Abstract
Objectives
Organophosphorus (OP) pesticides exert a tremendous health burden, particularly in the developing world. Limited resources, the severity of intentional OP ingestions, and a paucity of beneficial therapies all contribute to the morbidity and mortality of this broad class of chemicals. A novel theoretical treatment for OP poisoning is the use of an enzyme to degrade the parent OP in the circulation after poisoning. The aims of this study were to determine the pharmacokinetics and efficacy of an OP hydrolase (OpdA) in a rodent model of severe methyl-parathion poisoning.
Methods
Two animal models were used. First, Wistar rats were administered two different doses of the hydrolase (0.15 mg/kg and 1.5 mg/kg), and the ex vivo hydrolytic activity of plasma was determined by a fluorometric method. Second, an oral methyl-parathion animal poisoning model was developed to mimic severe human poisoning, and the efficacy of post-poisoning OpdA (as measured by survival to 4 hours and 24 hours) was determined.
Results
The half-life of OpdA in the Wistar rat was dependent upon the dose administered, and ranged between 45.0 and 57.9 minutes. The poisoning model of three times the lethal dose to 50% of the population (3×LD50) of methyl-parathion resulted in 88% lethality at 4 and 24 hours. Using a single dose of 0.15 mg/kg OpdA 10 minutes after poisoning resulted in 100% survival at 4 hours (p = 0.001 vs. placebo), but 0% at 24 hours post-poisoning (p = NS vs. placebo).
Conclusions
The OP hydrolase OpdA exhibits pharmacokinetics suitable for repeated dosing and increases short-term survival after severe methyl-parathion poisoning.
Keywords: organophosphate, pesticide, hydrolase
INTRODUCTION
As a leading cause of premature death in many developing countries, organophosphorus (OP) pesticide poisoning kills an estimated 200,000 people every year in the Asia-Pacific region alone.1 In North America, the situation is quite different. While occupational and suicidal pesticide poisoning do occur, the principal fear of large-scale OP poisoning is from terrorist attacks on civilian or military populations. Exposure through the release of OP nerve gases in crowded spaces, or the introduction of highly toxic pesticides into urban water supplies, is a potential terrorist scenario. The exposure of OPs through accidental industrial or agricultural release, or secondary to a natural disaster is also a possibility.
The acute toxicity of OPs is related primarily due to inhibition of central and peripheral acetylcholinesterase (AChE).2,3 Current therapy for OP poisoning consists primarily of resuscitation with oxygen and atropine, followed by administration of oximes to reactivate AChE, plus benzodiazepines to mitigate neurological sequelae.4,5 However, these antidotes have limited effectiveness. Depending on the responsible OP, between 10% and 40% of poisoned patients die, even in developed countries.6
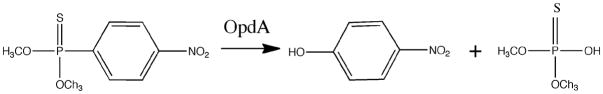
Several enzymatic systems are involved in the detoxification of OPs, including phosphotriesterases (PTEs).7,8 The products of this enzymatic hydrolysis lack any AChE phosphorylating properties, and thus do not inhibit AChE9–11 (Figure 1). Therefore, these enzymes have been proposed as candidates for therapy of acute OP exposure.12,13
Figure 1.
Schematic of methyl-parathion hydrolysis by OpdA to paranitrophenol and dimethyl phosphorothioic acid.
Bacterial PTEs hydrolyze a large number of OP substrates, including nerve agents,14 with higher efficacies than mammal PTEs. OpdA (a metal-dependent OP hydrolase) from Agrobacterium radiobacter has recently been characterized and shown to hydrolyze many chemically distinct OPs.8,15 Decreasing blood OP concentrations via the degradation of the OP pesticide in combination with current standard care could decrease OP-induced mortality. The purpose of these studies was to determine the pharmacokinetics of OpdA in a rat model, and to determine the efficacy of OpdA in a lethal rat model of OP poisoning with methyl-parathion.
