Abstract
Monoacylglycerols (MAGs) are short-lived intermediates of glycerolipid metabolism. Specific molecular species, such as 2-arachidonoylglycerol, which is a potent activator of cannabinoid receptors, may also function as lipid signaling molecules. In mammals, enzymes hydrolyzing MAG to glycerol and fatty acids, resembling the final step in lipolysis, or esterifying MAG to diacylglycerol, are well known; however, despite the high level of conservation of lipolysis, the corresponding activities in yeast have not been characterized yet. Here we provide evidence that the protein Yju3p functions as a potent MAG hydrolase in yeast. Cellular MAG hydrolase activity was decreased by more than 90% in extracts of Yju3p-deficient cells, indicating that Yju3p accounts for the vast majority of this activity in yeast. Loss of this activity was restored by heterologous expression of murine monoglyceride lipase (MGL). Since yju3Δ mutants accumulated MAG in vivo only at very low concentrations, we considered the possibility that MAGs are re-esterified into DAG by acyltransferases. Indeed, cellular MAG levels were further increased in mutant cells lacking Yju3p and Dga1p or Lro1p acyltransferase activities. In conclusion, our studies suggest that catabolic and anabolic reactions affect cellular MAG levels. Yju3p is the functional orthologue of mammalian MGL and is required for efficient degradation of MAG in yeast.
Abbreviations: MAG, monoacylglycerol; MGL, monoacylglycerol lipase; MGH, monoacylglycerol hydrolase; MGAT, acyl-CoA:monoacylglycerol acyltransferase; DGAT, acyl-CoA:diacylglycerol acyltransferase; EC, endocannabinoid; 2-AG, 2-arachachidonoyl glycerol; LD, lipid droplet; TAG, triacylglycerol; FFA, free fatty acid; DAG, diacylglycerol; GFP, green fluorescent protein; NAPE, N-acylphosphatidylethanolamine; NAE, N-acylethanolamide; AEA, N-arachidonoyl ethanolamide
Keywords: Monoacylglycerols, Monoglyceride lipase, MGAT activity, Yeast
1. Introduction
Monoacylglycerols (MAGs) are important intermediates in mammalian lipid metabolism. In the intestine, 2-MAG results from triacylglycerol (TAG) hydrolysis that is catalyzed by pancreatic lipase. MAG and free fatty acids (FFAs) are taken up by enterocytes and are re-esterified by monoacylglycerol acyltransferases (MGATs) to diacylglycerol (DAG) [1]. This process is essential for the efficient packaging of dietary fat into chylomicron lipoprotein particles [2]. In the circulation, TAG-rich lipoproteins are hydrolyzed by lipoprotein lipase (LPL), generating MAG and FFA [3]. These lipolytic products are internalized by tissues and further processed for lipid synthesis or energy conversion. Within cells, MAG may derive from the catabolism of TAG, which is catalyzed by the combined action of adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL) [4], or from glycerophospholipid degradation. In mammals, monoacylglycerol lipase (MGL or MAGL) is suggested to carry out the rate-limiting step of MAG hydrolysis. MGL activities have been detected in rat tissues already in the seventies, and the respective enzymes have also been subjected to purification [5]. MGL is a member of the serine hydrolase family and ubiquitously expressed [6]. The enzyme has a high substrate specificity for MAG, but low stereoselectivity. Mammalian MGL catalyzes the hydrolysis of rac-1(3)- and 2-MAG at equal rates, and has no activity against trioleoylglycerol, dioleoylglycerol, cholesteryl oleate, or lysophosphatidylcholine in vitro [5].
Specific MAG species, however, are not only intermediary products of lipid metabolism but also possess signaling function. In mammals, 2-arachidonoylglycerol (2-AG) belongs to the family of endocannabinoids (EC), which are endogenous agonists of cannabinoid receptors [7]. ECs are involved in the control of many biological processes including behavior, appetite regulation, pain, blood pressure, energy metabolism, inflammation, and cell growth. Their biological effect is mimicked by Δ9-tetrahydrocannabinol (THC), the major psychoactive component of marijuana. The broad spectrum of bioactivities of EC compounds has attracted great interest in pharmacological research; however, their clinical use is limited due to rapid cellular degradation. Thus, inhibiting 2-AG hydrolyzing enzymes, such as MGL, is considered a promising pharmacological approach to modulate EC levels [8]. Indeed, inhibition of MGL using a specific inhibitor in mice raised 2-AG levels and induced cannabinoid receptor-dependent effects like analgesia, hypomotility, and hypothermia [9]. These observations indicate that MGL degrades 2-AG and hence plays an important role in EC signaling.
A search in the yeast genome database unveiled the gene YJU3 (YKL094W) to encode a putative monoacylglycerol lipase, based on 23% sequence identity to mouse and human MGL, suggesting a high level of conservation of enzymes involved in EC metabolism [10]. Yju3p was previously identified by mass spectrometry as a lipid droplet protein, consistent with a role in lipid metabolism [10]. The enzymology of TAG catabolism in yeast has recently been characterized in greater detail [11–13], and it shows a high level of functional conservation to mammalian cells. Whereas Tgl4p functions as a TAG lipase, Tgl3p has both TAG and DAG lipase activities [13], in analogy to the catalytic cascade governed by adipose triglyceride lipase, ATGL, and hormone sensitive lipase, HSL [14]. However, no specific MGL has been described yet in yeast. Furthermore, alternative pathways for MAG metabolization, i.e., for the resynthesis of diacylglycerol, are uncharacterized in that organism.
Here we provide experimental evidence that Yju3p indeed functions as the major monoacylglycerol lipase in yeast. In addition, our data indicate that acyltransferases such as Dga1p and Lro1p play a role in MAG metabolism.
2. Material and methods
2.1. Strains and media
The yeast strains used in this study were BY4742 (MATa his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0), yju3Δ (MATa his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 YKL094W∷kanMX4), dga1Δ (MATa his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 YOR245C∷kanMX4), the dga1Δ lro1Δ double-mutant strain (MATa his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 YOR245C∷kanMX4 YNR008W∷kanMX4), and the dga1Δ lro1Δ yju3Δ triple-mutant strain (MATa his3Δ1 leu2Δ0 ura3Δ0 YOR245C∷kanMX4 YNR008W∷kanMX4 YKL094W∷kanMX4). Wild-type and single mutant strains were obtained from Euroscarf (Frankfurt, Germany), the double- and triple-mutant strains were generated by standard genetic crosses and tetrad dissection and were verified by colony PCR using gene deletion specific primers. The Escherichia coli strain TOP10F′ ([proAB, laqIq, lacZΔM15, Tn10(Tetr)], mcrAΔ(mrr-hsdRSM-mcrBC), phi80ΔlacZM15, ΔlacX74, deoR, recA1, ara-D139Δ(ara, leu), 7697galU, galK, d-rps(streptomycinr), endA1, nupG) was used for plasmid amplification and purification. Yeast cells were grown in YPD medium containing 1% yeast extract, 2% peptone, and 2% glucose or in YNB minimal medium containing 0.67% yeast nitrogen base, 2% glucose, and the respective amino acids and bases. Yeast transformants carrying expression plasmids were selected in uracil-free medium. Geneticin resistance was scored on YPD plates containing 200 mg/l geneticin (G418; Calbiochem, Merck, Whitehouse Station, USA). Sporulation medium contained 0.25% yeast extract, 0.1% glucose, and 1% potassium acetate. Ampicillin-resistant E. coli transformants were selected on LBA plates containing 0.5% yeast extract, 1% peptone, 0.5% NaCl, and 100 mg/l ampicillin (Roche, Basel, Switzerland). Yeast strains were grown at 30 °C on a rotary shaker with vigorous aeration. Cell growth was monitored with a Casy® TTC cell counter (Schärfe System, Reutlingen, Germany) or by measuring the optical density at 600 nm (OD).
