Abstract
Objective
Inflammatory cytokines may influence both labile anger and depression. Both psychiatric conditions can occur during interferon-alpha (IFN–α) based treatments. Evidence also indicates a central nervous system role for TNF-α, whose expression may be increased by IFN-α. A polymorphism in the promoter region of TNF-α has been associated with various inflammatory illnesses. We therefore hypothesized that this TNF-α polymorphism would influence susceptibility to psychiatric symptoms during IFN-α therapy.
Methods
105 patients with hepatitis C, initially without active major depression (MDD), were treated with IFN-α and then prospectively monitored using the Structured Clinical Interview for DSM-IV, the Beck Depression Inventory-II (BDI), the Anger Irritability and Assault Questionnaire, and circulating TNF-α levels. The A-308G polymorphism (rs1800629) was determined using the 5′-nuclease assay. Repeated-measure mixed-effect analyses compared changes in symptoms over time.
Result
BDI increased during IFN-α therapy (F = 6.2; p<0.001), with 27% developing MDD. The TNF-α A allele was associated with worsened labile anger (F = 2.5; p<0.05) and fatigue (F = 2.9; p<0.05) during treatment, but not with major depression incidence (X2 = 0.0; p=0.99) or increased BDI (F = 1.2; p=0.31). Labile anger was not predicted by the serotonin transporter polymorphism (F = 0.8; p=0.59).
Discussion
During treatment with an exogenous cytokine, vulnerability to worsening labile anger -- distinct from major depression -- is associated with genetic variability in TNF-α. This has implications both for patients being treated with IFN-α, as well as our understanding of genetic vulnerability for different subtypes of dysphoric and mood disorders.
Keywords: Cytokine, inflammation, genetic, polymorphism, anger
Introduction
Inflammation may be involved in the etiology of mood disorders.1 Genetic polymorphisms in the inflammatory system have been associated with major depressive disorder (MDD),2 and twins concordant for MDD have elevated levels of inflammatory activity.3 Additionally, inflammatory cytokines are implicated in the neurobiology of aggression,4 and mice bred for high aggression have an elevated pro-inflammatory response.5 Elevated production of tumor necrosis factor-α (TNF-α) has been associated with hostility in humans.6-8 ‘Anger attacks’ have been described during MDD9 and various other psychiatric diagnoses.10 Abnormal regulation of anger, with both genetic and neurobiologic influences, can be related to significant violence.11, 12 Labile anger can be measured by inquiring about having loss of control over switches in temper; sudden angry feelings with the urge to yell; or feeling so mad as to experience shaking, a pounding heart, and/or the urge to hit.
Consistent with a possible relationship between anger dysregulation and inflammatory cytokines, labile anger and hostility have been observed during exogenous interferon-alpha (IFN-α therapy.13-20 During treatment with IFN-α, between 15-40% of patients also develop MDD.21 However, worsened labile anger may be distinct from MDD.22, 23 IFN-α based therapies are the primary recommendation for chronic hepatitis C,20, 24 which affects over 170 million people and results in about 10,000 deaths per year in the U.S. 25, 26 Importantly, not everyone exposed to elevated inflammatory cytokines such as IFN-α develops worsened labile anger or MDD.
Genetic variation in the serotonergic system may be a source of resilience to developing MDD.27-29 Less is know about resilience or vulnerability for developing worsened labile anger. However, genetic variation in tumor necrosis factor-alpha (TNF-α may be a plausible influence. TNF-α is biologically active in the brain and can influence synaptic strength,30 ion channels,31 synaptic scaling,32 neurogenesis,33 and serotonin transporter function.34 It is produced throughout the limbic and hypothalamic regions, where it can be influenced by peripheral inflammatory cytokines.35-39 TNF-α can mediate the induction of indolamine deoxygenase and depression-like behaviors following peripheral inflammation.40 Peripherally administered TNF-α can affect both neurotransmitter and endocrine release.41, 42 In humans, anti-TNF-α therapy may alleviate some mood related symptoms.43-45 The ‘A’ allele in a promoter region of the TNF-α gene (A-308G) has been associated with higher TNF-α plasma levels,46-48 arthritis,49, 50 AIDS-associated dementia,51 Parkinson's disease,52 Alzheimer's,53 asthma,54-56 and metabolic syndrome.57 In elderly patients, the A allele is associated with cognitive dysfunction.58
To date however, evidence for an association between the A allele and mood disorders is equivocal. Small studies suggest an association.59-61 Conversely, other reports no association with MDD but a possible protective association with bipolar II disorder.62 Several larger studies have found no association with childhood mood disorder63-65 nor with post-partum mood disorder.66 Whether it is associated with risk for labile anger has not been examined. Therefore, in subjects with elevated states of peripheral inflammation as a result of IFN-α administration, we prospectively examined the association of this candidate polymorphism with the development of either labile anger or depression.
Materials and methods
We followed subjects prescribed IFN-α as described previously,28, 67 and as approved by the University of Pittsburgh Institutional Review Board. In brief, patients with chronic hepatitis C (n=105) who were initiating pegylated (PEG) IFN-α2 (PEG-IFN-α2a: 135 mg/week or PEG-IFN-α2b: 120 or 150 mg/week) and oral ribavirin treatment were examined pre-treatment and then for 16 weeks during therapy.68-72 Patients were excluded from these analyses if they had active mood, anxiety, psychotic, drug/alcohol use disorders within 6 months prior to starting IFN-α. As antidepressants can affect mood, labile anger,73 and/or cytokine levels,74 data from individuals on antidepressants were excluded.
MDD during IFN-α treatment was diagnosed using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I).75 The 21 item Beck Depression Inventory-II (BDI) was used to assess depressive symptoms at week two and during monthly visits (but could be returned by mail if the participant was unable to attend the scheduled appointment).76 The Anger Irritability and Assault Questionnaire (AIAQ), a 28 item self-report,77 was similarly employed to prospectively assess labile anger. Items on this questionnaire are scored 0 to 3, with subscales that include labile anger (range 0-18), irritability, and assault.
Monthly plasma samples were used for enzyme linked immunosorbent assays to determine TNF-α levels (Alpco, Salem, NH; sensitivity = 1.56pg/mL), and were run in duplicate. Genomic DNA was isolated from lymphocytes using the PureGene kit (Gentra Systems, Minneapolis, MN), or from whole blood using the QuickGene-Mini-80 kit (Fujifilm Life Science; www.autogen.com). A-308G (rs1800629) was genotyped using the 5′-nuclease (Taqman) assay using the ABI 7900 DNA detection system, employing Assays-on-Demand and Assays-by-Design (Applied Biosystems, Inc., Foster City, CA). The uncommon A/A genotype was combined with the A/G heterozygotes for statistical analyses. The serotonin transporter promoter length polymorphism (5-HTTLPR) was assessed as previously described.28
All statistics employed SPSS 17.0. Skewed TNF-α levels were corrected using a square root transformation. Repeated-measure mixed-effect analyses with an ante-dependence covariance structure78 were used to compare changes in either subjective symptoms over time (i.e., assessing an interaction between genotype and time). We confirmed the appropriate choice of covariance structure, as the ante-dependence model produced the smallest -2 log likelihood (data not shown). Kaplan-Meier survival analyses examining time until MDD development were compared using the Mantel-Cox log rank test. Results are presented as the mean +/- standard deviation, unless otherwise indicated.
