary encounter. Besides this capacity to acquire long-lived memory, the second cardinal feature of adaptive immunity is antigen specificity. It has been generally believed that only T and B cells can develop antigen-specific immunological memory, because these lymphocytes uniquely express recombination-activating gene (RAG) proteins, which are necessary for somatic rearrangement of V(D)J gene segments to assemble diverse antigen-specific receptors. However, recent work has uncovered discrete subsets of murine natural killer (NK) cells capable of mediating long-lived, antigen-specific recall responses to a variety of hapten-based contact sensitizers. These NK cells appear to use distinct, RAG-independent mechanisms to generate antigen specificity. Murine NK cells have also recently been shown to develop memory upon viral infection. Here, we review recent evidence indicating that at least some NK cells are capable of mediating what appears to be adaptive immunity and discuss potential mechanisms that may contribute to RAG-independent generation of antigenic diversity and longevity.
Keywords: natural killer cells, memory, liver
Introduction
The innate immune system distinguishes itself from the adaptive immune system by usage of a finite number of germline-encoded receptors to sense pathogens and tissue damage (1). Innate immune cells are not subject to clonal selection and are not thought to develop immunological memory. In contrast, the textbook view of adaptive immunity is that this system is restricted to T and B cells, which rely on recombination-activating gene (RAG)-dependent non-homologous end-joining and chromosomal DNA recombination of V(D)J gene segments to generate a vast repertoire of highly diverse T and B-cell receptors (2). Activation of T and B cells by a cognate antigen triggers proliferation and differentiation into short-lived effector cells, which give rise to a population of long-lived memory cells. The hallmark of immunological memory is the ability to mount accelerated and enhanced recall responses upon challenge with a previously encountered antigen. Vaccines exploit the body’s capacity to develop immunological memory, as vaccine-induced pathogen-specific memory cells can either prevent an infection from taking hold or substantially shorten and alleviate the course of an infection by the actual pathogen. Many key components of the adaptive immune system are shared among jawed vertebrates ranging from fish to humans, providing evidence that this system has evolved hundreds of millions of years ago (3). Innate pattern recognition receptors go even further back in evolutionary time and are found throughout the animal kingdom.
Unlike the B and T cells, the third lymphocyte subset, natural killer (NK) cells, have long been considered part of the innate immune system. Indeed, NK cells do not express RAG-dependent antigen receptors. Rather, a well-established function of NK cells is to distinguish healthy from abnormal somatic cells by screening them using an array of germline-encoded pattern recognition receptors (4). However, two recent studies have uncovered evidence that NK cell-mediated immune responses can have features reminiscent of adaptive immunity. In this article, we briefly review current knowledge of the classic ‘innate’ NK cell functions and the underlying molecular mechanisms, we discuss the available evidence for the adaptive properties of certain subsets of NK cells, and we consider potential mechanisms to reconcile these diverse activities.
Mechanisms of NK cell-mediated innate immunity
It is now clear that NK cells share a common progenitor with T cells, and they share with T cells some of their effector functions, such as interferon-γ (IFNγ) release and perforin/granzyme-mediated killing (5). Furthermore, many so-called ‘NK receptors’ are not actually NK cell specific but are also constitutively and/or inducibly expressed on γδ cells, NK T cells, and activated CD8+ T cells. However, NK cells are thought to be distinct from T cells in that their activity is not regulated by a T-cell antigen receptor (TCR) but rather through integration of a multitude of activating and dampening receptor signals, depending on the presence and quantity of ligands in their environment. Unlike T cells, NK cells also do not secrete interleukin-2 (IL-2) upon activation, and they can instantaneously lyse target cells that bear appropriate activating signals without the need to undergo effector differentiation (4). In this sense, mature NK cells appear to be in a constitutively primed state, as lysis of target cells does not require gene transcription. In fact, effector molecules, like INFγ, granzyme, and perforin, are pre-synthesized and stored in cytosolic granules and are rapidly released upon activation. This may explain why the ability of NK cells to develop memory has long been overlooked; one cardinal feature of T-cell-mediated memory is an accelerated effector response, which would not be apparent (and probably irrelevant) in the case of NK cells that are constitutively poised for immediate action.
In humans, the largest subset of germline encoded NK cell receptors belongs to the killer cell immunoglobulin (Ig)-like receptor family (KIR), which consists of a polymorphic collection of type I transmembrane proteins that belong to the Ig superfamily. In mice, the functional analogue to the human KIR receptors is the Ly49 family, a group of type II integral membrane, C-type lectin-like molecules (6). KIRs and Ly49s are constitutively expressed on virtually all mature NK cells, which can express just one or a random assembly of several of these receptors. Some of the KIR and Ly49 receptors recognize host-derived major histocompatibility complex class I (MHC-I), but this recognition involves constant regions and is mainly independent of any specific peptide presented in the MHC-I complex. Even though KIR or Ly49 receptor binding to MHC-I is highly relevant for NK cell biology in humans and mice, respectively, their genetic loci are not linked. Moreover, the fact that human KIRs and murine L49s are functional orthologues but are structurally unrelated implies that these molecules and their associated function may only have evolved very recently in mammals. However, it seems likely that the NK lineage itself predates the advent of these molecules, since NK-like nonspecific cytotoxic cells have been identified in more primitive vertebrates, such as teleost fish (7). Thus, it appears reasonable to assume that at least some NK cells may exert immune functions that are much ‘older’ and distinct from those associated with the modern KIR or Ly49 system.
Both human and mouse as well as fish NK cells also express a variety of more conserved pattern recognition receptors (8, 9). Mammalian examples include CD94, a lectin-like heterodimeric receptor coexpressed with members of the NKG2 family, and natural cytotoxicity receptors Nkp44, Nkp46, and FcR CD16 (8). NK cells can be activated through ligation of activating receptors by either endogenous or pathogen-derived glycoproteins, as well as by cytokines, such as type I IFN, IL-12, IL-15, and IL-18. Cytokines are sometimes presented to NK cells in trans by other leukocytes with which NK cells interact. Interestingly, NK cells can take up MHC-I from neighboring cells; as many as 20% of NK cell-expressed MHC-I complexes can be ‘acquired’ in this manner (10, 11).
NK cells in immunity, autoimmunity, and inflammatory disease
The importance of NK cell-mediated recognition of self-MHC-I has recently been highlighted in a number of studies focusing on mechanisms of self-tolerance in NK cells. This biological process referred to as ‘licensing’ occurs during NK cell development and is believed to assure that only NK cells capable of engaging self-MHC-I with one or more specific inhibitory Ly49 receptors are allowed to become functionally responsive to certain stimuli (12–14). Most NK cells express, on average, two or three Ly49-inhibitory receptors, and the expression levels of the MHC-I reactive Ly49 receptor(s) is modulated by the amount of MHC-I. Interestingly, Ly49 receptors can bind in either trans or cis to MHC-I, and it has been suggested that the signal transmitted by cis and trans interactions may be qualitatively distinct (15). It is currently not understood which precise signaling pathways mediate NK cell licensing, but there is strong evidence that NK cells that lack MHC-I-specific Ly49 receptors are hyporesponsive to certain activating stimuli.
