Abstract
Hydroxyurea (HU) is underutilized in adults with sickle cell disease (SCD) despite the Multicenter Study of Hydroxyurea in Sickle Cell Anemia (MSH) (1). Since little is known about HU utilization in children with SCD we sought to: 1) evaluate patterns of HU utilization; 2) elicit how providers define frequent pain when prescribing HU; and 3) identify barriers to HU use by surveying members of the American Society of Pediatric Hematology/Oncology. Data analysis included descriptive statistics and Chi-square. Of the 350 respondents, 63% care for SCD patients. Of these providers, only 9% have 50–90% of patients on HU, while 10% have <10% on HU. Criteria used to initiate HU included acute chest syndrome and frequent pain. Approximately half of providers account only for pain requiring hospitalization when prescribing HU. Those accounting for pain managed at home were more likely to have >30% of patients on HU (35.2% vs. 20%; p=0.023; Chi-square). Provider-related barriers to prescribing HU included compliance with: HU (86%), laboratory monitoring (85%), and contraception (85%). Our survey suggests substantial variation in HU utilization in children. Providers accounting for pain managed both in and out of the hospital had more patients on HU. Existing barriers to HU utilization should be addressed to optimize care for children with SCD.
Keywords: hydroxyurea, sickle cell disease, children, utilization, barriers
Pain is the most common complication of SCD accounting for over 80% of all hospitalizations for children. Home pain diary studies reveal pain is also frequently managed at home and goes underreported (2–4). Treatment of painful events primarily involves symptomatic care. Preventative measures are limited and HU, an oral drug taken once daily, is the only drug shown to decrease the frequency of SCD painful events (1).
The efficacy of HU was proven in adults in 1995 through a randomized controlled trial, the MSH (1). The MSH found HU significantly reduced the annual rate of painful events, acute chest syndrome episodes, and transfusions (1). The 9 year follow-up to MSH revealed HU was associated with significant reduction in mortality, minimal side effects, and was safe (5). A large trial mimicking the MSH in children was not conducted. However, efficacy studies in children include a randomized, placebo controlled, cross-over trial with small numbers of children and open-label, single-arm studies (6–8). These studies demonstrated a significant decrease in painful events in the HU arm (6–8) and led to the introduction of HU into pediatric practice for the prevention of pain. Subsequent studies have proven safety and hematologic efficacy in children (9–12).
Since the efficacy of HU was established in a controlled clinical trial environment, its effectiveness is dependent upon its utilization in real clinical practice. Despite the impressive findings of the MSH, HU is underutilized in adults limiting its effectiveness (13, 14). The National Institutes of Health (NIH) Consensus Development Conference Statement confirmed this underutilization (15). The utilization of HU in children has never been studied, thus no data exist to support whether its utilization will be better in pediatrics.
The NIH published recommendations for HU in children and adults in 2002 stating HU should be initiated in patients with “frequent pain episodes” (16). Currently, there are no national data addressing how pediatric SCD providers define “frequent pain episodes” and how they use this definition to recommend HU. Emerging SCD pain literature reveals majority of pain episodes are managed at home (2–4, 17) and it is unknown if providers incorporate these data into their definition of frequent pain.
To assess these important knowledge gaps we surveyed pediatric hematology/oncology providers and sought to achieve the following objectives: 1) evaluate practice patterns of HU utilization in children with SCD; 2) elicit how providers define frequent pain when prescribing HU; and 3) identify barriers to HU use.
Overall there was a 31% response rate (n=350). Of those that responded, 63% (n=220) take care of SCD patients. The majority of SCD providers were physicians (n=213; 96.8%), about half were female (n=103; 46.8%). The median years in practice was 12 (IQR 5–20). See online supplement for additional provider demographics and characteristics of practice and flow diagram of final study population (Table I, Figure 1).
Table I.