2.2. Construction of a plasmid encoding pGFP–MGL
A pcDNA4/Hismax C vector (Invitrogen, Carlsbad, CA) containing the murine MGL open reading frame was digested with BamHI and XhoI, and the MGL open reading frame was inserted into the BamHI/XhoI restriction sites of plasmid pUG36 (kindly provided by H. Hegemann, University of Duesseldorf, Germany) for the expression and subcellular localization of the fusion protein GFP–MGL in yeast. Plasmids were transformed into yeast using the lithium acetate method [15], and colonies were selected on plates lacking uracil.
2.3. Cloning, overexpression and purification of His-tagged Yju3p
For the overexpression of Yju3p in E. coli, the gene was excised from the yeast vector pYEX-4 T-1 using the restriction enzymes BamHI and EcoRI and ligated into the pET21a vector (Novagen, Merck, Whitehouse Station, USA), using standard protocols. To generate an N-terminal His-tag, the gene was again cleaved from pET21a using BamHI and XhoI and cloned into the vector pPROEX HTb (Invitrogen, Carlsbad, CA, USA), which provides the coding sequence for an N-terminal (His)6 tag. The clone pProExHtb-YJU3 was transformed into E. coli BL21 (DE3) and gene expression was induced in midlog phase at 37 °C for 4 hours using 0.5 mM IPTG. The harvested cells were lysed by sonication in a buffer containing 50 mM Tris–HCl (pH 8.0), 100 mM NaCl, and 0.5% NP-40. After centrifugation (27,000 × g at 4 °C, 30 min), the soluble fraction was loaded on to a HisTrap™ FF column (Pharmacia, GE Healthcare) and eluted using buffer containing 50 mM Tris (pH 8.0), 100 mM NaCl, 10% glycerol, and 240 mM imidazole. The protein sample was dialysed against a buffer containing 50 mM Tris (pH 8.0) 100 mM NaCl, 20% glycerol, 1 mM DTT, and concentrated in the presence of 8 mM Mega8.
2.4. Cell fractionation and isolation of lipid droplets
Yeast cells were harvested in the early stationary phase, washed in deionized water, and resuspended in 0.25 M sucrose with 1 mM EDTA containing 2 mg/l antipain, 1 mg/l pepstatin, 20 mg/l leupeptine as protease inhibitors. Cells were broken with glass beads in a Merckenschlager homogenizer (Braun Biotech International GmbH, Melsungen, Germany) under CO2 cooling. Cell debris was removed by centrifuging at 1000 × g for 10 min. The supernatant was transferred to centrifugation tubes, overlaid with 50 mM potassium phosphate buffer pH 7.5 containing 100 mM KCl and 1 mM EDTA ( buffer A), and centrifuged at 100,000 × g for 1 h to collect the floating lipid layer, cytosolic fraction, and crude membrane fraction. Lipid droplet (LD) and membrane fractions were purified by a subsequent step of centrifuging at 100,000 × g. For protein determination, aliquots of the cell fractions were mixed with a 4-fold volume of acetone and incubated overnight at −20 °C to precipitate proteins and to remove lipids. After centrifuging at 10,000 × g for 30 min, precipitated protein was dissolved in 0.1% SDS and 0.3 M NaOH and protein concentration was determined with a BCA protein assay according to the manufacturer's instructions (BCA™ Protein Assay Kit, Pierce, Illinois, USA), using BSA as a standard.
2.5. Triacylglycerol hydrolysis activity of isolated LD fractions
Triacylglycerol hydrolysis activity of isolated LDs was determined by using 25–50 μg of LD protein in a total volume of 100 μl of buffer A and incubation with 100 μl of [carboxyl-14C] trioleoylglycerol (final concentration of 300 μmol/l and a specific activity of 15 μCi/ml) for 1 h at 37 °C in a shaking water bath. The substrate was prepared as follows: trioleoylglycerol was dried under a stream of nitrogen, emulsified by sonication with 45 μmol/l phosphatidylcholine/phosphatidylinositol (PC/PI, 3:1) in 100 mM potassium phosphate buffer pH 7.5, and adjusted to 5% defatted BSA. The reaction was stopped by the addition of 1 ml of chloroform/methanol (2:1, vol./vol.) containing 1% acetic acid and lipids were extracted by vortexing. After centrifuging at 1000 × g for 10 min, the lower phase was collected, dried under a stream of nitrogen, and applied onto silica gel plates (silica gel 60, Merck, Whitehouse Station, USA). Lipids were separated using chloroform/acetone/acetic acid (92:6:1, vol./vol./vol.) as the solvent system, and radioactivity was detected after exposure to radiosensitive screens by scanning with a Storm™ 860 scanner (GE Healthcare, Piscataway, NJ). FFA, DAG, and MAG fractions were scraped off the plates, and radioactivity was measured by liquid scintillation counting.
2.6. Monoacylglycerol hydrolase activity assay
Monoacylglycerol hydrolase (MGH) activity was assayed with either purified recombinant Yju3p or cell homogenates. Purified Yju3p was dialyzed against a buffer containing 50 mM Tris (pH 7.4) and 100 mM NaCl before measurements to remove glycerol. Samples were incubated with 100 μl of substrate containing either 1 mM rac-1(3)-oleoylglycerol (rac-1(3)-OG) or 2-oleoylglycerol (2-OG; Sigma Aldrich, St. Louis, USA), complexed to defatted BSA at a molar ratio of 1:1 in 100 mM potassium phosphate buffer (pH 6–7.5), sodium acetate buffer (pH 4–5.5), or Tris–HCl buffer (pH 8–9). Reactions were carried out at 37 °C for 20 min, which is in the linear range of the reaction, and stopped by the addition of 100 μl of chloroform. Samples were centrifuged at 10,000 × g for 10 min, and aliquots of the upper phase were collected to determine the free glycerol concentration using a commercial kit (Sigma Aldrich, St. Louis, USA).