The importance of NK cell tolerance to self is highlighted in animal models of autoimmunity. Depending on circumstances, NK cells can either augment or ameliorate such diseases (16). It has been suggested that NK cells may initially protect against autoimmunity, but they may exacerbate disease severity once a certain level of inflammation has been reached. While this concept requires further investigation, it is noteworthy that in the majority of studies, NK cell depletion exacerbated disease, while adoptive transfer of bone marrow-derived naive NK cells reduced disease severity, for instance in mouse models of experimental autoimmune encephalitis (EAE), a condition resembling multiple sclerosis (MS) in humans, and type 1 diabetes. Accordingly, compared with healthy controls, patients with active MS present with fewer NK cells and impaired NK cell-mediated effector functions (16). Concurrent with these findings, treatment of patients suffering from MS or autoimmune uveitis with a monoclonal antibody specific for the IL-2Rα chain (17) increased the number of CD56bright blood NK cells, which then killed autologous activated T cells (18, 19). Amelioration of disease correlated with NK cell expansion in the blood of responder patients, while T-cell counts were only moderately affected (20). However, NK cells have also been shown to augment autoimmune diseases in some settings. For example, in non-obese-diabetic mice, autoimmune diabetes could be prevented after blockade of the activating NK cell receptor NKG2D, suggesting that at least in some settings activation of NK cells is required for disease induction (21, 22). In humans, predisposition to rheumatoid arthritis (23), psoriatic arthritis (24), scleroderma (25), and psoriasis vulgaris (26, 27) has also been linked to the expression of certain KIRs and human leukocyte antigen (HLA) alleles; however, the precise role of NK cells in these diseases is not clear. Another example of how chronically activated NK cells may pose a threat to human health are patients deficient in Tap-2 (transporter associated with antigen-2), who suffer from chronic respiratory infections and granulomatous lesions in the skin and respiratory tract caused by activated NK cells (28). As most endogenous signals that trigger NK cell function in these diseases are unknown, it is unclear whether NK cells employ innate or adaptive (or both) immune mechanisms to contribute to either the prevention or progression of autoimmune disorders.
There is increasing evidence that NK cells can regulate the quality and magnitude of both cellular and humoral adaptive immune responses. For example, NK cells can activate or kill antigen-presenting cells (APCs) and regulatory T cells (29, 30), augment cytotoxic T lymphocyte (CTL) generation and effector functions (31, 32), polarize or kill T-helper (Th) cells, and enhance B-cell activation and isotype switching (33). Immature dendritic cells (DCs) are particularly susceptible to lysis by NK cells that lack inhibitory KIRs/Ly49 receptors, while mature DCs are protected from lysis upon upregulation of MHC-I, particularly HLA-E/Qa-1 (34). Upon maturation, DCs also upregulate MHC-II, costimulatory molecules, and CCR7, required for DC migration to lymph nodes (35–39). Upon direct contact with mature DCs, NK cells secrete tumor necrosis factor-α (TNFα) and INFγ, which promotes Th1 polarization of activated Th cells. Once activated, T cells themselves can become susceptible to NK cell-mediated lysis, because they upregulate activating ligands for NKG2D. However, activated T cells can be protected by concomitant expression of Qa-1-Qdm, a ligand for CD94/NKG2A inhibitory NK receptors (40, 41). Activated macrophages and human microglial cells are also sensitive to NK cell-mediated lysis, via Nkp46 and NKG2D-mediated mechanisms (42). Hence, NK cells can indirectly modulate adaptive immune responses by either augmenting or dampening APC and T-cell effector functions through a variety of mechanisms.
A menagerie of NK cell subsets
There is mounting evidence indicating that myeloid and lymphoid leukocyte populations, including NK cells, are divisible into numerous distinct subsets that exert unique effector functions and are often identifiable by unique combinations of cell surface markers (43). For example, human NK cells are commonly subdivided into a CD56dim and a CD56bright subset (6). CD56dim NK cells make up ~90% of blood NK cells; they express high levels of KIRs and CD16 and are highly cytotoxic. In contrast, CD56bright NK cells are rare in the circulation, but they make up 75–90% and 50% of resident NK cells in lymph nodes and the spleen, respectively. Upon stimulation, CD56bright NK cells, which express CD94/NKG2D but not KIRs, produce cytokines and are poorly cytotoxic. Moreover, only CD56dim NK cells are thought to develop in the bone marrow, whereas their CD56bright cousins are believed to arise within lymph nodes or the thymus.
A subset of thymus-derived NK cells has also been described in mice. These cells are negative for Ly49 inhibitory receptors or CD16, but they express other NK markers, such as NK1.1, DX5, CD94, and CD127 (44). Aside from this population, murine NK cell subsets are not well defined. Mice harbor NK cells in various developmental stages in many different anatomic regions of the body, including the blood, bone marrow, thymus, lymph nodes, spleen, lung, intestine, and liver. As discussed above, individual NK cells express, on average, a random selection of two or three members of the Ly49 family (6). This is also true for NK cells that reside in different organs, but the relative frequency of subsets that express any given Ly49 molecule varies from tissue to tissue (45). Additionally, each subset may be further subdivided based on an assortment of non-uniformly expressed patterns of other surface markers, such as CD11b, CD11c, CD27, CD43, CD69, CD90, DX5, and c-kit (43). Some mature NK cells also express markers that are typically associated with DCs and macrophages, such as CD11b, CD11c, and MHC-II (46, 47). Currently, we do not have a good understanding of the development and function of murine NK cell subsets and their biological relevance. However, it should be noted that the enormous variability in the repertoire of germline-encoded surface receptors with activating and inhibitory (and in some cases functionally undefined) properties can create substantial diversity within the NK cell lineage, even in the absence of classic antigen receptors that require gene rearrangement and non-homologous end-joining. This diversity in hardwired pattern recognition receptors can account for the adaptive features displayed by a subset of NK cells responding to mouse cytomegalovirus virus (MCMV) (48) (see below), but it remains to be determined whether similar rules apply also to adaptive NK responses to other challenges.
NK cell-mediated adaptive immunity to viruses
MCMV has been used as a tool to study NK cell-mediated antiviral immune surveillance, as this virus has evolved elaborate mechanisms to evade T and B-cell responses (49–52). NK cells in C57BL6 mice express two activating receptors, Ly49H and Ly49P, which specifically recognize the MCMV-derived proteins m157 and m04, respectively (49–51). Ly49H does not contain an immunoreceptor tyrosine-based activating motif in its cytoplasmic domain and instead signals via its associated signaling molecule Dap12 (53). This signaling allows for the generation of mice that lack Ly49H+ NK cells, as genetic deficiency in Dap12 abolishes the development of this subset. A recent study by Lanier’s group (48) has demonstrated that upon MCMV infection of WT×Dap12 knockout (KO) mixed BMC mice, Ly49H+ NK cells undergo an initial period of proliferative expansion followed by contraction and, eventually, persistence of self-renewing memory cells. This expansion is dependent on MCMV-encoded m157 but is only observed when hosts are first rendered lymphopenic for Ly49H+ NK cells. Adoptively transferred MCMV sensitized Ly49H+ NK cells expanded also upon secondary challenge with MCMV, at least under conditions of Ly49H-lymphopenia. Adoptive transfer experiments showed that a 10-fold larger number of naive than memory NK cells was necessary to protect non-immune mice against MCMV challenge. Hence, NK cells, not unlike CTLs, are activated and proliferate in response to a specific antigen that must be recognized by discrete activating receptors. Following clearance of the pathogen, the expanded NK cell subset undergoes a contraction phase and gives rise to a memory pool, which upon rechallenge provides for enhanced immunity. As such, NK cells exhibit attributes of adaptive immunity (48) that they share with CTLs, including clonal proliferation and long-lived memory cells that can give rise to functionally enhanced effector responses upon reinfection. However, MCMV recognition by Ly49H is restricted to mice of the C57BL/6 background, while other inbred mouse strains and 90% of outbreed mice do not express NK cell receptors capable of direct recognition of MCMV-encoded antigens. Further work is required to establish if activating NK cell receptors generally promote NK cell memory differentiation upon their activation or if this function is restricted to specific cases, such as Ly49H where the receptor and its cognate pathogen have coevolved over eons. It is also not clear if this form of memory is molecularly restricted to the initially encountered challenge. Since Ly49H+ NK cells express a variety of other activating receptors, it will be important to determine whether the ligation of these coexpressed molecules on MCMV-primed NK cells can also result in enhanced NK cell responses or whether the memory response remains restricted to signals perceived via Ly49H.