Provider Demographics and Characteristics of Practice (n=220)
| Variable | N (%) |
|---|---|
| Race | |
| White, Non-Hispanic | 161 (73.2) |
| Black | 9 (4.1) |
| Hispanic | 8 (3.6) |
| Asian or Pacific Islander | 33 (15) |
| Native American | 1 (0.5) |
| Other | 8 (3.6) |
| Region of Practice (in US) | |
| Northeast | 52 (23.6) |
| Midwest | 45 (20.5) |
| South | 70 (31.8) |
| West | 36 (16.4) |
| Not in US | 17 (7.7) |
| Patient Type (pediatric vs. adult)* | |
| Pediatrics | 179 (84.4) |
| Adults | 1(0.5) |
| Both Pediatrics and Adults | 26 (12.3) |
| Practice Type | |
| Hematology | 48 (21.8) |
| Oncology | 1 (0.5) |
| Hematology/Oncology | 170 (77.7) |
| Hospital Type* | |
| Rural teaching | 8 (3.8) |
| Rural non-teaching | 5 (2.4) |
| Urban teaching | 178 (84) |
| Urban non-teaching | 14 (6.6) |
some missing data; US= United States
Figure 1.
Flow diagram of final study population.
Most providers (90%) felt HU was effective or very effective for the prevention of pain. Only 9% of providers have 50–90% of their patients on HU, 22% have 31–50% on HU, 54% have 10–30% on HU, and 10% have <10% on HU. Table II shows the most common criteria providers use to start HU. About half (54%) indicated they initiated HU in children <4 years of age.
Table II.
Criteria used by providers to start hydroxyurea
| Criteria | Proportion (%) |
|---|---|
| Acute Chest Syndrome | 88 |
| ≥3 painful episodes/year | 86 |
| Requiring hospitalization only | 42 |
| At home or requiring hospitalization | 44 |
| Chronic pain requiring frequent narcotic use | 70 |
| Priapism | 48 |
| Pulmonary Hypertension | 43 |
| Symptomatic Anemia | 40 |
| Stroke | 36 |
| Renal Failure | 15 |
| Ankle Ulcers | 12 |
| Low hemoglobin F levels | 9 |
| Elevated white cell count without evidence of infection | 6 |
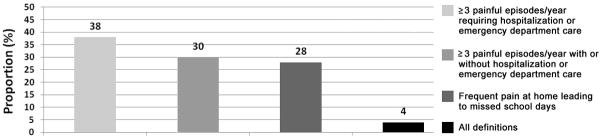
Figure 2 displays how providers define frequent pain in children with SCD. When asked about criteria used to initiate HU for pain, 42% used only pain requiring hospitalization whereas 44% accounted for pain both at home and in the hospital when prescribing HU. Importantly, providers incorporating pain at home into their definition of frequent pain were more likely to have >30% of their patients on HU (35.2% vs. 20%; p=0.023; Pearson Chi-square).
Figure 2.
Proportion of providers using various definitions for frequent pain in children with sickle cell disease.
The proportions of providers stating a particular factor influenced their decision to not prescribe HU to eligible patients are listed in Table III. The most common factors identified as barriers to prescribing HU revolved around the theme of compliance.
Table III.
Provider-related barriers to hydroxyurea use
| Barrier | Proportion (%) |
|---|---|
| Patient compliance with taking medication | 86 |
| Patient compliance with blood tests | 85 |
| Lack of contraception in females | 85 |
| Patient’s anticipation of side effects | 75 |
| Patient is too young | 68 |
| Concern for male infertility | 46 |
| Lack of formal guidelines for use in children | 30 |
| Provider discomfort with carcinogenic potential | 27 |
| Cost | 18 |
| Lack of time/resources to explain risks/benefits | 16 |
| Not FDA approved in children | 12 |
| Doubt effectiveness of hydroxyurea | 11 |
Twenty-six percent of providers reported >20% of their patients/families refused HU when it was offered. The most common reasons for patients’ refusal were fear of cancer (51%) and other side effects (62%), don’t want to take medication (49%), or don’t want required laboratory monitoring (28%). Interestingly, 17% refused HU because they didn’t think it would work.
To our knowledge, this is the first study to assess utilization of HU in children with SCD and to identify barriers to its use in children. Our survey suggests substantial variation exists in the use of HU in children with SCD. Very few providers have more than half of their patients on HU and 1 in 10 rarely use HU and have <10% of patients on HU.
