Abstract
The applicability of islet transplantation as treatment for type 1 diabetes is limited by renal and islet toxicities of currently available immunosuppressants. We describe a novel immunosuppressive regimen using the anti-leukocyte functional antigen-1 antibody efalizumab which permits long-term islet allograft survival while reducing the need for corticosteroids and calcineurin inhibitors (CNI).
Eight patients with type 1 diabetes and hypoglycemic unawareness received intraportal allogeneic islet transplants. Immunosuppression consisted of anti-thymocyte globulin induction followed by maintenance with efalizumab and sirolimus or mycophenolate. When efalizumab was withdrawn from the market in mid-2009, all patients were transitioned to regimens consisting of mycophenolate and sirolimus or mycophenolate and tacrolimus.
All patients achieved insulin independence and 4/8 patients became independent after single islet transplants. Insulin independent patients had no further hypoglycemic events, hemoglobin A1c levels decreased, and renal function remained stable. Efalizumab was well tolerated and no serious adverse events were encountered.
Although long-term follow-up is limited by discontinuation of efalizumab and transition to conventional imunnosuppression (including CNI in 4 cases), these results demonstrate that insulin independence after islet transplantation can be achieved with a CNI and steroid-free regimen. Such an approach may minimize renal and islet toxicity and thus further improve long-term islet allograft survival.
INTRODUCTION
Type 1 diabetes mellitus remains an important cause of death, blindness, kidney failure, and non-traumatic amputations [1, 2, 3], and establishing safe and effective methods of maintaining normal blood glucose levels can have substantial implications for the health and quality of life of individuals with diabetes. Currently, the most physiologic strategy for maintaining euglycemia without the associated risk of hypoglycemia is to restore islet function by pancreas transplantation or transplantation of isolated pancreatic islets. Simultaneous pancreas-kidney transplantation in uremic diabetic patients is an increasingly successful procedure, primarily because patients enjoy the benefits of being independent of dialysis [4–6]. Solitary pancreas transplantation in non-uremic patients, on the other hand, has received only limited acceptance due to the associated surgical complications, need for vigorous immunosuppression, and the nephrotoxic side effects of currently used immunosuppressive regimens in a patients already at risk for renal dysfunction [7–9].
Pancreatic islet transplantation offers a promising, minimally invasive approach to restore normoglycemia and insulin independence in type 1 diabetics without the surgical complications associated with whole organ transplantation [10, 11]. Although significant progress has been made in overcoming the technical difficulties surrounding islet isolation and transplantation, the outcomes in type I diabetic recipients of islet allografts remain compromised by the reliance on conventional immunosuppressive therapies that have significant islet and renal toxicities [12, 13].
Here we describe the results of pancreatic islet transplantation in non-uremic type 1 diabetics using a novel immunosuppressive protocol that is based on sirolimus and the anti-leukocyte functional antigen-1 (anti-LFA-1) antibody efalizumab. Efalizumab (Raptiva®) is a potent immunosuppressant that inhibits T-cell activation and trafficking by blocking the co-stimulatory attachment of the CD11a subunit of LFA-1 to the intercellular adhesion molecule-1 (ICAM-1) [14–17]. Recently, efalizumab was withdrawn from clinical use due to concerns about the development of progressive multifocal myeloencephalopathy (PML) in several patients who received the drug as treatment for psoriasis. Nonetheless, efalizumab lacks many of the toxicities of other currently employed drugs, including beta cell impairment and nephrotoxicity, and may represent an appropriate immunosuppressive agent for this patient population with type 1 diabetes.
METHODS
Patients
Patients were considered eligible for transplantation if they met the following criteria: 1) ≥ 5 years of insulin-dependent type 1 diabetes mellitus; 2) stimulated C-peptide levels < 0.5 ng/ml; 3) history of recurrent, severe hypoglycemic episodes requiring assistance by another person for treatment or hospitalization despite management by an experienced diabetologist; 4) body mass index (BMI) < 28 kg/m2 or weight < 80 kg; 5) insulin requirements < 55 units/day; 6) creatinine clearance > 60 ml/min/m2; and 7) no history of malignancy within 10 years (except treated basal or squamous cell carcinoma of the skin). All study procedures were reviewed and approved by the institutional review boards at the University of California, San Francisco (UCSF) or at the University of Minnesota (UM), and all subjects signed an informed consent.
Islet preparation and Transplantation
Islets were purified from deceased donor pancreata as described [18]. Both LIBERASE HI (Roche) and the Collagenase NB1 blend (Serva Electrophoresis) were used for digestion [19, 20]. Islets were maintained in culture for 36–48 hours prior to transplantation, assessed for sterility and viability, and suspended in transplant medium supplemented with heparin (70U/kg recipient body weight). Criteria for transplantation included: ≥ 4,000 islet equivalents (IE)/kg recipient body weight, viability ≥ 70%, purity ≥ 20,000 IEQ/ml settled tissue volume, settled tissue volume ≤ 15cc, glucose stimulated insulin release (GSIR) index ≥ 1.0, endotoxin levels ≤ 5.0 EU/kg recipient body weight, and negative gram stain of the islet culture fluid [21].
Islets were infused intraportally by percutaneous portal vein catheterization (UCSF) or minilaparotomy (UM) [22]. Recipients received intravenous heparin for 48 hours after transplantation followed by 5 days of twice daily subcutaneous enoxaparin injections. Recipients who were not insulin independent 2–3 months after transplantation but who had detectable C-peptide were listed for second islet transplants.
Immunosuppressive Protocol
Induction immunosuppression consisting of thymoglobulin and sirolimus was initiated 2 days prior to islet transplant. Patients received a total of 4 mg/kg thymoglobulin given intravenously (IV) in two divided doses on days −2 and −1 relative to transplant. One dose of methylprednisolone was used as a premedication prior to the first dose of thymoglobulin. Efalizumab therapy was initiated at 1mg/kg/week subcutaneously starting 1 day prior to transplant and reduced to 0.5mg/kg/wk 3 months after transplant. Target sirolimus serum levels were ≥ 8 ng/ml; however, many patients were unable to tolerate these and required dose reduction and addition of mycophenolic acid (MMF, 360–720 mg PO bid). Patients who required second islet transplants received induction immunotherapy with basiliximab (20 mg IV on days 0 and 4 relative to transplant) but otherwise continued their maintenance immunosuppressive regimen. Patients remained on a steroid-free, CNI-free maintenance regimen consisting of efalizumab, sirolimus, and/or MMF until efalizumab had to be discontinued in all patients on May 1, 2009 due to safety concerns. Since then, 3 patients have been maintained on sirolimus and MMF, one patient has been maintained on MMF monotherapy, and four patients have been maintained on tacrolimus and MMF.