NK cells have also been implicated in antiviral immunity in human, although it is unclear whether this activity should be categorized as innate or adaptive. Recent studies of patients with viral hepatitis and human immunodeficiency virus (HIV) infection have linked co-expression of certain KIR and HLA class I alleles in infected individuals with disease progression (54, 55). Co-expression of the KIR3DS1 allele in conjunction with HLA-Bw4 complexes that encoded an isoleucine at position 80 (HLA-Bw480I) resulted in significantly slower progression towards acquired immunodeficiency syndrome (AIDS) in HIV-infected individuals compared to individuals that had only one or neither of these two alleles (54, 55). KIR3DS1 expression was associated with a significantly elevated NK cell response against HIV-infected target cells. NK cell responses were strongest in individuals that encoded for both KIR3DS1 and HLA-Bw480I (54, 55). Another example of direct NK cell-mediated recognition of viruses is influenza (56). Influenza infection can directly activate NK cells via Nkp46 and Nkp44 ligation through virally expressed hemagglutinin (HA) (56–60), leading to rapid recruitment of NK cells and expansion of resident NK cells in lung tissue. NK cells make up about 10% of lymphocytes found in healthy lungs, and rare patients deficient in NK cells and CTLs suffer from severe respiratory and viral infections (61–66), demonstrating the crucial role NK cells play during this disease. Likewise, Nkp46 and NK cell-depleted mice also present with increases morbidity and mortality upon influenza challenge (67, 68). This outcome may be explained by a variety of factors, such as the critical contribution by NK cells to the activation of CTL responses, their role as INFγ-producers, and their ability to augment T and B-cell effector functions. Additionally, it should be noted that NK cells themselves might also mediate adaptive immune responses to viral infections other than MCMV, although current published work is insufficient to draw firm conclusions.
NK cell-mediated adaptive immunity to hapten-based contact sensitizers
A few years prior to the discovery of NK cell memory to MCMV, we reported (45) that NK cells exhibit features of adaptive immunity in a different experimental system. This work was prompted by the observation that different strains of T and B-cell-deficient mice (e.g. RAG knockout, SCID, and nu/nu) mounted vigorous recall responses to hapten-based contact sensitizers. Others independently reported similar findings (69). Haptens are reactive chemicals that covalently modify self-proteins, which are then recognized as foreign antigens. Through this mechanism, haptens induce allergic diseases referred to as contact hypersensitivity (CHS), a form of delayed type hypersensitivity (DTH), which can affect many organs and occurs in response to a wide variety of agents (70). Typically, a first exposure to a hapten results only in a minor local irritation at the site of exposure, most often the skin, but leads to sensitization in local draining lymph nodes resulting in the establishment of long-lived antigen-specific memory. Subsequent exposure to the same hapten generates a pathological CHS reaction that typically manifests itself as an itchy rash with fluid-filled blisters and hives (71).
In a series of experiments using 2,4-dinitro-1-fluorobenzene (DNFB) or 4-ethoxy-methylene-2-phenyl-3-oxazalin-5-one (OXA) or picryl chloride (PC) as experimental contact sensitizers and an extensive panel of mutant mice, we determined that NK cells are both required and sufficient to mediate hapten-specific CHS responses in the absence of T and B cells (Tables 1 and 2). The requirement for NK cells in these experiments was further corroborated by adoptive transfers of NK cell subsets to naive recipients (Table 3) and by antibody-mediated NK cell depletion (Table 4). Moreover, by using fluorescence activated cell sorting (FACS) analysis (Fig. 1) and confocal microscopy (45) of exposed tissues, we determined that NK cells are actively recruited to sites of CHS upon challenge of sensitized but not naive mice.
Table 1.
Genotypes of responder and non-responder mice upon hapten sensitization and challenge
| Genotype | T, B | NK | Sensitization | Challenge | CHS | References |
|---|---|---|---|---|---|---|
| RAG-KO C57BL/6 |
No | Yes | DNFB | DNFB | Yes | 1 |
| RAG-KO C57BL/6 |
No | Yes | Acetone | DNFB | No | 1 |
| RAG-KO C57BL/6 |
No | Yes | OXA | OXA | Yes | 1 |
| RAG-KO C57BL/6 |
No | Yes | Acetone | OXA | No | 1 |
| RAG-KO C57BL/6 |
No | Yes | OXA | DNFB | No | 1 |
| RAG-KO C57BL/6 |
No | Yes | DNFB | OXA | No | 1 |
| Rag/γcDKO B10 |
No | No | DNFB | DNFB | No | 1 |
| Rag/γcDKO B10 |
No | No | Acetone | DNFB | No | 1 |
| Rag/γcDKO B10 |
No | No | OXA | OXA | No | 1 |
| Rag/γcDKO B10 |
No | No | Acetone | OXA | No | 1 |
| SCID Balb/c | No | Yes | DNFB | DNFB | Yes | 1 |
| SCID Balb/c | No | Yes | Acetone | DNFB | No | 1 |
| SCID Balb/c | No | Yes | PC | PC | Yes | 1 |
| SCID Balb/c | No | Yes | Acetone | PC | No | 1 |
| SCID beige Balb/c |
No | not functional | DNFB | DNFB | No | 1 |
| SCID beige Balb/c |
No | not functional | Acetone | DNFB | No | 1 |
| SCID beige Balb/c |
No | not functional | PC | PC | No | 1 |
| SCID beige Balb/c |
No | not functional | Acetone | PC | No | 1 |
| Rag-KO Balb/c | No | Yes | FITC | FITC | Yes | a |
| Rag-KO Balb/c | No | Yes | Acetone | FITC | No | a |
Mice were sensitized on two consecutive days by painting of 0.5% DNFB, 0.5% FITC in acetone/dibutylphthalate, or 10% OXA on the shaved back; and on day 5, the right ear was challenged with 0.25% DNFB, 0.5% FITC, or 1% OXA, and the left ear was painted with vehicle, and ear thickness was measured 24 h later. To account for acute hapten-induced tissue irritation, background swelling was measured in parallel with that of nonsensitized mice. Hapten-specific ear swelling was calculated as follows: [treated ear thickness −control ear thickness] − background swelling.
unpublished observation.
CHS responses that are significantly greater than background (p<0.05) are indicated as positive.
Table 2.
Genotypes of memory responder and non-responder mice upon hapten sensitization and challenge
| Genotype | T, B | NK | Sensitization | Challenge | CHS | References |
|---|---|---|---|---|---|---|
| C57BL/6 | Yes | Yes | DNFB | DNFB | Yes | 1 |
| C57BL/6 | Yes | Yes | Acetone | DNFB | No | 1 |
| RAG-KO C57BL/6 |
No | Yes | DNFB | DNFB | Yes | 1 |
| RAG-KO C57BL/6 |
No | Yes | Acetone | DNFB | No | 1 |
Mice were sensitized on two consecutive days by painting of 0.5% DNFB or 10% OXA on the shaved back; 4 weeks later, the right ear was challenged with 0.25% DNFB or 1% OXA and the left ear was painted with vehicle, and ear thickness was measured 24 h later. To account for acute hapten-induced tissue irritation, background swelling was measured in parallel with that of nonsensitized mice. Hapten-specific ear swelling was calculated as follows: [treated ear thickness − control ear thickness] − background swelling. CHS responses that are significantly greater than background (p<0.05) are indicated as positive.