Our survey also found providers use HU for SCD-related complications without data to support its use for these complications, representing clinical drift (18). Over a third of providers use HU for secondary stroke prevention and almost half use HU for priapism and pulmonary hypertension; all complications currently lacking HU efficacy data. These data raise the need for continued and future funding of clinical trials to evaluate the unknown efficacy of HU for these complications which will prevent or promote the appropriate use of HU and ultimately avoid clinical drift (18).
Fortunately, majority of providers use frequent pain as criteria for starting HU, however, how providers define frequent pain varied. Our data show almost half of providers use only pain events requiring hospitalization as criteria for starting HU. If this strict definition is used, many children that may benefit from HU will not be considered eligible. Recent pain literature in SCD reveals majority of pain is managed at home (2–4, 17), goes underreported and impacts school attendance and children’s health-related quality of life (2–4, 19, 20). Our survey found providers accounting for pain at home were significantly more likely to have more children on HU, suggesting how providers define frequent pain may be a barrier to using the drug.
The identified barriers to HU use in children at the provider and patient level were similar to previously identified barriers in adults with SCD (13, 14, 21). The most common provider-related barriers involved the theme of patient compliance, including compliance with taking HU, required laboratory monitoring, and female contraception. Importantly, non-compliance may be a result of poor access to care, a systems-level barrier, or may stem from patients’ fears of side effects. In addition, patients’ access to HU may be limited if providers are not prescribing HU to eligible patients because of their own biases about the drug.
Other barriers included concerns for toxicities there may or may not be evidence to support, such as concern for carcinogenesis. Long-term follow-up data from MSH do not provide evidence supporting this concern in adults taking HU (5). The Agency for Healthcare Research and Quality systematic review about HU also stated “limited evidence suggests that HU treatment in adults with SCD does not increase the risk for leukemia” (22). Widespread provider and patient education regarding the limited or non-existent association between HU and cancer is imperative to eliminate this barrier. Concern for male infertility was a provider-related barrier in almost half. Currently, evidence is lacking to support or disprove this concern. Concern for teratogenesis was a fear for majority of providers and if the provider doubted compliance with female contraception, this was a barrier to the use of HU. Current MSH data reveal harm did not occur to offspring of women taking HU at the time pregnancy occurred (23). Finally, although some providers place children <4 years on HU, majority of providers reported age (patient too young) was a barrier to prescribing HU. Data from the trial “HU to Prevent Organ Damage in Children with Sickle Cell Anemia” (24) will confirm the safety of HU in young children and potentially reduce the barrier of age in the prescribing of HU to younger children that may benefit.
Ultimately, it is important to remember SCD carries with it significant morbidity and is associated with mortality. Based on proven efficacy and safety in children (6–12, 25, 26) , HU provides significant benefit to children suffering from a life-long debilitating disease and likely improves their health-related quality of life; thus the benefit of HU may outweigh potential risks.
Our study is limited in that survey responses may not reflect true practice. Individual charts would require auditing to verify this information. In addition, we do not have information about non-responders since the survey was anonymous. The overall response rate was 31%, however this is consistent with other published survey research (27–29).
In conclusion, our survey suggests substantial variation in HU utilization in children with SCD. HU is used for complications other than pain despite insufficient evidence for its efficacy for these complications, representing clinical drift (18). Studies to determine the efficacy of HU for SCD complications other than pain are urgently needed to prevent or promote the appropriate use of the drug. Although majority of providers report frequent pain as criteria for starting HU, almost half only account for pain bringing a child to the hospital. This strict definition likely misses many children experiencing significant pain at home that would benefit from HU. Finally, provider, patient, and systems-level barriers to HU utilization in children exist and need to be addressed. Future studies should be aimed at evaluating unknown toxicities of HU that influence practice, exploring whether access to care contributes to noncompliance, adherence research, and provider education about the extent of pain experienced by children with SCD.