Assessment of islet function
All patients were discharged 2–3 days after transplantation and asked to measure blood glucose levels at least 5 times daily while on insulin and 2–3 times/day after discontinuation of insulin. Islet graft function and glucose control were assessed at each visit by reviewing blood glucose data and measuring fasting plasma glucose, C-peptide (fasting and postprandial), and HbA1c levels. Prior to transplant and at 3–6 month intervals after each transplant, subjects underwent mixed meal tolerance testing (MMTT) and continuous glucose monitoring (CGM) using a 24-hour glucometer [23, 24].
Assessment of Renal Function
Glomerular filtration rates were determined using iohexol infusion before islet transplantation and every 6 months after transplantation [25]. Twenty-four hour urine collections were performed in all UCSF subjects (n=5) before transplant and every 6 months after transplant and analyzed for urine protein and albumin excretion and creatinine clearance [26, 27].
Immunologic Evaluation of Islet Recipients
Sample Preparation
Recipient peripheral blood mononuclear cells (PBMC) were prepared from samples obtained before and after transplant (days 7, 14, 28, 56, 75, 90, 120, 175, 270, 365 relative to transplant) using Ficoll gradient centrifugation and cryopreserved before analysis. For immune response assays, donor splenocytes were isolated using Ficoll gradient centrifugation and cryopreserved. PBMC from 20 random blood donors were pooled, irradiated at 30Gy, and cryopreserved for use as reference 3rd party allostimulator cells. Data for patients transplanted at UCSF are presented.
Flow cytometry
The fluorochrome-labeled anti-human antibodies to CD3, CD4, CD25, CD127, and IFN-γ were purchased from BD Biosciences (San Jose, CA). Alexa488-conjugated anti-human FoxP3 was purchased from BioLegend (San Diego, CA). Intracellular staining was performed according to the BioLegend protocol [28]. Stained cells were washed and analyzed with an LSR II (BD Biosciences) flow cytometer. All data analysis was performed with FlowJo software (Treestar, Ashland, OR).
Assessment of T cell immune responses
Cryopreserved donor splenocytes, 3rd party splenocytes, and recipient PBMC were thawed, washed twice and resuspended in complete RPMI (RPMI 1640 supplemented with 10% heat-inactivated human AB serum, 100U/mL penicillin, 100μg/mL streptomycin sulfate, and 2mM sodium glutamate). Patient-derived PBMCs obtained at various time points before and after transplant were cultured alone, with donor splenocytes, or with the 3rd party cells. Patient PBMCs were also cultured with Staphylococcus enterotoxin B (1μg/mL) to serve as a positive control. On day 5 of culture, brefeldin A (Epicentre, 10μg/mL) was added to cultures for 6 hours. Cells were then collected, washed twice with PBS, and permeabilized with FACSPerm Solution II (BD Biosciences) for 10 minutes. Cells were then washed twice and labeled with the following antibodies at room temperature for 1 hour: IL-4 PE, CD8 PerCP, IFNγ APC, and CD4 Pacific Blue (BD Biosciences). Cells were washed twice, and acquired on a LSR II cytometer (BD Biosciences). Data was analyzed using Flowjo Software (Treestar, Inc).
Statistical analysis
Data are expressed as a mean ± standard deviation (SD) unless otherwise stated. Differences between groups were examined using Student’s t-test. P values ≤ 0.05 were considered significant.
RESULTS
Donor and islet characteristics
Characteristics of the pancreas donors and the islet preparations are listed in Table 1. A total of 13 organs were processed to yield 12 preparations suitable for transplantation into 8 recipients. All islet preparations had greater than 85% viability and GSIR indices ≥ 1.0 at the time of transplant. Recipient 1 received islets combined from 2 donors at the time of his first transplant; all other transplants were performed using islets from single donors.
Table 1.
Donor and Islet Characteristics
| Donor # | 1* | 2* | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recipient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Mean ± SD | |||||
| Age (yrs) | 46 | 35 | 49 | 43 | 25 | 19 | 37 | 33 | 17 | 38 | 17 | 48 | 29 | 34 ± 11 |
| Gender (M, F) | M | M | F | F | M | M | M | M | M | M | M | M | F | |
| Weight (kg) | 114 | 95 | 99 | 88 | 143 | 109 | 112 | 97 | 91 | 94 | 114 | 139 | 101 | 107 ± 17 |
| BMI (kg/m2) | 35.9 | 32.8 | 34.4 | 29.4 | 40.0 | 45.4 | 35.8 | 27.2 | 26.9 | 24.5 | 37.2 | 42.9 | 31.5 | 34.1 ± 6.3 |
| Cause of Death | CVA# | Trauma | CVA | CVA | CVA | Trauma | CVA | Trauma | Trauma | CVA | Trauma | Trauma | CVA | |
| Cold Ischemic Time (hours) | 7.5 | 6.7 | 7.6 | 7.1 | 8.2 | 4.7 | 5.0 | 7.0 | 7.5 | 7.0 | 7.0 | 7.5 | 7.5 | 6.9 ± 1.0 |
| Total Purified IEQ | 303,893 | 223,782 | 502,980 | 408,598 | 470,605 | 320,864 | 542,777 | 600,900 | 661,409 | 482,050 | 630,165 | 574,950 | 351,195 | 467,244± 136,995 |
| IEQ/kg body weight | 12,328 | 14,067 | 6,291 | 17,226 | 11,023 | 8,034 | 11,670 | 13,620 | 11,782± 3,451 | |||||
| Viability (%) | 89 | 92 | 88 | 96 | 88 | 94 | 94 | 100 | 100 | 97 | 100 | 100 | 100 | 95 ± 5 |
| GSIR index | 1.8 | 1.01 | 1.3 | 1.3 | 4.9 | 5.4 | 6.9 | 5.2 | 1.2 | 1.3 | 1.9 | 2.6 | 2.3 | 2.8 ± 2.0 |
donors 1 and 2 were transplanted together.
cerebrovascular accident
Recipient characteristics and post transplant islet function
Eight patients with type 1 diabetes and documented hypoglycemic unawareness underwent islet transplantation at our center (n=5) and at the University of Minnesota (n=3). Their clinical characteristics and post-transplant courses are listed in Table 2. All patients had long-standing diabetes and reported severe hypoglycemic episodes in the 1 year prior to transplant. Five of 8 patients had pretransplant HbA1c levels >7.0%.