Table 3.
Specific subsets of hepatic sensitized NK cells mediate CHS responses and transfer sensitivity to naive T, B, and NK cell-deficient recipients
| NK phenotype | Sensitization | Donor Organ | Challenge | CHS | Reference |
|---|---|---|---|---|---|
| NK1.1+ | DNFB | Liver | DNFB | Yes | 1 |
| NK1.1+ | Acetone | Liver | DNFB | No | 1 |
| NK1.1+ | DNFB | Spleen | DNFB | No | 1 |
| NK1.1+ | Acetone | Spleen | DNFB | No | 1 |
| Thy1+NK1.1+ | DNFB | Liver | DNFB | Yes | a |
| Thy1+NK1.1+ | Acetone | Liver | DNFB | No | a |
| Thy1−NK1.1+ | DNFB | Liver | DNFB | No | a |
| Thy1−NK1.1+ | Acetone | Liver | DNFB | No | a |
| Thy1+NK1.1+ | DNFB | Spleen | DNFB | No | a |
| Thy1+NK1.1+ | Acetone | Spleen | DNFB | No | a |
| Thy1−NK1.1+ | DNFB | Spleen | DNFB | No | a |
| Thy1−NK1.1+ | Acetone | Spleen | DNFB | No | a |
| Thy1+NK1.1+ | OXA | Liver | OXA | Yes | a |
| Thy1+NK1.1+ | Acetone | Liver | OXA | No | a |
| Thy1−NK1.1+ | OXA | Liver | OXA | No | a |
| Thy1−NK1.1+ | Acetone | Liver | OXA | No | a |
| Thy1+NK1.1+ | OXA | Spleen | OXA | No | a |
| Thy1+NK1.1+ | Acetone | Spleen | OXA | No | a |
| Thy1−NK1.1+ | OXA | Spleen | OXA | No | a |
| Thy1−NK1.1+ | Acetone | Spleen | OXA | No | a |
| Thy1+NK1.1+ | OXA | Liver | DNFB | No | a |
| Thy1+NK1.1+ | DNFB | Liver | OXA | No | a |
| Thy1+NK1.1+CD27− | DNFB | Liver | DNFB | Yes | a |
| Thy1+NK1.1+CD27− | Acetone | Liver | DNFB | No | a |
| Thy1+NK1.1+CD27+ | DNFB | Liver | DNFB | No | a |
| Thy1+NK1.1+CD27+ | Acetone | Liver | DNFB | No | a |
| Thy1+NK1.1+Mac-1+ | DNFB | Liver | DNFB | Yes | a |
| Thy1+NK1.1+Mac-1+ | Acetone | Liver | DNFB | No | a |
| Thy1+NK1.1+Mac-1− | DNFB | Liver | DNFB | No | a |
| Thy1+NK1.1+Mac-1− | Acetone | Liver | DNFB | No | a |
| Thy1+NK1.1+ Mac-1+CD27+ | DNFB | Liver | DNFB | No | a |
| Thy1+NK1.1+ Mac-1+CD27+ | Acetone | Liver | DNFB | No | a |
| Thy1+NK1.1+Ly49C/I+ | DNFB | Liver | DNFB | Yes | 1 |
| Thy1+NK1.1+Ly49C/I+ | Acetone | Liver | DNFB | No | 1 |
| Thy1+NK1.1+Ly49C/I+ | OXA | Liver | OXA | Yes | a |
| Thy1+NK1.1+Ly49C/I+ | Acetone | Liver | OXA | No | a |
| Thy1+NK1.1+Ly49C/I− | DNFB | Liver | DNFB | No | 1 |
| Thy1+NK1.1+Ly49C/I− | Acetone | Liver | DNFB | No | 1 |
| Thy1+NK1.1+Ly49C/I− | OXA | Liver | OXA | No | a |
| Thy1+NK1.1+Ly49C/I− | Acetone | Liver | OXA | No | a |
Rag-KO donor mice were sensitized with either 0.5% DNFB in acetone or acetone on days 0 and 1. Indicated groups of NK cells were isolated by FACS sorting on day 4 or 5, and 80,000 NK cells were adoptively transferred into naive into Ragγc-DKO recipients. Recipient mice and mock recipients were challenged 24 h post adoptive transfer with 0.2% DNFB on one ear and acetone on the other, and ear swelling determined using a micrometer. To account for acute hapten-induced irritation, background swelling of mock recipients was subtracted from that of NK cell recipients. Hapten-specific ear swelling was calculated as follows: (treated ear thickness − control ear thickness) − background swelling. CHS responses that are significantly greater than background (p<0.05) are indicated as positive in Table 4.
unpublished observation.
Table 4.
Effect of treatment with NK cell depleting antibodies on the DNFB-induced CHS response in Rag-KO mice
| Genotype | Sensitization | Ab treatment | Challenge | CHS |
|---|---|---|---|---|
| RAGKO C57Bl/6 | DNFB | Isotype control | DNFB | Yes |
| RAGKO C57Bl/6 | Acetone | Isotype control | DNFB | No |
| RAGKO C57Bl/6 | DNFB | Anti-Thy1mAb | DNFB | No |
| RAGKO C57Bl/6 | DNFB | Anti-NK1.1 | DNFB | No |
| RAGKO C57Bl/6 | DNFB | Anti-asialoGM1 | DNFB | No |
| C57Bl/6 | DNFB | Isotype control | DNFB | Yes |
| C57Bl/6 | Acetone | Isotype control | DNFB | No |
| C57Bl/6 | DNFB | Anti-NK1.1 | DNFB | Reduced |
| C57Bl/6 | DNFB | Anti-asialoGM1 | DNFB | Yes |
Mice were sensitized with DNFB as described above. At 24 h before DNFB challenge, sensitized mice were depleted of NK cells with anti-asialo-GM1 or anti-NK1.1. Control mice received rabbit IgG or mouse IgG2a, respectively.
Fig. 1. Sensitized Thy1+ NK cells accumulate at sites of challenge.
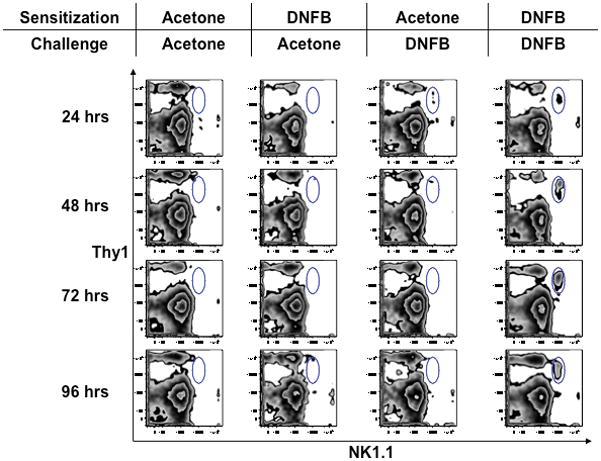
C57BL/6 mice were sensitized by abdominal painting with DNFB or hapten-free solvent (acetone) as a control on days 0 and 1, and one ear was challenged with DNFB on day 4, while the opposite ear was concomitantly treated with acetone. Ears were harvested at indicated time points after challenge, and single cell suspensions were analyzed by flow cytometry for the presence of NK cells. Thy1 and NK1.1 staining is shown after gating on CD45+TCRβ−cells.