Methods
An anonymous cross-sectional survey was emailed to 1316 pediatric hematology/oncology providers identified through the published 2008 American Society of Pediatric Hematology/Oncology (ASPHO) membership directory. ASPHO is an international professional society of pediatric hematology/oncology providers who conduct research in and treat children with cancer and other blood diseases. The survey was kept anonymous to encourage providers to be honest with their responses and the anonymity also permitted multiple respondents from the same institution to report their practice patterns as individuals allowing for variability of practice within a large program. This limited biasing responses of individuals towards the views and practices of the institution. The survey was adapted with permission from that done by adult SCD providers (13), pilot-tested amongst experts in pediatric SCD, modified based on their recommendations, and emailed using a web-based survey program (30). A brief introductory letter explained the study and stated informed consent was implied with survey completion. The initial email and five reminder emails were sent between February 12, 2009 and July 13, 2009 with reminder emails sent only to non-responders. The survey program allowed for completion of the survey only once by each member emailed. The survey began by identifying providers that care for SCD patients. If the provider did not care for SCD patients, the first question indicated this and the survey was complete. If the provider did care for SCD patients, he/she was directed to complete the remainder of the survey. The response rate includes all respondents, however, all other data includes only those that care for SCD patients. See online supplement for survey details addressing the main manuscript objectives. The study was approved by the Institutional Review Board of the Children’s Hospital of Wisconsin, which allowed for completion of the survey to serve as implied consent.
Statistical Analysis
Analyses were conducted with SPSS version 14.0 for Windows (SPSS, Chicago, IL). Respondent survey data was extracted, inputted into SPSS, and descriptive statistics were calculated. We report proportions, medians and interquartile ranges as appropriate. Chi-square analysis was used to test differences between proportions. A p-value of ≤ 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
The authors would like to acknowledge all of the providers who took the time to complete the survey. This work was supported in part by a grant from the National Institutes of Health National Heart, Lung, and Blood Institute U54 HL090503 (AB) and K23 HL80092 (JP).
Footnotes
Conflicts of Interest: None for any of the authors
Contributions: A.M. Brandow designed research, performed research, analyzed data, and wrote manuscript. D.L. Jirovec designed research, performed research and revised manuscript. J.A. Panepinto designed research and revised manuscript.
References
- 1.Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, McMahon RP, Bonds DR. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med. 1995;332:1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro BS, Dinges DF, Orne EC, Bauer N, Reilly LB, Whitehouse WG, Ohene-Frempong K, Orne MT. Home management of sickle cell-related pain in children and adolescents: natural history and impact on school attendance. Pain. 1995;61:139–144. doi: 10.1016/0304-3959(94)00164-A. [DOI] [PubMed] [Google Scholar]