Results
Participants were 67% male, 90% European-American, 47 +/-11 years (18-72), 87 +/- 17 Kg (46-144 Kg), 44% had any prior history of mood disorder, and 34% had any prior history of drug/alcohol use disorder. Demographics did not differ between those who developed MDD and those who did not (Table 1), nor did brand of IFN-α or dose of ribavirin. The TNF-α polymorphism was in Hardy-Weinberg Equilibrium with 4 A/A, 29 A/G, and 72 G/G. The A allele was more prevalent in African Americans (t(103) = 2.5); but otherwise demographics did not differ between genotypes (Table 1). Because of racial differences in the prevalence of the A-308G polymorphism, we specifically examined Caucasian Americans; but we then repeated some analyses in all subjects regardless of self-reported race.
Table 1.
| MDD (n=27) | No MDD (n=78) | AA or AG (n=33) | GG (n=72) | |||
|---|---|---|---|---|---|---|
| Gender (percent male) | 66.7% | 68.0% | ns | 72.7% | 65.3% | ns |
| Race (percent European-American) | 85.2% | 91.0% | ns | 78.8% | 94.4% | P=0.015 |
| Age (years) | 46.5 +/- 7.9 | 47.0 +/- 12.7 | ns | 45.7 +/- 10.6 | 47.4 +/- 12.2 | ns |
| Weight (Kg) | 87.0 +/- 13.7 | 86.5+/- 18.7 | ns | 85.4 +/- 15.3 | 87.2 +/- 18.4 | ns |
| History of any mood disorder | 48.2% | 42.3% | ns | 36.4% | 47.2% | ns |
| History of drug or alcohol disorder | 37.0% | 33.3% | ns | 30.3% | 36.1% | ns |
There were no statistically significant (ns) differences in demographics between subjects who developed major depressive disorder (MDD) and those who didn't. The A allele was more prevalent in self-identified African-Americans.
TNF-α polymorphism is associated with labile anger development
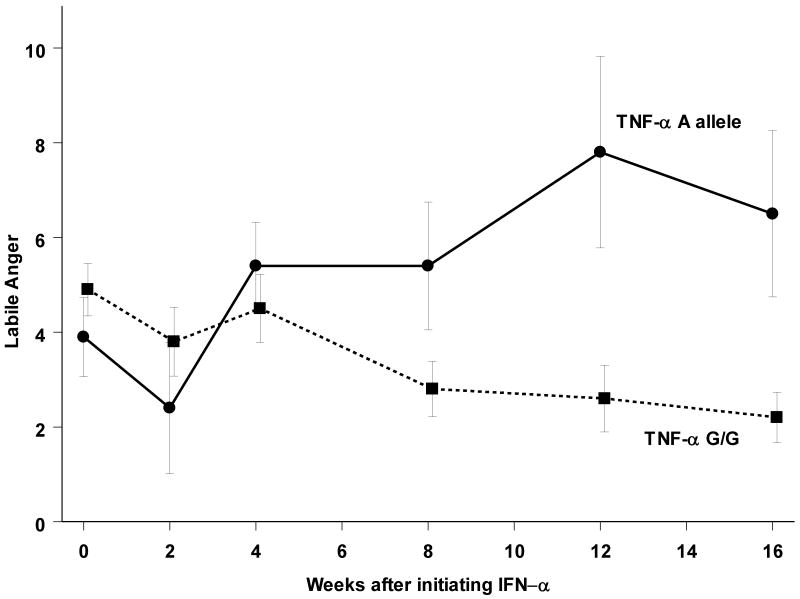
Among Caucasian subjects, labile anger increased more (Figure 1) in those with the A allele (F(5,66.4) = 2.5; p<0.05) compared to those with the G/G genotype. There was no significant relationship between race and the development of labile anger (F(5,79.4) = 1.9; p=0.09), and the genotype relationship with labile anger continued to be evident when including African-Americans in the analysis (F(5,70.2) = 3.1; p<0.05).
Figure 1.
Labile anger (mean +/- standard error the mean) increased more during IFN-α treatment in subjects with the A allele in the TNF-α upstream polymorphism.
TNF-α polymorphism is not associated with other mental health problems
Genotype was not associated with the development of irritability (p=0.45) or assault (p=0.72). Additionally, although sleep quality (as assessed with the Pittsburgh Sleep Quality Inventory) worsened over time (F(5,70.3) = 2.8; p<0.05), there was no relationship between genotype and worsening sleep quality (F(5,70.3) = 1.4; p=0.24). Time until development of MDD during IFN-α therapy, which occurred in 27 subjects, was also not associated with the TNF-α genotype in Caucasians (X(1)2 = 0.4; p=0.53) nor in all subjects combined (X(1)2 = 0.0; p=0.99). BDI increased over time during IFN-α therapy (F(5,87.4) = 6.2; p<0.001), but this was not associated with genotype (F(5,81.2) = 1.2; p=0.31). Nor was there any main effect of genotype on BDI (F(1,87.2) = 0.3; p=0.63). When including African-Americans in the analysis of BDI, there was a trend for those with the A allele to develop more depression symptoms (F(5,87.4) = 2.1; p<0.08), but this was not significant.
Exploratory analysis of TNF-α polymorphism and individual BDI items
Ten individual BDI items worsened during IFN-α treatment -- items #3,7,10,11,12,14,15,17,18, and 19 – past failure, self-dislike, crying, agitation, loss of interest, worthlessness, loss of energy, irritability, changes in appetite, and fatigue (results not shown). However, only two individual BDI measures worsened more in those with the A allele: loss of energy (question 15) (F(5,76.3) = 2.5; p<0.05) and fatigue (question 20) (F(5,86.5) = 2.7; p<0.05) (p values uncorrected for multiple testing).