RAG-deficient mice mounted T and B-cell-independent CHS responses only when they were challenged with the same hapten used during sensitization, but these mice remained unresponsive when exposed to a different hapten (45). Moreover, following sensitization (usually by hapten painting of shaved abdominal skin on two consecutive days), NK cells in RAG-deficient mice recalled the sensitizing hapten for at least several months (45, authors’ unpublished data) (Table 2). Thus, mice devoid of T and B cells are capable of learning, remembering, and discriminating between distinct antigens, which are the hallmark features of adaptive immunity.
Further work using adoptive transfers of highly purified leukocyte subsets from sensitized RAG-deficient donors led to the identification of a discrete population of liver-resident NK cells that could mediate hapten-specific recall responses upon transfer to naive hosts (45) (Table 3). The capacity to transfer a hapten-specific CHS response was enriched in a discrete population of NK1.1+ cells that expressed Thy1, Mac-1, Ly49C/I, and were negative for CD27 in the liver of sensitized donors, while naive or sensitized splenic NK cells did not mediate adaptive immune responses upon hapten rechallenge. Interestingly, the expression of Ly49C/I, a licensing receptor in C57Bl/6 mice, correlated with enhanced CHS activity upon adoptive transfer, suggesting that adaptive NK cell-mediated immune responses may depend on proper licensing during NK cell development. As expected and consistent with published results of anti-Thy1.2 treatment of wildtype mice in which the monoclonal antibody depletes both T cells and hapten-responsive NK cells (72), treatment of sensitized RAG-deficient mice with anti-Thy1.2 abrogated CHS responses (45). Similarly, treatment with a blocking antibody to NKG2D attenuated NK cell-mediated CHS responses. However, NKG2D itself is insufficient to identify the NK cell subset able to transfer CHS responses, since splenic NKG2D+ NK cells did not transfer CHS responses. None of the markers that were associated with the hepatic memory NK cells subset are sufficient to confer hapten specificity, since a sizable number of NK cells in the spleen of sensitized mice also coexpressed these markers, since all of them are found on splenic NK cells, which are always incapable of hapten recognition in vitro and in vivo (45, authors’ unpublished data). In this regard, hapten-specific NK cells are notably different from MCMV-specific memory NK cells, which were reportedly found in both liver and spleen (48).
Sensitization of RAG KO mice can be achieved not only by skin painting but also by subcutaneous or intravenous or administration of hapten-loaded antigen-presenting cells (authors’ unpublished results). Irrespective of the route of antigen entry, it is likely that sensitization of both T cells and NK cells occurs in secondary lymphoid tissues (73). Upon skin painting, hapten is taken up by migratory DCs in the skin and transported to the draining lymph nodes. Consequently, sensitization of both wildtype and RAG-deficient mice is markedly attenuated when animals are pretreated with an antibody that blocks L-selectin, a key homing receptor for lymphocyte trafficking to peripheral lymph nodes (45, 73). Mice deficient in L-selectin present with impaired CHS responses (74–76). Hence, we can piece together a tentative road map for hapten-specific memory NK cells (Fig. 2): NK cells are primed in peripheral lymph nodes of sensitized mice by DCs and/or other antigen-presenting cells and subsequently enter the blood to migrate to the liver where they persist as long-lived memory cells. During steady state, NK cell egress from lymph nodes into efferent lymphatics requires S1P5, the expression of which is regulated by T-bet (77), although it remains to be seen if this pathway is also utilized by hapten-sensitized NK cells. Once NK cells have accessed the blood and traveled to the liver, they likely respond to local signals that induce their retention and/or survival and/or further differentiation. The nature of these putative signals remains to be characterized. Upon hapten challenge of an ear, memory NK cells depart from the liver and are recruited to the dermal site of exposure, a migration step that does not rely on L-selectin but requires β2 integrins and endothelial P-and E-selectin (45)(Table 5). Once the NK cells reencounter a cognate hapten, they become activated and precipitate the recruitment of additional inflammatory leukocytes, such as neutrophils, macrophages and DCs. Interestingly, in human patients suffering from atopic eczema and dermatitis, NK cells are in close contact with DCs in skin lesions (78), suggesting DCs and NK cells may be engaged in continuous communications at effector sites.
Fig. 2.
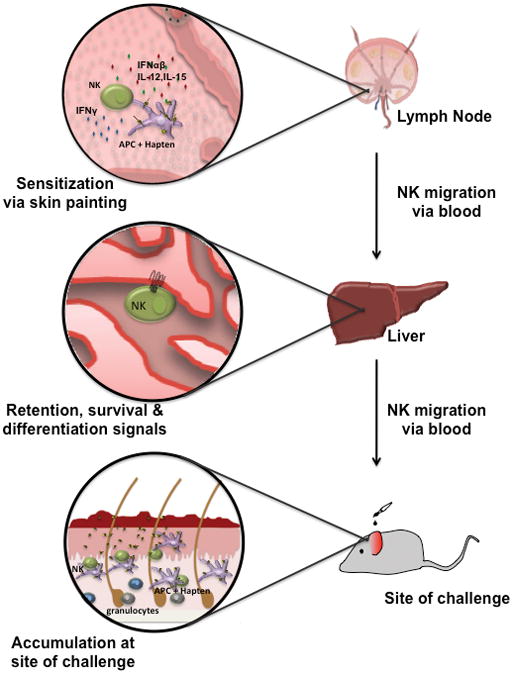
Schematic representation of the sequence of events leading to hapten-specific NK memory in CHS.
Table 5.
Role of adhesion receptors in NK cell mediated CHS
| Genotype | Sensitization | Ab treatment | Challenge | CHS |
|---|---|---|---|---|
| RAGKO B6 | DNFB | Isotype control | DNFB | Yes |
| RAGKO B6 | Acetone | Isotype control | DNFB | No |
| RAGKO B6 | DNFB | Anti-CD18 | DNFB | reduced |
| RAGKO B6 | DNFB | Anti-L-selectin | DNFB | reduced |
| RAGKO B6 | DNFB | Anti-P+E-selectin | DNFB | reduced |
Rag-KO mice were sensitized with DNFB and treated intravenously with monoclonal antibodies (mAbs) specific for L-selectin (MEL-14), CD18 (GAME-46), P-selectin (RB40.34), and E-selectin (9A9) before DNFB challenge. All mAbs were given at a dose of 100 mg/mouse; anti-L-selectin was given 24 h before sensitization, and all other mAbs were given 2 h before challenge.
Differential gene expression of NK cells in different anatomic compartments
One of the many open questions in the field are the mechanisms that render NK cells in the liver functionally distinct from those in the spleen. Such differences have also been noted by others (79, 80). For example, several investigators have reported that hepatic NK cells in rats and humans are more cytotoxic than blood NK cells against tumor cells (81–84). Differences in gene expression between hepatic and blood NK cells have been analyzed in the rat using microarrays (85). This analysis identified ~150 genes and expressed sequence tags that were differentially expressed between the two subsets, including a number of genes that could be functionally relevant for the observed differences. Examples include NKG2D, NKG2C, CD94, ecto-ATPase, phosphatidylinositol 3-kinase and granule-associated effector molecules such as granzymes and defensin NP3. Whether and to what extent these and the many other anatomically restricted gene products contribute to the adaptive properties of hepatic NK cells is currently under investigation.