METHODS
Study Design
This was a laboratory study utilizing a murine model. All animals were acquired and cared for in accordance with NIH guidelines. The Institutional Animal Care and Use Committee of the University of Massachusetts Medical School approved the study protocol.
Animal Handling
Male Wistar rats weighing 250 ±50 g (Charles River Laboratories, Wilmington, MA) were housed in pairs, maintained on 12:12 light:dark cycle, and provided food and water ad libitum except for 2 hours prior to experimentation. The details of OpdA generation have been published elsewhere.16
Kinetic Protocol
Animals were briefly anesthetized for 1–2 minutes with isoflurane while a 24-gauge intravenous (IV) lateral tail vein catheter was placed. OpdA at doses of 0.15 or 1.5 mg/kg were administered IV in a volume of 1.5 mL/kg to 16 rats at each dose. To determine the kinetics of OpdA, two rats were euthanized by cardiac puncture blood draw and bilateral pneumothoraces under isoflurane anesthesia at the following time points after OpdA injection: 5, 10, 20, 40, 80, 160, and 320 minutes. Two rats underwent phlebotomy just prior to OpdA administration in order to determine the baseline rate of hydrolysis in the blood. The blood was rapidly cooled to 4°C and centrifuged at this temperature for 5 minutes at 2,000 × g. The red blood cells were removed and the plasma frozen at −20°C until analyzed.
A fluorometric assay of the levels of bioactive OpdA was used to determine the half-life of OpdA in vivo. For the fluorometric assay, saturating concentrations of coumaphos (100μM) were added to plasma samples and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer. Formation of the fluorometric hydrolysis product of coumaphos, chlorferon, was measured in real time at 37°C using a fluorimeter (Fluoroskan Ascet, Thermo Fisher Scientific, Waltham, MA) using an excitation wavelength of 355 nm and emission intensity at 460 nm according to the method of Harcourt et al.17 The known specific activity of OpdA for coumaphos was used to calculate residual activity of the enzyme present in the blood. The half-life was obtained by analyzing the clearance data according to a non-compartmental pharmacokinetic model using WinNonlin software (Pharsight, St. Louis, MO).
Efficacy Protocol
Animals were anesthetized for 1–2 minutes with isoflurane while a 24-gauge IV lateral tail vein catheter was placed. Immediately upon awakening, three times the oral LD50 for methyl- parathion (LD50 = 10 mg/kg) (Sigma-Aldrich, St. Louis, MO) suspended in peanut oil was given to a total of 16 rats (n = 8 rats per group) via enteral gavage in a volume of 1.5 mL/kg. The LD50 for methyl-parathion was determined from literature reviews.
Rats were divided into two groups based upon treatment. Ten minutes after poisoning, negative control rats received normal saline placebo IV, and rats in the treatment group were given 0.15 mg/kg OpdA IV. All injections were given via a 24-gauge catheter (Surflo catheter, Terumo Corporation, Somerset, NJ) through a lateral tail vein in volumes of 0.5 mL. We chose 0.15 mg/kg of OpdA in order to give a margin of error based upon an estimated minimum effective dose of 0.05 mg/kg OpdA derived from in vitro data, while remaining within the range of larger OpdA doses given in preliminary safety dosing studies (data not shown). Nitrile gloves and chemical safety goggles were worn when working with the methyl-parathion.
Outcomes of interest were 4 hour and 24 hour survival, as assessed by a researcher blinded to the treatment received. Rats were observed at least hourly for 16 hours, then again 24 hours after poisoning.
Data Analysis
Grouped survival data were compared using a two-tailed Fisher’s exact test. Three times the LD50 of methyl-parathion was used in order to mimic severe human poisoning and to assure that all, or nearly all, control animals would die, thereby decreasing the number of animals needed to demonstrate statistical significance. For 80% power to detect a 50% reduction in mortality at any time point, assuming an alpha of 0.05, eight animals per group were required. Because there were two primary endpoints (4 hours and 24 hours), a Bonferroni correction to the type I error was performed. Therefore, a p value of less than 0.025 was considered significant. All statistical analyses were performed with GraphPad Prism software version 4 for Mac (GraphPad Software, Inc., San Diego, CA).