2.7. Monoacylglycerol acyltransferase activity assay
To determine MGAT activity, 50 μg of protein in a final volume of 10 μl were preincubated for 3 min at 25 °C with 170 μl of substrate containing 50 μmol/l 2-oleoylglycerol, 100 μmol/l PC/PI (3:1, emulsified by sonication), and 1 g/l defatted BSA in 100 mM Tris–HCl buffer (pH 7.0). The reaction was started by the addition of 20 μl 14C-labeled oleoyl-coenzyme A (final concentration 20 μmol/l with a specific activity of 55 μCi/μmol), and was stopped after 10 min at 25 °C by the addition of 1 ml of chloroform/methanol (2:1, vol./vol.) and vortexing. After centrifuging at 1000 × g for 10 min, the lower phase was collected, evaporated under a stream of nitrogen, and redissolved in chloroform. Lipid extracts were applied onto silica gel plates and separated using hexane/diethylether/acetic acid (70:30:1, vol./vol./vol.) as the solvent system. DAG and TAG spots were scraped off the plates, and radioactivity was determined by liquid scintillation counting.
2.8. Microscopy
yju3Δ cells harboring the plasmid pMGL-GFP were cultivated for 24 h in YNB medium without uracil. Microscopy was performed on a Leica SP2 confocal microscope using a 100× oil immersion objective (NA 1.4). GFP was excited at 488 nm, fluorescence emission was detected between 500 and 535 nm. Nile Red staining of yeast lipid droplets was performed according to Wolinski and Kohlwein [16], which allows for optimized color separation. Nile Red was excited at 543 nm, and fluorescence emission was detected between 550 and 575 nm. Transmission images were recorded using differential interference contrast (DIC) optics.
2.9. Lipid analysis
For analysis of neutral lipids, cells were broken with glass beads by vigorous shaking, and lipids were extracted with chloroform/methanol 2:1 (vol./vol.) as described [17]. Aliquots of the lipid extracts were applied onto silica gel plates and separated with chloroform/acetone/acetic acid (90:8:1, vol./vol./vol.) as the solvent system. Lipids were visualized by carbonization after spraying the plates with 10% CuSO4 (wt./vol.) and 10% H3PO4 (vol./vol.) and heating them to 120 °C for 30 min.
2.10. Metabolic labelling
Stationary phase cultures were inoculated into fresh medium at a density of 2 × 610 cells/ml and grown for 10 h to the log phase. For in vivo labelling, cells equivalent to 25 OD were harvested, resuspended in 5 ml of medium containing 1 μCi/ml 14C-palmitic acid (60 mCi/mmol), and incubated for 1 h at 30 °C. Labeled cells were harvested by centrifugation, washed 1× with 0.1% SDS and 3× with deionized water, and frozen in liquid nitrogen. Cells were broken with glass beads by vigorous shaking, and lipids were extracted with chloroform/methanol 2:1 (vol./vol.). Aliquots of the lipid extracts were applied onto silica gel plates and separated with chloroform/acetone/acetic acid (90:8:1, vol./vol./vol.) as the solvent system. Radioactivity was detected after exposure to radiosensitive screens by scanning with a Storm™ 860 scanner (GE Healthcare, Piscataway, NJ). For quantification, lipid fractions were scraped off the plates according to comigrating standards, and radioactivity was measured by liquid scintillation counting.
2.11. Statistical analysis
Statistical significance was determined by the Student's unpaired t test (two-tailed). Group differences are considered significant for p < 0.05 (*), p < 0.01 (**), and p < 0.001 (***).
3. Results
3.1. Identification of Yju3p as a monoacylglycerol lipase
A database search unveiled that yeast Yju3p (encoded by gene YKL094W) possesses more than 20% sequence identity to mammalian MAG lipases [18]. Yju3p consists of 313 amino acids and was predicted to be a serine hydrolase harboring a G-X-S-X-G motif and an α/β hydrolase fold, which are both characteristics for lipolytic enzymes [19]. Based on BLAST sequence homology analysis [20,21], the catalytic triad of Yju3p can be assigned to Ser123, Asp251, and His281 (Fig. 1).
Fig. 1.
Sequence alignment and structural comparison of human MGL (hMGL), mouse MGL (mMGL), and Yju3p. The sequences of MGL from human and mouse were aligned with the sequence from Yju3p using Clustal W2 [45]. Identical and highly similar residues are indicated with (*) or (:), respectively. A consensus sequence (cons.) was generated without turn-like residues as used in Bork et al. [46]. h, hydrophobic; a, aromatic; 1, aliphatic; 0, S/T; p, polar; c, charged; +, positive; −, negative; s, small; u, tiny. The conserved G-X-S-X-G motif and residues of the catalytic triad which are located on loop regions are highlighted in white and grey boxes, respectively. Secondary structure elements (helices: cylinders, strands: arrows) and residues within the cap-region (highlighted in italics) were assigned as observed in the crystal structure of hMGL [35].
To provide experimental evidence for the biological function of Yju3p as a MAG lipase, we tested cell extracts of the yju3Δ mutant and the wild-type strain for their activity in vitro. As shown in Fig. 2A, the mutant strain yju3Δ lacks detectable MGL activity at pH 7.5 against rac-1(3)-OG, and hydrolysis of 2-OG was reduced by ∼ 90% compared to wild-type. This demonstrates that Yju3p indeed functions as a MAG lipase in yeast. To investigate whether other lipases exist which are active at lower or higher pH, we performed MGH assays at different pH values. Wild-type cell lysates exhibited MGH activity over a broad pH spectrum (pH 4.5 to 8). In contrast, MGH activity was barely detectable in mutant lysates (Fig. 2B) demonstrating that Yju3p indeed represents the major monoacylglycerol lipase in yeast. To confirm Yju3p function, a His-tagged Yju3p fusion protein was expressed in E. coli and purified using affinity chromatography. The purified fusion protein with a molecular mass of 39 kDa hydrolyzed rac-1(3)-OG and 2-OG at similar rates (Fig. 2C). Saturation analysis of Yju3p activity revealed a Vmax of 10.3 mmol/h⋅mg (170 U/mg; Fig. 2D). The apparent Km of the enzyme was determined to be 260 μM which is close to the values reported for mouse and rat MGL (300 μM and 200 μM, respectively) [22]. No activity was observed using oleoyl-ethanolamine (OEA), diolein, or triolein as substrate (not shown). Together, these observations demonstrate that Yju3p specifically hydrolyzes MAG, and with a remarkably high specific activity.
Fig. 2.