Future directions
The phenomenon of antigen-specific NK memory is entirely unprecedented and suggests a mechanism of immune recognition and memory that may be fundamentally distinct from other cellular and molecular mechanisms of adaptive immunity. The finding that NK cells retained an intrinsic memory of prior activation and may do so in an antigen-specific manner, both functions until now attributed only to antigen-specific adaptive immune cells, suggests that NK cells combine attributes of innate and acquired immunity when responding to activating stimuli. Indeed, NK cells remembered prior activation to a variety of hapten antigens, and MCMV-derived m157. However, there are important and still unresolved differences between these two forms of NK memory: hapten-specific NK memory is not restricted to a specific MHC haplotype nor does it require the expression of a specific activating Ly49 receptor. By contrast, NK cell-mediated responses to MCMV depends on Ly49H and H2-db MHC-I. Moreover, MCMV-responsive Ly49H+ NK cells appear to confer memory regardless of the anatomic origin, while hapten-specific NK cell responses were restricted to the liver. Interestingly, while both splenic and hepatic NK cells respond to MCMV infection, they do so in an organ-specific manner (86, 87). In the spleen, perforin mediates viral clearance, while IFNγ mediates protection in the liver. It will therefore be informative to directly compare splenic versus hepatic memory NK cells with regard to their response to MCMV challenge using adoptive transfer strategies. It will also be important to test if ligation of other activating receptors on MCMV-primed Ly49+ NK cells triggers enhanced ‘recall’ responses, which would clarify whether these memory cells are truly antigen specific. Since the hapten-induced hepatic NK cells to not appear to rely on Ly49 molecules to mediate CHS responses (45), it seems likely that NK cells utilize more than one mechanism with which they mediate memory responses. Hence, NK cell-mediated effector functions during anti-tumor responses, allergic, and infectious disease may clinically be more important then initially appreciated, and NK cells may be attractive therapeutic targets for the treatment or prevention of such diseases. NK cell-directed vaccination strategies may be possible, particularly for patients with compromised T and B-cell function.
The molecular mechanism by which murine NK cells mediate antigen-specific responses in a RAG-independent manner is currently unknown. Evidence for a RAG-independent generation of a clonal repertoire of lymphocytes has been described in two jawless vertebrates, sea lampreys and hagfish, which use recombinatorial assembly of gene segments encoding leucine-rich repeats to generate diversified variable lymphocyte receptors (3, 88). These animals can generate adaptive, clonal immune responses to a variety of antigens, rejection of secondary skin allographs, and DTH responses. However, the numbers of immune gene homologs to those of mammalian are comparatively low relative to that of jawed vertebrates. It is tempting to speculate that the early vertebrates’ evolution of an adaptive immune system was key to their survival over the past ~500 million years. Whether other vertebrates have retained remnants of this ancient RAG-independent immune system is unknown.
Acknowledgments
This work was supported, in part, by fellowships from the Cancer Research Institute and the Ragon Institute to S.P. and by NIH grants AI069259, AI072252 and AI078897 to U.H.v.A.
References
- 1.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 2.Bassing CH, Swat W, Alt FW. The mechanism and regulation of chromosomal V(D)J recombination. Cell. 2002;109 (Suppl):S45–55. doi: 10.1016/s0092-8674(02)00675-x. [DOI] [PubMed] [Google Scholar]
- 3.Cooper MD, Alder MN. The evolution of adaptive immune systems. Cell. 2006;124:815–822. doi: 10.1016/j.cell.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 5.DiSanto JP, Muller W, Guy-Grand D, Fischer A, Rajewsky K. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc Natl Acad Sci USA. 1995;92:377–381. doi: 10.1073/pnas.92.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonsson AH, Yokoyama WM. Natural killer cell tolerance licensing and other mechanisms. Adv Immunol. 2009;101:27–79. doi: 10.1016/S0065-2776(08)01002-X. [DOI] [PubMed] [Google Scholar]
- 7.Shen L, et al. Channel catfish cytotoxic cells: a mini-review. Dev Comp Immunol. 2002;26:141–149. doi: 10.1016/s0145-305x(01)00056-8. [DOI] [PubMed] [Google Scholar]
- 8.Culley FJ, et al. Natural killer cell signal integration balances synapse symmetry and migration. PLoS Biol. 2009;7:e1000159. doi: 10.1371/journal.pbio.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoder JA. Form, function and phylogenetics of NITRs in bony fish. Dev Comp Immunol. 2009;33:135–144. doi: 10.1016/j.dci.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Zimmer J, Ioannidis V, Held W. H-2D ligand expression by Ly49A+ natural killer (NK) cells precludes ligand uptake from environmental cells: implications for NK cell function. J Exp Med. 2001;194:1531–1539. doi: 10.1084/jem.194.10.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sjostrom A, et al. Acquisition of external major histocompatibility complex class I molecules by natural killer cells expressing inhibitory Ly49 receptors. J Exp Med. 2001;194:1519–1530. doi: 10.1084/jem.194.10.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim S, et al. HLA alleles determine differences in human natural killer cell responsiveness and potency. Proc Natl Acad Sci USA. 2008;105:3053–3058. doi: 10.1073/pnas.0712229105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yokoyama WM, Kim S. Licensing of natural killer cells by self-major histocompatibility complex class I. Immunol Rev. 2006;214:143–154. doi: 10.1111/j.1600-065X.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 14.Yokoyama WM, Kim S. How do natural killer cells find self to achieve tolerance? Immunity. 2006;24:249–257. doi: 10.1016/j.immuni.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Dam J, et al. Variable dimerization of the Ly49A natural killer cell receptor results in differential engagement of its MHC class I ligand. J Mol Biol. 2006;362:102–113. doi: 10.1016/j.jmb.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Lunemann A, Lunemann JD, Munz C. Regulatory NK-cell functions in inflammation and autoimmunity. Mol Med. 2009;15:352–358. doi: 10.2119/molmed.2009.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schippling DS, Martin R. Spotlight on anti-CD25: daclizumab in MS. Int MS J. 2008;15:94–98. [PubMed] [Google Scholar]
- 18.Bielekova B, et al. Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL-2Ralpha-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci USA. 2006;103:5941–5946. doi: 10.1073/pnas.0601335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z, Lim WK, Mahesh SP, Liu S, Nussenblatt RB. Cutting edge: in vivo blockade of human IL-2 receptor induces expansion of CD56(bright) regulatory NK cells in patients with active uveitis. J Immunol. 2005;174:5187–5191. doi: 10.4049/jimmunol.174.9.5187. [DOI] [PubMed] [Google Scholar]
- 20.Saraste M, Irjala H, Airas L. Expansion of CD56Bright natural killer cells in the peripheral blood of multiple sclerosis patients treated with interferon-beta. Neurol Sci. 2007;28:121–126. doi: 10.1007/s10072-007-0803-3. [DOI] [PubMed] [Google Scholar]
- 21.Ogasawara K, et al. Impairment of NK cell function by NKG2D modulation in NOD mice. Immunity. 2003;18:41–51. doi: 10.1016/s1074-7613(02)00505-8. [DOI] [PubMed] [Google Scholar]