RESULTS
Pharmacokinetics of OpdA
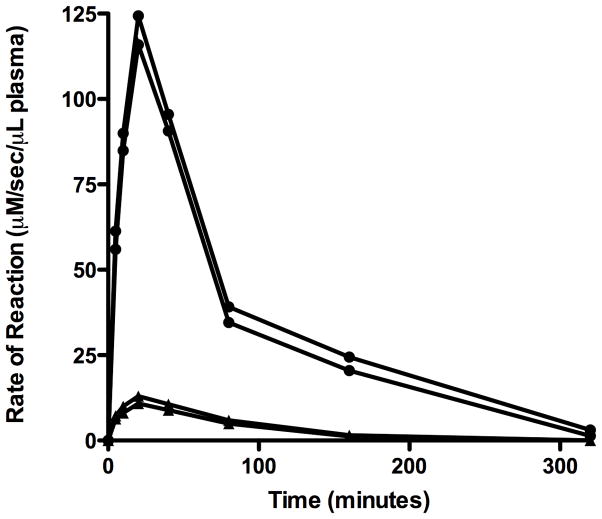
There was very little intrinsic in-vivo coumaphos hydrolytic activity of blood. Significant enzyme activity was apparent 5 minutes after injection, with peak enzymatic effect observed 20 minutes after administration of OpdA. Kinetic curves of the 0.15 and 1.5 mg/kg OpdA doses are shown in Figure 2. Using the time points of 20 and 160 minutes, at a dose of 0.15 mg/kg, the in vivo half-life of OpdA in this model was calculated to be 45.0 minutes. Using the same time points, at a dose of 1.5 mg/kg, the in vivo half-life was calculated to be 57.9 minutes.
Figure 2.
Kinetics of 0.15 m/kg (▲) and 1.5 mg/kg (●) OpdA given intravenously.
Methyl-parathion oral poisoning model
Seven of eight control rats developed muscarinic (salivation, urination, defecation) and nicotinic (fasciculations) signs within 30 minutes of methyl-parathion administration. Seven of these eight rats died by 159 minutes (median time to death 79 minutes) (Figure 3). One rat exhibited no signs of OP poisoning at any time.
Figure 3.
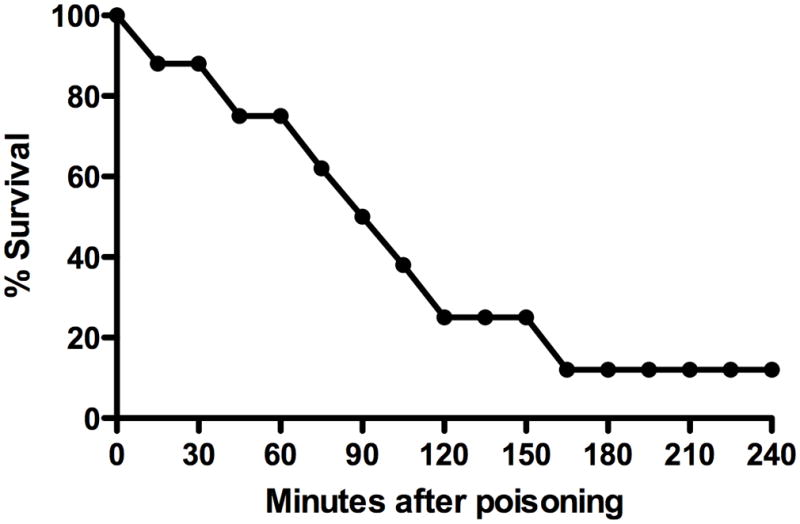
Time to death in control rats following 3×LD50 of oral methyl-parathion.