Enzymatic properties of Yju3p. (A) MGH activity of wild-type and yju3Δ cell lysates. Samples were incubated with rac-1(3)-OG and 2-OG as substrates at pH 7.5. (B) pH dependence of MGH activities in wild-type (closed squares) and yju3Δ (open squares) cell lysates. Samples were incubated with rac-1(3)-OG as substrate. The following buffer systems were used: 75 mM sodium acetate buffer for pH 4 to 5.5; 75 mM potassium phosphate buffer for pH 6 to 7.5; 75 mM Tris–HCl buffer for pH 8 to 9. (C) Left panel: Purification of His-tagged recombinant Yju3p. His-tagged Yju3p was expressed in E. coli, and the protein was purified by affinity chromatography. Purity of the protein was determined by means of SDS–PAGE. Lane 1: Molecular weight protein standard. Lane 2: Yju3p after purification. Right panel: Stereospecificity of purified Yju3p. Purified Yju3p was incubated with rac-1(3)-OG and 2-OG as substrates at pH 7.5. (D) Saturation curve of Yju3p activity and Lineweaver-Burk plot (insert). The purified enzyme was incubated with rac-1(3)-OG as substrate at pH 7.5. All experiments were performed in triplicates and are representative of at least two independent experiments. Data are presented as mean ± SD. ***p < 0.001.
3.2. Subcellular distribution of Yju3p activity and heterologous expression of murine MGL in yeast
Yju3p was previously detected on purified lipid droplets (LD) by mass spectrometric analysis [10] and was found by fluorescence microscopy of a GFP fusion protein to localize both to LD and to the endoplasmic reticulum [23]. To investigate the cellular distribution of MGH activity, cells were fractionated into cytosol, membranes, and lipid droplets for activity assays. As shown in Fig. 3A, MGH activity was not detectable in the cytosolic fraction. Compared to whole cell lysates, the specific activity in membranes and lipid droplets was enriched 3- and 10-fold, respectively, consistent with the subcellular distribution of the protein as determined by GFP fluorescence microscopy [23]. Subcellular fractions of Yju3p-deficient cells exhibited almost no detectable activity.
Fig. 3.
MGH activities in subcellular fractions and localization of heterologous expressed murine MGL. (A) A yju3Δ strain overexpressing murine MGL as well as a wild-type strain and a yju3Δ strain harbouring the empty plasmid were fractionated into cytosol, membranes, and lipid droplets, and MGH activities were determined. Specific activities are expressed relative to the MGH activity of wild-type cell lysate. Measurements were performed in triplicates and are representative of two independent experiments. Data are presented as mean ± SD; n. det. indicates that samples contained no detectable activity. (B) A MGL–GFP fusion protein was overexpressed in yju3Δ, and localization was determined by laser scanning microscopy. Nile red staining of LDs was used to visualize the colocalization of the MGL–GFP fusion protein with LDs.
Next, we introduced murine MGL for heterologous expression into the yju3Δ strain and determined its activity in subcellular fractions (Fig. 3A). Interestingly, MGH activity was detectable only in whole cell lysates and the LD fraction but was completely absent from the cytosol and membranes. The almost exclusive localization of the heterologous enzyme to LD in the yju3Δ cells was confirmed by fluorescence microscopy using GFP-tagged MGL (Fig. 3B).
3.3. Yju3p deficiency causes MAG accumulation in vitro and can be complemented by the expression of murine MGL
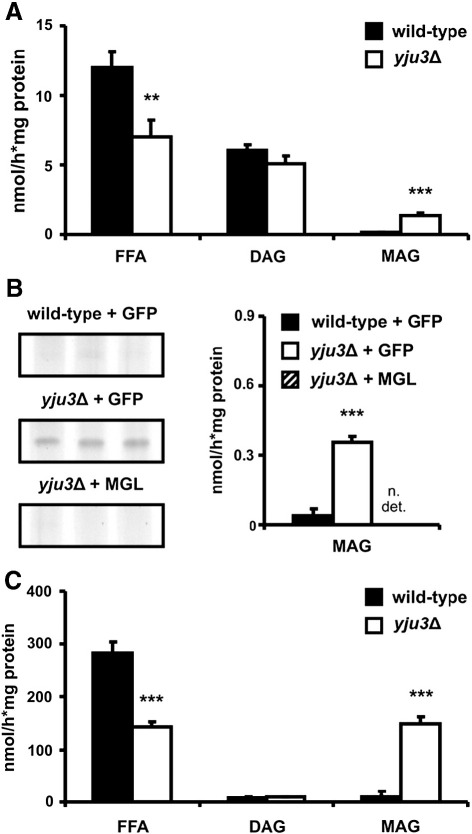
To investigate the role of Yju3p specifically in TAG hydrolysis, we incubated isolated LDs with radiolabeled TAG and separated the reaction products by TLC. Quantification of the reaction products by liquid scintillation counting revealed that FFA release in Yju3p-deficient LD preparations was decreased by 40%. DAG accumulated to equal levels in both strains and MAG levels were increased 10-fold in the absence of Yju3p (Fig. 4A). Overexpression of murine MGL in the yju3Δ strain prevented the accumulation of MAG in Yju3p-deficient preparations (Fig. 4B) demonstrating that Yju3p is the functional orthologue of murine MGL. To increase the formation of MAG, we added pancreatic lipase to yeast LD fractions. This mammalian lipase exhibits very low MGH activity in comparison to its capability of hydrolyzing TAG and DAG [24]. Addition of pancreatic lipase to wild-type LDs stimulated the lipolytic activity ∼ 30-fold and led to almost complete degradation of TAG into glycerol and FFA. In contrast, pancreatic lipase caused a massive accumulation of MAG in Yju3p-deficient preparations (Fig. 4C).
Fig. 4.
Yju3p deficiency causes MAG accumulation in vitro and can be complemented by the expression of murine MGL. Isolated LDs were incubated with radiolabeled trioleoylglycerol as substrate. Lipolytic products were extracted, separated by TLC. For quantification, FFA, DAG, and MAG comigrating with lipid standards was scraped off, and the radioactivity of samples was determined by liquid scintillation counting. (A) Generation of lipolytic products by LD preparations of wild-type and yju3Δ cells. (B) Left panel: TLC autoradiogram of MAG bands showing the signals obtained for wild-type and yju3Δ samples expressing GFP, and for yju3Δ samples expressing a GFP–MGL fusion protein. Right panel: Quantitation of MAG signals by liquid scintillation counting. (C) Generation of lypolytic products by LD preparations of wild-type and yju3Δ cells in the presence of pancreatic lipase. Measurements were performed in triplicates and are representative for at least two independent experiments. Data are presented as mean ± SD. **p < 0.01; ***p < 0.001; n. det., not detectable.