Table 2.
Recipient Characteristics and Post-transplant Course
| Recipient Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Mean ± SD |
|---|---|---|---|---|---|---|---|---|---|
| Age (yrs) | 44 | 44 | 50 | 47 | 58 | 48 | 58 | 40 | 49 ± 7 |
| Gender (M, F) | M | F | F | F | F | F | F | F | |
| Weight (kg) | 84 | 63 | 51 | 68 | 60 | 60 | 54 | 68 | 63 ± 10 |
| BMI (kg/m2) | 22.3 | 22 | 19.4 | 25.4 | 21.3 | 25.6 | 19.2 | 24.9 | 22.5 ± 2.6 |
| Duration of diabetes (yrs) | 21 | 21 | 24 | 40 | 50 | 45 | 30 | 19 | 31 ± 12 |
| Pretransplant HbA1c (%) | 6.4 | 8 | 7.7 | 6.7 | 8.2 | 7.3 | 8.0 | 6.7 | 7.4 ± 0.7 |
| Insulin use (U/d) | 28.8 | 35 | 21 | 40 | 30 | 30 | 22 | 40 | 31 ± 7 |
| Pretransplant hypoglycemia (episodes/yr) | 24 | 6 | 12 | 3 | 12 | 5 | 5 | 12 | |
| Complications of diabetes | None | Retinopathy | Hypertension | Hypertension | Hypertension Autonomic neuropathy Retinopathy | Autonomic neuropathy | Hypertension Autonomic neuropathy Peripheral neuropathy Retinopathy | Autonomic neuropathy | |
| Post-transplant course | |||||||||
| Post-transplant Hypoglycemic Episodes | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Duration of insulin independence after last islet transplant (days) | 32 | 157 | 395 | 202 | 731 | 797 | 797 | 534 | 456 ± 305 |
| Duration of efalizumab therapy (days) | 126 | 105 | 264 | 504 | 568 | 583 | 392 | 804 | 419 ± 243 |
| Duration off efalizumab (days) | 200 | 200 | 150 | 150 | 169 | 237 | 420 | 150 | 210 ± 91 |
| Immunosuppressive regimen since d/c of efalizumab | Tacrolimus MMF | Tacrolimus MMF | Tacrolimus MMF | Tacrolimus MMF | Sirolimus MMF | Sirolimus MMF | MMF | Sirolimus MMF | |
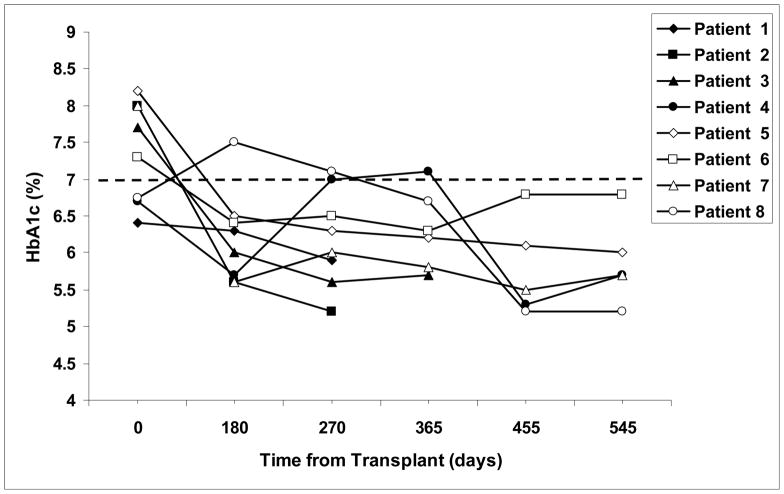
All patients became insulin independent after their last islet transplant and had complete resolution of their hypoglycemic episodes. Four of 8 patients achieved insulin independence after their first islet transplant with a mean time to independence of 30 ± 21 days. One of these (patient 6) resumed intermittent low dose insulin use (2–4 units/day 2–3 times/month) approximately 650 days after her transplant during a period of illness, but eventually stopped using insulin and is currently insulin independent. Her most recent HbA1c levels have risen however, suggesting worsening glycemic control (Figure 2).
Figure 2.
Hemoglobin A1c levels in study subjects before and after islet transplantation. Patients 1, 2, 4, and 8 received second islet transplants 243 days, 123 days, 442 days, and 400 days, respectively after their initial transplant. The dashed line indicates the top normal value of HbA1c at our institutions.
Four patients achieved insulin independence after 2 transplants (patients 1, 2, 4, 8). Patient 8 was insulin independent after her first transplant but then resumed low dose insulin use (8–10 units/day) approximately 150 days after her first transplant. She received a second transplant and has now been insulin independent for over 500 days.