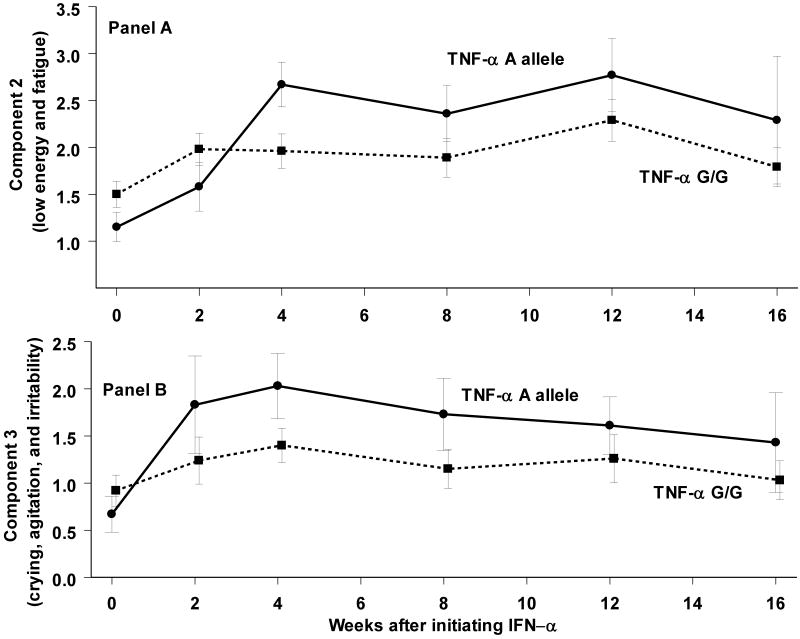
A principal component factor analysis of the 21 BDI items extracted 3 components with an eigenvalue >1. The first component correlated (>0.4) with most questions except #10 (crying), #18 (changes in appetite) and # 21 (loss of interest in sex). The second correlated (>0.4) with #15 (loss of energy) and #20 (fatigue). The third correlated (>0.4) with #10 (crying), #11 (agitation), and #17 (irritability). Genotype was associated with a combined measure of fatigue and loss of energy (F(5,78.0) = 2.9; p<0.05) (Figure 2a). An association between genotype and worsening of crying/agitation/irritability was not statistically significance (F(5,86.8) = 1.4; p=0.22) (Figure 2b).
Figure 2.
A composite measure of low energy and fatigue (Panel A) from the BDI (mean +/- standard error the mean) increased more during IFN-α treatment in subjects with the A allele in the TNF-α upstream polymorphism; this trend was similar for a composite measure of crying, agitation, and irritability (Panel B) but was not significant.
Association of TNF-α polymorphism and labile anger is not mediated by fatigue
The composite measure of fatigue/low energy was slightly correlated with labile anger (R=0.17; F=10.3; p<0.001), and both were associated with the TNF-α polymorphism. When including the composite fatigue/energy measure as a time-varying covariate in the repeated measure longitudinal mixed-effect analysis, the A allele continued to predict worsening of labile anger during IFN-α treatment (F(5,70.4) = 2.9; p<0.05), arguing against mediation by fatigue.
Peripheral TNF-α levels and psychiatric symptoms
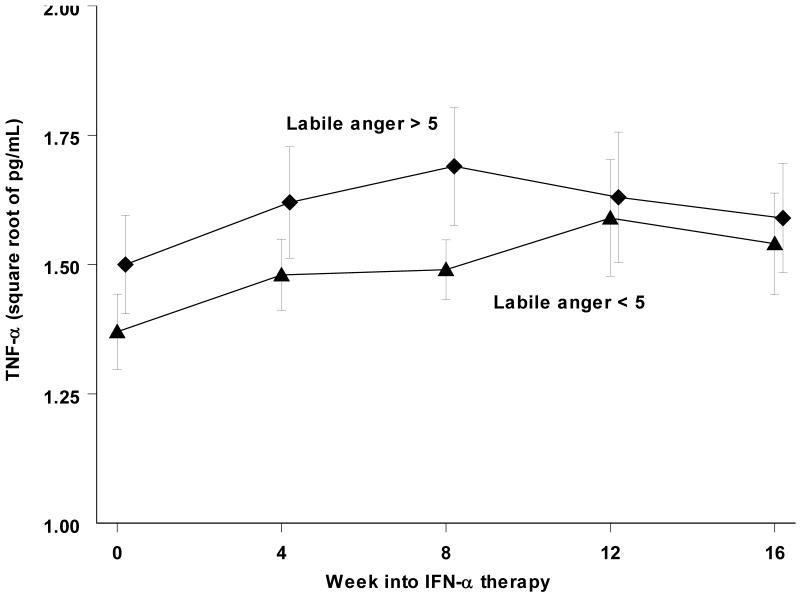
Monthly TNF-α levels (Figure 3) increased during IFN-α treatment (F(4,54.9) = 4.4; p<0.005). But this was not associated with genotype (F(4,45.8) = 0.4; p=0.78). Those who developed MDD trended towards greater TNF-α levels during treatment (F(4.47.8) = 2.3; p=0.08). We next compared TNF-α levels in those with labile anger scores anytime during treatment > 5 (the median split point) with those scoring 5 or less (Figure 3). The difference in levels was not significant (F(1,75.5) = 1.1; p=0.30).
Figure 3.
Systemic TNF-α levels (mean +/- standard error the mean) increased during treatment. In subjects with labile anger scores greater than 5 at any time during treatment, this elevation was not significantly higher from those whose scores remained 5 or less.
Including peripheral TNF-α levels as a covariate, we repeated the repeated-measure mixed-effect analysis of genotype on labile anger. The association between the A allele and increased labile anger remained (F(4,44.5) = 15.7; p<0.001). It is therefore unlikely that peripheral TNF-α levels mediate the relationship between the A-308G polymorphism and worsening labile anger.
5-HTTLPR is not associated with labile anger development
Because we have previously reported a relationship between the 5-HTTLPR genotype and MDD during IFN-α therapy in an overlapping set of these patients,28 we assessed an association between 5-HTTLPR and the development of labile anger. No relationship was found (F(10,52.3) = 0.8; p=0.59).