Efficacy of OpdA versus methyl-parathion
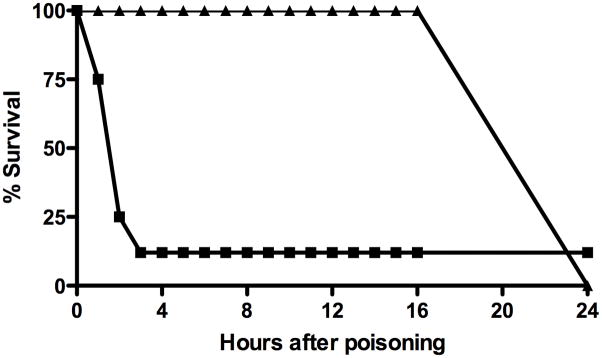
After a single dose of 0.15 mg/kg OpdA, all rats survived to 4 hours (p = 0.001 vs. placebo), but none survived to 24 hours (p = NS vs. placebo) (Figure 4). In the first 16 hours after poisoning, no rats exhibited signs of cholinergic excess.
Figure 4.
Survival following 3×LD50 of methyl-parathion with placebo (■) and 0.15 mg/kg intravenous OpdA (▲).
DISCUSSION
Data from these studies demonstrate that in this rat model, OpdA has pharmacokinetics favorable for use in OP poisoning. These kinetic parameters include rapid enzymatic effects after administration, and a half-life of approximately one hour at a dose of 1.5 mg/kg. Furthermore, decreased short-term lethality after a single dose when administered post-poisoning in this model provides proof of concept that OpdA is an adequate candidate therapeutic hydrolase.
OpdA is an excellent candidate enzyme for clinical use in acute OP exposures for a number of reasons. OpdA is a very efficient enzyme, performing at near diffusion-limited rates towards its favored substrates in vitro,18 thus allowing a small dose of enzyme to degrade a large amount of OP. Furthermore, OpdA is active against a wide range of OP pesticides, including substrates with a phosphoryl sulfur (rather than an oxygen), and substrates with both methoxy and ethoxy groups.16 There are several hundred different OP agents world-wide, and therapeutic modalities active against a narrow spectrum of OP agent would find limited clinical utility. Finally, although few people have ever been exposed to, or died from, nerve agents such as soman (GD) or VX, most current research on acetylcholinesterase inhibitors has focused on these military weapons. Recently, OpdA has been shown to possess catalytic activity towards the nerve agents,19 thus raising the possibility of developing OpdA as a therapy for OP and nerve agent poisoning.
Published kinetic data on other recombinant enzymes do not demonstrate broad substrate affinity towards many of the problematic OPs in the developing world. Because so many different OP pesticides are available around the world, and the causative agent would likely be unknown immediately after poisoning, a hydrolase for clinical use would need to be effective against a large number of chemically diverse OPs. The situation will not be very different after intentional contamination of water supplies, since the responsible pesticide is unlikely to be identified for several hours.
In the acute OP pesticide-poisoning scenario, it is unlikely that OpdA would be required after the first few hours or perhaps a couple of days. Although multiple doses of OpdA may be required, it is unlikely that prolonged treatment would be needed. Therefore, theoretical concerns regarding prolonged re-exposure to a bacterial OP hydrolase such as OpdA (e.g.: anaphylaxis) are not particularly relevant.
Despite very good in vitro efficacy against methyl-parathion, a single dose of 0.15 mg/kg OpdA did not eliminate mortality. The effect of OpdA was short lived, with 100% mortality at 24 hours post-poisoning. However, this could be explained by the physicochemical properties of methyl-parathion and the relatively short half-life of OpdA. Methyl-parathion requires metabolism from its native thion form to the biologically active oxon form, and also distributes to fat. The lipophilicity decreases the quantity of methyl-parathion available in circulation for OpdA hydrolysis, and also provides a depot for prolonged leaching of methyl-parathion back into circulation. However, a single dose of OpdA could potentially lower serum OP concentrations to the point where traditional therapies (e.g. atropine and pralidoxime) are sufficient. This theory is borne out in data from a Sri Lankan cohort of patients poisoned with dimethoate. In their study, Eddleston et al.20 found a linear relationship between serum dimethoate concentration and mortality. Like methyl-parathion, dimethoate is a dimethyl thion OP pesticide that requires bioactivation to its toxic oxon form. Therefore, simply decreasing the serum OP concentration to some limit, rather than total degradation, is likely to improve survival. The limit to which an OP would need to be decreased will depend on the individual OP itself.