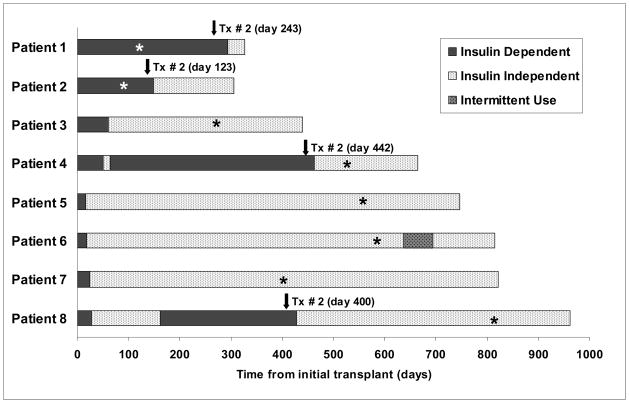
The mean duration of efalizumab therapy was 419 days, although two patients (patients 1 and 2) received efalizumab for only 126 and 105 days, respectively, and both received their second islet transplants after discontinuation of the drug. All patients were weaned from efalizumab between 150 and 420 days ago and remain on the immunosuppressive medications listed in Table 2. In patients transitioned to tacrolimus (patients 1–4), mean ± SD serum trough levels of tacrolimus were 6.4 ± 2.1 ng/ml. Mean ± SD serum trough levels of sirolimus in patients after discontinuation of efalizumab were 8.8 ± 3.6 ng/ml (range 3.7–12.7 ng/ml). The length of follow-up, timing of islet transplants, duration of independence, and time of efalizumab discontinuation are depicted in Figure 1.
Figure 1.
Duration of follow-up and insulin independence after islet transplantation in patients receiving an efalizumab-based immunosuppressive regimen.
*Time at which efalizumab was discontinued.
Glycemic control after transplantation
Glycemic control improved in all subjects after transplantation. None of the patients experienced any hypoglycemic episodes after their initial islet transplant. The mean pre-transplant HbA1c in our patients was 7.4 ± 0.7 %. Once insulin independence was achieved, mean HbA1c levels decreased in all patients (mean ± SD = 5.8 ± 0.4%; Figure 2). HbA1c levels in one patient (patient 6) who resumed insulin use for a brief period of time have increased from 6.3% at one year to 6.8% at 18 months, suggesting worsening glycemic control. MMTTs performed approximately one year after transplant in recipients of single islet grafts and between 75 and 180 days after final transplant in recipients of 2 grafts are depicted in Figure 3. The mean plasma glucose level in the recipients 2 hours after oral administration of Boost® (6 ml/kg body weight) was 166 ± 19 mg/dl.
Figure 3.
C-peptide levels during a mixed meal tolerance test in study subjects one year after islet transplantation. In subjects who received 2 islet transplants (patients 1, 2, 4 and 8), results from tests performed between 75 and 180 days after second transplant are depicted.
Renal function
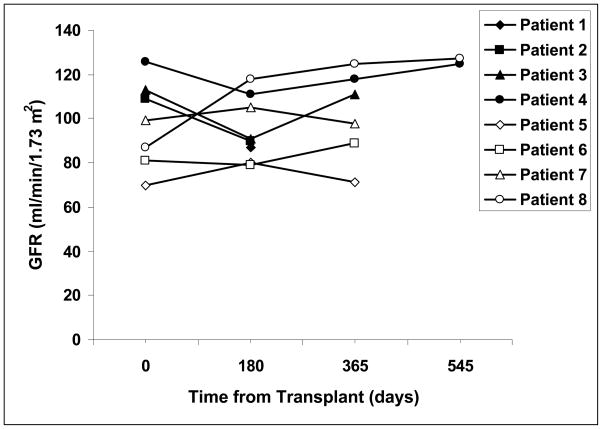
Renal function as determined by iohexol clearance remained stable in all patients for the duration of the study (Figure 4). The mean glomerular filtration rate was 98 ± 20 mg/min/1.73m2 prior to transplant, and was 103 ± 21 mg/min/1.73 m2 in patients who were one year or more after initial islet transplant (p= 0.3).
Figure 4.
Glomerular filtration rates in islet transplant recipients before (time 0) and at varying times after initial transplantation.
Urinary protein and albumin excretion as determined by 24 hour urine collections also remained stable or improved in all tested subjects. At baseline, mean protein excretion was 153 ± 40 mg/day, compared with 102 ± 29 mg/day 270 days after initial islet transplant (p= 0.049). Urine albumin excretion prior to transplant was 22.5 ± 15 mg/day and 9.5 ± 6 mg/day 270 days after initial transplant (p = 0.1).
Immunologic Studies
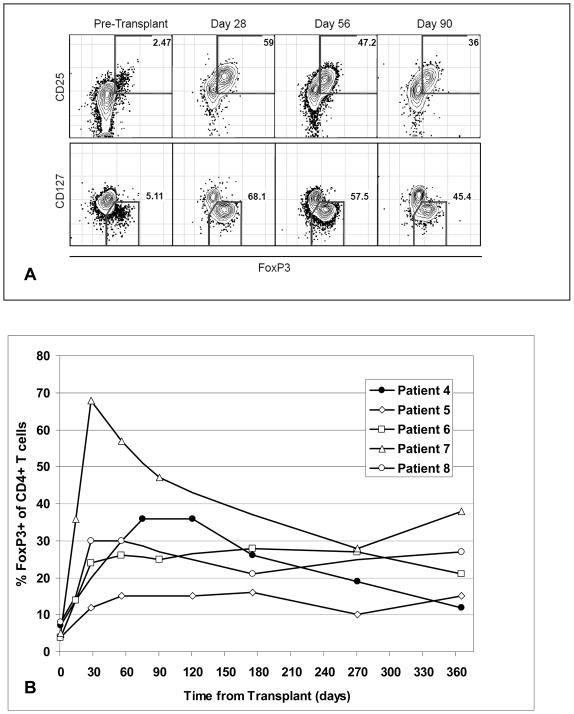
To examine the effects of islet transplantation and efalizumab-based immunosuppressive therapy on the patients’ circulating immune cells, PBMC from patients who received their transplants at UCSF were assessed before transplant and at varying times after transplant by flow cytometry. The percentage of circulating CD4+ FoxP3+ T cells significantly increased following transplant (Fig. 5A). The phenotype of these cells (CD25hi and CD127lo) was consistent with the phenotype of regulatory T cells. This increase was found in all patients and persisted out to 1 year (Fig. 5B).
Figure 5.
Flow cytometric analysis of the kinetics of FoxP3+ CD4+ T cells in islet transplant recipients. Cryopreserved PBMC obtained at the specified timepoints were batch analyzed by flow cytometry. PBMC were stained with fluorochrome-labeled anti-human antibodies to CD3, CD4, CD25, and CD127. Following fixation and permeabilization, PBMC were stained intracellularly with labeled anti-FoxP3 antibody.