3.4. Dga1p exhibits DGAT and MGAT activity
In mammals, the acyl-CoA dependent re-esterification of MAG to DAG represents an important step in lipid metabolism. So far, acyl-CoA:monoacylglycerol acyltransferase (MGAT) activity has not been described for yeast, which could counteract MAG accumulation in Yju3p-deficient cells (see below). Since mammalian monoacylglycerol acyltransferase 3 (MGAT3) also displays DGAT activity [25], we hypothesized that the orthologous yeast DGAT enzyme, Dga1p, might possess dual MGAT and DGAT activity. Dga1p is an LD-associated protein and, together with Lro1p, is essential for efficient TAG synthesis in yeast [26]. Indeed, MGAT activity was decreased by 60% in Dga1p-deficient preparations, compared to the wild-type control (1.93 ± 0.30 and 0.77 ± 0.13 nmol/h⋅mg protein respectively, p < 0.01), indicating that Dga1p also catalyzes the esterification of MAG.
3.5. Catabolic and anabolic reactions affect MAG levels in vivo
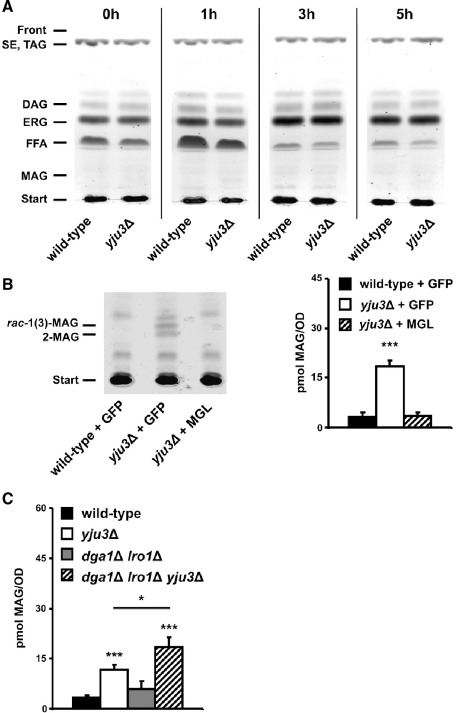
Our observations suggested that Yju3p complements the action of upstream lipases Tgl4p and Tgl3p in TAG and DAG hydrolysis and may also hydrolyze MAG derived from the degradation of phospholipids. To test whether Yju3p deficiency results in the accumulation of MAG in vivo, total lipids from wild-type and the yju3Δ mutant strain were extracted and analyzed by TLC. Yeast cells preferentially mobilize their TAG stores after transfer from a stationary culture into fresh glucose-containing medium [13]. Therefore, we focused our analysis on cells from lag-phase and early log-phase of growth (0–4 h of cultivation). As shown in Fig. 5A, DAG and FFA were readily detectable in both wild-type and mutant cells. In contrast, MAG were neither detectable in wild-type lipid extracts nor in extracts of Yju3p-deficient cells. To switch to a more sensitive detection system, cells were metabolically labelled with 14C-palmitic acid. Subsequently, lipids were extracted, subjected to TLC analysis, and exposed to a radiosensitive screen. Under these conditions, we were able to detect signals comigrating with MAG standards (Fig. 5B). Compared to the wild-type control, MAG levels were increased 5-fold in Yju3-deficient cells, which decreased to wild-type levels upon heterologous expression of murine MGL.
Fig. 5.
The effect of Yju3p deficiency on MAG levels in vivo. (A) Lipid analysis of wild-type and yju3Δ. Cells were harvested in different growth phases as indicated by the time points (0 h, stationary phase; 1 h and 3 h, lag phase; 5 h early log phase). Lipids were separated by TLC and visualized by charring at 120 °C. Lipids were identified by comigration with lipid standards. SE, steryl ester; ERG, ergosterol. (B) Detection of MAG in yju3Δ. Cells were labeled with 14C-palmitic acid, lipids were separated by TLC, and radioactivity was visualized by exposure to a radiosensitive screen. MAG was identified with comigrating lipid standards and the associated activity was quantified by liquid scintillation counting. Left panel: TLC autoradiogram showing MAG-associated signals of wild-type and yju3Δ expressing GFP, and of yju3Δ expressing a GFP-MGL fusion protein. Right panel: Quantitation of MAG signals by liquid scintillation counting. (C) MAG-associated activity of wild-type, yju3Δ, dga1Δ lro1Δ, and dga1Δ lro1Δ yju3Δ. Measurements were performed in triplicates and are representative for two independent experiments. Data are presented as mean ± SD. *p < 0.05; ***p < 0.001.
To test whether anabolic reactions affect MAG levels, we tested mutant cells lacking the acyltransferases Lro1p and Dga1p. Lro1p catalyzes the acyl transfer from phospholipids to DAG and was also shown to accept MAG as an acyl acceptor [27]. In the absence of both enzymes, TAG synthesis in yeast is virtually abolished [28]. Compared to wild-type cells, no significant changes in MAG levels were detected in dga1Δ lro1Δ double mutants. However, in the dga1Δ lro1Δ yju3Δ triple mutant, cellular MAG levels were increased by 60% compared to yju3Δ cells (Fig. 5C). This suggests that in the absence of Yju3p, MAG generated by the degradation of TAG or phospholipids are re-esterified to DAG and, subsequently, used for TAG or phospholipid synthesis.
4. Discussion
In this study, we identified Yju3p as the major yeast monoacylglycerol lipase sharing structural and functional homology with mammalian MGL. MGH activity of Yju3p was readily detectable in yeast lysates or in purified preparations of the enzyme. Deletion of the YJU3 gene abolished MGH activity in vitro using rac-1(3)-MAG as the substrate, whereas residual activity against 2-MAG remained detectable in the mutant. This observation suggests that at least one additional enzyme exists in yeast which preferentially hydrolyses MAG esterified at the sn-2 position.
Earlier studies identified Yju3p as a lipid droplet and membrane bound protein [10,23]. In accordance with these studies, we found Yju3p activity exclusively in isolated lipid droplet and membrane fractions, whereas cytosolic preparations did not exhibit detectable MGH activity. Interestingly, mouse GFP-MGL almost exclusively localized to LDs when expressed in yeast. To our knowledge, MGL was shown to bind to membrane structures and was never identified as LD-associated protein in mammalian cells so far. The presence of MGH activity on LDs suggests that TAG can be completely hydrolyzed into glycerol and FFA on the lipid droplet surface. In yeast, degradation of TAG is mainly catalyzed by the lipases Tgl3p and Tgl4p [13,29]. The lack of both enzymes causes a severe lipolytic defect and accumulation of TAG. Tgl3p was also reported to hydrolyze DAG [13] implicating that this enzyme is responsible for the generation of MAG. In mammalian cells, three enzymes are responsible for efficient TAG breakdown, namely ATGL, HSL, and MGL. Lack of ATGL and HSL activity in mice results in accumulation of TAG and DAG, respectively, in adipose tissue and in other tissues [30,31]. Currently, a mouse model lacking MGL does not exist. However, in vitro experiments and in vivo studies using a specific inhibitor for the enzyme demonstrate that MGL is required for efficient MAG hydrolysis [9,32,33].