Discussion
The A allele in the -308 promoter region of TNF-α was specifically associated with worsening labile anger during IFN-α treatment. Labile anger was based on questions in the AIAQ about having minimal control over switches in temper; feeling normally “OK” but then suddenly feeling angry or furious with the urge to yell; or feeling so mad as to experience shaking, a pounding heart, and/or the urge to hit something.77 Of note, the A allele was specifically associated labile anger and not with irritability or increases in assault behaviors (either verbal or physical). We also did not find any association between the TNF-α polymorphism and the development of categorical MDD or worsening BDI, consistent with most prior studies.62-65
Several groups have independently described increases in hostility-related complaints in patients receiving IFN-α treatment,13-20 something very disruptive for patients.17 This phenomenon occurs whether depression develops or not,22 and is orthogonally distinct from increased depression on the symptom checklist-90.23 Aggression and depression are under genetic influence in childhood twin studies, albeit via potentially distinct pathways.79 Also, 5-HTTLPR was associated with risk for MDD during IFN-α treatment,27, 28 but not risk for labile anger. There may be a relationship between elevated peripheral interleukin-6 (IL-6) levels and MDD during IFN-α therapy,67, 80 despite no relationship between TNF-α levels and MDD. This is consistent with reports of elevated systemic IL-6 but not TNF-α in “idiopathic” MDD.81
It is entirely speculative as to what this labile-anger syndrome represents. ‘Anger attacks’ could be related to a depression subtype,9 impulsive aggression,10 mixed mood disorder,70, 82 or an entirely distinct syndrome. Consistent with a ‘mixed mood disorder’ hypothesis, mood dysregulation may be related to risk for bipolar disorder;83-89 bipolar disorder and elevated TNF-α levels both may predict worse response to classical antidepressants; 90, 91 and elevated TNF-α levels may occur in bipolar disorder.92 Inconsistent with a ‘mixed mood disorder’ hypothesis, we very rarely observed grandiosity, decreased sleep requirements, or increased goal-directed activities during IFN-α treatment. Future work will be required to further delineate this labile anger ‘syndrome’. An important limitation of this study is that our assessment of labile anger was limited to this single self-report questionnaire, and thus the findings should be considered preliminary.
Anger during depression has been previously examined in genetic association analyses, with possible associations with CREB1,93 ABCG1 transporter,94 tryptophan hydroxylase,95 monoamine oxidase A,11 and catecholamine-O-methyl transferase.96 A limitation to this study is that we also did not examine these genes or several other polymorphisms that are upstream from the TNF-α transcription start site (e.g. positions -863, -376, -244, -238). Future work will be required to more fully examine the role of genetic variation across the TNF-α gene and other candidate genes in labile anger.
We did examine whether worsening fatigue mediates the increased labile anger. Fatigue and malaise are well-known symptoms of IFN-α treatment,23, 97 and TNF-α has been associated with malaise in animal models.98 Although TNF-α genotype was associated with increased fatigue, no mediation was found. Thus, the A allele may increase sensitivity to both fatigue as well as labile anger, albeit independently.
Also, circulating levels of TNF-α were neither associated with psychiatric symptoms nor genotype. This is inconsistent with higher plasma TNF-α levels being associated with the A allele during inflammation46-48 and increased depressive symptoms correlating with lymphocyte production of TNF-α.99 Thus, our lack of associations with circulating TNF-α levels should be interpreted with caution. Nonetheless, the A-308G genotype has been associated with cognitive dysfunction in elderly subjects despite no association between genotype and peripheral TNF-α levels,58 similar to our study. It is possible that central TNF-α may be of more relevance to psychiatric symptoms.
TNF-α levels in the brain can be influenced by stress or peripheral inflammation35, 36, 38 as well as peripheral cytokines.39 Similarly, IFN-α decreases cell proliferation in the hippocampus by induction of local cytokines.100 Therefore, it is plausible that the A-308G polymorphism could particularly influence localized central nervous systems expression of TNF-α during IFN-α treatment. Consistent with this speculation, the A-308G polymorphism may affect the binding of some transcriptional regulators but not others.101-105 Enhanced production of quinolinic acid may mediate some psychiatric effects of IFN-α106 and elevated central TNF-α could be influential on this pathway.40 Also, increased central nervous system TNF-α can increase norepinephrine and serotonin turnover,107 possibly mediated by its effect on c-jun-N-terminal kinase.108 As TNF-α can be induced in the limbic system, any of these central TNF-α influences could be a plausible mechanism leading to labile anger. Nonetheless, the pathway by which the A allele in the TNF-α promoter region specifically leads to enhanced labile anger requires future investigation.
In summary, the risk for inflammation-related labile anger may be enhanced in subjects with the A allele in the promoter region for TNF-α. However, it should be reiterated that this study was specifically performed in patients undergoing therapy with IFN-α. Whether TNF-α is associated with labile anger in other clinical situations requires examination. A strength of the IFN-α paradigm is the ability to prospectively assess the development of symptoms; however the limitation is the lack of generalization to other “stressful” or inflammatory scenarios. Also, although hypothesis driven, these analyses had no correction for multiple testing and require replication. Regardless, the results indicate that genetic variation upstream from TNF-α may influence labile anger during a state of heightened inflammation without concomitantly influencing risk for depression.
Acknowledgments
The authors thank Nancy Petro for assistance with genotyping. Funding for this study was provided by NIMH grant K23 MH074012 (FEL); the NIMH had no further role in study design; in the collection, analysis and interpretation
Contributor Information
Francis E. Lotrich, Department of Psychiatry, Western Psychiatric Institute and Clinic, University of Pittsburgh School of Medicine, Pittsburgh, PA
Robert E. Ferrell, Department of Human Genetics, University of Pittsburgh, Pittsburgh, PA
Mordechai Rabinovitz, Department of Medicine, Division of Gastroenterology, Hepatology, and Nutrition, University of Pittsburgh School of Medicine, Pittsburgh, PA