A) Representative analysis from a study patient. CD3+ CD4+ cells were gated upon and analyzed for CD25, 127, and FoxP3 staining.
B) Graph depicting changes in the percentage of FoxP3+ cells within the CD4+ T cell subset in islet recipients at various times relative to islet transplantation.
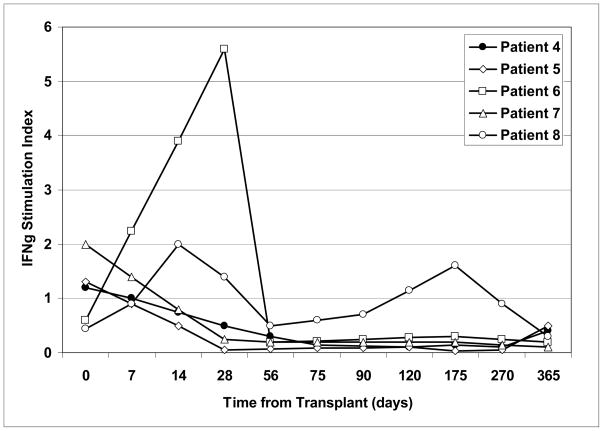
When the frequency of circulating donor-specific alloreactive T cells was estimated in these patients before and after transplantation by flow cytometry and intracellular cytokine staining, 4 of 5 patients had reduced IFNγ production to the donor over time (Fig. 6). In contrast, all patients had preserved responses to 3rd party stimulators. The one patient (Patient # 8) who continued to have significant responses to the islet donor became hyperglycemic at around the same time as an increase in donor-specific alloreactivity was observed (day 150–175), suggesting that some of the graft failure may have been due to immunologic reasons.
Figure 6.
Kinetics of alloreactive CD4+ T cells in islet recipients before (day 0) and at various times after transplant. Cryopreserved, patient-derived PBMC from the specified timepoints were batch analyzed for alloreactivity by intracellular IFNγ staining and flow cytometry. Patient PBMC were either incubated with irradiated patient-specific donor splenocytes or an irradiated pool of 20 random blood donors (3rd party allostimmulator). The % of CD4+ T cells producing IFNγ following 5 days of culture was measured. The IFNγ index represents the % of CD4+ T cells producing IFNγ in response to donor splenocytes) divided by the % of CD4+ T cells producing IFNγ in response to the 3rd party allostimmulator.
Adverse Events
All patients complained of nausea immediately after the islet cell infusion which resolved over 24–36 hours. One subject experienced intra-abdominal bleeding from the liver puncture site after the islet infusion but this resolved without the need for a transfusion. One subject developed a partial portal vein thrombosis that resolved with anticoagulation.
Efalizumab therapy was well tolerated by all study patients, with side effects being limited to the development of a rash at the injection site after the first efalizumab dose in 1 subject and transient redness at the injection site in another subject. Efalizumab was discontinued in one patient with back pain and fatigue since this agent could not conclusively be ruled out as a cause. Although her symptoms did not improve following efalizumab discontinuation, they resolved when sirolimus was stopped. This patient remains insulin independent on MMF monotherapy. One recipient developed detectable EBV levels, which resolved spontaneously without treatment.
In late 2008/early 2009, PML was identified in 3 patients out of over 40,000 who had received efalizumab as treatment for psoriasis and the drug was withdrawn from the market in June of 2009 [29–32]. Because of these concerns, efalizumab was discontinued in all patients and their maintenance immunosuppression was adjusted to include low dose sirolimus or prograf, and/or MMF (Table 2, Figure 1). Serial neurologic exams in all subjects have remained normal.
Additional side effects of the other immunosuppressive drugs included nausea (n=3), diarrhea (n=4), neutropenia (n=3) and thrombocytopenia (n=1). Symptomatic oral ulcers developed in most of our patients and were the main reason for reduction of sirolimus dosing and addition of MMF or conversion to tacrolimus.
DISCUSSION
The immunosuppressive protocol pioneered by the University of Alberta and used in most previous studies of clinical islet transplantation was in part successful because the investigators recognized that omission of corticosteroids was beneficial for islet function and survival [10, 33, 34]. Nonetheless, this and similar protocols that followed all relied on CNIs, particularly tacrolimus, for maintenance immunosuppression, and these agents can be problematic in diabetics not only due to their deleterious effect on renal function, but also due to their direct islet toxicity [13, 35, 36]. Recently, several biological agents without these side effects have been identified and successfully used in organ transplantation; however, none have been used in islet transplantation [16, 37, 38]. Efalizumab, an anti-LFA-1 antibody is one such agent that has been used extensively in the treatment of psoriasis and has recently been found to be effective in prolonging renal allograft survival [37]. We chose this agent for our trial due to its ease of administration (weekly subcutaneous injection), excellent overall tolerability by patients, and few initial reports of significant side effects despite widespread clinical use [39]. The efficacy of an efalizumab-based protocol was also seen in this trial, with all patients treated with efalizumab and sirolimus or MMF achieving stable insulin independence after 1 or 2 islet transplants, and five of eight patients now being independent for more than 1 year. Moreover, all patients have remained insulin independent after discontinuation of efalizumab, although one patient (patient 6) experienced a brief period of insulin dependence after efalizumab withdrawal and now has worsening glycemic control as evidenced by increasing HbA1c levels. It is possible that she will require an additional islet transplant in the near future. This decrease in function may be related to the withdrawal of efalizumab, but seems less likely given that the other patients have remained stable after drug withdrawal.
Several factors may be responsible for our current results. First, our donor selection, organ procurement, and islet isolation protocols were systematically restructured to improve islet quality and yields [21]. Second, avoidance of corticosteroids and tacrolimus minimized the toxic effects of these agents on the islet graft. Finally, it is possible that the use of a potent T-cell depleting induction agent combined with co-stimulation/migration blockade may provide better protection against allo - and autoimmune responses directed against the islets. In support of this, the percentage of CD4 T-lymphocytes which are phenotypic T-regulatory lymphocytes was consistently higher in our patients than in patients treated with co ventional immunosuppressive protocols [40–43]. In addition, the observation that 4/5 patients had decreased IFN-γ production to donor stimulators suggests that some degree of donor-specific hyporesponsiveness may has occurred. Together, these findings suggest that this immunosuppressive protocol may have some tolerogenic properties, though clearly more extensive studies are required to confirm this finding.