Very recently, the three-dimensional structure of human MGL was determined [34,35] and revealed an α/β-hydrolase fold, as predicted. As observed in many members of the α/β-hydrolase fold family, the catalytic triad is covered by a cap (ranging from residues 151 to 225 in human MGL). The channel in proximity of the catalytic site allows accommodation of long lipid chains and widens towards the surface of the protein. In the case of human MGL, the cap region is of wide U-shaped architecture which is attributed to allow access to the catalytic site for a broad range of substrates. Notably, a surface-exposed hydrophobic region was identified in this lid region and is speculated to either facilitate localization on the membrane to get in contact with its lipophilic substrates. Although the corresponding residues are not highly conserved in Yju3p, the hydrophobic nature of this protein domain (Q165-V178) is well preserved.
Remarkably, MGH-specific activities measured in yeast preparations were more than 1000-fold higher compared to enzyme activities reported for the TAG lipases [11,13]. Moreover, LD-associated Yju3p efficiently prevented MAG accumulation in vitro when lipolysis was stimulated ∼ 30-fold by the addition of pancreatic lipase. This implies an overrepresentation of MGH activity on LDs. Mammalian MGL is also known to possess very high specific activity compared to other acylglycerol hydrolases. The reason for these high MGH activities in both mammalian cells and in yeast remains elusive. Presumably, MAGs are rapidly metabolized as soon as these lipid intermediates are formed. Reis et al. [36] suggested that 2-MAG can inhibit lipolysis by displacing lipases from the lipid–water interface due to an interfacial competition. However, we did not observe TAG accumulation or a substantial reduction in LD-associated lipolytic activity in Yju3p-deficient cells. MAGs were also shown to possess antimicrobial activity [37] and saturated monoacylglycerols are highly toxic when fed to mice [38]. Thus, it is reasonable to assume that high MGH activities are necessary to protect cells from toxic concentrations of MAG.
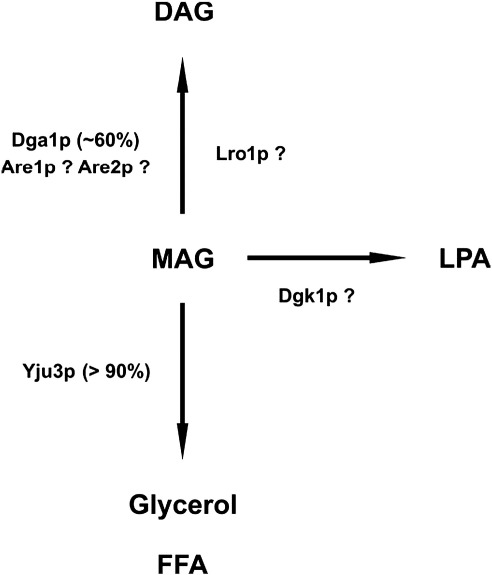
Interestingly, the lack of MGH activity was only associated with minor MAG accumulation in yju3Δ cells. We therefore considered the possibility that MAG may be secreted into the culture media, but this was not the case (data not shown). These observations suggest that alternative mechanisms exist for metabolizing MAG. In mammals, the acyl-CoA dependent esterification of MAG by MGATs is known to play an important role in lipid metabolism and energy homeostasis, but no such activity was reported for yeast before. Our data show that MGAT activity is clearly present in yeast and that Dga1p, the homologue of mammalian DGAT2 [26,39], mediates ∼ 60% of this activity in yeast. Are1p and Are2p might be responsible for the residual acyl-CoA-dependent acyltransferase activity [40]. It remains to be shown, however, whether these enzymes also accept MAG as a substrate. Lro1p, which catalyzes the transfer of an acyl group from phospholipids to diacylglycerol [28], might also play a role in MAG metabolism. In triple-mutant cells, lacking Yju3p, Dga1p, and Lro1p, MAG levels were increased compared to Yju3p-deficient cells, indeed suggesting that acyltransferase or transacylase reactions contribute to MAG metabolism in vivo. In addition, the diacylglycerol kinase Dgk1p [41], could potentially utilize MAG for the synthesis of lysophosphatidic acid, which is subsequently further acylated to phosphatidic acid and channeled into phospholipid or TAG metabolism. Thus, additional metabolic pathways exist, such as transacylation or phosphorylation, which allow an efficient metabolization of MAG (Fig. 6).
Fig. 6.
Hypothetical pathways of MAG metabolism. MAG is generated by the catabolism of phospholipids and TAG and can be further metabolized by several reactions. Yju3p catalyzes the hydrolysis of MAG to glycerol and FFA. In addition, MAG can be esterified to DAG in an acyl CoA-dependent manner by Dga1p. Furthermore, Lro1p, Are1p, and Are2p, which are known to be involved in neutral lipid synthesis, might contribute to the esterification of MAG. Finally, lipid kinases such as Dgk1p could mediate the phosphorylation of MAG to lysophosphatidic acid (LPA).
MGL was recognized in recent years as a potential player in EC metabolism [42]. 2-AG and anandamide (N-arachidonoylethanolamide; AEA) are considered as the main endogenous agonists of cannabinoid receptors [7]. Currently, it is not known whether MAG or N-acylethanolamines also possess a signaling function in yeast. Notably, several yeast proteins display homology to mammalian enzymes involved in the synthesis or in the degradation of 2-AG and AEA [43]. Yeast also expresses a phospholipase D encoded by the gene YPL103C, which cleaves N-acylphosphatidylethanolamine (NAPE) [44], the precursor of N-acylethanolamines. Thus, studying molecular mechanisms that control MAG and N-acylethanolamine metabolism in yeast might unveil new strategies for therapeutic interventions modulating the endocannabinoid system.
In summary, we have identified Yju3p as the functional orthologue of mammalian monoglyceride lipase in yeast. However, Yju3p-deficient cells accumulated MAG only at low concentrations implicating that other cellular activities exist, such as acyltransferase or phosphorylation reactions, which contribute to the metabolization of MAG. As Yju3p localizes to LDs and cellular membranes, we propose that it is required for efficient hydrolysis of MAG generated from the catabolism of TAG and glycerophospholipids. The absence of a scorable phenotype for the Yju3p-deficient mutant under multiple conditions is puzzling given its 1000-fold higher specific activity compared to other lipid hydrolases, such as Tgl3p or Tgl4p, and requires further investigation.
Acknowledgments
This research was supported by grants of the Austrian Science Funds, FWF, projects P21296 to R.Z. and P19041 to R.L., the DK Molecular Enzymology (W901-B05 DK) to S.D.K., M.O., and R.Z., and by the Austrian Federal Ministry for Science and Research, project “GOLD - Genomics of Lipid-Associated Disorders” in the framework of the Austrian Genome Project “GEN-AU Genome research in Austria” to R.L., S.D.K., M.O., and R.Z.