Bruce G. Pollock, Rotman Research Institute and the Centre for Addiction and Mental Health, University of Toronto, Canada
References
- 1.Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain, Behavior, and Immunity. 2007;21:374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Wong ML, Dong C, Maestre-Mesa J, et al. Polymorphisms in inflammation-related genes are associated with susceptibility to major depression and antidepressant response. Molecular Psychiatry. 2008;13:800–812. doi: 10.1038/mp.2008.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaccarino V, Brennan ML, Miller AH, et al. Association of major depressive disorder with serum myeloperoxidase and other markers of inflammation: a twin study. Biological Psychiatry. 2008;64:476–483. doi: 10.1016/j.biopsych.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zalcman SS, Siegel A. The neurobiology of aggression and rage: role of cytokines. Brain, Behavior, & Immunity. 2006;20:507–514. doi: 10.1016/j.bbi.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Petitto JM, Gariepy JL, Gendreau PL, et al. Differences in NK cell function in mice bred for high and low aggression: genetic linkaeg between complex behavioral and immunological traits? Brain, Behavior, & Immunity. 1994;13:175–186. doi: 10.1006/brbi.1998.0539. [DOI] [PubMed] [Google Scholar]
- 6.Suarez EC, Lewis JG, Kuhn C. The relation of aggression, hostility, and anger to lipopolysaccharide-stiumlated tumor necrosis factor (TNF)-a by blood monocytes from normal men. Brain Behavior and Immunology. 2002;16:675–684. doi: 10.1016/s0889-1591(02)00019-3. [DOI] [PubMed] [Google Scholar]
- 7.Suarez EC, Lewis JG, Krishnan RR, et al. Enhanced expression of cytokines and chemokines by blood monocytes to in vitro lipopolysaccharide stimulation are associated with hostility and severity of depressive symptoms in healthy women. Psychoneuroendocrinology. 2004;29:1119–1128. doi: 10.1016/j.psyneuen.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Kiecolt-Glaser JK, Loving TJ, Stowell JR, et al. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Archives of General Psychiatry. 2005;62:1377–1384. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- 9.Painuly N, Sharan P, Mattoo SK. Relationship of anger and anger attacks with depression: a brief review. European Archives of Psychiatry & Clinical Neuroscience. 2005;255:215–222. doi: 10.1007/s00406-004-0539-5. [DOI] [PubMed] [Google Scholar]
- 10.Jensen PS, Youngstrom EA, Steiner H, et al. Consensus report on impulsive aggression as a symptom across diagnostic categories in child psychiatry: implications for medication studies. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46:309–322. doi: 10.1097/chi.0b013e31802f1454. [DOI] [PubMed] [Google Scholar]
- 11.Alia-Klein N, Goldstein RZ, Tomasi D, et al. Neural mechanisms of anger regulation as a function of genetic risk for violence. Emotion. 2009;9:385–396. doi: 10.1037/a0015904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation - A possible prelude to violence. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- 13.Kronenberger B, Berg T, Herrmann E, et al. Efficacy of amantadine on quality of life in patients with chronic hepatitis C treated with interferon-alpha and ribavirin: results from a randomized, placebo-controlled, double-blind trial. European Journal of Gastroenterology & Hepatology. 2007;19:639–646. doi: 10.1097/MEG.0b013e3281ac20ca. [DOI] [PubMed] [Google Scholar]
- 14.Kraus MR, Schafer A, Csef H, et al. Psychiatric side effects of pegylated interferon alfa-2b as compared to conventional interferon alfa-2b in patients with chronic hepatitis C. World Journal of Gastroenterology. 2005;11:1769–1774. doi: 10.3748/wjg.v11.i12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kraus MR, Schafer A, Faller H, et al. Psychiatric symptoms in patients with chronic hepatitis C receiving interferon alfa-2b therapy. Journal of Clinical Psychiatry. 2003;64:708–714. doi: 10.4088/jcp.v64n0614. [DOI] [PubMed] [Google Scholar]
- 16.McHutchison JG, Gordon SC, Schiff ER, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. New England Journal of Medicine. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 17.Preau M, Marcellin F, Spire B, et al. Impaired anger control as an underappreciated side effect of treatments for chronic HCV infection in HIV-HCV coinfected patients. Journal of Clinical Gastroenterology. 2008;42:92–96. doi: 10.1097/01.mcg.0000225645.75651.b8. [DOI] [PubMed] [Google Scholar]
- 18.Dan AA, Crone C, Wise TN, et al. Anger experiences among hepatitis C patients: relationship to depressive symptoms and health-related quality of life. Psychosomatics. 2007;48:223–229. doi: 10.1176/appi.psy.48.3.223. [DOI] [PubMed] [Google Scholar]
- 19.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon afla-2a plus ribavirin for chronic hepatitis C virus infection. New England Journal of Medicine. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 20.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 21.Raison CL, Borisov AS, Broadwell SD, et al. Depression during pegylated interferon-alpha plus ribarin therapy: prevalence and prediction. Journal of Clinical Psychiatry. 2005;66:41–48. doi: 10.4088/jcp.v66n0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franzen PL, Buysse DJ, Rabinovitz M, et al. Poor sleep quality predicts onset of either major depression or subsyndromal depression with irritability during interferon-alpha treatment. Journal of Psychiatric Research. 2009 doi: 10.1016/j.psychres.2009.02.011. e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lotrich FE, Rabinovitz F, Gironda P, et al. Depression following pegylated interferon-alpha: characteristics and vulnerability. Journal of Psychosomatic Research. 2007;63:131–135. doi: 10.1016/j.jpsychores.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witthoft T, Moller B, Wiedmann KH, et al. Safety, tolerability and efficacy of peginterferon alpha-2a and ribavirin in chronic hepatitis C in clinical practice: The German Open Safety Trial. Journal of Viral Hepatitis. 2007;14:788–798. doi: 10.1111/j.1365-2893.2007.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Memon MI, Memon MA. Hepatitis C: an epidemiological review. Journal of Viral Hepatitis. 2002;9:84–100. doi: 10.1046/j.1365-2893.2002.00329.x. [DOI] [PubMed] [Google Scholar]
- 26.Williams I. Epidemiology of hepatitis C in the United States. American Journal of Medicine. 1999;107:2S–9S. doi: 10.1016/s0002-9343(99)00373-3. [DOI] [PubMed] [Google Scholar]