LIMITATIONS
Rodent models are commonly used in OP pesticide and nerve agent research. The high degree of toxicity, the large number of animals required to demonstrate statistical significance, and tremendous costs generally preclude the use of larger animals or non-human primates. Thus, the results obtained here may not be fully applicable to other animal models or humans.
To eliminate the confounding effects of anesthesia, our studies were performed on awake, unanesthetized animals. However, because the experiments were performed on awake animals, we were unable to obtain extensive physiologic data such as invasive blood pressure, arterial blood gas measurements, or electroencephalography monitoring to support our findings. Similarly, because of the limited blood volume, we could not perform serial phlebotomy necessary to quantitate the methyl-parathion or its metabolite paranitrophenol.
Because of our small sample size and brief survival in the negative control animals, we could not reliably employ survival analyses such as Cox proportional hazards regression to compare time to death between the two groups, and instead used a simple Fisher’s exact test. The Cox proportional hazards regression method computes a coefficient for each variable that indicates the direction and amount of benefit (or harm) that the variable exerts on survival. Future studies using longer survival times and other treatment variables (such as atropine or pralidoxime) would be strengthened by the use of such survival analyses.
CONCLUSIONS
The in vivo half-life of the OP hydrolase OpdA is dependent upon the dose administered in this rodent model, with larger doses of the hydrolase exhibiting a longer half-life. Use of a single dose of OP hydrolase significantly reduced the 4-hour lethality of methyl-parathion in this rodent model, with no effect on 24-hour survival. The tremendous public health burden caused by OP pesticides, the potential use of OP pesticides against military and civilian targets, and the encouraging preclinical studies of OP hydrolases should stimulate accelerated research and human trials of broadly active and low-cost therapeutic OP hydrolases.
Acknowledgments
This was an NIH-funded study: NIEHS R21 ES014019.
References
- 1.Jeyaratnam J. Acute pesticide poisoning: a major global health problem. World Health Stat Q. 1990;43:139–44. [PubMed] [Google Scholar]
- 2.Sidell FR. Clinical effects of organophosphorus cholinesterase inhibitors. J Appl Toxicol. 1994;14:111–3. doi: 10.1002/jat.2550140212. [DOI] [PubMed] [Google Scholar]
- 3.Thiermann H, Szinicz L, Eyer P, Zilker T, Worek F. Correlation between red blood cell acetylcholinesterase activity and neuromuscular transmission in organophosphate poisoning. Chem Biol Interact. 2005;157–158:345–7. doi: 10.1016/j.cbi.2005.10.102. [DOI] [PubMed] [Google Scholar]
- 4.Bird SB, Gaspari RJ, Dickson EW. Early death due to severe organophosphate poisoning is a centrally mediated process. Acad Emerg Med. 2003;10:295–8. doi: 10.1111/j.1553-2712.2003.tb01338.x. [DOI] [PubMed] [Google Scholar]
- 5.Eddleston M, Singh S, Buckley N. Organophosphorus poisoning (acute) Clin Evid (BMJ) 2005;13:1744–55. [PubMed] [Google Scholar]
- 6.Eyer F, Meischner V, Kiderlen D, et al. Human parathion poisoning. A toxicokinetic analysis. Toxicol Rev. 2003;22:143–63. doi: 10.2165/00139709-200322030-00003. [DOI] [PubMed] [Google Scholar]