References
- 1.Chon S.H., Zhou Y.X., Dixon J.L., Storch J. Intestinal monoacylglycerol metabolism: developmental and nutritional regulation of monoacylglycerol lipase and monoacylglycerol acyltransferase. J. Biol. Chem. 2007;282:33346–33357. doi: 10.1074/jbc.M706994200. [DOI] [PubMed] [Google Scholar]
- 2.Yen C.L., Cheong M.L., Grueter C., Zhou P., Moriwaki J., Wong J.S., Hubbard B., Marmor S., Farese R.V., Jr Deficiency of the intestinal enzyme acyl CoA:monoacylglycerol acyltransferase-2 protects mice from metabolic disorders induced by high-fat feeding. Nat. Med. 2009;15:442–446. doi: 10.1038/nm.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang H., Eckel R.H. Lipoprotein lipase: from gene to obesity. Am. J. Physiol. Endocrinol. Metab. 2009;297:E271–E288. doi: 10.1152/ajpendo.90920.2008. [DOI] [PubMed] [Google Scholar]
- 4.Bezaire V., Langin D. Regulation of adipose tissue lipolysis revisited. Proc. Nutr. Soc. 2009;68:350–360. doi: 10.1017/S0029665109990279. [DOI] [PubMed] [Google Scholar]
- 5.Tornqvist H., Belfrage P. Purification and some properties of a monoacylglycerol-hydrolyzing enzyme of rat adipose tissue. J. Biol. Chem. 1976;251:813–819. [PubMed] [Google Scholar]
- 6.Karlsson M., Reue K., Xia Y.R., Lusis A.J., Langin D., Tornqvist H., Holm C. Exon–intron organization and chromosomal localization of the mouse monoglyceride lipase gene. Gene. 2001;272:11–18. doi: 10.1016/s0378-1119(01)00559-5. [DOI] [PubMed] [Google Scholar]
- 7.Di Marzo V. The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol. Res. 2009;60:77–84. doi: 10.1016/j.phrs.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Bari M., Battista N., Fezza F., Gasperi V., Maccarrone M. New insights into endocannabinoid degradation and its therapeutic potential. Mini Rev. Med. Chem. 2006;6:257–268. doi: 10.2174/138955706776073466. [DOI] [PubMed] [Google Scholar]
- 9.Long J.Z., Li W., Booker L., Burston J.J., Kinsey S.G., Schlosburg J.E., Pavon F.J., Serrano A.M., Selley D.E., Parsons L.H., Lichtman A.H., Cravatt B.F. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat. Chem. Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Athenstaedt K., Zweytick D., Jandrositz A., Kohlwein S.D., Daum G. Identification and characterization of major lipid particle proteins of the yeast Saccharomyces cerevisiae. J. Bacteriol. 1999;181:6441–6448. doi: 10.1128/jb.181.20.6441-6448.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Athenstaedt K., Daum G. YMR313c/TGL3 encodes a novel triacylglycerol lipase located in lipid particles of Saccharomyces cerevisiae. J. Biol. Chem. 2003;278:23317–23323. doi: 10.1074/jbc.M302577200. [DOI] [PubMed] [Google Scholar]
- 12.Athenstaedt K., Daum G. Tgl4p and Tgl5p, two triacylglycerol lipases of the yeast Saccharomyces cerevisiae are localized to lipid particles. J. Biol. Chem. 2005;280:37301–37309. doi: 10.1074/jbc.M507261200. [DOI] [PubMed] [Google Scholar]
- 13.Kurat C.F., Natter K., Petschnigg J., Wolinski H., Scheuringer K., Scholz H., Zimmermann R., Leber R., Zechner R., Kohlwein S.D. Obese yeast: triglyceride lipolysis is functionally conserved from mammals to yeast. J. Biol. Chem. 2006;281:491–500. doi: 10.1074/jbc.M508414200. [DOI] [PubMed] [Google Scholar]
- 14.Schweiger M., Schreiber R., Haemmerle G., Lass A., Fledelius C., Jacobsen P., Tornqvist H., Zechner R., Zimmermann R. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J. Biol. Chem. 2006;281:40236–40241. doi: 10.1074/jbc.M608048200. [DOI] [PubMed] [Google Scholar]
- 15.Gietz R.D., Schiestl R.H., Willems A.R., Woods R.A. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast. 1995;11:355–360. doi: 10.1002/yea.320110408. [DOI] [PubMed] [Google Scholar]
- 16.Wolinski H., Kohlwein S.D. Microscopic analysis of lipid droplet metabolism and dynamics in yeast. Meth. Mol. Biol. 2008;457:151–163. doi: 10.1007/978-1-59745-261-8_11. [DOI] [PubMed] [Google Scholar]
- 17.Schneiter R., Daum G. Extraction of yeast lipids. Meth. Mol. Biol. 2006;313:41–45. doi: 10.1385/1-59259-958-3:041. [DOI] [PubMed] [Google Scholar]
- 18.McPartland J.M., Matias I., Di Marzo V., Glass M. Evolutionary origins of the endocannabinoid system. Gene. 2006;370:64–74. doi: 10.1016/j.gene.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Baxter S.M., Rosenblum J.S., Knutson S., Nelson M.R., Montimurro J.S., Di Gennaro J.A., Speir J.A., Burbaum J.J., Fetrow J.S. Synergistic computational and experimental proteomics approaches for more accurate detection of active serine hydrolases in yeast. Mol. Cell. Proteomics. 2004;3:209–225. doi: 10.1074/mcp.M300082-MCP200. [DOI] [PubMed] [Google Scholar]
- 20.Altschul S.F., Madden T.L., Schaffer A.A., Zhang J., Zhang Z., Miller W., Lipman D.J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altschul S.F., Wootton J.C., Gertz E.M., Agarwala R., Morgulis A., Schaffer A.A., Yu Y.K. Protein database searches using compositionally adjusted substitution matrices. FEBS J. 2005;272:5101–5109. doi: 10.1111/j.1742-4658.2005.04945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karlsson M., Tornqvist H., Holm C. Expression, purification, and characterization of histidine-tagged mouse monoglyceride lipase from baculovirus-infected insect cells. Protein Expr. Purif. 2000;18:286–292. doi: 10.1006/prep.1999.1194. [DOI] [PubMed] [Google Scholar]
- 23.Kals M., Natter K., Thallinger G.G., Trajanoski Z., Kohlwein S.D. YPL.db2: the Yeast Protein Localization database, version 2.0. Yeast. 2005;22:213–218. doi: 10.1002/yea.1204. [DOI] [PubMed] [Google Scholar]
- 24.Lowe M.E. The triglyceride lipases of the pancreas. J. Lipid Res. 2002;43:2007–2016. doi: 10.1194/jlr.r200012-jlr200. [DOI] [PubMed] [Google Scholar]