- 27.Bull SJ, Huezo-Diaz P, Binder EB, et al. Functional polymorphisms in the interleukin-6 and serotonin transporter genes, and depression and fatigue induced by interferon-a and ribavirin treatment. Molecular Psychiatry. 2008;14:1095–1104. doi: 10.1038/mp.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lotrich FE, Ferrell RE, Rabinovitz M, et al. Risk for depression during interferon-alpha treatment is affected by the serotonin transporter polymorphism. Biological Psychiatry. 2009;65:344–348. doi: 10.1016/j.biopsych.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kraus MR, Al-Taie O, Schefer A, et al. Serotonin-1A receptor gene (HTR1A) vairation predicts interferon-induced depression chronic hepatitis C. Gastroenterology. 2007;132:1279–1286. doi: 10.1053/j.gastro.2007.02.053. [DOI] [PubMed] [Google Scholar]
- 30.Beattie EC, S D, Morishita W, Bresnahan JC, H BK, et al. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- 31.Viviani B, Gardoni F, Marinovich M. Cytokines and neuronal ion channels in health and disease. International Review of Neurobiology. 2007;82 doi: 10.1016/S0074-7742(07)82013-7. [DOI] [PubMed] [Google Scholar]
- 32.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 33.Iosif RE, Ekdahl CT, Ahlenius H, et al. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. Journal of Neuroscience. 2006;26:9703–9712. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foley KF, Pantano C, Ciolino A, et al. IFN-gamma and TNF-alpha decrease serotonin transporter function and expression in Caco2 cells. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2007;292:G779–G784. doi: 10.1152/ajpgi.00470.2006. [DOI] [PubMed] [Google Scholar]
- 35.de Pablos RM, Villaran RF, Arguelles S, et al. Stress increases vulnerability to inflammation in the rat prefrontal cortex. Journal of Neuroscience. 2006;26:5709–5719. doi: 10.1523/JNEUROSCI.0802-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibb J, Hayley S, Gandhi R, et al. Synergistic and additive actions of a psychosocial stressor and endotoxin challenge: Circulating and brain cytokines, plasma corticosterone and behavioral changes in mice. Brain, Behavior, & Immunity. 2008;22:573–589. doi: 10.1016/j.bbi.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Churchill L, Taishi P, Wang M, et al. Brain distribution of cytokine mRNA induced by systemic administration of interleukin-1beta or tumor necrosis factor alpha. Brain Research. 2006;1120 doi: 10.1016/j.brainres.2006.08.083. [DOI] [PubMed] [Google Scholar]
- 38.Tonelli LH, Postolache TT. Tumor necrosis factor alpha, interleukin-1 beta, interleukin-6 and major histocompatibility complex molecules in the normal brain and after peripheral immune challenge. Neurological Research. 2006;27:679–684. doi: 10.1179/016164105X49463. [DOI] [PubMed] [Google Scholar]
- 39.Anisman H, Gibb J, Hayley S. Influence of continuous infusion of interleukin-1beta on depression-related processes in mice: corticosterone, circulating cytokines, brain monoamines, and cytokine mRNA expression. Psychopharmacology. 2008;199:231–244. doi: 10.1007/s00213-008-1166-z. [DOI] [PubMed] [Google Scholar]
- 40.O'Connor JC, Andre C, Wang Y, et al. Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. Journal of Neuroscience. 2009;29:4200–4209. doi: 10.1523/JNEUROSCI.5032-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dunn AJ, Wang J, Ando T. Effects of cytokines on cerebral neurotransmission. Advances in Experimental Medicine and Biology. 1999;461:117–127. doi: 10.1007/978-0-585-37970-8_8. [DOI] [PubMed] [Google Scholar]
- 42.McCann SM, Kimura M, Karanth S, et al. The mechanism of action of cytokines to control the release of hypothalamic and pituitary homrones in infection. Annals of the New York Academy of Science. 2000;917:4–18. doi: 10.1111/j.1749-6632.2000.tb05368.x. [DOI] [PubMed] [Google Scholar]
- 43.Uguz F, Akman C, Kucuksarac S, et al. Anti-tumor necrosis factor-alpha therapy is associated with less frequent mood and anxiety disorders in patients with rheumatoid arthritis. Psychiatry & Clinical Neurosciences. 2009;63:50–55. doi: 10.1111/j.1440-1819.2008.01905.x. [DOI] [PubMed] [Google Scholar]
- 44.Loftus EV, Feagan BG, Colombel JF, et al. Effects of adalimumab maintenance therapy on health-related quality of life of patients with Crohn's disease: patient-reported outcomes of the CHARM trial. American Journal of Gastroenterology. 2008;103:3132–3141. doi: 10.1111/j.1572-0241.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- 45.Krishnan R, Cella D, Leonardi C, et al. Effects of etanercept therapy on fatigue and symptoms of depression in subjects treated for moderate to severe plaque psoriasis for up to 96 weeks. British Journal of Dermatology. 2007;157:1275–1277. doi: 10.1111/j.1365-2133.2007.08205.x. [DOI] [PubMed] [Google Scholar]
- 46.Sallakci N, Akcurin G, Koksoy S, et al. TNF-alpha G-308A polymorphism is associated with rheumatic fever and correlates with increased TNF-alpha production. Journal of Autoimmunity. 2005;25:150–154. doi: 10.1016/j.jaut.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 47.Marotte H, Arnaud B, Diasparra J, et al. Association between the level of circulating bioactive tumor necrosis factor alpha and the tumor necrosis factor alpha gene polymorphism at -308 in patients with rheumatoid arthritis treated with a tumor necrosis factor alpha inhibitor. Arthritis & Rheumatism. 2008;58:1258–1263. doi: 10.1002/art.23430. [DOI] [PubMed] [Google Scholar]
- 48.Sohail M, Kaul A, Bali P, et al. Alleles -308A and -1031C in the TNF-alpha gene promoter do not increase the risk but associated with circulating levels of TNF-alpha and clinical features of vivax malaria in Indian patients. Molecular Immunology. 2008;45:1682–1692. doi: 10.1016/j.molimm.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Popko K, Gorska E, Potapinska O, et al. Frequency of distribution of inflammatory cytokines IL-1, IL-6 and TNF-alpha gene polymorphism in patients with obstructive sleep apnea. Journal of Physiology & Pharmacology. 2008;59:607–614. [PubMed] [Google Scholar]
- 50.Bhushan B, Guleria R, Misra A, et al. TNF-alpha gene polymorphism and TNF-alpha levels in obese Asian Indians with obstructive sleep apnea. Respiratory Medicine. 2009;103:386–392. doi: 10.1016/j.rmed.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 51.Pemberton LA, Stone E, Price P, et al. The relationship between ApoE, TNFA, IL1a, IL1b and IL12b genes and HIV-1-associated dementia. HIV Medicine. 2008;9:677–680. doi: 10.1111/j.1468-1293.2008.00614.x. [DOI] [PubMed] [Google Scholar]
- 52.Bialecka M, Klodowska-Duda G, Kurzawski M, et al. Interleukin-10 (IL10) and tumor necrosis factor alpha (TNF) gene polymorphisms in Parkinson's disease patients. Parkinsonism & Related Disorders. 2008;14:636–640. doi: 10.1016/j.parkreldis.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 53.Yang L, Lu R, Jiang L, et al. Expression and genetic analysis of tumor necrosis factor-alpha (TNF-alpha) G-308A polymorphism in sporadic Alzheimer's disease in a Southern China population. Brain Research. 2009;1247:178–181. doi: 10.1016/j.brainres.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 54.Kumar V, Khosla R, Gupta V, et al. Differential association of tumour necrosis factor-alpha single nucleotide polymorphism (-308) with tuberculosis and bronchial asthma. National Medical Journal of India. 2008;21:120–122. [PubMed] [Google Scholar]