- 22.Ogasawara K, et al. NKG2D blockade prevents autoimmune diabetes in NOD mice. Immunity. 2004;20:757–767. doi: 10.1016/j.immuni.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Namekawa T, et al. Killer cell activating receptors function as costimulatory molecules on CD4+CD28null T cells clonally expanded in rheumatoid arthritis. J Immunol. 2000;165:1138–1145. doi: 10.4049/jimmunol.165.2.1138. [DOI] [PubMed] [Google Scholar]
- 24.Martin MP, et al. Cutting edge: susceptibility to psoriatic arthritis: influence of activating killer Ig-like receptor genes in the absence of specific HLA-C alleles. J Immunol. 2002;169:2818–2822. doi: 10.4049/jimmunol.169.6.2818. [DOI] [PubMed] [Google Scholar]
- 25.Momot T, et al. Association of killer cell immunoglobulin-like receptors with scleroderma. Arthritis Rheum. 2004;50:1561–1565. doi: 10.1002/art.20216. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki Y, et al. Genetic polymorphisms of killer cell immunoglobulin-like receptors are associated with susceptibility to psoriasis vulgaris. J Invest Dermatol. 2004;122:1133–1136. doi: 10.1111/j.0022-202X.2004.22517.x. [DOI] [PubMed] [Google Scholar]
- 27.Luszczek W, et al. Gene for the activating natural killer cell receptor, KIR2DS1, is associated with susceptibility to psoriasis vulgaris. Hum Immunol. 2004;65:758–766. doi: 10.1016/j.humimm.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Hanna J, et al. Functional aberrant expression of CCR2 receptor on chronically activated NK cells in patients with TAP-2 deficiency. Blood. 2005;106:3465–3473. doi: 10.1182/blood-2005-03-0855. [DOI] [PubMed] [Google Scholar]
- 29.Ferlazzo G, et al. The interaction between NK cells and dendritic cells in bacterial infections results in rapid induction of NK cell activation and in the lysis of uninfected dendritic cells. Eur J Immunol. 2003;33:306–313. doi: 10.1002/immu.200310004. [DOI] [PubMed] [Google Scholar]
- 30.Brillard E, et al. Natural killer cells prevent CD28-mediated Foxp3 transcription in CD4+CD25-T lymphocytes. Exp Hematol. 2007;35:416–425. doi: 10.1016/j.exphem.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Nocentini G, Ronchetti S, Cuzzocrea S, Riccardi C. GITR/GITRL: more than an effector T cell co-stimulatory system. Eur J Immunol. 2007;37:1165–1169. doi: 10.1002/eji.200636933. [DOI] [PubMed] [Google Scholar]
- 32.Jinushi M, et al. Natural killer cell and hepatic cell interaction via NKG2A leads to dendritic cell-mediated induction of CD4 CD25 T cells with PD-1-dependent regulatory activities. Immunology. 2007;120:73–82. doi: 10.1111/j.1365-2567.2006.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao N, Dang T, Yuan D. IFN-gamma-dependent and -independent initiation of switch recombination by NK cells. J Immunol. 2001;167:2011–2018. doi: 10.4049/jimmunol.167.4.2011. [DOI] [PubMed] [Google Scholar]
- 34.Della Chiesa M, Vitale M, Carlomagno S, Ferlazzo G, Moretta L, Moretta A. The natural killer cell-mediated killing of autologous dendritic cells is confined to a cell subset expressing CD94/NKG2A, but lacking inhibitory killer Ig-like receptors. Eur J Immunol. 2003;33:1657–1666. doi: 10.1002/eji.200323986. [DOI] [PubMed] [Google Scholar]
- 35.Iijima N, Yanagawa Y, Clingan JM, Onoe K. CCR7-mediated c-Jun N-terminal kinase activation regulates cell migration in mature dendritic cells. Int immunol. 2005;17:1201–1212. doi: 10.1093/intimm/dxh297. [DOI] [PubMed] [Google Scholar]
- 36.Jang MH, et al. CCR7 is critically important for migration of dendritic cells in intestinal lamina propria to mesenteric lymph nodes. J Immunol. 2006;176:803–810. doi: 10.4049/jimmunol.176.2.803. [DOI] [PubMed] [Google Scholar]
- 37.Ohl L, et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–288. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 38.Rivas-Caicedo A, Soldevila G, Fortoul TI, Castell-Rodriguez A, Flores-Romo L, Garcia-Zepeda EA. Jak3 is involved in dendritic cell maturation and CCR7-dependent migration. PloS one. 2009;4:e7066. doi: 10.1371/journal.pone.0007066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yanagihara S, Komura E, Nagafune J, Watarai H, Yamaguchi Y. EBI1/CCR7 is a new member of dendritic cell chemokine receptor that is up-regulated upon maturation. J Immunol. 1998;161:3096–3102. [PubMed] [Google Scholar]
- 40.Lu L, Kim HJ, Werneck MB, Cantor H. Regulation of CD8+ regulatory T cells: Interruption of the NKG2A-Qa-1 interaction allows robust suppressive activity and resolution of autoimmune disease. Proc Natl Acad Sci USA. 2008;105:19420–19425. doi: 10.1073/pnas.0810383105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu L, Ikizawa K, Hu D, Werneck MB, Wucherpfennig KW, Cantor H. Regulation of activated CD4+ T cells by NK cells via the Qa-1-NKG2A inhibitory pathway. Immunity. 2007;26:593–604. doi: 10.1016/j.immuni.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lunemann A, et al. Human NK cells kill resting but not activated microglia via NKG2D- and NKp46-mediated recognition. J Immunol. 2008;181:6170–6177. doi: 10.4049/jimmunol.181.9.6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Santo JP. Natural killer cell developmental pathways: a question of balance. Annu Rev Immunol. 2006;24:257–286. doi: 10.1146/annurev.immunol.24.021605.090700. [DOI] [PubMed] [Google Scholar]
- 44.Vosshenrich CA, et al. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat Immunol. 2006;7:1217–1224. doi: 10.1038/ni1395. [DOI] [PubMed] [Google Scholar]
- 45.O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 46.Vosshenrich CA, et al. CD11cloB220+ interferon-producing killer dendritic cells are activated natural killer cells. J Exp Med. 2007;204:2569–2578. doi: 10.1084/jem.20071451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blasius AL, Barchet W, Cella M, Colonna M. Development and function of murine B220+CD11c+NK1.1+ cells identify them as a subset of NK cells. J Exp Med. 2007;204:2561–2568. doi: 10.1084/jem.20070991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith HR, et al. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc Natl Acad Sci USA. 2002;99:8826–8831. doi: 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bubic I, et al. Gain of virulence caused by loss of a gene in murine cytomegalovirus. J Virol. 2004;78:7536–7544. doi: 10.1128/JVI.78.14.7536-7544.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Voigt V, et al. Murine cytomegalovirus m157 mutation and variation leads to immune evasion of natural killer cells. Proc Natl Acad Sci USA. 2003;100:13483–13488. doi: 10.1073/pnas.2233572100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.French AR, et al. Escape of mutant double-stranded DNA virus from innate immune control. Immunity. 2004;20:747–756. doi: 10.1016/j.immuni.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 53.Gosselin P, Mason LH, Willette-Brown J, Ortaldo JR, McVicar DW, Anderson SK. Induction of DAP12 phosphorylation, calcium mobilization, and cytokine secretion by Ly49H. J Lleuk Biol. 1999;66:165–171. doi: 10.1002/jlb.66.1.165. [DOI] [PubMed] [Google Scholar]
- 54.Alter G, et al. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J Exp Med. 2007;204:3027–3036. doi: 10.1084/jem.20070695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alter G, Altfeld M. NK cells in HIV-1 infection: evidence for their role in the control of HIV-1 infection. J Intern Med. 2009;265:29–42. doi: 10.1111/j.1365-2796.2008.02045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mandelboim O, et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409:1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 57.Culley FJ. Natural killer cells in infection and inflammation of the lung. Immunology. 2009;128:151–163. doi: 10.1111/j.1365-2567.2009.03167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meijer J, Ogink J, Kreike B, Nuyten D, de Visser KE, Roos E. The chemokine receptor CXCR6 and its ligand CXCL16 are expressed in carcinomas and inhibit proliferation. Cancer Res. 2008;68:4701–4708. doi: 10.1158/0008-5472.CAN-08-0482. [DOI] [PubMed] [Google Scholar]