Avoiding CNI’s was an important goal of the study, particularly since these agents have renal toxicity that should be minimized in this patient population already at risk for renal insufficiency due to their diabetes [3]. Unfortunately, CNI therapy had to be initiated in some of our patients after efalizumab withdrawal, thus making interpretation of long-term renal function difficult. Nonetheless, measurements of renal function with iohexol during the CNI-free period were stable or improved in our patients, suggesting that this regimen, at last in the short term, has little nephrotoxicity. It remains to be seen whether long-term renal function in patients on a CNI-free regimen will remain stable and whether reinstitution of low-dose CNI therapy in some patients after efalizumab discontinuation will have adverse effects.
An important aspect of this study was to determine the safety and efficacy of the immunosuppressive regimen used in these patients. The overall tolerability of the regimen was excellent, and many of the adverse events were attributable to sirolimus. Several patients were unable to tolerate full doses of sirolimus due to development of oral ulcers or leg swelling, and as a result required dose reduction and supplementation with or conversion to mycophenolate (Cellcept® or Myfortic®). Overall tolerability of efalizumab was outstanding, with side effects being limited to localized, transient skin irritation at the injection site in 2 patients. Previous studies with efalizumab have shown that CD11a coating and modulation by the drug can occur at doses significantly lower than those routinely used when treating psoriasis [44]. In addition, studies in renal transplant recipients found an increased incidence of post-transplant lymphoproliferative disease (PTLD) in patients treated with high doses of the drug (2 mg/kg/week) in conjunction with sirolimus and high dose mycophenolate [37]. Based on these findings, we selected a low dose of the drug (1 mg/kg/week) for our studies and reduced the dose further (to 0.5 mg/kg/week) when patients were 3 months from their final islet transplant. Using this regimen, we did not observe any cases of PTLD throughout the follow-up period. One patient did develop detectable EBV levels, but these resolved spontaneously without treatment. Pharmacokinetic studies in selected patients showed appropriate coating and modulation of CD11a at the doses used (data not shown).
Recent reports have described the development of progressive multifocal leukoencephalopathy (PML) in 4 patients (3 confirmed, 1 suspected) out of approximately 46,000 who had received efalizumab as treatment for psoriasis [45] These patients had all received standard doses of the drug and had been treated for >3 years [32]. Although several other immunosuppressive agents have also been associated with PML yet continue to be used clinically, these reports led to withdrawal of the drug from clinical use and prompted us to discontinue the medication to minimize long-term exposure [46–48]. We have weaned all of the patients off efalizumab and are now treating them with a maintenance regimen of mycophenolate and/or sirolimus or low-dose tacrolimus. Serial neurologic examinations in all patients have been normal and we continue to monitor these patients closely for any new developments.
There are several drawbacks to this study. First, the lack of a control group treated with sirolimus and/or MMF makes it difficult to evaluate the additive immunosuppressive effect of efalizumab therapy. Although there is evidence from other studies that maintenance immunosuppression consisting of sirolimus and MMF but without CNI results in early islet failure, a control population in our study would be important to clarify these issues and to address the possibility that other factors such as islet isolation play a role in our outcomes [49]. Second, efalizumab had to be discontinued in all of the patients at varying times after transplant (and in two instances before the second transplant), so the long-term effects of this protocol on islet survival and renal function cannot be evaluated.
A recent editorial called for a “sober reassessment of the clinical applicability” of islet transplantation as the result of the relatively poor glycemic control following islet transplantation, nephrotoxicity of the immunosuppressive agents, and the sensitization of recipients resulting from multiple infusions and failed islet allografts [50, 51]. It was these important concerns that prompted the current trial using lymphocyte depletion and co-stimulatory/migration blockade to provide effective immmunosuppression to prevent early islet loss, minimize the number of infusions required to obtain insulin independence, and prevent nephrotoxicity in this vulnerable group of Type I diabetic recipients. This study provides preliminary evidence that clinical pancreatic islet transplantation can be successfully achieved using a novel immunosuppressive regimen based on efalizumab that does not require concomitant corticosteroids and minimizes CNI use. Furthermore, insulin independence was achieved with single donor pancreata in 4/8 patients – an important consideration in light of concerns for sensitization from multiple donors as well as the shortage of donor organs and efficacy of whole organ transplantation. Finally, this regimen minimizes the use of beta cell toxic agents, and in the short-term appears to reduce the risk of renal toxicity associated with CNI therapy. These have been important steps in the right direction, although the long-term consequences of this immunosuppressive protocol and the effects of efalizumab discontinuation on graft function remain to be determined and should be carefully monitored and reported. The strategy of avoiding beta-cell toxic and nephrotoxic immunosuppressive agents to protect beta cells will likely also have a role in future replacement protocols, as the sources of beta cells is expanded from adult islets to stem cell derived beta cells [52, 53].
Acknowledgments
Funding agency: JDRF, NIH
This work was supported by a grant from the Juvenile Diabetes Research Foundation (4-2004-372). The UCSF islet facility is supported in part by the National Institutes of Health grants P30 DK63720, UO1 AIO65193, and CRC grant UL1 RR024131.
We are indebted to the many individuals without whose enthusiastic participation and help this study would never have been accomplished: the study nurse coordinators (Joan McElroy and Debbie Ramos at UCSF; Janet Bricher, Sandra White, and Joyce Sinding at UM); the administrative/regulatory staff (Tina Johnson and Tara Rojas at UCSF; Jean Witson at UM); the islet isolation teams (Florinna Dekovic, Jiena Lang, Michael Lee, Pavel Koudria, Vihn Nguyen at UCSF; A.N Balamurugan and his staff at UM); the interventional radiology staff at UCSF; and the capable staff of the Clinical Research Centers at each institution.