- 55.Gentile DA, Doyle WJ, Zeev iA, et al. Association between TNF- and TGF- genotypes in infants and parental history of allergic rhinitis and asthma. Human Immunology. 2004;65:347–351. doi: 10.1016/j.humimm.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 56.Aoki T, Hirota T, Tamari M, et al. An association between asthma and TNF-308G/A polymorphism: meta-analysis. Journal of Human Genetics. 2006;51:677–685. doi: 10.1007/s10038-006-0007-3. [DOI] [PubMed] [Google Scholar]
- 57.Sookoian SC, Gonzalez C, Pirola CJ. Meta-analysis on the G-308A tumor necrosis factor alpha gene variant and phenotypes associated with the metabolic syndrome. Obesity Research. 2005;132:2122–2131. doi: 10.1038/oby.2005.263. [DOI] [PubMed] [Google Scholar]
- 58.Baune BT, Ponath G, Rothermundt M, et al. Association between genetic variants of IL-1beta, IL-6 and TNF-alpha cytokines and cognitive performance in the elderly general population of the MEMO-study. Psychoneuroendocrinology. 2008;33:68–76. doi: 10.1016/j.psyneuen.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 59.Jun TY, Pae CU, Han Hoon, et al. Possible association between -G308A tumour necrosis factor-a gene polymorphism and major depressive disorder in the Korean population. Psychiatric Genetics. 2003;13:179–181. doi: 10.1097/00041444-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 60.Pae CU, Lee KU, Han H, et al. Tumor necrosis factor- gene-G308A polymorphism associated with bipolar I disorder in the Korean population. Psychiatry Research. 2004;125:65–68. doi: 10.1016/j.psychres.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 61.Czerski PM, Rybakowski F, Kapelski P, et al. Association of tumor necrosis factor -308G/A promoter polymorphism with schizophrenia and bipolar affective disorder in a Polish population. Neuropsychobiology. 2008;57:88–94. doi: 10.1159/000135642. [DOI] [PubMed] [Google Scholar]
- 62.Clerici M, Arosio B, Mundo E, et al. Cytokine polymorphisms in the pathophysiology of mood disorders. CNS Spectrums. 2009;14:419–425. doi: 10.1017/s1092852900020393. [DOI] [PubMed] [Google Scholar]
- 63.Brambilla F, Maggioni M. Blood levels of cytokines in elderly patients with major depressive disorder. Acta Psychiatrica Scandinavia. 1998;97:309–313. doi: 10.1111/j.1600-0447.1998.tb10005.x. [DOI] [PubMed] [Google Scholar]
- 64.Brambilla F, Monteleone P, Maj M. Interleukin-1b and tumor necrosis factor-a in children with major depressive disorder or dysthymia. Journal of Affective Disorders. 2004;78:273–277. doi: 10.1016/S0165-0327(02)00315-4. [DOI] [PubMed] [Google Scholar]
- 65.Misener VL, Gomez L, Wigg KG, et al. Cytokine Genes TNF, IL1A, IL1B, IL6, IL1RN and IL10, and childhood-onset mood disorders. Neuropsychobiology. 2008;58:71–80. doi: 10.1159/000159775. [DOI] [PubMed] [Google Scholar]
- 66.Middle F, Jones I, Robertson E, et al. Tumour necrosis factor alpha and bipolar affective puerperal psychosis. Psychiatric Genetics. 2000;10:195–198. doi: 10.1097/00041444-200010040-00008. [DOI] [PubMed] [Google Scholar]
- 67.Prather AA, Rabinovitz M, Pollock BG, et al. Cytokine-induced depression during IFN-α treatment: the role of IL-6 and sleep quality. Brain, Behavior, & Immunity. 2009;23:1109–1116. doi: 10.1016/j.bbi.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dieperink E, Ho SB, Thuras P, et al. A prospective study of neuropsychiatric symptoms associated with interferon-a-2b and ribavirin therapy for patients with chronic hepatitis C. Psychosomatics. 2003;44:104–112. doi: 10.1176/appi.psy.44.2.104. [DOI] [PubMed] [Google Scholar]
- 69.Reichenberg A, Gorman JM, Dieterich DT. Interferon-induced depression and cognitive impairment in hepatitis C virus patients: a 72 week prospective study. AIDS. 2005;19:S174–S178. doi: 10.1097/01.aids.0000192087.64432.ae. [DOI] [PubMed] [Google Scholar]
- 70.Castera L, Constant A, Henry C, et al. Impact on adherence and sustained viological response of psychiatric side effects during peginterferon and ribavirin therapy for chronic hepatitis C. Alimentary Pharmacology and Therapeutics. 2006;24:1223–1230. doi: 10.1111/j.1365-2036.2006.03107.x. [DOI] [PubMed] [Google Scholar]
- 71.Hauser P, Khosla J, Aurora H, et al. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Molecular Psychiatry. 2002;7:942–947. doi: 10.1038/sj.mp.4001119. [DOI] [PubMed] [Google Scholar]
- 72.Schaefer M, Schmidt F, Horn M, et al. Depression during treatment with interferon alpha. Psychosomatics. 2004;45:176. doi: 10.1176/appi.psy.45.2.176. [DOI] [PubMed] [Google Scholar]
- 73.Coccaro EF, Kavoussi RJ. Fluoxetine and impulsive-aggressive behavior in personality disordered subjects. Archives of General Psychiatry. 1997;54:1081–1088. doi: 10.1001/archpsyc.1997.01830240035005. [DOI] [PubMed] [Google Scholar]
- 74.Castanon N, Leonard BE, Neveu PJ, et al. Effects of antidepressants on cytokine production and actions. Brain Behavior and Immunology. 2002;16:569–574. doi: 10.1016/s0889-1591(02)00008-9. [DOI] [PubMed] [Google Scholar]
- 75.Ventura J, Liberman RP, Green MF, et al. Training and quality assurance with the Structured Clinical Interview for DSM-IV (SCID-I/P) Psychiatry Research. 1998;79:163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- 76.Beck A, Steer R, Garbin M. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- 77.Coccaro EF, Harvey PD, Kupsaw-Lawrence E, et al. Development of neuropharmacologically based behavioral assessments of impulsive aggressive behavior. Journal of Neuropsychiatry & Clinical Neurosciences. 1991;3:S44–S51. [PubMed] [Google Scholar]
- 78.Kenward M. A method for comparing profiles of repeated measurements. Applied Statistics. 1987;36:296–308. [Google Scholar]
- 79.Hudziak JJ, Rudiger LP, Neale MC, et al. A twin study of inattentive, aggressive, and anxious/depressed behaviors. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:469–476. doi: 10.1097/00004583-200004000-00016. [DOI] [PubMed] [Google Scholar]
- 80.Wichers MC, Kenis G, Koek G, et al. Interferon-a-induced depressive symptoms are related to changes in the cytokine network but not to cortisol. Journal of Psychosomatic Research. 2007;62:207–214. doi: 10.1016/j.jpsychores.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 81.Dinan T, Siggins L, Scully P, et al. Investigating the inflammatory phenotype of major depression: focus on cytokines and polyunsaturated fatty acids. Journal of Psychiatric Research. 2009;43:471–476. doi: 10.1016/j.jpsychires.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 82.Constant A, Castera L, Dantzer R, et al. Mood alterations during interferon-alfa therapy in patients with chronic hepatitis C: evidence for an overlap between manic-hypomanic and depressive symptoms. Journal of Clinical Psychiatry. 2005;66:1050–1057. doi: 10.4088/jcp.v66n0814. [DOI] [PubMed] [Google Scholar]