- 25.Cheng D., Nelson T.C., Chen J., Walker S.G., Wardwell-Swanson J., Meegalla R., Taub R., Billheimer J.T., Ramaker M., Feder J.N. Identification of acyl coenzyme A:monoacylglycerol acyltransferase 3, an intestinal specific enzyme implicated in dietary fat absorption. J. Biol. Chem. 2003;278:13611–13614. doi: 10.1074/jbc.C300042200. [DOI] [PubMed] [Google Scholar]
- 26.Sorger D., Daum G. Synthesis of triacylglycerols by the acyl-coenzyme A:diacyl-glycerol acyltransferase Dga1p in lipid particles of the yeast Saccharomyces cerevisiae. J. Bacteriol. 2002;184:519–524. doi: 10.1128/JB.184.2.519-524.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghosal A., Banas A., Stahl U., Dahlqvist A., Lindqvist Y., Stymne S. Saccharomyces cerevisiae phospholipid:diacylglycerol acyl transferase (PDAT) devoid of its membrane anchor region is a soluble and active enzyme retaining its substrate specificities. Biochim. Biophys. Acta. 2007;1771:1457–1463. doi: 10.1016/j.bbalip.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Oelkers P., Tinkelenberg A., Erdeniz N., Cromley D., Billheimer J.T., Sturley S.L. A lecithin cholesterol acyltransferase-like gene mediates diacylglycerol esterification in yeast. J. Biol. Chem. 2000;275:15609–15612. doi: 10.1074/jbc.C000144200. [DOI] [PubMed] [Google Scholar]
- 29.Athenstaedt K., Daum G. The life cycle of neutral lipids: synthesis, storage and degradation. Cell. Mol. Life Sci. 2006;63:1355–1369. doi: 10.1007/s00018-006-6016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haemmerle G., Zimmermann R., Hayn M., Theussl C., Waeg G., Wagner E., Sattler W., Magin T.M., Wagner E.F., Zechner R. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J. Biol. Chem. 2002;277:4806–4815. doi: 10.1074/jbc.M110355200. [DOI] [PubMed] [Google Scholar]
- 31.Haemmerle G., Lass A., Zimmermann R., Gorkiewicz G., Meyer C., Rozman J., Heldmaier G., Maier R., Theussl C., Eder S., Kratky D., Wagner E.F., Klingenspor M., Hoefler G., Zechner R. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 32.Fredrikson G., Tornqvist H., Belfrage P. Hormone-sensitive lipase and monoacylglycerol lipase are both required for complete degradation of adipocyte triacylglycerol. Biochim. Biophys. Acta. 1986;876:288–293. doi: 10.1016/0005-2760(86)90286-9. [DOI] [PubMed] [Google Scholar]
- 33.Long J.Z., Nomura D.K., Cravatt B.F. Characterization of monoacylglycerol lipase inhibition reveals differences in central and peripheral endocannabinoid metabolism. Chem. Biol. 2009;16:744–753. doi: 10.1016/j.chembiol.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertrand T., Auge F., Houtmann J., Rak A., Vallee F., Mikol V., Berne P.F., Michot N., Cheuret D., Hoornaert C., Mathieu M. Structural basis for human monoglyceride lipase inhibition. J. Mol. Biol. 2010;396:663–673. doi: 10.1016/j.jmb.2009.11.060. [DOI] [PubMed] [Google Scholar]
- 35.Labar G., Bauvois C., Borel F., Ferrer J.L., Wouters J., Lambert D.M. Crystal structure of the human monoacylglycerol lipase, a key actor in endocannabinoid signaling. Chembiochem. 2010;11:218–227. doi: 10.1002/cbic.200900621. [DOI] [PubMed] [Google Scholar]
- 36.Reis P., Holmberg K., Miller R., Kragel J., Grigoriev D.O., Leser M.E., Watzke H.J. Competition between lipases and monoglycerides at interfaces. Langmuir. 2008;24:7400–7407. doi: 10.1021/la800531y. [DOI] [PubMed] [Google Scholar]
- 37.Kabara J.J., Vrable R. Antimicrobial lipids: natural and synthetic fatty acids and monoglycerides. Lipids. 1977;12:753–759. doi: 10.1007/BF02570908. [DOI] [PubMed] [Google Scholar]
- 38.Siddhanti S.R., Trumbo P.R., Schnitzer-Polokoff R., King M.W., Tove S.B. Toxicity of palmitoyl glycerol to mice: hypothermia and reversal of the toxicity. J. Nutr. 1987;117:1671–1675. doi: 10.1093/jn/117.10.1671. [DOI] [PubMed] [Google Scholar]
- 39.Oelkers P., Cromley D., Padamsee M., Billheimer J.T., Sturley S.L. The DGA1 gene determines a second triglyceride synthetic pathway in yeast. J. Biol. Chem. 2002;277:8877–8881. doi: 10.1074/jbc.M111646200. [DOI] [PubMed] [Google Scholar]
- 40.Zweytick D., Leitner E., Kohlwein S.D., Yu C., Rothblatt J., Daum G. Contribution of Are1p and Are2p to steryl ester synthesis in the yeast Saccharomyces cerevisiae. Eur. J. Biochem. 2000;267:1075–1082. doi: 10.1046/j.1432-1327.2000.01103.x. [DOI] [PubMed] [Google Scholar]
- 41.Han G.S., O'Hara L., Siniossoglou S., Carman G.M. Characterization of the yeast DGK1-encoded CTP-dependent diacylglycerol kinase. J. Biol. Chem. 2008;283:20443–20453. doi: 10.1074/jbc.M802866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dinh T.P., Carpenter D., Leslie F.M., Freund T.F., Katona I., Sensi S.L., Kathuria S., Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc. Natl Acad. Sci. USA. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muccioli G.G., Sia A., Muchowski P.J., Stella N. Genetic manipulation of palmitoylethanolamide production and inactivation in Saccharomyces cerevisiae. PLoS ONE. 2009;4:e5942. doi: 10.1371/journal.pone.0005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Merkel O., Schmid P.C., Paltauf F., Schmid H.H. Presence and potential signaling function of N-acylethanolamines and their phospholipid precursors in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta. 2005;1734:215–219. doi: 10.1016/j.bbalip.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 45.Larkin M.A., Blackshields G., Brown N.P., Chenna R., McGettigan P.A., McWilliam H., Valentin F., Wallace I.M., Wilm A., Lopez R., Thompson J.D., Gibson T.J., Higgins D.G. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 46.Bork P., Brown N.P., Hegyi H., Schultz J. The protein phosphatase 2C (PP2C) superfamily: detection of bacterial homologues. Protein Sci. 1996;5:1421–1425. doi: 10.1002/pro.5560050720. [DOI] [PMC free article] [PubMed] [Google Scholar]