References
- 1.Nathan DM. Long-Term Complications of Diabetes-Mellitus. New England Journal of Medicine. 1993;328(23):1676–1685. doi: 10.1056/NEJM199306103282306. [DOI] [PubMed] [Google Scholar]
- 2.Shamoon H, et al. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes-Mellitus. New England Journal of Medicine. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.Shannon H, et al. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. New England Journal of Medicine. 2000;342(6):381–389. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh RP, Stratta RJ. Advances in immunosuppression for pancreas transplantation. Current Opinion in Organ Transplantation. 2008;13(1):79–84. doi: 10.1097/MOT.0b013e3282f2fd91. [DOI] [PubMed] [Google Scholar]
- 5.Sutherland DER, Gruessner AC, Gruessner RWG. Pancreas transplantation: A review. Transplantation Proceedings. 1998;30(5):1940–1943. doi: 10.1016/s0041-1345(98)00489-8. [DOI] [PubMed] [Google Scholar]
- 6.Sutherland DER, et al. Pretransplant immunosuppression for pancreas transplants alone in nonuremic diabetic recipients. Transplantation Proceedings. 2001;33(1–2):1656–1658. doi: 10.1016/s0041-1345(00)02629-4. [DOI] [PubMed] [Google Scholar]
- 7.Sutherland DER, et al. Lessons learned from more than 1,000 pancreas transplants at a single institution. Annals of Surgery. 2001;233(4):463–501. doi: 10.1097/00000658-200104000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutherland DER, et al. Solitary pancreas transplants: A new era. Transplantation Proceedings. 1998;30(2):280–281. doi: 10.1016/s0041-1345(97)01266-9. [DOI] [PubMed] [Google Scholar]
- 9.Gruessner RW, et al. Solitary pancreas transplantation for nonuremic patients with labile insulin-dependent diabetes mellitus. Transplantation. 1997;64(11):1572–7. doi: 10.1097/00007890-199712150-00011. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro AMJ, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. New England Journal of Medicine. 2000;343(4):230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 11.Ricordi C, et al. beta-cell transplantation for diabetes therapy. Lancet. 2008;372(9632):27–28. doi: 10.1016/S0140-6736(08)60984-8. [DOI] [PubMed] [Google Scholar]
- 12.Gruessner RW, et al. A prospective, randomized, open-label study of steroid withdrawal in pancreas transplantation-a preliminary report with 6-month follow-up. Transplant Proc. 2001;33(1–2):1663–4. doi: 10.1016/s0041-1345(00)02632-4. [DOI] [PubMed] [Google Scholar]
- 13.Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4(2):481–508. doi: 10.2215/CJN.04800908. [DOI] [PubMed] [Google Scholar]
- 14.Nicolls MR, Gill RG. LFA-1 (CD11a) as a therapeutic target. Am J Transplant. 2006;6(1):27–36. doi: 10.1111/j.1600-6143.2005.01158.x. [DOI] [PubMed] [Google Scholar]
- 15.Corbascio M, et al. CTLA4Ig combined with anti-LFA-1 prolongs cardiac allograft survival indefinitely. Transpl Immunol. 2002;10(1):55–61. doi: 10.1016/s0966-3274(02)00014-x. [DOI] [PubMed] [Google Scholar]
- 16.Vincenti F, et al. A phase I/II randomized open-label multicenter trial of efalizumab, a humanized anti-CD11a, anti-LFA-1 in renal transplantation. Am J Transplant. 2007;7(7):1770–7. doi: 10.1111/j.1600-6143.2007.01845.x. [DOI] [PubMed] [Google Scholar]
- 17.Frampton JE, Plosker GL. Efalizumab: a review of its use in the management of chronic moderate-to-severe plaque psoriasis. Am J Clin Dermatol. 2009;10(1):51–72. doi: 10.2165/0128071-200910010-00009. [DOI] [PubMed] [Google Scholar]
- 18.Ricordi C, et al. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37(4):413–20. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 19.Bucher P, et al. Serva collagenase NB1: A new enzyme preparation for human islet isolation. Transplantation Proceedings. 2004;36(4):1143–1144. doi: 10.1016/j.transproceed.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 20.Brandhorst H, et al. The importance of tryptic-like activity in purified enzyme blends for efficient islet isolation. Transplantation. 2009;87(3):370–5. doi: 10.1097/TP.0b013e31819499f0. [DOI] [PubMed] [Google Scholar]
- 21.Szot GL, et al. Successful Clinical Islet Isolation Using a GMP-Manufactured Collagenase and Neutral Protease. Transplantation. 2009;88(6):753–756. doi: 10.1097/TP.0b013e3181b443ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Owen RJ, et al. Percutaneous transhepatic pancreatic islet cell transplantation in type 1 diabetes mellitus: radiologic aspects. Radiology. 2003;229(1):165–70. doi: 10.1148/radiol.2291021632. [DOI] [PubMed] [Google Scholar]
- 23.Faradji RN, et al. Simple measures to monitor beta-cell mass and assess islet graft dysfunction. Am J Transplant. 2007;7(2):303–8. doi: 10.1111/j.1600-6143.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- 24.Paty BW, et al. Assessment of glycemic control after islet transplantation using the continuous glucose monitor in insulin-independent versus insulin-requiring type 1 diabetes subjects. Diabetes Technol Ther. 2006;8(2):165–73. doi: 10.1089/dia.2006.8.165. [DOI] [PubMed] [Google Scholar]
- 25.Brandstrom E, et al. GFR measurement with iohexol and 51Cr-EDTA. A comparison of the two favoured GFR markers in Europe. Nephrol Dial Transplant. 1998;13(5):1176–82. doi: 10.1093/ndt/13.5.1176. [DOI] [PubMed] [Google Scholar]
- 26.Polkinghorne KR. Detection and measurement of urinary protein. Curr Opin Nephrol Hypertens. 2006;15(6):625–30. doi: 10.1097/01.mnh.0000247502.49044.10. [DOI] [PubMed] [Google Scholar]
- 27.Leischner MP, et al. Evaluation of proteinuria in healthy living kidney donor candidates. Transplant Proc. 2006;38(9):2796–7. doi: 10.1016/j.transproceed.2006.08.126. [DOI] [PubMed] [Google Scholar]