- 7.Jokanovic M. Biotransformation of organophosphorus compounds. Toxicology. 2001;166:139–60. doi: 10.1016/s0300-483x(01)00463-2. [DOI] [PubMed] [Google Scholar]
- 8.Horne I, Sutherland TD, Harcourt RL, Russell RJ, Oakeshott JG. Identification of an opd (organophosphate degradation) gene in an Agrobacterium isolate. Appl Environ Microbiol. 2002;68:3371–6. doi: 10.1128/AEM.68.7.3371-3376.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sogorb MA, Vilanova E. Enzymes involved in the detoxification of organophosphorus, carbamate and pyrethroid insecticides through hydrolysis. Toxicol Lett. 2002;128:215–28. doi: 10.1016/s0378-4274(01)00543-4. [DOI] [PubMed] [Google Scholar]
- 10.Vilanova E, Sogorb MA. The role of phosphotriesterases in the detoxication of organophosphorus compounds. Crit Rev Toxicol. 1999;29:21–57. doi: 10.1080/10408449991349177. [DOI] [PubMed] [Google Scholar]
- 11.Sutherland TD, Horne I, Weir KM, et al. Enzymatic bioremediation: from enzyme discovery to applications. Clin Exp Pharmacol Physiol. 2004;31:817–21. doi: 10.1111/j.1440-1681.2004.04088.x. [DOI] [PubMed] [Google Scholar]
- 12.Bird SB, Dawson A, Ollis D. Enzymes and bioscavengers for prophylaxis and treatment of organophosphate poisoning. Front Biosci. 2010;S2:209–20. doi: 10.2741/s58. [DOI] [PubMed] [Google Scholar]
- 13.Sogorb MA, Vilanova E, Carrera V. Future applications of phosphotriesterases in the prophylaxis and treatment of organophosporus insecticide and nerve agent poisonings. Toxicol Lett. 2004;151:219–33. doi: 10.1016/j.toxlet.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Raushel FM. Bacterial detoxification of organophosphate nerve agents. Curr Opin Microbiol. 2002;5:288–95. doi: 10.1016/s1369-5274(02)00314-4. [DOI] [PubMed] [Google Scholar]
- 15.Yang H, Carr PD, McLoughlin SY, et al. Evolution of an organophosphate-degrading enzyme: a comparison of natural and directed evolution. Protein Eng. 2003;16:135–45. doi: 10.1093/proeng/gzg013. [DOI] [PubMed] [Google Scholar]
- 16.Jackson CJ, Foo JL, Tokuriki N, et al. Conformational sampling, catalysis, and evolution of the bacterial phosphotriesterase. Proc Natl Acad Sci USA. 2009;106(51):21631–6. doi: 10.1073/pnas.0907548106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harcourt RL, Horne I, Sutherland TD, Hammock BD, Russell RJ, Oakeshott JG. Development of a simple and sensitive fluorimetric method for isolation of coumaphos-hydrolysing bacteria. Lett Appl Microbiol. 2002;34:263–8. doi: 10.1046/j.1472-765x.2002.01078.x. [DOI] [PubMed] [Google Scholar]
- 18.Jackson CJ, Weir K, Herlt A, et al. Structure-based rational design of a phosphotriesterase. Appl Environ Microbiol. 2009;75:5153–6. doi: 10.1128/AEM.00629-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dawson RM, Pantelidis S, Rose HR, Kotsonis SE. Degradation of nerve agents by an organophosphate-degrading agent (OpdA) J Hazard Mater. 2008;157:308–14. doi: 10.1016/j.jhazmat.2007.12.099. [DOI] [PubMed] [Google Scholar]
- 20.Eddleston M, Eyer P, Worek F, Sheriff MH, Buckley NA. Predicting outcome using butyrylcholinesterase activity in organophosphorus pesticide self-poisoning. QJM. 2008;101:467–74. doi: 10.1093/qjmed/hcn026. [DOI] [PMC free article] [PubMed] [Google Scholar]