- 3.Dampier C, Ely E, Brodecki D, O’Neal P. Home management of pain in sickle cell disease: a daily diary study in children and adolescents. J Pediatr Hematol Oncol. 2002;24:643–647. doi: 10.1097/00043426-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Wang WC, Wang WC. Pain at home in sickle cell disease: an underrecognized problem. Journal of Pediatric Hematology/Oncology. 2002;24:610–612. doi: 10.1097/00043426-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Steinberg MH, Barton F, Castro O, Pegelow CH, Ballas SK, Kutlar A, Orringer E, Bellevue R, Olivieri N, Eckman J, Varma M, Ramirez G, Adler B, Smith W, Carlos T, Ataga K, DeCastro L, Bigelow C, Saunthararajah Y, Telfer M, Vichinsky E, Claster S, Shurin S, Bridges K, Waclawiw M, Bonds D, Terrin M. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA. 2003;289:1645–1651. doi: 10.1001/jama.289.13.1645. [DOI] [PubMed] [Google Scholar]
- 6.Scott JP, Hillery CA, Brown ER, Misiewicz V, Labotka RJ. Hydroxyurea therapy in children severely affected with sickle cell disease. Journal of Pediatrics. 1996;128:820–828. doi: 10.1016/s0022-3476(96)70335-9. [DOI] [PubMed] [Google Scholar]
- 7.Jayabose S, Tugal O, Sandoval C, Patel P, Puder D, Lin T, Visintainer P. Clinical and hematologic effects of hydroxyurea in children with sickle cell anemia. Journal of Pediatrics. 1996;129:559–565. doi: 10.1016/s0022-3476(96)70121-x. [DOI] [PubMed] [Google Scholar]
- 8.Ferster A, Vermylen C, Cornu G, Buyse M, Corazza F, Devalck C, Fondu P, Toppet M, Sariban E. Hydroxyurea for treatment of severe sickle cell anemia: a pediatric clinical trial. Blood. 1996;88:1960–1964. [PubMed] [Google Scholar]
- 9.Zimmerman SA, Schultz WH, Davis JS, Pickens CV, Mortier NA, Howard TA, Ware RE, Zimmerman SA, Schultz WH, Davis JS, Pickens CV, Mortier NA, Howard TA, Ware RE. Sustained long-term hematologic efficacy of hydroxyurea at maximum tolerated dose in children with sickle cell disease. Blood. 2004;103:2039–2045. doi: 10.1182/blood-2003-07-2475. [DOI] [PubMed] [Google Scholar]
- 10.Hankins JS, Ware RE, Rogers ZR, Wynn LW, Lane PA, Scott JP, Wang WC, Hankins JS, Ware RE, Rogers ZR, Wynn LW, Lane PA, Scott JP, Wang WC. Long-term hydroxyurea therapy for infants with sickle cell anemia: the HUSOFT extension study. Blood. 2005;106:2269–2275. doi: 10.1182/blood-2004-12-4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kinney TR, Helms RW, O’Branski EE, Ohene-Frempong K, Wang W, Daeschner C, Vichinsky E, Redding-Lallinger R, Gee B, Platt OS, Ware RE. Safety of hydroxyurea in children with sickle cell anemia: results of the HUG-KIDS study, a phase I/II trial. Pediatric Hydroxyurea Group. Blood. 1999;94:1550–1554. [PubMed] [Google Scholar]
- 12.Heeney MM, Ware RE. Hydroxyurea for children with sickle cell disease. Pediatr Clin North Am. 2008;55:483–501. x. doi: 10.1016/j.pcl.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Zumberg MS, Reddy S, Boyette RL, Schwartz RJ, Konrad TR, Lottenberg R. Hydroxyurea therapy for sickle cell disease in community-based practices: a survey of Florida and North Carolina hematologists/oncologists. Am J Hematol. 2005;79:107–113. doi: 10.1002/ajh.20353. [DOI] [PubMed] [Google Scholar]
- 14.Lanzkron S, Haywood C, Jr, Hassell KL, Rand C. Provider barriers to hydroxyurea use in adults with sickle cell disease: a survey of the Sickle Cell Disease Adult Provider Network. J Natl Med Assoc. 2008;100:968–973. [PubMed] [Google Scholar]
- 15.Brawley OW, Cornelius LJ, Edwards LR, Gamble VN, Green BL, Inturrisi C, James AH, Laraque D, Mendez M, Montoya CJ, Pollock BH, Robinson L, Scholnik AP, Schori M, Brawley OW, Cornelius LJ, Edwards LR, Gamble VN, Green BL, Inturrisi C, James AH, Laraque D, Mendez M, Montoya CJ, Pollock BH, Robinson L, Scholnik AP, Schori M. National Institutes of Health Consensus Development Conference statement: hydroxyurea treatment for sickle cell disease. Annals of Internal Medicine. 2008;148:932–938. doi: 10.7326/0003-4819-148-12-200806170-00220. [DOI] [PubMed] [Google Scholar]
- 16.The Management of Sickle Cell Disease. NIH; 2002. pp. 161–165. [Google Scholar]