- 28.Grant J, et al. Validated protocol for FoxP3 reveals increased expression in type 1 diabetes patients. Cytometry B Clin Cytom. 2009;76(2):69–78. doi: 10.1002/cyto.b.20446. [DOI] [PubMed] [Google Scholar]
- 29.Major EO. Progressive Multifocal Leukoencephalopathy in Patients on Immunomodulatory Therapies. Annu Rev Med. 2009 doi: 10.1146/annurev.med.080708.082655. [DOI] [PubMed] [Google Scholar]
- 30.Korman BD, Tyler KL, Korman NJ. Progressive multifocal leukoencephalopathy, efalizumab, and immunosuppression: a cautionary tale for dermatologists. Arch Dermatol. 2009;145(8):937–42. doi: 10.1001/archdermatol.2009.175. [DOI] [PubMed] [Google Scholar]
- 31.Carson KR, et al. Monoclonal antibody-associated progressive multifocal leucoencephalopathy in patients treated with rituximab, natalizumab, and efalizumab: a Review from the Research on Adverse Drug Events and Reports (RADAR) Project. Lancet Oncol. 2009;10(8):816–24. doi: 10.1016/S1470-2045(09)70161-5. [DOI] [PubMed] [Google Scholar]
- 32.Pugashetti R, Koo J. Efalizumab discontinuation: a practical strategy. J Dermatolog Treat. 2009;20(3):132–6. doi: 10.1080/09546630902984596. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro AMJ, et al. International trial of the edmonton protocol for islet transplantation. New England Journal of Medicine. 2006;355(13):1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 34.Zeng YJ, et al. The Effect of Prednisone on Pancreatic-Islet Autografts in Dogs. Surgery. 1993;113(1):98–102. [PMC free article] [PubMed] [Google Scholar]
- 35.Kaufman DB, et al. Sequential kidney/islet transplantation using prednisone-free immunosuppression. American Journal of Transplantation. 2002;2(7):674–677. doi: 10.1034/j.1600-6143.2002.20715.x. [DOI] [PubMed] [Google Scholar]
- 36.Drachenberg CB, et al. Islet cell damage associated with tacrolimus and cyclosporine: morphological features in pancreas allograft biopsies and clinical correlation. Transplantation. 1999;68(3):396–402. doi: 10.1097/00007890-199908150-00012. [DOI] [PubMed] [Google Scholar]
- 37.Vincenti F, et al. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005;353(8):770–81. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- 38.Venetz JP, Pascual M. New treatments for acute humoral rejection of kidney allografts. Expert Opin Investig Drugs. 2007;16(5):625–33. doi: 10.1517/13543784.16.5.625. [DOI] [PubMed] [Google Scholar]
- 39.Scheinfeld N. Efalizumab: a review of events reported during clinical trials and side effects. Expert Opin Drug Saf. 2006;5(2):197–209. doi: 10.1517/14740338.5.2.197. [DOI] [PubMed] [Google Scholar]
- 40.Ramirez E, et al. Peripheral blood regulatory T cells in long-term kidney transplant recipients. Transplant Proc. 2009;41(6):2360–2. doi: 10.1016/j.transproceed.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 41.Boros P, Bromberg JS. Human FOXP3+ regulatory T cells in transplantation. Am J Transplant. 2009;9(8):1719–24. doi: 10.1111/j.1600-6143.2009.02704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim SH, et al. Clinical significance of monitoring circulating CD4+CD25+ regulatory T cells in kidney transplantation during the early posttransplant period. J Korean Med Sci. 2009;24(Suppl):S135–42. doi: 10.3346/jkms.2009.24.S1.S135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adams AB, et al. Calcineurin inhibitor-free CD28 blockade-based protocol protects allogeneic islets in nonhuman primates. Diabetes. 2002;51(2):265–70. doi: 10.2337/diabetes.51.2.265. [DOI] [PubMed] [Google Scholar]
- 44.Joshi A, et al. An overview of the pharmacokinetics and pharmacodynamics of efalizumab: a monoclonal antibody approved for use in psoriasis. J Clin Pharmacol. 2006;46(1):10–20. doi: 10.1177/0091270005283282. [DOI] [PubMed] [Google Scholar]
- 45.http://www.gene.com/gene/products/information/pdf/raptiva-prescribing.pdf.
- 46.Neff RT, et al. Progressive multifocal leukoencephalopathy and use of mycophenolate mofetil after kidney transplantation. Transplantation. 2008;86(10):1474–8. doi: 10.1097/TP.0b013e31818b62c8. [DOI] [PubMed] [Google Scholar]
- 47.Morgenstern LB, Pardo CA. Progressive multifocal leukoencephalopathy complicating treatment for Wegener’s granulomatosis. J Rheumatol. 1995;22(8):1593–5. [PubMed] [Google Scholar]
- 48.Piccinni C, et al. Stronger association of drug-induced progressive multifocal leukoencephalopathy (PML) with biological immunomodulating agents. Eur J Clin Pharmacol. 2009 doi: 10.1007/s00228-009-0739-z. [DOI] [PubMed] [Google Scholar]
- 49.Hering BJ, et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA. 2005;293(7):830–5. doi: 10.1001/jama.293.7.830. [DOI] [PubMed] [Google Scholar]
- 50.Bromberg JS, et al. The islet transplant experiment: Time for a reassessment. American Journal of Transplantation. 2007;7(10):2217–2218. doi: 10.1111/j.1600-6143.2007.01957.x. [DOI] [PubMed] [Google Scholar]
- 51.Campbell PM, et al. High risk of sensitization after failed islet transplantation. American Journal of Transplantation. 2007;7(10):2311–2317. doi: 10.1111/j.1600-6143.2007.01923.x. [DOI] [PubMed] [Google Scholar]
- 52.Guo T, Hebrok M. Stem cells to pancreatic beta-cells: new sources for diabetes cell therapy. Endocr Rev. 2009;30(3):214–27. doi: 10.1210/er.2009-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liew CG, Andrews PW. Stem cell therapy to treat diabetes mellitus. Rev Diabet Stud. 2008;5(4):203–19. doi: 10.1900/RDS.2008.5.203. [DOI] [PMC free article] [PubMed] [Google Scholar]