- 83.Kim B, Wang HR, Son JI, et al. Bipolarity in depressive patients without histories of diagnosis of bipolar disorder and the use of the Mood Disorder Questionnaire for detecting bipolarity. Comprehensive Psychiatry. 2008;49:469–475. doi: 10.1016/j.comppsych.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 84.O'Donovan C, Garnham JS, Hajek T, et al. Antidepressant monotherapy in pre-bipolar depression; predictive value and inherent risk. Journal of Affective Disorders. 2008;107:293–298. doi: 10.1016/j.jad.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 85.Utsumi T, Sasaki T, Shimada I, et al. Clinical features of soft bipolarity in major depressive inpatients. Psychiatry & Clinical Neurosciences. 2006;60:611–615. doi: 10.1111/j.1440-1819.2006.01566.x. [DOI] [PubMed] [Google Scholar]
- 86.Akiskal HS, Kilzieh N, Maser JD, et al. The distinct temperament profiles of bipolar I, bipolar II and unipolar patients. Journal of Affective Disorders. 2006;92:19–33. doi: 10.1016/j.jad.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 87.Benazzi F. Mood patterns and classification in bipolar disorder. Current Opinion in Psychiatry. 2006;19:1–8. doi: 10.1097/01.yco.0000194145.03895.bc. [DOI] [PubMed] [Google Scholar]
- 88.Bowden CL. A different depression: clinical distinctions between bipolar and unipolar depression. Journal of Affective Disorders. 2005;84:117–125. doi: 10.1016/S0165-0327(03)00194-0. [DOI] [PubMed] [Google Scholar]
- 89.Benazzi F. Inter-episode mood lability in mood disorders: residual symptom or natural course of illness? Psychiatry & Clinical Neurosciences. 2004;58:480–486. doi: 10.1111/j.1440-1819.2004.01289.x. [DOI] [PubMed] [Google Scholar]
- 90.Eller T, Vasar V, Shlik J, et al. Pro-inflammatory cytokines and treatment response to escitalopram in major depressive disorder. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2008;32:445–450. doi: 10.1016/j.pnpbp.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 91.O'Brien SM, Scully P, Fitzgerald P, et al. Plasma cytokine profiles in depressed patients who fail to respond to selective serotonin reuptake inhibitor therapy. Journal of Psychiatric Research. 2007:413–4. doi: 10.1016/j.jpsychires.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 92.Ortiz-Dominguez A, Hernandez ME, Berlanga C, et al. Immune variations in bipolar disorder: phasic differences. Bipolar Disorders. 2007;9:596–602. doi: 10.1111/j.1399-5618.2007.00493.x. [DOI] [PubMed] [Google Scholar]
- 93.Perlis RH, Purcell S, Fagerness J, et al. Clinical and genetic dissection of anger expression and CREB1 polymorphisms in major depressive disorder. Biological Psychiatry. 2007;62:536–540. doi: 10.1016/j.biopsych.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 94.Gietl A, Giegling I, Hartmann AM, et al. ABCG1 gene variants in suicidal behavior and aggression-related traits. European Neuropsychopharmacology. 2007;17:410–416. doi: 10.1016/j.euroneuro.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 95.Rujescu D, Giegling I, Bondy B, et al. Association of anger-related traits with SNPs in the TPH gene. Molecular Psychiatry. 2002;7:1023–1029. doi: 10.1038/sj.mp.4001128. [DOI] [PubMed] [Google Scholar]
- 96.Rujescu D, Giegling I, Gietl A, et al. A functional single nucleotide polymorphism (V158M) in the COMT gene is associated with aggressive personality traits. Biological Psychiatry. 2003;54:34–39. doi: 10.1016/s0006-3223(02)01831-0. [DOI] [PubMed] [Google Scholar]
- 97.Capuron L, Gumnick JF, Musselman DL, et al. Neurobehavioral effects of interferon-a in cancer patients: Phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 98.Jiang Y, Deacon R, Anthony DC, et al. Inhibition of peripheral TNF can block the malaise associated with CNS inflammatory diseases. Neurobiology of Disease. 2008;32:125–132. doi: 10.1016/j.nbd.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 99.Loftis JM, Huckans M, Ruimy S, et al. Depressive symptoms in patients with chronic hepatitis C are correlated with elevated plasma levels of interleukin-1beta and tumor necrosis factor-alpha. Neuroscience Letters. 2008;430:264–268. doi: 10.1016/j.neulet.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kaneko N, Kudo K, Mabuchi T, et al. Suppression of cell proliferation by interferon-alpha through interleukin-1 production in adult rat dentate gyrus. Neuropsychopharmacology. 2006;31:2619–2626. doi: 10.1038/sj.npp.1301137. [DOI] [PubMed] [Google Scholar]
- 101.van Heel DA, Udalova IA, De Silva AP, et al. Inflammatory bowel disease is associated with a TNF polymorphism that affects an interaction between the OCT1 and NF(--)B transcription factors. Human Molecular Genetics. 2002;11:1281–1289. doi: 10.1093/hmg/11.11.1281. [DOI] [PubMed] [Google Scholar]
- 102.Hohjoh H, Tokunaga K. Allele-specific binding of the ubiquitous transcription factor OCT-1 to the functional single nucleotide polymorphism (SNP) sites in the tumor necrosis factor- gene (TNFA) promoter. Genes Immunology. 2001;2:105–109. doi: 10.1038/sj.gene.6363721. [DOI] [PubMed] [Google Scholar]
- 103.Bayley JP, Ottenhoff TH, Verweij C. Is there a future for TNF promoter polymorphisms? Genes Immun. Genes Immunology. 2004;5:315–329. doi: 10.1038/sj.gene.6364055. [DOI] [PubMed] [Google Scholar]
- 104.Baseggio L, Bartholin L, Chantome A, et al. Allele-specific binding to the -308 single nucleotide polymorphism site in the tumour necrosis factor- promoter. European Journal of Immunogenetics. 2004;31:15–19. doi: 10.1111/j.1365-2370.2004.00440.x. [DOI] [PubMed] [Google Scholar]
- 105.Kroeger KM, Steer JH, Joyce DA, et al. Effects of stimulus and cell type on the expression of the -308 tumour necrosis factor promoter polymorphism. Cytokine. 2000;12:110–119. doi: 10.1006/cyto.1999.0529. [DOI] [PubMed] [Google Scholar]
- 106.Muller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Molecular Psychiatry. 2007;12:988–1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- 107.Hayley S, Wall P, Anisman H. Sensitization to the neuroendocrine, central monoamine and behavioural effects of murine tumor necrosis factor-a: peripheral and central mechanisms. European Journal of Neuroscience. 2002;15:1061–1076. doi: 10.1046/j.1460-9568.2002.01936.x. [DOI] [PubMed] [Google Scholar]
- 108.Palin K, McCusker RH, Strle K, et al. Tumor necrosis factor-alpha-induced sickness behavior is impaired by central administration of an inhibitor of c-jun N-terminal kinase. Psychopharmacology. 2008;197:629–635. doi: 10.1007/s00213-008-1086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]