- 17.Smith WR, Penberthy LT, Bovbjerg VE, McClish DK, Roberts JD, Dahman B, Aisiku IP, Levenson JL, Roseff SD. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008;148:94–101. doi: 10.7326/0003-4819-148-2-200801150-00004. [DOI] [PubMed] [Google Scholar]
- 18.Debaun MR, Field JJ. Limitations of clinical trials in sickle cell disease: a case study of the Multi-center Study of Hydroxyurea (MSH) trial and the Stroke Prevention (STOP) trial. Hematology Am Soc Hematol Educ Program. 2007:482–488. doi: 10.1182/asheducation-2007.1.482. [DOI] [PubMed] [Google Scholar]
- 19.Brandow AM, Brousseau DC, Pajewski NM, Panepinto JA, Brandow AM, Brousseau DC, Pajewski NM, Panepinto JA. Vaso-occlusive painful events in sickle cell disease: impact on child well-being. Pediatric Blood & Cancer. 2010;54:92–97. doi: 10.1002/pbc.22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brandow AM, Brousseau DC, Panepinto JA, Brandow AM, Brousseau DC, Panepinto JA. Postdischarge pain, functional limitations and impact on caregivers of children with sickle cell disease treated for painful events. British Journal of Haematology. 2009;144:782–788. doi: 10.1111/j.1365-2141.2008.07512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thornburg CD, Calatroni A, Telen M, Kemper AR. Adherence to hydroxyurea therapy in children with sickle cell anemia. J Pediatr. 156:415–419. doi: 10.1016/j.jpeds.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segal JB, Strouse JJ, Beach MC, Haywood C, Witkop C, Park H, Wilson RF, Bass EB, Lanzkron S. Hydroxyurea for the treatment of sickle cell disease. Evid Rep Technol Assess (Full Rep) 2008:1–95. [PMC free article] [PubMed] [Google Scholar]
- 23.Ballas SK, McCarthy WF, Guo N, DeCastro L, Bellevue R, Barton BA, Waclawiw MA, Ballas SK, McCarthy WF, Guo N, DeCastro L, Bellevue R, Barton BA, Waclawiw MA Multicenter Study of Hydroxyurea in Sickle Cell A. Exposure to hydroxyurea and pregnancy outcomes in patients with sickle cell anemia. Journal of the National Medical Association. 2009;101:1046–1051. doi: 10.1016/s0027-9684(15)31072-5. [DOI] [PubMed] [Google Scholar]
- 24.National Heart, Lung, and Blood Institute (NHLBI) Clinical Trialsgov [Internet] Bethesda (MD): National Library of Medicine (US); Hydroxyurea to Prevent Organ Damage in Children with Sickle Cell Anemia. p available from: http://clinicaltrials.gov/show/NCT00006400 NLM Identifier: NCT00006400. [Google Scholar]
- 25.Ferster A, Tahriri P, Vermylen C, Sturbois G, Corazza F, Fondu P, Devalck C, Dresse MF, Feremans W, Hunninck K, Toppet M, Philippet P, Van Geet C, Sariban E. Five years of experience with hydroxyurea in children and young adults with sickle cell disease. Blood. 2001;97:3628–3632. doi: 10.1182/blood.v97.11.3628. [DOI] [PubMed] [Google Scholar]
- 26.Wang WC, Wynn LW, Rogers ZR, Scott JP, Lane PA, Ware RE. A two-year pilot trial of hydroxyurea in very young children with sickle-cell anemia. Journal of Pediatrics. 2001;139:790–796. doi: 10.1067/mpd.2001.119590. [DOI] [PubMed] [Google Scholar]
- 27.Streiff MB, Smith B, Spivak JL. The diagnosis and management of polycythemia vera in the era since the Polycythemia Vera Study Group: a survey of American Society of Hematology members’ practice patterns. Blood. 2002;99:1144–1149. doi: 10.1182/blood.v99.4.1144. [DOI] [PubMed] [Google Scholar]
- 28.Lee SJ, Joffe S, Kim HT, Socie G, Gilman AL, Wingard JR, Horowitz MM, Cella D, Syrjala KL. Physicians’ attitudes about quality-of-life issues in hematopoietic stem cell transplantation. Blood. 2004;104:2194–2200. doi: 10.1182/blood-2003-07-2430. [DOI] [PubMed] [Google Scholar]
- 29.Wong EC, Perez-Albuerne E, Moscow JA, Luban NL. Transfusion management strategies: a survey of practicing pediatric hematology/oncology specialists. Pediatr Blood Cancer. 2005;44:119–127. doi: 10.1002/pbc.20159. [DOI] [PubMed] [Google Scholar]
- 30.Constant Contact. www.constantcontact.com. Waltham, MA.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.