- 59.Draghi M, et al. NKp46 and NKG2D recognition of infected dendritic cells is necessary for NK cell activation in the human response to influenza infection. J Immunol. 2007;178:2688–2698. doi: 10.4049/jimmunol.178.5.2688. [DOI] [PubMed] [Google Scholar]
- 60.Nogusa S, Ritz BW, Kassim SH, Jennings SR, Gardner EM. Characterization of age-related changes in natural killer cells during primary influenza infection in mice. Mech Ageing Dev. 2008;129:223–230. doi: 10.1016/j.mad.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 61.Welliver TP, Reed JL, Welliver RC., Sr Respiratory syncytial virus and influenza virus infections: observations from tissues of fatal infant cases. Pediatr Infect Dis J. 2008;27:S92–96. doi: 10.1097/INF.0b013e318168b706. [DOI] [PubMed] [Google Scholar]
- 62.Welliver TP, et al. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J Infect Dis. 2007;195:1126–1136. doi: 10.1086/512615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Komiyama A, et al. Natural killer cell immunodeficiency in siblings: defective killing in the absence of natural killer cytotoxic factor activity in natural killer and lymphokine-activated killer cytotoxicities. Pediatrics. 1990;85:323–330. [PubMed] [Google Scholar]
- 64.Komiyama A, et al. A killing defect of natural killer cells with the absence of natural killer cytotoxic factors in a child with Hodgkin’s disease. Blood. 1987;69:1686–1690. [PubMed] [Google Scholar]
- 65.Eidenschenk C, et al. A novel primary immunodeficiency with specific natural-killer cell deficiency maps to the centromeric region of chromosome 8. Am J Hum Genet. 2006;78:721–727. doi: 10.1086/503269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eidenschenk C, et al. Familial NK cell deficiency associated with impaired IL-2- and IL-15-dependent survival of lymphocytes. J Immunol. 2006;177:8835–8843. doi: 10.4049/jimmunol.177.12.8835. [DOI] [PubMed] [Google Scholar]
- 67.Gazit R, et al. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat Immunol. 2006;7:517–523. doi: 10.1038/ni1322. [DOI] [PubMed] [Google Scholar]
- 68.Stein-Streilein J, Guffee J. In vivo treatment of mice and hamsters with antibodies to asialo GM1 increases morbidity and mortality to pulmonary influenza infection. J Immunol. 1986;136:1435–1441. [PubMed] [Google Scholar]
- 69.Boehncke WH, et al. Leukocyte extravasation as a target for anti-inflammatory therapy – Which molecule to choose? Exp Dermatol. 2005;14:70–80. doi: 10.1111/j.0906-6705.2005.290a.x. [DOI] [PubMed] [Google Scholar]
- 70.Gober MD, Gaspari AA. Allergic contact dermatitis. Curr Dir Autoimmun. 2008;10:1–26. doi: 10.1159/000131410. [DOI] [PubMed] [Google Scholar]
- 71.Sicherer SH, Leung DY. Advances in allergic skin disease, anaphylaxis, and hypersensitivity reactions to foods, drugs, and insects in 2007. J Allergy Clin Immunol. 2008;121:1351–1358. doi: 10.1016/j.jaci.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 72.Biedermann T, et al. Mast cells control neutrophil recruitment during T cell-mediated delayed-type hypersensitivity reactions through tumor necrosis factor and macrophage inflammatory protein 2. J Exp Med. 2000;192:1441–1452. doi: 10.1084/jem.192.10.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Catalina MD, Carroll MC, Arizpe H, Takashima A, Estess P, Siegelman MH. The route of antigen entry determines the requirement for L-selectin during immune responses. J Exp Med. 1996;184:2341–2351. doi: 10.1084/jem.184.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Catalina MD, Carroll MC, Arizpe H, Takashima A, Estess P, Siegelman MH. The route of antigen entry determines the requirement for L-selectin during immune responses. J Exp Med. 1996;184:2341–2351. doi: 10.1084/jem.184.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Catalina MD, Estess P, Siegelman MH. Selective requirements for leukocyte adhesion molecules in models of acute and chronic cutaneous inflammation: participation of E- and P- but not L-selectin. Blood. 1999;93:580–589. [PubMed] [Google Scholar]
- 76.Diacovo TG, Catalina MD, Siegelman MH, von Andrian UH. Circulating activated platelets reconstitute lymphocyte homing and immunity in L-selectin-deficient mice. J Exp Med. 1998;187:197–204. doi: 10.1084/jem.187.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jenne CN, et al. T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J Exp Med. 2009;206:2469–2481. doi: 10.1084/jem.20090525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Buentke E, et al. Natural killer and dendritic cell contact in lesional atopic dermatitis skin-- Malassezia-influenced cell interaction. J Invest Dermatol. 2002;119:850–857. doi: 10.1046/j.1523-1747.2002.00132.x. [DOI] [PubMed] [Google Scholar]
- 79.Vermijlen D, et al. High-density oligonucleotide array analysis reveals extensive differences between freshly isolated blood and hepatic natural killer cells. Eur J Immunol. 2004;34:2529–2540. doi: 10.1002/eji.200324712. [DOI] [PubMed] [Google Scholar]
- 80.Andrews DM, Smyth MJ. A potential role for RAG-1 in NK cell development revealed by analysis of NK cells during ontogeny. Immunol Cell Biol. 2010;88:107–116. doi: 10.1038/icb.2009.94. [DOI] [PubMed] [Google Scholar]
- 81.Vanderkerken K, Bouwens L, Wisse W. Characterization of a phenotypically and functionally distinct subset of large granular lymphocytes (pit cells) in rat liver sinusoids. Hepatology. 1990;12:70–75. doi: 10.1002/hep.1840120112. [DOI] [PubMed] [Google Scholar]
- 82.Hata K, Zhang XR, Iwatsuki S, Van Thiel DH, Herberman RB, Whiteside TL. Isolation, phenotyping, and functional analysis of lymphocytes from human liver. Clin Immunol Immunopathol. 1990;56:401–419. doi: 10.1016/0090-1229(90)90160-r. [DOI] [PubMed] [Google Scholar]
- 83.Hata K, Van Thiel DH, Herberman RB, Whiteside TL. Natural killer activity of human liver-derived lymphocytes in various liver diseases. Hepatology. 1991;14:495–503. [PubMed] [Google Scholar]
- 84.Vermijlen D, et al. Hepatic natural killer cells exclusively kill splenic/blood natural killer-resistant tumor cells by the perforin/granzyme pathway. J Leuk Biol. 2002;72:668–676. [PubMed] [Google Scholar]
- 85.Vermijlen D, et al. High-density oligonucleotide array analysis reveals extensive differences between freshly isolated blood and hepatic natural killer cells. Eur J Immunol. 2004;34:2529–2540. doi: 10.1002/eji.200324712. [DOI] [PubMed] [Google Scholar]
- 86.Tay CH, Welsh RM. Distinct organ-dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells. J Virol. 1997;71:267–275. doi: 10.1128/jvi.71.1.267-275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Loh J, Chu DT, O’Guin AK, Yokoyama WM, Virgin HW. Natural killer cells utilize both perforin and gamma interferon to regulate murine cytomegalovirus infection in the spleen and liver. J Virol. 2005;79:661–667. doi: 10.1128/JVI.79.1.661-667.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Amemiya CT, Saha NR, Zapata A. Evolution and development of immunological structures in the lamprey. Curr Opin Immunol. 2007;19:535–541. doi: 10.1016/j.coi.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


